Abstract
Secretory carcinoma (SC) of salivary gland, previously known as mammary analogue secretory carcinoma, is a rare low-grade malignancy harboring a diagnostic ETV6-NTRK3 gene fusion. SC of salivary gland shares histopathological, immunohistochemical and genetic characteristics with SC of the breast. There are several previous cytomorphological characterizations of SC of salivary gland reported in the literature. The most commonly reported patterns are of epithelial clusters with papillary architectural features, or of single dispersed epithelial cells on a background of abundant histiocytes. Tumor cells exhibit vacuolated eosinophilic cytoplasm and round to oval nuclei with regular nuclear contours and inconspicuous or small nucleoli. The cytomorphology of SC may closely mimic that of acinic cell carcinoma or low-grade mucoepidermoid carcinoma. Moreover, when cohesive epithelial clusters do not appear on the smears, it may be very difficult to distinguish dispersed tumor cells from histiocytes. In this article, we review the literature pertaining to SC cytomorphology and we report a fine needle aspiration biopsy case of SC in salivary gland showing well-defined intracytoplasmic hyaline globules, a feature that has not been previously reported. This novel cytomorphological feature may be helpful in distinguishing the tumor cells of SC from histiocytes and from other low-grade salivary gland tumors.
Keywords: Salivary gland, Secretory carcinoma, FNA cytology, Intracytoplasmic hyaline globular structure, ETV6-NTRK3
Introduction
In 2010, a salivary gland counterpart of secretory carcinoma (SC) of the breast was described by Skalova et al. who termed it “mammary analogue secretory carcinoma” [1]. In the 2017 World Health Organization Classification of Head and Neck Tumors this entity was redesignated “secretory carcinoma” [2]. SC occurs in both children and adults with an average age at diagnosis of 44.2 years, and is more common in males. SC usually presents as a slowing-growing mass in the parotid, submandibular or minor salivary glands. Microscopically, SC typically has papillary and microcystic architecture, a pattern uncommon in acinic cell carcinoma. The neoplastic cells of SC have a moderate amount of vacuolated eosinophilic cytoplasm with extracellular and intracytoplasmic mucin, and lack basophilic zymogen granules. Nuclei are round to ovoid with inconspicuous nucleoli. SC is typically positive for S100, mammaglobin, GCDFP-15, vimentin, and SOX-10, and negative for DOG-1 [3]. Like secretory carcinoma of breast and other tumors such as infantile fibrosarcoma and congenital mesoblastic nephroma, SC harbors a characteristic recurrent t(12;15)(p13;q25), leading to an ETV6-NTRK3 fusion [1].
Fine needle aspiration (FNA) biopsy has become the frontline diagnostic tool for salivary gland tumors. Due to its rarity and its significant morphologic overlap with other entities, FNA diagnosis of SC is challenging. In this article, we review the literature pertaining to SC cytomorphology and we report a fine needle aspiration biopsy case of SC in salivary gland showing well-defined intracytoplasmic hyaline globules, a feature that has not been previously reported.
Case Presentation
A 26-year-old man presented with a two-month history of a slow-growing asymptomatic right cheek mass. Soft tissue computerized tomography (CT) with contrast displayed a 1.7 cm peripherally enhancing lesion in the medial aspect of the superficial lobe of the right parotid gland. The radiographic differential diagnosis included abscess, suppurative lymphadenitis, and primary salivary gland neoplasm.
