Abstract
Background
Monoclonal antibodies (mAb) that neutralize SARS-CoV-2 decrease hospitalization and death compared to placebo in patients with mild to moderate COVID-19; however, comparative effectiveness is unknown. We report the comparative effectiveness of bamlanivimab, bamlanivimab-etesevimab, and casirivimab-imdevimab.
Methods
A learning health system platform trial in a U.S. health system enrolled patients meeting mAb Emergency Use Authorization criteria. An electronic health record-embedded application linked local mAb inventory to patient encounters and provided random mAb allocation. Primary outcome was hospital-free days to day 28. Primary analysis was a Bayesian model adjusting for treatment location, age, sex, and time. Inferiority was defined as 99% posterior probability of an odds ratio < 1. Equivalence was defined as 95% posterior probability the odds ratio is within a given bound.
Findings
Between March 10 and June 25, 2021, 1935 patients received treatment. Median hospital-free days were 28 (IQR 28, 28) for each mAb. Mortality was 0.8% (1/128), 0.8% (7/885), and 0.7% (6/922) for bamlanivimab, bamlanivimab-etesevimab, and casirivimab-imdevimab, respectively. Relative to casirivimab-imdevimab (n = 922), median adjusted odds ratios were 0.58 (95% credible interval [CI] 0.30–1.16) and 0.94 (95% CI 0.72–1.24) for bamlanivimab (n = 128) and bamlanivimab-etesevimab (n = 885), respectively. These odds ratios yielded 91% and 94% probabilities of inferiority of bamlanivimab versus bamlanivimab-etesevimab and casirivimab-imdevimab, and an 86% probability of equivalence between bamlanivimab-etesevimab and casirivimab-imdevimab.
Interpretation
Among patients with mild to moderate COVID-19, bamlanivimab-etesevimab or casirivimab-imdevimab treatment resulted in 86% probability of equivalence. No treatment met prespecified criteria for statistical equivalence. Median hospital-free days to day 28 were 28 (IQR 28, 28) for each mAb.
Funding and registration
This work received no external funding. The U.S. government provided the reported mAb. This trial is registered at ClinicalTrials.gov, NCT04790786.
1. Introduction
In clinical trials, anti-SARS-CoV-2 neutralizing monoclonal antibodies (mAb) bamlanivimab-etesevimab and casirivimab-imdevimab decreased hospitalization, compared to placebo, in patients with mild to moderate COVID-19 [[1], [2], [3], [4], [5], [6]]. These results led to Emergency Use Authorization (EUA) by the U.S. Food and Drug Administration (FDA) and free supply to healthcare systems by the federal government [[7], [8], [9], [10]]. However, uptake was limited due to lack of access and comparative effectiveness of the mAb was unknown [11,12].
In February 2021, UPMC partnered with the U.S. Federal COVID-19 Response Team to expand mAb use to all EUA-eligible patients and evaluate the comparative effectiveness of available mAbs using a learning health system approach. This approach synergizes knowledge generation with daily practice to seek continuous improvement in care; randomization was embedded into the electronic health record (EHR) and aligned with routine clinical care across the system [13,14]. We set two objectives: to equitably treat as broad a proportion of mAb-eligible patients as possible, and to compare the effectiveness between mAbs overall and over time as SARS-CoV-2 variants emerged. Initially, there were three mAbs available (bamlanivimab, bamlanivimab-etesevimab, casirivimab-imdevimab). However, the U.S. government halted distribution of bamlanivimab on March 24, 2021 and of bamlanivimab-etesevimab on June 25, 2021. We have previously published the full details of the trial infrastructure, implementation, and outreach efforts [15,16]. Here, we report the first results of the OPTIMISE-C19 (OPtimizing Treatment and Impact of Monoclonal antIbodieS through Evaluation for COVID-19) trial, evaluating monoclonal antibody use through June 25, 2021 [17].
2. Methods
2.1. Setting and expansion of monoclonal antibody access
Prior to U.S. federal partnership, UPMC developed a mAb treatment infrastructure with outpatient infusion centers in response to the bamlanivimab EUA issued November 9, 2020. The first patient was treated within this infrastructure on December 9, 2020 [16,18]. After observational results of patients treated through March 3, 2021 demonstrated a 60% reduction in hospitalization and death compared to those who did not receive mAb, UPMC launched the OPTIMISE-C19 platform in partnership with the U.S. federal government. These efforts included expansion of mAb infusion capacity to 31 emergency departments (ED), increased infusion center staffing, and a multifaceted outreach campaign. UPMC partnered with government public health bodies, community leaders, and neighboring health systems to increase awareness and referrals, and with a home infusion company to provide treatment for homebound patients (Chartwell Pennsylvania, Oakdale, PA).
Prior to trial launch, the UPMC Pharmacy and Therapeutics Committee developed a therapeutic interchange policy on November 21, 2020 in response to the EUA for casirivimab-imdevimab. The policy considered all available mAb equivalent; a patient could receive any mAb based on local inventory. The policy was updated to include bamlanivimab-etesevimab on February 9, 2021, and to remove bamlanivimab on March 31, 2021 [8,19]. All pharmacies supplying all infusion sites had equal opportunity to order any available mAb from a central supply facility. After trial launch, all mAb were ordered as a generic referral order (embedded into multiple EHRs for outpatient and ED prescribing) and provided as per EUA guidance (Table S1 in Supplement, p 1). Prescribers were required to provide and review all mAb EUA Fact Sheets with the patient or patient guardian/representative and explain the patient could receive any EUA-governed mAb under the therapeutic interchange policy.
