Abstract
Background
Coronavirus disease-2019 (COVID-19) cholangiopathy is a recently known entity. There are very few reports of liver transplantation (LT) for COVID-19-induced cholangiopathy. It is well known that vaccines can prevent severe disease and improve outcomes. However, there are no reports on the impact of COVID-19 vaccines on cholestasis. Therefore, we aimed to compare the course and outcome of patients who developed cholestasis following COVID-19 infection among vaccinated and unvaccinated individuals. Methods: Patients diagnosed with post-COVID cholestasis during the pandemic were included in the study after excluding other causes of cholestasis.
Results
Eight unvaccinated and seven vaccinated individuals developed cholestasis following COVID-19 infection. Baseline demographics, presentation, severity, and management of COVID-19 were similar in both groups. However, patients in the unvaccinated group had a protracted course. The peak ALP was 312 (239–517) U/L in the vaccinated group and 571.5 (368–1058) U/L in the unvaccinated group (P = 0.02). Similarly, the peak γ-glutamyl transpeptidase values were lower in the vaccinated (325 [237–600] U/L) than in the unvaccinated group (832 [491–1640] U/L; P = 0.004). However, the peak values of total bilirubin, transaminases, and INR were similar in both groups. Five patients developed ascites gradually in the unvaccinated group whereas none in the vaccinated group developed ascites. Plasma exchange was done in five patients, and two were successfully bridged to living donor LT in the unvaccinated group. Only two patients recovered with conservative management in the unvaccinated group, whereas all recovered with conservative management in the vaccinated group. The other four patients in the unvaccinated group were planned for LT.
Conclusion
Post-COVID-19 cholestasis is associated with high morbidity and mortality, meriting early identification and appropriate management. Vaccination can modify the course of severe COVID-19 infection and improve outcomes.
Keywords: vaccination, liver function test, liver transplantation, plasma exchange
Abbreviations: ALP, alkaline phosphatase; AST, aspartate transaminase; ALT, alanine transaminase; COVID-19, coronavirus disease-2019; DDLT, deceased donor living transplantation; GGT, γ-glutamyl transpeptidase; LDLT, living donor liver transplantation; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; UDCA, ursodeoxycholic acid; ULN, upper limit of normal
Coronavirus disease-2019 (COVID-19) can have a myriad of symptoms. Furthermore, it is now known that most patients recovered from COVID-19 can have multiple symptoms (long COVID) attributed either to the persistent chronic inflammation in the convalescent phase, sequelae of critical illness, organ damage (lung injury resulting in pulmonary fibrosis), and non-specific effects from the hospitalization and social isolation (psychological trauma, nutritional anemia, muscle wasting).1,2 Liver injury is also common in COVID-19.3, 4, 5 Multiple factors lead to liver injury in COVID-19.3,6 Elevation in alkaline phosphatase (ALP) and γ-glutamyl transpeptidase (GGT) is less common in patients with COVID-19 at initial presentation noted in approximately 6–20% of patients.3 COVID-19-induced cholestasis (ALP ≥3 ULN [upper limit of normal]) is reported among 1% of severely infected COVID-19 patients at admission and is associated with a poor prognosis.7 Persistently elevated ALP and GGT are noted in 28% and 53% of patients, respectively, even after 2 months of COVID-19 infection.8 However, the data on the outcomes of these patients are still evolving.
COVID-19 cholangiopathy is a recently known entity.9,10 Roth et al. reported three patients developing features similar to secondary sclerosing cholangitis (SSC) in patients recovering from severe COVID-19 disease.9 Unfortunately, none of the patients had recovered completely. The last reported serum bilirubin and ALP in these patients ranged between 7.6 and 15.2 mg/dl and 1256 to 2018 IU/L, respectively.9 In addition, Durazo et al. reported successful liver transplantation (LT) for a similar patient developing end-stage liver disease following COVID-19 cholangiopathy.10 Currently, there is a lack of literature on the management of COVID-19 patients who develop chronic cholestasis after recovery. Furthermore, it is well known that vaccinations can prevent severe COVID-19 and improve outcomes.11,12 However, the effect of vaccines on COVID-19-induced cholestasis is unknown. Therefore, we aimed to compare the course and outcome of patients who developed cholestasis following COVID-19 infection among vaccinated and unvaccinated individuals. To the best of our knowledge, there are no studies comparing the outcomes of vaccinated and unvaccinated patients developing post-COVID-19 cholestasis.
Methods
Patients diagnosed with post-COVID-19 cholestasis during the pandemic were included in the study. COVID-19-induced cholestasis was defined as a rise in ALP by ≥ 1.5 times the ULN with serum bilirubin (≥2 ULN) and GGT (≥3 ULN) in the absence of active sepsis.13 Following tests were confirmed to be negative before labeling as COVID-19-induced cholestasis in all patients to exclude other causes of altered liver chemistries: viral hepatitis markers including hepatitis A (IgM), hepatitis B (surface antigen), hepatitis C (antibody), hepatitis E (IgM), dengue (IgM), Epstein–Barr virus (IgM), cytomegalovirus (DNA), and herpes simplex virus (RNA); autoimmune markers, including anti-nuclear antibody, anti-mitochondrial antibody, and a normal serum total immunoglobulin G (IgG). Serum procalcitonin <0.5 ng/mL and negative blood and urine cultures were also confirmed before labeling COVID-19 cholestasis. Magnetic resonance cholangiopancreatography (MRCP) was done in all patients to rule out any obstructive cause. The severity of COVID-19 was defined by Indian Council of Medical Research (ICMR) guidelines. Severe COVID-19 was defined as a respiratory rate >30/minute, dyspnea and/or SpO2 < 90% on room air. Moderate disease was defined as respiratory rate ≥24/minute, dyspnea and/or SpO2 between 90 and 93%. The first abnormal liver function test reported to the physician at our tertiary care center after COVID-19 illness, that is, from polymerase chain reaction (PCR) positivity, is mentioned here as first total bilirubin, first ALP, first aspartate transaminase (AST), alanine transaminase (ALT), and first GGT. The peak value reached during the course is mentioned here as peak values of abnormal liver chemistry.
