Abstract
Peripheral ossifying fibromas (POFs) and peripheral odontogenic fibromas (POdFs) appear clinically similar but of different histogenesis. The novel marker SATB2 is involved in regulation of osteoblastic differentiation and phenotype. However, SATB2 expression has not been previously explored in POFs and POdFs. Given the potential for mineralized tissue formation in POFs and POdFs, and to more clarify the phenotype of the lesional cells, this study was aimed to immunohistochemically investigate SATB2 expression in POFs and POdFs. Fourteen cases of POF and POdF (7 cases each) were selected, stained for SATB2 immunohistochemically, and scored according to the percentage of positive lesional cells (0, no staining; 1 +, < 5%; 2 +, 5–25%; 3 +, 26–50%; 4 +, 51–75%; and 5 +, 76–100%), and the intensity of staining was graded as weak, moderate, or strong. The control group included the inflammatory fibrous hyperplasia-like area present in two cases, 1 case fibroma, and 1 case giant cell fibroma. Moderate to strong, and diffuse SATB2 nuclear immunoreactivity was detected in the lesional cells of all cases of POFs and POdFs with variable scores; 3–5 + for the POFs and 3–4 + for the POdFs (P = 0.101). The distribution of staining was more prominent in those lesional cells associated with the osteoid/calcification in the cases of POFs. No staining was noted in the control group. The lesional cells in both POFs and POdFs express SATB2 and may exhibit the osteoblastic-like phenotype. SATB2 staining may be useful for diagnosis of subsets of POFs with minimal or absent calcification and some POdFs with unidentifiable odontogenic epithelium.
Keywords: Ossifying fibroma, Odontogenic tumors, Calcification, Cell differentiation, Gingival neoplasms, Staining and labeling, Phenotype
Introduction
Special AT-rich sequence binding protein 2 (SATB2) is a nuclear, multifunctional DNA-binding protein which is mainly used as a diagnostic marker for lower gastrointestinal tract adenocarcinomas [1–3]. SATB2 protein also regulates multiple transcription factors involved in skeletal development and osteoblastic differentiation/osteoblasts lineage commitment [4]. Recently, SATB2 expression has been shown in some bone-forming bone/soft tissue tumors as a novel maker for osteoblastic differentiation [5, 6].
Peripheral ossifying fibromas (POFs) and peripheral odontogenic fibromas (POdFs) are relatively common lesions exclusively seen in the gingiva and appear clinically similar, but of different histopathogenesis [7]. POFs are inflammatory/reactive lesions, predominantly seen in young adults and females. The anterior of maxilla is the most common site of involvement [8, 9]. Histopathologically, POF presents as a nodule composed of spindled-shaped fibroblasts with variable amounts of mineralized tissue including bone, cementum-like material, and dystrophic calcification [9].
POdFs are benign odontogenic neoplasm of ectomesenchymal origin [10]. Clinically, there is a prediction for the anterior region. POdFs occur over a wide range of age with a peak incidence in the second and forth decades. Histopathologically, POdF is characterized by a nodule of fibrous and/or myxoid connective tissue covered by intact oral mucosa. The tumor is composed of varying numbers of spindled-shaped cells and variable amounts of inactive-looking rests or strands of odontogenic epithelium with or without calcification in the form of bone/osteoid, cementum-like calcification, or dentinoid [11, 12].
Given the potential for mineralized tissue formation in POFs and POdFs, and to more clarify the phenotype of the lesional cells, this study intends to immunohistochemically investigate SATB2 expression in POFs and POdFs.
Materials and Methods
This study was fully approved by Institutional Review Board (19-06666-XP). A total of 14 cases diagnosed as POF and POdF (7 cases each) in the Oral Pathology Biopsy Services of UTHSC, College of Dentistry were selected based on the quality and quantity of the H & E-stained histopathologic sections. The normal-looking or inflammatory fibrous hyperplasia-like, non-lesional tissue present in one case of POF and one case of POdF, and 1 case of gingival fibroma and 1 case of gingival giant cell fibroma were considered as the control group. Formalin-fixed, paraffin-embedded sections (4–5 µm) were provided from each tissue block, deparaffinized in xylene, and rehydrated through a series of alcohol (from 100% ethanol to 50% ethanol). The sections were incubated in hydrogen peroxidase and absolute alcohol for 30 min to block endogenous peroxidase activity. Antigen retrieval was performed using pressure cooker pretreatment in citrate buffer (pH 6.0). Immunohistochemical staining was performed using the Envision Plus/Horseradish Peroxidase system (Dako, Carpinteria, CA), a polyclonal antibody to SATB2 (Sigma, Cat# HPA-001042) at 1:1000 titer. Tissue sections were subsequently incubated with the primary antibody for 40 min at room Temperature. Following TBS rinses, the tissue was incubated using the Envision Plus secondary antibody for 30 min followed by diaminobenzidine for 5 min. Appropriate positive (normal colon) and negative (incubation with secondary antibody only) controls were stained in parallel for each round of immunohistochemistry. First the hematoxylin and eosin-stained sections of each case were studied, and the presence or absence of ulceration, inflammation, and calcification were evaluated. Then SATB2 stained sections were assessed both qualitatively and semi-quantitatively by 2 of the authors according to the previously reported scoring system [6]. The extent of nuclear staining for SATB2 was scored according to the percentage of positive lesional cells (0, no staining; 1 +, < 5%; 2 +, 5–25%; 3 +, 26–50%; 4 +, 51–75%; and 5 +, 76–100%), and the intensity of staining was graded as weak, moderate, or strong.
