Abstract
Secretory carcinoma of the thyroid gland is histologically and genetically similar to its mammary and salivary gland counterparts. Unlike differentiated thyroid carcinomas of follicular cell origin, thyroid SC is not a thyroglobulin-producing tumor and would not be amenable to radioactive iodine therapy. Instead, these carcinomas may respond to targeted therapy with TRK inhibitors, which further emphasizes the importance of their recognition among morphologically similar thyroid entities. Based on eleven cases reported to date, most primary thyroid SC tend to present as locally advanced malignancies and are characterized by frequent recurrences and long-term survival. High-grade histologic features, increased mitotic count and necrosis have been described but their impact on clinical course and outcome remains unclear. We hereby report the case of a primary SC with high-grade features arising in the thyroid of a 49-year-old man, who was treated with Larotrectinib for his second recurrence. The patient achieved a durable response that lasted for 18 months but then he continued to progress and died of disease 181 months after the diagnosis.
Supplementary Information
The online version contains supplementary material available at 10.1007/s12105-021-01386-6.
Keywords: Secretory carcinoma, Thyroid, ETV6-NTRK3, TRK inhibitor
Introduction
The thyroid gland has been long known to occasionally give rise to salivary gland tumors, such as mucoepidermoid carcinoma, sclerosing mucoepidermoid thyroid carcinoma with eosinophilia and pleomorphic adenoma [1–3]. Secretory carcinoma (SC) is a relatively recent addition to the list and is gaining attention as a possibly underrecognized and misdiagnosed primary thyroid malignancy. In 2016, Reynolds et al. reported the first case of primary thyroid SC and our group provided a detailed genetic, morphologic and clinical analysis of three case series [4, 5]. To date, a total of 11 cases have been reported in the literature [4–11]. SCs arising in any of their potential anatomic locations are genetically distinguished by the presence of a hallmark ETV6-NTRK3 gene rearrangement in the vast majority of cases. Histologically, primary thyroid SC resembles SC arising in other locations, namely salivary glands, and can display microcystic, cystic, cribriform, papillary, micropapillary and solid growth patterns [4–12]. The tumor cells exhibit eosinophilic, slightly granular to bubbly cytoplasm and are associated with the production of variably dense eosinophilic secretions. Considering that the histological features of SC can significantly overlap with those of papillary thyroid carcinoma (PTC), an immunohistochemical profile including positive S100 and mammaglobin stains and negative thyroglobulin is an essential pre-requisite for accurate pathologic diagnosis. Primary thyroid SC can arise in association with a PTC component, which can further complicate the cytologic and histologic diagnosis [5, 13, 14]. Clinically, thyroid SC occur in adult patients, typically presenting as locally aggressive, large masses with extrathyroidal extension and frequent lymph node metastases [4–11]. Though most thyroid SCs are characterized by long-term survival despite frequent recurrences, fatal outcome can occur in a minor proportion of cases [6, 9]. Various ETV6-NTRK3 fusion-positive cancers can respond to targeted therapy with TRK inhibitors, which further emphasizes the importance of an accurate pathologic diagnosis of SC [15]. We hereby report the case of a 49-year-old man with primary thyroid SC and long-term survival despite the presence of high-grade histologic features.
Case
Initial Presentation
The patient presented to us as a 49-year-old male, never-smoker with no relevant medical history, no prior cancer history, and no history of radiation seeking medical assistance for hoarseness and the left vocal cord paralysis. Ultrasound assessment showed a large solitary hypoechoic and heterogeneous infiltrating mass in the inferior aspect of the left lobe. The mass extended into the substernal space and measured approximately 6.0 × 3.9 × 3.9 cm. A chest X-ray showed the left thyroid mass to slightly displace the trachea to the right. On chest CT scan, the mass showed inferior extension into the superior mediastinum and invasion of the tracheal wall was suspected. Fine needle aspiration (FNA) of the left thyroid lobe mass was interpreted as “suspicious for PTC” and the subsequent left thyroid lobe core biopsy was diagnosed as adenocarcinoma. The patient first underwent left hemithyroidectomy and tracheal resection for a widely infiltrating tumor and the completion right hemithyroidectomy and laryngopharyngectomy were performed four months later. Pathology showed a tumor infiltrating skeletal muscle, tracheal cartilage, respiratory mucosa, as well as muscularis and lamina propria of the esophagus. The distal esophageal margin was focally positive for tumoral involvement. There was multifocal lymphovascular invasion with one positive paratracheal lymph node and twenty-five benign left neck lymph nodes. The tumors from both surgeries were interpreted as intermediate-grade adenocarcinoma of minor salivary gland originating from the trachea. Details on the clinical course are summarized in Table 1. Of note, the patient was discovered to have a right kidney mass and received right partial nephrectomy two years following the surgical management of the thyroid mass. The kidney tumor was found to represent an unrelated papillary renal cell carcinoma, type 1.
