Abstract
Tumour heterogeneity in oral cancer is attributed to the presence of cancer stem cells (CSCs). CSCs are the most migratory and metastatic cellular subpopulation within tumours. Assessment of CSC markers as significant predictors of lymph node metastasis may prove valuable in the clinical setting. Furthermore, analysis of this panel of putative stem cell markers in oral dysplasia may additionally inform of the likelihood for oral potentially malignant disorders (OPMDs) to progress to oral squamous cell carcinoma (OSCC). The present study aims to assess the significance of CSC markers in the progression of OPMDs to OSCC and assessment of lymph node metastasis in OSCC. CD44 and ALDH1 were assessed immunohistochemically in 25 normal, 30 OPMDs, and 24 OSCCs. CD44 is a membranous marker and ALDH1 is a cytoplasmic marker. The immunohistochemical expression of these markers were compared between OPMDs with and without dysplasia, as well as between low-risk and high-risk dysplasias. Similarly, expression was compared between OSCC with and without lymph node metastasis and among grades of OSCC. Positive CD44 expression was seen in all normal mucosal tissues. The expression decreased from normal epithelium to OPMDs but increased in OSCC. CD44 expression was positive in 21 cases of OSCC (87.5%) and reduced from well-differentiated to poorly differentiated OSCC. CD44 staining index was higher in OSCC without lymph node metastasis (3.59) when compared with OSCC with lymph node metastasis (1.33). There was a statistically significant difference observed in the ALDH1 staining index among three groups (p < 0.05), with highest expression seen in OSCC. Within OPMDs, the ALDH1 staining index was statistically higher in OPMDs with dysplasia as compared to OPMDs without dysplasia. Furthermore, the expression was higher in OPMDs with high-risk dysplasia when compared with low-risk dysplasia, but this was not statistically significant (p = 0.82). In conclusion, The CD44 positive population possesses properties of CSCs in head and neck carcinoma, and continuous shedding could be found after CD44 down-regulation. The present study reports differences in ALDH1 expression between OPMDs with and without dysplasia, dysplastic and non-dysplastic epithelia, and low-risk and high-risk dysplasia. These findings may suggest ALDH1 as a specific marker for dysplasia. CD44 demonstrated a difference in staining index in OSCC without lymph node metastasis versus OSCC with lymph node metastasis. These findings may suggest CD44 as a marker for lymph node metastasis. Both proteins may play key roles in the tumorigenicity of CSCs in OPMDs and OSCC.
Keywords: Cancer stem cells; CD44; ALDH1, Cancer stem cell markers, Oral potentially malignant disorders, Hyperkeratosis, Oral submucous fibrosis, Dysplasia; Oral squamous cell carcinoma; Lymph node metastasis
Introduction
Despite treatment advances to improve quality of life, survival rates of oral cancer have not changed significantly in more than 30 years. Mortality from OSCC remains high due to metastases and therapy-resistant local and regional recurrences [1]. After standard therapy, a subset of patients fails to respond to treatment and/or experiences recurrence. The current OSCC progression model has limitations in explaining the heterogeneity observed between cases. Recent studies have shown that heterogeneity among tumours may be attributed to the presence of cancer stem cells (CSCs), tumour cells that have characteristics of both stem cells and cancer cells [2]. Presence of CSCs and their ability to escape treatment modalities may be a key mechanism that determines treatment failure. Presence and maintenance of CSCs, the most migratory and metastatic cellular subpopulation within the tumour, is associated with metastasis. A worse prognosis of OSSC is associated with metastasis to cervical lymph nodes. Assessment of these markers as significant predictor of lymph node metastasis could be highly valuable in the clinical setting.
While OSCCs may arise de novo, most are preceded by the presence of clinically apparent changes of oral mucosa, termed oral potentially malignant disorders (OPMDs) [3]. As numerous etiologies may mimic OPMDs, there is a need for better predictors of malignant transformation in OPMDs. Analysis of a panel of putative stem cell markers in dysplastic oral tissues may provide more informed assessment of OPMDs and their progression to OSCC. To facilitate the identification and purification of normal stem cells and CSCs, specific cell surface markers have been investigated. The main surface marker phenotypes associated with stem cell characteristics include Cluster of Differentiation 44 (CD44) and Aldehyde Dehydrogenase1 (ALDH1) [4]. CD44 is a cell surface glycoprotein receptor for hyaluronic acid (HA), and is involved in cancer cell adhesion, migration, and metastasis [5]. It is strongly involved in migration of neoplastic cells and thus metastatic spread of malignant tumours. CD44 cell-surface marker has been used to identify putative CSCs in various tumour types including breast, prostate, pancreatic, and head and neck carcinomas [6–9].
The cytosolic enzyme ALDH1 is a putative CSC marker in a variety of malignancies and is involved in the oxidation of retinol to retinoic acid in early stem cell differentiation. ALDH1 activity was recently discovered to be an identifying marker of CSC-like cells in head and neck squamous cell carcinomas (HNSCC) [10]. ALDH1 positivity also corresponds to the number of cells undergoing epithelial–mesenchymal transition, a process that is considered a key prerogative for the formation of metastases. However, correlations between ALDH1 and the clinicopathologic features of OSCC, as well as its prognostic value, are still subject to debate. We evaluated these CSC markers as predictors of lymph node metastasis to identify at risk OPMDs for progression to OSCC. We aimed to determine the prognostic significance of CSC markers CD44 and ALDH1 in OPMDs and OSCC.
Materials and Methods
This in-vitro, tissue-based, immunohistochemical study was carried out in the departments of Oral Pathology and Microbiology at our institution. Paraffin-embedded tissue sections of clinically and histopathologically diagnosed cases of OPMDs and OSCCs were used for CD44 and ALDH1 assessment. Samples of non-inflammatory oral mucosa tissues, obtained during minor oral surgical procedures in healthy individuals, were used as normal controls. Diagnosis of the selected samples was confirmed by routine haematoxylin and eosin stain, followed by immunohistochemical staining, data analysis, interpretation, and statistical analysis.
Study Sample
The study comprised of 25 normal cases (Group I), 30 OPMD cases (Group II), and 24 OSCC cases. The OPMDs comprised histopathological diagnoses of hyperkeratosis (18 cases) and oral submucous fibrosis (OSMF, 12 cases). These were assessed histologically for dysplasia and graded as high-risk or low-risk dysplasia per criteria of Kujan et al. [11]. Out of 30 cases of OPMDs, 22 cases showed dysplasia. Of these, 7 were low-risk and 15 were high-risk dysplasia. The remaining 8 were without dysplasia. Group III comprised histopathologically diagnosed cases of OSCC and were assessed for tumour grade. Fifteen cases were well-differentiated OSCC (WDOSCC), 6 moderately-differentiated (MDSCC), and 3 were poorly-differentiated (PDSCC). Lymph node metastases were confirmed histopathologically. Fifteen OSCCs were negative for lymph node metastasis and nine cases were positive.
Immunohistochemistry
A total three, 3.5 μm sections were taken from formalin fixed paraffin embedded (FFPE) blocks of tissues of selected cases. One section was stained with haematoxylin & eosin (H&E) for histopathological assessment. The other two sections were placed on silane coated slides and treated for immunohistochemical analysis of CD44 (DF 1485, ready to use mouse monoclonal antibody in PBS with carrier protein and preservative—Biogenex) and ALDH1A1/ALDH1 (Clone EP168, ready to use mouse monoclonal antibody in PBS with carrier protein and preservative—Bio SB)].
