Abstract
Laryngeal neuroendocrine neoplasms (NENs) are rare and heterogeneous, encompassing well-differentiated neuroendocrine tumors (NETs; grade 1, 2, and 3), neuroendocrine carcinomas (NECs, small cell and large cell types), and mixed neuroendocrine non-neuroendocrine neoplasms (MiNEN). We aimed to study the clinicopathologic spectrum of these neoplasms. A retrospective review of all primary laryngeal NENs diagnosed from 2005 to 2017 was undertaken. Mitotic index was divided into < 2, ≥ 2–10, and > 10 mitoses/2 mm2, with a Ki-67 labelling index of < 2%, ≥ 2–20%, and > 20% for the NET grade 1, 2 and 3 categories, respectively. A total of 27 patients were included. The median age at presentation was 60 years; the male-to-female ratio was 8:1. Supraglottis (n = 22) was the most frequently affected subsite. There were 9 NETs grade 2 (G2), and 18 NECs cases. There were no NET grade 1 or 3 cases in our cohort. Among the NETs G2, the morphology was epithelioid (2), plasmacytoid (3), clear (2), oncocytic (1), and rhabdoid (1). Unique ‘glomeruloid structures’ (n = 5), calcification (n = 3), lymphoid aggregates (n = 5), intranuclear inclusions (n = 2), hyaline globules (n = 3), and Leisegang rings (n = 2) were identified. NECs comprised 16 small cell neuroendocrine carcinoma and 2 large cell neuroendocrine carcinoma. On immunohistochemistry, tumor cells expressed AE1/AE3 (86%), synaptophysin (100%), chromogranin (100%), INSM1 (100%), calcitonin (33.3%). In the NEC group, p53 aberrant expression (87.5%), Retinoblastoma (Rb) loss (88.2%), and diffuse p16 immunoreactivity (66.7%) were additionally observed. Lymph-node metastasis was detected in 62.5% and 85.7%, while distant metastasis in 55.6% and 76.9%, respectively in NET G2 and NEC. Laryngeal NENs are aggressive neoplasms with a high rate of nodal and distant metastasis. Awareness of the wide pathologic spectrum of laryngeal NENs and appropriate use of IHC is needed to render an accurate diagnosis. Ki67 assessment is strongly recommended for laryngeal NEN prognostication
Supplementary Information
The online version contains supplementary material available at 10.1007/s12105-021-01367-9.
Keywords: Larynx, Neuroendocrine, Ki-67, Small cell, Large cell neuroendocrine carcinoma, Head and neck
Introduction
Laryngeal neuroendocrine neoplasms (NENs) are a rare and heterogeneous group of malignant neoplasms exhibiting neuroendocrine (NE) differentiation. These tumors are unified by shared histologic, immunohistochemical, and ultrastructural characteristics while displaying a broad morphologic spectrum [1, 2]. Laryngeal NENs are extremely rare, comprising < 1% of all laryngeal neoplasms [3].
From the first description of laryngeal NENs by Goldman in 1969 [1], the nomenclature and classifications of laryngeal NENs have undergone substantial evolution over time (Table 1) [2, 4–7]. Based on the histology and clinical behavior, NENs are classified into 3 broad categories: (1) Well-differentiated neuroendocrine tumors (NET), grade 1–3 (G1/G2/G3). (2) Neuroendocrine carcinoma (NEC); this further encompasses two morphologic subtypes: (a) Small cell neuroendocrine carcinoma (SCNEC), and (b) Large cell neuroendocrine carcinoma (LCNEC). (3) Mixed neuroendocrine non-neuroendocrine neoplasms (MiNEN).
Table 1.
Classifications of laryngeal neuroendocrine neoplasms
| Woodruff et al. 1985 [4] | Wenig et al. 1988 [2] | WHO 1991 [5] | WHO 2005 [6]a | WHO 2017 [7] |
|---|---|---|---|---|
|
Small cell Large cell |
Well differentiated Moderately differentiated Poorly differentiated |
Typical carcinoid Atypical carcinoid Small cell (oat cell) Intermediate cell Large cell |
Typical carcinoid Atypical carcinoid Small cell Combined small cell and non-small cell |
Well differentiated Moderately differentiated Poorly differentiated -Small cell -Large cell |
aLarge cell included in the atypical carcinoid category
As clinical behaviour and prognosis of laryngeal NEN differ widely from the more common squamous cell carcinomas (SCC), an accurate diagnosis and grading of these tumors are of paramount importance. Owing to their rarity, there is a lack of familiarity among pathologists with the pathologic spectrum of laryngeal NEN. As a consequence, these tumors are susceptible to erroneous diagnosis, particularly in a small biopsy, and thereafter to incorrect management [8]. Finally, clinical biology remains poorly understood and therefore, the treatment algorithms are not yet standardized. Shifting classifications and taxonomy in the past have made a meaningful comparison of rare global data difficult, contributing to the paucity of knowledge about these neoplasms. Hence, there is an urgent need to gather cogent data on laryngeal NEN based on standardized terminology. We aimed to evaluate the clinical and pathologic spectrum of the laryngeal NEN at our tertiary-care oncology institute to expand our understanding of these rare neoplasms.
Methods
Clinicopathologic data of all primary laryngeal NEN diagnosed between 2005 and 2017 were retrieved from the archives of the Department of Pathology at a tertiary-care oncology center. The search terms were: ‘carcinoid’, ‘atypical carcinoid’, ‘neuroendocrine’, ‘head and neck’, ‘small cell carcinoma’, ‘neuroendocrine carcinoma’, and ‘large cell neuroendocrine carcinoma’. Hematoxylin and eosin stained slides and immunohistochemistry (IHC) studies were reviewed and the diagnosis was confirmed [7]. However, based on a more harmonized approach to NEN classification as suggested by the International Agency for Research on Cancer (IARC) and World Health Organization (WHO) [9], the terminology and grading used in the present study to further classify laryngeal NENs was as follows:
Neuroendocrine tumor (NET) for well-differentiated epithelial neoplasms that displayed morphologic and immunohistochemical features of NE differentiation, most typically exhibiting cellular monotony, stippled/granular chromatin, and organoid architecture or varied patterns such as nests, trabeculae, cords, or rosettes. NETs were graded into 3 grades based on mitotic rate: G1, G2, and G3 with a mitotic rate of < 2/2 mm2, 2–10/2 mm2, and > 10/2 mm2., Ki-67 labelling index (performed manually on a digital image in the region of most intense labelling or hotspots, counting 2000 tumor cell nuclei in a resection specimen, and at least 500 tumor cell nuclei in a biopsy specimen, using MIB1 antibody) at < 2%, ≥ 2–20%, and > 20%, respectively. Tumor necrosis (apoptotic cells to coagulative necrosis) was identified, and used to separate between grade 1 and 2/3 tumours (present in grade 2 and 3 tumors).
Neuroendocrine carcinoma (NEC) for poorly differentiated epithelial neoplasms with histologic and immunohistochemical evidence of NE differentiation, showing tumor necrosis, and mitotic rate of > 10/2 mm2. A Ki-67 proliferation index was not required, but was determined for each tumor (method as described above). NECs were further classified into: (a) small cell neuroendocrine carcinoma (SCNEC) exhibiting hyperchromatic nuclei, finely granular chromatin, inconspicuous nucleoli, scant cytoplasm, and nuclear molding, with the cell smaller than the diameter of 3 lymphocytes; and (b) large cell neuroendocrine carcinoma (LCNEC) displaying organoid, nested, or trabecular architecture, rounded vesicular nuclei, with prominent nuclei, and moderate to abundant amounts of cytoplasm, larger than the diameter of 3 lymphocytes.
Mixed neuroendocrine non-neuroendocrine neoplasm (MiNEN) for epithelial neoplasms composed of dual components with morphologically and immunohistochemically distinct NE and non-NE components.
