Abstract
Syphilis is a sexually transmitted infectious disease caused by Treponema pallidum and characterized by a complex and variable clinical presentation. Cases of unexpected oral syphilis presenting as non-healing ulcers are uncommonly reported. We report 3 cases (one female and two males, aged 35, 35, and 56 years, respectively) in which patients presented with non-healing oral ulcers. Biopsies revealed surface ulceration and a significant neutrophilic infiltrate rather than the more conventional plasma cell infiltrate seen with most reported syphilis infections, potentially leading to an inaccurate diagnosis. Treponema pallidum immunohistochemistry highlighted spirochetes within the epithelium, with additional diagnostic confirmation by serum T. pallidum particle agglutination assay. Sexual history documentation by the clinician with nonspecific oral ulcers is paramount to aiding diagnosis and leading to proper management. Further, it is important to perform immunohistochemistry for T. pallidum in oral biopsies from non-healing ulcers, especially when clinical history raises the differential diagnosis or when other clinical manifestations may support this consideration.
Keywords: Oral ulcer, Syphilis, Treponemal pallidum, Mouth diseases, Sexual behavior, Spirochaetales, Immunohistochemistry, Differential diagnosis
Introduction
Syphilis is an infectious disease of the spirochete Treponema pallidum, and is transmitted by sexual contact in most cases (congenital cases are not considered herein). Infection is divided into four stages: primary, secondary, latent, and tertiary [1]. The first sign of primary syphilitic infection is the appearance of a small, painless papule that develops into a hard chancre with nontender lymphadenopathy 1–3 weeks after exposure. Chancres are usually identified on the external genitalia, but oral sex may lead to syphilis transmission, with the lips most commonly affected [2]. There is a wide diversity of etiologies for oral ulcers, and thus recognition of oral syphilis can be challenging [3]. However, the incidence of oral syphilis has been increasing, and thus oral lesions may represent an important diagnostic indicator of infection [4].
Mucosal ulcers are a common patient complaint, with syphilitic infection only one of many different etiologies to be considered [5]. The objectives of this study were to report three cases of syphilis with primarily oral manifestations, highlighting the importance of an accurate sexual history and maintaining a high index of suspicion for syphilis. Histopathological studies of oral syphilis commonly report a significant subepithelial plasma cell infiltrate [6, 7]. However, cases of oral syphilis with a predominant neutrophilic infiltrate are rarely reported. This study discusses this uncommon histology which can lead to misdiagnosis of primary or secondary oral syphilis.
Case Representation
Case 1
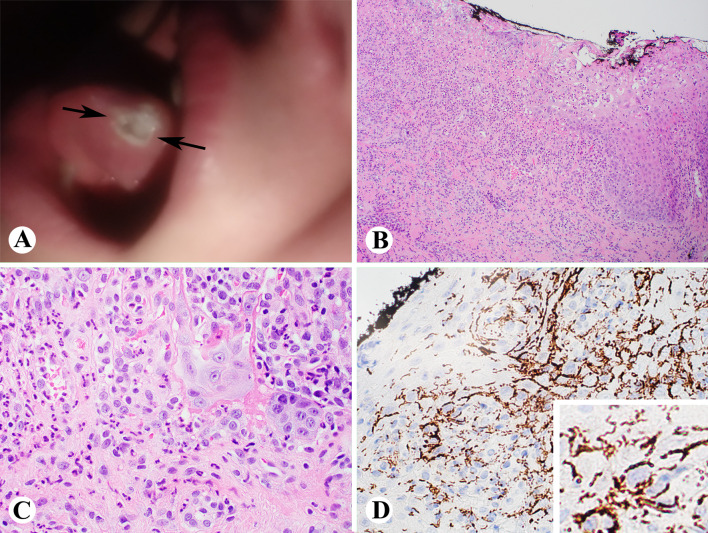
A 35-year-old female presented with a 2-month long nonhealing ulcer on the right lateral tongue with an associated burning sensation. She noted changing her diet to avoid spicy and hot foods due to pain. The patient had done nothing to attempt to alleviate her symptoms. Significant past medical history included diabetes mellitus, chlamydia infection, and gastroesophageal reflux disease (GERD). She was a non-smoker. Clinical examination revealed a 1 cm right tongue ulcer (Fig. 1a). An unrelated right torus palatini was noted.
Fig. 1.