2.2. Trial design and oversight
OPTIMISE-C19 was designed as an open-label, pragmatic, comparative effectiveness, platform trial with response-adaptive randomization [15]. The existing organizational architecture of a therapeutic interchange policy allowed for rapid generation of practice-based evidence within the context of a quality improvement initiative evaluating EHR-embedded routine care [15]. The three mAbs (bamlanivimab, bamlanivimab-etesevimab, and casirivimab-imdevimab) evaluated in this report were supplied by the U.S. federal government. The trial was approved by the UPMC Quality Improvement Committee and launched March 10, 2021 (Project ID 3280). The University of Pittsburgh Institutional Review Board and study investigators considered provision of mAb therapy quality improvement and only the additional data collection and analyses represented research (STUDY21020179).
2.3. Patients
A centralized operations team confirmed patient eligibility upon referral. Patients were eligible if they met EUA criteria of a positive SARS-CoV-2 polymerase chain reaction or antigen test, mild or moderate symptoms for ≤10 days, and risk factor(s) for progression to severe COVID-19. EUA criteria excluded patients who required oxygen above baseline requirements, weighed <40 kg, were < 12 years of age, or were hospitalized for COVID-19.
2.4. Procedures and treatment assignment
A custom application embedded into the EHR linked local mAb inventory to patient encounters and provided a random mAb allocation (bamlanivimab, bamlanivimab-etesevimab, or casirivimab-imdevimab) at time of mAb referral, within the therapeutic interchange policy [18,20]. Patients provided verbal consent to receive mAb therapy as part of routine care within the EUA. Prescribers and/or patients could request a specific mAb. Consistent with a learning health system approach, trial procedures were embedded within routine care and all other aspects of care were provided per each site's clinicians.
2.5. Outcomes and measures
To evaluate mAb use expansion, the EHR-screen eligible population was defined as all outpatients with a positive SARS-CoV-2 polymerase chain reaction or antigen test performed within the health system and an EUA risk factor. Race was derived from registration system data using categories consistent with the Centers for Medicare & Medicaid Services EHR meaningful use dataset and the American Medical Association Manual of Style [21,22]. Pre-specified categories included non-Hispanic Black, non-Hispanic White, and Other. Individuals were considered Other due to small sample sizes for Hispanic, American Indian, and other races and ethnicities. Geographic distribution of mAb treatment was illustrated using the zip code of patient residence [23,24].
To evaluate comparative effectiveness, the primary outcome was hospital-free days up to day 28 after mAb treatment. This outcome is an ordinal endpoint with death up to day 28 as the worst outcome (assigned −1 hospital-free days), then the length of time alive and free of hospital (all hospitalizations), such that the best outcome is 28 hospital-free days. If a patient had intervening days free of hospital and was re-hospitalized, the patient was credited for the intervening days as free of the hospital. Secondary outcomes included 28-day mortality. Rates of hospitalization by infusion location and incidence of adverse events were also evaluated (Fig. S2 in Supplement, p 7). SARS-CoV-2 variant prevalence in the Pennsylvania catchment were assessed over time using Global Initiative on Sharing All Influenza Data [25].
2.6. Data collection
A data analytics team built a system for automated data extraction from the UPMC Clinical Data Warehouse to synthesize EHR-embedded data feeds from EHRs across the inpatient and outpatient care continuum. All extracted data underwent validation by a clinical pharmacist and were reviewed by a system Quality Center nurse to ensure appropriate patient capture. The primary outcome was ascertained by linking inpatient (Cerner, Kansas City, Missouri) and outpatient (Epic, Madison, Wisconsin) EHRs, as in prior work [20]. Patient-directed phone calls were conducted at day 28 to ascertain health care encounters outside our health system, along with Social Security Administration Death Master File queries [26]. Adverse events were collected in a secure electronic application completed by infusion center nurses on day of treatment, and a patient safety reporting system completed by clinical staff in infusion centers and EDs. Adverse event severity was adjudicated blinded to mAb type.
2.7. Statistical analysis
To determine the epidemiology of mAb infusions, we measured the proportion of EHR-screen eligible patients treated with mAb, stratified by demographics, geography, and prior to (December 9, 2020–March 9, 2021) or after (March 10–June 25, 2021) trial launch (Fig. S1 in Supplement, p 7).
To analyze comparative effectiveness, the statistical analysis plan was written by blinded investigators prior to data lock and analysis (in Supplement statistical analysis plan, p 20–29) and applied to treated patients March 10–June 25, 2021. The platform continuously evaluates multiple mAb, with randomization continuing until pre-determined statistical thresholds are met. The trial launched with equal randomization and planned interim analyses for adaptive randomization where mAb performing better would be given higher randomization probabilities. The mAb arm at the first adaptive analysis with the largest sample size was specified as the referent, as there was no non-mAb control and all patients received treatment. Methods and results are reported as per the CONSORT Pragmatic Extension checklist (in Supplement, p 118) [27]. An unblinded statistical analysis committee conducted analyses with R version 4.0.5 using the RStan package version 2.21.0 (R Foundation, Vienna, Austria) and reported results to the UPMC Chief Medical Officer who functioned as data and safety monitor.
The primary analysis population was the “as-infused” population of patients randomly allocated mAb and treated. As all arms included mAb, there was no anticipated relationship between lack of infusion and assigned arm. The primary analysis model was a Bayesian cumulative logistic model that adjusted for treatment location (infusion center or ED), age (<30, 30–39, 40–49, 50–59, 60–69, 70–79, and ≥ 80 years), sex, and time (2-week epochs). Comparisons between individual mAb were based on the relative odds ratio between a given two arms for the primary outcome. An odds ratio for an arm to a comparator >1 implies improved outcomes. A sliding scale with different levels of equivalence bounds was pre-defined (in Supplement statistical analysis plan, p 20–29). Equivalence between two arms was defined as 95% posterior probability the odds ratio is within a given bound. Inferiority of one arm compared to another was defined as 99% posterior probability of an odds ratio < 1.