Tissue sections were incubated at 56 °C for 2 h before dewaxing. Dewaxed sections were deparaffinized twice in xylene, treated with a graded series of alcohol (100%, 95%, 85% and 75%) then water, and then bathed in freshly prepared phosphate buffered saline (PBS, pH 7.4) for 5–10 min. For both CD44 and ALDH1, heat induced antigen retrieval was performed by immersion in 10 mM citrate buffer at pH 6 at 600 W in an autoclave under 15 psi pressure at 121 °C for 30 min. The slides were bench cooled for 20 min and washed with PBS working solution. The excess buffer was wiped using absorbent wipes. Endogenous peroxidase blocking was performed using 3% hydrogen peroxide for 10 min followed by a PBS wash. The tissue sections were covered with prediluted primary antibodies to CD44 and ALDH1 for 60 min in a humidifying chamber. Lastly, after PBS washing, tissue sections were treated with 3,3′-diaminobenzidine-tetrahydrochloride (DAB) in chromogen as a substrate chromogen and counterstained with Mayer’s haematoxylin for 3 min. Tonsils and breast carcinoma sections were used as positive controls for CD44 and ALDH1, respectively. One section was used as negative control by omitting the primary antibody. The slides were mounted, observed, and evaluated using Leica DM LB2 (Leica microscope) at ×100 magnification followed by ×400 magnification. Sections stained with CD44 antibody and ALDH1 antibody were observed under a research microscope with a computer assisted image analyser (Motic microscope attached to a computer with Motic advanced images 3.2 software), to assess expression in all three groups. The presence of brown coloured membranous staining for CD44 and brown cytoplasmic staining for ALDH1 was interpreted as positive staining. The IHC stained slides were evaluated by the staining pattern in the basal and suprabasal cells of OPMDs and in the tumour cells in the stroma for OSCC at ×400 magnification.
Evaluation of CD44 and ALDH1 Expression
The expression of CD44 staining was evaluated by the presence or absence of brown staining (intensity) on cell membranes, and expression was graded based on the intensity and percentage of positive cells [12]. ALDH1 positive cells showed cytoplasmic staining, and the intensity of staining and percentage of positive cells was evaluated [13]. The whole slide was scanned and 1000 cells per specimen were counted at 400× magnification. The percentage of expression of CD44 and ALDH1 protein was determined using the number of positive cells/total number of cells (minimum of 1000 cells) in chosen high power fields using grid-aided image analysis software (Nikon). The percentage of positive cells (P) was assessed (0% = no positive cells, 1 = 1–25% positive cells, 2 = 26–50% positive cells, 3 = 50–75% positive cells, and 4 = 75–100% positive cells). Intensity (I) of staining was also evaluated: 0 = no cells positive, 1 = pale brown staining, 2 = dark brown staining. The total immunoscore for CD44 and ALDH1 was calculated based on percentage of immunopositive cells (A) multiplied by intensity score (B) (i.e. A × B = staining index). This staining index was classified as: 0 = Zero (Absent), 1, 2 = Low, 3, 4, and 6–8 = High.
All H&E and immunohistochemically stained sections were evaluated by two observers to eliminate inter-observer bias. Any disagreements were resolved. The data were subjected to statistical analysis using the Statistical Package for Social Sciences (SPSS v 21.0, IBM).
Statistical Analysis and Results
Non-parametric statistical analysis was performed using SPSS Statistics V.20 software. The CD44 and ALDH1 staining indices were compared between groups I, II and III, as well as subgroups IIa, IIb, IIIa and IIIb. Pairwise comparison of CD44 and ALDH1 staining index was done in study groups using Tukey’s Post Hoc Tests.
A total of 79 samples were included in the present study. These were categorized in three groups: group I normal (n = 25), group II OPMDs (n = 30), and group III OSCC (n = 24). In group II, there were 18 cases of hyperkeratosis and 12 cases of OSMF. OPMDs were histopathologically assessed for dysplasia and categorised into high-risk (15 cases) and low-risk dysplasias (7 cases). There were 22 OPMD cases with dysplasia and 8 without dysplasia. Group III had 6 WDSCC, 15 MDSCC, and 3 PDSCC. 15 OSCCs were negative for lymph node metastasis and 9 had lymph node metastasis.
The mean age of occurrence in group II was 49.53 years and in group III was 54.54 years. In group II, the male to female ratio was 2:1, and in group III there were 22 males and 2 females. The male predominance suggests increased male indulgence in high-risk habits such as the use of tobacco, betel nut, and alcohol. In group III, the most common site for occurrence was buccal mucosa (41.66%), followed by tongue (33.33%), and gingiva-buccal-sulcus (25%). Buccal mucosa as the most common site of occurrence for oral cancer may be due to tobacco chewing and betel quid habits common in Indians.
CD44 Results
All 25 cases in group I showed positive CD44 staining. Staining in normal epithelium was mostly present in basal and suprabasal cell layers; however, a few cases demonstrated positive staining throughout the entire thickness of epithelium (Fig. 1). In group II, 24 cases showed CD44 positivity a at the basal and suprabasal layers of the epithelium. In low-risk OPMD cases, CD44 expression was seen up to parabasal cell layers with intense staining of basal cells. In high-risk dysplasias, CD44 expression was less intense in basal cell layers although positivity was observed in basal and parabasal cell layers. In group III, 21 of 24 cases showed membranous positivity in malignant epithelial cells (Figs. 2, 3, and 4).
Fig. 1.
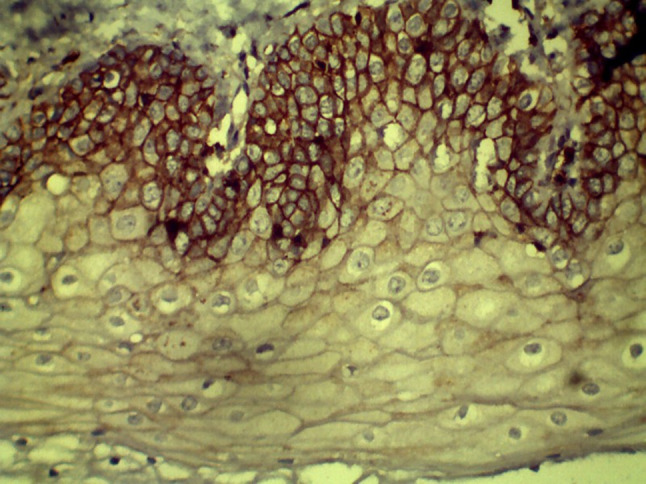
CD44 expression in Group I (NOM) (40×)
Fig. 2.

CD44 expression in hyperkeratosis with low risk (10×)
Fig. 3.
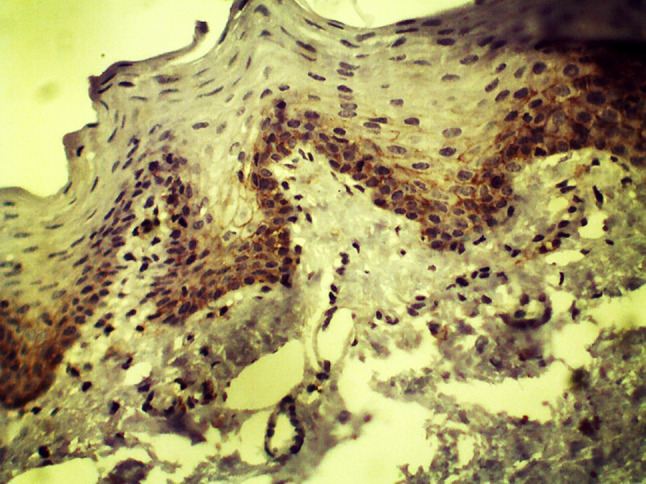
CD44 expression in hyperkeratosis without dysplasia (10×)
Fig. 4.