Data on age, sex, symptomatology, tobacco consumption, tumor localization, clinical stage, treatment, recurrence, metastasis, and the status at the last follow-up (FU) visit were recorded from the hospital’s electronic medical records. Pathologic features recorded were: tumor size, differentiation (well-differentiated vs poorly-differentiated), histologic type (NET vs NEC small cell type vs NEC large cell type vs MiNEN), tumor grade (G1, G2, G3), morphologic patterns, mitotic rate (per 2 mm2 determined by counting a minimum of 10 mm2), necrosis, lymphovascular invasion (LVI), perineural invasion (PNI), resection margins, lymph node (LN) status, lymph node ratio (LNR; the ratio of affected lymph node number to total lymph node number), pathologic staging (AJCC 8th edition) [10], and immunohistochemistry profile (Supplementary table: list of antibodies).
The optimal cut-off value for Ki-67 percentage to predicting survival and metastasis was assessed by receiver operating characteristic (ROC) curve analysis and Youden’s index. All analyses were carried out using SPSS version 25.0 statistical software (SPSS Inc, Chicago, IL, USA).
Results
A total of 27 cases of laryngeal NEN were analyzed. The laryngeal NENs accounted for 0.9% of all laryngeal neoplasms reported in our institute (21 in-house cases out of 2526 laryngeal neoplasms) over 13 years. Of the 27 cases, 5 had an incorrect pre-operative biopsy diagnosis of basaloid carcinoma (n = 1), squamous cell carcinoma (n = 1), and salivary gland neoplasm (n = 3), subsequently recognized as laryngeal NEN on resection.
Clinical Findings
The details of clinical findings are provided in Table 2. Briefly, the median age was 60 years with a striking male preponderance. The majority (91.3%) patients were tobacco users; tobacco use was in the form of cigarette/bidi smoking and smokeless betel-quid chewing over a duration ranging from 10 to 35 years. There was no family history, syndrome or genetic association documented. Symptoms included hoarseness (47%), difficulty swallowing (29.4%), difficulty breathing (23.5%), pricking-sensation in the throat (11.8%), irritative cough (11.8%), and painful swallowing (11.8%) experienced over a duration of 10 days to 2 years (median 8 months).
Table 2.
Clinical features of laryngeal neuroendocrine neoplasms (n = 27)
| Clinical features | Total | NET G2 | SCNEC | LCNEC |
|---|---|---|---|---|
| Total number of cases | 27 | 9 (33.3%) | 16 (59.3%) | 2 (7.4%) |
| Median age, years (range) | 60 (31–84) | 56 (44–64) | 64 (31–84) | 60 (49–60) |
| Male: female ratio | 8:1 | 7:2 | 15:1 | 2:0 |
| Tobacco consumption (n = 23)a | 21/23 (91.3%) | 7/9 (77.8%) | 12/12 (100%) | 2/2 (100%) |
| Subsites | ||||
| Supraglottis | 22 (81.5%) | 9 (100%) | 13 (81.3%) | 0 (0%) |
| Glottis | 2 (7.4%) | 0 (0%) | 2 (12.5%) | 0 (0%) |
| Subglottis | 3 (11.1%) | 0 (0%) | 1 (6.2%) | 2 (100%) |
| Distant metastasis at presentation (n = 22)a | 8/22 (36.4%) | 2/9 (22.2%) | 4/11 (36.4%) | 2/2 (100%) |
| Clinical staging (n = 23)a | ||||
| I and II | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
| III | 4/23 (17.4%) | 2/8 (25%) | 2/13 (15.4%) | 0/2 (0%) |
| IV: | 19/23 (82.6%) | 6/8 (75%) | 11/13 (30.8%) | 2/2 (100%) |
| IVA | 7/23 (30.4%) | 3/8 (37.5%) | 4/13 (30.8%) | 0/2 (0%) |
| IVB | 4/23 (17.4%) | 1/8 (12.5%) | 3/13 (20.0%) | 0/2 (0%) |
| IVC | 8/23 (34.8%) | 2/8 (25%) | 4/13 (30.8%) | 2/2 (100%) |
| Specimen | ||||
| Biopsy onlyb | 15 (55.6%) | 1 (11.1%) | 12 (75%) | 2 (100%) |
| Excision specimen | 12 (44.4%) | 8 (88.9%) | 4 (25%) | 0 (0%) |
| Treatment (n = 22)a,c | ||||
| Surgery alone | 4 | 4 | – | – |
| Surgery plus adjuvant RT | 1 | 1 | – | – |
| Surgery plus CRT | 3 | 3 | – | – |
| Surgery plus CT | 4 | – | 4 | – |
| CRT | 4 | – | 4 | – |
| Palliative RT | 1 | – | 1 | – |
| Palliative Chemotherapy | 5 | – | 3 | 2 |
| Follow-up number (range in months) | 21 (2–74) | 8 (6–74) | 11 (2–25) | 2 (5–6.5) |
| Local recurrence (n = 21)a | 6/21 (28.6%) | 2/8 (25%) | 4/11 (36.4%) | 0/2 (0) |
| Distant metastasis (n = 22)a | 15/22 (68.2%) | 5/9 (55.6%) | 8/11 (72.7%) | 2/2 (100%) |
| Lung | 7 | 3 | 4 | 2 |
| Liver | 6 | 1 | 3 | 2 |
| Bone | 7 | 2 | 3 | 2 |
| Cutaneous | 6 | 4 | 2 | 0 |
| Peritoneal | 2 | 1 | 1 | 0 |
| Breast | 1 | 1 | 0 | 0 |
| Brain | 1 | 0 | 1 | 0 |
| Outcome (n = 21)a | ||||
| Alive no evidence of disease | 4/21 (19.0%) | 2/8 (25%) | 2/11 (18.2%) | 0/2 (0%) |
| Alive with disease | 8/21 (38.1%) | 4/8 (50%) | 3/11 (27.3%) | 1/2 (50%) |
| Died of disease | 9/21 (42.9%) | 2/8 (25%) | 6/11 (54.5%) | 1/2 (50%) |
NET G2 neuroendocrine tumor, Grade 2; SCNEC small cell neuroendocrine carcinoma, LCNEC large cell neuroendocrine carcinoma, RT radiotherapy, CRT chemoradiation, CT chemotherapy
aWhere information was available
bPatients with unresectable or metastatic disease had only biopsy samples as the pathologic material
cTwo patients underwent neck dissection at recurrence while another 2 received peptide receptor radionuclide therapy (PRRT) at progression
Tumors were most frequently localized to the supraglottis (81.5%), with these tumors either arising from or involving the aryepiglottic region (aryepiglottic fold [AEF], epiglottis, arytenoids), followed by the subglottis (11.1%), and the glottis (7.4%). Direct laryngoscopy findings (available in 19 patients), reported a polypoid smooth-surfaced lobulated, pedunculated, globular mass (n = 6), while an ulcerated lesion (n = 2), or an ulceroproliferative growth (n = 11) was noted.
Eight (36.4%) patients had evidence of distant metastasis (DM) at first clinical presentation. Initial clinical staging was available for 23 patients. There were no stage I or II patients; 17.4% stage III, and 82.6% stage IV (Table 2). Twelve (44.4%) patients underwent resection: endoscopic micro-laryngeal surgery (n = 6); partial laryngectomy (n = 2); and total laryngectomy (n = 4). Of the remaining patients, surgery was not performed due to refusal (n = 2), an unresectable primary (n = 4), or DM (n = 9).
Pathology findings
The pathologic findings are detailed in Table 3. Briefly, the average tumor size was 3.8 cm. Macroscopy findings were recorded as pedunculated/polypoid/globular (n = 7), ulcerative lesion (n = 3), ulceroproliferative (n = 4), and infiltrative (n = 1).
