Abstract
The aim of this study is to present an elusive case of primary thyroid lymphoma (PTL), initially thought to be anaplastic thyroid carcinoma, then Rosai Dorfman disease, before the final diagnosis of PTL was made. An elderly female with hypothyroidism presented with compressive airway symptoms secondary to an enlarging neck mass. Imaging was suggestive of undifferentiated thyroid cancer. The initial biopsy was unexpectedly consistent with a lymphoproliferative disorder such as Rosai-Dorfman disease. A repeat biopsy with immunohistochemical analysis yielded a diagnosis of diffuse large B-cell lymphoma of germinal center subtype. The patient was spared thyroid surgery and started on appropriate chemotherapy. PTL is within the differential diagnosis that physicians must consider in a patient with a rapidly-enlarging neck mass. A clinical index of suspicion and early accurate diagnosis may spare the patient from unnecessary surgery that is required of most other non-hematopoeitic thyroid malignancies.
Keywords: Thyroid malignancy, Rosai Dorfman, Lymphoma, Head and neck, Otolaryngology, Endocrine
Introduction
Primary thyroid lymphoma (PTL) is a rare type of thyroid cancer (TC) that comprises only 0.5–5% of all thyroid malignancies [1]. It typically presents as a rapidly-enlarging mass that causes compressive symptoms, which mimics the classical clinical presentation of undifferentiated (anaplastic) thyroid carcinoma (ATC) or poorly-differentiated thyroid carcinoma (PDTC). Though PTL is rare, the importance of its early recognition lies in that PTL can often be treated successfully without surgery that is usually required for most other TCs [2].
This case report demonstrates an elderly female patient who was diagnosed with PTL and spared unnecessary thyroid surgery. Her investigative workup is interesting in that a diagnosis of Rosai-Dorfman disease with thyroid involvement, which is exceedingly rare, was considered based on initial fine needle biopsy findings prior to a final diagnosis of PTL.
Case History
A 66-year-old-female with a history of hypothyroidism, hypertension, hyperlipidemia, Type II diabetes mellitus, and uterine cancer presented to the emergency department (ED) for acute exacerbation of compressive airway symptoms. Her sole complaint was dyspnea with no other associated symptoms such as hoarseness, dysphagia, odynophagia, cough, fevers, chills, night sweats, or unintentional weight loss. Physical examination demonstrated a large anterior left-sided neck mass with extension toward the midline. Flexible nasolaryngoscopy examination demonstrated anterior displacement of the arytenoids from posterior mass effect. Cervical spine radiography showed a 4 cm calcified neck mass with soft tissue swelling around C6 and an anteriorly displaced trachea. A 6.3 × 4.9 × 9.4 cm mass of the left lobe of the thyroid gland, with partial encasement of the trachea and mass effect on the esophagus, was observed on contrast-enhanced computed tomography (CT) (Fig. 1). Non-contrast CT of the neck and chest re-demonstrated an enlarged left hemithyroid with invasion of the posterior trachea and encasement of the left common carotid artery, suggestive of a thyroid malignancy. Left tracheal, paratracheal, and mediastinal lymphadenopathy was also present.
Fig. 1.
Axial and sagittal CT demonstrating an enlarged thyroid mass with posterior tracheal encasement
Given the patient’s clinical presentation and imaging findings, thyroid malignancy, particularly an undifferentiated thyroid carcinoma, was suspected; however, a fine-needle aspiration (FNA) of the neck performed at bedside yielded results inconclusive for malignancy. Rather, numerous polymorphic lymphoid cells and large form, tingible body macrophages with emperipolesis were identified. These findings were suggestive of Rosai-Dorfman disease or another lymphoproliferative process (Fig. 2). A core needle biopsy of the cervical mass with immunohistochemical and flow cytometric studies was also inconclusive. An open surgical biopsy of the cervical mass was subsequently performed. The histologic features of this biopsy included fibroadipose tissue with multiple foci of diffuse lymphoid infiltrates. The infiltrates composed of large, neoplastic lymphoid cells with irregular nuclei, vesicular chromatin, prominent nucleoli, and a moderate amount of cytoplasm. Apoptotic bodies and mitotic figures were occasionally seen, and intravascular infiltration was noted. Immunohistochemical and FISH results were consistent with a diffuse large B-cell lymphoma, germinal center type (Table 1).
Fig. 2.