Table 1.
Clinical course
| Initial presentation | 6 years follow-up | 12 years follow-up | 14 years follow-up | |
|---|---|---|---|---|
| Clinical presentation | 6.0 cm left thyroid mass with extrathyroidal extension and tracheal invasion | Left neck subcutaneous/soft tissue nodule with distant metastases to the lung | Multiple new lung and neck metastases | Progression of cervical disease with development of spinal metastases |
| Laboratory tests | Normal serum TG (2.9 ng/mL); normal serum TSH (1.54 mcUnits/mL); normal free T4 (1.24 ng/mL) | Low serum TSH (0.48 mlU/L); serum TG not performed | Normal serum TSH (0.88 mlU/L); normal free T4 (1.47 ng/dL); serum TG not performed | High serum TSH (10.24 mlU/L); low free serum T3 (1.9 pg/mL) |
| Original pathologic diagnosis | Adenocarcinoma of minor salivary glands likely originating from the trachea | Metastatic salivary gland adenocarcinoma of probable tracheal origin | Metastatic SC of probable tracheal origin | Metastatic SC of probable tracheal origin |
| Revised pathologic diagnosis | SC of the thyroid | Metastatic SC of the thyroid | Metastatic SC of the thyroid | Metastatic SC of the thyroid |
| Stage | T4aN1M0 | |||
| Treatment | Initial left hemithyroidectomy with limited tracheal wall resection, followed by completion right thyroidectomy with laryngopharyngectomy and external beam RT (6000 cGy) | Therapeutic bilateral lung wedge resections and close surveillance of recurrent cervical disease | First generation TRK inhibitor (Larotrectinib) | RT Investigational second generation TRK inhibitor (5 months) followed by chemotherapy |
RT Radiation therapy
Pathology
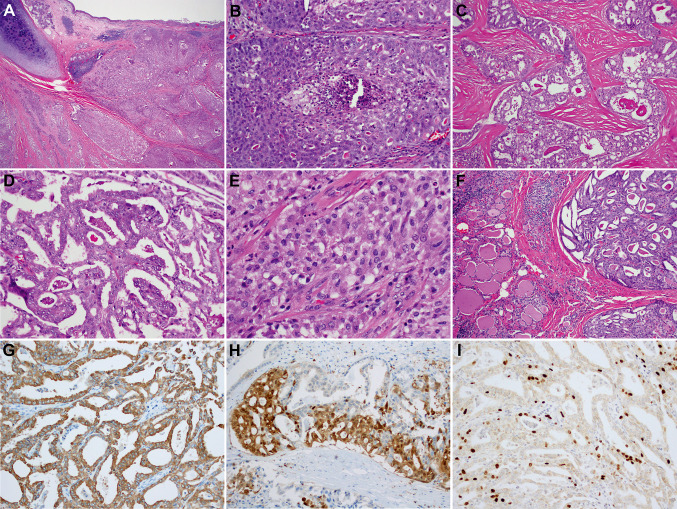
The initial left thyroid lobe core biopsy showed a cribriform, and focally micropapillary, infiltrative malignant epithelial process and was diagnosed as adenocarcinoma. The tumor resected on the left thyroidectomy was interpreted as an intermediate-grade adenocarcinoma measuring at least 1.9 cm and postulated to have originated from tracheal minor salivary glands. In fact, most of the tumor involved peri-thyroidal fibrous tissue and skeletal muscle. The tumor was infiltrative, comprised of cribriform, microcystic and solid areas surrounded by dense fibrotic and hyalinized stroma. Epithelial tufting and papillae were also present. The tumor cells showed a variable eosinophilic to vacuolated cytoplasm, mildly enlarged nuclei with open chromatin, subtle nuclear membrane irregularities and conspicuous nucleoli. Although occasional nuclear grooves were noted, pseudoinclusions were not identified. Foci of tumor comedo-type necrosis were present in nested and solid areas. Three mitotic figures per 10 high power fields (400× magnification) were noted and Ki-67 proliferation index labeled around 10% of tumor nuclei. There was no associated PTC component. Apart for mild chronic inflammation near the tumor periphery, the adjacent thyroid parenchyma was unremarkable, without chronic lymphocytic thyroiditis or nodular hyperplasia (Fig. 1). An extensive immunohistochemical work-up performed at the time of diagnosis showed the tumor to be positive for cytokeratins, S100 and mammaglobin, while negative for PAX8 and TTF1, among others (Table S1).
Fig. 1.