Case 1 showing a 1 cm right tongue ulcer (a); biopsy showing ulceration, granulation tissue and active inflammation (b and c, H&E stain); and T. Pallidum immunostain showing innumerable T. Pallidum spirochetes (d and inset) (b, 100x; c and d, 400x)
An excisional biopsy was performed. Sections revealed ulcerated squamous epithelium with associated granulation tissue (Fig. 1b, c). Acute inflammation was seen extending into the subjacent stroma. Based on these findings, a Gomori Methamine silver stain and herpes simplex virus (HSV) and T. pallidum spirochete immunohistochemistry were performed. Innumerable T. pallidum organisms were highlighted via immunohistochemistry (Fig. 1d). No fungal organisms were identified. Serologic testing confirmed a diagnosis of syphilis (positive T. Pallidum IgG and IgM by T. Pallidum particle agglutination assay (TP-PA) with a Rapid Plasma Reagin (RPR) titer 1:128). The patient was treated with penicillin-G (2.4 million units) and has not returned for additional evaluation.
Case 2
A 35-year-old male presented with a three month-long history of left-sided tongue discomfort. He reported oral sex with females only. Past medical history was significant for alcoholic cirrhosis. Physical exam revealed white plaques of varying sizes on the hard palate, bilateral tonsillar fossa, and on the left lateral tongue and lower lip mucosa (Fig. 2a). Upon further examination, similar lesions were identified on his penis.
Fig. 2.
Case 2 showing a 1.4 cm lip white plaque (a); biopsy showing ulcerated squamous mucosa with adjacent pseudoepitheliomatous hyperplasia and a rich acute inflammatory infiltrate (b and c, H&E stain); and immunostain of T. Pallidum showing numerous T. Pallidum spirochetes (d and inset) (b, 100x; c and d, 400x)
An excisional biopsy was performed on both the tongue and lower lip. Histologic sections showed ulcerated squamous mucosa with adjacent pseudoepitheliomatous hyperplasia and a rich acute inflammatory infiltrate (Fig. 2b, c). Special studies included a negative Grocott’s methenamine silver stain and acid-fast bacillus (AFB) stains for fungal organisms and mycobacteria, respectively, while numerous organisms were identified by immunohistochemistry stain of T. pallidum (Fig. 2d). Human immunodeficiency virus (HIV) and HSV serology screens were negative. Serologic testing confirmed a diagnosis of syphilis (positive T. Pallidum IgG and IgM by TP-PA with a RPR titer 1:32). The patient was treated with penicillin-G (2.4 million units) and all lesions resolved by the time he was seen one month later.
Case 3
A 56-year-old male presented with an 8-week-long indentation on the left lateral tongue. He reported it was of a sudden onset and associated with soreness. He denied trauma. He smoked cigars occasionally. Pertinent past medical history included anorectal pain. Previous serology screens for sexually acquired infections were negative within the past 2 years. Physical exam revealed a 5 mm ulcer on the left lateral tongue (Fig. 3a), without additional findings.
Fig. 3.
Case 3 showing a 5 mm lateral tongue ulcer (a); biopsy showing surface ulceration with both acute and chronic inflammation and adjacent squamous epithelial hyperplasia (b and c, H&E stain); and immunostain of T. Pallidum showing many T. Pallidum spirochetes (d and inset) (b, 100x; c and d, 400x)
The biopsy showed surface ulceration with both acute and chronic inflammation and adjacent squamous epithelial hyperplasia (Fig. 3b, c). Immunohistochemistry for T. pallidum spirochetes (Fig. 3d) was positive. Serologic testing confirmed a diagnosis of syphilis (positive T. Pallidum IgG and IgM by TP-PA with a RPR titer 1:64). The patient was treated with doxycycline due to a penicillin allergy. No lesion remained at 3-month follow-up.
Discussion
Syphilis has experienced an increased incidence in the 21st century [1, 4], compared to levels in the previous century [8]. Yet, recognition of the disease through its many various presentations challenges even the most experienced of clinicians [7–10].
Primary syphilis often presents as an isolated papule at the site of inoculation, that rapidly erodes and becomes an indurated chancre, with surrounding erythema. A classic chancre is usually not painful but can become so if suprainfected with bacteria. The vast majority of chancres present as anogenital lesions, but chancres may present extragenitally usually after orogenital contact, most often in the oral cavity or on the lips [11]. Secondary syphilis presents in the oral cavity in many forms, including multiple white mucous patches, condyloma lata, and split papules [12]. Oral ulcers are a relatively common clinical presentation, due to a wide variety of causes, which makes recognition of oral syphilis challenging. The differential diagnoses include infections (bacterial, viral fungal), trauma, neoplasm, autoimmune diseases, and allergies, etc. Factors such as duration, pattern of recurrence, clinical appearance, mucosal location, and presence or absence of systemic symptoms are useful clues to determining an ulcer’s cause [13]. The clinical manifestations of ulcers are nonspecific, requiring detailed clinical history, including social and sexual history, along with history of trauma, dental appliance use, food or additive allergies, aphthous history, among other causes, with supporting laboratory investigations to reach a definitive diagnosis.