In addition to the primary analysis, to determine potential differential treatment effects of mAbs by COVID-19 variant type, patients were categorized into four time epochs relative to variant prevalence in 2021 (March 10–31, April 1–30, May 1–31, and June 1–25). Treatment effects of mAbs by variant were estimated by measuring the effect within the four epochs (Figs. S3 and S4 in Supplement, p 8–9).
2.8. Decision to publish interim results
The U.S. Department of Health and Human Services halted distribution of bamlanivimab alone on March 24, 2021, and of bamlanivimab-etesevimab on June 25, 2021, due to concern of lack of efficacy against variants predominant in the U.S. at those times [19]. As further treatment with these mAbs was not possible, we unblinded and analyzed patients allocated through June 25, 2021, with follow up completed July 23, 2021.
3. Results
3.1. Expansion of monoclonal antibody treatment
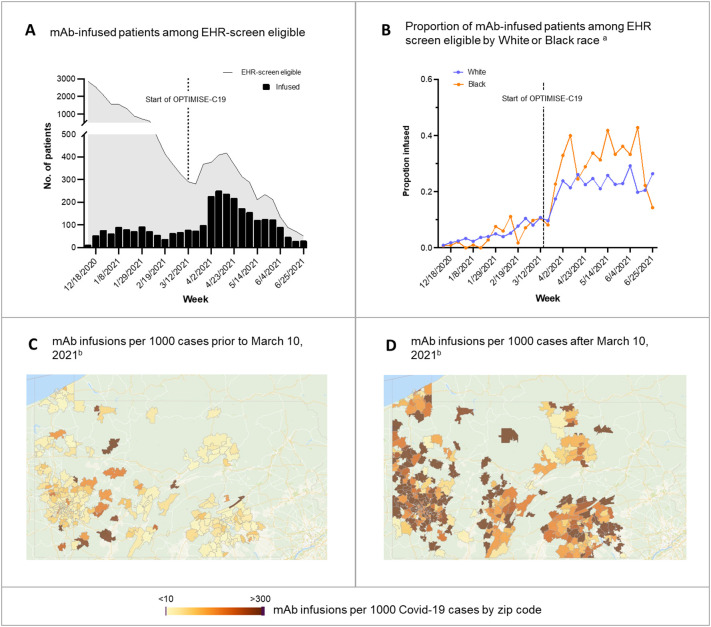
Prior to the trial, 16,345 patients were EHR-screen eligible. Of these, 502 patients (3.1%) received mAb, of whom the proportion of EHR-screen eligible patients who were treated were similar between patients who identified as Black (2.6%) and White (3.1%). After trial launch, 1201 of 5173 EHR-screen eligible patients (23.2%) were treated. Every patient who received mAb received it in the context of the trial; there was no use outside the trial. The proportions of eligible White patients receiving mAb increased from 3.1 to 21.6% and eligible Black patients receiving mAb increased from 2.6 to 29.9% during the trial. Broad geographic expansion was evident across the catchment (Fig. 1 ).
Fig. 1.
Epidemiology of Monoclonal Antibody Infusions. Panel A shows the number of mAb-infused patients among the EHR screen eligible. Panel B shows the proportion of mAb-infused patients among EHR screen eligible by White or Black race. Hispanic ethnicity, Other, and Unknown race are not shown due to small sample sizes. Panel C shows the number of mAb infusions per 1000 cases prior to March 10, 2021. Panel D shows the number of mAb infusions per 1000 cases after March 10, 2021. Zip codes with <10 COVID-19 cases or those outside of Pennsylvania are not shown.
Interpretive example: Prior to the trial (panel A), mAb infusion was low. In panel B, the proportion of White and Black race infused among EHR-screen eligible patients increased. In the UPMC catchment in Pennsylvania, mAb infusions also increased in amount (darker, panel C, D) and in geographic distribution (more zip code areas colored) during OPTIMISE-C19.
3.2. Trial patients
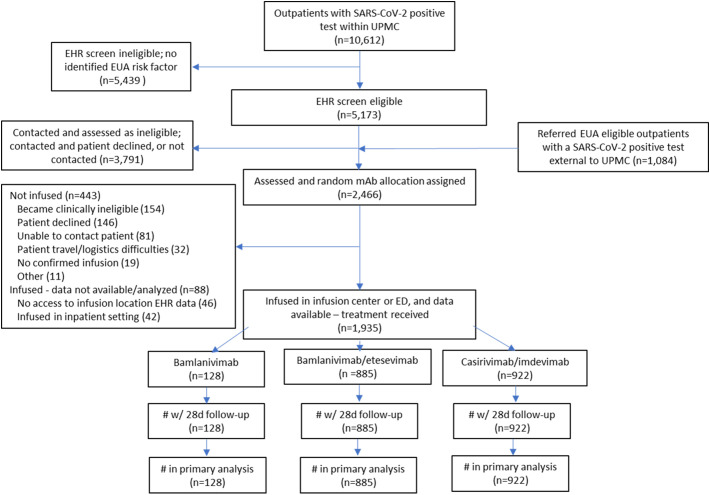
Between March 10 and June 25, 2021, 5173 outpatients were EHR-screen eligible, of whom 1382 were referred and underwent random mAb allocation. One thousand and eighty-four EUA-eligible patients with a positive SARS-CoV-2 test from outside UPMC were also referred, yielding a total of 2466 patients who were assigned a random mAb allocation. Of these, 1935 (78%) were infused and comprised the primary analysis cohort (bamlanivimab (n = 128), bamlanivimab-etesevimab (n = 885), casirivimab-imdevimab (n = 922), Fig. 2 ). Of the 531 patients assigned a random mAb allocation and excluded from primary analysis, most (300, 56%) were not infused due to becoming clinically ineligible at time of infusion scheduling (n = 154, 29%) or patient declining treatment (n = 146, 27%). There were 88 patients (17%) who received mAb at a location without available EHR data or while hospitalized for non-COVID-19 reasons. No patient or provider requested a mAb different than randomized.