Results
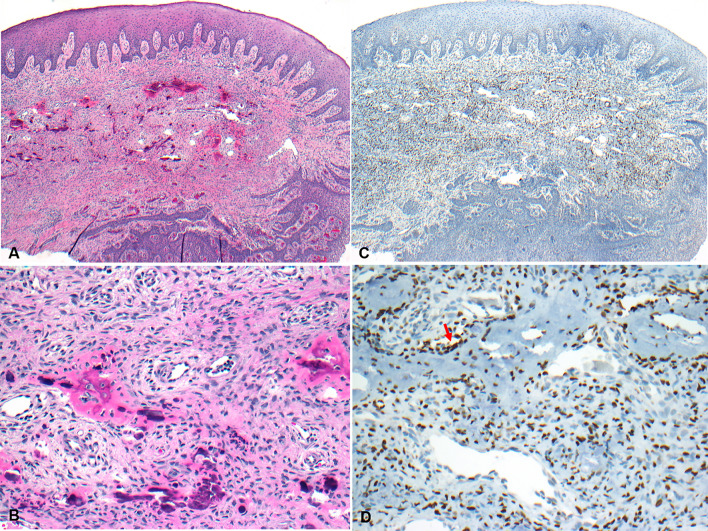
All but one of the cases of POF were female; aged 11–62 years (median, 15 years). Histopathologically, the cases were characterized as nodules, covered by stratified squamous epithelium which was focally ulcerated in 4 cases. The nodules were composed of a spindled-shaped cellular proliferation, containing calcification in the forms of bone/osteoid; except for one case without calcific material production. A patchy inflammatory cell infiltrate was noted in 3 cases. Diffuse and moderate to strong SATB2 nuclear immunoreactivity was detected in all the cases of POF which was variably scored from 3 + to 5 +. The distribution of staining was more prominent in those lesional cells in close association with the osteoid/bone areas (Fig. 1).
Fig. 1.
Photomicrographs showing a POF. A Photomicrograph showing a nodule of POF, covered by surface mucosa (H&E*, X 20). B The lesion is composed of a proliferation of spindled-shaped cells with bone formation (H&E*, X 200). C Photomicrograph showing the nodule of POF shown in A (IHC**, X 20) with nuclear staining of the lesional cells with SATB2 (D, IHC**, X 200), more prominently in those cells adjacent to the area of calcification (red arrow)
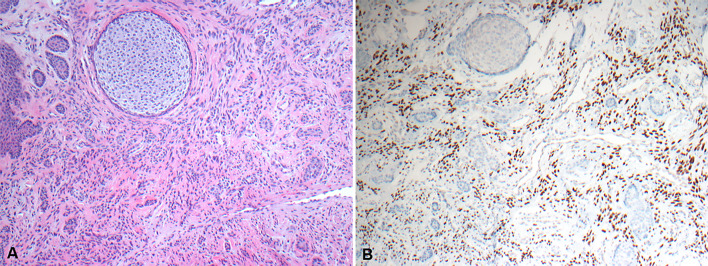
The cases of POdF were related to 3 females and 4 males, aged 27–78 (median, 42 years). Histopathologically, all the cases were nodules of fibrous and/or myxoid connective tissue covered by intact oral mucosa. The tumors were composed of varying numbers of spindled-shaped cells and variable amounts of inactive-looking rests or strands of odontogenic epithelium. Three out of 7 cases exhibited calcification or dentinoid or hyalinized regions around, within, or in close association with the rests of odontogenic epithelium, demonstrating an inductive effect. Immunohistochemically, a diffuse and moderate to strong SATB2 nuclear immunoreactivity was detected in all cases of POdF which varied in intensity from 3 + to 4 + (Fig. 2). Using t-test, no statistically significant difference in SATB2 expression was obtained between the cases of POF and POdFs (P = 0.101).