Morphology and immunophenotype of primary thyroid SC with high-grade features. At the invasive front, the tumor formed solid sheets and nests and invaded the tracheal cartilage and respiratory mucosa (A, 40x). Multiple foci of comedo-type necrosis were identified (B, 200x). Cribriform areas with dense pink-red luminal secretions were divided by thick, dense, hypocellular and hyalinized stroma (C, 100x). Areas of epithelial tufting, papillae and bridges were noted (D, 200x). Tumor cells had ample amount of delicate eosinophilic vacuolated cytoplasm, mildly enlarged nuclei with open chromatin, and conspicuous nucleoli (E, 400x). Except for the minimal chronic infiltrate at the interface with the tumor, the adjacent thyroid parenchyma was unremarkable (F, 200x). SC was positive for mammaglobin (G, 200x) and S100 protein (H, 200x). Proliferation index labeled by Ki-67 was about 10% (I, 200x)
First Recurrence
Six years following surgical removal, the patient was confirmed to have regional recurrence to the left neck and distant metastases to the lung, which were histologically similar to the primary thyroid tumor. Five years following the first recurrence, the lung metastasis was profiled by MSK-IMPACT™ assay and ETV6-NTRK3 rearrangement was detected. At that time, the pathology slides were retrospectively reviewed, and the tumor was found to be compatible with SC of probable tracheal origin.
Second Recurrence and Progression of Disease
Twelve years post initial diagnosis, new lung and neck metastases were identified. At that time, the patient was placed on systemic therapy with a TRK inhibitor (Larotrectinib), which resulted in clinically and radiographically improved neck disease [15]. The treatment provided relatively durable clinical benefit for approximately 18 months. However, the patient thereafter complained of increased neck pain on the account of cervical disease progression with new-onset spinal lesions. A repeat biopsy of the left neck lymph node was performed and confirmed the diagnosis of recurrent disease. The material was forwarded to the molecular diagnostic pathology lab with the aim of identifying potential resistance mechanisms but was deemed insufficient for molecular profiling. He received radiation therapy. An investigational second generation TRK inhibitor was then administered and initially led to disease stabilization. Of note, the last five years of his disease course were also marked by multiple complications including esophageal stricture, epidural abscess and jaw trauma, all of which required surgical intervention. After 5 months of treatment with a second generation TRK inhibitor, he continued to progress and was placed on chemotherapy. However, there was no evidence of further improvement and the patient succumbed to his disease 15 years and one month after the first diagnosis.
Molecular Diagnosis
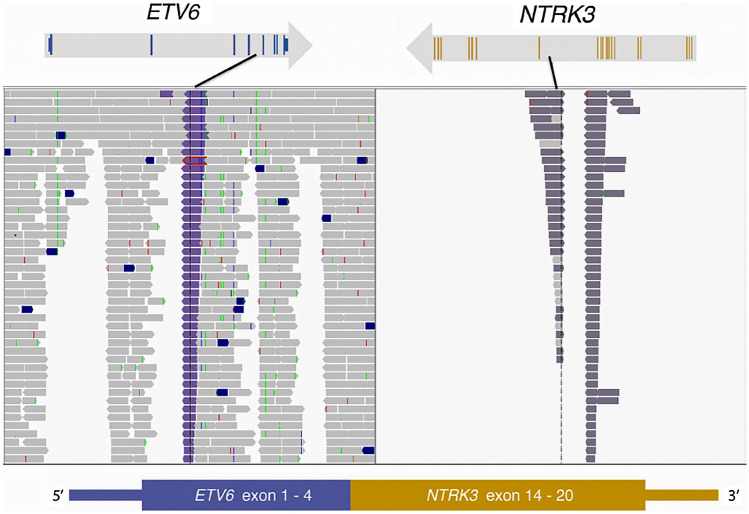
Targeted exome sequencing assay (MSK-IMPACT™) was performed on the lung metastasis and revealed an in-frame ETV6-NTRK3 fusion involving ETV6 exons 1–4 and NTRK3 exons 14–20 (Fig. 2) [16, 17]. A few other concurrent variants were detected including ETV6 R399Pfs*26 and NKX2-1 Y215_L216del variants. ETV6-NTRK3 fusion was further confirmed by MSK-Fusion (Archer) assay [18].
Fig. 2.