Table 3.
Pathologic features of the laryngeal neuroendocrine neoplasms (n = 27)
| Pathologic features | NET G2 (n = 9) | SCNEC (n = 16) | LCNEC (n = 2) |
|---|---|---|---|
| Initial erroneous biopsy diagnosis | Salivary gland tumor (n = 3) | SCC (n = 1), basaloid carcinoma (n = 1) | – |
| Median tumor size, cm (range) | 1.8 (0.6–3.6) | 3.8 (1.4–9.8) | 4.8 (4.5–11.1) |
| Macroscopy | Polypoid/globular | Ulcerated; ulceroproliferative | Ulcerated; ulceroproliferative |
| Microscopy | |||
| Architectural patterns | Trabeculae, nests, organoid | Diffuse solid sheets | Nests, trabeculae |
| Fibrovascular stroma | Prominent | Absent | Absent |
| Cellular monotony | Present | Present | Present |
| Cells | Plasmacytoid, infrequently clear, rhabdoid | Closely packed, <3 times the lymphocyte size; hyperchromatic, round to elongate nuclei, scant cytoplasm | Large, >3 times the lymphocyte size polygonal, basaloid to undifferentiated, vesicular nuclei, moderate cytoplasm |
| Prominent nucleoli | Absent | Absent | Present |
| Crushing artifacts | Absent | Present | Absent |
| Rosettes | 3 (33%) | 1 (6.3%) | 2 (100%) |
| Glomeruloid structures | 5 (55.5%) | 0 (0%) | 0 (0%) |
| Mitotic count/2 mm2 | 2–5 | 5-55a | 10–22 |
| Necrosis | 2 (22%) | 10 (63%) | 2 (100%) |
| Lymphovascular invasion | 1/8 (12.5%) | 3/4 (75%) | NA |
| Perineural invasion | 0/8 (0%) | 0/4 (0%) | NA |
| Involved resection margin | 2/8 (25%) | 0/4 (0%) | NA |
| Pathologic T stage | |||
| pT1-2 | 8/8 (100%) | 0/4 (0%) | NA |
| pT3-4b | 0/8 (0%) | 4/4 (100%) | NA |
| Lymph node metastasis | 5/8 (62.5%) | 10/12 (83.3%) | 2/2 (100%) |
| Immunohistochemistry | |||
| AE1/AE3 (n = 21) | 7/7 (100%) | 9/12 (75%) | 2/2 (100%) |
| Synaptophysin (n = 27) | 9/9 (100%) | 16/16 (100%) | 2/2 (100%) |
| Chromogranin (n = 27) | 9/9 (100%) | 13/16 (81.3%) | 2/2 (100%), focal |
| INSM1 (n = 12) | 6/6 (100%) | 4/4 (100%) | 2/2 (100%) |
| Rb loss (n = 23) | 0/6 (0%) | 13/15 (86.7%) | 2/2 (100%) |
| p53 aberrant (n = 22)b | 0/6 (0%) | 12/14 (80%) | 2/2 (100%) |
| Carcinoembryonic antigen (n = 14) | 3/7 (42.9%) | 2/5 (40%) | 0/2 (0%) |
| p40 (n = 7) | 0/1 (0%) | 1/4 (25%), focal | 0/2 (0%) |
| CK20 (n = 3) | ND | 0/3 (0%) | ND |
| GATA3 (n = 4) | 0/4 (0%) | ND | ND |
| Calcitonin (n = 15) | 3/9 (33.3%) | 0/6 (0%) | ND |
| CD99 (n = 8) | ND | 2/8 (25%) | ND |
| TTF1 (n = 11) | 3/6 (50%); focal | 3/3 (100%) | 1/2 (50%), focal |
| SMARCB1 loss (n = 5) | ND | 0/3 (0%) | 0/2 (0%) |
| ATRX loss (n = 5) | 0/3 (0%) | 0/2 (0%) | ND |
| S100/SOX10 (n = 5) | 0/2 (0%) | 0/1 (0%) | 0/2 (0%) |
| HMB45 (n = 1) | ND | ND | 0/1 (0%) |
| p16 (n = 7) | 0/1 (0%) | 4/4 (100%) | 0/2 (0%) |
| Ki-67 Labelling Index | 5–18% | 55–80% | 75 and 80% |
NET G2 neuroendocrine tumor, Grade 2, SCNEC small cell neuroendocrine carcinoma, LCNEC large cell neuroendocrine carcinoma, SCC squamous cell carcinoma, NA not applicable, ND Not done
aExtensive crushing artefacts in 4 cases precluded accurate mitotic counting
bAberrant p53 staining was seen as diffuse nuclear staining in the tumor cells (> 75% positive cells) in 12 cases and as a complete absence of p53 staining in the tumor cells with positive internal control (null-type staining) in 2 cases (1 case each of SCNEC and LCNEC)
On microscopy, there were 9 (33%) NETs G2 and 18 (67%) NECs; the latter group further comprised 16 SCNEC and 2 LCNEC cases. There was no case of NET G1 or G3 or MiNEN in this cohort.
Neuroendocrine Tumor, Grade 2 (n = 9)
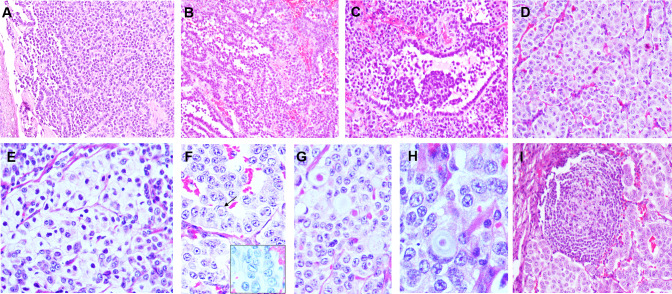
Histologic features of NET G2 are detailed in Table 3 and illustrated in Fig. 1. The majority of tumors were centered in the subepithelium and were striking for their uniformity at low magnification. Cellular monotony, random anisonucleosis, round to ovoid nuclei with stippled nuclear chromatin were common features of NE differentiation seen in all the cases. A variety of architectural patterns including organoid, trabeculae, cords, glandular, sheets, and pseudopapillary, with a variable admixture of more than one pattern, were observed (Fig. 1A–D). Remarkably, 5 (56%) tumors revealed cystic spaces lined by neoplastic cells, with an abortive papilla or a cellular nub protruding into the lumen, forming peculiar ‘glomeruloid-structures’ (Fig. 1C). The cystic spaces were either filled with pale fluid or with blood. These ‘glomeruloid-structures’ were frequently present, ranging from 3 to 8 per case. Neoplastic cells were epithelioid to plasmacytoid with eosinophilic cytoplasm. In a few cases, a dominant clear cell (n = 2) (Fig. 1E), oncocytic (n = 1), and rhabdoid (n = 1) morphology of neoplastic cells was observed. Nuclear grooves and intranuclear inclusions were identified focally in a few cases (Fig. 1F). Additional findings of calcification (n = 3), hyaline globules (n = 3) (Fig. 1G), Liesegang rings (n = 2) (Fig. 1H), and lymphoid aggregates (n = 5) (Fig. 1I) were also noted. Two cases showed spotty necrosis. The mitotic rate ranged from 2 to 5 per 2 mm2 (average 3.5/2 mm2). Ki-67 labelling index ranged from 5 to 18% (average 10.4%; median 10%).
Fig. 1.
