Abstract
A complex pathogenesis involving several physiological systems is theorized to underline the development of depressive disorders. Depression is accompanied by circadian regulation disruption and interaction with the functioning of both central and peripheral oscillators. Many aspects of melatonin function unite these systems. The use of drugs for circadian rhythm disorders could inspire a potential treatment strategy for depression. Melatonin plays an essential role in the regulation of circadian rhythms. It exerts effect by activating two types of melatonin receptors, type 1A (MT1) and 1B (MT2). These are G-protein-coupled receptors, predominantly located in the central nervous system. MT1/MT2 agonists could be a useful treatment approach according to all three prevalent theories of the pathogenesis of depression involving either monoamines, synaptic remodeling, or immune/inflammatory events. MT1/MT2 receptors can be a potential target for novel antidepressants with impact on concentrations of neurotrophins or neurotransmitters, and reducing levels of pro-inflammatory cytokines. There is an interesting cross-talk mediated via the physical association of melatonin and serotonin receptors into functional heteromers. The antidepressive and neurogenetic effects of MT1/MT2 agonists can also be caused by the inhibition of the acid sphingomyelinase, leading to reduced ceramide, or increasing monoamine oxidase A levels in the hippocampus. Compounds targeting MT1 and MT2 receptors could have potential for new anti-depressants that may improve the quality of therapeutic interventions in treating depression and relieving symptoms. In particular, a combined effect on MT1 and/or MT2 receptors and neurotransmitter systems may be useful, since the normalization of the circadian rhythm through the melatonergic system will probably contribute to improved treatment. In this review, we discuss melatonergic receptors as a potential additional target for novel drugs for depression.
Keywords: Depression, Melatonin, Receptors, Clock genes, Neurogenesis, Circadian rhythm
Introduction
The neurohormone melatonin (N-acetyl-5-methoxytryptamine), which plays an important role in the circadian clock system, is primarily synthesized in the pineal gland. The disturbances of the diurnal cycle are reflected in melatonin’s circulating levels. The circadian clock is an evolutionarily highly conservative feature of bacteria, plants, and animals, allowing organisms to adapt physiological processes to the time of day. This internal synchronization mechanism coordinates biochemical, physiological, and behavioral processes to maintain synchronization with light, temperature, and nutrient intake cycles. In mammals, including humans, circadian rhythms are regulated by a temporary control system consisting of the primary pacemaker in the suprachiasmatic nuclei (SCN) of the hypothalamus and peripheral oscillators located throughout the body. Independent circadian oscillators exist in every cell of any tissue and organ [1].
Melatonin mainly controls the sleep–wake cycle, regulates the immune system in mammals (pro- and anti-inflammatory states), and energy metabolism [2]. Melatonin exerts its effect by acting primarily on activating two types of high-affinity rhodopsin transmembrane melatonin receptors type 1A (MT1) and 1B (MT2) [3–7]. MT1 and MT2 receptors are predominantly located in the central nervous system (CNS), being more in areas that belong to the brain’s dopaminergic system, purveying a correlation between the monoaminergic and melatonergic receptors pathways [8]. MT1 receptors are distributed more frequently outside the CNS, while MT2s are predominantly in the CNS [3]. Interestingly, the expression of both of these receptors exhibits photo-regulation since it was seen that there is an augmentation in the messenger ribonucleic acid (mRNA) expression of MT1 receptors in rodents during daytime [9]. Therefore, melatonin forms a basis for exploring various neuropsychiatric conditions by playing a significant role in maintaining the sleep–wake cycle and its association with monoaminergic pathways [10].
The treatment of neuropsychiatric disorders has recently gained particular importance due to the coronavirus disease 2019 (COVID-19) pandemic. Many researchers note that mental health has deteriorated sharply due to the pandemic. The most common mental disorders are mood disorders, especially depression and anxiety, circadian rhythm, and sleep disorders. The last meta-analysis showed that pooled prevalence of depression was 45% in patients after COVID-19 [11]. It is assumed that the development of mental disturbances may be associated with biorhythmology. The most studied is the relationship between the development of mental illness and circadian dysregulation, which can self-regulate and is very sensitive to any stressful influences [12].
Depression is one of the most common mood disorders in the world. The absolute number of new cases of depression worldwide increased by almost 50% from 1990 to 2017 [13]. Despite the constant stream of research on mood disorders and the emergence of new antidepressants, depression remains a severe problem. Depression affects a person’s well-being and behavior. According to the American Psychiatric Association, every 15th inhabitant of our planet suffers from depression every year, and every 6th person feels depression at least once in their life [14]. The pathogenesis of the development of depression is multicomponent and complex because it includes changes at various stages of the body’s functioning, from the genome to metabolic processes. Depression is associated with multiple neurobiological targets, from altered neuroplasticity to disturbances in the body’s circadian rhythms [15]. This is due to genetic, physiological, psychological, and social factors. In depression, symptoms range from loss of motivation and energy to suicidal thoughts. Moreover, with depression, there may also be changes in the sleep–wake cycle and the circadian rhythms of hormonal secretion [16].
The relationship between major depressive disorders (MDD) and melatonergic pathways is complex, as is shown by disparity in the measured melatonin levels in depressed individuals. Most studies indicate MDD as a low melatonin syndrome, thus conceptualizing inadequate melatonin secretion as a biological marker for depression [17]. MDD has many underlying mechanisms associated with its pathogenesis, of which impairment in the central monoaminergic system remains the most acknowledged [18]. The relation between MT1/MT2 activation and monoaminergic pathways may suggest its role in the pathophysiology of MDD. One of the classic symptoms of MDD is mood decline in the morning, and this time-linked symptom has encouraged proposals about the role of the circadian oscillator in its development [19]. Literature suggests that MDD is not a disturbance of a particular rhythm, but a deterioration in the function of the central circadian clock [20], which causes a decrease in the amplitude of core body temperature, thyroid and glucocorticosteroid hormonal status, and motor activity in MDD [21]. The correlations mentioned above provide substantial evidence for avenues of clinical interventions and research on chronotherapy as one of the novel drug therapies for depression.
Given the complex multifactorial pathophysiology of MDD, new antidepressants should have targets that simultaneously affect several pathogenesis pathways. The purpose of this article is to describe how melatonergic receptor agonists could act as a potential additional target of novel drugs for depression by elucidating the role of melatonin, circadian rhythm, clock genes, and their relationships with the monoaminergic system, neuronal remodeling, and inflammation in the pathophysiology of depression.
Melatonin Production
Melatonin is an endogenous hormone of the human organism, initially discovered in 1958 [22]. The molecular structure of melatonin is derived from tryptamine, rendering it similar to 5-HT (5-hydroxytryptamine) in structure. Chemically, it can be classified as an acetamide or indolamine. Melatonin is produced in the pineal gland located centrally in the neuroendocrine system of the brain and secreted into the circulation exerting numerous effects on the body. Melatonin receptors are found on many tissues throughout the body, where the hormone exerts its effect. The biosynthesis of melatonin involves various steps, resulting in its precursor tryptophan being processed into serotonin, N-acetyl serotonin, and finally, into melatonin [23] (Fig. 1). Synthesis of melatonin is regulated by the suprachiasmatic nucleus of the anterior hypothalamus, through multi-synaptic descending pathways, which releases norepinephrine (NE) [24] and entails photoperiodic variation [25]; this results in the modulation of N-acetyl transferase activity which transforms serotonin (5-HT) into melatonin [26]. The neurohormone is implicated in the circadian rhythm as a key regulator for the sleep–wake- cycle. Additionally, melatonin participates in immunity processes, hemostasis regulation, aging, and stress. The effects of melatonin are harnessed in therapeutic melatonin, which is often taken to promote sleep. Further, therapeutic usage of melatonin has been used in treatment attempts for depression, which has an essential link to melatonin-related pathology [27].
Fig. 1.
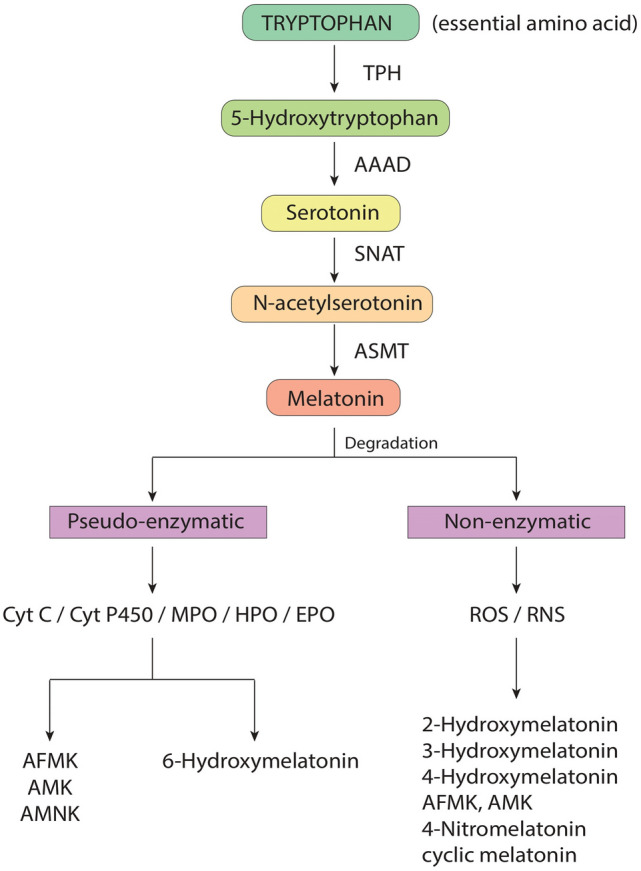
Scheme of Melatonin metabolism. TPH, tryphtophan hydroxylase; AAAD, aromatic amino-acid decarboxylase; SNAT, serotonin N-acetyltransferase; ASMT, N-acetylserotonin O-methyltransferase; Cyt C, cytochrome C; Cyt P450, cytochrome P450; MPO, myeloperoxidase; HPO, horseradish peroxidase; EPO, eosinophil peroxidase; ROS, reactive oxygen species; RNS, reactive nitrogen species; AFMK, N1-acetyl-N2-formyl-5-methoxykynuramine; AMK, N1-Acetyl-5-Methoxykynuramine; AMNK, N-Acetyl-5-Methoxy-3-nitrokynuramine
Production and secretion of melatonin occur in the pineal gland, though it can also be synthesized in peripheral tissues throughout the body. It is also found in plants and other organisms such as bacteria. Melatonin production decreases in aged people, and structural changes in the pineal gland can be associated with calcification [28]. The synthesis of melatonin is based on the structure of serotonin, a related neurohormone, which derives its configuration from the amino acid tryptophan. The chemical reaction follows several steps from 5-HT to the intermediate N-acetylserotonin by the enzyme arylalkylamine N-acetyltransferase, converted to melatonin by the enzyme hydroxyl-indole-O-methyltransferase. Serotonin, N-acetylserotonin, and acetylserotonin-O-methyltransferase are key melatonin level regulation factors. The onset of neural stimulation triggers these steps to melatonin synthesis [29].