CD44 expression in OSMF with low risk dysplasia (40×)
A statistically significant difference was seen with CD44 expression across groups (p = 0.000) (Table 1). CD44 expression was highest in group I (mean staining index of 4.68). Expression reduced significantly from group I to group II (mean staining index of 1.67, p = 0.000, Table 2). CD44 expression was increased in group III (mean staining index of 2.67) as compared to group II, but the expression was lower when compared with group I (p = 0.006). When CD44 expression was compared between hyperkeratosis and OSMF within group II, CD44 positivity was seen in 14 cases (77.77%) of hyperkeratosis, with expression in basal and suprabasal cell layers (mean staining index of 1.16). 83.33% cases of OSMF showed CD44 positivity and staining was noted in basal and parabasal cell layers (mean staining index of 2.41). Staining was observed to be more intense in OSMF with dysplasia as compared to hyperkeratosis with dysplasia (Fig. 4). In OPMDs, cases without dysplasia showed more CD44 positivity (mean staining index of 3.00) when compared with OPMDs with dysplasia (mean staining index as 1.18). The difference was statistically significant (p < 0.05). When expression was compared between grades of dysplasia, CD44 staining index was increased in low risk dysplasia (1.57) as compared to high risk dysplasia (1.00), but the difference was not statistically significant (Fig. 5).
Table 1.
CD44 staining index in Group I, II and III
| Groups | N | Mean | Std. deviation | Std. error | Minimum | Maximum | p value of one way ANOVA | |
|---|---|---|---|---|---|---|---|---|
| Staining index of CD44 (Intensity × percentage) | I | 25 | 4.68 | 2.610 | .522 | 0 | 8 | .000** |
| II | 30 | 1.67 | 1.422 | .260 | 0 | 6 | ||
| III | 24 | 2.67 | 2.565 | .524 | 0 | 8 | ||
| Total | 79 | 2.92 | 2.531 | .285 | 0 | 8 |
**Highly significant
Table 2.
Pair wise comparison using Tukey’s Post Hoc tests
| Dependent variable | (I) group | (J) group | Mean Difference (I-J) | Std. Error | p value |
|---|---|---|---|---|---|
| Staining index of CD44 (Intensity × percentage) | 1 | 2 | 3.013* | .600 | .000** |
| 1 | 3 | 2.013* | .633 | .006** | |
| 2 | 3 | − 1.000 | .607 | .232# |
**Highly significant
#Less/not significant
Fig. 5.
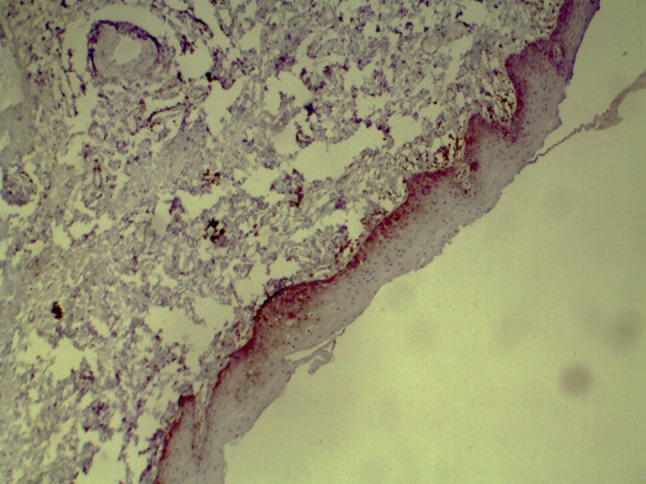
CD44 expression in OSMF without dysplasia (10×)
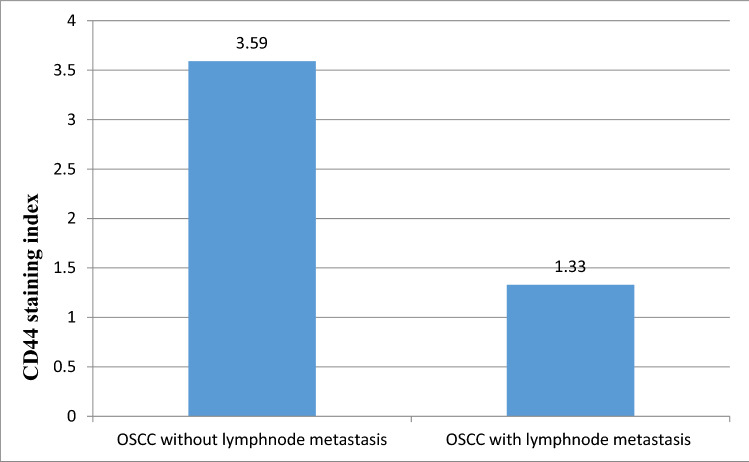
In group III, CD44 expression was correlated with histopathological grade and found to be highest and most intense in WDSCC (Figs. 6 and 7; mean staining index of 2.85). This was followed by MDSCC (Fig. 8; mean staining index of 2.84), and it was least in PDSCC (Figs. 9 and 10; mean staining index of 1.33). Furthermore, CD44 expression was related to lymph node status and higher in OSCC without lymph node metastasis (mean staining index of 3.59) as compared to OSCC with lymph node metastasis (mean staining index of 1.33) The difference was significant (p < 0.05) (Bar Diagram 1).
Fig. 6.
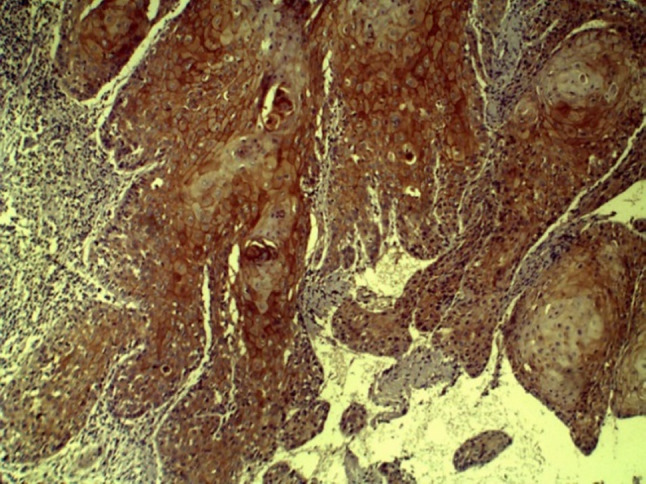
CD44 expression in WDSCC (10×)
Fig. 7.
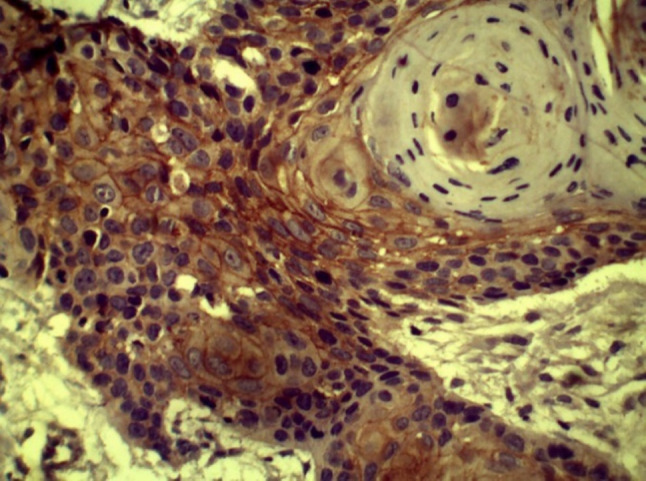
CD44 expression within keratin pearl in WDSCC
Fig. 8.