Acute oral ulcers develop due to a wide variety of etiologies. The most common causes include trauma but may also be the oral manifestations of autoimmune disorders, connective tissue disease, gastrointestinal tract disease (Crohn disease; ulcerative colitis), and the nonspecific aphthous stomatitis, along with contact allergies or reactions (amalgam, toothpaste sensitivity, cinnamon allergy, among others) [14]. Past reports have highlighted the many protean manifestations of syphilis, with presentations that mimic oral tuberculosis, HIV-associated Kaposi sarcoma, recurrent aphthous ulcer, and oral squamous cell carcinoma [15]. However, when the history is of painless oral ulceration, the differential diagnosis is narrowed to syphilis, lupus erythematosus, traumatic ulceration from neuropathy, and/or carcinoma [16]. However, syphilitic oral chancres develop at inoculation sites and often spontaneously resolve within 3–8 weeks, unlike oral tuberculosis, which often includes constitutional symptoms for a significant time frame [15].
Most syphilis cases are diagnosed based on a combination of clinical, microscopic, immunohistochemical, and serologic findings. Histology of a subepithelial plasma cell-predominant infiltrate is the most common finding that prompts T. pallidum immunohistochemistry studies [7]. The immunohistochemistry study is specific for T. pallidum, helping to exclude the spirochete commensals normally identified in the oral cavity, and potentially difficult to separate on Warthin-Starry stains, or other non-specific spirochete histochemistry studies. Further serologic testing is generally advocated to document disease. In our reported cases, all patients had chancres affecting the oral mucosa (tongue and lip). Microscopic exam highlighted acute inflammation rather than a plasmacytic cell population, making the diagnosis more challenging. Syphilis was confirmed with the immunohistochemistry reaction for T. pallidum and serology tests of TP-PA and RPR. Thus, in nonspecific oral ulcers, careful consideration must be given to infectious etiologies, using a panel approach of fungal stains along with selected immunohistochemistry studies (HSV, T. pallidum, cytomegalovirus) directed by possible additional histologic features. This targeted approach is especially important in potentially at-risk patients (immunocompromised, past sexually transmitted infection history, multiple sexual partners, males who have sex with males, and sex worker contact).
A relatively low 21% of patients with syphilis are diagnosed at an early stage, and the high proportion of patients with early latent syphilis, suggests that primary and secondary syphilis frequently goes undiagnosed or misdiagnosed [8, 17]. Diagnosis of syphilis is frequently based on clinical and serological findings. However, biopsies may provide an important histologic basis to further direct specific serologic testing. In these reported cases, syphilis was not suspected clinically until microscopic evaluation and additional special studies were performed to identify the causative T. pallidum agent. It is most helpful to have a complete clinical history that may provide some presumptive evidence to aid in recognizing and confirming the diagnosis of syphilis and thus improve health outcomes.
Treatment of syphilis is determined by disease stage, but penicillin-G remains the drug of first choice in allergy-free patients for all stages [11]. Alternative therapies are used for penicillin-allergic patients.
Conclusion
Oral ulcers represent a wide diversity of etiologies. For any nonhealing oral ulcer, a broad differential diagnosis must include infectious etiologies, including fungal, mycobacterial, viral and spriochetal infections. There are important public health related concerns when managing sexually transmitted infections, and so a broader awareness of syphilis as it presents in the oral cavity may aid it better detection and management [1, 18–20]. Our presentation highlights the broad spectrum of histologic features that can be seen with oral syphilis, which underscores the need for a high index of suspicion for syphilis when presented with oral ulceration, whether showing classical plasmacytic infiltration or acute inflammation.
Authors’ Contributions
Each author contributed important intellectual content during manuscript drafting or revision and accepts accountability for the overall work by ensuring that questions pertaining to the accuracy or integrity of any portion of the work are appropriately investigated and resolved.
Funding
No external funding was obtained for this study.
Declarations
Conflict of interest
All authors declare that they have no conflict of interest regarding this study.
Ethical approval
This clinical investigation was conducted in accordance and compliance with all statutes, directives, and guidelines of an Internal Review Board authorization involving a retrospective data analysis involving human subjects in research with appropriate informed consent.