Secretion of melatonin follows into the peripheral circulation from the pineal gland, which lies externally to the blood–brain barrier. It is distributed in the body, taking on several regulatory roles in peripheral tissues. Interestingly, melatonin also reaches the suprachiasmatic nuclei of the hypothalamus, which control the rhythm of melatonin secretion, leading to auto-regulation and synchronization of production of the hormone. Melatonin is metabolized by the liver; its primary metabolite being sulfatoxymelatonin, is excreted through the renal route [29, 30].
In the sleep–wake cycle secretion of melatonin occurs in a rhythm termed nycthohemeral rhythm. Melatonin production is directly linked to the amount of light perceived through the eyes. Thus, melatonin plays an important intermediary and coordination role between the body’s state and the body’s organs’ functions and could give it an important coordination role. It is routinely produced during the night or at dark, whereas light negatively influences production. Changes in the sleep–wake cycle (or light–dark cycle) can change melatonin secretion patterns and regulate its secretion. Similar effects can be perceived through seasonal changes. Melatonin is subject to a complex biological regulatory system. Systems that play a role in regulating production and secretion can be found both in the central and autonomic nervous systems [30]. The secretion rhythm depends on stimuli from the suprachiasmatic nuclei of the hypothalamus, termed the body’s biological clock. These nuclei receive direct information on light and dark through the retinohypothalamic tract, a monosynaptic neural pathway leading from the retina to the hypothalamus. The primary neurotransmitters of this tract have been proposed to be glutamate and adenylate cyclase-activating polypeptide, which create the sensation of light when present. Neural pathways from the suprachiasmatic nuclei of the hypothalamus then project toward the pineal gland, where the received input is transmitted toward melatonin synthesis [31]. The regulation of melatonin production at the pineal gland mainly depends on sympathetic, beta-adrenergic receptor input, resulting from noradrenaline stimulation. The subsequent elevation of intracellular cyclic adenosine monophosphate activates the aforementioned enzyme arylalkylamine N-acetyltransferase, triggering melatonin production in the pineal gland cells [29]. Due to its complex regulation and various functions in the body, disturbance thereof can be an essential pathophysiological finding [30]. Melatonin’s implication in the sleep–wake cycle makes it a critical therapeutic hormone.
Several neuropsychiatric illnesses have been implicated in melatonin dysfunction, including sleep disturbances, schizophrenia, and depression. Specifically, mood disorders are highly relevant with the link to this pathology. The vital link between disease pathology and the neurohormone is the disturbance of the circadian rhythm [16]. Depression is linked explicitly to melatonin dysfunction, where disruption of hormone secretion is seen in the context of circadian rhythm alterations. This pathological change can cause sleep disturbances and changes in a daily rhythm, and the loss of motivation and mood disturbances seen in the disease. New antidepressants targeting this system, such as therapeutic melatonin and melatonin analogs, have shown promising effects in symptom relief of depression [16, 32].
Circadian Rhythms and Depression
Circadian clocks, present in almost all multicellular organisms, control many biological processes and regulatory mechanisms. Studies have found the organization of the circadian clocks to be hierarchical, with SCN being the master clock controlling all other oscillators in mammals [33]. Chronobiologists later discovered circadian rhythms in tissues other than SCN, called peripheral oscillators [34]. Scientific investigations have revealed the self-autonomous nature of peripheral oscillators. For instance, a study conducted using cultured Drosophila body segments where a clock gene per promoter was fused to luciferase reported reporter gene expression throughout all body parts. Moreover, this study reported bioluminescence to be rhythmic [35].
In contrast, another study found the rhythms of peripheral tissues to dampen over time in vitro compared to the robust rhythmic activity of SCN, which questions the self-sustainability of the peripheral tissues [36]. However, several factors could have influenced these findings. This could be due to a molecular defect in the oscillator or only some cells partially dampened due to in vitro conditions. Additionally, it is also possible that the rhythms of these peripheral oscillators are no longer synchronized after a certain period, leading to a drop-in amplitude as they cancel each other out. A study using Rat-1 fibroblasts revealed that peripheral tissues and SCN neurons in vitro could become out-of-phase and have different oscillation periods [34, 37]. Considering such literature findings, one can be conclude that even though the possibility of peripheral oscillators being self-sustaining cannot be ruled out, more in-depth analysis accounting for various factors needs to be done to better understand the properties of peripheral oscillators.
Clock genes are responsible and involved directly in regulating circadian rhythm. These genes have their expression and outcome loop system that directs the levels of mRNAs and proteins [38, 39]. These mRNAs can be in the SCN in the hypothalamus region, master clock, and other brain regions [40]. With mammals, the circadian locomotor output cycles kaput (Clock) gene encoding for the transcription factor CLOCK, and the brain and muscle aryl hydrocarbon receptor nuclear translocator-like 1 (Bmal1) gene encode for BMAL1 [41]. Their heterodimer activates the transcription of three periods (PER1, 2, and 3) and two cryptochromes (CRY1, 2) genes [42]. The Period and cryptochrome move to the cytoplasm, where they get translated to respective heterodimers. The neuronal PAS domain protein 2 acts as an alternative dimer partner for BMAL1, responsible for circadian rhythmicity [43, 44]. Multiple interlocking transcriptional and translational loops contain both positive and negative transcription factors that help adjust the rhythms to the 24-h cycle [45]. The phosphorylation process is carried out by the casein kinase I epsilon [46]. The ubiquitinase complex degrades F-Box and Leucine-Rich Repeat Protein 3, which regulates the period of circadian oscillation, thus controlling the accumulation and translocation of Period and cryptochrome genes [47, 48]. Polymorphism observed in human CLOCK genes causes alteration in the sleep–wake cycle [49]. The presence of one or more copies of the CLOCK 31 11C allele causes an increase in eveningness and reduction in morningness, while the presence of 3111 T/T causes a reversal mechanism. The Japanese population has reported that the 31111C/C genotype causes a delay in sleeping timing [51] and more significant daytime sleepiness in the Japanese population [50]. The association of CRY1 rs2287161 and CRY2 rs10838524 polymorphisms has been investigated in relation to depression symptoms in patients with Parkinson’s disease[52]. In the case of patients with bipolar disorders and schizoaffective patients, more eveningness scores by Horne-Ostberg’s Morningness-Eveningness scale are observed. The bipolar patients showed an eveningness score probably based on age, while schizoaffective patients showed eveningness in all age groups [53]. The circadian phototransduction process involves the photosensitive retinal cells known as intrinsically photosensitive retinal ganglion cells. Though they are pretty low, their expression of melanopsin makes them photosensitive. Melanopsin is a retinal photopigment that is not involved in image formation, but rather may mediate non-visual photoreceptive tasks such as regulation of circadian rhythms and suppression of melatonin secretion. The visible blue light stimulates the light intensely, while the red light has minimal stimulatory power [54, 55]. The sensitivity spectrum of melanopsin depends on the natural solar cycle, and its disruption causes an alteration in circadian rhythm [56]. The cells transfer the neural impulses using the retinohypothalamic tract and later to the circadian master clock in the hypothalamus. The release of an amino acid called glutamate conveys this information. Activation of the suprachiasmatic nucleus causes the influx of Ca2 + ions and activates the intracellular signaling pathway [57]. This complete process causes the expression of Period genes and sets the molecular clock. With depressed patients, the increase in mean core temperature and decrease in amplitude of circadian changes are observed. Change in temperature causes the release of thyroid-stimulating hormones and melatonin [58].
In people with MDD, circadian regulation disturbances are noted, manifest by internal desynchronosis, disturbances in daily functioning and metabolic disorders. These circadian rhythm disruptions often return to normal after an episode of depression. Modulation of the 5-HT system is one of the possible ways through which the circadian regulation system affects susceptibility to depression. Some circadian rhythm-based treatment strategies for depression alter circadian rhythm, improve mood, and maybe effective as SSRIs [59]. The pacemaker centers affect the serotonergic nuclei arranged to direct push reactions and neuroimmunological capacities. The course and the level of the chronobiological desynchronization may well be utterly dissimilar in the case of the distinctive emotional disarranges. Distinctive chronobiological intercessions are required subsequently within the case of progressed and deferred rest disarranges. Resting disarranges are considered the earliest recognized signs of chronobiological desynchronization in misery, still, these indications are as if they were the tip of the iceberg since other chronobiological side effects might be displayed due to the covered-up physiological anomalies [22, 60]. The use of melatonin in mice with bipolar affective disorder altered the expression of genes for serotonergic neurotransmission in the dorsal suture and the content of serotonin in the amygdala. Melatonin treatment showed modest changes in the amplitude and phase of transcriptional oscillators in the SCN, which may explain the association with the effects observed in the brain’s serotonergic system and an improvement in depressive-like behavior [61].
Autopsy histological peculiarities additionally show the potential role of the circadian clock within the pathophysiology of depression within the SCN of the depressive population. An appealing aspect is the recognition of the commonality between circadian oscillator genes and ones that increase the risk of mood disorders. For example, Per3 polymorphisms have been associated with age at onset of mood disorders (bipolar disorder, anxiety, and depression), response to treatment, and diurnal mood variations. Research is underway to see if the same genetic factors that predict response to anti-depressants can also predict response to specific chronotherapy [62].
Pathogenesis Theories of Depression
Monoaminergic Theories
Monoaminergic theories associate the development of depression with a decrease in monoamines in the brain, primarily 5-HT, NE, and dopamine (DA). The catecholamine theory arose about 50 years ago and has not lost its relevance [63]. The pathophysiological basis of this theory has been elucidated empirically using effective antidepressants that act on these neurotransmitters [64]. Recent studies argue that a deficiency in serotonergic activity increases vulnerability to MDD. Disorders of both peripheral and central 5-HT metabolism play an essential role in the pathogenesis of MDD. While abnormality of the one monoamine cannot sufficiently explain the pathophysiological process of MDD, 5-HT is probably the most significant of these. Disorders of the 5-HT system in depressive patients can occur at different levels, for example, decreased availability of L-tryptophan, impaired synthesis, release, reuptake or metabolism of 5-HT, and abnormalities of 5-HT receptors [65].
Biological changes in patients with depression are caused by as physiopathology as morphology modifications.. Postmortem studies of the brains of depressed suicide victims and platelets in depressed patients have shown a decrease in the density of α2-adrenergic receptors [66]. Depletion studies provide some of the most convincing evidence that a deficiency of catecholamines is directly linked to depressive symptoms. The studies show that rapid α-methyl-p-tyrosine-induced depletion of catecholamines in antidepressant-free euthymic subjects with a history of MDD results in a rapid relapse into depression. In contrast, catecholamine depletion in psychiatrically normal subjects does not elicit depressive symptoms [67, 68].
Dysfunction of DA systems is also associated with the pathogenesis of depressive symptoms. Many symptoms of depression, such as anhedonia and lack of motivation, are more consistently associated with dysfunction of the dopaminergic system. Anhedonia, one of the most difficult depressive symptoms to treat, is associated with dysfunctions in the reward system, especially the DA system. The dopaminergic system regulates the processes of motivational excitation and responses to conditioned stimuli. Depression and anhedonia are associated with decreased striatal response to reward. We also found lower DA transporter binding in depressed patients compared to healthy subjects. This suggests downregulation of DA concentrations, as previous studies show lower DA transporter density when DA is chronically depleted [69, 70].