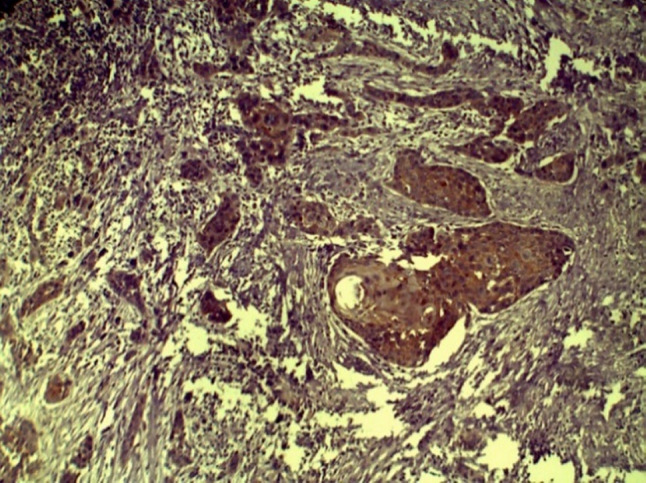
CD44 expression in MDSCC (10×)
Fig. 9.

CD44 expression in PDSCC (10×)
Fig. 10.

CD44 expression in PDSCC (40×)
Bar Diagram 1.
CD44 staining index in OSCC without lymph node metastasis and with lymph node metastasis
ALDH1 Results
One-way ANOVA showed a statistically significant difference for ALDH1 staining index among all the groups (p = 0.029) with highest expression seen in OSCC (Table 3). Normal mucosal tissues (group I) were negative for expression of ALDH1 (Fig. 11). In group II, 14 (46.66%) cases showed ALDH1 positivity with a mean staining index of 0.57. Immunopositivity for ALDH1 was observed as scattered patches of positive cells in basal and parabasal cell layers of the epithelium in hyperkeratosis and OSMF. In group III, 7 cases were positive for ALDH1 with a mean staining index of 0.67. Cytoplasmic ALDH1 positivity was observed in dysplastic epithelium as well as in invasive, malignant epithelial cells in OSCC. Pairwise comparison of ALDH1 staining index using Tukey’s post hoc test showed a statistically significant difference between group I and group III (p = 0.039) (Table 4). When ALDH1 expression was assessed in OPMDs, it was increased in OPMDs with dysplasia as compared to OPMDs without dysplasia. In low risk dysplasia, ALDH1 expression was lower than high risk dysplasia; however, the difference was not statistically significant. Hyperkeratosis with dysplasia and OSMF with dysplasia showed more ALDH1 expression than hyperkeratosis without dysplasia (p = 0.004, Figs. 12 and 13) and OSMF without dysplasia (p = 0.009, Figs. 14 and 15) respectively (Tables 5 and 6).
Table 3.
ALDH1 staining index in Group I, II and III
| Groups | N | Mean | Std. deviation | Std. error | Minimum | Maximum | p value of one way ANOVA | |
|---|---|---|---|---|---|---|---|---|
| Staining index of ALDH1 | 1 | 25 | .00 | .000 | .000 | 0 | 0 | .029* |
| 2 | 30 | .57 | .774 | .141 | 0 | 3 | ||
| 3 | 24 | .67 | 1.465 | .299 | 0 | 6 | ||
| Total | 79 | .42 | .969 | .109 | 0 | 6 |
Fig. 11.
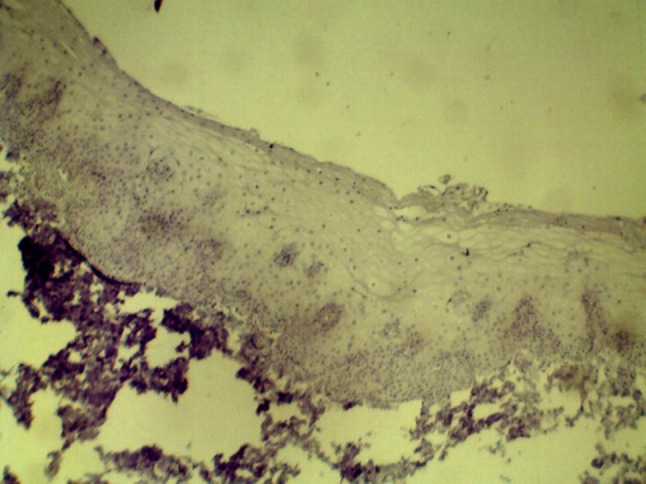
ALDH1 expression in Normal mucosa (10× and 40×)
Table 4.
Pair wise comparison of ALDH1 staining index using Tukey’s Post Hoc Tests
| Dependent Variable | (I) group | (J) group | Mean difference (I − J) | Std. error | p value |
|---|---|---|---|---|---|
| Staining index of ALDH1 | 1 | 2 | − .567 | .254 | .072# |
| 1 | 3 | − .667* | .268 | .039* | |
| 2 | 3 | − .100 | .257 | .920# |
#Less/ not significant
Fig. 12.

ALDH1 expression in hyperkeratosis with high risk dysplasia (10×)
Fig. 13.
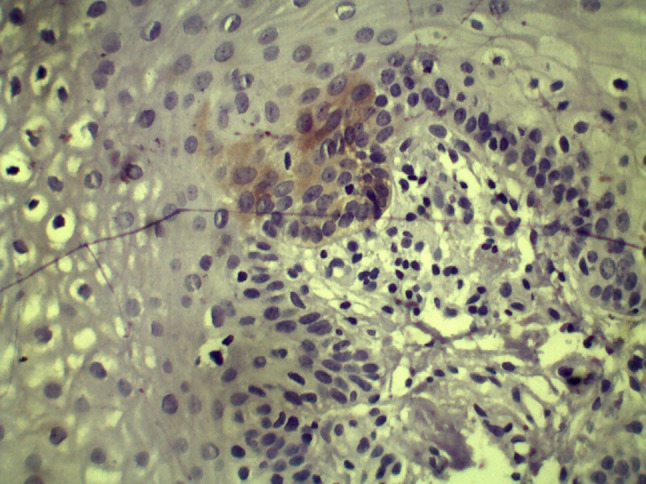
ALDH1 expression in hyperkeratosis with low risk dysplasia (10×)
Fig. 14.
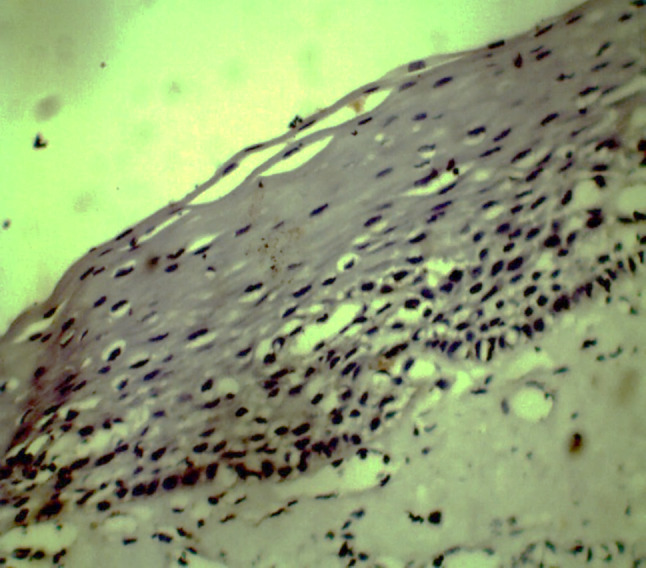
ALDH1 expression in OSMF without dysplasia (40×)
Fig. 15.

ALDH1 expression in OSMF with dysplasia (40×)
Table 5.
Comparison of ALDH1 staining index between hyperkeratosis without dysplasia and hyperkeratosis with dysplasia
| Groups | N | Mean | Std. deviation | Std. error mean | T value | p value of t test | |
|---|---|---|---|---|---|---|---|
| ALDH1 staining index | Hyperkeratosis without dysplasia | 3 | .00 | .000 | .000 | − 3.500 | .004** |
| Hyperkeratosis with dysplasia | 15 | .47 | .516 | .133 |
**Highly significant
Table 6.
