Abstract
Background
Lower respiratory tract infections are among the main causes of death. Although there are many respiratory viruses, diagnostic efforts are focused mainly on influenza. The Respiratory Viruses Network (RespVir) collects infection data, primarily from German university hospitals, for a high diversity of infections by respiratory pathogens. In this study, we computationally analysed a subset of the RespVir database, covering 217,150 samples tested for 17 different viral pathogens in the time span from 2010 to 2019.
Methods
We calculated the prevalence of 17 respiratory viruses, analysed their seasonality patterns using information-theoretic measures and agglomerative clustering, and analysed their propensity for dual infection using a new metric dubbed average coinfection exclusion score (ACES).
Results
After initial data pre-processing, we retained 206,814 samples, corresponding to 1,408,657 performed tests. We found that Influenza viruses were reported for almost the half of all infections and that they exhibited the highest degree of seasonality. Coinfections of viruses are frequent; the most prevalent coinfection was rhinovirus/bocavirus and most of the virus pairs had a positive ACES indicating a tendency to exclude each other regarding infection.
Conclusions
The analysis of respiratory viruses dynamics in monoinfection and coinfection contributes to the prevention, diagnostic, treatment, and development of new therapeutics. Data obtained from multiplex testing is fundamental for this analysis and should be prioritized over single pathogen testing.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12889-022-13555-5.
Keywords: Respiratory viruses, Coinfection, Seasonality, Surveillance, Viral exclusion
Background
The current COVID-19 pandemic prominently demonstrates the serious threat posed by respiratory infections, not only for the health of individuals, but also for the stability of modern society, in general. While SARS-CoV-2 infections are currently extensively recorded and analysed, future studies must encompass the full breadth of respiratory viruses as has been done in the past. Even before the pandemic, lower track respiratory infections were among the main causes of death in children and adults [1, 2]. Influenza infection killed between 250,000 and 500,000 people annually, 152,000 deaths were reported in Europe in the 2017–2018 season [3]. In Germany, during the 2018–2019 season, 182,000 influenza-positive tests were confirmed, including 40,000 from inpatients [4].
In 2009 the Respiratory Viruses Network (RespVir www.clinical-virology.net) was founded as an initiative of a Clinical Virology group within the German Virology Society (GfV). The purpose of RespVir is to record respiratory infections in an online database [5], providing clinicians with up-to-date information about circulating pathogens. The RespVir database contains mainly registries from inpatients data reported by 47 laboratories from university hospitals and a few private. These institutions are located primarily in Germany, Austria, and Switzerland, collecting data from central Europe. Over 12 years, RespVir has analysed more than 280,000 samples with respect to 25 respiratory pathogens (17 viruses and 8 bacteria). Among these years RespVir had obtained data on causal agents of respiratory infections.
RespVir includes records of samples from all patients with respiratory symptoms, sent in by clinicians requesting a diagnosis. Independent of the diagnostic hypothesis of the clinician, each sample was tested in a multiplex manner covering a maximum of 17 respiratory viruses, depending on test availability of each laboratory.
In this study, our aims were (i) to describe the prevalence and seasonal variation (seasonality) for each pathogen, (ii) to assess the prevalence of coinfections and (iii) to determine the rate of exclusion or affinity for pairwise coinfections.
After filtering registries with incomplete data and a post-hoc data quality control. To accomplish our objectives, we performed the analysis in a subset of the RespVir database including 17 different viral pathogens covering the time span from 2010 to 2019.
We observed that 48.64% of all reported respiratory infections are caused by influenza virus. We found four general seasonality patterns. Each of the 17 viruses belongs to one of these patterns. Stratification across years shows biennial seasonality patterns for some viruses, indicating infection peaks every other season. We further observed that coinfections do not occur statistically independently, but that for most virus pairs coinfection is far less frequent than expected by chance.
Methods
Samples and data collection
Since November 2009, the RespVir network (Fig. 1) has collected multiplex test records for 17 virus infections from patients that showed respiratory infection symptoms. The records stem from various 47 sites, according to the sites’ test availability. The tests used by some sites do not differentiate between certain virus types or subtypes: (i) FLUA-generic, which cannot distinguish between influenza A H1N1 and H3N2, (ii) HPIV-generic, which cannot differentiate between parainfluenzas 1, 2, 3 or 4, (iii) HCoV-generic which cannot differentiate between the human coronaviruses HCoV-OC43, HCoV-NL63, HCoV-229E, and HCoV-HKU1, and (iv) RV/EV which does not differentiate between rhinovirus and enterovirus (Table 1). Each RespVir member site submitted a file that includes the date of sampling, the tests performed and their results to a data base manager, who fed the data to the database.
Fig. 1.
Distribution of RespVir Network. The figure shows a European map with the location of the 47 laboratory members of RespVir Network. These laboratories are located in the following countries: Germany, Austria, Switzerland, Netherlands, and Spain
Table 1.