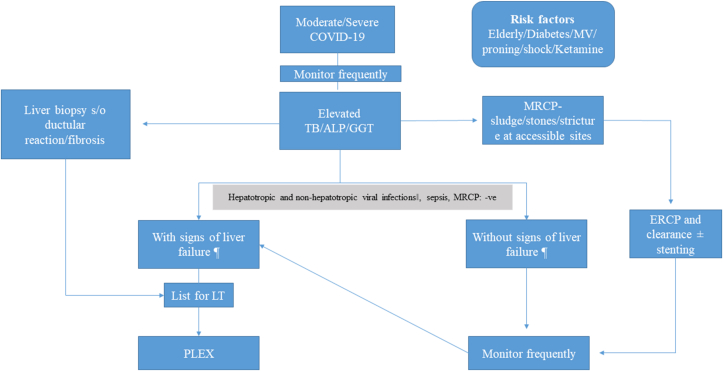
The management of COVID-19 cholestasis has been depicted in Figure 1. Ursodeoxycholic acid (UDCA) (15 mg/kg) was initiated in all cases. Steroid therapy was initiated when the enzyme elevation (>2 ULN) persisted three consecutive times, with liver biopsy demonstrating inflammatory infiltrates. Plasma exchange was offered for patients with serum bilirubin >20 mg/dl and INR >1.5 with or without pruritus. LT was suggested when the serum bilirubin and INR did not subside despite plasma exchange with or without the development of ascites/hepatic encephalopathy.
Figure 1.
Management of COVID-19-induced cholestasis. Hepatotropic viruses-hepatitis A IgM, hepatitis B surface antigen, anti-hepatitis C antibody, hepatitis E IgM. Non-hepatotropic viruses-cytomegalovirus deoxyribonucleic acid (DNA), Epstein–Barr virus IgM, herpes simplex virus ribonucleic acid (RNA), dengue (IgM or NS1). ¶signs of liver cell failure-INR >1.5 or development of ascites or encephalopathy.‖ Plasma exchange was offered for patients with serum bilirubin >20 mg/dl and INR >1.5 with or without pruritus.‡ LT was suggested when the serum bilirubin, and INR did not subside despite plasma exchange with or without the development of ascites/hepatic encephalopathy. Medical management included UDCA (15 mg/kg) and steroid therapy was initiated when the enzyme elevation (>2 ULN) persisted three consecutive times, with liver biopsy demonstrating inflammatory infiltrates. Footnotes: MV, mechanical ventilation; TB, total bilirubin; ALP, alkaline phosphatase; GGT-γ, glutamyl transpeptidase; MRCP, magnetic resonance cholangiopancreatography; ERCP, endoscopic retrograde cholangiopancreatography; INR, international normalized ratio; LT, liver transplantation; PLEX, plasma exchange.
Statistical Analysis
Continuous data have been expressed as median (range) and compared using Student's T-test, while categorical data have been expressed as n (%) and compared using Chi-square test or Fischer's exact test. Statistical tests have been performed using SPSS ver. 25.
Results
During the past year and half, 15 patients were diagnosed with post-COVID-19 cholestasis. Eight patients were unvaccinated (case #1–8), and seven were vaccinated (case #9–15). All except two in the vaccinated group were diagnosed with severe COVID-19 according to ICMR guidelines. The details of each case are mentioned in Supplementary Table 1 for the unvaccinated group and in Supplementary Table 2 for the vaccinated group. The median (lower limit – upper limit) age was 59 (24–67) years in the unvaccinated group versus 52 (29–67) in the vaccinated group (P = 0.38). Only two in the vaccinated group were females. The median time from vaccination to infection was 23 (14–34) days. Six patients after Covishield and one after Covaxin inoculation were diagnosed with Severe Acute Respiratory Syndrome Coronavirus-2 (SARS CoV-2) infection. A similar proportion of patients in each group had comorbidities. Four patients in the vaccinated group and five patients in the unvaccinated group had diabetes mellitus. Common symptoms of COVID-19 were fever, fatigue, dyspnea, and cough in both groups. More patients in the unvaccinated group (87.5%) required intensive care unit admission than in the vaccinated group (57.1%), although there was no statistical significance (P = 0.23). A higher proportion of patients in the unvaccinated group required invasive mechanical ventilation (37.5% vs. 14.3%; P = 0.56) while a higher proportion in the unvaccinated group required non-invasive ventilation (50% vs. 71.43%; P = 0.6). The median hospitalization duration was lower in the vaccinated group (12 days) than in the unvaccinated group (24 days; P = 0.11). Remdesivir, intravenous steroids, and antibiotics were administered for COVID-19 disease in all the patients. None of the patients received favipiravir. There was no history of any intake of complementary and alternative medicines. Only one patient was on vasopressor therapy in the unvaccinated group (case #4). Readmission and hospitalization duration was longer in the unvaccinated group (38 [21–124] days) than in the vaccinated group (9.5 [8–12] days; P = 0.04) (Table 1).