Comparison of ALDH1 expression between OSMF without dysplasia and OSMF with dysplasia
| Group | N | Mean | Std. deviation | Std. error mean | T value | p value of t test | |
|---|---|---|---|---|---|---|---|
| ALDH1 staining index | OSMF without dysplasia | 5 | .00 | .000 | .000 | − 3.227 | .009** |
| OSMF with dysplasia | 7 | 1.43 | .976 | .369 |
**Highly significant
ALDH1 expression was found to be higher in hyperkeratosis with high-risk dysplasia as compared to low-risk dysplasia (p = 0.001, Figs. 12 and 13, Table 7). Similarly, the expression was increased in OSMF with high-risk when compared to OSMF with low-risk dysplasia (p = 0.003, Figs. 16 and 17, Table 8).
Table 7.
Comparison of ALDH1 expression between hyperkeratosis with low risk dysplasia and hyperkeratosis with high risk dysplasia
| Groups | N | Mean | Std. deviation | Std. error mean | Mean difference | ||
|---|---|---|---|---|---|---|---|
| ALDH1 immunoscore | Group IIai-Hyperkeratosis with low risk dysplasia | 4 | .75 | .500 | .250 | 1.636* | .001* |
| Group IIaii-Hyperkeratosis with high risk dysplasia | 11 | .36 | .5.0 | .152 |
**Highly significant
Fig. 16.

ALDH1 expression in OSMF with low risk dysplasia (10×)
Fig. 17.

ALDH1 expression in OSMF with high risk dysplasia (10×)
Table 8.
Comparison of ALDH1 expression between OSMF with low risk dysplasia and OSMF with high risk dysplasia
| N | Mean | Std. deviation | Std. error mean | Mean difference | P value | ||
|---|---|---|---|---|---|---|---|
| ALDH1 immunoscore | OSMF with low risk dysplasia | 3 | .67 | .577 | .333 | − 1.33* | 0.033* |
| OSMF with high risk dysplasia | 4 | 2 | .816 | .408 |
In group III, ALDH1 expression was positive in seven cases of OSCC and expression did not correlate with histopathologic grade of OSCC. An important observation was the expression of ALDH1 in overlying dysplastic epithelium of OSCC tissues (Fig. 18). ALDH1 expression was found to be increased in OSCC with lymph node metastasis as compared to OSCC without lymph node metastasis; however, the difference was not statistically significant.
Fig. 18.
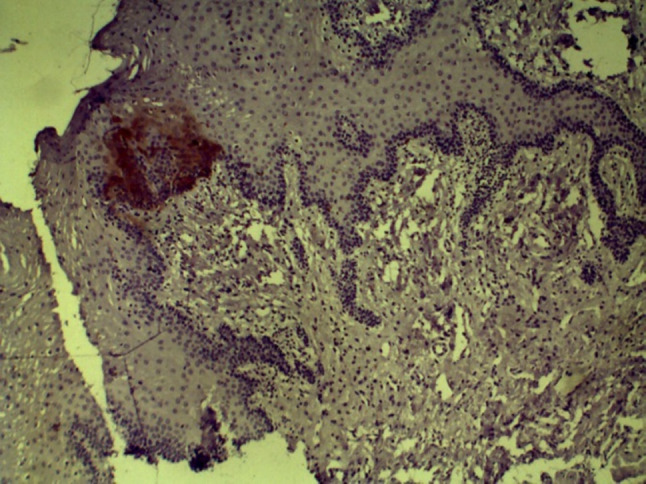
ALDH1 expression in overlying dysplastic epithelium of MDSCC (10×)
Discussion
Accumulating evidence has demonstrated that recurrence and metastasis in cancer is associated with the presence and maintenance of CSCs, the most migratory and highly metastatic cellular subpopulation within tumours. CSCs are essential to tumour initiation, maintenance, recurrence, and metastasis; and their identification has been proposed as a targeted therapeutic strategy against cancer. Identifying and quantifying CSCs could be used to determine the relative aggression of cancer and for development of targeted anticancer drugs. By targeting CSCs, potential significant side effects caused by inhibition of normal stem cell function may be alleviated.
Studies have shown that CSCs can be isolated and enhanced by cell surface markers, such as CD44, ALDH1, BMI1, Nanog, and Oct4 in various solid tumours. However, a universal marker for CSCs has not been identified and associations among these have not been confirmed in oral carcinoma. The most widely known marker of CSCs is CD44, a transmembrane glycoprotein and receptor for hyaluronan (HA). CD44 expression is associated with cell adhesion, migration, proliferation, and angiogenesis in tumorigenesis. Although previous studies could not prove role of CD44 in tumour progression, some reports have found a positive prognostic value of CD44 in oral cancer [14]. ALDH1, a detoxifying enzyme involved in cell differentiation and drug resistance, is highly expressed in normal and CSCs. ALDH1 activity was recently shown to identify CSC-like cells in HNSCC. ALDH1 is also involved in the epithelial–mesenchymal transition of cancer cells.
OSCC is often preceded by clinically visible changes in oral mucosa categorised as OPMDs. The progression of OPMD to OSCC is a multistep process that provides an opportunity for early cancer detection. Early interception improves treatment outcomes and reduces the disease burden of OSCC. Research has focused on stem cells and their behaviour in cancer; however, very few studies have been devoted to the analysis of stem cells in OPMDs. Study of CSCs in dysplastic oral tissues may provide for a more informed assessment of progression of OPMDs and serve as a prognosticator of malignant transformation in OPMDs.
Neck metastasis of OSCC is associated with a high recurrence rate, increased likelihood of distant metastasis, and poor prognosis. The present study compared the expression of putative cancer stem cell biomarkers, ALDH1 and CD44, in OSCC with and without lymph node metastasis. Additionally, the immune score was correlated with clinicopathologic data and prognosis to understand the biologic behaviour of tumour cells in OSCC and malignant transformation in OPMDs.
CD44 and ALDH1 expression was evaluated in normal mucosal tissues (group I), OPMDs (group II), and OSCC (group III) with and without lymph node metastasis. CD44 expression was positive in all normal mucosal tissues. In most normal mucosal tissues, two-thirds of the thickness of epithelium showed CD44 positivity. Few cases showed full thickness epithelial positivity. A strong, primarily plasma membrane-associated expression of CD44 was observed in the basal layer and suprabasal layers. The staining pattern became progressively weaker or negative in the upper granular layers in normal mucosal tissues (Fig. 1). The positive CD44 expression seen in basal and spinous cell layers of normal mucosa indicates the relationship of CD44 expression with cellular differentiation in normal squamous epithelium. CD44 is a multistructural and multifunctional transmembrane glycoprotein which acts as a receptor for hyaluronan/HA, a major component of the extracellular matrix, and a coreceptor for various growth factors and cytokines. It has attracted considerable attention because of its important function in mediating cell‐cell and cell‐matrix interactions, as well as its association with malignancy, particularly cancer dissemination [15]. The present study relates CD44 expression with pathologic variance in OPMDs. There was a difference in CD44 staining intensity and immunoscore between hyperkeratosis and OSMF. Expression of CD44 in OSMF was increased compared to hyperkeratosis. In OSMF, the expression was more intense in both the basal and parabasilar layer of epithelium. There was a statistically significant difference in CD44 expression between OPMDs with and without dysplasia. In hyperkeratosis with dysplasia and OSMF with dysplasia, the mean CD44 staining index was decreased as compared to non-dysplastic epithelia in OSMF and hyperkeratosis, although the difference was not statistically significant. These results could be related to differences in thickness, proportions of basal cell layers and squamous layers, and patterns of epithelial differentiation between OSMF and hyperkeratosis. Due to the atrophy of epithelium, epithelial cells of OSMF may be more differentiated, leading to intense staining of CD44. These findings beg the question of whether there are differences in epithelial maturation between OSMF and hyperkeratosis or if expression suggests malignant potential. In dysplasia, CD44 expression is reduced, as seen in a study by Kuo et al., who found decreased CD44 immunostaining in dysplastic epithelium as compared to normal epithelium [5]. When grades of dysplasia were compared, expression was found to be less in high-risk than low-risk. In epithelium with low-risk dysplasia, CD44 expression was more intense and observed up to suprabasal cell layers. In high-risk dysplasia, epithelium showed less CD44 staining in intensity and number of positive cell layers. Thus, CD44 expression decreased with increasing grade of dysplasia. AbdulMajeed et al. and Kosunen et al. reported decreased and irregular CD44 expression in dysplastic epithelium overlying invasive cancer nests [16, 17]. CD44 isoforms play an important role in cell–cell and cell-ECM interactions [18]. Decreased CD44 in dysplastic epithelium may suggest loss of cell adhesiveness which is a step towards invasion [19].