Prevalence of Circulating Viruses
| Prevalence of Circulating Viruses | ||||||||
|---|---|---|---|---|---|---|---|---|
| Tests Used for Viral Infection Diagnostic | Tested | Negatives | Positives | |||||
| tested virus | Abbreviation | Number | Proportion | Number | Percentage | Number | Percentage | Proportion |
| Influenza A (H3N2) | FLUA(H3N2) | 93,848 | 6.66% | 81,711 | 87.07% | 12,137 | 12.93% | 7.93% |
| Influenza A (H1N1) | FLUA(H1N1) | 65,468 | 4.65% | 60,602 | 92.57% | 4,866 | 7.43% | 3.18% |
| Non-differentiated Influenza A (H1N1 and H3N2) | FLUA-generic | 96,711 | 6.87% | 64,907 | 67.11% | 31,804 | 32.89% | 20.79% |
| Influenza B | FLUB | 168,628 | 11.97% | 143,013 | 84.81% | 25,615 | 15.19% | 16.74% |
| Parainfluenza 1 | HPIV-1 | 77,011 | 5.47% | 75,848 | 98.49% | 1,163 | 1.51% | 0.76% |
| Parainfluenza 2 | HPIV-2 | 76,406 | 5.42% | 75,431 | 98.72% | 975 | 1.28% | 0.64% |
| Parainfluenza 3 | HPIV-3 | 77,981 | 5.54% | 73,380 | 94.10% | 4,601 | 5.90% | 3.01% |
| Parainfluenza 4 | HPIV-4 | 45,825 | 3.25% | 44,978 | 98.15% | 847 | 1.85% | 0.55% |
| Non-differentiated Parainfluenza (1,2,3, and 4) | HPIV-generic | 21,949 | 1.56% | 20,882 | 95.14% | 1,067 | 4.86% | 0.70% |
| Metapneumovirus | HMPV | 86,107 | 6.11% | 80,858 | 93.90% | 5,249 | 6.10% | 3.43% |
| Respiratory Syncytial Virus | HRSV | 97,976 | 6.96% | 78,015 | 79.63% | 19,961 | 20.37% | 13.05% |
| Rhinovirus | RV | 74,061 | 5.26% | 53,150 | 71.77% | 20,911 | 28.23% | 13.67% |
| Enterovirus | EV | 63,444 | 4.50% | 59,377 | 93.59% | 4,067 | 6.41% | 2.66% |
| Non-differentiated Picornaviruses (Rhinovirus and Enterovirus) | RV/EV | 8,604 | 0.61% | 6,823 | 79.30% | 1,781 | 20.70% | 1.16% |
| Adenovirus | HAdV | 80,593 | 5.72% | 73,611 | 91.34% | 6,982 | 8.66% | 4.56% |
| Coronavirus OC43 | HCoV-OC43 | 63,523 | 4.51% | 60,818 | 95.74% | 2,705 | 4.26% | 1.77% |
| Coronavirus E229 | HCoV-E229 | 59,369 | 4.21% | 58,132 | 97.92% | 1,237 | 2.08% | 0.81% |
| Coronavirus NL63 | HCoV-NL63 | 61,455 | 4.36% | 59,922 | 97.51% | 1,533 | 2.49% | 1.00% |
| Coronavirus HKU1 | HCoV-HKU1 | 17,013 | 1.21% | 16,812 | 98.82% | 201 | 1.18% | 0.13% |
| Non-differentiated Coronaviruses (OC43, E229, NL63 and HKU1) | HCoV-generic | 7,628 | 0.54% | 6,909 | 90.57% | 719 | 9.43% | 0.47% |
| Bocavirus | HBoV | 65,057 | 4.62% | 60,474 | 92.96% | 4,583 | 7.04% | 3.00% |
| Total | 1,408,657 | 100% | 1,255,653 | 153,004 | 100% | |||
Table 1 Prevalence of Circulating Viruses. The table describes the 21 tests to detect 17 respiratory viruses, indicating the name of the test performed, the abbreviation for this study. The table also shows the number of tests performed, tests with negatives outcome and tests with positive outcome of each specific test type. The proportion indicates the percentage of all tests in each category (tested and positives). The percentage indicates the negativity and positivity percentage of each test type
Database pre-processing
The database required a pre-processing of the data. This process consisted in 1) filtering all incomplete data records before further analysis, and 2) a post-hoc curation.
After manual inspection of the data records, we found that most of the sites reported coinfection rates below 0.01 of any virus pairs. There were few sites (up to 4 out of 47, depending on the analysed virus pairs) reporting coinfection rates above 0.01. Due to the possibility of typographic errors and to avoid overestimation of the coinfection rates, we analysed only coinfections for each virus pairs reported below 0.01 threshold per site. Of note, this threshold also did not significantly affect the results.
After the pre-processing we split the database in two for the analysis. The first part of the analysis was performed on data records from monoinfections. Therefore, we filtered the data records for monoinfection. The second analysis was coinfections. For the coinfection analysis we took the records from samples tested for multiple viruses and exclusively evaluated coinfections with two viruses.
Seasonality profiles
We computed the seasonality profile of a virus by stratifying the number of positive tests from 2010 to 2019 by month and normalizing these month-specific infection counts. To quantify the degree of the seasonality of each virus, we computed the Kullback–Leibler divergence (KLD) of the seasonality profile to a uniform distribution [6]. Hence, a value of zero corresponds to uniform prevalence over the year, i.e., no seasonal variation. To compare the seasonality profiles of the 17 pathogens among each other, we computed the Jensen-Shannon divergence (JSD) [7] and applied agglomerative clustering with average linkage.
We also computed year-specific seasonality profiles for each year from 2010 to 2019. We clustered these profiles according to their JSD to compare the seasonality profiles over different years. In addition, we repeated the year-specific analysis using the positivity percentage instead of the absolute counts of positive tests.
Coinfections prevalence and virus pair relations
For studying coinfections, we considered the 17 viruses corresponding to 21 tests including the non-differentiating tests (FLUA-generic, HPIV-generic, RV/EV and HCoV-generic). While theoretically 210 combinations of the 21 tests are possible, we excluded combinations of non-differentiating tests with their more specific counterparts, such as FLUA(H3N2) combined with FLUA-generic. Finally, 197 valid combinations remained.
For assessing the tendency of each virus pair to exclude each other or coinfect the patient, we created a coinfection exclusion score (CES), see Supplementary material, Sect. 1.1, for the precise definition.
To exclude bias due to seasonal effects, we carried out this procedure for the entire data set stratified by months and averaged the resulting values over the statistically significant CES per virus pairs and months (Supplementary material, Sect. 1.2), yielding the average coinfection exclusion score (ACES). The CES and ACES scores assume a value of 1 if coinfections are ten times less likely than expected by chance and a value of -1 if they are ten times more likely.
Results
Database pre-processing
From 2010 to 2019, RespVir database registered 217,150 samples. After the initial filtering of the records with incomplete data, we retained 213,131 (98.14%) sample records.