Fig. 2.
CONSORT Diagram. Due to pharmacy logistics, five patients who received bamlanivimab-etesevimab had been randomly assigned to casirivimab-imdevimab, and seven patients who received casirivimab-imdevimab had been randomly assigned to bamlanivimab-etesevimab. All infused patients who received bamlanivimab monotherapy had been randomly assigned to bamlanivimab monotherapy. The FDA mAb policies changed over time, resulting in varying mAb availability and EUA eligibility criteria over time (Table S1 in Supplement, p 1). .
Baseline characteristics were similar across groups (Table 1 , Table S3 in Supplement, p 4). Mean age was 55–57 years, half were female (range, 53–54%), 18% were Black (range 12–19%), and the most common risk factors were advanced age, high body mass index, and hypertension. Mean duration of symptom onset to referral was 5 (2.1) days.
Table 1.
Baseline characteristics.
| Variable | No. (%) |
|||
|---|---|---|---|---|
| Bamlanivimab (n = 128) |
Bamlanivimab -etesevimab (n = 885) |
Casirivimab -imdevimab (n = 922) |
Entire Cohort (n = 1935) |
|
| Age, mean (SD), years | 57 (17) | 56 (16) | 55 (16) | 56 (16) |
| Female sexa | 69 (54) | 470 (53) | 502 (54) | 1041 (54) |
| Raceb | ||||
| White | 104 (81) | 693 (78) | 701 (76) | 1498 (77) |
| Black | 15 (12) | 148 (17) | 173 (19) | 336 (17) |
| Otherc | 1 (0.8) | 23 (2.6) | 27 (2.9) | 51 (2.6) |
| Unknown | 8 (6.3) | 21 (2.4) | 21 (2.3) | 50 (2.6) |
| Vaccine status | ||||
| Fully vaccinated | 2 (1.6) | 43 (4.9) | 19 (2.1) | 64 (3.3) |
| Partially vaccinated | 26 (20) | 39 (4.4) | 55 (6.0) | 120 (6.2) |
| Unvaccinated | 0 (0) | 27 (3.1) | 30 (3.3) | 57 (2.9) |
| Unknown | 100 (78) | 776 (88) | 818 (89) | 1694 (88) |
| Body mass index, mean (SD)d | 35.6 (9.1) | 34.5 (8.4) | 35.1 (8.4) | 34.8 (8.4) |
| Days from randomization to infusion, mean (SD) |
0.6 (0.9) | 0.4 (0.7) | 0.4 (0.8) | 0.4 (0.8) |
| Days from symptoms to randomization, mean (SD) | 5.0 (2.0) | 5.4 (2.0) | 4.9 (2.0) | 5.0 (2.1) |
| Days from symptoms to infusion, mean (SD) | 6.3 (1.8) | 6.0 (1.9) | 6.2 (2.0) | 6.1 (1.9) |
| Location | ||||
| Infusion center | 103 (81) | 463 (52) | 462 (50) | 1028 (53) |
| Emergency department | 25 (20) | 422 (48) | 460 (50) | 907 (47) |
| Qualifying EUA criteria | ||||
| March 10, 2021–May 23, 2021 | 128 (100) | 753 (85) | 783 (85) | 1664 (86) |
| Age ≥ 65 years | 39 (31) | 223 (30) | 236 (30) | 498 (30) |
| Body mass index ≥ 35d | 33 (47) | 238 (47) | 257 (49) | 528 (48) |
| Chronic kidney disease | 4 (4.0) | 43 (7.7) | 51 (8.8) | 98 (7.9) |
| Diabetes | 24 (24) | 154 (28) | 154 (27) | 332 (27) |
| Immunosuppressive disease or treatmente | 26 (26) | 158 (28) | 155 (27) | 339 (27) |
| Sickle cell disease | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Age ≥ 55 years and | ||||
| Cardiovascular disease | 29 (26) | 112 (17) | 117 (17) | 258 (18) |
| Hypertension | 43 (39) | 224 (35) | 223 (33) | 490 (34) |
| Respiratory condition | 29 (26) | 124 (19) | 119 (18) | 272 (19) |
| Age 12–17 years with qualifying criterion | 1 (0.8) | 13 (1.7) | 6 (0.8) | 20 (1.2) |
| May 24, 2021–June 25, 2021f | 0 (0) | 132 (15) | 139 (15) | 271 (14) |
| Age ≥ 65 years | .. | 30 (23) | 37 (27) | 67 (25) |
| Body mass index >25d | .. | 88 (89) | 90 (90) | 178 (89) |
| Chronic kidney disease | .. | 7 (6.6) | 7 (6.5) | 14 (6.5) |
| Diabetes | .. | 29 (27) | 25 (23) | 54 (25) |
| Down syndrome | .. | 0 (0) | 0 (0) | 0 (0) |
| Current or former smoker | .. | 25 (31) | 32 (37) | 57 (34) |
| Current or history of substance abuse | .. | 0 (0) | 2 (2.3) | 2 (1.2) |
| Immunosuppressive disease or treatmente | .. | 29 (27) | 27 (25) | 56 (26) |
| Sickle cell disease | .. | 0 (0) | 0 (0) | 0 (0) |
| Cardiovascular disease | .. | 24 (23) | 14 (13) | 38 (18) |
| Hypertension | .. | 61 (58) | 52 (48) | 113 (53) |
| Respiratory condition | .. | 42 (40) | 40 (37) | 82 (38) |
| Age 12–17 years with qualifying criterion | .. | 1 (0.8) | 2 (1.4) | 3 (1.1) |
Abbreviations: SD, standard deviation; EUA, emergency use authorization.