Fig. 2.
Photomicrographs showing a POdF. A A POdF with proliferation of spindled-shaped cells and small islands of odontogenic epithelium (H&E*, X 100). B SATB2 nuclear positivity of the lesional cells (IHC**, X 100)
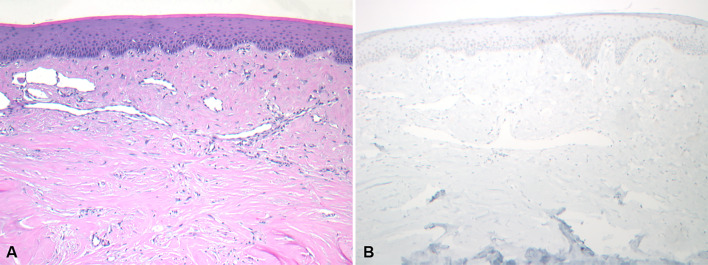
A scattered week SATB2 nuclear positivity (1 +) was seen in the inflammatory fibrous hyperplasia-like, non-lesional tissue in the two cases of POF and POdF. There was a sharp delineation in SATB2 staining between lesional and non-lesional tissues with almost no staining in non-lesional tissue. Cases of fibroma and giant cell fibroma showed no immunoreactivity with SATB2 immunostaining (Fig. 3).
Fig. 3.
Photomicrographs showing a giant cell fibroma. A A giant cell fibroma characterized by large and stellate-shaped fibroblasts within the superficial connective tissue (H&E*, X 100) and B Negative SATB2 immunoreactivity in the lesional cells (IHC**, X 100). *Hematoxylin and eosin. **Immunohistochemistry
Discussion
In our study, expression of the novel marker of SATB2 was found in two groups of gingival lesions with potential for mineralized tissue formation. To date, these results have not been reported in the English literature.
SATB2 is an essential nuclear factor in the development of osteoblastic cell lineage. The gene is located on chromosome 2 and originally was identified in some isolated cleft palate patients [13, 14]. SATB2 gene serves as a regulator of several genes and it is critical in multiple developmental processes [4]. An abnormal SATB2 gene may be associated with some craniofacial and skeletal abnormalities (SATB2-associated syndrome) such as isolated clefting, micrognathia, maxillary hypoplasia, dental anomalies, and osteoporosis [15]. For terminal differentiation of osteoblasts, Runx2 would be a key gene in addition of its role as a regulator in production of bone matrix proteins [16]. It has been shown SATB2 is downstream of Runx2 and, not only may participate in DNA replication of pre-osteoblasts, but also is involved in subsequent differentiation of those cells [17]. In diagnostic pathology, Conner et al. showed SATB2 to be a specific marker for osteoblastic differentiation in some benign and malignant mesenchymal tumors (including some bone and soft tissue tumors), but not specific for osteosarcoma [6]. SATB2 expression by lesional cells has been reported not only in those tumors of osteoblastic origin with observable calcific component, but also in some without evidence of osteoblastic lineage or calcific material formation such as chondromyxoid fibromas, giant cell tumors, unclassified pleomorphic sarcomas, and synovial sarcomas [6]. These findings raise the possibility of a primitive osteoblastic phenotype of the cell population in the mentioned tumors.
Although both POFs and POdFs are not diagnostically challenging in practical pathology, expression of SATB2 observed in our study could be discussed from the biological and histogenesis standpoints. SATB2 expression of the lesional cells in our cases of POF provides more insight about the phenotype of the cells and confirms previously published data. Previous studies suggested the spindle-shaped cells in peripheral ossifying fibromas (POFs) have features of mineralized tissue-forming cells since expression of some proteins such as Runx-2, bone morphogenic protein 2 (BMP-2), and cementum attachment protein (CAP) have been shown in POFs [18]. The cells also express osterix which is an essential transcription factor required for osteoblasts differentiation [19]. Since POFs are seen in tooth-bearing regions, periodontal ligament has been suggested as the source of the cells and the presence of the stem cells with potential for osteoblastic and cementoblastic differentiation has been demonstrated in previous studies [20].