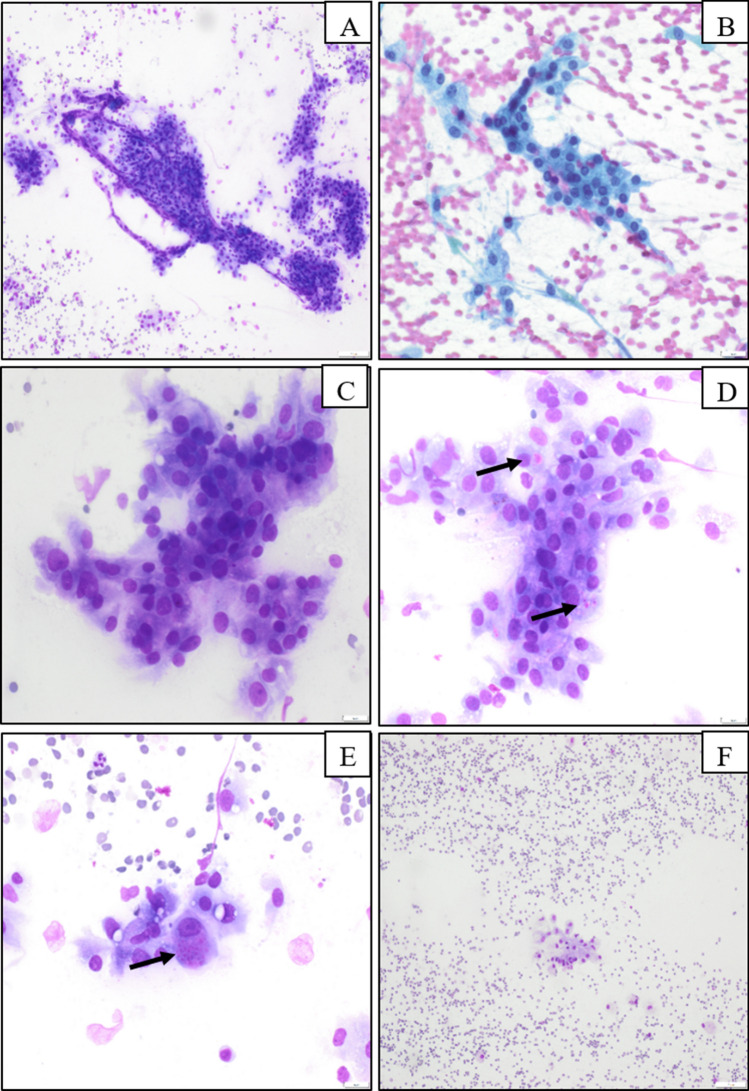
The patient underwent an FNA procedure, and direct smear were prepared with Papanicolaou and Diff-Quik stains. The direct smears were highly cellular, with monomorphic tumor cells arranged in loosely cohesive papillary-like groups or flat sheets and as singly dispersed cells with ill-defined cytoplasmic borders (Fig. 1a). The neoplastic cells contained a moderate amount of vacuolated or granular cytoplasm (Fig. 1b) with an amphophilic appearance on Diff-Quik-stained smears (Fig. 1c). Well-defined intracytoplasmic hyaline globules were present in approximately 5% of neoplastic cells, best visualized on the Diff-Quik-stained smears given their bright pink color and contrast with the amphophilic cytoplasm (Fig. 1d, e) and inconspicuous on Papanicolau-stained smears. The hyaline globules were intracellular only, and were not present in the smear background. Nuclei were round to ovoid with a fine chromatin pattern, and there was mild anisonucleosis. Occasional neoplastic cells with signet-ring-like morphology were present, and a few mitotic figures were present. The background was hemorrhagic and contained histiocytes and scant mucinous material (Fig. 1f). The cytological differential diagnosis included mucoepidermoid carcinoma, acinic cell carcinoma, oncocytoma, and other low-grade salivary gland carcinomas.
Fig. 1.