Neuronal/Synaptic Remodeling Theories
Brain-derived neurotrophic factor (BDNF) is a growth factor that affects neuronal maturation, synaptic plasticity, synapse formation, and neurogenesis. There is a hypothesis that a decrease in BDNF concentration directly participates in the pathophysiology of depression, and its recovery reflects the effectiveness of anti-depressant treatment [71]. BDNF has a high affinity for the tropomyosin receptor kinase B (TrkB) receptor and regulates neurotransmitter transmission and postsynaptic transmission. Its interaction with TrkB regulates the intracellular pathways of the following messengers: protein kinase C, mitogen-activated protein kinase, and phosphatidylinositol-3’OH-kinase. Some anti-depressants mediate their effects through TrkB. Phosphorylation of TrkB increases with chronic anti-depressant treatment, resulting in increased activity in the hippocampus and anterior cingulate cortex, suggesting that BDNF-TrkB signaling in these brain regions is significant for mediating anti-depressant effects. Antidepressants also interfere with BDNF intracellular signaling via monoamines, as their depletion prevents TrkB activation [72, 73].
Data on vascular endothelial growth factor (VEGF) concentration in MDD patients is inconsistent. Analysis of 8 studies has shown increasing VEGF levels in the plasma or serum of depressed patients compared to the control group; however, there are five other studies demonstrating decreased plasma or serum VEGF levels or the absence of significant differences between depressed patients and healthy people [74]. While some researchers suggest high VEGF levels in MDD patients, there is no clarity about the reflection of neurotrophins’ brain level by blood or serum concentration. However, selective 5-HT reuptake inhibitors-treated MDD showed higher VEGF-mediated neurogenesis in the hippocampus. This factor also plays a vital role in the proliferation of hippocampal nerve cells following electroconvulsive therapy treatment and in the therapeutic effects of antidepressants [75].
Glial cell line-derived neurotrophic factor (GDNF) is widely recognized as a survival factor for dopaminergic neurons. Still, GDNF has also been shown to promote the development, differentiation, and protection of other central nervous system neurons and to play an essential role in various neuropsychiatric disorders. It is one of the recognized vital mediators of structural plasticity with a supporting role in depression. Many studies have been conducted on the decreases observed in plasma and blood GDNF levels and mRNA expression in MDD [75], which is also confirmed by a recent meta-analysis [76]. In contrast, treatment with antidepressants or electroconvulsive therapy increases its expression [77].
Immune and Inflammation Theories
Neuroinflammation is associated with synaptic neuroplasticity and depression via pro-inflammatory cytokines. Activation of the immune system associated with cell proliferation, neurogenesis, gliogenesis, and apoptosis plays an essential role in the pathogenesis of MDD. Recent studies demonstrate the antidepressant effects of anti-inflammatory drugs. Potential biomarkers of MDD are interleukin-6 (IL-6), interleukin-1β (IL-1β), and tumor necrosis factor-alpha (TNF-α) [78], which is confirmed by several meta-analyses [79, 80]. The association between neuroinflammation and depression is demonstrated in either experimental animals or depressed individuals. Pro-inflammatory cytokines can induce depressive-like behavior, while antidepressants can reduce their levels [81, 82].
There is contradictory evidence of increased IL-6 and IL-1 in patients with MDD. Macrophages and monocytes synthesize IL-6 in response to a molecular pathogen. Increased concentration in the periphery is associated with decreased BDNF concentration and alterations in synaptic neurotransmission in depressed patients. It is also clearly established that pro-inflammatory cytokines such as IL-6 stimulate the indoleamine 2,3-dioxygenase, which may leads to decrease in the central availability of tryptophan for 5-HT synthesis, which confirms the role of a decrease in the central activity of 5-HT in depression [83]. A depressive-like behavior can be induced by chronic light deprivation, which leads to the activation of NF-B signaling and an increase in the concentration of IL-6 [84, 85].
Nuclear factor kappa B (NF-kB) is a transcription factor with various immune and metabolic effects. The NF-κB pathway is considered the major pro-inflammatory signaling pathway, mainly based on the activation of NF-κB by pro-inflammatory cytokines followed by transcriptional activation of sensitive genes [86]. MDD is associated with an imbalance between several components regulating NF-kB, such as high nitric oxide and reactive oxygen species, leading to oxidative stress [87]. It is supposed that NF-κB regulates BDNF expression and that BDNF can induce NF-κB activation. This mechanism can be used to implement antidepressant effects through increased neurogenesis, plasticity, and, as a result, the restoration of synaptic transmission [88].
Another pro-inflammatory cytokine, IL-1β crosses the blood–brain barrier and is a significant risk factor for the development of MDD. An increase in its level in the blood reduces the reuptake of 5-HT from the synaptic cleft. The administration of IL-1β in animal models caused depressive behavior and neuroinflammation [84, 89]. Maturation of IL-1β activates inflammasomes containing cryopyrin. The increased co-location of cryopyrin and the protein expression of ionized calcium-binding adapter molecule 1 suggests that microglia in glial cells are the main contributors to the activation of the cryopyrin inflammasome in rats. These changes suggest a new therapeutic target for antidepressants [90].
Depression can develop as a consequence of morphofunctional changes provoked by TNF-α. It is part of the 5-HT metabolic pathway, which is recognized as a vital component of the pathophysiology of MDD. TNF-α stimulates the uptake of 5-HT in synaptosomes, can reduce its bioavailability and activity of the neuronal transporter of 5-HT and DA, which leads to symptoms of depression [91]. New studies link the increase in TNF-α concentration to disturbances in tryptophan metabolism. In MDD, tryptophan metabolism changes from 5-HT to kynurenine pathways [92]. The immune system links the three theories discussed in this section, because both neurotrophin and monoamine levels decrease in response to an increase in pro-inflammatory cytokines.
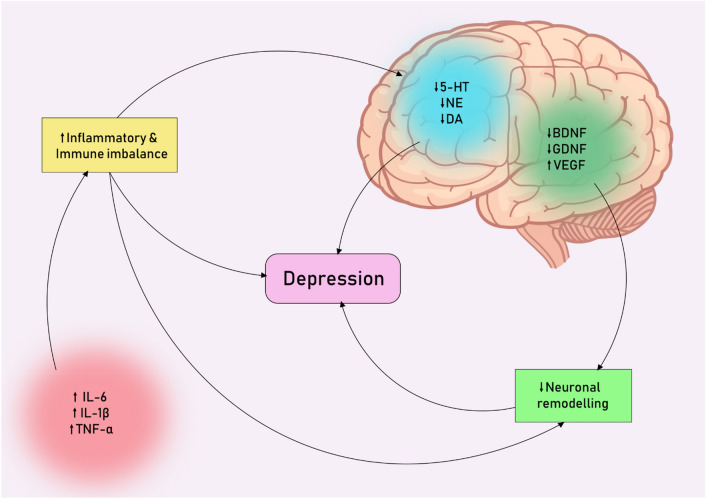
One of the main players in MDD development are microglia—immune cells of the nervous system. Microglia dysfunction is associated with many neuropsychiatric disorders, commonly referred to as microgliopathies. Patients with MDD have a chronic inflammatory response. Previous studies indicate a relationship between the severity of a depressive episode and microglia activation in different brain areas, such as the prefrontal cortex and anterior cingulate cortex [93, 94]. In studies of animal models of depression, hyperactivation of microglia with a high level of pro-inflammatory cytokines was established [95, 96], inhibition of which reduces the severity of symptoms [97]. To sum up these three different pathways of the pathogenesis of depression, we can schematically create pathological circles, which are shown in Fig. 2.
Fig. 2.
Pathological circles of interactions between three main theories of depression pathogenesis
As discussed above, immune imbalance and the inflammatory process play an important role in the pathogenesis of depression, and may also affect the concentration of monoamines, which form the basis of another theory of its pathogenesis. At the same time, neuronal remodeling occupies an important place in the pathogenesis of depression, which can also decrease against the background of inflammatory reactions and an imbalance of neurotrophic brain factors.
Melatonergic Receptors and Their Functions
Melatonin regulates a variety of physiological functions through interacting with intracellular proteins and activating MT1 and MT2 [98]. MT1 and MT2 receptors are localized mostly in the CNS, but their anatomical distributions are different. Animal studies show that the MT1 receptors are present in the hippocampus, the SCN, the substantia nigra pars compacta, the dorsal raphe nucleus, and the pars tuberalis of the pituitary gland. On the other hand, the MT2 receptor is localized as in the CNS, namely in the hippocampus, the reticular thalamic nucleus, the supraoptic nucleus, the inferior colliculus, the substantia nigra pars reticulata, and the ventrolateral periaqueductal gray [99] as inretina, kidney, and heart tissue [100]. Both melatoninergic receptors are observed in the retina, brown and white adipose tissue, pancreatic alpha and beta cells, granulosa cells, myometrium, and testis [101, 102].
MT1 and MT2 receptors pertain to class A of G-protein-coupled receptors (GPCR) located in the SCN and cardiac vessels. The MT1 receptor is coupled with Gαi2, Gαi3, insensitive Gq/11 G proteins and encoded by melatonin receptor 1A gene on chromosome 4q35.1. It increases potassium conductance through Kir inwardly rectifying channels. MT1 regulates the rhythmic expression of the clock genes, such as Per1, Per 2, Bmal1 and Cry1 [103]. MT1 receptor has anti-inflammatory, antiapoptotic, and antioxidative effects through sirtuin-mediated impact on nuclear factor erythroid 2–related factor 2 or NF-kB, etc. There is increased neuronal survival and, as a result, adult neurogenesis. Melatonin shows an anti-inflammatory effect in different tissues. It reduces neuroinflammation and releases the inflammatory factors IL-1β, IL-6, TNF-α in the nervous system by both MT1 and MT2 receptors [104–108].
The MT2 receptor is coupled with Gi/o‐type proteins and encoded by the melatonin receptor 1B gene on chromosome 11q21‐q22. It inhibits cyclic guanosine monophosphate formation and decreases calcium-dependent dopamine release in the retina [103, 109]. Melatonin receptors are studied as neuroprotective drug targets. Melatonin has a neurogenic effect also through MT2 receptors [110]. Both receptors activate protein kinase C in the SCN and increase phosphorylation of extracellular signal-regulated kinase, which regulates dendritogenesis [111] and transcription of clock gene expression, such as PER1, and inhibit forskolin-stimulated cyclic adenosine monophosphate production.