Histomorphology of laryngeal neuroendocrine tumor (NET G2). a–d Tumors were monotonous in appearance and displayed a variety of patterns: trabeculae, cords, glandular, pseudopapillary, nests in a fibrovascular stroma; c Glomeruloid structures seen as cystic spaces lined by neoplastic cells, with abortive papillae protruding into the lumen, resembling renal glomeruli were identified in some cases; e clear cell morphology; f intranuclear grooves (inset) and inclusions (arrow); g hyaline globules; h Leisegang rings; i lymphoid aggregates
LVI were identified in 12.5% while PNI was absent. Thyroid cartilage invasion was not identified. Positive resection margins were identified in two cases. Pathologic T staging was T1 and T2 in 5 and 3 cases, respectively. The rate of LN metastasis was 62.5% in the primary resection. Pathologic N staging was Nx, N0, N1, N2b, and N3b in 1, 2, 1, 3, and 1 patient, respectively. One case had extranodal extension (< 2 mm). LNR ranged from 0.13 to 0.6 (median 0.4).
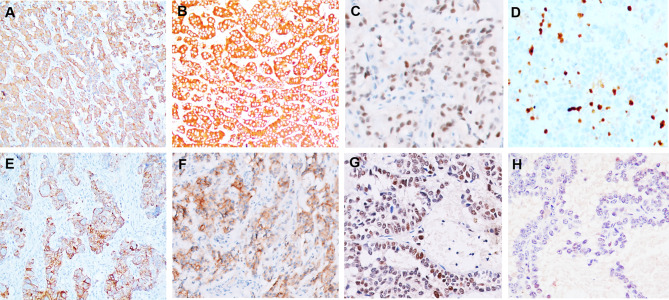
IHC results are provided in Table 3 (Fig. 2A–H). In addition to the NE markers, the tumor cells expressed cytoplasmic calcitonin (33.3%), carcinoembryonic antigen (CEA; 42.9%; diffuse in 1, and focal, moderate in 2 cases), and TTF1 (50%; weak, occasional to focal nuclear expression). p53 expression was identified as scattered, weakly positive tumor cells i.e., wild-type pattern (100%) while retinoblastoma (Rb) protein expression was retained in all the cases (100%; focal expression in 50% cases).
Fig. 2.
Immunohistochemical profile of laryngeal neuroendocrine tumor (NET G2). Neuroendocrine markers- synaptophysin (a), chromogranin (b), and INSM1 (c) were identified in all cases; d Ki67 labeling index of 18% in a case; e: calcitonin expression was present in 33% cases; f carcinoembryonic antigen immunoreactivity varied from focal to diffuse and was detected in 42.9%; g Tumor nuclei show positivity with Retinoblastoma antibody; h weak and focal expression of p53 in the tumor nuclei (wild-type pattern of staining)
Neuroendocrine Carcinoma (n = 18)
Small Cell Neuroendocrine Carcinoma (n = 16)
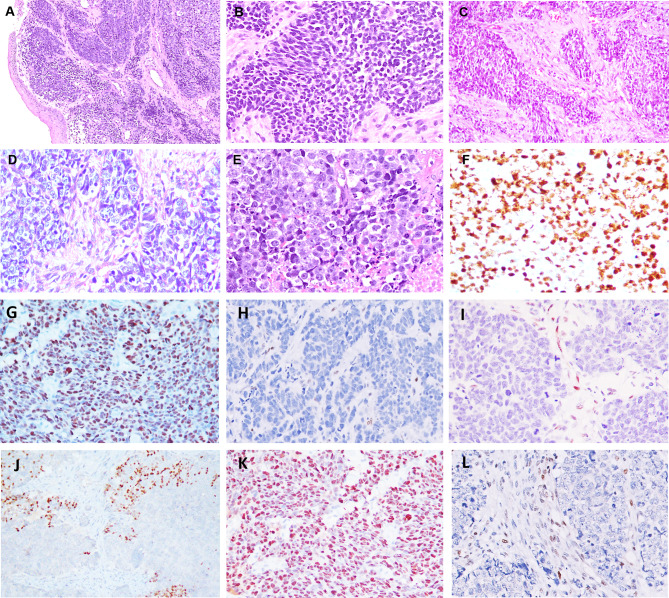
The tumors in this cohort were highly cellular and closely packed imparting a blue tumor appearance at low magnification (Fig. 3A). Surface ulceration was noted in the majority, although the bulk of tumor volume tended to be subepithelial. Tumor cells possessed round to elongate hyperchromatic nuclei, devoid of conspicuous nucleoli and surrounded by scant cytoplasm (Fig. 3A, B). Crush artifacts and confluent areas of necrosis were frequent. Mitoses (average 25/2 mm2) and apoptosis were brisk. The Ki-67 labelling index ranged from 55 to 80%, with a median of 65% and average of 70.5%). Four SCNEC cases had a mitotic rate < 10/2 mm2, but with Ki-67 > 55%; these tumors revealed extensive crushing artifacts which precluded accurate histologic counting of mitoses. Pathologic staging was only possible in 4 patients who were treated by total laryngectomy. All were advanced stage: pT3 N1 (n = 1), pT3 N3b (n = 1), and pT4a N0 (n = 2).
Fig. 3.
Laryngeal neuroendocrine carcinoma. a, b Small cell neuroendocrine carcinoma (SCNEC). a Highly cellular tumor imparting a blue tumor appearance at low magnification. b Tumor cells possess round to elongate hyperchromatic nuclei, devoid of conspicuous nucleoli and scant cytoplasm; c–e Large cell neuroendocrine carcinoma (LCNEC). c, d Tumors cells show nests and irregular islands of basaloid cells in a desmoplastic stroma; e Tumor with undifferentiated morphology showing monomorphic cells with prominent nucleoli amidst necrosis; f Ki 67 labeling index of 95% in small cell carcinoma; g diffuse and strong overexpression of p53 (80% of positive cells) in SCNEC; h completely absent immunoexpression of p53 (null-type expression) in SCNEC with weakly positive stromal cells serving as an internal positive control; i complete loss of Rb staining in SCNEC with stromal cells serving as internal positive control; j Focal TTF1 immunoreactivity in LCNEC; k p53 overexpression in LCNEC (85% of positive cells); l Complete loss of Rb in LCNEC with positive internal control of stromal cells
Large Cell Neuroendocrine Carcinoma (n = 2)
Histologic features are elaborated in Table 3 and illustrated in Fig. 3C–E. Tumors were cellular with an epithelial appearance and formed large solid nests, trabeculae, and rosettes. Peripheral palisading was noted focally. Tumors were composed of a uniform population of cells with rounded, vesicular nuclei, conspicuous nucleoli, and moderate eosinophilic cytoplasm; the cells appeared basaloid in one case and undifferentiated in the other. Although lacking morphologic differentiation, the cells were monotonous and devoid of overt pleomorphism. Scattered tumor giant cells were identified. The tumor stroma was scant and fibrotic. Large areas of necrosis and apoptosis were noted, with easily identified mitoses (10 to 22/2 mm2). The Ki-67 labelling index was 75 and 80%.
IHC results for the NEC cohort are detailed in Table 3 and Fig. 3F–L. Additional to the NE markers, the tumor cells expressed cytoplasmic CD99 (25%), TTF1 (80%), strong and diffuse nuclear and cytoplasmic p16 (66.7%), focal CEA (28.6%), and scattered p40 (25%) positivity. Aberrant p53 immunoreactivity (mutant-type pattern of staining) was observed in 87.5% cases. This aberrant staining was seen as diffuse p53 staining (in > 75% cells) in 85.7% and alternately as a complete absence (null-type) of p53 staining (with focally and weakly staining stromal cells serving as internal controls) in 14.3% of cases. A complete loss of nuclear Rb protein was observed in 88.2% while weak, scattered tumor cell positivity was detected in 11.8% cases. Calcitonin, SMARCB1 (INI1) loss, or ATRX loss was not detected. IHC for lymphoma (LCA), melanoma (SOX10, S100, HMB45), NUT carcinoma, Merkel cell carcinoma (CK20) were selectively performed during initial evaluation in some cases and were non-reactive.