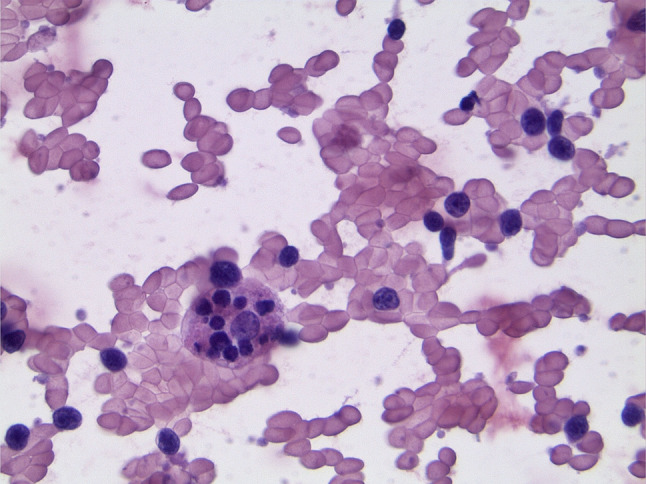
FNA biopsy of the thyroid demonstrating emperipolesis
Table 1.
Immunohistochemical testing results for large B-cell lymphoma
| Positive | Negative | |
|---|---|---|
| CD20 | CD3 | Cyclin D1 |
| PAX5 | CD5 | Pan keratin |
| BCL6 | CD10 | EBV-EBER |
| Ki67 (90-95% positive) | MUM1 (<30% positive) | FISH studiesa |
| c-Myc | BCL2 | |
aFISH studies for large B-cell lymphoma included testing for rearrangements of BCL2, BCL6, MYC and amplification of MYC
Discussion
Thyroid cancer (TC) is the most common malignancy of the endocrine system and comprises 3.4% of all diagnosed cancers worldwide [3]. TC can be categorized into three main histologic types: differentiated, undifferentiated, and medullary. Differentiated TC accounts for more than 90% of thyroid malignancies and includes papillary thyroid carcinoma (PTC) and follicular thyroid carcinoma (FTC). Aggressive thyroid malignancies include undifferentiated (anaplastic) TC and poorly-differentiated thyroid carcinoma (PDTC), which account for 5% and 1% of all TC, respectively. Medullary thyroid carcinoma (MTC) accounts for 5% of all TC [4]. Other uncommon types of TC include primary thyroid lymphoma (PTL), which accounts for 0.5–5% of TC, and primary thyroid sarcoma (PTS), which accounts for 0.01–1.5% of TC [1, 5].
PTL comprises 3% of all non-Hodgkin lymphomas and usually presents in the sixth or seventh decades in females with chronic lymphocytic thyroiditis [6, 7]. The most common type of PTL is diffuse large B-cell lymphoma (DLBCL), followed by marginal zone lymphoma of mucosa-associated lymphoid tissue (MALT) lymphoma [6]. PTL typically presents as a rapidly-enlarging, painless goiter that causes compressive symptoms including dyspnea, dysphagia, stridor, and hoarseness [8, 9]. The definitive diagnosis is determined through combined analyses of morphology, immunophenotypic and cytogenetic studies [10]. Tissue samples are typically be obtained via FNA or core needle biopsy. Open surgical biopsy, which was ultimately performed in this patient, may also be performed if the aforementioned, less invasive methods cannot adequately provide a subtype of lymphoma. Management of PTL is generally non-surgical with treatment modalities focusing on chemotherapy, radiotherapy, and monoclonal antibody therapy [7, 11]. The prognosis of PTL, as determined from the Survival Epidemiology and End Results (SEER) database, depends on several factors with advanced stage, lack of radiation or surgery, older age, and certain histologic subtypes of PTL portending a poorer prognosis. Median overall survival of PTL is 9.3 years and 5-year survival is 66%. Disease-specific 5-year survival is 96% for MALT lymphoma, 87% for follicular lymphoma, 86% for small lymphocytic lymphoma, and 75% for DLBCL [12].