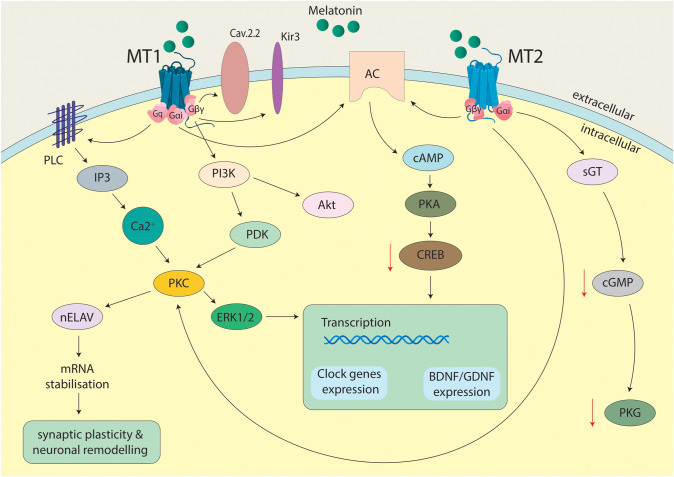
MT1 and MT2 are both GPCRs receptors. The interaction of these receptors with melatonin involves different secondary messengers. The MT1 complex linked Gq subunits to PLC, IP3 and intracellular accumulation of Ca + 2 which phosphorylates both PKC and ERK1/2. PKC activation leads to activation of nELAV proteins and, as a result, mRNAs stabilization that provides synaptic plasticity and neuronal remodeling. The Gαi subunit of MT1 decreases level of cAMP and causes inactivation of phosphorylation of CREB. Melatonin activation of Gβγ subunit of MT1 leads to activation of the PI3K/Akt pathway. Modulation of neuronal action potential is mediated by activation Kir3 channels and inhibition Cav2.2 channels. Activation of MT2 receptors triggers Gαi-dependent activation of cAMP and PKA acting through membrane adenyl cyclase and following inactivation of phosphorylation of CREB too; cGMP levels are reduced, that is caused lower activity of PKG because of interaction with guanylyl cyclase. Melatonin-dependent activation of MT2 impacts PKC and ERK1/2 complexes. ERK1/2 and CREB pathways converge onto transcription of clock genes and neurotrophic factors genes (e.g. BDNF, GDNF) [108, 112–114] (Fig. 3).
Fig. 3.
Receptor transduction mechanisms for melatonin. MT1, melatonergic receptor 1; MT 2, melatonergic receptor 2; Cav2.2, voltage-gated calcium channel; Kir3, G protein-coupled inwardly rectifying potassium channel; sGC, soluble guanylate cyclase, AC, membrane adenylate cyclase; cAMP, cyclic adenosine monophosphate; cGMP, cyclic guanosine monophosphate; PKC, protein kinase C; PKA, protein kinase A; PKG, protein kinase G; CREB, cAMP-responsive element binding protein; PLC: phospholipase C; IP3, inositol trisphosphate; Akt, serine/threonine-specific protein kinases; PI3K, phosphoinositide 3-kinase; PDK, pyruvate dehydrogenase kinase; nELAV, neuronal embryonic lethal abnormal vision; ERK1/2, extracellular signal-regulated kinases 1/2; mRNA, messenger ribonucleic acid; BDNF, brain-derived neurotrophic factor; GDNF, Glial cell derived neurotrophic factor
Recent studies have demonstrated that GPCRs can assemble into heteromeric complexes. Several GPCRs heteromers are associated with psychiatric and neurological disorders [115]. The cross-talk mediated via the physical association of melatonin MT2 and 5-HT2c receptors into functional heteromers is discussed in recent research. The MT2 receptor can allosterically transactivate 5‐HT2c‐dependent Gq signaling and form the heterodimer MT2/5-HT2c that amplifies the 5-HT-mediated Gq/phospholipase C response [116]. The antidepressive and neurogenic effects of MT1/MT2 agonists also can be caused by the inhibition of the acid sphingomyelinase, leading to reduced ceramide or increasing monoamine oxidase A levels in the hippocampus [117]. In mice with deletion of MT1 and/or MT2 receptors, there are increased mood disturbances [118]. As known also, patients with depression often suffer from circadian rhythm disruption that can be caused by increasing MT1, but not MT2, receptors in SCN during the disease. It contributes to the possibility that MT1/MT2 will target new anti-depressants [119].
Electrophysiological recordings in MT1 receptor knockout mice have shown decreased impulse activity of locus coeruleus NE neurons during the dark phase and the elimination of circadian oscillations of spontaneous impulse activity of both NE and 5-HT neurons dorsal suture. It has been suggested that the MT1 receptor may induce melancholic depression and be a potential pharmacological target for this mental state [120]. MT1 activation may induce the expression of GDNF in neural stem cells [121]. Therefore, both MT1 and MT2 receptors have links with different theories of depression pathogenesis, as highlighted in Table 1. This may point to potential pharmacological targets for melatonergic drugs in depression pathophysiology.
Table 1.
Roles of MT1/2 receptors in theories of depression pathogenesis
| Type of theories of depression pathogenesis | Type of melatonergic receptors | |
|---|---|---|
| MT1 | MT2 | |
| Monoaminergic theories | Knockout leads to decrease in dorsal raphe nucleus 5-HT and locus coeruleus NE neuronal bursts activity [122] | Capacity to assemble into functional heteromers with 5-HT2C receptors [122, 123] |
| Agonists increase monoamine oxidase A levels [124] | ||
| Neuronal/synaptic remodeling theories | Activation may induce the expression of GDNF [121] | Activation enhances neurogenesis [110] |
| Immune and inflammation theories | Reduces neuroinflammation and releases IL-1β, IL-6, TNF-α [108, 125–127] | |
Interestingly it is clear that luzindole, despite its melatonin antagonism, exerts antidepressant-like activity through actions at the MT2 receptor. Chronic luzindole administration increases hippocampal neurogenesis in mice. It is suggested that the rapid MT2 receptor desensitization kinetics could facilitate melatonin-mediated antidepressant-like effects following therapy with an MT1/MT2 receptor agonist [128]. Research has shown that the absence of MT2 but not MT1 receptors enables melatonin to increase cerebellar and cerebellar granule cells’ BDNF content. Nanomolar melatonin concentration was neuroprotective in MT2-knockout mice [129]. At the same time MT2 genetic inactivation impacts 5-HT neurotransmission and interferes with mood disorders and social interactions [130, 131]. The selective knocking out of MT2 caused cognitive impairments and elevation anxiety levels, together with induced depression-like behaviors[132]. It was discovered that genetic deletion of the MT1 and/or MT2 receptors is associated with depression- and anxiety-like behaviors in mice [118].
Exogenous Melatonin and Other Melatonergic Drugs
Exogenous melatonin has been investigated to treat various medical and surgical conditions [133], including but not limited to insomnia, hypertension, metabolic disease, chronic obstructive pulmonary disease, and inflammatory bowel disease [134]. It has been available in the market for more than five decades [134] and has effectively reduced sleep onset latency, increased total sleep time, and improved sleep quality [135]. It has also shown beneficial effects in treating jet lag and shift work symptoms and neurological and psychiatric conditions like attention deficit hyperactivity disorder and MDD [134, 136].
In the United States of America, exogenous melatonin is available as an over-the-counter non-prescription drug, marketed as a nutraceutical supplement, and classified as a dietary supplement by the Federal Drug Administration hence exempted from the need to be subject to the regulatory processes applied to pharmaceuticals [134, 135]. However, in most European countries, it continues to be sold as a prescription drug approved for treating primary insomnia in people above 55 [133, 134]. However, off-licensing prescriptions in dosages ranging from 0.3 to 3 mg have shown equivalent efficacy in resetting the circadian rhythm [134]. Conversely, doses of 10 mg or more have displayed reduced effectiveness in treating intrinsic disorders of the light–dark cycle [135].
The known half-life of all melatonin-containing drugs is about 30–50 min [135]. As a chronobiotic, melatonin, even at doses as low as 0.5–1 mg maintains supraphysiological levels in the body and, when administered acutely, decreases core body temperature, diminishes alertness, and encourages sleep propensity [22, 137]. Furthermore, in treating sleep initiation and maintenance disorders, its soporific effects are utilized; in fact, various studies have reported these effects [135]. A recent meta-analysis investigating sleep latency, quality, and sleep time in patients suffering from primary sleep disorders showed improvement in each assessed component of sleep on exogenous melatonin administration. The analysis included 19 randomized placebo-controlled studies (n = 1683), and the patients received melatonin from 7 days up to 182 days [138]. Moreover, therapeutic administration of exogenous melatonin in fully-blind volunteers with desynchronized free-running circadian rhythms—has improved sleep–wake cycles due to their inability to perceive external time cues [137]. In addition to that, studies suggest that melatonin might play a “neuroprotective” role in REM-behavior disorder [135]. Melatonin also shows antidepressant-like effects in a rodent model of depression [139, 140].
In summary, the physiological functions of endogenous melatonin can be simulated by low-dose exogenous melatonin that is carefully administered in a specially timed manner to correct circadian rhythm disturbances. In addition, it can be used as a soporific agent to treat various sleep disorders at higher doses since it does not affect or suppress the normal rapid eye movement (REM) sleep or its onset[137, 141]. Thus, exogenous melatonin administration can be beneficial in treating disorders of sleep initiation and maintenance and circadian phase abnormalities. Melatonin treatment significantly inhibited cytokine production and NF-κB activation in the cortex and hippocampus. Further, melatonin treatment significantly reduced oxidative stress and inhibited glial cell activation, suggesting that melatonin can act as an anti-depressant through MT1 and MT2 agonism by rescuing neuroinflammation [142].
Several melatonin receptor agonists have been introduced, including MT1 and MT2 melatonin receptor agonists. One of such agents, Ramelteon, has been approved to treat insomnia;it alters the expression of the circadian genes, the levels of inflammatory cytokines and neurotrophins in patients with insomnia comorbid anxiety and depression [143]. It has been demonstrated that ramelteon can increase the content of the neuronal protein BDNF, GDNF level, and pro-inflammatory cytokine concentrations (IL-6, IL-1b, TNF-ɑ) in patients with a diagnosis of primary insomnia comorbid with depression [143, 144]. Ramelteon has been shown as effective in maintaining stability also in bipolar disorder due to the prevention of depressive phase relapse, which is explained by its effect on the normalization of circadian rhythms through selective activation of MT1 and MT2 receptors [145, 146]. Its properties may indicate a potential usage for anti-depressant augmentation, which has been demonstrated as a clinical case [147].
Agomelatine, alongside being an agonist of MT1 and MT2 receptors, is also a serotonin 5-HT2C antagonist and has been approved in Europe and Australia to treat depression. Agomelatine also restored the diurnal rhythm of motor activity, accompanied by increased MT1 receptor and BDNF expression in the hippocampus in rats [148]. It is the only melatonergic drug approved for the treatment of depression combining the effect of melatonin receptors agonism with antagonism of the serotonergic system, which may indicate a new therapeutic target in a combination of substances influencing both the melatonergic system and other neurotransmitters. Another agent, Tasimelteon, has been on the market to treat the non-24-h sleep–wake disorder in the USA since 2014 [135]. To the best of our knowledge, presently, there appear to be no trials that have been conducted to compare one formulation with another in assessing their effects on resetting circadian rhythm or improving sleep onset, efficiency, and total time, respectively. A trial, however, was performed to determine the impact in totally blind people of Tasimelteon in non-24-h sleep–wake disorder. The drug was effective in regulating circadian rhythm. However, its continuous administration was required to maintain the effect [149].
Many experimental and clinical studies performed on infants, children, and adult patients have demonstrated no serious adverse effects of administering exogenous melatonin in low doses. However, minor adverse effects like dizziness, headaches, drowsiness, and nausea have been reported [134, 137]. Following exogenous melatonin administration, no tolerance, dependence, or hangover effects have been reported. Nevertheless, melatonin has shown impaired glucose tolerance at higher doses (5 mg and more). Additionally, it might affect prepubertal development in children and is classified as unsafe to be consumed during pregnancy and breastfeeding. In overdose, however, it is not life-threatening [135].