POdF is the most common peripheral odontogenic tumor in which bone and/or osteoid, cementum-like calcifications, dentinoid, and dystrophic calcifications may be formed, particularly around, within or in close association with rests of odontogenic epithelium. This is explained by an inductive effect. The exact source of the fibroblastic component of POdFs is still unknown, but the periodontal ligament has been suggested. This seems unlikely, however, since POdFs are sometimes seen on edentulous ridges [11, 12]. Regardless of the whether the origin of the ectomesenchymal component of the tumor is of PDL or the ectomesenchyme of the gingiva, expression of SATB2 in these cells may indicate an osteoblastic-like property of these cells. It is known that the neural crest is the origin of oral and maxillofacial ectomesenchymal tissue. SATB2 expression has been reported in neural crest progenitors which delineates a developmental jaw module and its role in the regulating variation in jaw size has been suggested [21–23].
In summary, this study showed the lesional cells in both POFs and POdFs express SATB2 and may exhibit an osteoblastic-like phenotype. SATB2 staining may be useful for diagnosis of subsets of POFs with minimal or absent calcification and some POdFs with unidentifiable odontogenic epithelium. Regardless of the presence of surface epithelium in both POFs and POdFs, where there is minimal or absent calcification, the lesional cells may mimic other odontogenic lesions, including a hyperplastic dental follicle and odontogenic fibromyxoma. Therefore, SATB2 immunostaining may be also helpful in distinguishing POFs and POdFs from their histopathologic mimics. Further studies using larger samples are needed to clarify SATB2 expression in other odontogenic processes with or without calcific material formation and also in dental stem cells to see its possible role in expression of other osteogenic-related proteins such as bone matrix proteins.
Author Contributions
All authors contributed to this work. All authors reviewed and approved the manuscript for submission.
Funding
UTHSC College of Dentistry, Department of Diagnostic Sciences, Oral and Maxillofacial Pathology Lab. The authors have no funding, financial relationships.
Declarations
Conflict of interest
No conflict of interest to disclose.
Ethical Approval
This study was fully approved by Institutional Review Board (19-06666-XP).
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Shokoufeh Shahrabi-Farahani, Email: sfarahan@uthsc.edu.
David M. Pencarinha, Email: dpencari@uthsc.edu
Mark Anderson, Email: kander20@uthsc.edu.
References
- 1.Magnusson K, de Wit M, Brennan DJ, Johnson LB, McGee SF, Lundberg E, et al. SATB2 in combination with cytokeratin 20 identifies over 95% of all colorectal carcinomas. Am J Surg Pathol. 2011;35(7):937–948. doi: 10.1097/PAS.0b013e31821c3dae. [DOI] [PubMed] [Google Scholar]
- 2.Lin F, Shi J, Zhu S, Chen Z, Li A, Chen T, et al. Cadherin-17 and SATB2 are sensitive and specific immunomarkers for medullary carcinoma of the large intestine. Arch Pathol Lab Med. 2014;138(8):1015–1026. doi: 10.5858/arpa.2013-0452-OA. [DOI] [PubMed] [Google Scholar]
- 3.Dragomir A, de Wit M, Johansson C, Uhlen M, Ponten F. The role of SATB2 as a diagnostic marker for tumors of colorectal origin: results of a pathology-based clinical prospective study. Am J Clin Pathol. 2014;141(5):630–638. doi: 10.1309/ajcpww2urz9jkqju. [DOI] [PubMed] [Google Scholar]
- 4.Dobreva G, Chahrour M, Dautzenberg M, Chirivella L, Kanzler B, Farinas I, et al. SATB2 is a multifunctional determinant of craniofacial patterning and osteoblast differentiation. Cell. 2006;125(5):971–986. doi: 10.1016/j.cell.2006.05.012. [DOI] [PubMed] [Google Scholar]
- 5.Righi A, Gambarotti M, Longo S, Benini S, Gamberi G, Cocchi S, et al. Small cell osteosarcoma: clinicopathologic, immunohistochemical, and molecular analysis of 36 cases. Am J Surg Pathol. 2015;39(5):691–699. doi: 10.1097/pas.0000000000000412. [DOI] [PubMed] [Google Scholar]