Sex was reported by the patients.
Race was reported by the patients.
Other includes Chinese, Filipino, Hawaiian, American Indian/Alaskan Native, Asian, Hawaiian/other Pacific Islander, Middle Eastern, Native American, or Pacific Islander.
Calculated as weight in kilograms divided by height in meters squared.
Immunosuppressive disease or treatment was defined as a history of HIV, cancer, transplant (solid organ, stem cell, bone marrow), chemotherapy treatment, lupus, rheumatoid arthritis, or liver disease.
EUA criteria were expanded May 14, 2021, and operationalized at UPMC May 24, 2021.
3.3. Primary outcome
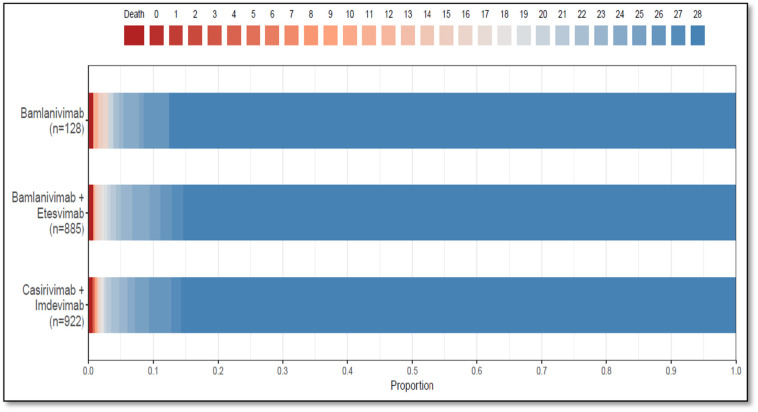
Median hospital-free days were 28 (IQR, 28–28) for each mAb (Table 2 , Fig. 3 ). Relative to casirivimab-imdevimab, the posterior median adjusted odds ratios were 0.58 (95% credible interval, 0.30–1.16) and 0.94 (95% credible interval, 0.72–1.24) for bamlanivimab and bamlanivimab-etesevimab, respectively. The probabilities of inferiority for bamlanivimab versus bamlanivimab-etesevimab and casirivimab-imdevimab were 91% and 94% respectively. The probability of equivalence between bamlanivimab-etesevimab and casirivimab-imdevimab was 86% at the first prespecified bound. No comparison met prespecified criteria for statistical inferiority or equivalence.
Table 2.
Primary outcome.
| Outcome/analysis | No. (%) |
||
|---|---|---|---|
| Bamlanivimab (n = 128) |
Bamlanivimab -etesevimab (n = 885) |
Casirivimab -imdevimab (n = 922) |
|
| Primary outcome, hospital-free days, median (IQR) | 28 (28–28) | 28 (28–28) | 28 (28–28) |
| Patients with 28 hospital-free days | 112 (87.5) | 755 (85.3) | 790 (85.7) |
| Subcomponents of hospital-free days | |||
| Deaths | 1 (0.8) | 7 (0.8) | 6 (0.7) |
| Hospital length of stay among hospitalized patients, median days (IQR) |
4 (2−10) | 4 (3–7) | 3 (2–6) |
| Hospitalized | 16 (12.5) | 130 (14.7) | 132 (14.3) |
| After mAb infusion in ED | 8/25 (32.0) | 100/422 (23.7) | 100/460 (21.7) |
| After mAb infusion in IC | 8/103 (7.8) | 30/463 (6.5) | 32/462 (6.9) |
| Primary analysis of the primary outcome | |||
| Adjusted odds ratio | |||
| mean (SD) | 0.62 (0.22) | 0.95 (0.13) | 1 [reference] |
| median (95% credible interval) | 0.58 (0.30–1.16) | 0.94 (0.72–1.24) | 1 [reference] |
| Probability of inferiority to bamlanivimab-etesevimab, % | 91% | .. | 34% |
| Probability of inferiority to casirivimab-imdevimab, % | 94% | 66% | .. |
| Probability of equivalence between bamlanivimab-etesevimab and casirivimab-imdevimab, prespecified bounds, % | |||
| 0.25 | .. | 86% | |
| 0.20 | .. | 77% | |
| 0.15 | .. | 64% | |
| 0.10 | .. | 47% | |
| 0.05 | .. | 25% | |
Abbreviations: IQR, interquartile range; mAb, monoclonal antibodies; ED, emergency department; IC, infusion center; SD, standard deviation.
Fig. 3.
Hospital-Free Days to Day 28. Primary outcome is displayed as horizontally stacked proportions by monoclonal antibody type. Red represents worse values and blue represents better values. The median adjusted odds ratio from the primary analysis, using a Bayesian cumulative logistic model, were 0.58 (95% credible interval, 0.30–1.16) and 0.94 (95% credible interval, 0.72–1.24) for the bamlanivimab and bamlanivimab-etesevimab groups compared with the casirivimab-imdevimab group. These odds ratios yielded 91% and 94% probabilities of inferiority of bamlanivimab versus bamlanivimab-etesevimab and casirivimab-imdevimab respectively, and an 86% probability of equivalence between bamlanivimab-etesevimab and casirivimab-imdevimab at the first prespecified bound. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
3.4. Secondary outcomes
The 28-day mortality rates were 0.8% (1/128), 0.8% (7/885), and 0.7% (6/922) and hospitalization rates were 12.5% (16/128), 14.7% (130/885), and 14.3% (132/922), in the bamlanivimab, bamlanivimab-etesevimab, and casirivimab-imdevimab groups, respectively.