There is little evidence regarding the effects of long-term use of exogenous melatonin. A trial that spanned over 6 months and studied the daily use of melatonin in human subjects reported no adverse effects or withdrawal [150]. Similarly, another study that assessed the outcomes of the use of melatonin 2 mg 1–2 h before bedtime orally in older patients (> 55 years) suffering from primary insomnia concluded improved quality of sleep and remarkable tolerance of melatonin for up to 13–14 weeks [151]. However, large doses of melatonin administered over a more extended period can have drug-to-drug interactions and suppress reproductive hormones [135]. Melatonin acts as a neuroprotective agent that abolishes lipopolysaccharide effects on oxidative stress, NF-κB activation, redox-sensitive signaling, and depressive-like behaviors in a receptor-dependent manner [106].
Studies assessing the interactions of exogenous melatonin with other drugs in humans have documented an increment in the plasma levels of drugs metabolized by CYP liver enzymes, including fluvoxamine, caffeine, and oral contraceptives following the administration of melatonin. According to Andersen et al., non-published data [133] suggest that drugs like psoralen and cimetidine may raise plasma melatonin levels; however, others like carbamazepine and rifampicin may drop levels of plasma melatonin. Interestingly, smoking has been depicted to increase the levels of plasma melatonin. However, there is no documented clinical impact of these variations of plasma levels on the risk of precipitating adverse effects. Nevertheless, additive adverse effects such as psychomotor impairment and memory changes are reported when melatonin is administered in combination with hypnotics. Due to its easy availability as an over-the-counter drug in many countries, the authorities do not routinely monitor good manufacturing practices. This raises a concern for an increment in the risk of diminished or variable product quality and potential contamination [133].
Conclusion and Perspectives
Melatonergic receptors play an essential role regulating the circadian rhythm, which affects many human body functions. Considering circadian regulation is important in trying to understand any of the three prevailing concepts of the pathogenesis of depression, whether it is the widely accepted theory involving monoamines, or the neurotrophic remodeling concept, or the immune/inflammatory theory. Depression is accompanied by disorders of circadian regulation and interaction with the functioning of both central and peripheral oscillators. To date, melatonin receptor agonists are presented in the form of 4 drugs: melatonin, ramelteon, tasimelteon, and agomelatine. Among them, the antidepressant effect has been proven in melatonin and agomelatine. At the same time, there is not enough research that discusses the possibility of the influence of ramelteon or tasimelteon on depressive-like behavior. The issue of melatonin and depression is unclear, as there are not enough findings relating to the effectiveness of researched MT1 and MT2 agonists in depression. Studies often have a low sample size and different results among them.
We can distinguish main possible ways of the effect of melatonergic drugs on depression. The normalization of sleep and circadian rhythm, leads to a decrease in the severity of clinical signs, and the impact on various points in the pathogenesis of depression. The simultaneous influence of melatoninergic drugs on three theories of the pathogenesis of depression provides opportunities for further research in this area. This is due to the melatonin agonists increasing the concentration of BDNF and necessary neurotransmitters, especially NE and 5-HT, and reducing the release of pro-inflammatory cytokines, such as IL-1, IL-6, and TNF-α. By the way, we should consider that these theories are related. The inflammatory theory acts as a link between the others because both neurotrophin and monoamine levels decrease in response to an increase in the concentration of pro-inflammatory cytokines.
At the same time, there is an interesting cross-talk mediated via the physical association of melatonin MT2 and 5-HT2c receptors into functional heteromers. The antidepressive and neurogenic effects of MT1/MT2 agonists can also be caused by the inhibition of the acid sphingomyelinase, leading to reduced ceramide or increasing monoamine oxidase A levels in the hippocampus. More clinical research is needed on the potential effect of melatoninergic drugs on depressive-like behavior, as this could open new horizons for the pharmacological treatment of depression. In particular, a combined effect on MT1 and/or MT2 receptors and neurotransmitter systems may be useful, since the normalization of the circadian rhythm through the melatonergic system will probably contribute to improved treatment.
Acknowledgements
Research in the authors’ laboratories in Ukraine was supported by Poltava State Medical University (research project No. 0121U108235). The authors sincerely acknowledge the intellectual support by the Prof. Dr. Ihor P. Kaidashev.
Author Contributions
Conceptualization, DIB. and ADS; methodology, DIB., ADS. and MMH; validation, DIB, ADS and AAB; investigation, DIB, ADS and MMH; resources, DIB, ADS, MMH and AAB; data curation, DIB and ADS; writing—original draft preparation, DIB, ADS, MMH, MB, SKK, HC, PB; writing—review and editing, DIB, ADS, AMS, MMH and AAB; visualization, DIB and ADS; supervision, DIB, ADS, AMS, MMH and AAB; project administration, DIB, ADS, MMH and AAB; funding acquisition, HC. All authors have read and saagreed to the published version of the manuscript.
Declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Dmytro I. Boiko, Email: bojko998@gmail.com
Anastasiia D. Shkodina, Email: ad.shkodina@gmail.com
Mohammad Mehedi Hasan, Email: mehedi.bmb.mbstu@gmail.com.
Mainak Bardhan, Email: bardhan.mainak@gmail.com.
Syeda Kanza Kazmi, Email: skanzakazmi@gmail.com.
Hitesh Chopra, Email: chopraontheride@gmail.com.
Prerna Bhutra, Email: prernabhutra@gmail.com.
Atif Amin Baig, Email: atifamin@unisza.edu.my.
Andrii M. Skrypnikov, Email: skrypnikovandrii@gmail.com
References
- 1.Kaidashev IP. Poль мoлeкyляpныx чacoв циpкaдиaнныx pитмoв в пaтoгeнeзe мeтaбoличecкoгo cиндpoмa. Eндoкpинoлoгiя. 2020;25:158–170. doi: 10.31793/1680-1466.2020.25-2.158. [DOI] [Google Scholar]
- 2.Hardeland R. Melatonin metabolism in the central nervous system. Curr Neuropharmacol. 2010 doi: 10.2174/157015910792246164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dubocovich ML, Markowska M. Functional MT1 and MT2 melatonin receptors in mammals. Endocrine. 2005;27:101–10. doi: 10.1385/ENDO:27:2:101. [DOI] [PubMed] [Google Scholar]
- 4.Benítez-King G. Melatonin as a cytoskeletal modulator: Implications for cell physiology and disease. J. Pineal Res. 2006;40:1–9. doi: 10.1111/j.1600-079X.2005.00282.x. [DOI] [PubMed] [Google Scholar]
- 5.Macías M, Escames G, Leon J, et al. Calreticulin-melatonin: an unexpected relationship. Eur J Biochem. 2003 doi: 10.1046/j.1432-1033.2003.03430.x. [DOI] [PubMed] [Google Scholar]
- 6.Ferry G, Hecht S, Berger S, et al. Old and new inhibitors of quinone reductase 2. Chem Biol Interact. 2010 doi: 10.1016/j.cbi.2010.04.006. [DOI] [PubMed] [Google Scholar]
- 7.Nosjean O, Ferro M, Cogé F, et al. Identification of the melatonin-binding site MT3 as the quinone reductase 2. J Biol Chem. 2000 doi: 10.1074/jbc.M005141200. [DOI] [PubMed] [Google Scholar]
- 8.Wu YH, Zhou JN, Balesar R, et al. Distribution of MT1 melatonin receptor immunoreactivity in the human hypothalamus and pituitary gland: Colocalization of MT1 with vasopressin, oxytocin, and corticotropin-releasing hormone. J Comp Neurol. 2006 doi: 10.1002/cne.21152. [DOI] [PubMed] [Google Scholar]
- 9.Masson-Pévet M, Gauer F, Schuster C, Guerrero HY. Photic regulation of mt1melatonin receptors and 2-iodomelatonin binding in the rat and Siberian hamster. Neurosignals. 2000 doi: 10.1159/000014638. [DOI] [PubMed] [Google Scholar]
- 10.De Berardis D, Marini S, Fornaro M, et al. The melatonergic system in mood and anxiety disorders and the role of agomelatine: Implications for clinical practice. Int. J. Mol. Sci. 2013;14:12458–83. doi: 10.3390/ijms140612458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Deng J, Zhou F, Hou W, et al. The prevalence of depression, anxiety, and sleep disturbances in COVID-19 patients: a meta-analysis. Ann NY Acad Sci. 2021 doi: 10.1111/nyas.14506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boiko DI, Zhyvotovska LV, Sonnik GT, Skrypnikov AM. Clinical and psychopathological characteristics of the autoagressive behavior in patients with the first psychotic episode with considering circadian rhythms. Wiad Lek. 2017;70:553–557. [PubMed] [Google Scholar]
- 13.Liu Q, He H, Yang J, et al. Changes in the global burden of depression from 1990 to 2017: findings from the global burden of disease study. J Psychiatr Res. 2020;126:134–140. doi: 10.1016/J.JPSYCHIRES.2019.08.002. [DOI] [PubMed] [Google Scholar]
- 14.Chand SP, Arif H, Kutlenios RM. Depression (nursing) StatPearls; 2021. [PubMed] [Google Scholar]
- 15.Wittmann M, Schreiber W, Landgrebe M, Hajak G. Störung zirkadianer Rhythmen im Kontext depressiver Erkrankungen. Fortschritte der Neurol Psychiatr. 2018;86:308. doi: 10.1055/s-0043-123069. [DOI] [PubMed] [Google Scholar]
- 16.Mendoza J. Circadian insights into the biology of depression: Symptoms, treatments and animal models. Behav. Brain Res. 2019;376:112186. doi: 10.1016/j.bbr.2019.112186. [DOI] [PubMed] [Google Scholar]
- 17.Wetterberg L. Clinical importance of melatonin. Prog Brain Res. 1979 doi: 10.1016/S0079-6123(08)62962-3. [DOI] [PubMed] [Google Scholar]
- 18.Bondy B. Pathophysiology of depression and mechanisms of treatment. Dialogues Clin. Neurosci. 2002;4:7–20. doi: 10.31887/DCNS.2002.4.1/bbondy. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wirz-Justice A. Diurnal variations of depressive symptoms. Dialog Clin Neurosci. 2008;10:337–343. doi: 10.31887/dcns.2008.10.3/awjustice. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Courtet P, Olié E. Circadian dimension and severity of depression. Eur Neuropsychopharmacol. 2012 doi: 10.1016/j.euroneuro.2012.07.009. [DOI] [PubMed] [Google Scholar]