To the monoinfection analysis, we selected samples with one or no positively tested pathogen. Regarding coinfections we selected all samples tested for at least two pathogens. To filter out sample records that are likely erroneous, for the coinfection analysis, we performed an additional post-hoc curation by setting a coinfection rate threshold of 0.01, for each of the virus pair per site. We discarded all coinfection patient records that yielded confections rates above this coinfection rate threshold.
After all filters were applied to the data base, we retained 206,814 sample records, of which 126,808 (61.31%) were monoinfections, 6781 (3.27%) coinfections, and 72,335 (34.97%) negatives. The majority of the samples, 26.69%, belonged to patients in ages between 0 < 6 years (26.69%), followed by patients between 45 < 65 years (23.96%) (Table 2). The retained samples correspond to 1,408,657 tests performed. For coinfection, we analysed 7,790,879 tests results combinations corresponding to the 197 senseful viral pairs (Supplementary table S1).
Table 2.
Age distribution by age goup
| Age group | # of samples | Percentage |
|---|---|---|
| 0 < 6 | 55,199 | 26.69% |
| 6 < 13 | 14,684 | 7.1% |
| 13 < 19 | 10,382 | 5.02% |
| 19 < 46 | 44,486 | 21.51% |
| 46 < 65 | 49,553 | 23.96% |
| 65 + | 32,511 | 15.72% |
| Total | 209,814 | 100% |
Table 2 The table shows the age distribution and percentage of samples per age group of retained samples after filtering the database
Infections prevalence
The 17 different respiratory viruses were not tested equally frequently. For example, while the 30.15% of the samples were tested only for the Orthomyxoviridae family, consisting of with FLUA (18.18%) and FLUB (11.97%). Members of the Coronaviridae family were tested significantly less frequently, with 4.51% HCoV-OC43, 4.36% HCoV-NL63, 4.21% HCoV-229E, 1.21% HCoV-HKU1, and 0.54% HCoV-generic (Table 1).
Influenza viruses represent 48.64% of all positive reports, of which 31.90% pertain to FLUA, and 16.74% to FLUB. The remaining 51.36% cover the remaining 14 viruses.
FLUA-generic test is the test with the highest positivity percentage (32.89%), followed by rhinovirus (28.23%), RV/EV (20.70%), human respiratory syncytial virus (HRSV) (20.37%), and influenza B (15.19%). The tests for coronaviruses and parainfluenza families were the ones with the lowest positivity percentage (Table 1).
Seasonal variations of viruses circulation
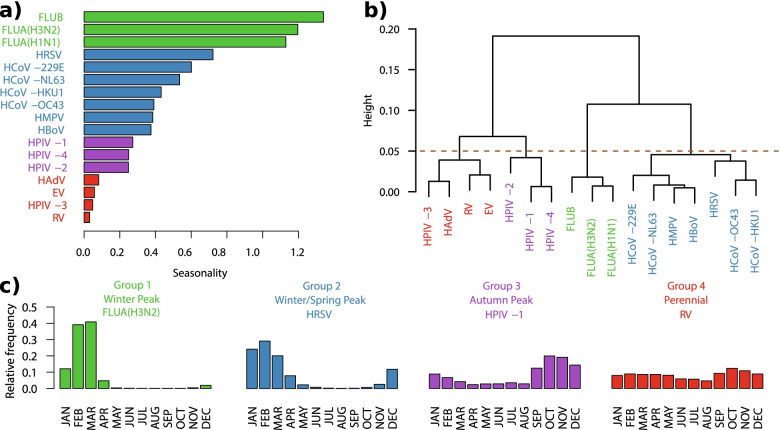
To study the seasonality of the respiratory viruses, we excluded the four tests that do not differentiate virus subtypes. The influenza viruses (FLUB, FLUA(H3N2), and FLUA(H1N1) exhibit the highest degree of seasonality, whereas RV, HPIV-3, enterovirus, and adenovirus exhibit the lowest (Fig. 2a).
Fig. 2.
Seasonality Profile of the Respiratory Viruses. The figure shows the seasonality profile of the 17 respiratory viruses studied. a) Degree of seasonality of each virus calculated by Kullback–Leibler divergence, where zero indicates no seasonality (see Methods, seasonality profile). b) Average linkage clustering of the 17 viruses according to their seasonality profile. c) The seasonal four groups according to the similarities of the 17 viruses, the figure shows the seasonal profile of one virus per group and the group name
We determined the similarity of the seasonality profiles among the 17 viruses and carried out hierarchical clustering with average linkage. After applying a cut-off of 0.05 to the dendrogram, we obtained four groups (Fig. 2b), each of which we assigned an interpretive label: (1) “Winter Peak” comprises FLUB, FLUA(H3N2) and FLUA(H1N1)), (2) “Winter/Spring Peak” comprises HRSV, HCoV-229E, HCoV-NL63, HCoV-HKU1, HCoV-OC43, HMPV, and HBoV, (3) “Autumn Peak” comprises HPIV-1, HPIV-2, and HPIV-4, and (4) “Perennial” comprises HAdV, EV, HPIV-3, RV (Fig. 2c and Supplementary figure S1). We repeated this analysis using the positivity percentage, obtaining similar results. (Supplementary figure S2).
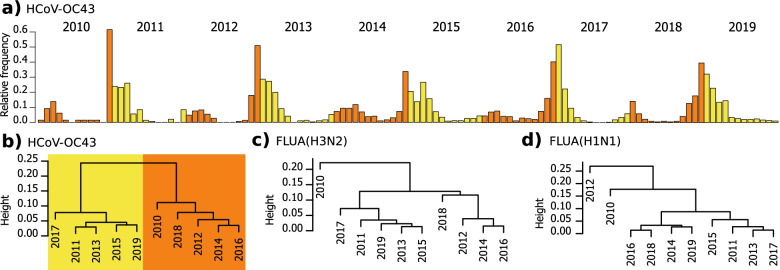
To analyse seasonality variation of each virus among the 10 years of the study, we stratified the frequency of positive tests for each pathogen by year and calculated their year-specific seasonality profiles (Supplementary figure S3). We found that HCoV-OC43, exhibiting a clear biennial pattern, that is, high infection numbers at the end of even and beginning of odd years (Fig. 3a). To obtain a more concise representation, we applied hierarchical clustering with average linkage to the ten profiles of each virus (Supplementary figure S4). We found that the year-specific seasonality profiles of HCoV-OC43 resolve into two groups that contain the odd and even years respectively (Fig. 3b), and a similar biennial variation becomes apparent for HPIV-1, HPIV-3, and HRSV (Supplementary figure S4). For FLUA(H3N2) (Fig. 3c) and FLUA(H1N1) (Fig. 3d) a biennial pattern can be presumed, but it is not consistently true all years.