Table 1.
Baseline Characteristics and Outcomes of Unvaccinated and Vaccinated Patients Developing COVID-19-Induced Cholestasis.
| Variables | Unvaccinated (n = 8) | Vaccinated (n = 7) | P value |
|---|---|---|---|
| Age | 59 (24–67) | 52 (29–67) | 0.38 |
| Male | 8 (100%) | 5 (71.43%) | 0.2 |
| Comorbidities (n) | 0.33 | ||
| Diabetes mellitus | 5 | 4 | |
| Hypertension | 1 | 2 | |
| Hypothyroidism | 0 | 1 | |
| CAD | 1 | 0 | |
| None | 3 | 3 | |
| Fatty liver (in a prior ultrasonography) | 2 | 4 | 0.23 |
| Symptoms of COVID-19 | |||
| Fever | 7 | 7 | 0.53 |
| Cough | 5 | 2 | 0.21 |
| Fatigue | 4 | 3 | 0.6 |
| Dyspnea | 4 | 3 | 0.6 |
| Treatment received | |||
| Remdesivir | 8 (100%) | 6 (85.7%) | 0.67 |
| Antibiotics | 8 (100%) | 7 (100%) | – |
| Steroids | 8 (100%) | 6 (85.7%) | 0.67 |
| Vasopressor therapy | 1 (12.5%) | 0 | 0.53 |
| ICU care (n, %) | 7 (87.5%) | 4 (57.1%) | 0.23 |
| MV (n, %) | 3 (37.5%) | 1 (14.3%) | 0.56 |
| NIV (n,%) | 4 (50%) | 5 (71.43%) | 0.6 |
| Time from vaccination to infection (days) | – | 23 (14–34) | |
| Number of readmissions | 1.5 (0–3) | 1 (0–2) | 0.09 |
| Duration of hospitalization for COVID-19 (days) | 24 (17–124) | 12 (7–17) | 0.11 |
| Time to rise in ALP, GGT, and bilirubin after PCR positivity (days) | 39.5 (27–57) | 35 (19–44) | 0.15 |
| Duration of readmission (days) | 38 (21–124) | 9.5 (8–12) | 0.04 |
| Baseline TB (mg/dl) | 0.85 (0.7–1.6) | 1.2 (0.9–1.5) | 0.33 |
| Baseline AST (U/L) | 33.5 (23–54) | 36 (23–56) | 0.54 |
| Baseline ALT (U/L) | 39 (23–44) | 32 (20–55) | 0.62 |
| Baseline ALP (U/L) | 99.5 (87–132) | 114 (67–154) | 0.63 |
| Baseline GGT (U/L) | 34 (12–87) | 45 (33–88) | 0.36 |
| Baseline serum creatinine¶ | 0.96 ± 0.33 | 1.01 ± 0.25 | 0.78 |
| First TB (mg/dl) | 8.35 (3.3–26.1) | 12.4 (3.1–32.4) | 0.29 |
| First AST (U/L) | 117 (21–452) | 170 (33–1256) | 0.39 |
| First ALT (U/L) | 117 (35–896) | 106 (19–627) | 0.71 |
| First ALP (U/L) | 323 (217–383) | 312 (177–489) | 0.85 |
| First GGT (U/L) | 300.5 (230–570) | 312 (71–532) | 0.59 |
| Peak TB (mg/dl) | 22.95 (4.2–48.5) | 17 (8.3–32.4) | 0.23 |
| Peak AST (U/L) | 306 (83–757) | 199 (89–1256) | 0.71 |
| Peak ALT (U/L) | 250 (111–2190) | 134 (67–627) | 0.32 |
| Peak ALP (U/L) | 571.5 (368–1058) | 312 (239–517) | 0.02 |
| Peak GGT (U/L) | 832 (491–1640) | 325 (237–600) | 0.004 |
| Lowest albumin¶ (g/dl) | 2.83 ± 0.28 | 3.25 ± 0.32 | 0.02 |
| First INR¶ | 1.19 ± 0.21 | 1.4 ± 0.24 | 0.1 |
| Peak INR¶ | 1.62 ± 0.25 | 1.7 ± 0.22 | 0.6 |
Continuous data are expressed as median (minimum–maximum) except that marked ¶ which are expressed as mean ± standard deviation.
COVID-19, coronavirus disease-2019; CAD, coronary artery disease; ICU, intensive care unit; NIV, non-invasive ventilation; IV; invasive ventilation; ALP, alkaline phosphatase; GGT, γ glutamyl transpeptidase; PCR, polymerase chain reaction; TB, total bilirubin; AST, aspartate transaminase; ALT, alanine transaminase; INR, international normalized ratio.
Baseline liver function tests are the values at diagnosis or during the index COVID-19 admission.
The first abnormal test reported to physician at the tertiary care center after COVID-19 illness (from SARS-CoV-2 positivity) are depicted here as the first TB/AST/ALT/ALP/GGT.