Treatment and Follow-Up
The treatment modalities among the laryngeal NEN patients were varied and decided in a multidisciplinary clinic; treatment and follow-up details are in Table 2. Among the NET G2 cohort, 2 (25%) patients developed locoregional recurrence and 5 (55.6%) DM. The most frequent sites of DM were skin and lung; DM to > 1 site was noted in 3 patients. At the last follow-up (median 24 months), 2 patients were alive with no evidence of disease (ANED; follow-up of 21 and 24 months, respectively), 4 were alive with disease (AWD; median follow up 21 months), and 2 patients had died of disease (DOD) (follow up of 40 and 74 months). While Ki-67 labelling index did not yield a specific cut-off that correlated with overall survival, a cut-off of 9.5% was statistically significant for discriminating metastatic and non-metastatic patients (p = 0.018, Fisher’s exact test).
Among the SCNEC, 8 (72.7%) patients developed DM including 4 (36.4%) patients with concomitant locoregional recurrence. The most frequent sites of DM were lung, liver, and skeleton; DM to > 1 site was frequent. Follow-up was available in only 11 patients: 2 patients were ANED (follow up of 6 and 7 months, respectively), 3 were AWD (median follow up 9 months), and 6 had DOD (median follow up 6 months).
Both patients of LCNEC had DM at first clinical presentation. Sites of DM were lung, liver, and skeleton. One patient died of disease 5 months after diagnosis, while the other patient is AWD 6.5 months after initial presentation.
Discussion
Laryngeal NENs are very rare malignancies comprising less than 1% of all laryngeal neoplasms [2, 3]. The commonest laryngeal NEN reported is the SCNEC (41%) followed by NET G2 (37%) with the NET G1 being exceedingly rare (5.3%) [11, 12]. In the present study, 2/3rds of our cases were NEC while 1/3rd were NET G2. However, we did not encounter any case of NET G1/G3 or MiNENs in our series, confirming the extreme rarity of these types among laryngeal NEN.
Laryngeal NENs typically affect middle-aged to elderly (median, 64 years) patients with a history of heavy smoking [2, 9, 12]. The median age of our cohort was 60 years with a striking male preponderance, with SmCC occurring almost exclusively in males. Concordant with other studies [2, 11], > 90% of our patients had a long-standing history of tobacco consumption, including the smokeless forms. As reported in the literature [2, 11], a strong predilection for the supraglottis (82%) location, with AEF being the primary subsite, was observed in our series. While all our NET G2 cases involved the AEF, all LCNEC arose in the subglottis. Hoarseness of voice, dysphagia, cough, or dyspnoea is common. Contrasting with the conventional SCC, laryngeal NENs tend to present as submucosal polypoid or nodular masses with tan-white cut-surface [12, 13]. Tumor size usually ranges from a few mm to 4 cm [9]; the largest tumor in our series reached up to 11 cm. All tumors in our cohort were at an advanced stage: 82.6% were stage IV with 36.4% of patients showing evidence of DM at their first clinical presentation.
Pathologic features varied considerably with the tumor grade. The NET G2 tumors were characterized by cellular monotony, stippled chromatin, and a variety of architectural patterns in a fibrovascular stroma. Interestingly, peculiar ‘glomeruloid structures,’ unique to the NET G2 cohort, were identified in 56% of our tumors (Fig. 1C) and we believe the presence of these may serve as a diagnostic clue in a small biopsy. Infrequently, we encountered a predominance of clear cells, oncocytic, and rhabdoid cells. Although clear cell morphology is reported in paraganglioma or with the VHL syndrome, it is not common in laryngeal NEN. [14] Occasionally, intranuclear inclusions, nuclear grooves, Leisegang rings, and hyaline globules were also noted in our cases. Liesegang rings (laminated, ring-like structures, seen infrequently with benign cysts or inflammatory lesions) have been reported as an exceptionally rare occurrence in medullary thyroid carcinoma (MTC) [16]. However, we didn’t come across any reports in laryngeal NEN and believe the present series to be the first to report the presence of Leisegang rings in laryngeal NEN.
Little is known about the histopathologic prognostic factors of laryngeal NEN. The NET G2 category is defined by a mitotic rate of 2–10/2 mm2, and/or the presence of necrosis [7]. In the present study, the mitotic rate ranged from 2 to 5/2 mm2 while necrosis was present in 22% of the NET G2 cases. In the present study, albeit with small numbers, no correlation was found between the mitotic count or necrosis with the outcome of NET G2. Wenig et al. found LVI in only 4% NET G2 [2]. In contrast, LVI was identified in 12.5% of our cases. PNI remains an uncommon occurrence in NET G2 [2]. While laryngeal tumors in the NET G2 cohort were small (pT1-T2), LN metastasis was seen 62.5% in their primary resection with a high LNR (median 0.4). Similar to our experience, a high LN metastasis rate of 71% was reported by Wenig et al. [2].
SCNEC accounts for approximately 41% of all laryngeal NEN [11, 12]. In the present series, SCNEC formed the majority (59%) of laryngeal NENs. Laryngeal SmCC is morphologically indistinguishable from pulmonary or extra-laryngeal SmCC. Glomeruloid structures and fibrovascular stroma found in NET G2 were absent in this subset. Diagnosis of LCNEC necessitates stringent criteria [17–19]: (1) tumor with NE morphology: organoid nesting, palisading, rosettes, trabeculae; (2) mitotic rate: > 10/2 mm2; (3) necrosis; (4) moderate to abundant cytoplasm with vesicular nuclei containing coarse chromatin and prominent nucleoli; and (5) diffuse NE differentiation using either INSM1 or using chromogranin and synaptophysin together, or a combination of diffuse INSM1 with either diffuse chromogranin or synaptophysin. Consonant with published reports [11], LCNEC formed 7% of all laryngeal NEN in our series. Tumor cells exhibited basaloid and undifferentiated morphology with high mitotic and apoptotic rate.
On IHC, NE expression is mandatory for a diagnosis of laryngeal NEN. The NET G2 cases in our cohort displayed an absence of aberrant p53 immunostaining and an intact Rb expression. Although necessitating molecular confirmation, this finding indirectly hints at an underlying intact p53/Rb pathway similar to their NET G2 gastrointestinal (GI) counterparts. It is now well-recognized that NET G2 may express calcitonin (60–80%) and less commonly TTF1 (~ 15%) in a subset of cases [12]. A third of our NET G2 showed calcitonin positivity and half the cases revealed TTF1 positive cells. Notably, the TTF1 staining in all our NET G2 cases was weak and never diffuse. If unaware, this may lead to an erroneous diagnosis, particularly at a metastatic site, of MTC resulting in an unwarranted thyroidectomy. Awareness of this phenomenon, normal serum calcitonin, and thyroid ultrasonogram usually help in excluding an MTC diagnosis. Although exceedingly rare, instances of raised serum calcitonin are reported [20]. In such rare scenarios, functional imaging detecting somatostatin receptor expression i.e., somatostatin receptor scintigraphy, 68 Ga-DOTA positron emission tomography/computed tomography, can help to exclude a thyroid primary. ATRX loss, identified in a subset of well-differentiated pancreatic NET with unfavorable biology [21], was not identified in the NET G2s cases we tested.
In addition to the NE marker expression, our cases of SCNEC expressed TTF1 (100%) similar to other extra-pulmonary counterparts. The majority of NEC cases were characterized by Rb loss and aberrant p53 expression, suggesting mutations involving the p53/Rb pathway. Our findings are concordant with p53 overexpression identified in 92% of cases in Kao’s study [18]. Strong, diffuse p16 positivity was frequent. In SCNEC, p16 overexpression as an indirect upregulation following dysregulation of the Rb pathway has been demonstrated [22]. While HPV-related oropharyngeal SCNECs are documented [23, 24], the role of HPV in laryngeal NEN remains currently unclear [12, 23]. Two cases of HPV positive LCNEC reported in Halmos et al. series were negative for p16 indicating either contamination or passenger DNA [25]. HPV testing was not available in our p16 positive cases.