In group III, CD44 expression was less than in normal mucosal tissues but increased when compared to OPMDs. Ue et al. and Kosunen et al. also found decreased CD44 expression in OSCC as compared to normal mucosal tissues [17, 20]. However, two studies by Kokko et al. and Mohanta et al. found increased immunostaining in OSCC as compared to normal mucosa [21, 22]. In OSCC, CD44 positivity was observed in malignant epithelial cells in the stroma. The present investigation found positive expression of ALDH1 mainly localized to the cytoplasm of OSCC at the rate of 29.16%, whereas expression of CD44 was mainly localized to the membrane at 87.5%. CD44 was also strongly present on the plasma membrane of lymphocytic cells in the tumour stroma with variable intensity in other stromal cells.
There was difference in staining pattern and intensity of CD44 staining among grades of OSCC. The intensity was found to be increased in WDSCC, less in MDSCC, and least in PDSCC. The percentage of tumour cell positivity also decreased with increasing grades of OSCC. It was 75–100% in well-differentiated OSCCs. MDSCC cases showed 50–75% positivity, and poorly differentiated OSCCs showed 25–50% positivity. CD44 expression was higher in WDSCC, with a pattern that resembled normal oral epithelium. The reaction was most obvious at the periphery of malignant islands, with a weak or negative expression inside keratin pearls. The pattern of positivity was confined to plasma membrane in a continuous manner in WDSCC. In MDSCC, the intensity of CD44 positivity was decreased in tumour islands. In PDSCC, the positivity of CD44 was cytoplasmic and discontinuous in the plasma membranes of cells. Decreased expression of CD44 with increasing grades of OSCC is in accordance with the studies by Mărgăritescu et al., Fonseca et al., B´ankfalvi et al., Carinci et al., Gonzalez-Moles et al., and Kosunen et al. [17, 23–27]. Reduced CD44 expression with increasing grades of OSCC may reflect loss of cellular adhesion and enhanced invasive potential of malignant epithelial cells. Our findings support the theory that CD44 may mediate adhesive properties along with orientation signals for epithelial cells that differentiate and migrate upwards. In OSCC, CD44 showed intense, irregular, and disorganised staining in all 24 cases. There was complete absence of cytoplasmic staining of CD44 in tumour cells as compared to normal mucosa where the expression was membranous as well as cytoplasmic. This finding may point towards a more efficient shedding of the CD44 ectodomain which is seen in PDSCC.
CD44 expression was found to be significantly reduced in OSCC with lymph node metastasis as compared to OSCC without lymph node metastasis. Wang et al. found reduced expression of CD44 in metastatic OSCC as compared to primary OSCC [28]. Masuda et al., however, found decreased CD44 expression in patients with late nodal metastasis as compared to high expression of CD44 in immediate lymph node metastasis. These findings correlated with reduced survival rate, but this could be related to the difference in CD44 isoforms studied in HNSCC [29]. Kawano et al. found positive correlation between CD44 v6 expression and tumour volume, lymph node metastasis, and shorter survival in HNSCC [30]. Others have reported a correlation between downregulation of various CD44 variant isoforms with worse prognosis. Kanke et al. reported that down-regulation of CD44 v2 correlates with poorer differentiation and shorter overall survival [31]. The negative relationship between CD44 expression and prognosis in OSCC could be due to reduced adhesion between cells and between cells and the basement membrane, resulting in easy detachment from the rigid constitution. Thus, decreased expression of CD44 in OSCC tissues may be an indicator of high metastatic potential and be related to lymph node metastasis and poor prognosis. These findings suggest CD44 as an important molecular marker to predict metastasis in OSCC. Its role in monitoring the treatment and prognosis of patients with OSCC and should be investigated further.
ALDH1 expression was negative in all samples of normal mucosal tissues. This finding is in accordance with those of Visus et al., while the study by Asareh et al. showed 10% immunostaining of ALDH1 in normal mucosa [32, 33]. In group II, 14 (46.66%) cases showed ALDH1 positivity with mean staining index of 0.57. In group III, seven cases showed positivity for ALDH1. Increased expression and altered distribution of ALDH1 was observed in OSCC, in accordance with studies by Asareh et al. and Tsai et al. [33, 34]
In group II, there were 18 cases of hyperkeratosis and 12 cases of OSMF. Eight cases of hyperkeratosis showed ALDH1 positivity, with a mean staining index of 0.87, while six OSMF cases were positive for ALDH1 expression, with mean staining index of 1.66. Immunopositivity for ALDH1 was observed as scattered patches of positive cells in the basal and parabasal epithelial cell layers in hyperkeratosis and OSMF. When group II was assessed for dysplasia, ALDH1 was negative in all cases of OPMDs without dysplasia, while 63.6% of cases with dysplasia showed ALDH1 (mean staining index of 1.21). This difference was statistically significant (p < 0.05). In OPMD tissues with dysplasia, immunoreactivity for ALDH1 was distributed in basal and parabasal layers. ALDH1 expression was found to be higher in OSMF and OSMF with dysplasia as compared to hyperkeratosis and hyperkeratosis with dysplasia. Also, there was a statistically significant increase in ALDH1 expression for high-risk dysplasia in OSMF which was not identified within the hyperkeratosis group. Visus et al. reported ALDH1 as a marker for distinguishing malignant tissues from premalignant [32]. The present study reports a difference in ALDH1 expression between OSMF and hyperkeratosis, dysplastic and non-dysplastic epithelia in OPMDs, as well as a difference between low-risk and high-risk dysplasia. These findings suggest an association of ALDH1 expression with pathological variance in OPMDs, and that it can be used as a putative marker for predicting malignant transformation in OPMDs.
The present study reports 29.16% OSCC cases to be positively stained with ALDH1, with mean staining index of 2.28 and 6 as a maximum total immunoscore. Immunopositivity for ALDH1 showed scattered patches of positive cells and a notable absence of immune-expression within keratin pearls. ALDH1 expression was increased in OSCC with lymph node metastasis as compared to OSCC without lymph node metastasis, although the difference was not statistically significant. Among seven ALDH1 expression positive cases of OSCC, five were with lymph node metastasis and two were without lymph node metastasis. Chen et al., Jayasooriya et al., and Zhou & Sun found that ALDH1-positive OSCC patients had worse prognosis corresponding to poor tumour differentiation, lymph node metastasis, decreased overall survival, and decreased disease-free survival [13, 35, 36]. However, in our study, there was a smaller number of OSCC cases that showed positive ALDH1 expression which constrained the association between ALDH1 and OSCC prognosis. One important observation in our study, however, was the ALDH1 positivity observed in overlying dysplastic epithelium above the invading malignant cells in tissues of OSCC (Fig. 18). This may suggest that ALDH1 can be associated with malignant transformation of oral epithelial cells.
Due to the significant difference seen in CD44 expression in OSCC with and without cervical lymph node metastasis, it is valuable to associate CD44 as a CSC marker for OSCC and prognosticator in OSCC. In OPMDs, ALDH1 showed altered expression with the pathological variance of OPMDs, and its significant increase in expression in dysplastic epithelia in OPMDs and OSCC may suggest its value in assessing cancer risk in OPMDs (Figs. 19 and 20).