MSK-IMPACT assay was performed on the lung metastasis: exons 1–4 of ETV6 gene are fused with the exons 14–20 of NTRK3 gene resulting in ETV6–NTRK3 fusion
Summary of the Literature
The clinicopathologic characteristics of reported primary thyroid SC cases in the literature are summarized in Table 2. This cumulative group of 12 primary thyroid SC cases (11 published cases plus the current case) shows this cancer occurs in adults at the median age of 56.5 years (range 36–74), more often in women (9/12, 75%) than in men (3/12, 25%). Tumors are relatively large with an average size of 4.5 cm (range 2.4–7.6 cm) and mostly present as T3 or T4 stage tumors (9/12, 75%) as per eight edition of the American Joint Committee on Cancer (AJCC) staging manual for follicular-cell derived thyroid carcinomas [19]. More than half of the patients (7/12, 58%) had lymph node metastases at presentation and 42% of patients (5/12) developed distant metastases during the course of disease; mostly in the lungs (n = 4) and liver (n = 3), followed by bone, kidney and soft tissue (n = 1, each). Median follow-up time was 29.5 months (range, 1–238 months). Four patients (33.3%) died of disease, one (17.6%) patient dead of other causes and 6 (50%) were alive with no evidence of disease at the last follow-up. Mitotic count ranged from 1 to 10 mitoses (median 2) per 10 HPF. Most cases (80%) had 1–3 mitoses per 10 HPF, one case had 7 mitoses per 10 HPF and another had 10 mitoses per 10 HPF, with the latter corresponding to a patient who died of disease after 12 months. Tumor necrosis was noted in 40% (4/10) of cases, three of which occurred in patients who died of disease.
Table 2.
Clinicopathologic characteristics of patients with primary thyroid SC
| Year | Author | Age (years) | Sex | Size (cm) | Clinical presentation | Mitoses | Necrosis | Stage | Margins | Distant metastases | Local or regional recurrence | Outcome | Follow-up (months) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2016 | Reynolds | 36 | F | 4.5 | Bilateral thyroid mass with enlarged neck LNs and mediastinal extension | 1/30 HPF | Absent | T4aN1 | Positive | Yes, at recurrence (soft tissue, lung and liver) | Regional recurrence × 2 | DOD | 107 |
| 2016 | Dogan | 72 | F | 2.9 | Right thyroid mass with gross ETE and tracheal invasion | 1/10 HPF | Absent | T4aN0 | Focally positive | No | Regional recurrence × 2 | DOOC | 238 |
| 47 | M | 7.0 | Left thyroid mass with gross ETE and enlarged left neck LN | 1 per 10 HPF | Absent | T3N1b | Negative | No | Regional recurrence × 3 | NED | 230 | ||
| 65 | F | 6.5 | Isthmic thyroid mass with ETE and tracheal invasion | 1/10 HPF | Absent | T4aN0 | Positive | No | None | NED | 24 | ||
| 2017 | Dettloff | 52 | F | 2.4 | Left thyroid mass, No gross ETE | 3/10 HPF | Absent | T2N1a | NS | No | None | NED | 26 |
| 55 | F | 2.6 | Infiltrative thyroid mass involving perilaryngeal soft tissues | 2/10 HPF | Absent | T3N1b | NS | No | None | NED | 43 | ||
| 74 | M | 4.0 | Right thyroid mass, No gross ETE | 10/10 HPF | Present | T2N1b | NS | Yes, at recurrence (liver) | None | DOD | 12 | ||
| 2017 | Common case to Wu and Liao | 58 | F | 4.0 | Left thyroid mass with gross ETE, tracheal involvement and left recurrent laryngeal nerve impingement causing progressive hoarseness | 7/10 HPF | Present | T4aN0 | Positive | No | None | NED | 14 |
| 2018 | Asa | 72 | F | 2.5 | Left thyroid mass with gross ETE | NS | NS | T3N0 | NS | No | None | NED | 18 |
| 2019 | Desai | 74 | F | 7.6 | Thyromegaly with progressive dyspnea requiring intubation, ETE with tracheal compression | 2/10 HPF | Present | T4bN1b | Positive | Yes, at diagnosis (liver, lung, kidney, bone) | None (DOD) | DOD | 20 days |
| 2020 | Huang | 36 | F | 5.0 | Right thyroid mass with tumor thrombus in jugular vein | NS | NS | T3aN0 | Yes, 11 months later (lung) | None | AWD | 33 | |
| 2021 | Current case | 49 | M | 4.7 | Presented for hoarseness, found to have a left thyroid mass with left recurrent nerve and tracheal involvement | 3/10 HPF | Present | T4Nx | Focally positive | Yes, at recurrence (lung) | Regional recurrence × 3 | DOD | 181 |
NS Not specified, AWD Alive with disease, DOD Died of disease, DOOC Died of other causes, NED No evidence of disease, ETE Extrathyroidal extension, LN Lymph node
Discussion
We hereby report the case of a 49-year-old man with a long history of primary thyroid SC. Consistent with the prior reports this case was characterized by an extensive, locally aggressive disease and a prolonged clinical course with multiple recurrences [4, 5]. Despite the lacking thyroid markers, in view of the similarities to the previously reported thyroid SC [4–6, 8, 9]. The clinical and morphologic features in the current case were consistent with the primary thyroid origin. After the treatment with TRK inhibitor, the patient achieved a durable response but experienced disease progression after 18 months.