While Ki-67, a proliferation marker, is an integral part of the GI and pancreatic NEN grading system [26], definite cut-offs for laryngeal NEN have not been validated yet. Emerging data show a growing role of Ki-67 in laryngeal NEN prognostication [18, 25], however, Ki67 being a dynamic continuous variable, determining the optimal thresholds to risk stratify laryngeal NEN remains a challenge. In our series, the Ki-67 labelling index ranged from 5 to 18% in the NET G2. No significant cut-off correlating with overall survival could be derived. However, Ki-67 > 9.5% was found to correlate with distant metastasis within the NET G2 group. In contrast, the Ki-67 labelling index in the NEC cohort was uniformly high, all > 55%, reflecting high proliferative activity. Four SCNEC cases had mitotic rate < 10/2 mm2 but with Ki-67 > 55%; extensive crushing artifacts in these cases precluded accurate mitotic counting. Unlike mitotic rate which is a function of tissue preservation, Ki-67 is a more stable indicator of proliferative potential. A few studies have identified discordance between the mitotic rate and Ki-67 [17, 25]. Our results strongly confirm the utility of Ki-67 in laryngeal NEN prognostication; however, larger cohort studies are necessary to confirm this finding.
Histologic features of laryngeal NENs may overlap and be mistaken for commoner pathologic entities, particularly on a small biopsy. Table 4 highlights the distinguishing IHC features between NETs G2 and its morphologic mimics. The differential diagnoses (DD) of NET G2 include diverse entities such as SCC, salivary gland neoplasms (SGN), melanoma, MTC, paraganglioma (PGL), and rarely plasmacytoma, and glomus tumor. NET G2 may be misdiagnosed as SCC, the commonest laryngeal malignancy, if careful attention to uniformity of neoplastic cells and variety in architectural patterns (nested, trabecular, cords, glomeruloid-structures) is missed on low power examination. Contrarily, low-grade SGN viz. hyalinizing clear cell carcinoma, myoepithelial carcinoma, mucoepidermoid carcinoma, and adenocarcinoma, may mimic NET G2 due to cellular uniformity and diversity of growth patterns. Three of our cases were diagnosed as low-grade SGN on a pre-operative biopsy. Although not reported at this site, secretory carcinoma, theoretically, may also enter the list of DDs due to its lack of pleomorphism. Nested architecture and NE marker positivity of NENs overlap with PGL. Unlike NENs, laryngeal PGLs are predominantly seen in females, in the subglottic region and have favorable clinical outcomes [3, 14]. Compared to NET G2, a different set of DDs need consideration in the context of NEC (Table 5). For SmCC, the commonest pitfall is the basaloid or poorly differentiated SCC. Other malignancies that need distinction include solid adenoid cystic carcinoma, melanoma, lymphoma, and Merkel cell carcinoma. In the present era of new entities being increasingly recognized in the head and neck region, the list of DDs for NECs has expanded. In this regard, NUT carcinoma [27], or malignant round cell tumors such as Ewing sarcoma, Adamantinoma-like Ewing sarcoma, rhabdomyosarcoma (RMS), and poorly differentiated synovial sarcoma may need exclusion. It is important to note that NE marker positivity may be aberrantly detected in 30–40% of RMS leading to a diagnostic pitfall [28]. Expression of SMARCA4 in ovarian small cell carcinoma, hypercalcemic type, is reported [29]. However, there are no reports, yet, of SMARCA4 expression in laryngeal SCNEC. LCNEC can be a particularly challenging diagnosis primarily because it can remain unsuspected on morphology. LCNEC may be easily mistaken for basaloid SCC if IHC is not employed. On the other hand, LCNEC with undifferentiated appearance may need distinction from NUT carcinoma [27], and SMARCB1- [30] or /SMARCA4-deficient carcinoma. As reports of NE marker positivity are emerging in the latter, it would be prudent to include SMARCA4 in the IHC panel when considering a diagnosis of LCNEC. Infrequently, plasmablastic lymphoma may require exclusion, as LCA and CD20 may be negative; a history of retroviral infection is a vital clue to the diagnosis. Rarely, an infiltration by an undifferentiated variant of anaplastic thyroid carcinoma may also mimic LCNEC wherein the tumor epicenter is in the thyroid (Table 5).
Table 4.
Immunohistochemistry in differential diagnoses of laryngeal neuroendocrine tumor (NET, G2) [8, 10, 18, 19]
| Antibody | NET G2 | SCC | CCC | MyoECa | MEC | ADCa | Melanoma | PGL | MTC | Plasmacytoma | Glomus |
|---|---|---|---|---|---|---|---|---|---|---|---|
| AE1/AE3 | + | + | + | + | + | + | − | − | + | − | − |
| Synaptophysin | + | _ | − | − | − | − | − | + | + | − | − |
| Chromogranin | + | _ | − | − | − | − | − | + | + | − | − |
| INSM1 | + | _ | − | − | − | − | − | + | + | − | − |
| CD56 | + | _ | − | − | − | − | − | + | + | − | − |
| p40 | − | + + | + + | + | + + | − | − | − | − | − | − |
| GATA3 | − | − | − | − | − | − | − | + | − | − | − |
| Calcitonin | ± | − | − | − | − | − | − | − | + | − | − |
| CD99 | ∓ | − | − | − | − | − | − | − | − | − | − |
| TTF1 | ∓ | − | − | − | − | − | − | − | ± | − | − |
| SMA | − | − | − | + | − | − | − | − | − | − | + |
| MUM1 | − | − | − | − | − | − | − | − | − | + | − |
| S-100 | − | − | − | + | − | + a | + | + ; STC | − | − | − |
| Melan A; HMB45 | − | − | − | − | − | − | + | − | − | − | − |
NET G2 Neuroendocrine tumor, grade 2, SCC Squamous cell carcinoma, CCC Hyalinizing clear cell carcinoma, MyoECa Myoepithelial carcinoma, MEC Mucoepidermoid carcinoma, ADCa Adenocarcinoma, PGL Paraganglioma, MTC Medullary thyroid carcinoma, STC Sustentacular cells
+ + : strong, diffuse
∓ : more frequently negative than positive
± : more frequently positive than negative
aIn the Salivary Polymorphous adenocarcinoma type
Table 5.