Fig. 3.
Annual variation of seasonality. The figure shows the annual variation of seasonality and the biennial pattern discovered. a) Biennial pattern of HCoV-OC43 exhibiting high infection numbers at the end of even and beginning of odd years, but low infection numbers at the end of odd and beginning of even years. b) Hierarchical clustering with average linkage of the annual variation of seasonality of HCoV-OC43. c) Hierarchical clustering with average linkage of the annual variation of seasonality of FLUA(H3N2). d) Hierarchical clustering with average linkage of the annual variation of seasonality of FLUA(H1N1)
Coinfections prevalence and virus pair relations
To describe the coinfection prevalence, we analysed 7,790,879 tests results for the 197 virus test pairs.
We found that RV/HBoV, RV/HAdV, HRSV/HBoV, HRSV/HAdV and HRSV/HCoV-OC43 coinfections were the most prevalent coinfections, and together with HRSV/RV had the highest positivity percentage. For 19 out of the 197 studied virus pairs, we did not find any coinfection case (Supplementary table S1).
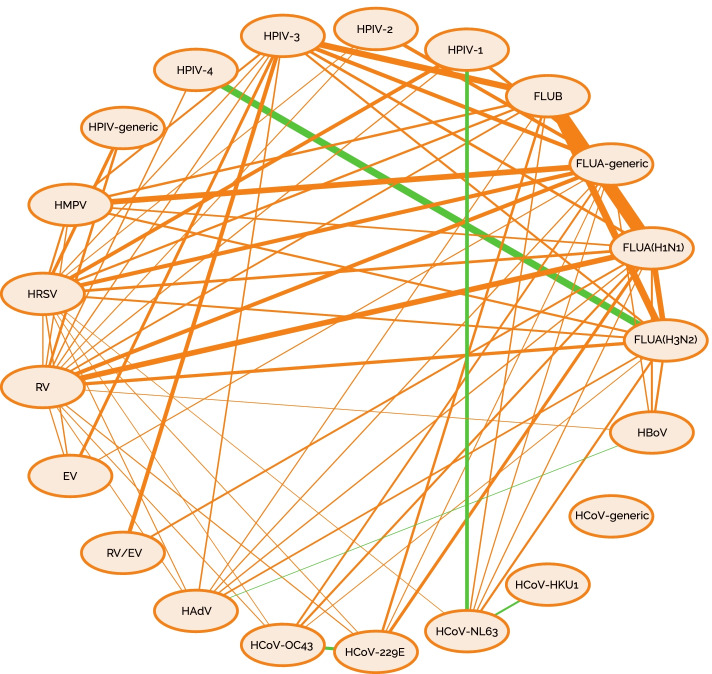
For 73 of the 197 virus pairs, we obtained a coinfection exclusion score (CES), which implies a statistically significant dependence of both test results, for at least one month. The great majority of test pairs yield a positive ACES (Average coinfection exclusion score), indicating coinfection exclusion. The pair FLUA(H1N1)/FLUB shows the highest coinfection exclusion (ACES = 1.67), followed by FLUA(H3N2)/FLUB (ACES = 1.39), FLUB/HPIV-3 (ACES = 1.34), FLUA-generic/HMPV (ACES = 1.30), and FLUA(H1N1)/RV and FLUA(H3N2)/FLUA(H1N1) with an ACES = 1.29 for both cases. We found negative ACES values in only five test pairs, indicating affinity of the viral pairs like FLUA(H3N2)/HPIV-4 with ACES = -1.39 (Fig. 4 and Supplementary table S2).
Fig. 4.
Interaction strength between the 17 virus pairs regarding coinfection. The figure shows the 17 studied viruses linked by lines. Orange lines indicate an exclusion interaction while green lines an affinity interaction. The thickness of the lines indicates the strength of the interaction
Discussion
In the present study, we present data from the respiratory pathogens network that has been in place since 2009, based on multicenter, wide-spectrum collection rather than collection of data based on narrowly defined selection criteria. In principle it could be taken as a limitation due to the different clinical criteria by applied by physicians as they request specific diagnostic test. The multiplex test approach in our analysis reduces a possible bias because the samples are tested not only for a single suspected pathogen, but for the 17 respiratory viruses of the multiplex panel.
One disadvantage of this strategy is a lack of clinical historal data. For example, we cannot determine the influence of vaccination rate in our cohort. Nevertheless, the broad coverage (nationwide) of our data allows to assume that the vaccination rate is representative in our cohort.
Filtering and post-hoc curation was required. This was partly necessary due to non-curated data entering the database. To overcome this disadvantage, it is important to use quality control mechanisms during data collection in the future to reject the collection of false data mainly regarding coinfections.
We analysed the frequency of 17 respiratory pathogens with respect to monoinfections and coinfections, their seasonal variation, and the affinity to coinfect with other viruses, spanning 10 years (2010–2019). To our knowledge, this study reports on the largest volume of data of its kind [4, 8–15]. Nevertheless, another limitation in terms of global health is that our samples come mainly from Germany, Austria and Switzerland, these samples constitute a good basis to analyze the Central Europe (continental) region and therefor the the results about saisonality should not be extrapolated to other hemisphere regions, nor even to north Europe, Spain or United kingdom.