Cytomorphology of secretory carcinoma of salivary gland. Highly cellular smear with cohesive, papillary-like clusters (a) with background histiocytes or singly dispersed neoplastic cells (f). The neoplastic cells have moderate amount of vacuolated cytoplasm and round to oval nucleus and smooth nuclear contour (b, c). Well-defined intracytoplasmic hyaline globules are noted in a portion of neoplastic cells (d, e by arrow)
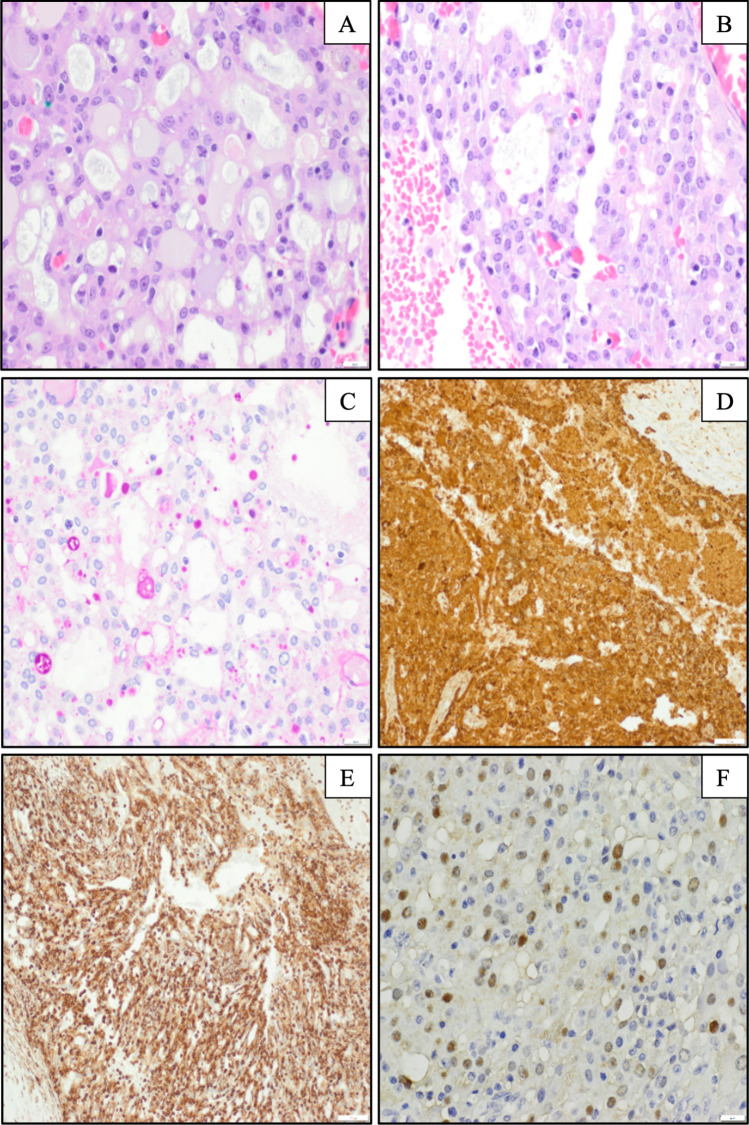
Right parotidectomy and modified radical neck dissection were performed three weeks after the FNA procedure. The parotid specimen contained a 1.7 × 1.0 × 1.0 cm red-brown, hemorrhagic and cystic mass. Histological sections showed a neoplasm with an infiltrative border and microcystic architecture. The microcysts contained amorphous pale eosinophilic secretions and scant mucin. The neoplastic cells contained a moderate amount of vacuolated or granular eosinophilic cytoplasm on hematoxylin and eosin stained-sections (Fig. 2a). Well-defined hyaline globules were present in both intracytoplasmic and intracystic spaces, highlighted on periodic acid-Schiff with diastase (PAS-D)-stained sections (Fig. 2b, c) and partially highlighted on mucicarmine stained-sections. Nuclei were round to ovoid with a fine chromatin pattern, and there was mild anisonucleosis. The neoplastic cells were positive for mammaglobin and vimentin (Fig. 2d, e), focally positive for S100 (Fig. 2f), GCDFP-15 and GATA3, and negative for p63 and DOG-1. Next-generation sequencing using the Archer FusionPlex Comprehensive Thyroid & Lung (CTL) panel performed on the surgical specimen showed a fusion of the ETV6-NTRK3 genes, and the diagnosis of secretory carcinoma was confirmed. The neck dissection specimen showed twenty-four lymph nodes with no evidence of metastatic disease.
Fig. 2.