(Table 2). For patients receiving mAb in an infusion center, rates of hospitalization after treatment were 7.8% (bamlanivimab), 6.5% (bamlanivimab-etesevimab), and 6.9% (casirivimab-imdevimab). For patients receiving mAb in an ED, rates of hospitalization after treatment were 32.0% (bamlanivimab), 23.7% (bamlanivimab-etesevimab), and 21.7% (casirivimab-imdevimab).
3.5. Adverse events
There were 21 infusion-related adverse events that occurred in 0% (0/128), 1.4% (12/885), and 1.0% (9/922) of patients treated with bamlanivimab, bamlanivimab-etesevimab, and casirivimab-imdevimab, respectively. Of these, 5 events (0 bamlanivimab, 1 bamlanivimab-etesevimab, 4 casirivimab-imdevimab) were adjudicated as serious (Table S2 in Supplement, p 3).
3.6. Differences in treatment over time
During the trial, the Alpha variant was the dominant variant of concern, while the Delta variant became more prevalent in the final time epoch (Fig. S2 in Supplement, p 7). We found no relative difference in mAb treatment effects over time and no comparisons reached a pre-specified statistical threshold (in Supplement statistical analysis plan, p 20–29).
4. Discussion
In an EHR-embedded, open-label, randomized trial of mAb for mild to moderate COVID-19, treatment of EHR-screen eligible patients increased 7.5-fold, particularly among historically and geographically underserved populations. OPTIMISE-C19 is proof of concept for a learning health system approach to knowledge generation, demonstrating that electronic integration of a trial into routine care, paired with a data warehouse and quality improvement initiative, can rapidly generate answers to key clinical questions. There was a 91% and 94% probability of inferiority of bamlanivimab respectively to bamlanivimab-etesevimab and casirivimab-imdevimab, and an 86% probability of equivalence between bamlanivimab-etesevimab and casirivimab-imdevimab. However, bamlanivimab and bamlanivimab-etesevimab use was stopped early, and the identified probabilities did not meet prespecified statistical triggers for conclusions of inferiority or equivalence.
Systematic inequities have exacerbated racial health disparities throughout the COVID-19 pandemic, and equitable access to treatments is crucial for population health [28]. This trial was able to increase access using a multifaceted approach: 1) infrastructure with infusion locations in all regions, 2) electronic screening of all patients within the system with a positive SARS-CoV-2 test followed by direct-to-patient phone calls, 3) paper referral forms for patients without access to an in-system provider, 4) community outreach, 5) a provider phone line for patients, and 6) home infusions for patients without transportation.
The finding of potential inferiority of bamlanivimab is similar to mechanistic studies suggesting a waning efficacy of bamlanivimab due to variants. It supports the FDA decision to revoke the bamlanivimab EUA [19]. A recent observational study reached a different conclusion and found similar effectiveness between bamlanivimab and casirivimab-imdevimab [28]. However, this study analyzed patients treated prior to widespread emergence of variants and U.S. federal decisions to halt bamlanivimab distribution.
SARS-CoV-2 variant epidemiology changes rapidly and this report addresses a period when Alpha was dominant, and Delta was not widespread. Notably, the U.S. federal government reinstated circulation of bamlanivimab-etesevimab on August 27, 2021 in areas where variant resistance to this mAb is ≤5%, based on in vitro data of activity against Delta, and lack of activity against the Beta, Gamma, Delta plus, and B.1.621 variants [29,30]. The similar effectiveness of bamlanivimab-etesevimab and casirivimab-imdevimab in the current trial supports this decision. Also aligning with these data is an observational study of 165 patients that found no difference in hospitalization or death between bamlanivimab-etesevimab and casirivimab-imdevimab in patients infected with Alpha, but worse outcomes with bamlanivimab-etesevimab in patients infected with Gamma [31].
OPTIMISE-C19 is designed to continuously compare all available mAb for COVID-19 and can stop mAb arms based on statistical triggers or external factors such as U.S. federal decisions. Had federal decisions not prompted unblinding, the internal action would have been to generate updated randomization proportions and continue enrollment. The trial continued to evaluate casirivimab-imdevimab and sotrovimab while preparing this report. Due to the August 27, 2021 federal decision to resume bamlanivimab-etesevimab, the trial resumed evaluation of bamlanivimab-etesevimab on September 16, 2021.
Trial strengths include capture of nearly all mAb treated patients from 49 sites across a large geographic region, enhancing generalizability. An advantage of Bayesian design is that any data, including data following unplanned cessation of a trial arm, can be analyzed and quantified as posterior probabilities, which is potentially more useful and is more quantitative than a frequentist conclusion of failure to reject a null hypothesis possibly because of lack of power. Lastly, the trial was embedded into usual care to enhance patient and provider engagement [13].
4.1. Limitations
A trial limitation is results are presented before any prespecified statistical trigger was reached. Nonetheless, to our knowledge, this trial represents the largest randomized comparative effectiveness data of mAb for COVID-19. Second, lack of patient-level variant data limited ability to assess comparative effectiveness relative to variant strains. Alpha was also the dominant variant during the trial. Using regional data as a surrogate for variant data in the Pennsylvania population, we found no difference in treatment effect over time. Third, we primarily relied on EHR data to capture death and hospitalization, and patients may have accessed care outside our health system after mAb treatment. We conducted patient calls and national death registry queries to address this concern. Fourth, the EHR eligibility screen identified most, but not all, EUA risk factors and could not identify if a patient was asymptomatic or severely ill. Also, despite significantly increased treatment of EHR-screen eligible patients during the trial, most did not receive mAb, underscoring how critical continued outreach and access efforts are. Finally, vaccination status was unable to be ascertained for this cohort which may impact effectiveness of monoclonal antibody therapy.