Fig. 19.

ALDH1 expression in overlying dysplastic epithelium of PDSCC (10×)
Fig. 20.
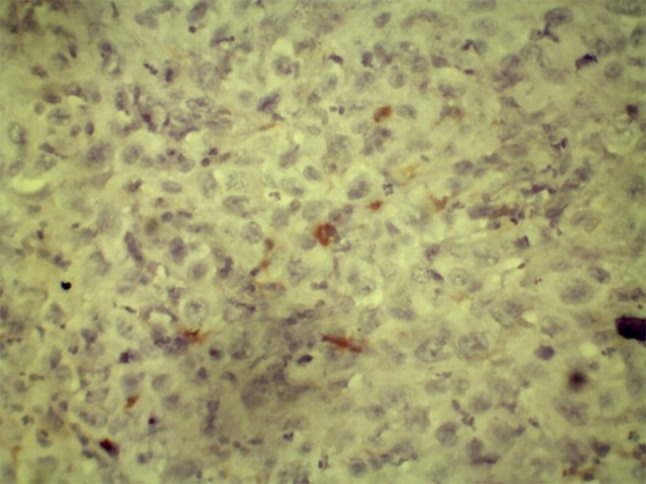
Positive expression in malignant epithelial cells within connective tissue (40×)
Conclusion
The CD44 population possesses the properties of CSCs in head and neck carcinoma, and continuing shedding may be found after CD44 down-regulation. Data from the literature demonstrates CD44 as the most used surface marker to identify CSCs from OSCC. Our study points out a strong counter-argument for using CD44 as a surface marker of oral CSCs, as it is expressed in both normal oral epithelium and in premalignant and malignant lesions. Additionally, the percentage of carcinomatous positive cells varied with the degree of tumour differentiation. We found that the mean of CD44 immunopositive cells was more in normal mucosa when compared to OPMDs without dysplasia, low-risk dysplasia, high-risk dysplasia, and OSCC. High-grade dysplasia and OSCC cases showed down-regulated expression of CD44. The correlation between the degree of dysplasia and CD44 down-regulation has been related to proliferation as well as the grade of cellular differentiation, implicated in motility and invasion of the lesion.
The overexpression of ALDH1 in OPMDs with high-risk dysplasia as compared to OPMDs with low-risk dysplasia was a significant predictor of malignant transformation of OPMDs. Therefore, it is reasonable to hypothesize an association between the expression of ALDH1 in primary tumours and the progression of OSCC to cervical lymph nodes.
Author Contributions
Dr. SND—Original concept, Performed the study, manuscript writing, manuscript editing. Dr. SKC—Manuscript writing, manuscript editing. Dr. SP—Manuscript review. Dr. SSG—Manuscript editing. Dr. YBJ—Images, Tables editing, Manuscript reviewing. Dr. SM—Manuscript reviewing.
Funding
The authors have no relevant financial or non-financial interests to disclose.
Data Availability
Not applicable.
Code availability
Not applicable.
Declarations
Conflict of interest
No any conflict of interest.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Ethical Approval
Ethical approval was waived by the local Ethics Committee of Dr. G.D. Pol Foundation’s YMT Dental college and Hospital, in view of the retrospective nature of the study and all the procedures being performed were part of the routine care.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Prince ME, Ailles LE. Cancer stem cells in head and neck squamous cell cancer. J Clin Oncol. 2008;26(17):2871–2875. doi: 10.1200/JCO.2007.15.1613. [DOI] [PubMed] [Google Scholar]
- 2.Michor F, Polyak K. The origins and implications of intratumor heterogeneity. Cancer Prev Res (Phila) 2010;3:1361–1364. doi: 10.1158/1940-6207.CAPR-10-0234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Van der Waal I. Potentially malignant disorders of the oral and oropharyngeal mucosa; terminology, classification and present concepts of management. Oral Oncol. 2009;45(4–5):317–323. doi: 10.1016/j.oraloncology.2008.05.016. [DOI] [PubMed] [Google Scholar]
- 4.Krishnamurthy S, Nör JE. Head and neck cancer stem cells. J Dent Res. 2012;91(4):334–340. doi: 10.1177/0022034511423393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kuo MY, Cheng SJ, Chen HM, Kok SH, Hahn LJ, Chiang CP. Expression of CD44s, CD44v5, CD44v6 and CD44v7-8 in betel quid chewing-associated oral premalignant lesions and squamous cell carcinomas in Taiwan. J Oral Pathol Med. 1998;27(9):428–433. doi: 10.1111/j.1600-0714.1998.tb01980.x. [DOI] [PubMed] [Google Scholar]
- 6.Blick T, Hugo H, Widodo E, Waltham M, Pinto C, Mani SA, Weinberg RA, Neve RM, Lenburg ME, Thompson EW. Epithelial mesenchymal transition traits in human breast cancer cell lines parallel the CD44 hi/CD24 lo/-stem cell phenotype in human breast cancer. J Mammary Gland Biol Neoplasia. 2010;15(2):235–252. doi: 10.1007/s10911-010-9175-z. [DOI] [PubMed] [Google Scholar]
- 7.Collins AT, Berry PA, Hyde C, Stower MJ, Maitland NJ. Prospective identification of tumorigenic prostate cancer stem cells. Cancer Res. 2005;65(23):10946–10951. doi: 10.1158/0008-5472.CAN-05-2018. [DOI] [PubMed] [Google Scholar]
- 8.Fitzgerald TL, McCubrey JA. Pancreatic cancer stem cells: association with cell surface markers, prognosis, resistance, metastasis and treatment. Adv Biol Regul. 2014;1(56):45–50. doi: 10.1016/j.jbior.2014.05.001. [DOI] [PubMed] [Google Scholar]
- 9.Prince ME, Sivanandan R, Kaczorowski A, Wolf GT, Kaplan MJ, Dalerba P, Weissman IL, Clarke MF, Ailles LE. Identification of a subpopulation of cells with cancer stem cell properties in head and neck squamous cell carcinoma. Proc Natl Acad Sci. 2007;104(3):973–978. doi: 10.1073/pnas.0610117104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.(http://refhub.elsevier.com/S1368-8375(14)00260-7/h0015).