Histopathology and selected immunostains of secretory carcinoma of salivary gland. Predominant microcystic architecture with intraluminal eosinophilic secretions and scant mucin are present a. Well-defined intracytoplasmic globular structures are present b and are highlighted by PAS-D stain (c). The tumor cells are diffusely positive for mammaglobulin (d) and vimentin (e), and focally positive for S100 (f), supporting the diagnosis of SC
After eighteen months of follow up, the patient showed no clinical or radiographic evidence of residual or recurrent disease.
Discussion
SC of salivary gland is a recently recognized entity, and its biological behavior has not been fully characterized. SC is generally considered a low-grade malignancy, though some cases have shown aggressive features including extracapsular/intraglandular extension and perineural invasion [4]. One case of SC displaying high-grade cytology, a solid and microcystic growth pattern, and comedonecreosis has been reported [5].
SC may resemble acinic cell carcinoma (AciCC) or low-grade mucoepidermoid carcinoma (MEC) both histologically and cytologically. However, careful examination reveals diagnostic clues. SC typically displays microcystic architecture and intraluminal eosinophilic secretions. Intraluminal eosinophilic secretions are rare or absent in AciCC. AciCC typically shows closely packed or overlapping acinar structures on both histology and FNA smears, a feature not seen in SC. The neoplastic cells of AciCC often possess microvesiculated basophilic cytoplasm with zymogen granules [6]. SC may display bubbly eosinophilic cytoplasm, but lacks zymogen granules. The distinct intracytoplasmic hyaline globules seen in our case of SC are not a feature of AciCC. Low-grade MEC generally has cystic architecture with abundant intracellular and extracellular mucin that is readily visible on both histological and cytological preparations. In contrast, if mucin is present in SC, it is typically scant. MEC contains squamoid cells with dense cytoplasm that are not seen in SC [7].
Ancillary studies may also be helpful in distinguishing these entities. SC is generally positive for S100, SOX-10, vimentin, and mammaglobin, but is negative for p63 and DOG-1. AciCC is typically positive for S0X-10, DOG1 and NR4A3 but negative for p63 and mammaglobin. Low-grade MEC is typically positive for p63 and cytokeratin, and negative for S100. Molecular characterization may also be very helpful, as SC harbors a defining EVT6-NTRK3 translocation [1], whereas low-grade MEC carries its own characteristic CRTC1 (MECT1)-MAML2 translocation [8]. Recently, it was reported that AciCC harbors recurrent and specific rearrangement involving the upstream elements of transcription factor Nuclear Receptor Subfamily 4 Group A member 3 (NR4A3) at 9q31 [9].
SC typically presents as a slowly enlarging and painless nodule, found incidentally on physical examination. The imaging findings are non-specific. SC possesses similar clinical behavior to AciCC as evidenced by comparable mean disease-free survival and disease involvement of regional lymph nodes between these two entities [3, 6]. Surgical intervention including neck dissection is the standard treatment for SC, which is the same as other low-grade salivary gland malignancies. A small minority of patients also receive postoperative radiotherapy and chemotherapy. Tropomyosin receptor kinase (Trk) inhibitors have been tested in the SC patients, but their efficacy is not established. Some advanced cases showed initial response but subsequently acquired resistance [10, 11].
So far, approximately fifteen reports have been published regarding the cytology of SC. The patient age range in reported cases is 9 to 78 years old, with a median age of 47. These tumors are generally small to medium-sized, averaging 2 cm in greatest dimension. 81% of reported SCs occurred in the parotid gland, and 11% of them were found in submandibular glands. The remainder were in minor salivary glands or accessory parotid glands. A non-specific diagnosis of low-grade neoplasm was rendered in 34% of reported FNA cases. Diagnoses of AciCC and pleomorphic adenoma were rendered in 19% and 11% of cases, respectively. The remaining cases were diagnosed with benign salivary gland, MEC or high-grade neoplasm. Only 2% of reported cases were correctly and specifically diagnosed with SC by FNA [5–7, 12–24]. In previous reports, direct smears are typically highly cellular, and hemosiderin-laden macrophages are a common finding. Tumor cells are loosely cohesive and are arranged in papillary or follicular patterns. Single polygonal neoplastic cells of variable size with abundant vacuolar cytoplasm and round to oval nuclei are present. Cytoplasmic vacuoles containing colloid-like substance were reported in one published case [21]. Compared to the colloid-like substance reported previously, the intracytoplasmic hyaline globules in our case are well-delineated and display a bright pink color. No zymogen granules or other diagnostic features of acinic differentiation are present. Nuclear contours are smooth, with fine chromatin and indistinct or small nucleoli. Extracellular and intracytoplasmic mucin with signet ring-like cells has also been noted [12, 16, 23, 25]. Bishop et al. reviewed five cases of SC and described two distinct cytomorphological patterns: (1) Hypercellular smears composed of cellular fragments of variable size with sheet-like or papillary architecture together with occasional singly dispersed neoplastic cells, and (2) A predominance of single neoplastic cells admixed with histiocytes and granular debris. Tumor cells can mimic histocytes, which may hinder accurate classification of SC of FNA [12].