The median time to the development of cholestasis was similar in both groups, that is, 35 (19–44) days and 39.5 (27–57) in the unvaccinated group (P = 0.15). All patients except cases 4 and 8 in the unvaccinated group (who were incidentally detected to have abnormal LFT) presented with complaints of jaundice and were diagnosed to have cholestasis. The first abnormal liver chemistries reported after COVID-19 PCR positivity was similar in both groups (Table 1). However, the peak values of ALP and GGT were lower in the vaccinated group than in the unvaccinated group. The peak ALP was 312 (239–517) U/L in the vaccinated group and 571.5 (368–1058) U/L in the unvaccinated group (P = 0.02). Similarly, the peak GGT values were lower in the vaccinated (325 [237–600] U/L) than in the unvaccinated group (832 [491–1640] U/L; P = 0.004). None of the patients had active sepsis during the peak values of ALP and GGT. Case #5 and #6 developed bacterial sepsis much later after recovery from COVID-19. However, the peak values of total bilirubin, AST, ALT, and INR were similar in both groups (Table 1).
Liver biopsy
Five patients underwent transjugular liver biopsy (#1–3, #6, and #7) in the unvaccinated group. Architectural distortion, fibrosis, cholestasis, and ductular reaction with ductopenia were noted in these patients. However, only two patients (Case #11 and 13) in the vaccinated group underwent liver biopsy, which demonstrated cholestasis with ductular reaction and mild lymphocytic infiltrates.
Management
Case #1 and #2 underwent high-volume plasma exchange, but the bilirubin did not normalize, and patients developed moderate ascites. Ascitic fluid analysis revealed high serum-ascites albumin gradient (SAAG), low protein, and normal leucocyte counts. Gastroscopy performed on both patients demonstrated mild portal hypertensive gastropathy. The trend of liver function tests in cases #1 and 2 are shown in Supplementary Table 3 and 4. After four months of PCR positivity, case #1 underwent living donor liver transplantation (LDLT). Similarly, case #2 underwent LDLT 5 months after COVID-19 diagnosis. There was no residual lung damage or fibrosis in both the patients on pre-operative computed tomographic scans of the chest. Post-operative recovery was uneventful. Both the patients were started on triple immunosuppression (steroids, mycophenolate mofetil, and tacrolimus). At last follow-up, that is, 5 (case #1) and eight months (case #2) post-transplant, both patients have normal liver function tests and have resumed work. The explant of case #1 demonstrated cholestasis, ductular reaction with ductopenia, and architectural distortion with the formation of incomplete nodules (Figure 2). The explant of case #2 revealed diffuse cholestasis with bile lakes, macrovesicular steatosis, and complete cirrhotic nodule formation (Figure 3). Prior ultrasonography at the time of COVID-19 diagnosis in cases #1 and 2 had revealed grade I fatty liver.
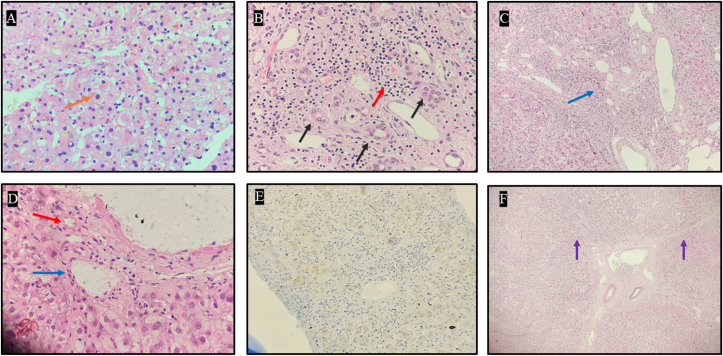
Figure 2.
Liver explant histopathology of case #1. A: Light microscopy (40x) using Hematoxylin and Eosin stain demonstrating intracytoplasmic cholestasis (orange arrow). B: Light microscopy (40x) using Hematoxylin and Eosin stain demonstrating bile ductular proliferation (black arrow) with moderate portal inflammation (red arrow). C: Light microscopy (20x) using Hematoxylin and Eosin stain demonstrating profound bile ductular reaction (blue arrow). D: Light microscopy (40x) using Hematoxylin and Eosin stain demonstrating portal tract with arteriole (red arrow) and vein (blue arrow) and absence of bile duct. E: Immunohistochemistry (20x) targeting cytokeratin (CK) 7 stain demonstrating lack of bile ducts. F: Light microscopy (20x) using Hematoxylin and Eosin stain demonstrating incomplete nodule formation surrounded by fibrous septae (purple arrow).
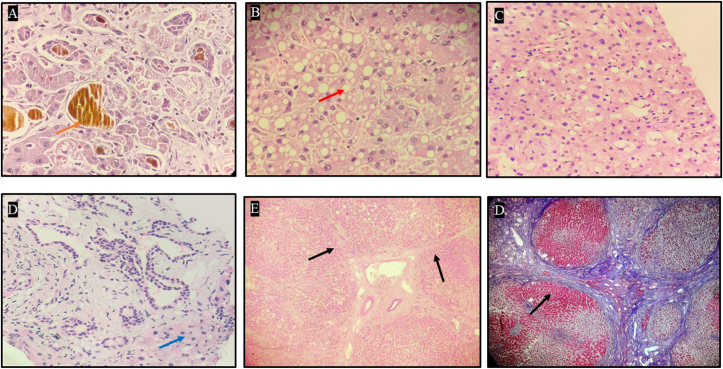
Figure 3.