- 11.Kujan O, Oliver RJ, Khattab A, Roberts SA, Thakker N, Sloan P. Evaluation of a new binary system of grading oral epithelial dysplasia for prediction of malignant transformation. Oral Oncol. 2006;42(10):987–993. doi: 10.1016/j.oraloncology.2005.12.014. [DOI] [PubMed] [Google Scholar]
- 12.Kosunen A. CD44 expression and its relationship with MMP-9, clinicopathological factors and survival in oral squamous cell carcinoma. Oral Oncol. 2007;43:51–59. doi: 10.1016/j.oraloncology.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 13.Zhou C, Sun B. The prognostic role of the cancer stem cell marker aldehyde dehydrogenase 1 in head and neck squamous cell carcinomas: a meta-analysis. Oral Oncol. 2014;50:1144. doi: 10.1016/j.oraloncology.2014.08.018. [DOI] [PubMed] [Google Scholar]
- 14.Prffko J, Bànkfalvi A, Klauke K, Dreier R, Joos U, Böcker W, Schmid KW. Unaltered strong immunohistochemical expression of CD44-v6 and-v5 isoforms during development and progression of oral squamous cell carcinomas. J Oral Pathol Med. 1996;25(9):502–506. doi: 10.1111/j.1600-0714.1996.tb00305.x. [DOI] [PubMed] [Google Scholar]
- 15.Kuhn S, Koch M, Nubel T, Ladwein M, Antolovic D, et al. A complex of EpCAM, claudin-7, CD44 variant isoforms, and tetraspanins promotes colorectal cancer progression. Mol Cancer Res. 2007;5:553–567. doi: 10.1158/1541-7786.MCR-06-0384. [DOI] [PubMed] [Google Scholar]
- 16.AbdulMajeed AA, Dalley AJ, Farah CS. Putative cancer stem cell marker expression in oral epithelial dysplasia and squamous cell carcinoma. J Oral Pathol Med. 2013;42(10):755–760. doi: 10.1111/jop.12073. [DOI] [PubMed] [Google Scholar]
- 17.Kosunen A, Pirinen R, Ropponen K, Pukkila M, Kellokoski J, Virtaniemi J, Sironen R, Juhola M, Kumpulainen E, Johansson R, Nuutinen J. CD44 expression and its relationship with MMP-9, clinicopathological factors and survival in oral squamous cell carcinoma. Oral Oncol. 2007;43(1):51–59. doi: 10.1016/j.oraloncology.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 18.Plaks V, Kong N, Werb Z. The cancer stem cell niche: how essential is the niche in regulating stemness of tumor cells? Cell Stem Cell. 2015;16:225–238. doi: 10.1016/j.stem.2015.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen J, Zhou J, Lu J, Xiong H, Shi X, Gong L. Significance of CD44 expression in head and neck cancer: a systemic review and meta-analysis. BMC Cancer. 2014;14(1):15. doi: 10.1186/1471-2407-14-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ue T, Yokozaki H, Kagai K, Higashikawa K, Yasui W, Sugiyama M, Tahara E, Ishikawa T. Reduced expression of the CD44 variant exons in oral squamous cell carcinoma and its relationship to metastasis. J Oral Pathol Med. 1998;27(5):197–201. doi: 10.1111/j.1600-0714.1998.tb01941.x. [DOI] [PubMed] [Google Scholar]
- 21.Kokko LL, Hurme S, Maula SM, Alanen K, Grénman R, Kinnunen I, Ventelä S. Significance of site-specific prognosis of cancer stem cell marker CD44 in head and neck squamous-cell carcinoma. Oral Oncol. 2011;47(6):510–516. doi: 10.1016/j.oraloncology.2011.03.026. [DOI] [PubMed] [Google Scholar]
- 22.Mohanta S, Siddappa G, Valiyaveedan SG, Dodda Thimmasandra Ramanjanappa R, Das D, Pandian R, Khora SS, Kuriakose MA, Suresh A. Cancer stem cell markers in patterning differentiation and in prognosis of oral squamous cell carcinoma. Tumor Biol. 2017;39(6):1010428317703656. doi: 10.1177/1010428317703656. [DOI] [PubMed] [Google Scholar]
- 23.Mărgăritescu C, Pirici D, Simionescu C, Stepan A. The utility of CD44, CD117 and CD133 in identification of cancer stem cells (CSC) in oral squamous cell carcinomas (OSCC) Rom J Morphol Embryol. 2011;52(3 Suppl):985–993. [PubMed] [Google Scholar]
- 24.Fonseca I, Pereira T, Rosa-Santos J, Soares J. Expression of CD44 isoforms in squamous cell carcinoma of the border of the tongue: a correlation with histological grade, pattern of stromal invasion and cell differentiation. J Surg Oncol. 2001;76(2):115–120. doi: 10.1002/1096-9098(200102)76:2<115::AID-JSO1021>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 25.Bánkfalvi A, Kraßort M, Buchwalow IB, Végh A, Felszeghy E, Piffkó J. Gains and losses of adhesion molecules (CD44, E-cadherin, and β-catenin) during oral carcinogenesis and tumour progression. J Pathol J Pathol Soc Great Britain Ireland. 2002;198(3):343–351. doi: 10.1002/path.1204. [DOI] [PubMed] [Google Scholar]
- 26.Kochiwa H, Suzuki R, Washio T, Saito R, Bono H, Carninci P, Okazaki Y, Miki R, Hayashizaki Y, Tomita M. Inferring alternative splicing patterns in mouse from a full-length cDNA library and microarray data. Genome Res. 2002;12(8):1286–1293. doi: 10.1101/gr.220302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gonzalez-Moles MA, Bravo M, Ruiz-Avila I, Esteban F, Rodriguez-Archilla A, Gonzalez-Moles S, Arias B. Adhesion molecule CD44 as a prognostic factor in tongue cancer. Anticancer Res. 2003;23(6D):5197–5202. [PubMed] [Google Scholar]
- 28.Wang SJ, Wong G, de Heer AM, Xia W, Bourguignon LY. CD44 variant isoforms in head and neck squamous cell carcinoma progression. Laryngoscope. 2009;119(8):1518–1530. doi: 10.1002/lary.20506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Masuda M, Kuratomi Y, Shiratsuchi H, Nakashima T, Naonobu K, Komiyama S. Decreased CD44H expression in early-stage tongue carcinoma associates with late nodal metastases following interstitial brachytherapy. Head Neck J Sci Spec Head Neck. 2000;22(7):662–665. doi: 10.1002/1097-0347(200010)22:7<662::AID-HED4>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 30.Kawano T, Nakamura Y, Yanoma S, Kubota A, Furukawa M, Miyagi Y, Tsukuda M. Expression of E-cadherin, and CD44s and CD44v6 and its association with prognosis in head and neck cancer. Auris Nasus Larynx. 2004;31:35–41. doi: 10.1016/j.anl.2003.09.005. [DOI] [PubMed] [Google Scholar]
- 31.Kanke M, Fujii M, Kameyama K, Kanzaki J, Tokumaru Y, Imanishi Y, Tomita T, Matsumura Y. Clinicopathological significance of expression of CD44 variants in head and neck squamous cell carcinoma. Jpn J Cancer Res. 2000;91(4):410–415. doi: 10.1111/j.1349-7006.2000.tb00960.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Visus C, Ito D, Amoscato A, Maciejewska-Franczak M, Abdelsalem A, Dhir R, Shin DM, Donnenberg VS, Whiteside TL, DeLeo AB. Identification of human aldehyde dehydrogenase 1 family member A1 as a novel CD8+ T-cell–defined tumor antigen in squamous cell carcinoma of the head and neck. Can Res. 2007;67(21):10538–10545. doi: 10.1158/0008-5472.CAN-07-1346. [DOI] [PubMed] [Google Scholar]
- 33.Herrera Costa F, Narana Ribeiro El Achkar V, Costa V, Paladini I, Kowalski LP, Rodarte Carvalho Y, Kaminagakura E. Different expression of aldehyde dehydrogenases 1A1 and 2 in oral leukoplakia with epithelial dysplasia and in oral squamous cell carcinoma. Appl Immunohistochem Mol Morphol. 2010;27(7):537–42. [DOI] [PubMed]
- 34.Tsai MS, Chen WC, Lai CH, Chen YY, Chen MF. Epigenetic therapy regulates the expression of ALDH1 and immunologic response: relevance to the prognosis of oral cancer. Oral Oncol. 2017;1(73):88–96. doi: 10.1016/j.oraloncology.2017.08.007. [DOI] [PubMed] [Google Scholar]
- 35.Chen YC, Chen YW, Hsu HS, Tseng LM, Huang PI, Lu KH, Chen DT, Tai LK, Yung MC, Chang SC, Ku HH. Aldehyde dehydrogenase 1 is a putative marker for cancer stem cells in head and neck squamous cancer. Biochem Biophys Res Commun. 2009;385(3):307–313. doi: 10.1016/j.bbrc.2009.05.048. [DOI] [PubMed] [Google Scholar]
- 36.Jayasooriya P, Fernando C, Suraweera A, Dissanayake U. Stem cell markers as a resource to predict prognosis of betel quid induced oral squamous cell carcinoma: an immunohistochemical investigation. Chemotherapy. 2017;3:4. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.
Not applicable.