Thyroidectomy was initially considered in this patient given the mass effect and clinical suspicion for an undifferentiated TC, which can pose a rapid threat to the airway and esophagus if not emergently palliated [13]. However, given its relative ease and accessibility, an FNA of the neck mass was obtained at bedside to elucidate a clearer diagnosis. Interestingly enough, findings were not suggestive of a carcinoma as suspected, but rather of a lymphoproliferative disorder including Rosai-Dorfman disease (RDD), also known as sinus histiocytosis with massive lymphadenopathy (SHML). RDD is a non-neoplastic, non-Langerhans cell histiocytosis that primarily involves lymph nodes. There are only a few hundred cases of RDD in the literature with less than 3% of cases reporting extranodal involvement of the thyroid [14]. Emperipolesis, which was suspected on the patient’s FNA biopsy, occurs when one cell is penetrated by another cell, but both remain undamaged. This is distinctly different from phagocytosis where the engulfed cell or cell fragments are often dead or dying and are eventually broken down by lysosomal activity. Upon review of the literature, emperipolesis has been noted in various normal tissue samples (fetal liver, bone marrow megakaryocytes) in addition to non-malignant processes (autoimmune hemolytic anemia, idiopathic thrombocytopenic purpura, post-radiation) and malignant processes (multiple myeloma, myeloproliferative disorders, neuroblastoma, lymphoma, leukemia, etc.). Emperipolesis is considered a hallmark feature of RDD but has also been recognized physiologically and pathologically as noted above. Regarding lymphoma, emperipolesis is more often noted in non-Hodgkin’s lymphomas than Hodgkin’s lymphoma, though there are case reports including both. Emperipolesis has been noted in B-cell lymphomas and rarely in T-cell lymphoma [15–17]. Disseminated RDD can be treated non-surgically like PTL, often with corticosteroids or immunosuppressants [16].
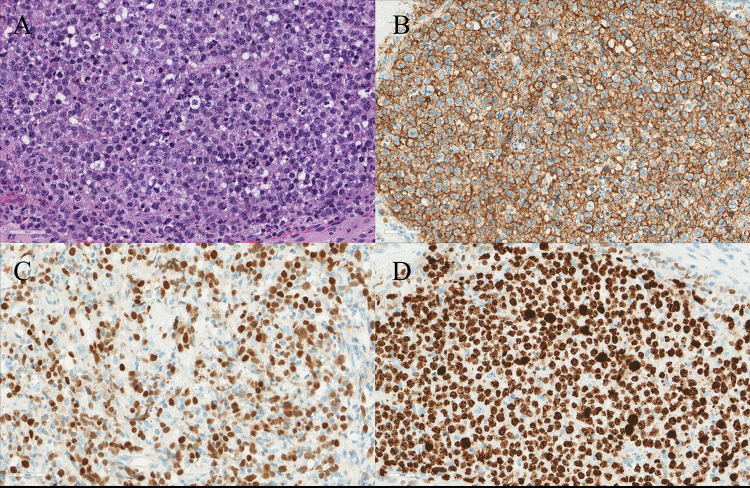
The final diagnosis was obtained via open biopsy histologic sections which demonstrated diffuse infiltrate of large cells containing irregular nuclei, vesicular chromatin, prominent nucleoli, and a moderate amount of cytoplasm (Figure 3). In addition, numerous apoptotic bodies and mitotic figures were evident, which was further supported by a high Ki-67 proliferation index (90–95%). The high rate of cellular turnover resulting in abundant tangible body macrophages with engulfed cells and debris may explain the findings suggestive of emperipolesis noted in the initial FNA. Histologic sections, combined with immunohistochemistry and cytogenetic studies, resulted in a final diagnosis of DLBCL. Based on the final diagnosis, the initial surgical plans were aborted and medical management commenced, sparing the patient a potentially unnecessary thyroid surgery. Although surgical resection has been described in the literature for the management of PTL, its utility remains controversial as surgery alone is considered incomplete treatment for PTL [17]. As depicted by this patient’s case, the similarities in clinical presentation between PTL and undifferentiated TC can create a diagnostic challenge where the need for an accurate diagnosis is weighed against the time constraints posed by a threatening, enlarging neck mass. The differential diagnosis of RDD, PTL, and TC can be clinically difficult; however, the clinical, radiologic, and histologic findings are vital for precise diagnosis and subsequent treatment (Tables 1 and 2).
Fig. 3.
A Histologic examination demonstrated diffuse large cells with irregular nuclei, vesicular chromatin, and prominent nucleoli in a background of apoptotic bodies (hematoxylin and eosin). B B-cells with diffuse positivity for CD20. C B-cells positive for Bcl-6. D High proliferation index by KI-67 (> 90%), indicating high cellular turnover
Table 2.