- 21.Coogan AN, Thome J. Chronotherapeutics and psychiatry: setting the clock to relieve the symptoms. World J Biol Psychiatry. 2011 doi: 10.3109/15622975.2011.598389. [DOI] [PubMed] [Google Scholar]
- 22.Arendt J, Skene DJ. Melatonin as a chronobiotic. Sleep Med Rev. 2005 doi: 10.1016/j.smrv.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 23.Wurtman RJ, Larin F, Axelrod J, et al. Formation of melatonin and 5-hydroxyindole acetic acid from 14C-tryptophan by rat pineal glands in organ culture. Nature. 1968;217:953–954. doi: 10.1038/217953a0. [DOI] [PubMed] [Google Scholar]
- 24.Chattoraj A, Liu T, Zhang LS, et al. Melatonin formation in mammals: in vivo perspectives. Rev. Endocr. Metab. Disord. 2009;10:237–243. doi: 10.1007/s11154-009-9125-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Grosse J, Davis FC. Melatonin entrains the restored circadian activity rhythms of Syrian hamsters bearing fetal suprachiasmatic nucleus grafts. J Neurosci. 1998 doi: 10.1523/jneurosci.18-19-08032.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cardinali DP, Pévet P. Basic aspects of melatonin action. Sleep Med. Rev. 1998;2:175–90. doi: 10.1016/S1087-0792(98)90020-X. [DOI] [PubMed] [Google Scholar]
- 27.Melatonin | C13H16N2O2—PubChem. https://pubchem.ncbi.nlm.nih.gov/compound/Melatonin (accessed 23 Oct 2021)
- 28.Starchenko II, Grinko RM, Shkodina AD, et al. The degree of pineal gland calcification in the aged people is associated with changes in the internal structure. J Int Dent Med Res. 2021;14:841–844. [Google Scholar]
- 29.Seithikurippu RAM. Melatonin, the hormone of darkness: from sleep promotion to Ebola treatment. Brain Disord Ther. 2015 doi: 10.4172/2168-975x.1000151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Claustrat B, Leston J. Melatonin: physiological effects in humans. Neurochirurgie. 2015;61:77–84. doi: 10.1016/j.neuchi.2015.03.002. [DOI] [PubMed] [Google Scholar]
- 31.Hannibal J. Neurotransmitters of the retino-hypothalamic tract. Cell Tissue Res. 2002;309:73–88. doi: 10.1007/s00441-002-0574-3. [DOI] [PubMed] [Google Scholar]
- 32.Satyanarayanan SK, Su H, Lin Y-W, Su K-P. Circadian rhythm and melatonin in the treatment of depression. Curr Pharm Des. 2018 doi: 10.2174/1381612824666180803112304. [DOI] [PubMed] [Google Scholar]
- 33.Yamazaki S, Numano R, Abe M, et al. Resetting central and peripheral circadian oscillators in transgenic rats. Science. 2000;288:682–685. doi: 10.1126/science.288.5466.682. [DOI] [PubMed] [Google Scholar]
- 34.Welsh DK, Yoo SH, Liu AC, et al. Bioluminescence imaging of individual fibroblasts reveals persistent, independently phased circadian rhythms of clock gene expression. Curr Biol. 2004 doi: 10.1016/j.cub.2004.11.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Plautz JD, Kaneko M, Hall JC, Kay SA. Independent photoreceptive circadian clocks throughout Drosophila. Science. 1997;278:1632–1635. doi: 10.1126/science.278.5343.1632. [DOI] [PubMed] [Google Scholar]
- 36.Yamazaki S, Straume M, Tei H, et al. Effects of aging on central and peripheral mammalian clocks. Proc Natl Acad Sci USA. 2002 doi: 10.1073/pnas.152318499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Balsalobre A, Damiola F, Schibler U. A serum shock induces circadian gene expression in mammalian tissue culture cells. Cell. 1998 doi: 10.1016/S0092-8674(00)81199-X. [DOI] [PubMed] [Google Scholar]
- 38.Ando H, Yanagihara H, Hayashi Y, et al. Rhythmic messenger ribonucleic acid expression of clock genes and adipocytokines in mouse visceral adipose tissue. Endocrinology. 2005;146:5631–5636. doi: 10.1210/en.2005-0771. [DOI] [PubMed] [Google Scholar]
- 39.Schibler U. The daily timing of gene expression and physiology in mammals. Dialog Clin Neurosci. 2007;9:257–272. doi: 10.31887/dcns.2007.9.3/uschibler. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Reghunandanan V, Reghunandanan R. Neurotransmitters of the suprachiasmatic nuclei. J Circadian Rhythms. 2006;4:2. doi: 10.1186/1740-3391-4-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hamada T, LeSauter J, Venuti JM, Silver R. Expression of period genes: rhythmic and nonrhythmic compartments of the suprachiasmatic nucleus pacemaker. J Neurosci. 2001;21:7742–7750. doi: 10.1523/jneurosci.21-19-07742.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Walker WH, Walton JC, DeVries AC, Nelson RJ. Circadian rhythm disruption and mental health. Transl Psychiatry. 2020;10:1–13. doi: 10.1038/s41398-020-0694-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.DeBruyne JP, Weaver DR, Reppert SM. CLOCK and NPAS2 have overlapping roles in the suprachiasmatic circadian clock. Nat Neurosci. 2007;10:543–545. doi: 10.1038/nn1884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Reppert SM, Weaver DR. Molecular analysis of mammalian circadian rhythms. Annu Rev Physiol. 2001;63:647–676. doi: 10.1146/annurev.physiol.63.1.647. [DOI] [PubMed] [Google Scholar]
- 45.Yoo SH, Kojima S, Shimomura K, et al. Period2 3′-UTR and microRNA-24 regulate circadian rhythms by repressing PERIOD2 protein accumulation. Proc Natl Acad Sci USA. 2017 doi: 10.1073/pnas.1706611114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Eide EJ, Kang H, Crapo S, et al. Casein kinase I in the mammalian circadian clock. Methods Enzymol. 2005;393:408–418. doi: 10.1016/S0076-6879(05)93019-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yoo SH, Mohawk JA, Siepka SM, et al. Competing E3 ubiquitin ligases govern circadian periodicity by degradation of CRY in nucleus and cytoplasm. Cell. 2013;152:1091–1105. doi: 10.1016/j.cell.2013.01.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Xing W, Busino L, Hinds TR, et al. SCF FBXL3 ubiquitin ligase targets cryptochromes at their cofactor pocket. Nature. 2013;496:64–68. doi: 10.1038/nature11964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Charrier A, Olliac B, Roubertoux P, Tordjman S. Clock genes and altered sleep–wake rhythms: their role in the development of psychiatric disorders. Int J Mol Sci. 2017 doi: 10.3390/ijms18050938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Katzenberg D, Young T, Finn L, et al. A CLOCK polymorphism associated with human diurnal preference. Sleep. 1998;21:569–576. doi: 10.1093/sleep/21.6.569. [DOI] [PubMed] [Google Scholar]
- 51.Mishima K, Tozawa T, Satoh K, et al. The 3111T/C polymorphism ofhClock is associated with evening preference and delayed sleep timing in a Japanese population sample. Am J Med Genet. 2005;133:101–104. doi: 10.1002/ajmg.b.30110. [DOI] [PubMed] [Google Scholar]
- 52.Shkodina AD, Tan SC, Hasan MM, et al. Roles of clock genes in the pathogenesis of Parkinson’s disease. Ageing Res Rev. 2022 doi: 10.1016/J.ARR.2021.101554. [DOI] [PubMed] [Google Scholar]
- 53.Mansour HA, Wood J, Chowdari KV, et al. Circadian phase variation in bipolar I disorder. Chronobiol Int. 2005;22:571–584. doi: 10.1081/CBI-200062413. [DOI] [PubMed] [Google Scholar]
- 54.Dacey DM, Liao HW, Peterson BB, et al. Melanopsin-expressing ganglion cells in primate retina signal colour and irradiance and project to the LGN. Nature. 2005;433:749–754. doi: 10.1038/nature03387. [DOI] [PubMed] [Google Scholar]
- 55.Do MTH, Yau KW. Intrinsically photosensitive retinal ganglion cells. Physiol Rev. 2010;90:1547–1581. doi: 10.1152/physrev.00013.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Blume C, Garbazza C, Spitschan M. Effects of light on human circadian rhythms, sleep and mood. Somnologie. 2019;23:147–156. doi: 10.1007/s11818-019-00215-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Song Z, Wang Y, Zhang F, et al. Calcium signaling pathways: key pathways in the regulation of obesity. Int. J. Mol. Sci. 2019;20:2766. doi: 10.3390/ijms20112768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ikegami K, Refetoff S, Van Cauter E, Yoshimura T. Interconnection between circadian clocks and thyroid function. Nat Rev Endocrinol. 2019;15:590–600. doi: 10.1038/s41574-019-0237-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Daut RA, Fonken LK. Circadian regulation of depression: a role for serotonin. Front. Neuroendocrinol. 2019 doi: 10.1016/j.yfrne.2019.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kálmán J, Kálmán S. Depression as chronobiological illness. Neuropsychopharmacol Hung. 2009;11:69–81. [PubMed] [Google Scholar]
- 61.Nagy AD, Iwamoto A, Kawai M, et al. Melatonin adjusts the expression pattern of clock genes in the suprachiasmatic nucleus and induces antidepressant-like effect in a mouse model of seasonal affective disorder. Chronobiol Int. 2015 doi: 10.3109/07420528.2014.992525. [DOI] [PubMed] [Google Scholar]
- 62.Hickie IB, Naismith SL, Robillard R, et al. Manipulating the sleep-wake cycle and circadian rhythms to improve clinical management of major depression. BMC Med. 2013 doi: 10.1186/1741-7015-11-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mulinari S. Monoamine theories of depression: Historical impact on biomedical research. J. Hist. Neurosci. 2012;21:366–392. doi: 10.1080/0964704X.2011.623917. [DOI] [PubMed] [Google Scholar]
- 64.Delgado PL. Depression: The case for a monoamine deficiency. J Clin Psych. 2000;61:7–11. [PubMed] [Google Scholar]
- 65.Dell’Osso L, Carmassi C, Mucci F, Marazziti D. Depression, serotonin and tryptophan. Curr Pharm Des. 2016 doi: 10.2174/1381612822666151214104826. [DOI] [PubMed] [Google Scholar]
- 66.Moret C, Briley M. The importance of norepinephrine in depression. Neuropsychiatr Dis Treat. 2011 doi: 10.2147/NDT.S19619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Berman RM, Narasimhan M, Miller HL, et al. Transient depressive relapse induced by catecholamine depletion. Arch Gen Psychiatry. 1999 doi: 10.1001/archpsyc.56.5.395. [DOI] [PubMed] [Google Scholar]
- 68.Krahn LE, Lin SC, Klee GG, et al. The effect of presynaptic catecholamine depletion on 6-hydroxymelatonin sulfate: a double blind study of α-methyl-para-tyrosine. Eur Neuropsychopharmacol. 1999 doi: 10.1016/S0924-977X(98)00006-6. [DOI] [PubMed] [Google Scholar]