Liver explant histopathology of case #2. A: Light microscopy (40x) using Hematoxylin and Eosin stain demonstrating intracytoplasmic and canalicular cholestasis with bile plugs (orange arrow: golden yellow pigment). B: Light microscopy (40x) using Hematoxylin and Eosin stain demonstrating macrovesicular steatosis (red arrow). C: Light microscopy (40x) using Hematoxylin and Eosin stain demonstrating the absence of lobular inflammation. D: Light microscopy (40x) using Hematoxylin and Eosin stain demonstrating minimal portal inflammation (blue arrow). E: Light microscopy (20x) using Hematoxylin and Eosin stain demonstrating architectural distortion with nodule formation surrounded by fibrous septae (black arrow). F: Light microscopy (20x) using Masson Trichrome stain demonstrating bridging fibrosis with complete nodule formation (black arrow).
Case #3 also developed mild ascites. However, he has no suitable donor and is unwilling for liver transplant. Therefore, he is being managed conservatively with 15 mg/kg/day of UDCA. His last serum bilirubin and INR are 19.7 mg/dl and 1.58, respectively, on day 208 (from PCR positivity) (Supplementary Table 5).
Case #4 had a peak serum bilirubin of 4.2 mg/dl and peak INR of 1.27 (Supplementary Table 6). He recovered with conservative management (UDCA 15 mg/kg/day) though he had a turbulent COVID-19 course requiring extracorporeal membrane oxygenation and prolonged hospitalization.
Case #5 had a peak serum bilirubin and INR of 4.6 mg/dl and 1.22, respectively. His bilirubin and ALP levels decreased with UDCA (15 mg/kg). However, he developed pulmonary mucormycosis, underwent lobectomy, and later succumbed to secondary bacterial sepsis on the 12th post-operative day. His last serum bilirubin was 1.2 mg/dl, and ALP was 176 U/L (Supplementary Table 7).
The bilirubin levels of case #6 reached a peak of 48.5 mg/dl. He underwent plasma exchange five times and was discharged when his serum bilirubin was 12.5 mg/dl. He was also started on 20 mg of prednisolone and tapered off in a week. Two weeks later, he was readmitted for severe septic shock secondary to urinary tract infection and succumbed while waiting for LT. His last serum bilirubin and INR were 27.8 mg/dl and 1.77, respectively (Supplementary Table 8).
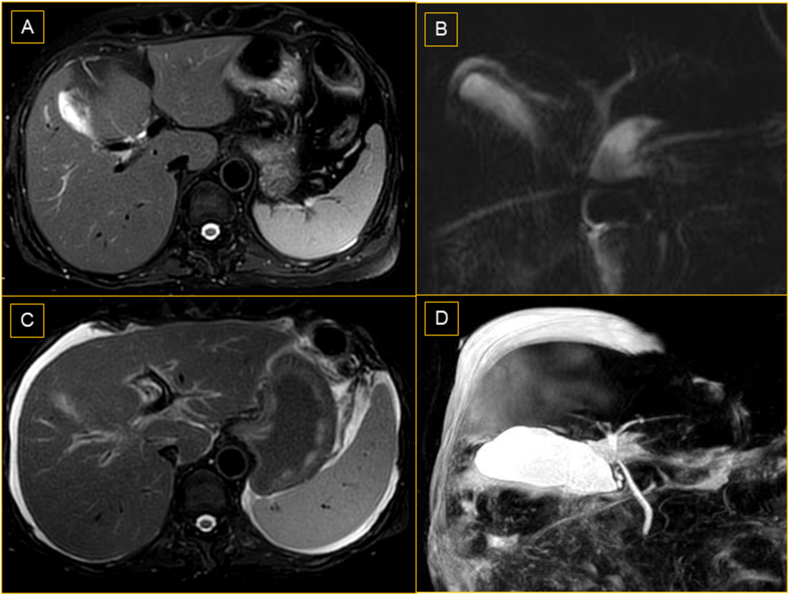
Case #7 continues to visit regularly. He underwent plasma exchange twice and had transiently stabilized post plasma exchange. He is also on high-dose UDCA (30 mg/kg). He has been listed for deceased donor LT (DDLT) as he has no suitable donor. The latest bilirubin and INR have peaked at 28.1 mg/dl and 1.77, respectively (Supplementary Table 9). He also has moderate ascites, which is high SAAG and low protein. He is on salt restriction and diuretics for the same. MRCP at diagnosis and at the last follow-up (day 317 from PCR positivity) did not reveal any biliary abnormalities (Figure 4).
Figure 4.
Magnetic resonance cholangiopancreatography (MRCP) of case #7. (A) T2 weighted imaging and (B) MRCP-3D Maximum intensity projection (MIP) images demonstrate normal biliary ducts at the initial presentation (C) T2 weighted imaging and (D) MRCP-3D MIP images demonstrated ascites with normal biliary ducts at the last follow-up.
Case #8 was referred during the 4th week of COVID-19 illness. His peak serum bilirubin and INR were 37.8 mg/dl and 1.7, respectively. He underwent plasma exchange thrice and was planned for LDLT. He was also started on high-dose UDCA (30 mg/kg). However, he developed pulmonary fibrosis and succumbed to bacteremia. His last serum bilirubin was 24.5 mg/dl.
On the contrary, none of the patients in the vaccinated group developed ascites (Case #9–15). UDCA was initiated in all patients at a dose of 15 mg/kg. In addition, three patients (#11, 13, and 15) also received a short course of oral prednisolone. The median time to normalization of liver function tests was 37 (29–57) days. Liver function tests of cases #9–15 have been tabulated in the supplementary material Tables 11–17.