Immunohistochemistry in differential diagnoses of laryngeal neuroendocrine carcinoma [8, 10, 16, 20–23]
| Antibody | NEC | SCC/BSCCC | Melanoma | Solid ACC | NUTa | SMARCB-1 | NHL | PBL | MCA | ALES | EWS | RMS | PDSS | ATC |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Keratin | + | + | − | + | + | + | − | − | + | + | ∓ | − | + | + |
| Synaptophysin | + | − | − | − | − | − | − | − | + | ± | ± | ∓ | − | − |
| Chromogranin | + | − | − | − | − | − | − | − | + | − | − | ∓ | − | − |
| INSM1 | + | − | − | − | − | − | − | − | + | − | ± | ± | − | − |
| CD56 | + | − | − | − | − | − | − | − | + | + | − | + | − | − |
| NKX2.2 | ± | − | − | − | − | − | − | − | ∓ | + | + | − | − | − |
| p40 | ∓ f | + | − | + | + | + | − | − | − | + | − | − | − | − |
| CD117 | ± | − | ± | + | − | − | − | − | − | ∓ | − | − | − | − |
| MYB | − | − | − | + | − | − | − | − | − | − | − | − | − | − |
| S100/SOX10 | − | − | + | + | − | − | − | − | − | − | − | − | ∓ | − |
| Melan A/ HMB45 | − | − | + | − | − | − | − | − | − | − | − | − | − | − |
| LCA | − | − | − | − | − | − | + | ∓ | − | − | − | − | − | − |
| MUM1; LCR | − | − | − | − | − | − | ± | + | − | − | − | − | − | − |
| CK20 | − | − | − | − | − | − | − | − | + | − | − | − | − | − |
| Polyoma virus | − | − | − | − | − | − | − | − | + | − | − | − | − | − |
| p16 | + | − | − | + f | − | − | − | − | − | − | − | − | − | − |
| NUT | − | − | − | − | + | − | − | − | − | − | − | − | − | − |
| Desmin | − | − | − | − | − | − | − | − | − | − | − | + | − | |
| SMA | − | − | − | − | − | − | − | − | − | − | − | − | − | − |
| CD99 | ∓ | − | − | − | − | − | − | − | ∓ | + | + | ± cy | + | − |
| Fli1 | − | − | − | − | − | − | − | − | ∓ | + | + | − | − | − |
| TTF1 | + | − | − | − | − | − | − | − | − | − | − | − | − | − |
| PAX8 | − | − | − | − | − | − | − | − | − | − | − | − | − | + |
| SMARCB1 | + | + | + | + | + | − | + | + | + | + | + | + | f* | + |
NEC neuroendocrine carcinoma, SCC squamous cell carcinoma, BSCC basaloid squamous cell carcinoma, ACC adenoid cystic carcinoma, NUT NUT carcinoma, SMARCB1 SMARCB1 (INI1) deficient carcinoma, NHL non-hodgkin lymphoma, PBL plasmablastic lymphoma, MCA Merkel cell carcinoma, ALES adamantinoma-like Ewing Sarcoma, EWS Ewing sarcoma, RMS Rhabdomyosarcoma, PDSS poorly differentiated synovial sarcoma, ATC anaplastic thyroid carcinoma, f focal, cy cytoplasmic, f* mosaic pattern of loss
∓ : More frequently negative than positive
± : More frequently positive than negative
aRare cases show focal expression of neuroendocrine markers
Owing to their rarity, and limited experience, treatment algorithms for laryngeal NEN are not standardized. Surgery remains the mainstay of treatment of NET G2 while the role of radiotherapy and chemoradiotherapy or PRRT remains poorly defined. On the other hand, chemotherapy (usually a cisplatin–etoposide combination), with or without RT is the primary treatment for the majority of NEC patients [13].
While we had no experience with NET G1, sparse data on the NET G1 suggest a favorable short-term outcome with late DM [11]. The clinical behavior of the laryngeal NEN patients in our series was aggressive; all patients were at an advanced stage; there was a high rate of LN metastasis (63%, 83%, and 100% in NET G2, SCNEC, and LCNEC, respectively) and DM (56%, 73%, 100%, in NET G2, SmCC, LCNEC, respectively). Remarkably, cutaneous/subcutaneous sites were the most frequent site of DM in NET G2, whereas lung and liver predominated in the NEC groups. These patterns of DM are similar to those reported by others [2, 10]. Five-year survival in NET G2 patients is reported to be 53% [10], with 38% of patients in a series of 54 NET G2 by Wenig et al. had DOD, similar to the 25% of NET G2 in our series dying of disease; half the patients were alive but with the disease at last follow-up. Our cohort of NEC patients had a fulminant course with more than half of SCNEC and LCNEC patients dying of their disease. Sparse literature on LCNEC suggests a similar aggressive course with a 5 year-DSS of 15.3% [10]; 90% of patients in the 10 patients reported by Lewis et al. had stage IV disease, and 60% patients DOD [16].
Our analysis had limitations of a retrospective study, a small sample size, inadequate follow-up, and lack of molecular characterization. Nonetheless, the present study provides rare data on a relatively large cohort of a rare malignancy that further expands the knowledge of the morphologic and clinical spectrum of laryngeal NEN while underscoring the need to avoid diagnostic errors, more so, in the light of newer entities being recognized in the head and neck region. To the best of our knowledge, this is the largest study from the South-Asian region in the Indian population. We also report for the first time the presence of Leisegang rings in laryngeal NET and describe the presence of ‘glomeruloid structures as a unique finding that may aid in the diagnosis of laryngeal NENs. Importantly, our study illustrates contrasting p53/Rb immunophenotypes of NET and NEC that are reflective of divergent genotypes and hence, supports the distinction of laryngeal NEN into the two types. Further, our study reports a Ki-67 labelling index of greater than 9.5% as a risk factor for distant metastasis in the NET G2 category.
Conclusions
Laryngeal NENs are extremely rare and heterogenous malignancies. SCNECs form the bulk of laryngeal NEN cases followed by NET G2. LCNEC is an uncommon and potentially under-recognized subtype. Contrasting with the GI and pulmonary neuroendocrine tumors, NET G1 tumors are exceedingly rare in the larynx. Awareness of the wide pathologic spectrum of laryngeal NEN and judicious use of IHC is imperative to avoid treacherous pitfalls and render an accurate diagnosis which in turn is essential for the optimal treatment. The majority of the laryngeal NEN in our series displayed aggressive biology with a high rate of nodal and distant metastases. Distinction of laryngeal NEN into NET and NEC categories is supported by divergent p53/Rb immunophenotypes. Our results strongly confirm the utility of Ki-67 in refining laryngeal NEN prognostication; however, larger cohort studies are needed to determine the optimal thresholds for risk stratification in laryngeal NEN. Further, elucidating the underlying molecular mechanisms may unravel actionable therapeutic targets. Finally, larger, prospective, multi-institutional studies are warranted to provide further insights into the clinical biology of this rare malignancy.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We sincerely thank Dr Jay Mehta, Center of Oncopathology, Mumbai, for his generous support in IHC standardization, and Shirsat laboratory, ACTREC, Ms. Priti Shenoy and Ms. Neelam for their technical assistance during the study.
Author Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by [AS], and [MB]. The first draft of the manuscript was written by [MB] and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Funding
No funding obtained.
Declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical Approval
All procedures performed in the study were in accordance with the ethical standards of the Institutional Ethics Committee.
Informed Consent
Not required as per institutional ethics committee’s policy for retrospective case series. The authors declare that all information is anonymized and the submission does not include images that may identify any patient.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Goldman NC, Hood CI, Singleton GT. Carcinoid of the larynx. Arch Otolaryngol. 1969;90:64–67. doi: 10.1001/archotol.1969.00770030066013. [DOI] [PubMed] [Google Scholar]
- 2.Wenig BM, Hyams VJ, Heffner DK. Moderately differentiated neuroendocrine carcinoma of the larynx: a clinicopathologic study of 54 cases. Cancer. 1988;62:2658–2676. doi: 10.1002/1097-0142(19881215)62:12<2658::AID-CNCR2820621235>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 3.Ferlito A, Devaney KO, Rinaldo A. Neuroendocrine neoplasms of the larynx: advances in identification, understanding, and management. Oral Oncol. 2006;42:770–788. doi: 10.1016/j.oraloncology.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 4.Woodruff JM, Huvos AG, Erlandson RA, et al. Neuroendocrine carcinomas of the larynx: a study of two types, one of which mimics thyroid medullary carcinoma. Am J Surg Pathol. 1985;9:771–790. doi: 10.1097/00000478-198511000-00001. [DOI] [PubMed] [Google Scholar]
- 5.Shanmugaratnam K. Histological typing of tumours of the upper respiratory tract and ear: World Health Organization International histological classification of tumors. 2. Berlin: Springer-Verlag; 1991. [Google Scholar]
- 6.Barnes L, Eveson JW, Reichart P, et al. Pathology and genetics of head and neck tumours: World Health Organization classification of tumors. Lyon: IARC Press; 2005. [Google Scholar]
- 7.Perez-Ordonez B, Bishop JA, Gnepp DR, et al. et al. Neuroendocrine tumors. In: El-Naggar AK, Chan JKC, Grandis JR, et al.et al., editors. WHO classification of head neck tumours. Lyon: IARC; 2017. pp. 95–98. [Google Scholar]
- 8.Strosberg C, Ferlito A, Triantafyllou A, et al. Update on neuroendocrine carcinomas of the larynx. Am J Clin Pathol. 2019;152:686–700. doi: 10.1093/ajcp/aqz106. [DOI] [PubMed] [Google Scholar]
- 9.Rindi G, Klimstra DS, Abedi-Ardekani B, et al. A common classification framework for neuroendocrine neoplasms: an International Agency for Research on Cancer (IARC) and World Health Organization (WHO) expert consensus proposal. Mod Pathol. 2018;31(12):1770–86. [DOI] [PMC free article] [PubMed]
- 10.Patel SG, Lydiatt WM, Glastonbury CM, et al. Larynx. In: Amin MB, editor., et al., AJCC cancer staging manual. 8th ed. New York, NY: Springer; 2017.