Following the FNA, the diagnostic work-up was performed to rule out PTC in the resected tumor. The absence of the associated PTC component, a lack of TTF-1 and PAX-8 immunoexpression and a large portion of tumor occupying the extrathyroidal space were all contributing elements leading to the initial diagnostic interpretation of adenocarcinoma, likely originating from the tracheal minor salivary glands. However, while we cannot entirely exclude the aforementioned possibility, the clinical presentation is rather consistent with a primary thyroid SC. In fact, imaging showed a primary thyroid-based tumor deviating the trachea to the contralateral side, rather than obstructing the tracheal lumen. No intraluminal endotracheal tumor growth was identified, as would normally be expected in primary tracheal neoplasms originating form submucosal minor salivary glands [20]. Primary adenoid cystic carcinomas of the trachea, for instance, typically present as an intraluminal mass and have a tendency to mimic obstructive pulmonary disorders [21]. Primary mucoepidermoid carcinomas appear as sharply demarcated, ovoid or lobulated intraluminal nodules that adapt to the branching features of the airways [20]. Our case, on the other hand, is rather consistent with the compression and infiltration of the trachea by an extrinsic tumor. Worth mentioning, primary SC of the pulmonary airway has yet to be documented. Prior studies on SC involving the thyroid have not been entirely definitive on whether this lesion primarily arose from the thyroid and suggested ectopic intrathyroidal/adjacent to thyroid minor salivary gland tissue as a possible cell of origin [8, 22]. However, no ectopic breast or salivary tissue was identified within/around these tumors to further support this hypothesis. Furthermore, should these cases truly arise from ectopic salivary gland tissue, one would expect both TTF-1 and PAX8 to be consistently negative. Previous reports have demonstrated the possibility of limited staining for TTF-1 and/or PAX8 [5, 6, 9]. Finally, the reported SC cases arising in association with PTC component could support the thyroid follicular-cell origin suggesting a divergent differentiation into a salivary gland phenotype [5]. This was further supported by both components sharing the ETV6-NTRK fusion and by a coexistence of both, PTC and SC components in a lymph node metastasis [5].
As for mammary SC, SC of salivary glands has been reported to be an indolent tumor with an overall good prognosis and prolonged disease-free survival [23]. In fact, with the exception of the rare cases that underwent high-grade transformation, the majority of salivary gland SCs typically behave as low grade carcinomas with overall low local recurrence and distant metastasis rate [12, 23]. Despite the genetic, morphologic and immunohistochemical similarities between SC arising in the breast and those arising in the salivary glands, primary thyroid SC stands apart by its higher tendency to present as locally aggressive malignancy, with frequent locoregional and distant metastases [4–9, 11]. This fact lends support to the possibility that the biological behavior of SC is determined by its anatomic site/cell of origin.
Although histologically SC is more likely to be confused with a PTC, its clinical presentation is rather unusual for most well-differentiated thyroid carcinomas. Of the eleven primary thyroid SCs reported in the literature, nine patients presented with compressive symptoms caused by tumors exhibiting gross extrathyroidal extension (staged T3b or more as per AJCC. 8th ed.), with most measuring 4 cm or more in greatest dimension [4–9, 11, 19]. In contrast, only 10.9% of papillary thyroid carcinomas showed gross extrathyroidal extension in one study on 596 patients [24]. Therefore, in terms of clinical and radiologic assessment, primary SC of the thyroid is more likely to be mistaken for a poorly differentiated thyroid carcinoma (PDTC) or anaplastic thyroid carcinoma, in which invasion of central neck structures is much more common than in well-differentiated follicular cell-derived thyroid carcinomas [25, 26].
Even though most primary SCs of the thyroid show a protracted disease progression, some cases assume a significantly more aggressive clinical course [4, 6, 9]. Given the small number of reported cases as well as the variability of follow-up period length and treatment approach, it is difficult to do more than speculate on the prognostic significance of clinicopathologic parameters. The high recurrence rate observed in these tumors is, in part, due to their invariably infiltrative nature, making complete surgical removal and negative surgical margins difficult to achieve. One other contributing factor would be the initial administration of inadequate systemic therapy (such as radioactive iodine) due to misdiagnosis and confusion with PTC. Alternatively, one could ponder on the significance of high-grade histologic features such as increased mitotic count and/or necrosis. It seems reasonable to suspect that the presence of these high-grade features in thyroid SCs would predict more aggressive biologic behavior, similar to what has been demonstrated in their salivary gland counterparts or in other thyroid carcinomas of follicular cell origin [12, 27]. Although the numbers are small, two out of three reported patients who died of disease within one year of diagnosis harbored tumors with focal necrosis and/or increased mitotic activity (10/10 HPF). Nevertheless, the case presented here suggests that the mere presence of necrosis may not necessarily portend a dismal prognosis and short-term survival. We speculate that the combination of necrosis and advanced patient age could raise a red flag for more aggressive biologic behavior and may possibly be associated with poorer outcome. Both patients who died of disease within one year of diagnosis had tumors with necrosis, and both were elderly (74 years old) [6, 9]. It could be that the overall biology of primary thyroid SC mirrors that of other follicular cell-derived thyroid tumors, in the sense that the patient’s age plays a significant role in the overall outcome [28, 29].