Discussion
In this unique study, we report for the first time that vaccines can effectively prevent severe cholestasis and lead to better outcomes. This is the largest Indian series on COVID-19-induced cholestasis. Post-COVID-19 cholestasis has been reported in a few studies previously.9,10 Typically cholestasis occurs in the early disease course, and cholangiopathy develops later.14 In a retrospective study of approximately 2047 COVID-19 hospitalized patients, only 12 patients developed a cholangiopathy characterized by cholestasis and biliary tract abnormalities.15
In this case series, we report prolonged cholestasis following severe COVID-19 infection in the unvaccinated group. Two of the patients could undergo LT due to the development of ascites and lack of decline in bilirubin levels. Only two patients with preserved synthetic function (normal INR) recovered from cholestasis in the unvaccinated group. Conversely, although they had moderate to severe disease, vaccinated individuals recovered rapidly from cholestasis.
A total of eight studies have been published to date on post-COVID cholestasis7,9,10,15, 16, 17, 18, 19 (Table 2). Previous studies included patients with severe COVID-19 disease who developed diffuse intrahepatic strictures (beading appearance) similar to SSC of the critically ill patient (SSC–CIP). Diabetes mellitus was the most common comorbidity in patients who developed SSC-CIP.18 However, biliary tree abnormalities were not documented in all patients. Initial studies reported biliary tree abnormalities in 20–44% of patients. Only one study reported intrahepatic strictures in 92% (11/12) of patients hospitalized for severe COVID-19 disease. Furthermore, two studies have also reported persistently elevated ALP and GGT without biliary tract abnormalities in COVID-19 infected patients.7,8 In our series, only one patient (in unvaccinated group) is following up. He has no evidence of strictures (although his bilirubin is high) even after nearly a year of developing COVID-19 infection.
Table 2.
Published Literature on COVID-19-Induced Cholestasis.
| Serial no. | First author ref, month, year | N | Age/gender | Comorbidities | Complications during COVID-19 illness | Time to rise in ALP | MRCP | Liver biopsy | ERCP | Outcome |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Edwards et al.16 Nov. 2020, UK | 1 | 59Y/M | – | MV/bacterial sepsis/vasopressor | Time to elevation-15 days. Peak at 79 days (4000 U/L)) |
Beading of intrahepatic ducts | Not done | ERCP for sludge clearance | Alive ¶ ? LT after liver biopsy |
| 2 | Sadeghi et al.19 2020. Iran | 1 | 67Y/F | None | – | At admission (966 U/L). Peak at day 3 (1353 U/L) |
Normal | Diffuse cholestasis with fibrosis and inflammatory infiltrates | – | Alive |
| 3 | Roth et al.9 January 2021, USA. | 3 | Age: 25, 38, 40 Y Males: 2 |
1/3 had DM | MV/AKI/Vasopressor support-100%. RRT-2/3 Secondary bacterial infections-3/3 |
– | Beading, with multiple short segmental strictures and intervening dilatation in 1/3 (33%) patients | Ductopenia-2/3 patients. Ductular reaction, portal tract inflammation and portal and periportal fibrosis-100% |
ERCP for 2 patients-sludge and stone extracted | Alive ¶ |
| 4 | The Keta-Cov research group17∗ May 2021, France. |
5 | Median age: 59Y Males: 3 |
HTN-3 DM-2 HBV-2 |
MV/AKI/vasopressor support −100% All had received intravenous ketamine |
– | Strictures and dilatations of intrahepatic bile ducts in 1/5 (20%) patient | Cholangiolar proliferation, biliary plugs, portal inflammation, extensive biliary fibrosis, and cirrhosis | ERCP-1 patient for removal of bile duct cast. | Two died (one died while waiting for LT) |
| 5 | Butikofer et al.18 May 2021. Switzerland | 20/34 severe cases: 11—mild and 9— severe cholestasis.ǁ | Median age: 59Y Males: 16 |
DM-1 in mild and 7 in severe group. HTN-8 in mild and 7 in severe group. |
Vasopressors: mild: 100%; severe: 89%; and MV: 100% |
6–20 days after admission to ICU. | Diffuse irregularities of the bile ducts with dilatations and strictures—4/9 (44%) severe cholestasis patients | Portal inflammation with pericellular fibrosis. | UDCA for all four patients who developed SSC. 1—listed for LT (MELD-17) |
Four died in the mild group. Five died in the severe group. Four developed SSC. |
| 6 | Durazo et al.10 May 2021, USA | 1 | 47Y/M | HTN | MV AKI required RRT |
At day 58 ALP 1644 U/L. At day 81–2730 U/L |
Diffuse intrahepatic biliary stricture | Hilar bile duct injury with hepatic abscess | ERCP removal of choledocholithiasis. | LT on day 108 (MELD-37) |
| 7 | Faruqui et al.15 July 2021.USA | 12 | Mean age: 58Y Males: 11 |
DM: 4/12 HTN: 8/12 |
MV—100% Vasopressors—10/12. Thrombosis—8/12 Sepsis—12/12 All had received intravenous ketamine |
118 days | Beading of intrahepatic ducts in 11/12 (92%) | Fibrosis and ductular reaction in four patients. No ductopenia. | 4-ERCP | 4—died. 1—LDLT 7—alive. (1—listed for LT and 2 declined LT) |
| 8 | Da et al.7 August. 2021. USA | 72 | 65Y Males: 43 |
DM: 31/72 HTN: 41/72 CLD: 4/72 |
MV: 20.8% Vasopressor: 22.2% |
– | – | – | – | 33.3% (24/72)—died versus 19.2% (23/120) in the control group |
| 9 | Current study. India | 15 | Median age-56Y Males-13 |
DM-9/15 | MV + NIV-13/15 Vasopressors-1/15 |
36 days | Normal | Architectural distortion, fibrosis, cholestasis, and ductular reaction with ductopenia in unvaccinated group. Cholestasis and inflammation in vaccinated group | Plasma exchange- 5. Oral steroids- 4 |
2-died 2-LT 2-listed for LT 1-declined LT 2-recovered. All 7 in vaccinated group recovered |
COVID-19, coronavirus disease 2019; ALP, alkaline phosphatase; MRCP, magnetic resonance cholangiopancreatography, ERCP, endoscopic retrograde cholangiopancreatography; Y, years; MV, mechanical ventilation; LT, liver transplantation; DM, diabetes mellitus; AKI, acute kidney injury; RRT, renal replacement therapy; HTN, hypertension; HBV, hepatitis B virus; ICU, intensive care unit; UDCA, ursodeoxycholic acid; MELD, model for end-stage liver disease, NIV, non-invasive ventilation.