We have reported a case of SC with a potentially underappreciated cytological feature. Overall morphological findings were similar to those described in previous reports, but our case showed prominent well-defined intracytoplasmic globules best visualized on Diff-Quik stained smears and highlighted by PAS-D on the histological sections. Interestingly, a subset of intracytoplasmic hyaline globules were highlighted by mucicarmine stain on the histological sections, which suggests that the globules in the neoplastic cells may represent small mucus containing vacuoles. Although the reason that not all intracytoplasmic globules were highlighted by mucicarmine stain is not clear, we presume the intracytoplasmic globules may contain other elements besides mucus. To our knowledge, this is the first report of this finding in SC. In cases of low-grade salivary neoplasia with a differential diagnosis including SC, AciCC, and MEC, accurate identification of intracytoplasmic material may aid in the distinction between these entities (Table 1). Additionally, recognition of these intracytoplasmic globules in FNA specimens may aid in the distinction between neoplastic cells and histiocytes when the tumor cells are singly dispersed.
Table 1.
Major features in the differential diagnosis of SC
| SC | AciCC | Low-grade MEC | |
|---|---|---|---|
| Architecture | Papillary, flat sheets, microcystic, or singly dispersed cells | Closely packed or overlapping acinar structures, microcystic on histology | Cystic |
| Background | Hemorrhagic with histiocytes, or sparsely mucinous. Intraluminal eosinophilic secretions on histology | None | Extracellular mucin may be abundant |
| Cytoplasm | Moderate amount of vacuolated eosinophilic cytoplasm | Granular basophilic cytoplasm | Mucinous, intermediate and squamoid and dense |
| Cytoplasmic contents | Mucin, rare signet ring-like cells, hyaline globules | Zymogen granules | Mucus-containing goblet cells |
| Nucleus | Round to oval nuclei with smooth nuclear contours, small or inconspicuous nucleoli, low mitotic activity | Round to oval nuclei with smooth nuclear contours, small or inconspicuous nucleoli, low mitotic activity | Round to oval nuclei with smooth nuclear contours, small or inconspicuous nucleoli, low mitotic activity |
| Immunophenotype | Positive for S100, SOX-10, vimentin, and mammaglobin; Negative for p63 and DOG-1 | Positive for SOX-10, DOG-1 and NR4A3; Negative for p63 and mammaglobin | Positive for p63; Negative for S100 |
| Molecular characteristics | ETV6-NTRK3 | Recurrent translocations involving the upstream elements of NR4A3 at 9q31 | CRTC1 (MECT1)-MAML2 |
AciCC: acinic cell carcinoma; MEC: mucoepidermoid carcinoma, PAS-D: periodic acid-Schiff with diastase; NR4A3: Nuclear receptor subfamily 4 group A member 3
Conclusion
In summary, SC is a malignant neoplasm of salivary gland which should be in the differential diagnosis when FNA direct smears are highly cellular with papillary and sheet-like architecture and display a background of hemorrhage, abundant histocytes, and/or mucin. In SC, the neoplastic cells have a moderate amount of vacuolated cytoplasm that may contain intracytoplasmic hyaline globules, a feature which can aid in the challenging morphological distinction between SC, AciCC, and MEC. The Diff-Quik stain is particularly helpful because it facilitates visualization of the intracytoplasmic globules, the pink color of which contrasts with the amphophilic cytoplasm. When SC is among differential diagnostic considerations, collection of sufficient material for molecular and immunohistochemical characterization is recommended.