Our results confirm previous reports that influenza A viruses, HRSV, and RV were detected most frequently in our cohort [4, 13, 14, 16, 17]. Nevertheless, almost one quarter (24.65%) of the infections are caused by other respiratory viruses. Although influenza tests are the most frequently performed assays, 51.36% of all positive tests derived from other respiratory viruses. This supports the importance of testing for multiple pathogens for diagnostic purposes [18–20]. A disadvantage of our approach is that relies on routine diagnostic test, therefore influenza typing is restricted to Influenza A (H1N1 and H3N2) and no further data on the subtypes of influenza B are given, Consequently, no detailed specific description of the viruses' behaviour or more specific coinfection relations can be evaluated.
To detect patterns of seasonal variation worldwide, corresponding worldwide and long-term studies are needed [18, 19]. Our study is robust and covers a long-term period for the central European area. This study allows us to confirm previously reported seasonal patterns [18–22], but also to propose a new seasonal classification of the studied viruses into four groups. Furthermore, we found a typical seasonal pattern repeated every other years for HPIV-1, HPIV-3, HMPV, HRSV, and HCoV-OC43; also, for FLUA(H3N2) except for the years 2010 and 2018 as well as for FLUA(H1N1) except for years 2010 and 2012, respectively. This confirms the constancy of the biennial patterns except for years near to a pandemic event due to new viruses appearance.
The SARS-CoV-2 outbreak has raised questions regarding the seasonal pattern of this virus. Studies on seasonal patterns of endogenous viruses could help to solve these questions. We found a slight difference in the seasonal profile within the coronaviruses (Figure S1). SARS-CoV-2 belongs to beta-coronaviruses which season usually starts in November–December and has its peak in December-January. Thus, a similar seasonality could be expected for SARS-CoV-2 in the future.
Coinfection modifies the natural history of diseases caused by single infections. Thus, deeper understanding of coinfections, especially the exclusion mechanisms could help the development of antivirals [23]. Only a few large-scale data analyses on virus-virus interactions exist, in contrast to numerous studies on bacterial coinfections and virus-bacteria studies [24–28]. We characterized the coinfection prevalence and the interactions between 17 different viruses and analysed 7,790,879 tests combinations, within the ten year observation period. To our knowledge, our study provides the analysis of virus-virus interaction with the largest diversity of respiratory viruses, the longest surveillance period, and the largest number of tests performed.
As expected, the most prevalent coinfection virus pairs and the highest positivity percentage (Table S1) had also a high monoinfection prevalence and a seasonal overlap. To compare the propensity of a virus pair to coinfect, we introduced a coinfection exclusion score (CES). To exclude bias due to seasonal effects, we calculated an average coinfection exclusion score (ACES).
One of the most relevant studies of virus-virus interaction has been performed by Nickbakhsh et. al. [29], who analysed 44,230 respiratory illness cases tested for 11 viruses over nine years and classified the viral pairs interactions. Our data confirm a strong exclusion of any of the influenza A strains (H1N1 or H3N2) to coinfect with rhinovirus. This exclusion has been confirmed also in an animal model [30]. Our data also confirm an exclusion between FLUB and HAdV. In contrary to Nickbakhsh et al. [29], our data suggest strong exclusion for HRSV and HMPV coinfection and no significant interaction between HPIV-2 with HPIV-3. Nickbakhsh et al. [29] did not report any other interaction, while our data shows the strongest exclusion for FLUA(H1N1) and FLUB as well as for FLUA(H3N2) and FLUB. For the other virus pairs the numbers are too small to test for significance. So, further studies are needed to get more insight into the frequency ant role of virus co-infections.
Conclusions
The deeper understanding on virus dynamics will contribute to improve diagnostics, the prevention of infection, and potentially, the development of therapies for viral infections. We show the advantages of multiplex testing to identify the causative agent for a respiratory disease. Our approach shows the usefulness for collecting data with such real world data bases. Analysis of data on (co-) infections, seasonality, and interactions of viruses can be performed much faster compared to prospective clinical studies.
Supplementary Information
Additional file 1. Supplementary file 1.
Acknowledgements
The RespVir-Network is supported by the Gesellschaft für Virology, Deutsche Vereinigung zur Bekämpfung der Viruskrankheiten e.V., and Paul Ehrlich Gesellschaft für Chemotherapie e.V. Respiratory Virus Network Rolf Kaiser, PhD1, Barbara C. Gärtner, MD8, Benedikt Weissbrich, PhD9, Ortwin Adams, MD11, Annemarie Berger, Dr. med. vet.12, Katrin Palupsky13, Daniela Huzly, MD14, Carsten Tiemann, PhD15, Wegene Borena, PhD16, Andreas Lindauer, PhD17, Uwe Gerd Liebert, PhD18, Hans-Joachim Siemens, MD19, Jörg Hofmann20, Anna-Maria Eis-Hübinger, PhD21, Hajo Grundmann, MD22, Astrid Kehlen, PhD23, Albrecht Oehme, MD23, Paul Schnitzler, PhD24, Joachim Kühn, MD25, Albert Heim, PhD26, Andreas Sauerbrei, MD27, Barbara Schmidt, MD28, Robert Beck, MD29, Dieter Hoffmann, MD30, Detlef Michel, PhD31, Hans Nitschko, PhD32, Christian Aepinus,PhD33, Jens Dreier,PhD34, Elisabeth Puchhammer-Stoeckl, PhD35, Theresa Popow Kaup, PhD35, Monika Redlberger,PhD35, Harald Kessler,MD36, Martin Obermeier, MD37, Kerstin Weise,PhD38, Patricia Bartsch39, Annette Devide, MD40, Bert Niesters, PhD41, Michael Kleines, PhD42, Andi Krumbholz, MD43, Thomas Meyer,, PhD44, Peter Gohl,, PhD45, Christian G. Schüttler, MD46, Meri Gorgievski, MD47, Andres Anton Pagarolas, PhD48, Wolfgang Gulich49, Thomas Ziegler,PhD50, Babett Wintsche,PhD51, Marcena Griego52, Walter Bossart, PhD53. 