5. Conclusion
In non-hospitalized patients with mild to moderate COVID-19, bamlanivimab, compared to bamlanivimab-etesevimab and casirivimab-imdevimab, resulted in 91% and 94% probabilities of inferiority with regards to odds of improvement in hospital-free days within 28 days. There was an 86% probability of equivalence between bamlanivimab-etesevimab and casirivimab-imdevimab. Median hospital-free days to day 28 were 28 (IQR 28, 28) for each mAb. However, the trial was stopped early and no treatment met prespecified criteria for statistical equivalence, precluding definitive conclusions.
Contributors
All authors met the criteria for authorship set forth by the International Committee for Medical Editors. All authors were involved in drafting or critically revising the manuscript, and all authors approved the final version and are accountable for the accuracy and integrity of the manuscript. All authors had full access to all the data in the study and had final responsibility for the decision to submit for publication. At least two authors (EKM and DTH) have accessed and verified the data.
Data sharing
The study protocol and statistical analysis plan are provided in appendix 2. De-identified, participant data will be made available 1 year following publication, upon requests directed to the corresponding author; data access is subject to a methodologically sound proposal and the necessary data sharing agreements.
Declaration of Competing Interest
None of the authors have conflicts of interest to report.
Acknowledgements
The authors thank the clinical staff of the UPMC monoclonal antibody infusion centers as well as the support and administrative staff behind this effort, including but not limited to: Michelle Adam, Jodi Ayers, Ashley Beyerl, Trudy Bloomquist, Mikaela Bortot, Jonya Brooks, Sherry Casali, Jeana Colella, Jennifer Dueweke, Jesse Duff, Janice Dunsavage, Jessica Fesz, Kathleen Flinn, Daniel Gessel, Amy Helmuth, Erik Hernandez, Larry Hruska, Allison Hydzik, Le Ann Kaltenbaugh, LuAnn King, Jim Krosse, Sheila Kruman, Amy Lukanski, Hilary Maskiewicz, Katelyn Mayak, Rebecca Medva, Theresa Murillo, Teressa Polcha, Kevin Pruznak, Debra Rogers, Rozalyn Russell, Sarah Sakaluk, Heather Schaeffer, Robert Shulik, Libby Shumaker, Susan Spencer, Betsy Tedesco, Ken Trimmer, Jennifer Zabala, and their entire teams.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.cct.2022.106822.
Appendix A. Supplementary data
Supplementary material
References
- 1.Dougan M., Nirula A., Azizad M., et al. Bamlanivimab plus etesevimab in mild or moderate Covid-19. N. Engl. J. Med. July 14 2021;385:1382–1392. doi: 10.1056/NEJMoa2102685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Weinreich D.M., Sivapalasingam S., Norton T., et al. REGN-COV2, a neutralizing antibody cocktail, in outpatients with Covid-19. N. Engl. J. Med. January 21 2021;384:238–251. doi: 10.1056/NEJMoa2035002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bariola J.R., McCreary E.K., Wadas R.J., et al. Impact of bamlanivimab monoclonal antibody treatment on hospitalization and mortality among nonhospitalized adults with severe acute respiratory syndrome Coronavirus 2 infection. Open Forum Infect Dis. July 2021;8 doi: 10.1093/ofid/ofab254. ofab254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen P., Nirula A., Heller B., et al. SARS-CoV-2 neutralizing antibody LY-CoV555 in outpatients with Covid-19. N. Engl. J. Med. January 21 2021;384:229–237. doi: 10.1056/NEJMoa2029849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jiang S., Hillyer C., Du L. Neutralizing antibodies against SARS-CoV-2 and other human coronaviruses. Trends Immunol. May 2020;41:355–359. doi: 10.1016/j.it.2020.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Weinreich D.M., Sivapalasingam S., Norton T., et al. REGEN-COV antibody cocktail clinical outcomes study in Covid-19 outpatients. medRxiv. 2021 doi: 10.1101/2021.05.19.21257469. 2021.05.19.21257469. [DOI] [Google Scholar]
- 7.U.S. Food and Drug Administration Fact Sheet for Health Care Providers Emergency Use Authorization (EUA) of Bamlanivimab and Etesevimab. November 9, 2020. https://www.fda.gov/media/145802/download (accessed July 20, 2021)
- 8.U.S. Food and Drug Administration Fact sheet for Health Care Providers Emergency Use Authorization (EUA) of Bamlanivimab. November 9, 2021. https://www.fda.gov/media/143603/download (accessed July 20, 2021)
- 9.U.S. Food and Drug Administration Coronavirus (COVID-19) Update: FDA Authorizes Monoclonal Antibody for Treatment of COVID-19. November 9, 2020. https://bit.ly/2HesBBs (accessed July 20, 2021)
- 10.U.S. Food and Drug Administration Fact Sheet for Health Care Providers Emergency Use Authorization (EUA) of REGEN-COVTM. 2021. https://www.regeneron.com/downloads/treatment-covid19-eua-fact-sheet-for-hcp.pdf (accessed July 20, 2021)
- 11.LML Bernstein. The Washington Post; August 20, 2021. Monoclonal Antibodies are Free and Effective against COVID-19, but Few People are Getting Them.https://www.washingtonpost.com/health/covid-monoclonal-abbott/2021/08/19/a39a0b5e-0029-11ec-a664-4f6de3e17ff0_story.html (accessed August 28, 2021) [Google Scholar]