Funding
No funding was received to assist with the preparation of this manuscript.
Declarations
Conflict of interest
The authors have no financial or proprietary interests to disclose.
Ethical Approval
This is a case report. Baylor Scott & White Health Research Ethics Committee has confirmed that no ethical approval is required.
Informed Consent
The images in this article are from the pathology slides with no identifying information. The consent is not necessary in this case according to the “instruction for authors”.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Skalova A, Vanecek T, Sima R, et al. Mammary analogue secretory carcinoma of salivary glands, containing the ETV6-NTRK3 fusion gene: a hithertoundescribed salivary gland tumor entity. Am J Surg Path. 2010;34(5):599–608. doi: 10.1097/PAS.0b013e3181d9efcc. [DOI] [PubMed] [Google Scholar]
- 2.WHO Classification of Head and Neck Tumors. 4th Edition, Volume 9. 2017.
- 3.Chiosea SI, Griffith C, Assaad A, et al. Clinicopathological characterization of mammary analogue secretory carcinoma of salivary glands. Histopathology. 2012;61(3):387–394. doi: 10.1111/j.1365-2559.2012.04232.x. [DOI] [PubMed] [Google Scholar]
- 4.Connor A, Perez-Ordonez B, Shago M, et al. Mammary analog secretory carcinoma of salivary gland origin with the ETV6 gene rearrangement by FISH: expanded morphologic and immunohistochemical spectrum if a recently described entity. Am J Surg Pathol. 2012;36:27–34. doi: 10.1097/PAS.0b013e318231542a. [DOI] [PubMed] [Google Scholar]
- 5.Jung MJ, Song JS, Kim SY, et al. Finding and characterizing mammary analogue secretory carcinoma of the salivary gland. Korean J Pathol. 2013;47:36–43. doi: 10.4132/KoreanJPathol.2013.47.1.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sethi R, Kozin E, Remenschneider A, et al. Mammary analogue secretory carcinoma: update on a new diagnosis of salivary gland malignancy. Laryngoscope. 2014;124:188–195. doi: 10.1002/lary.24254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Oza N, Sanghvi K, Shet T, et al. Mammary analogue secretory carcinoma of parotid, is preoperative cytological diagnosis possible? Diagn Cytopathol. 2016;44:519–525. doi: 10.1002/dc.23459. [DOI] [PubMed] [Google Scholar]
- 8.EI-Naggar AK, Lovell M, Killary AM, et al. A mucoepidermoid carcinoma of minor salivary gland with t(11;19)(q21;p13.1) as the only karyotypic abnormality. Cancer Genet Cytogenet. 1996;87:29–33. doi: 10.1016/0165-4608(95)00266-9. [DOI] [PubMed] [Google Scholar]
- 9.Haller F, Bieg M, Will R, et al. Enhancer hijacking activates oncogenic transcription factor NR4A3 in acinic cell carcinomas of the salivary glands. Nature Commun. 2019;10:368. doi: 10.1038/s41467-018-08069-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cipriani N, Blair EA, Finkle J, et al. Salivary gland secretory carcinoma with high-grade transformation, CDKN2A/B loss, distant metastasis, and lack of sustained response to Crizotinib. Int J Pathol. 2017;25(7):613–618. doi: 10.1177/1066896917709350. [DOI] [PubMed] [Google Scholar]
- 11.Drilon A, Li G, Dogan S, et al. What hides behind the MASC: clinical response and acquired resistance to entrectinib after ETV6-NTRK3 identification in a mammary analogue secretory carcinoma (MASC) Ann Oncol. 2016;27(5):920–926. doi: 10.1093/annonc/mdw042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bishop JA, Yonescu R, Batista DAS, et al. Cytopathologic features of mammary analogue secretory carcinoma. Cancer (Cancer Cytopathol) 2013;121:228–233. doi: 10.1002/cncy.21245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kai K, Minesaki A, Suzuki K, et al. Difficulty in the cytodiagnosis of mammary analogue secretory carcinoma: survey of 109 cytologists with a case originating from a minor salivary gland. Acta Cytol. 2017;61:469–476. doi: 10.1159/000477390. [DOI] [PubMed] [Google Scholar]