- 11.van der Laan TP, Plaat BEC, van der Laan BFAM, Halmos GB. Clinical recommendations on the treatment of neuroendocrine carcinoma of the larynx: a meta-analysis of 436 reported cases. Head Neck. 2015;37:707–715. doi: 10.1002/hed.23666. [DOI] [PubMed] [Google Scholar]
- 12.Perez-Ordoñez B. Neuroendocrine carcinomas of the larynx and head and neck: challenges in classification and grading. Head Neck Pathol. 2018;12:1–8. doi: 10.1007/s12105-018-0894-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhu Y, Gao L, Meng Y, Diao W, Zhu X, Li G, Gao Z, Chen X. Laryngeal neuroendocrine carcinomas: a retrospective study of 14 cases. Biomed Res Int. 2015;2015:832194. doi: 10.1155/2015/832194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hunt JL, Ferlito A, Hellquist H, Rinaldo A, Skálová A, Slootweg PJ, et al. Differential diagnosis in neuroendocrine neoplasms of the larynx. Adv Anat Pathol. 2017;24:161–168. doi: 10.1097/PAP.0000000000000147. [DOI] [PubMed] [Google Scholar]
- 15.Gavin K, Banville N, Gibbons D, Quinn CM. Liesegang rings in inflammatory breast lesions. J Clin Pathol. 2005;58:1343–1344. [PMC free article] [PubMed] [Google Scholar]
- 16.Sancheti S, Jain S. Liesegang rings: extremely rare structures in malignant lesions. Int J Surg Pathol. 2018;26:39–40. doi: 10.1177/1066896917722389. [DOI] [PubMed] [Google Scholar]
- 17.Lewis JS, Spence DC, Chiosea S, Barnes EL, Brandwein-Gensler M, El-Mofty SK. Large cell neuroendocrine carcinoma of the larynx: definition of an entity. Head Neck Pathol. 2010;4:198–207. doi: 10.1007/s12105-010-0188-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kao H-L, Chang W-C, Li W-Y, Chia-Heng Li A, Fen-Yau LA. Head and neck large cell neuroendocrine carcinoma should be separated from atypical carcinoid on the basis of different clinical features, overall survival, and pathogenesis. Am J Surg Pathol. 2012;36:185–192. doi: 10.1097/PAS.0b013e318236d822. [DOI] [PubMed] [Google Scholar]
- 19.Kusafuka K, Abe M, Iida Y, et al. Mucosal large cell neuroendo-crine carcinoma of the head and neck regions in Japanese patients: a distinct clinicopathological entity. J Clin Pathol. 2012;65:704–709. doi: 10.1136/jclinpath-2012-200801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Feola T, Puliani G, Sesti F, Modica R, Biffoni M, Di Gioia C, Carletti R, Anastasi E, Di Vito V, Centello R, Lenzi A, Isidori AM, Faggiano A, Giannetta E. Laryngeal neuroendocrine tumor with elevated serum calcitonin: a diagnostic and therapeutic challenge. Case report and review of literature. Front Endocrinol. (Lausanne) 2020;11:397 [DOI] [PMC free article] [PubMed]
- 21.Chou A, Itchins M, de Reuver PR, Arena J, Clarkson A, Sheen A, Sioson L, Cheung V, Perren A, Nahm C, Mittal A, Samra JS, Pajic M, Gill AJ. ATRX loss is an independent predictor of poor survival in pancreatic neuroendocrine tumors. Hum Pathol. 2018;82:249–257. doi: 10.1016/j.humpath.2018.07.032. [DOI] [PubMed] [Google Scholar]
- 22.Alos L, Hakim S, Larque AB, et al. p16 overexpression in high-grade neuroendocrine carcinomas of the head and neck: potential diagnostic pitfall with HPV-related carcinomas. Virchows Arch. 2016;469:277–284. doi: 10.1007/s00428-016-1982-1. [DOI] [PubMed] [Google Scholar]
- 23.Bishop JA, Westra WH. Human papillomavirus-related small cell carcinoma of the oropharynx. Am J Surg Pathol. 2011;35:1679–1684. doi: 10.1097/PAS.0b013e3182299cde. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kraft S, Faquin WC, Krane JF. HPV-associated neuroendo- crine carcinoma of the oropharynx: a rare new entity with potentially aggressive clinical behavior. Am J Surg Pathol. 2012;36:321–330. doi: 10.1097/PAS.0b013e31823f2f17. [DOI] [PubMed] [Google Scholar]
- 25.Halmos GB, van der Laan TP, van Hemel BM, et al. Is human papillomavirus involved in laryngeal neuroendocrine carci- noma? Eur Arch Otorhinolaryngol. 2013;270:719–725. doi: 10.1007/s00405-012-2075-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Klimstra DS, Klöppel G, La Rosa S, Rindi G. Classification of neuroendocrine neoplasms of the digestive system. In: WHO Classification of Tumours Editorial Board, editors. WHO clas-sification of tumours. Digestive system tumours, 5th ed. Lyon: IARC; 2019. pp. 16–19
- 27.Hellquist H, French CA, Bishop JA, Coca-Pelaz A, Propst EJ, PaivaCorreia A, Ngan BY, Grant R, Cipriani NA, Vokes D, Henrique R, Pardal F, Vizcaino JR, Rinaldo A, Ferlito A. NUT midline carcinoma of the larynx: an international series and review of the literature. Histopathology. 2017;70:861–868. doi: 10.1111/his.13143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bahrami A, Gown AM, Baird GS, Hicks MJ, Folpe AL. Aberrant expression of epithelial and neuroendocrine markers in alveolar rhabdomyosarcoma: a potentially serious diagnostic pitfall. Mod Pathol. 2008;21:795–806. doi: 10.1038/modpathol.2008.86. [DOI] [PubMed] [Google Scholar]
- 29.Conlon N, Silva A, Guerra E, Jelinic P, Schlappe BA, Olvera N, Mueller JJ, Tornos C, Jungbluth AA, Young RH, Oliva E, Levine D, Soslow RA. Loss of SMARCA4 expression is both sensitive and specific for the diagnosis of small cell carcinoma of ovary, Hypercalcemic type. Am J Surg Pathol. 2016;40:395–403. doi: 10.1097/PAS.0000000000000558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Neves-Silva R, Almeida LY, Silveira HA, Colturato CBN, Duarte A, Ferrisse TM, Silva EV, Vanzolin BF, Bufalino A, Ribeiro-Silva A, León JE. SMARCB1 (INI-1) and NUT immunoexpression in a large series of head and neck carcinomas in a Brazilian reference center. Head Neck. 2020;42:374–384. doi: 10.1002/hed.26008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.