- 12.National Academies of Sciences, Engineering, and Medicine News Release Strategies to Allocate Scarce COVID-19 Monoclonal Antibody Treatments to Eligible Patients Examined in New Rapid Response to Government. January 29, 2021. https://www.nationalacademies.org/news/2021/01/strategies-to-allocate-scarce-COVID-19-monoclonal-antibody-treatments-to-eligible-patients-examined-in-new-rapid-response-to-government (accessed July 20, 2021)
- 13.Angus D.C. Optimizing the trade-off between learning and doing in a pandemic. JAMA. May 19 2020;323:1895–1896. doi: 10.1001/jama.2020.4984. [DOI] [PubMed] [Google Scholar]
- 14.Institute of Medicine Roundtable on Evidence-Based Medicine: The Learning Healthcare System Workshop Summary. July 2007. https://pubmed.ncbi.nlm.nih.gov/21452449/ (accessed July 20, 2021)
- 15.McCreary E.K., Bariola J.R., Minnier T., et al. Launching a comparative effectiveness adaptive platform trial of monoclonal antibodies for COVID-19 in 21 days. Contemp. Clin. Trials. Feb 2022:113. doi: 10.1016/j.cct.2021.106652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bariola J.R., EK McCreary, Khadem T., et al. Establishing a distribution network for COVID-19 monoclonal antibody therapy across a large health system during a global pandemic. Open Forum Infect Dis. July 2021;8 doi: 10.1093/ofid/ofab151. ofab151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huang D.T., McCreary E.K., Bariola J.R., et al. The UPMC OPTIMISE-C19 (OPtimizing Treatment and Impact of Monoclonal antIbodieS through Evaluation for COVID-19) trial: a structured summary of a study protocol for an open-label, pragmatic, comparative effectiveness platform trial with response-adaptive randomization. Trials. May 25 2021;22:363. doi: 10.1186/s13063-021-05316-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Angus D.C. Fusing randomized trials with big data: the key to self-learning health care systems? JAMA. August 25, 2015;314:767–768. doi: 10.1001/jama.2015.7762. [DOI] [PubMed] [Google Scholar]
- 19.U.S. Department of Health and Human Services Coronavirus (COVID-19) Update: FDA Revokes Emergency Use Authorization for Monoclonal Antibody Bamlanivimab. April 16, 2021. https://www.fda.gov/news-events/press-announcements/coronavirus-COVID-19-update-fda-revokes-emergency-use-authorization-monoclonal-antibody-bamlanivimab (accessed July 20, 2021)
- 20.Reitz K.M., Seymour C.W., Vates J., et al. Strategies to promote ResiliencY (SPRY): a randomised embedded multifactorial adaptative platform (REMAP) clinical trial protocol to study interventions to improve recovery after surgery in high-risk patients. BMJ Open. September 29 2020;10 doi: 10.1136/bmjopen-2020-037690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Christainsen S.L., Cintron M.Y., Desai A., et al. Oxford University Press; 2020. American Medical Association Manual of Style, 11th Edition, a Guide for Authors and Editors.https://www.amamanualofstyle.com (accessed September 3, 2021) [Google Scholar]
- 22.Centers for Medicare and Medicaid Services Meaningful Use Data; Public Use Files - CMS. https://www.cms.gov (accessed September 3, 2021)
- 23.University of Wisconsin School of Medicine Public Health 2015 Area Deprivation Index v2.0. 2015. https://www.neighborhoodatlas.medicine.wisc.edu/ (accessed August 1, 2021)
- 24.Kind A.J.H., Buckingham W.R. Making neighborhood-disadvantage metrics accessible - the neighborhood atlas. N. Engl. J. Med. June 28, 2018;378:2456–2458. doi: 10.1056/NEJMp1802313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Global Initiative on Sharing All Infuenza Data Tracking of variants. July 20, 2021. https://www.gisaid.org/ (accessed July 20, 2021)
- 26.Social Security Administration Social Security Master File of Social Security Number Holders and Applications: Death Information. https://www.ssa.gov/dataexchange/request_dmf.html (accessed August 1, 2021)
- 27.Zwarenstein M., Treweek S., Gagnier J.J., et al. Improving the reporting of pragmatic trials: an extension of the CONSORT statement. BMJ. Nov 11 2008;337 doi: 10.1136/bmj.a2390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ganesh R., Philpot L.M., Bierle D.M., et al. Real-world clinical outcomes of bamlanivimab and casirivimab-imdevimab among high-risk patients with mild to moderate Coronavirus disease 2019. J. Infect. Dis. July 19, 2021 doi: 10.1093/infdis/jiab377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Connor B.A., Couto-Rodriguez M., Barrows J.E., et al. Monoclonal antibody therapy in a vaccine breakthrough SARS-CoV-2 hospitalized Delta (B.1.617.2) variant case. Int. J. Infect. Dis. September 2021;110:232–234. doi: 10.1016/j.ijid.2021.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Planas D., Veyer D., Baidaliuk A., et al. Reduced sensitivity of SARS-CoV-2 variant Delta to antibody neutralization. Nature. August 2021;596:276–280. doi: 10.1038/s41586-021-03777-9. [DOI] [PubMed] [Google Scholar]
- 31.Falcone M., Tiseo G., Valoriani B., et al. Efficacy of bamlanivimab/etesevimab and casirivimab/imdevimab in preventing progression to severe COVID-19 and role of variants of concern. Infect. Dis. Ther. August 25, 2021:1–10. doi: 10.1007/s40121-021-00525-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material