- 69.Grace AA. Dysregulation of the dopamine system in the pathophysiology of schizophrenia and depression. Nat. Rev. Neurosci. 2016;17:524–532. doi: 10.1038/nrn.2016.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Belujon P, Grace AA. Dopamine system dysregulation in major depressive disorders. Int. J. Neuropsychopharmacol. 2017;20:1036–1046. doi: 10.1093/ijnp/pyx056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Groves JO. Is it time to reassess the BDNF hypothesis of depression? Mol. Psychiatry. 2007;12:1079–1088. doi: 10.1038/sj.mp.4002075. [DOI] [PubMed] [Google Scholar]
- 72.Björkholm C, Monteggia LM. BDNF—a key transducer of antidepressant effects. Neuropharmacology. 2016;102:72–79. doi: 10.1016/j.neuropharm.2015.10.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Rantamäki T, Hendolin P, Kankaanpää A, et al. Pharmacologically diverse antidepressants rapidly activate brain-derived neurotrophic factor receptor TrkB and induce phospholipase-Cγ signaling pathways in mouse brain. Neuropsychopharmacology. 2007 doi: 10.1038/sj.npp.1301345. [DOI] [PubMed] [Google Scholar]
- 74.Sharma AN, Da Costa E, Silva BFB, Soares JC, et al. Role of trophic factors GDNF, IGF-1 and VEGF in major depressive disorder: a comprehensive review of human studies. J. Affect. Disord. 2016;197:9–20. doi: 10.1016/j.jad.2016.02.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Levy MJF, Boulle F, Steinbusch HW, et al. Neurotrophic factors and neuroplasticity pathways in the pathophysiology and treatment of depression. Psychopharmacology (Berl). 2018;235:2195–2220. doi: 10.1007/s00213-018-4950-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lin PY, Tseng PT. Decreased glial cell line-derived neurotrophic factor levels in patients with depression: a meta-analytic study. J Psychiatr Res. 2015 doi: 10.1016/j.jpsychires.2015.02.004. [DOI] [PubMed] [Google Scholar]
- 77.Mahmoudi Asl A, Mehdizadeh M, Kulisevsky J, et al. Reliability, validity, and diagnostic accuracy of Parkinson’s disease-cognitive rating scale in Iranian patients with idiopathic Parkinson’s disease. Disabil Rehabil. 2020 doi: 10.1080/09638288.2020.1813337. [DOI] [PubMed] [Google Scholar]
- 78.Ménard C, Hodes GE, Russo SJ. Pathogenesis of depression: Insights from human and rodent studies. Neuroscience. 2016;321:138–162. doi: 10.1016/j.neuroscience.2015.05.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Valkanova V, Ebmeier KP, Allan CL. CRP, IL-6 and depression: a systematic review and meta-analysis of longitudinal studies. J. Affect. Disord. 2013;150:736–755. doi: 10.1016/j.jad.2013.06.004. [DOI] [PubMed] [Google Scholar]
- 80.Dowlati Y, Herrmann N, Swardfager W, et al. A meta-analysis of cytokines in major depression. Biol Psych. 2010 doi: 10.1016/j.biopsych.2009.09.033. [DOI] [PubMed] [Google Scholar]
- 81.Kubera M, Obuchowicz E, Goehler L, et al. In animal models, psychosocial stress-induced (neuro)inflammation, apoptosis and reduced neurogenesis are associated to the onset of depression. Prog. Neuro-Psychopharmacol Biol. Psych. 2011;35:744–759. doi: 10.1016/j.pnpbp.2010.08.026. [DOI] [PubMed] [Google Scholar]
- 82.Dantzer R, O’Connor JC, Freund GG, et al. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat. Rev. Neurosci. 2008;9:46–56. doi: 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ting EYC, Yang AC, Tsai SJ. Role of interleukin-6 in depressive disorder. Int. J. Mol. Sci. 2020;21:2194. doi: 10.3390/ijms21062194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ng A, Tam WW, Zhang MW, et al. IL-1β, IL-6, TNF- α and CRP in elderly patients with depression or Alzheimer’s disease: systematic review and meta-analysis. Sci Rep. 2018 doi: 10.1038/s41598-018-30487-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Maes M, Anderson G, Kubera M, Berk M. Targeting classical IL-6 signalling or IL-6 trans-signalling in depression? Expert Opin. Ther. Targets. 2014;18:495–512. doi: 10.1517/14728222.2014.888417. [DOI] [PubMed] [Google Scholar]
- 86.Akimov OY, Vetkina AY, Malyk AI, et al. Role of transcriptional factor NF-κB in development of oxidative stress in heart of rats during systemic inflammatory response induced by bacterial lipopolysaccharide. Aктyaльнi пpoблeми cyчacнoї мeдицини: Bicник Укpaїнcькoї мeдичнoї cтoмaтoлoгiчнoї aкaдeмiї. 2019;19:108–112. doi: 10.31718/2077-1096.19.3.108. [DOI] [Google Scholar]
- 87.Vaváková M, ɰuračková Z, Trebatická J. Markers of oxidative stress and neuroprogression in depression disorder. Oxid. Med. Cell. Longev. 2015;2015:898393. doi: 10.1155/2015/898393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Caviedes A, Lafourcade C, Soto C, Wyneken U. BDNF/NF-κB signaling in the neurobiology of depression. Curr Pharm Des. 2017 doi: 10.2174/1381612823666170111141915. [DOI] [PubMed] [Google Scholar]
- 89.Maes M, Song C, Yirmiya R. Targeting IL-1 in depression. Expert Opin. Ther. Targets. 2012;16:1097–1112. doi: 10.1517/14728222.2012.718331. [DOI] [PubMed] [Google Scholar]
- 90.Pan Y, Chen XY, Zhang QY, Kong LD. Microglial NLRP3 inflammasome activation mediates IL-1β-related inflammation in prefrontal cortex of depressive rats. Brain Behav Immun. 2014 doi: 10.1016/j.bbi.2014.04.007. [DOI] [PubMed] [Google Scholar]
- 91.Ma K, Zhang H, Baloch Z. Pathogenetic and therapeutic applications of tumor necrosis factor-α (TNF-α) in major depressive disorder: a systematic review. Int. J. Mol. Sci. 2016;17:733. doi: 10.3390/ijms17050733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hochstrasser T, Ullrich C, Sperner-Unterweger B, Humpel C. Inflammatory stimuli reduce survival of serotonergic neurons and induce neuronal expression of indoleamine 2,3-dioxygenase in rat dorsal raphe nucleus organotypic brain slices. Neuroscience. 2011 doi: 10.1016/j.neuroscience.2011.03.070. [DOI] [PubMed] [Google Scholar]
- 93.Deng S-L, Chen J-G, Wang F. Microglia: a central player in depression. Curr Med Sci. 2020;40:391–400. doi: 10.1007/s11596-020-2193-1. [DOI] [PubMed] [Google Scholar]
- 94.Setiawan E, Wilson AA, Mizrahi R, et al. Role of translocator protein density, a marker of neuroinflammation, in the brain during major depressive episodes. JAMA Psychiat. 2015 doi: 10.1001/jamapsychiatry.2014.2427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wohleb ES, Fenn AM, Pacenta AM, et al. Peripheral innate immune challenge exaggerated microglia activation, increased the number of inflammatory CNS macrophages, and prolonged social withdrawal in socially defeated mice. Psychoneuroendocrinology. 2012 doi: 10.1016/j.psyneuen.2012.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Tynan RJ, Naicker S, Hinwood M, et al. Chronic stress alters the density and morphology of microglia in a subset of stress-responsive brain regions. Brain Behav Immun. 2010 doi: 10.1016/j.bbi.2010.02.001. [DOI] [PubMed] [Google Scholar]
- 97.Zhao Y, Wang Q, Jia M, et al. (+)-Sesamin attenuates chronic unpredictable mild stress-induced depressive-like behaviors and memory deficits via suppression of neuroinflammation. J Nutr Biochem. 2019 doi: 10.1016/j.jnutbio.2018.10.006. [DOI] [PubMed] [Google Scholar]
- 98.Pandi-Perumal SR, Trakht I, Srinivasan V, et al. Physiological effects of melatonin: role of melatonin receptors and signal transduction pathways. Prog. Neurobiol. 2008;85:335–353. doi: 10.1016/j.pneurobio.2008.04.001. [DOI] [PubMed] [Google Scholar]
- 99.Lacoste B, Angeloni D, Dominguez-Lopez S, et al. Anatomical and cellular localization of melatonin MT1 and MT2 receptors in the adult rat brain. J Pineal Res. 2015 doi: 10.1111/jpi.12224. [DOI] [PubMed] [Google Scholar]
- 100.Gunata M, Parlakpinar H, Acet HA. Melatonin: a review of its potential functions and effects on neurological diseases. Rev. Neurol. (Paris) 2020;176:148–165. doi: 10.1016/j.neurol.2019.07.025. [DOI] [PubMed] [Google Scholar]
- 101.Stauch B, Johansson LC, Cherezov V. Structural insights into melatonin receptors. FEBS J. 2020;287:1496–1510. doi: 10.1111/febs.15128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Slominski RM, Reiter RJ, Schlabritz-Loutsevitch N, et al. Melatonin membrane receptors in peripheral tissues: distribution and functions. Mol. Cell. Endocrinol. 2012;351:152–166. doi: 10.1016/j.mce.2012.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Jockers R, Delagrange P, Dubocovich ML, et al. Update on melatonin receptors: IUPHAR Review 20. Br. J. Pharmacol. 2016;173:2702–25. doi: 10.1111/bph.13536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Zhang Y, He F, Chen Z, et al. Melatonin modulates IL-1β-induced extracellular matrix remodeling in human nucleus pulposus cells and attenuates rat intervertebral disc degeneration and inflammation. Aging (Albany NY) 2019;11:10499–10512. doi: 10.18632/aging.102472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Arias J, Melean E, Valero N, et al. Effect of melatonin on lymphocyte proliferation and production of interleukin-2 (IL-2) and interleukin-1 beta (IL-1 beta) in mice splenocytes. Invest Clin. 2003;44:41–50. [PubMed] [Google Scholar]
- 106.Lin JJ, Lin Y, Zhao TZ, et al. Melatonin suppresses neuropathic pain via MT2-dependent and -independent pathways in dorsal root ganglia neurons of mice. Theranostics. 2017 doi: 10.7150/thno.19500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ali T, Hao Q, Ullah N, et al. Melatonin act as an antidepressant via attenuation of neuroinflammation by targeting Sirt1/Nrf2/HO-1 signaling. Front Mol Neurosci. 2020 doi: 10.3389/fnmol.2020.00096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Lv WJ, Liu C, Yu LZ, et al. Melatonin alleviates neuroinflammation and metabolic disorder in DSS-induced depression rats. Oxid Med Cell Longev. 2020 doi: 10.1155/2020/1241894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Cecon E, Oishi A, Jockers R. Melatonin receptors: molecular pharmacology and signalling in the context of system bias. Br. J. Pharmacol. 2018;175:3263–3280. doi: 10.1111/bph.13950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Wongprayoon P, Govitrapong P. Melatonin receptor as a drug target for neuroprotection. Curr Mol Pharmacol. 2020 doi: 10.2174/1874467213666200421160835. [DOI] [PubMed] [Google Scholar]
- 111.Shukla M, Sotthibundhu A, Govitrapong P. Role of melatonin in regulating neurogenesis: Implications for the neurodegenerative pathology and analogous therapeutics for Alzheimer’s disease. Melatonin Res. 2020 doi: 10.32794/mr11250059. [DOI] [Google Scholar]