ǁ (ALP ≥1.5 ULN and GGT≥3 ULN with bilirubin≥ 2 ULN).
¶At last follow-up patients still had elevated bilirubin and ALP.
Ischemia due to hypoxia in mechanically ventilated patients, microthrombi in the peribiliary plexus vascular, and hypotension have been reported to lead to intrahepatic biliary strictures in severe COVID-19.9,18. In addition, toxic bile injury, secondary bacterial sepsis, and drugs such as ketamine are also implicated in the development of SSC-CIP.15, 16, 17 SARS-CoV-2 enters cells through the angiotensin-converting enzyme 2 (ACE2) receptor, which is predominantly expressed on cholangiocytes and cholangiocytes progenitor TROP2+ cells.3,6 Therefore, direct cholangiocyte injury can lead to cholestatic liver injury by SARS CoV-2. Favipiravir is also known to cause cholestatic liver injury; however, none of the patients in this series had a history of favipiravir intake.20
In contrast to the previous studies, we found no evidence of intrahepatic strictures or biliary tree abnormalities in our patients. Instead, all our patients developed cholestasis and progressive liver failure. Few of the anti-COVID-19 drugs (lopinavir, ritonavir, remdesivir) are known to inhibit hepatocyte transporters, including bile salt export pump, multidrug resistance-associated protein 4 (MRP4), and organic anion transporting polypeptide (OATP) 1B1/B3 that may lead to cholestasis.21 It is unknown whether COVID-19 can directly inhibit these hepatocyte transporters and lead to cholestasis without affecting the biliary anatomy. Interleukin (IL)-6, the main driver of the cytokine storm in COVID-19, can also lead to cholestasis.22 Furthermore, given the severity of the disease and rapid progression, we could not repeat MRCP in many patients, which may have missed the strictures in the unvaccinated group.
The liver biopsy features in our series are similar to the previously reported series on post-COVID cholestasis.9,15,17 Roth et al. reported mild to moderate bile duct paucity in 2 out of 3 patients.9 The causes of vanishing bile duct syndrome (VBDS) are multiple. Drugs are also known to cause VBDS. We cannot conclusively determine the cause; however, we assume VBD in these patients may be due to the virus. Direct cholangiocyte injury through ACE-2 receptors may be the reason for the destruction of bile ducts. Furthermore, lack of pruritus, eosinophilia, immunoallergic features, and a lack of intake of commonly implicated notorious drugs, including amoxicillin-clavulanate, herbal and dietary supplements, and macrolides are features against drug-induced VBDS. Conversely, in the vaccinated group, there was no evidence of VBDS.
SARS CoV-2 can lead to the development of acute-on-chronic liver failure (ACLF), meriting LT in patients with cirrhosis.23, 24, 25 It may be argued that diabetes mellitus may have contributed to pre-existing fibrosis/liver disease in these patients, and COVID-19 led to the development of liver failure. However, in our series, patients gradually progressed over 2–3 months to develop ascites compared with patients who developed ACLF at presentation or during the initial hospital stay for COVID-19.24,26 Furthermore, the presence of ductular reaction, ductopenia, and fibrosis with a cholestatic pattern of liver injury is a feature suggestive of progression to biliary cirrhosis rather than the development of ACLF.
Plasma exchange has a proven role in cholestatic liver diseases.27 Previous studies have reported the use of plasma exchange for patients with post-COVID-19 cholestasis.15,19 The mechanism by which plasma exchange aids in recovery is unclear. However, we speculate that the removal of cytokines and reduction in hyperbilirubinemia plays a significant role in the resolution of cholestasis.27,28 Further studies should also evaluate cytokine profile of these patients pre- and post-plasma exchange in COVID-19 cholestasis.
The major limitation of our retrospective study is the small sample size and lack of a comparison cohort of severe COVID-19 patients who did not develop cholestasis. The severity of COVID-19 was lesser in the vaccinated group than in the unvaccinated group although the P-value was not significant. Lesser severity of COVID-19 may have led to better outcomes in vaccinated group. Furthermore, it may be argued that the cause of cholestasis is different in each group, which is challenging to demonstrate in this study. Secondary sepsis is frequent in COVID-19 and can present with cholestatic features; however, we ruled out sepsis at inclusion. Pro-cholestatic polymorphisms in hepatocyte transporters ATP-binding-cassette (ABC) B4/B11 can lead to prolonged cholestatic features in acute viral hepatitis.29 Whether genetic polymorphisms in hepatocyte transporters predispose certain individuals to prolonged cholestasis in COVID-19 needs to be assessed in further studies. In conclusion, COVID-19-induced cholestasis, although uncommon, is associated with high morbidity and mortality, meriting early identification and appropriate management. Vaccination can reduce the severity of COVID-19 and alter the course of COVID-19-induced cholestasis, reaffirming that vaccination is a must for all.