Clinical, radiological, and histological differences between primary thyroid lymphoma, undifferentiated thyroid carcinoma, and Rosai Dorfman disease
| Primary thyroid lymphoma (PTL) | Undifferentiated thyroid carcinoma | Rosai Dorfman disease (RDD) | |
|---|---|---|---|
| Clinical presentation |
Rapidly enlarging neck mass History of lymphocytic thyroiditis ~ 10-12% have compressive symptoms (dyspnea, hoarseness, stridor) and/or B-symptoms (fever, weight loss, night sweats) Firm and smooth lesion on physical examination [18] |
Rapidly enlarging neck mass Frequent compressive symptoms Older age (>50) Extrathyroidal extension and local organ invasion [19] |
Nodal form Massive bilateral lymphadenopathy (often cervical) that is painless May present with B-symptoms Additional lymph node involvement common (inguinal, axillary) Extranodal form Head and neck location can present with local mass effect, including respiratory symptoms [22] Younger age at presentation (mean ~20 years old) [23] |
| Radiology |
Ultrasound Nodular, diffuse, or mixed type with enhanced posterior echoes [18] Computed topography Homogenous attenuation without necrosis or calcification |
Ultrasound Solid, irregular, hypoechoic, internal calcifications and lymph node involvement [20] Computed topography Solid, ill-defined border, internal calcification, necrosis, and heterogenous attenuation Local organ invasion frequent [19] |
Ultrasound Focal hypoechoic or mixed echogenic nodule (similar to thyroid carcinoma) Diffusely enlarged, heterogeneously hypoechoic echotexture (similar to thyroiditis) [23] Computed topography isolated or diffuse lymphadenopathy with enhancement, infiltrative appearance if in organ [23] |
| Pathology |
Most common subtypes: DLBCL, Mucosa-associated lymphoid tissue (MALT), follicular, small lymphocytic, and Hodgkin’s lymphoma DLBCL: Diffuse infiltrate of medium to large atypical lymphoid cells with B-cell markers as shown in Table 1 [18] |
Spectrum of spindle cell, giant cell, and squamoid Hypercellularity with widespread invasion, angiotropism, and necrosis Large pleomorphic cells with frequent bizarre nuclei and prominent nucleoli [21] |
Emperipolesis is characteristic within histiocytes containing abundant cytoplasm (clear, eosinophilic) Background inflammation by lymphocytes and plasma cells Sinus histiocytosis in lymph nodes Immunohistochemistry: S100(+), CD68(+), CD1a(−), Factor XIIIa(−) [23] |
Conclusion
Though PTL is rare, the importance of its early recognition lies in that PTL can often be treated successfully without surgery as required for most other TC. Therefore, physicians should remain mindful of these more uncommon diagnoses, as their clinical presentation may mimic that of more aggressive thyroid diseases and possibly result in unnecessary surgical intervention.
Authors Contributions
All authors contributed to substantially to, and approve of, this manuscript. Conceptualization: SK, WPL. Supervision: MNP, YL, SCL. Writing—original draft: SK, ALG, WPL. Writing—review & editing: MNP, YL, SCL.
Funding
No financial support was provided for the creation of this manuscript.
Data Availability
Not applicable.
Code Availability
Not applicable.
Declarations
Conflict of interest
None of the authors have any conflicts of interests.
Ethical Approval
As part of the publishing ethics requirements, this case presentation was approved by the institutional review board of Loma Linda University.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Dündar HZ, Sarkut P, Kırdak T, Korun N. Primary thyroid lymphoma. Ulus Cerrahi Derg. 2016;32:75–77. doi: 10.5152/UCD.2015.2935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Peixoto R, Correia Pinto J, Soares V, Koch P, Taveira Gomes A. Primary thyroid lymphoma: a case report and review of the literature. Ann Med Surg (Lond) 2017;13:29–33. doi: 10.1016/j.amsu.2016.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chmielik E, Rusinek D, Oczko-Wojciechowska M. Heterogeneity of thyroid cancer. Pathobiology. 2018;85:117–129. doi: 10.1159/000486422. [DOI] [PubMed] [Google Scholar]
- 4.Prete A, Borges de Souza P, Censi S, Muzza M, Nucci N, Sponziello M. Update on fundamental mechanisms of thyroid cancer. Front Endocrinol (Lausanne) 2020;11:102. doi: 10.3389/fendo.2020.00102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Surov A, Gottschling S, Wienke A, Meyer HJ, Spielmann RP, Dralle H. Primary thyroid sarcoma: a systematic review. Anticancer Res. 2015;35:5185–5191. [PubMed] [Google Scholar]