An accurate diagnosis of thyroid SC is as important as it is rare, both for prognostication and therapeutic purposes. Considering the tumor’s intrinsic propensity for local aggressiveness, regional spread and potentially fatal outcome, a misdiagnosis will undermine the gravity of the disease. Furthermore, an incorrect diagnosis of either PTC or PDTC could lead to unnecessary radioactive iodine therapy, on one hand, and to a missed opportunity for TRK inhibitor targeted therapy, on the other [4, 30]. Several diagnostic approaches are used in modern pathology practice for molecular characterization of SC. Most FISH assays utilize ETV6 break-apart probes and this may be sufficient for a mere confirmation of SC pathologic diagnosis. However, should the patient become a candidate for TKR-inhibitor therapy, the fusion kinase gene must be detected. This can be achieved by FISH using NTRK3 probes, by RNA-based assays which can identify both fusion gene partners, such as MSK-Fusion (Archer) assay, or by select DNA-based NGS assays, such as MSK-IMPACT [5]. In recurrent SC, however, resistance mutations are commonly single nucleotide variants and these are detected DNA-based molecular assays, such as MSK-IMPACT.
Various cancers harboring ETV6-NTRK3 fusion show durable response to TRK inhibitors, this was also demonstrated in the current case [15, 31]. Many of these patients will eventually experience a relapse due to acquired molecular mechanisms of resistance to a first-generation inhibitor (Larotrectinib, Entrectinib) [30]. Nevertheless, the availability of second generation TRK inhibitors may represent an effective treatment alternative for cases with NTRK on-target resistance mutations. Other potential mechanisms of resistance such as off-target somatic alterations in BRAF, KRAS or MET might require a different treatment approach [30, 32]. Therefore, molecular profiling of the recurrent/metastatic tumor is essential to identify potential mechanisms of resistance and to select the best targeted treatment alternative for those patients. Unfortunately, in the case we present here, the biopsy material on the tumor recurrence was insufficient for molecular profiling and the potentially new, acquired mutation status remained unknown.
To summarize, thyroid SC is a rare type of primary thyroid carcinoma, which should be considered in the differential diagnosis of thyroid based tumors with non-classical morphologic features, especially in those with advanced locoregional spread at presentation. These cases can easily be misinterpreted as PTC or as an adenocarcinoma of tracheal origin secondarily involving the thyroid gland. High-grade histologic features i.e. tumor necrosis, are not uncommon and they alone may not necessarily indicate a relatively more aggressive clinical course. Larger studies are warranted to confidently assess the prognostic impact of high-grade histologic tumor features and that of other clinicopathological parameters. While an adequate recognition of primary thyroid SC will prevent the administration of ineffective treatment options and make these cases amenable for TRK targeted therapy, molecular assessment of each subsequent thyroid SC recurrence treated with TRK inhibitors would be needed to guide optimal treatment strategies.
Supplementary Information
Below is the link to the electronic supplementary material.
Funding
Supported by the Memorial Sloan Kettering Cancer Center Support Grant of the National Institutes of Health/National Cancer Institute under award number P30CA008748.
Data availability
Not applicable.
Code availability
Not applicable.
Declarations
Conflict of interest
None.
Ethical approval
The study was approved by the Institutional Review Board at MSKCC.
Consent to participate
Yes.