Informed consent
Patient granted consent for publication of their data. The consent form has been filed and can be produced upon request.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Peeling R, Mabey D, Kamb M, Chen X, Radolf J, Benzaken A. Syphilis. Nat Rev Dis Primers. 2017;3:7073. doi: 10.1038/nrdp.2017.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kelner N, Rabelo G, da Cruz Perez D, Assuncao J, Jr, Witzel A, Migliari D, Alves F. Analysis of nonspecific oral mucosal and dermal lesions suggestive of syphilis: a report of 6 cases. Oral Surg Oral Med Oral Pathol Oral Radiol. 2014;117(1):1–7. doi: 10.1016/j.oooo.2012.04.028. [DOI] [PubMed] [Google Scholar]
- 3.Fitzpatrick S, Cohen D, Clark A. Ulcerated Lesions of the Oral Mucosa: Clinical and Histologic Review. Head Neck Pathol. 2019;13(1):91–102. doi: 10.1007/s12105-018-0981-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Matias M, Jesus A, Resende R, Caldeira P, Aguiar M. Diagnosing acquired syphilis through oral lesions: the 12 year experience of an Oral Medicine Center. Braz J Otorhinolaryngol. 2020;86(3):358–63. doi: 10.1016/j.bjorl.2018.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fourie J, Boy S. Oral mucosal ulceration - a clinician’s guide to diagnosis and treatment. S Afr Dent J. 2016;71:500–8. [Google Scholar]
- 6.Barrett A, Villarroel Dorrego M, Hodgson T, Porter S, Hopper C, Argiriadou A, Speight P. The histopathology of syphilis of the oral mucosa. J Oral Pathol Med. 2004;33(5):286–91. doi: 10.1111/j.0904-2512.2004.00099.x. [DOI] [PubMed] [Google Scholar]
- 7.Ficarra G, Carlos R. Syphilis: the renaissance of an old disease with oral implications. Head Neck Pathol. 2009;3(3):195–206. doi: 10.1007/s12105-009-0127-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ghanem K, Ram S, Rice P. The Modern Epidemic of Syphilis. N Engl J Med. 2020;382(9):845–54. doi: 10.1056/NEJMra1901593. [DOI] [PubMed] [Google Scholar]
- 9.Carbone P, Capra G, Nelson B. Oral Secondary Syphilis. Head Neck Pathol. 2016;10(2):206–8. doi: 10.1007/s12105-015-0623-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Smith MH, Vargo RJ, Bilodeau EA, Anderson KM, Trzcinska A, Canterbury CR, Fantasia JE, Rawal YB. Oral Manifestations of Syphilis: a Review of the Clinical and Histopathologic Characteristics of a Reemerging Entity with Report of 19 New Cases. Head Neck Pathol. 2021 doi: 10.1007/s12105-020-01283-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Siu A, Landon K, Ramos D. Differential diagnosis and management of oral ulcers. Semin Cutan Med Surg. 2015;34(4):171–7. doi: 10.12788/j.sder.2015.0170. [DOI] [PubMed] [Google Scholar]
- 12.Little J. Syphilis: an update. Oral Surg Oral Med Oral Pathol. Oral Radiol Endod. 2005;100(1):3–9. doi: 10.1016/j.tripleo.2005.03.006. [DOI] [PubMed] [Google Scholar]
- 13.Bruce AJ, Dabade TS, Burkemper NM. Diagnosing oral ulcers. JAAPA. 2015;28(2):1–10. doi: 10.1097/01.JAA.0000459826.63026.67. [DOI] [PubMed] [Google Scholar]
- 14.Scully C, Shotts R. ABC of oral health. Mouth ulcers and other causes of orofacial soreness and pain. Br Med J. 2000;321(7254):162–5. doi: 10.1136/bmj.321.7254.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huang S, Lu R, Yang J, Zhou G. A nonspecific ulcer on upper lip presented as the first and sole sign of syphilis. Journal of infection chemotherapy: official journal of the Japan Society of Chemotherapy. 2020;26(12):1309–12. doi: 10.1016/j.jiac.2020.07.010. [DOI] [PubMed] [Google Scholar]
- 16.Lehman J, Rogers R. 3rd: Acute oral ulcers. Clin Dermatol. 2016;34(4):470–4. doi: 10.1016/j.clindermatol.2016.02.019. [DOI] [PubMed] [Google Scholar]
- 17.Wong N, Huang S, Zheng H, Chen L, Zhao P, Tucker J, Yang L, Goh B, Yang B. Stages of syphilis in South China – a multilevel analysis of early diagnosis. BMC Public Health. 2017;17(1):135. doi: 10.1186/s12889-016-4004-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hertel M, Matter D, Schmidt-Westhausen A, Bornstein M. Oral syphilis: a series of 5 cases. J Oral Maxillofac Surg. 2014;72(2):338–45. doi: 10.1016/j.joms.2013.07.015. [DOI] [PubMed] [Google Scholar]
- 19.Streight K, Paranal R, Musher D. The oral manifestations of syphilitic disease: a case report. J Med Case Rep. 2019;13(1):227. doi: 10.1186/s13256-019-2171-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Strieder L, Leon J, Carvalho Y, Kaminagakura E. Oral syphilis: report of three cases and characterization of the inflammatory cells. Ann Diagn Pathol. 2015;19(2):76–80. doi: 10.1016/j.anndiagpath.2015.01.003. [DOI] [PubMed] [Google Scholar]