- 6.Conner JR, Hornick JL. SATB2 is a novel marker of osteoblastic differentiation in bone and soft tissue tumours. Histopathology. 2013;63(1):36–49. doi: 10.1111/his.12138. [DOI] [PubMed] [Google Scholar]
- 7.Kenney JN, Kaugars GE, Abbey LM. Comparison between the peripheral ossifying fibroma and peripheral odontogenic fibroma. J Oral Maxillofac Surg. 1989;47(4):378–382. doi: 10.1016/0278-2391(89)90339-x. [DOI] [PubMed] [Google Scholar]
- 8.Cuisia ZE, Brannon RB. Peripheral ossifying fibroma—a clinical evaluation of 134 pediatric cases. Pediatr Dent. 2001;23(3):245–248. [PubMed] [Google Scholar]
- 9.Buchner A, Hansen LS. The histomorphologic spectrum of peripheral ossifying fibroma. Oral Surg Oral Med Oral Pathol. 1987;63(4):452–461. doi: 10.1016/0030-4220(87)90258-1. [DOI] [PubMed] [Google Scholar]
- 10.El-Naggar AK, Chan JKC, Jennofer R, Takata T, Slootweg PJ. WHO classification of head and neck tumours. Lyon: International Agency for Research on Cancer; 2017. [Google Scholar]
- 11.Ritwik P, Brannon RB. Peripheral odontogenic fibroma: a clinicopathologic study of 151 cases and review of the literature with special emphasis on recurrence. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2010;110(3):357–363. doi: 10.1016/j.tripleo.2010.04.018. [DOI] [PubMed] [Google Scholar]
- 12.Daley TD, Wysocki GP. Peripheral odontogenic fibroma. Oral Surg Oral Med Oral Pathol. 1994;78(3):329–336. doi: 10.1016/0030-4220(94)90064-7. [DOI] [PubMed] [Google Scholar]
- 13.FitzPatrick DR, Carr IM, McLaren L, Leek JP, Wightman P, Williamson K, et al. Identification of SATB2 as the cleft palate gene on 2q32-q33. Hum Mol Genet. 2003;12(19):2491–2501. doi: 10.1093/hmg/ddg248. [DOI] [PubMed] [Google Scholar]
- 14.Brewer CM, Leek JP, Green AJ, Holloway S, Bonthron DT, Markham AF, et al. A locus for isolated cleft palate, located on human chromosome 2q32. Am J Hum Genet. 1999;65(2):387–396. doi: 10.1086/302498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zarate YA, Fish JL. SATB2-associated syndrome: mechanisms, phenotype, and practical recommendations. Am J Med Genet A. 2017;173(2):327–337. doi: 10.1002/ajmg.a.38022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Komori T. Roles of Runx2 in skeletal development. Adv Exp Med Biol. 2017;962:83–93. doi: 10.1007/978-981-10-3233-2_6. [DOI] [PubMed] [Google Scholar]
- 17.Dowrey T, Schwager EE, Duong J, Merkuri F, Zarate YA, Fish JL. Satb2 regulates proliferation and nuclear integrity of pre-osteoblasts. Bone. 2019;127:488–498. doi: 10.1016/j.bone.2019.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tsiligkrou IA, Tosios KI, Madianos PN, Vrotsos IA, Panis VG. Oxytalan-positive peripheral ossifying fibromas express runt-related transcription factor 2, bone morphogenetic protein-2, and cementum attachment protein. An immunohistochemical study. J Oral Pathol Med. 2015;44(8):628–33. doi: 10.1111/jop.12275. [DOI] [PubMed] [Google Scholar]
- 19.El Achkar VNR, Medeiros RDS, Longue FG, Anbinder AL, Kaminagakura E. The role of Osterix protein in the pathogenesis of peripheral ossifying fibroma. Braz Oral Res. 2017;31:e53. doi: 10.1590/1807-3107BOR-2017.vol31.0053. [DOI] [PubMed] [Google Scholar]
- 20.Nagatomo K, Komaki M, Sekiya I, Sakaguchi Y, Noguchi K, Oda S, et al. Stem cell properties of human periodontal ligament cells. J Periodontal Res. 2006;41(4):303–310. doi: 10.1111/j.1600-0765.2006.00870.x. [DOI] [PubMed] [Google Scholar]
- 21.Fish JL, Villmoare B, Köbernick K, Compagnucci C, Britanova O, Tarabykin V, et al. Satb2, modularity, and the evolvability of the vertebrate jaw. Evol Dev. 2011;13(6):549–564. doi: 10.1111/j.1525-142X.2011.00511.x. [DOI] [PubMed] [Google Scholar]
- 22.Fish JL. Developmental mechanisms underlying variation in craniofacial disease and evolution. Dev Biol. 2016;415(2):188–197. doi: 10.1016/j.ydbio.2015.12.019. [DOI] [PubMed] [Google Scholar]
- 23.Britanova O, Depew MJ, Schwark M, Thomas BL, Miletich I, Sharpe P, et al. Satb2 haploinsufficiency phenocopies 2q32-q33 deletions, whereas loss suggests a fundamental role in the coordination of jaw development. Am J Hum Genet. 2006;79(4):668–678. doi: 10.1086/508214. [DOI] [PMC free article] [PubMed] [Google Scholar]