1Institute of Virology, Faculty of Medicine and University Hospital Cologne, Germany 8Institute of Medicine Microbiology and Hygiene, University of the Saarland Kirrberger Homburg/Saar, Germany 9Faculty of Medicine, Institute for Virology and Immunobiology, Würzburg University, Würzburg, Germany, 11University of Düsseldorf, Medical Faculty, Institute for Virology, Düsseldorf, Germany, 12 Institut für Medizinische Virologie - Universitätsklinikum Frankfurt, Frankfurt, Germany, 13Institut für Virologie – Universitätsklinikum Essen, Essen, Germany, 14Institut für Virologie - Universitätsklinikum Freiburg, Freiburg, Germany, 15Labor Krone GbR - Medizinal-Untersuchungsstelle im Regierungsbezirk Detmold, Bad Salzuflen, Germany, 16 Institut für Virologie - Medizinische Universität Innsbruck, Innsbruck, Austria, 17SYNLAB Medizinisches Versorgungszentrum Weiden GmbH, Weiden in der Oberpfalz, Germany, 18 Institut für Virologie - Universität Leipzig, Leipzig, Germany, 19MVZ Institut für Medizinische Mikrobiologie, Infektiologie, Hygiene und Tropenmedizin GmbH, Germany, 20Institut für Virologie, Charité - Universitätsmedizin Berlin, Berlin Germany, 21Institut für Virologie - Universitätsklinikum Bonn, Bonn Germany, 22Institut für Infektionsprävention und Krankenhaushygiene - Universitätsklinikum Freiburg, Freiburg Germany, 23Institut für Medizinische Mikrobiologie - Martin-Luther-Universität Halle-Wittenberg, Halle (Saale), Germany, 24Department für Infektiologie, Virologie - Universitätsklinikum Heidelberg, Heidelberg, Germany, 25Institut für Medizinische Mikrobiologie, Klinische Virologie - Universitätsklinikum Münster, Münster, Germany, 26Institut für Virologie und Seuchenhygiene - Medizinische Hochschule Hannover, Hannover, Germany, 27Institut für Virologie und Antivirale Therapie - Universitätsklinikum Jena, Jena, Germany, 28Institut für Mikrobiologie und Hygiene - Universitätsklinikum Regensburg, Regensburg, Germany, 29Institut für Medizinische Virologie und Epidemiologie der Viruskrankheiten - Uniklinikum Tübingen, Tübingen, Germany, 30Institut für Virologie - Technische Universität München, München, Germany, 31Institut für Virologie - Universitätsklinikum Ulm, 32Max-von-Pettenkofer-Institut für Hygiene und Medizinische Mikrobiologie - Universität München, München, Germany, 33Institut für Virologie - Universität Marburg, Marburg, Germany, 34Institut für Laboratoriums- und Transfusionsmedizin - Universitätsklinik Bochum, Bochum, Germany, 35Department für Virologie - Medizinische Universität Wien, Vienna, Austria, 36Institut für Hygiene, Mikrobiologie und Umweltmedizin - Medizinische Universität Graz, Graz, Austria, 37MIB, Medizinisches Infektiologiezentrum Berlin, Berlin, Germany, 38Institut für Virologie - Universitätsmedizin Mainz, Mainz, Germany, 39Medizinisches Versorgungszentrum Dr. Eberhard & Partner Dortmund, Dortmund, Germany, 40Institut für Medizinische Mikrobiologie, Virologie und Hygiene - Universitätsmedizin Rostock, Rostock, Germany, 41Laboratory of Clinical Virology - University of Gröningen, Gröningen, Germany, 42Labordiagnostisches Zentrum - Uniklinik RWTH Aaachen, Aachen, Germany, 43Labor Dr. Krause und Kollegen MVZ GmbH, Kiel, Germany, 44Medizinische Mikrobiologie, Virologie und Hygiene - Universitätsklinikum Hamburg-Eppendorf, Hamburg, Germany, 45Bioscientia Institut für Medizinische Diagnostik GmbH - Labor Ingelheim,, Ingelheim, Germany, 46Medizinische Virologie - Universitätsklinikum Giessen und Marburg GmbH, Marburg, Germany, 47Institut für Infektionskrankheiten - Universität Bern, Bern, Switzerland, 48Microbiology Department - Hospital Universitari Vall d‘Hebron, Barcelona, Spain, 49Medizinisches Labor Ostsachsen - Labor Görlitz, Görlitz, Germany, 50Institut für Medizinische Diagnostik Berlin-Potsdam MVZ GbR, Berlin, Germany, 51 Gemeinschaftslabor Cottbus MVZ GbR, Cottbus, Germany, 52Praxis für Labormedizin und Mikrobiologie, Bochum, Germany, 53Institut für Medizinische Virologie - Universität Zürich, Zürich, Switzerland.
Abbreviations
- RespVir
Respiratory Viruses Network
- ACES
Average coinfection exclusion score
- COVID-19
Coronavirus disease 2019
- SARS-CoV-2
Severe acute respiratory disease-coronavirus-2
- GfV
German Virology Society
- FLUA
Generic: influenza A generic test
- HPIV
Generic: human parainfluenza virus-generic test
- HCoV
Human coronavirus
- HCoV-OC43
Human coronavirus OC43
- HCoV-NL63
Human coronavirus NL63
- HCoV-229E
Human coronavirus 229E
- HCoV-HKU1
Human coronavirus HKU1
- RV/EV
Rhinovirus/enterovirus
- KLD
Kullback-Leibler divergence
- CES
Coinfection exclusion score
- FLUA
Influenza A
- FLUB
Influenza B
- HRSV
Human respiratory syncytial virus
- HMPV
Human metapneumovirus
- HBoV
Human bocavirus
- HPIV-1
Human parainfluenza virus 1
- HPIV-2
Human parainfluenza virus 2
- HPIV-4
Human parainfluenza virus 4
- HAdV
Human adenovirus
- EV
Enterovirus
- HPIV-3
Human parainfluenza virus 3
- RV
Rhinovirus
Authors' contributions
GHR*: concept of the current analysis, analysis of the data, and, writing the manuscript, RE*: bioinformatics analysis, Writing of supplementary appendix, NP: bioinformatics analysis, NS: database management, TL: interpretation of the data, and preparation of the manuscript, BG: co-coordination of the RespVir network and data collection, CP: co-coordination of the RespVir network and data collection, MK: patient recruitment and clinical validation of the RespVir network, CS: patient recruitment and clinical validation of the RespVir network, OA**: concept and coordination of the network, database structure, analyses of the virologic results; contributed RK**: concept and coordination of the network, database structure, analyses of the virologic results and concept of the current analysis. All authors contributed to the writing of the manuscript and accepted the final version *Contributed equally **Also contributed equally. The authors read and approved the final manuscript.