Consent for publication
Yes.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Chan JK, Albores-Saavedra J, Battifora H, Carcangiu ML, Rosai J. Sclerosing mucoepidermoid thyroid carcinoma with eosinophilia. A distinctive low-grade malignancy arising from the metaplastic follicles of Hashimoto’s thyroiditis. Am J Surg Pathol. 1991;15(5):438–48. doi: 10.1097/00000478-199105000-00003. [DOI] [PubMed] [Google Scholar]
- 2.Rhatigan RM, Roque JL, Bucher RL. Mucoepidermoid carcinoma of the thyroid gland. Cancer. 1977;39(1):210–214. doi: 10.1002/1097-0142(197701)39:1<210::AID-CNCR2820390133>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 3.Levy GH, Marti JL, Cai G, Kayne RD, Udelsman R, Hammers LW, et al. Pleomorphic adenoma arising in an incidental midline isthmic thyroid nodule: a case report and review of the literature. Hum Pathol. 2012;43(1):134–137. doi: 10.1016/j.humpath.2011.02.025. [DOI] [PubMed] [Google Scholar]
- 4.Reynolds S, Shaheen M, Olson G, Barry M, Wu J, Bocklage T. A case of primary mammary analog secretory carcinoma (MASC) of the thyroid masquerading as papillary thyroid carcinoma: potentially more than a one off. Head Neck Pathol. 2016;10(3):405–413. doi: 10.1007/s12105-016-0715-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dogan S, Wang L, Ptashkin RN, Dawson RR, Shah JP, Sherman EJ, et al. Mammary analog secretory carcinoma of the thyroid gland: A primary thyroid adenocarcinoma harboring ETV6-NTRK3 fusion. Mod Pathol. 2016;29(9):985–995. doi: 10.1038/modpathol.2016.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dettloff J, Seethala RR, Stevens TM, Brandwein-Gensler M, Centeno BA, Otto K, et al. Mammary analog secretory carcinoma (MASC) involving the thyroid gland: a report of the first 3 cases. Head Neck Pathol. 2017;11(2):124–130. doi: 10.1007/s12105-016-0741-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wu EY, Lebastchi J, Marqusee E, Lorch JH, Krane JF, Barletta JA. A case of primary secretory carcinoma of the thyroid with high-grade features. Histopathology. 2017;71(4):665–669. doi: 10.1111/his.13268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Asa SL, Mete O. An unusual salivary gland tumor mimicking papillary thyroid carcinoma: mammary analog secretory carcinoma. Front Endocrinol (Lausanne) 2018;9:555. doi: 10.3389/fendo.2018.00555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Desai MA, Mehrad M, Ely KA, Bishop JA, Netterville J, Aulino JM, et al. Secretory carcinoma of the thyroid gland: report of a highly aggressive case clinically mimicking undifferentiated carcinoma and review of the literature. Head Neck Pathol. 2019;13(4):562–572. doi: 10.1007/s12105-018-0995-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liao H, Khan A, Miron PM, Cornejo KM. Mammary analogue secretory carcinoma of the thyroid mimicking locally advanced papillary thyroid carcinoma: a rare case report. Int J Surg Pathol. 2018;26(5):459–463. doi: 10.1177/1066896917747076. [DOI] [PubMed] [Google Scholar]
- 11.Huang NS, Cao YM, Lu ZW, Guan Q, Chen JY, Ma B, et al. Mammary analog secretory carcinoma of the thyroid gland: a rare cancer harboring TRK fusion. Oral Oncol. 2021;115:105092. doi: 10.1016/j.oraloncology.2020.105092. [DOI] [PubMed] [Google Scholar]
- 12.Skálová A, Vanecek T, Majewska H, Laco J, Grossmann P, Simpson RH, et al. Mammary analogue secretory carcinoma of salivary glands with high-grade transformation: report of 3 cases with the ETV6-NTRK3 gene fusion and analysis of TP53, β-catenin, EGFR, and CCND1 genes. Am J Surg Pathol. 2014;38(1):23–33. doi: 10.1097/PAS.0000000000000088. [DOI] [PubMed] [Google Scholar]
- 13.Rupp AP, Bocklage TJ. Mammary analog secretory carcinoma of thyroid: a case report. Diagn Cytopathol. 2017;45(1):45–50. doi: 10.1002/dc.23608. [DOI] [PubMed] [Google Scholar]
- 14.Rodríguez-Urrego PA, Dogan S, Lin O. Cytologic findings of mammary analogue secretory carcinoma arising in the thyroid. Diagn Cytopathol. 2017;45(6):552–556. doi: 10.1002/dc.23692. [DOI] [PubMed] [Google Scholar]
- 15.Drilon A, Laetsch TW, Kummar S, DuBois SG, Lassen UN, Demetri GD, et al. Efficacy of larotrectinib in TRK fusion-positive cancers in adults and children. N Engl J Med. 2018;378(8):731–739. doi: 10.1056/NEJMoa1714448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cheng DT, Mitchell TN, Zehir A, Shah RH, Benayed R, Syed A, et al. Memorial sloan kettering-integrated mutation profiling of actionable cancer targets (MSK-IMPACT): a hybridization capture-based next-generation sequencing clinical assay for solid tumor molecular oncology. J Mol Diagn. 2015;17(3):251–264. doi: 10.1016/j.jmoldx.2014.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zehir A, Benayed R, Shah RH, Syed A, Middha S, Kim HR, et al. Mutational landscape of metastatic cancer revealed from prospective clinical sequencing of 10,000 patients. Nat Med. 2017;23(6):703–713. doi: 10.1038/nm.4333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Benayed R, Offin M, Mullaney K, Sukhadia P, Rios K, Desmeules P, et al. High yield of RNA sequencing for targetable kinase fusions in lung adenocarcinomas with no mitogenic driver alteration detected by DNA sequencing and low tumor mutation burden. Clin Cancer Res. 2019;25(15):4712–4722. doi: 10.1158/1078-0432.CCR-19-0225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Surgeons ACo. AJCC Cancer Staging Manual. 8 ed: Springer International Publishing; 2017. XVII, 1032 p.