- 112.Lanfumey L, Mongeau R, Hamon M. Biological rhythms and melatonin in mood disorders and their treatments. Pharmacol. Ther. 2013;138:176–84. doi: 10.1016/j.pharmthera.2013.01.005. [DOI] [PubMed] [Google Scholar]
- 113.Talman V, Pascale A, Jäntti M, et al. Protein kinase C activation as a potential therapeutic strategy in Alzheimer’s disease: is there a role for embryonic lethal abnormal vision-like proteins? Basic Clin Pharmacol. Toxicol. 2016;119:149–160. doi: 10.1111/bcpt.12581. [DOI] [PubMed] [Google Scholar]
- 114.Amini H, Rezabakhsh A, Heidarzadeh M, et al. An examination of the putative role of melatonin in exosome biogenesis. Front. Cell Dev. Biol. 2021;9:686551. doi: 10.3389/fcell.2021.686551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Milligan G. G protein-coupled receptor hetero-dimerization: contribution to pharmacology and function. Br. J. Pharmacol. 2009;158:5–14. doi: 10.1111/j.1476-5381.2009.00169.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Kamal M, Gbahou F, Guillaume JL, et al. Convergence of melatonin and serotonin (5-HT) signaling at MT2/5-HT2C receptor heteromers. J Biol Chem. 2015 doi: 10.1074/jbc.M114.559542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Leung JWH, Cheung KK, Ngai SPC, et al. Protective effects of melatonin on neurogenesis impairment in neurological disorders and its relevant molecular mechanisms. Int. J. Mol. Sci. 2020;21:5645. doi: 10.3390/ijms21165645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Liu J, Clough SJ, Dubocovich ML. Role of the MT1 and MT2 melatonin receptors in mediating depressive- and anxiety-like behaviors in C3H/HeN mice. Genes, Brain Behav. 2017 doi: 10.1111/gbb.12369. [DOI] [PubMed] [Google Scholar]
- 119.Wu YH, Ursinus J, Zhou JN, et al. Alterations of melatonin receptors MT1 and MT2 in the hypothalamic suprachiasmatic nucleus during depression. J Affect Disord. 2013 doi: 10.1016/j.jad.2012.12.025. [DOI] [PubMed] [Google Scholar]
- 120.Comai S, Ochoa-Sanchez R, Dominguez-Lopez S, et al. Melancholic-like behaviors and circadian neurobiological abnormalities in melatonin MT1 receptor knockout mice. Int J Neuropsychopharmacol. 2015 doi: 10.1093/ijnp/pyu075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Niles LP, Armstrong KJ, Rincón Castro LM, et al. Neural stem cells express melatonin receptors and neurotrophic factors: Colocalization of the MT1 receptor with neuronal and glial markers. BMC Neurosci. 2004 doi: 10.1186/1471.2202-5-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Comai S, Ochoa-Sanchez R, Dominguez-Lopez S, et al. Melancholic-like behaviors and circadian neurobiological abnormalities in melatonin MT1 receptor knockout mice. Int J Neuropsychopharmacol. 2015;18:1–10. doi: 10.1093/IJNP/PYU075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Liu J, Clough SJ, Hutchinson AJ, et al. MT1 and MT2 melatonin receptors: a therapeutic perspective. Annu Rev Pharmacol Toxicol. 2016;56:361–383. doi: 10.1146/ANNUREV-PHARMTOX-010814-124742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Leung JWH, Cheung KK, Ngai SPC, et al. Protective effects of melatonin on neurogenesis impairment in neurological disorders and its relevant molecular mechanisms. Int J Mol Sci. 2020;21:1–30. doi: 10.3390/IJMS21165645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Zhang Y, He F, Chen Z, et al. Melatonin modulates IL-1β-induced extracellular matrix remodeling in human nucleus pulposus cells and attenuates rat intervertebral disc degeneration and inflammation. Aging. 2019;11:10499–10512. doi: 10.18632/AGING.102472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Lin JJ, Lin Y, Zhao TZ, et al. Melatonin suppresses neuropathic pain via MT2-dependent and -independent pathways in dorsal root ganglia neurons of mice. Theranostics. 2017;7:2015–2032. doi: 10.7150/THNO.19500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Ali T, Hao Q, Ullah N, et al. Melatonin act as an antidepressant via attenuation of neuroinflammation by targeting Sirt1/Nrf2/HO-1 signaling. Front Mol Neurosci. 2020;13:96. doi: 10.3389/FNMOL.2020.00096/BIBTEX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Liu J, Clough SJ, Hutchinson AJ, et al. MT1 and MT2 melatonin receptors: a therapeutic perspective. Annu. Rev. Pharmacol. Toxicol. 2016;56:361–83. doi: 10.1146/annurev-pharmtox-010814-124742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Imbesi M, Uz T, Manev H. Role of melatonin receptors in the effects of melatonin on BDNF and neuroprotection in mouse cerebellar neurons. J Neural Transm. 2008 doi: 10.1007/s00702-008-0066-z. [DOI] [PubMed] [Google Scholar]
- 130.Comai S, De Gregorio D, Posa L, et al. Dysfunction of serotonergic activity and emotional responses across the light-dark cycle in mice lacking melatonin MT2 receptors. J Pineal Res. 2020 doi: 10.1111/jpi.12653. [DOI] [PubMed] [Google Scholar]
- 131.Thomson DM, Mitchell EJ, Openshaw RL, et al. Mice lacking melatonin MT2 receptors exhibit attentional deficits, anxiety and enhanced social interaction. J Psychopharmacol. 2021 doi: 10.1177/02698811211032439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Wang Y-q, Jiang Y-j, Zou M-s, et al. Antidepressant actions of melatonin and melatonin receptor agonist: Focus on pathophysiology and treatment. Behav. Brain Res. 2022;420:113724. doi: 10.1016/j.bbr.2021.113724. [DOI] [PubMed] [Google Scholar]
- 133.Andersen LPH, Gögenur I, Rosenberg J, Reiter RJ. The Safety of Melatonin in Humans. Clin. Drug Investig. 2016;36:169–175. doi: 10.1007/s40261-015-0368-5. [DOI] [PubMed] [Google Scholar]
- 134.Andersen LPH. The analgesic effects of exogenous melatonin in humans. Dan. Med. J. 2016;63:B5289. [PubMed] [Google Scholar]
- 135.Riha RL. The use and misuse of exogenous melatonin in the treatment of sleep disorders. Curr. Opin. Pulm. Med. 2018;24:543–548. doi: 10.1097/MCP.0000000000000522. [DOI] [PubMed] [Google Scholar]
- 136.Herxheimer A, Petrie KJ. Melatonin for the prevention and treatment of jet lag. Cochrane Database Syst Rev. 2002 doi: 10.1002/14651858.cd001520. [DOI] [PubMed] [Google Scholar]
- 137.Auld F, Maschauer EL, Morrison I, et al. Evidence for the efficacy of melatonin in the treatment of primary adult sleep disorders. Sleep Med. Rev. 2017;34:10–22. doi: 10.1016/j.smrv.2016.06.005. [DOI] [PubMed] [Google Scholar]
- 138.Ferracioli-Oda E, Qawasmi A, Bloch MH. Meta-analysis: melatonin for the treatment of primary sleep disorders. PLoS ONE. 2013 doi: 10.1371/journal.pone.0063773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Ramirez-Rodriguez G, Ortíz-Lõpez L, Domínguez-Alonso A, et al. Chronic treatment with melatonin stimulates dendrite maturation and complexity in adult hippocampal neurogenesis of mice. J Pineal Res. 2011 doi: 10.1111/j.1600-079X.2010.00802.x. [DOI] [PubMed] [Google Scholar]
- 140.Liu J, Somera-Molina KC, Hudson RL, Dubocovich ML. Melatonin potentiates running wheel-induced neurogenesis in the dentate gyrus of adult C3H/HeN mice hippocampus. J Pineal Res. 2013 doi: 10.1111/jpi.12023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Zhdanova IV, Wurtman RJ, Lynch HJ, et al. Sleep-inducing effects of low doses of melatonin ingested in the evening. Clin Pharmacol Ther. 1995 doi: 10.1016/0009-9236(95)90040-3. [DOI] [PubMed] [Google Scholar]
- 142.Ali T, Rahman SU, Hao Q, et al. Melatonin prevents neuroinflammation and relieves depression by attenuating autophagy impairment through FOXO3a regulation. J Pineal Res. 2020 doi: 10.1111/jpi.12667. [DOI] [PubMed] [Google Scholar]
- 143.Satyanarayanan SK, Chien YC, Chang JPC, et al. Melatonergic agonist regulates circadian clock genes and peripheral inflammatory and neuroplasticity markers in patients with depression and anxiety. Brain Behav Immun. 2020 doi: 10.1016/j.bbi.2019.03.003. [DOI] [PubMed] [Google Scholar]
- 144.Imbesi M, Uz T, Dzitoyeva S, Manev H. Stimulatory effects of a melatonin receptor agonist, ramelteon, on BDNF in mouse cerebellar granule cells. Neurosci Lett. 2008 doi: 10.1016/j.neulet.2008.04.099. [DOI] [PubMed] [Google Scholar]
- 145.Kishi T, Nomura I, Sakuma K, et al. Melatonin receptor agonists—ramelteon and melatonin—for bipolar disorder: a systematic review and meta-analysis of double-blind, randomized, placebo-controlled trials. Neuropsychiatr Dis Treat. 2019;15:1479–1486. doi: 10.2147/NDT.S198899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Norris ER, Burke K, Correll JR, et al. A double-blind, randomized, placebo-controlled trial of adjunctive ramelteon for the treatment of insomnia and mood stability in patients with euthymic bipolar disorder. J Affect Disord. 2013;144:141–147. doi: 10.1016/J.JAD.2012.06.023. [DOI] [PubMed] [Google Scholar]
- 147.Furuya M, Kunishige K, Miyaoka T, et al. Augmentation with ramelteon to achieve remission in geriatric major depression. Psychiatry Clin Neurosci. 2012;66:81–82. doi: 10.1111/J.1440-1819.2011.02298.X. [DOI] [PubMed] [Google Scholar]
- 148.Tchekalarova J, Kortenska L, Ivanova N, et al. Agomelatine treatment corrects impaired sleep-wake cycle and sleep architecture and increases MT1 receptor as well as BDNF expression in the hippocampus during the subjective light phase of rats exposed to chronic constant light. Psychopharmacology. 2020 doi: 10.1007/s00213-019-05385-y. [DOI] [PubMed] [Google Scholar]
- 149.Lockley SW, Dressman MA, Licamele L, et al. Tasimelteon for non-24-hour sleep-wake disorder in totally blind people (SET and RESET): Two multicentre, randomised, double-masked, placebo-controlled phase 3 trials. Lancet. 2015 doi: 10.1016/S0140-6736(15)60031-9. [DOI] [PubMed] [Google Scholar]
- 150.Wade AG, Ford I, Crawford G, et al. Nightly treatment of primary insomnia with prolonged release melatonin for 6 months: a randomized placebo controlled trial on age and endogenous melatonin as predictors of efficacy and safety. BMC Med. 2010 doi: 10.1186/1741-7015-8-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Wade AG, Ford I, Crawford G, et al. Efficacy of prolonged release melatonin in insomnia patients aged 55–80 years: quality of sleep and next-day alertness outcomes. Curr Med Res Opin. 2007 doi: 10.1185/030079907X233098. [DOI] [PubMed] [Google Scholar]