Funding
Open Access funding enabled and organized by Projekt DEAL. No specific funding for this project.
Availability of data and materials
Data and source code are available upon reasonable request from Rolf Kaiser and Gibran Horemheb-Rubio (data) and Ralf Eggeling (source code).
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Gibran Horemheb-Rubio, Ralf Eggeling and Rolf Kaiser contributed equally to this work.
Contributor Information
Rolf Kaiser, Email: rolf.kaiser@uk-koeln.de.
Respiratory Virus Network:
Rolf Kaiser, Barbara C. Gärtner, Benedikt Weissbrich, Ortwin Adams, Annemarie Berger, Katrin Palupsky, Daniela Huzly, Carsten Tiemann, Wegene Borena, Andreas Lindauer, Uwe Gerd Liebert, Hans-Joachim Siemens, Jörg Hofmann, Anna-Maria Eis-Hübinger, Hajo Grundmann, Astrid Kehlen, Albrecht Oehme, Paul Schnitzler, Joachim Kühn, Albert Heim, Andreas Sauerbrei, Barbara Schmidt, Robert Beck, Dieter Hoffmann, Detlef Michel, Hans Nitschko, Christian Aepinus, Jens Dreier, Elisabeth Puchhammer-Stoeckl, Theresa Popow Kaup, Monika Redlberger, Harald Kessler, Martin Obermeier, Kerstin Weise, Patricia Bartsch, Annette Devide, Bert Niesters, Michael Kleines, Andi Krumbholz, Thomas Meyer, Peter Gohl, Christian G. Schüttler, Meri Gorgievski, Andres Anton Pagarolas, Wolfgang Gulich, Thomas Ziegler, Babett Wintsche, Marcena Griego, and Walter Bossart
References
- 1.World Health Organization. The top 10 causes of death. 2014. https://www.who.int/news-room/fact-sheets/detail/the-top-10-causes-of-death. Accessed 30 Aug 2020.
- 2.Nielsen J, Vestergaard LS, Richter L, Schmid D, Bustos N, Asikainen T, et al. European all-cause excess and influenza-attributable mortality in the 2017/18 season: should the burden of influenza B be reconsidered? Clin Microbiol Infect. 2019;25:1266–1276. doi: 10.1016/j.cmi.2019.02.011. [DOI] [PubMed] [Google Scholar]
- 3.Paget J, Spreeuwenberg P, Charu V, Taylor RJ, Iuliano AD, Bresee J, Simonsen L, Viboud C. Global Seasonal Influenza-associated Mortality Collaborator Network and GLaMOR Collaborating Teams*. Global mortality associated with seasonal influenza epidemics: New burden estimates and predictors from the GLaMOR Project. J Glob Health. 2019;9(2):020421. 10.7189/jogh.09.020421. [DOI] [PMC free article] [PubMed]
- 4.Silke Buda, Viktoria Streib, Ute Preuß, Ralf Dürrwald, Barbara Biere JS, Udo Buchholz, Matthias an der Heiden WH, Aufgeführt. K 6 bis 9: DA einzelner B sind im jeweiligen A bzw. K. Bericht zur Epidemiologie der Influenza in Deutschland Saison 2018/19, Kap. 1 bis 5, 10. 2019. 10.25646/6232. [DOI]
- 5.Home - Clinical Virology. https://clinical-virology.net/en. Accessed 30 Aug 2020.
- 6.Kullback S, Leibler RA. On Information and Sufficiency. Ann Math Stat. 1951;22(1):79–86. doi: 10.1214/aoms/1177729694. [DOI] [Google Scholar]
- 7.Lin J. Divergence Measures Based on the Shannon Entropy. IEEE Trans Inf Theory. 1991;37:145–151. doi: 10.1109/18.61115. [DOI] [Google Scholar]
- 8.Visseaux B, Burdet C, Voiriot G, Lescure F-X, Chougar T, Brugière O, et al. Prevalence of respiratory viruses among adults, by season, age, respiratory tract region and type of medical unit in Paris, France, from 2011 to 2016. PLoS ONE. 2017;12:e0180888. doi: 10.1371/journal.pone.0180888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Weigl JAII, Puppe W, Gröndahl B, Schmitt H-JJ. Epidemiological investigation of nine respiratory pathogens in hospitalized children in Germany using multiplex reverse-transcriptase polymerase chain reaction. Eur J Clin Microbiol Infect Dis. 2000;19:336–43. doi: 10.1007/s100960050490. [DOI] [PubMed] [Google Scholar]
- 10.Macfarlane J, Holmes W, Gard P, Macfarlane R, Rose D, Weston V, et al. Prospective study of the incidence, aetiology and outcome of adult lower respiratory tract illness in the community. Thorax. 2001;56:109–114. doi: 10.1136/thorax.56.2.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Creer DD, Dilworth JP, Gillespie SH, Johnston AR, Johnston SL, Ling C, et al. Aetiological role of viral and bacterial infections in acute adult lower respiratory tract infection (LRTI) in primary care. Thorax. 2006;61:75–79. doi: 10.1136/thx.2004.027441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Topoulos S, Giesa C, Gatermann S, Fussen R, Lemmen S, Ewig S. Analysis of acute respiratory infections due to influenza virus A, B and RSV during an influenza epidemic 2018. Infection. 2019;47:425–433. doi: 10.1007/s15010-018-1262-x. [DOI] [PubMed] [Google Scholar]