- 20.Elnayal A, Moran CA, Fox PS, Mawlawi O, Swisher SG, Marom EM. Primary salivary gland-type lung cancer: imaging and clinical predictors of outcome. AJR Am J Roentgenol. 2013;201(1):W57–63. doi: 10.2214/AJR.12.9579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Singh M, Sharma M, Bhardwaj M, Gupta P, Ahuja A. Primary salivary gland malignancy of trachea: a clinical masquerader. J Clin Diagn Res. 2016;10(9):ed26–ed7. doi: 10.7860/JCDR/2016/21735.8589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stevens TM, Kovalovsky AO, Velosa C, Shi Q, Dai Q, Owen RP, et al. Mammary analog secretory carcinoma, low-grade salivary duct carcinoma, and mimickers: a comparative study. Mod Pathol. 2015;28(8):1084–1100. doi: 10.1038/modpathol.2015.64. [DOI] [PubMed] [Google Scholar]
- 23.Chiosea SI, Griffith C, Assaad A, Seethala RR. Clinicopathological characterization of mammary analogue secretory carcinoma of salivary glands. Histopathology. 2012;61(3):387–394. doi: 10.1111/j.1365-2559.2012.04232.x. [DOI] [PubMed] [Google Scholar]
- 24.Danilovic DLS, Castroneves LA, Suemoto CK, Elias LO, Soares IC, Camargo RY, et al. Is there a difference between minimal and gross extension into the strap muscles for the risk of recurrence in papillary thyroid carcinomas? Thyroid. 2020;30(7):1008–1016. doi: 10.1089/thy.2019.0753. [DOI] [PubMed] [Google Scholar]
- 25.Xu B, Fuchs T, Dogan S, Landa I, Katabi N, Fagin JA, et al. Dissecting anaplastic thyroid carcinoma: a comprehensive clinical, histologic, immunophenotypic, and molecular study of 360 cases. Thyroid. 2020;30(10):1505–1517. doi: 10.1089/thy.2020.0086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ibrahimpasic T, Ghossein R, Carlson DL, Chernichenko N, Nixon I, Palmer FL, et al. Poorly differentiated thyroid carcinoma presenting with gross extrathyroidal extension: 1986–2009 Memorial Sloan-Kettering Cancer Center experience. Thyroid. 2013;23(8):997–1002. doi: 10.1089/thy.2012.0403. [DOI] [PubMed] [Google Scholar]
- 27.Hiltzik D, Carlson DL, Tuttle RM, Chuai S, Ishill N, Shaha A, et al. Poorly differentiated thyroid carcinomas defined on the basis of mitosis and necrosis: a clinicopathologic study of 58 patients. Cancer. 2006;106(6):1286–1295. doi: 10.1002/cncr.21739. [DOI] [PubMed] [Google Scholar]
- 28.Gilliland FD, Hunt WC, Morris DM, Key CR. Prognostic factors for thyroid carcinoma. A population-based study of 15,698 cases from the Surveillance, Epidemiology and End Results (SEER) program 1973–1991. Cancer. 1997;79(3):564–73. doi: 10.1002/(SICI)1097-0142(19970201)79:3<564::AID-CNCR20>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 29.Kauffmann RM, Hamner JB, Ituarte PHG, Yim JH. Age greater than 60 years portends a worse prognosis in patients with papillary thyroid cancer: should there be three age categories for staging? BMC Cancer. 2018;18(1):316. doi: 10.1186/s12885-018-4181-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Drilon A. TRK inhibitors in TRK fusion-positive cancers. Ann Oncol. 2019;30(Suppl_8):viii23-viii30. [DOI] [PMC free article] [PubMed]
- 31.Drilon A, Li G, Dogan S, Gounder M, Shen R, Arcila M, et al. What hides behind the MASC: clinical response and acquired resistance to entrectinib after ETV6-NTRK3 identification in a mammary analogue secretory carcinoma (MASC) Ann Oncol. 2016;27(5):920–926. doi: 10.1093/annonc/mdw042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cocco E, Schram AM, Kulick A, Misale S, Won HH, Yaeger R, et al. Resistance to TRK inhibition mediated by convergent MAPK pathway activation. Nat Med. 2019;25(9):1422–1427. doi: 10.1038/s41591-019-0542-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Not applicable.
Not applicable.