- 14.Bajaj J, Gimenez C, Slim F, et al. Fine-needle aspiration cytology of mammary analog secretory carcinoma masquerading as low-grade mucoepidermoid carcinoma: case report with a review of the literature. Acta Cytol. 2014;58:501–510. doi: 10.1159/000368070. [DOI] [PubMed] [Google Scholar]
- 15.Pisharodi L. Mammary analog secretory carcinoma of salivary gland: cytologic diagnosis and differential diagnosis of an unreported entity. Diagn Cytopathol. 2013;41:239–241. doi: 10.1002/dc.21766. [DOI] [PubMed] [Google Scholar]
- 16.Levine P, Fried K, Krevitt LD, et al. Aspiration biopsy of mammary analogue secretory carcinoma of accessory parotid gland: another diagnostic dilemma in matrix-containing tumors of the salivary glands. Diagn Cytopathol. 2014;42:49–53. doi: 10.1002/dc.22886. [DOI] [PubMed] [Google Scholar]
- 17.Takeda M, Kasai T, Morita K, et al. Cytopathological features of mammary analogue secretory carcinoma-review of literature. Diagn Cytopathol. 2015;43:131–137. doi: 10.1002/dc.23146. [DOI] [PubMed] [Google Scholar]
- 18.Troncone G. MASC is indistinguishable from acinic cell carcinoma, papillary-cystic variant on salivary gland FNA cytomorphology: case report with histological and immunohistochemical correlates. Cytopathology. 2014;25:342–344. doi: 10.1111/cyt.12107. [DOI] [PubMed] [Google Scholar]
- 19.Griffith CC, Stelow EB, Saqi A, et al. The cytological features of mammary analogue secretory carcinoma: a series of 6 molecular confirmed cases. Cancer Cytopathol. 2013;121:234–241. doi: 10.1002/cncy.21249. [DOI] [PubMed] [Google Scholar]
- 20.Peterson F, Lian D, Chau YP, et al. Mammary analogue secretory carcinoma: the first submandibular case reported including findings on fine needle aspiration cytology. Head and Neck Pathol. 2012;6:135–139. doi: 10.1007/s12105-011-0283-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miesbauerova M, Tommola S, Steiner P, et al. Cytophatological features of secretory carcinoma of salivary glands and ancillary techniques in its diagnostics: impact of new Milan system for reporting salivary gland cytopathology. APMIS. 2019;127:491–502. doi: 10.1111/apm.12950. [DOI] [PubMed] [Google Scholar]
- 22.Samulski TD, Livolsi VA, Baloch Z. The cytopathologic features of mammary analog secretory carcinoma and its mimics. CytoJournal. 2014;11:24. doi: 10.4103/1742-6413.139726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Higuchi K, Urano M, Takahashi RH, et al. Cytological features of mammary analogue secretory carcinoma of salivary gland: fine-needle aspiration of seven cases. Diagn Cytopathol. 2014;42:846–855. doi: 10.1002/dc.23139. [DOI] [PubMed] [Google Scholar]
- 24.Kim YA, Joung JW, Lee S-J, et al. Cytopathologic features of secretory carcinoma of salivary gland: report of two cases. J Pathol Transl Med. 2019;53:70–74. doi: 10.4132/jptm.2018.11.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Inaba T, Fukumura Y, Saito T, et al. Cytological features of mammary analogue secretory carcinoma of the parotid gland in a 15-year-old girl: a case report with review of the literature. Case Rep Pathol. 2015 doi: 10.1155/2015/656107. [DOI] [PMC free article] [PubMed] [Google Scholar]