- 13.Meerhoff TJ, Simaku A, Ulqinaku D, Torosyan L, Gribkova N, Shimanovich V, Chakhunashvili G, Karseladze I, Yesmagambetova A, Kuatbayeva A, Nurmatov Z, Otorbaeva D, Lupulescu E, Popovici O, Smorodintseva E, Sominina A, Holubka O, Onyshchenko O, Brown CS, Gross D. Surveillance for severe acute respiratory infections (SARI) in hospitals in the WHO European region - an exploratory analysis of risk factors for a severe outcome in influenza-positive SARI cases. BMC Infect Dis. 2015;15:1. 10.1186/s12879-014-0722-x. [DOI] [PMC free article] [PubMed]
- 14.Caini S, de Mora D, Olmedo M, Portugal D, Becerra MA, Mejía M, et al. The epidemiology and severity of respiratory viral infections in a tropical country: Ecuador, 2009–2016. J Infect Public Health. 2019;12:357–363. doi: 10.1016/j.jiph.2018.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Walter JM, Wunderink RG. Severe Respiratory Viral Infections: New Evidence and Changing Paradigms. Infect Dis Clin North Am. 2017;31:455–474. doi: 10.1016/j.idc.2017.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Weigl JAI, Puppe W, Schmitt HJ. The incidence of influenza-associated hospitalizations in children in Germany. Epidemiol Infect. 2002;129:525–533. doi: 10.1017/S0950268802007707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Steppuhn H, Buda S, Wienecke A, Kraywinkel K, Tolksdorf K, Haberland J. Time trends in incidence and mortality of respiratory diseases of high public health relevance in Germany. J Heal Monit J Heal Monit Focus Respir Dis · LUNG Dis · Incid · Mortal · Surveill J Heal Monit · Robert Koch-Institut, Epidemiologie und Gesundheitsberichterstattung. 2017;2. 10.17886/RKI-GBE-2017-061. [DOI] [PMC free article] [PubMed]
- 18.Heraud J-M, Razanajatovo NH, Viboud C. Global circulation of respiratory viruses: from local observations to global predictions. Lancet Glob Heal. 2019;7:e982–e983. doi: 10.1016/S2214-109X(19)30277-3. [DOI] [PubMed] [Google Scholar]
- 19.Li Y, Reeves RM, Wang X, Bassat Q, Brooks WA, Cohen C, et al. Global patterns in monthly activity of influenza virus, respiratory syncytial virus, parainfluenza virus, and metapneumovirus: a systematic analysis. Lancet Glob Heal. 2019;7:e1031–e1045. doi: 10.1016/S2214-109X(19)30264-5. [DOI] [PubMed] [Google Scholar]
- 20.Monto AS, DeJonge PM, Callear AP, Bazzi LA, Capriola SB, Malosh RE, et al. Coronavirus Occurrence and Transmission Over 8 Years in the HIVE Cohort of Households in Michigan. J Infect Dis. 2020;222:9–16. doi: 10.1093/infdis/jiaa161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fisman D. Seasonality of viral infections: Mechanisms and unknowns. Clin Microbiol Infect. 2012;18:946–954. doi: 10.1111/j.1469-0691.2012.03968.x. [DOI] [PubMed] [Google Scholar]
- 22.Moriyama M, Hugentobler WJ, Iwasaki A. Seasonality of Respiratory Viral Infections. Annu Rev Virol. 2020;7(1):83–101. 10.1146/annurev-virology-012420-022445. Epub 2020 Mar 20. [DOI] [PubMed]
- 23.Kumar N, Sharma S, Barua S, Tripathi BN, Rouse BT. Virological and immunological outcomes of coinfections. Clin Microbiol Rev. 2018;31(4):e00111–17. 10.1128/CMR.00111-17. [DOI] [PMC free article] [PubMed]
- 24.Loosli CG. Influenza and the interaction of viruses and bacteria in respiratory infections1. Med (United States) 1973;52:369–384. doi: 10.1097/00005792-197309000-00001. [DOI] [PubMed] [Google Scholar]
- 25.Wiegers HMG, Van Nijen L, Van Woensel JBM, Bem RA, De Jong MD, Calis JCJ. Bacterial co-infection of the respiratory tract in ventilated children with bronchiolitis; A retrospective cohort study. BMC Infect Dis. 2019;19:938. doi: 10.1186/s12879-019-4468-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bakaletz LO. Viral–bacterial co-infections in the respiratory tract. Curr Opin Microbiol. 2017;35:30–35. doi: 10.1016/j.mib.2016.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ånestad G. INTERFERENCE BETWEEN OUTBREAKS OF RESPIRATORY SYNCYTIAL VIRUS AND INFLUENZA VIRUS INFECTION. The Lancet. 1982;319:502. doi: 10.1016/S0140-6736(82)91466-0. [DOI] [PubMed] [Google Scholar]
- 28.Zhu X, Ge Y, Wu T, Zhao K, Chen Y, Wu B, et al. Co-infection with respiratory pathogens among COVID-2019 cases. Virus Res. 2020;285. [DOI] [PMC free article] [PubMed]
- 29.Nickbakhsh S, Mair C, Matthews L, Reeve R, Johnson PCD, Thorburn F, et al. Virus-virus interactions impact the population dynamics of influenza and the common cold. Proc Natl Acad Sci U S A. 2019;116:27142–27150. doi: 10.1073/pnas.1911083116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gonzalez AJ, Ijezie EC, Balemba OB, Miura TA. Attenuation of Influenza A Virus Disease Severity by Viral Coinfection in a Mouse Model. J Virol. 2018;92:1–21. doi: 10.1128/JVI.00881-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1. Supplementary file 1.
Data Availability Statement
Data and source code are available upon reasonable request from Rolf Kaiser and Gibran Horemheb-Rubio (data) and Ralf Eggeling (source code).