- 6.Hirokawa M, Kudo T, Ota H, Suzuki A, Kobayashi K, Miyauchi A. Preoperative diagnostic algorithm of primary thyroid lymphoma using ultrasound, aspiration cytology, and flow cytometry. Endocr J. 2017;64:859–865. doi: 10.1507/endocrj.EJ17-0111. [DOI] [PubMed] [Google Scholar]
- 7.Walsh S, Lowery AJ, Evoy D, McDermott EW, Prichard RS. Thyroid lymphoma: recent advances in diagnosis and optimal management strategies. Oncologist. 2013;18:994–1003. doi: 10.1634/theoncologist.2013-0036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sinha S, Aish L, Oo TH. Primary thyroid lymphoma presenting with stridor. Am J Clin Oncol. 2005;28:531–533. doi: 10.1097/01.coc.0000160066.97299.a6. [DOI] [PubMed] [Google Scholar]
- 9.Matsuzuka F, Miyauchi A, Katayama Set. Clinical aspects of primary thyroid lymphoma: diagnosis and treatment based on our experience of 119 cases. Thyroid. 1993;3:93–99. doi: 10.1089/thy.1993.3.93. [DOI] [PubMed] [Google Scholar]
- 10.Lanham T, Lanham E, Sullivan A, Magaji V. Non-Hodgkin lymphoma of the thyroid in a patient with hyperthyroidism. J Community Hosp Intern Med Perspect. 2021;11:79–80. doi: 10.1080/20009666.2020.1829403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alzouebi M, Goepel JR, Horsman JM, Hancock BW. Primary thyroid lymphoma: the 40 year experience of a UK lymphoma treatment centre. Int J Oncol. 2012;40:2075–2080. doi: 10.3892/ijo.2012.1387. [DOI] [PubMed] [Google Scholar]
- 12.Graff-Baker A, Roman SA, Thomas DC, Udelsman R, Sosa JA. Prognosis of primary thyroid lymphoma: demographic, clinical, and pathologic predictors of survival in 1,408 cases. Surgery. 2009;146:1105–1115. doi: 10.1016/j.surg.2009.09.020. [DOI] [PubMed] [Google Scholar]
- 13.Chintakuntlawar AV, Foote RL, Kasperbauer JL, Bible KC. Diagnosis and management of anaplastic thyroid cancer. Endocrinol Metab Clin North Am. 2019;48:269–284. doi: 10.1016/j.ecl.2018.10.010. [DOI] [PubMed] [Google Scholar]
- 14.Gianella P, Dulguerov N, Arnoux G, Pusztaszeri M, Seebach JD. Thyroid Rosai-Dorfman disease with infiltration of IgG4-bearing plasma cells associated with multiple small pulmonary cysts. BMC Pulm Med. 2019;19:83. doi: 10.1186/s12890-019-0847-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rastogi V, Sharma R, Misra SR, Yadav L, Sharma V. Emperipolesis—a review. J Clin Diagn Res. 2014;8:Zm01-02. doi: 10.7860/JCDR/2014/10361.5299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Palmas Candia F, Porras Ledantes JA, Raventós Estellé A, Simón Muela I, Vendrell Ortega J, Näf Cortés S. Thyroid involvement by Rosai-Dorfman disease. Endocrinol Diabetes Nutr. 2017;64:280–281. doi: 10.1016/j.endinu.2017.01.002. [DOI] [PubMed] [Google Scholar]
- 17.Pavlidis ET, Pavlidis TE. A review of primary thyroid lymphoma: molecular factors, diagnosis and management. J Invest Surg. 2019;32:137–142. doi: 10.1080/08941939.2017.1383536. [DOI] [PubMed] [Google Scholar]
- 18.Stein SA, Wartofsky L. Primary thyroid lymphoma: a clinical review. J Clin Endocrinol Metab. 2013;98(8):3131–3138. doi: 10.1210/jc.2013-1428. [DOI] [PubMed] [Google Scholar]
- 19.Ahmed S, Ghazarian MP, Cabanillas ME, et al. Imaging of anaplastic thyroid carcinoma. AJNR Am J Neuroradiol. 2018;39:547–551. doi: 10.3174/ajnr.A5487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Suh HJ, Moon HJ, Kwak JY, Choi JS, Kim EK, et al. Anaplastic thyroid cancer: ultrasonographic findings and the role of ultrasonography-guided fine needle aspiration biopsy. Yonsei Med J. 2013;54(6):1400–1406. doi: 10.3349/ymj.2013.54.6.1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ragazzi M, Ciarrocchi A, Sancisi V, Gandolfi G, Bisagni A, Piana S. Update on anaplastic thyroid carcinoma: morphological, molecular, and genetic features of the most aggressive thyroid cancer. Int J Endocrinol. 2014;2014:790834. doi: 10.1155/2014/790834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Abla O, Jacobsen E, Picarsic J, et al. Consensus recommendations for the diagnosis and clinical management of Rosai-Dorfman-Destombes disease. Blood. 2018;131(26):2877–2890. doi: 10.1182/blood-2018-03-839753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mar WA, Yu JH, Knuttinen MG, et al. Rosai-Dorfman disease: manifestations outside of the head and neck. AJR Am J Roentgenol. 2018;208(4):721–732. doi: 10.2214/AJR.15.15504. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.
Not applicable.