Credit authorship contribution statement
Study concept and design by AVK and PNR; Data collection by AVK, RR, ST and BAG; Figures by ASK, AS, AS, JR and MS; critical inputs by BM, DNR and PNR.
Conflicts of interest
None.
Acknowledgements
None
FUNDING
None.
Informed consent
Obtained.
Article guarantor
Dr. Anand V Kulkarni and Prof. P N Rao.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jceh.2022.06.004.
Appendix ASupplementary data
The following is the supplementary data to this article:
References
- 1.Halpin S.J., McIvor C., Whyatt G., et al. Postdischarge symptoms and rehabilitation needs in survivors of COVID-19 infection: a cross-sectional evaluation. J Med Virol. 2021;93:1013–1022. doi: 10.1002/jmv.26368. [DOI] [PubMed] [Google Scholar]
- 2.Rao G.V., Gella V., Radhakrishna M., et al. Post-COVID-19 symptoms are not uncommon among recovered patients-a cross-sectional online survey among the Indian population. medRxiv. 2021:2021. doi: 10.1101/2021.07.15.21260234. 2007.2015.21260234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kulkarni A.V., Kumar P., Tevethia H.V., et al. Systematic review with meta-analysis: liver manifestations and outcomes in COVID-19. Aliment Pharmacol Ther. 2020;52:584–599. doi: 10.1111/apt.15916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kulkarni A.V., Tevethia H.V., Premkumar M., et al. Impact of COVID-19 on liver transplant recipients-a systematic review and meta-analysis. EClinicalMedicine. 2021;38 doi: 10.1016/j.eclinm.2021.101025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kumar K., Kulkarni A. Elevated liver chemistries in COVID-19-is it not a concern? Am J Gastroenterol. 2021;116:424. doi: 10.14309/ajg.0000000000000914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kumar P., Sharma M., Kulkarni A., Rao P.N. Pathogenesis of liver injury in coronavirus disease 2019. J Clin Exp Hepatol. 2020;10:641–642. doi: 10.1016/j.jceh.2020.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Da B.L., Suchman K., Roth N., et al. Cholestatic liver injury in COVID-19 is a rare and distinct entity and is associated with increased mortality. J Intern Med. 2021;290:470–472. doi: 10.1111/joim.13292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nayagam J.S., Jeyaraj R., Mitchell T., et al. Patterns and prediction of liver injury with persistent cholestasis in survivors of severe SARS-CoV-2 infection. J Infect. 2021;82:e11–e13. doi: 10.1016/j.jinf.2021.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Roth N.C., Kim A., Vitkovski T., et al. Post-COVID-19 cholangiopathy: a novel entity. Am J Gastroenterol. 2021;116:1077–1082. doi: 10.14309/ajg.0000000000001154. [DOI] [PubMed] [Google Scholar]
- 10.Durazo F.A., Nicholas A.A., Mahaffey J.J., et al. Post-COVID-19 cholangiopathy-a new indication for liver transplantation: a case report. Transplant Proc. 2021;53:1132–1137. doi: 10.1016/j.transproceed.2021.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jagadeesh Kumar V., Sowpati D.T., Munigela A., et al. Clinical outcomes in vaccinated individuals hospitalized with delta variant of SARS-CoV-2. medRxiv. 2021 doi: 10.1101/2021.07.13.21260417. 2021.2007.2013.21260417. [DOI] [Google Scholar]
- 12.Moon A.M., Webb G.J., García-Juárez I., et al. SARS-CoV-2 infections among patients with liver disease and liver transplantation who received COVID-19 vaccination. Hepatol Commun. 2022;6(4):889–897. doi: 10.1002/hep4.1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.EASL clinical practice guidelines: management of cholestatic liver diseases. J Hepatol. 2009;51:237–267. doi: 10.1016/j.jhep.2009.04.009. [DOI] [PubMed] [Google Scholar]
- 14.Fix O.K., Blumberg E.A., Chang K.M., et al. American association for the study of liver diseases expert panel consensus statement: vaccines to prevent coronavirus disease 2019 infection in patients with liver disease. Updated: v10.0 released november 2, 2021. Hepatology. 2021;74:1049–1064. doi: 10.1002/hep.31751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Faruqui S., Okoli F.C., Olsen S.K., et al. Cholangiopathy after severe COVID-19: clinical features and prognostic implications. Am J Gastroenterol. 2021;116:1414–1425. doi: 10.14309/ajg.0000000000001264. [DOI] [PubMed] [Google Scholar]
- 16.Edwards K., Allison M., Ghuman S. Secondary sclerosing cholangitis in critically ill patients: a rare disease precipitated by severe SARS-CoV-2 infection. BMJ Case Rep. 2020;13 doi: 10.1136/bcr-2020-237984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Intravenous ketamine and progressive cholangiopathy in COVID-19 patients. J Hepatol. 2021;74:1243–1244. doi: 10.1016/j.jhep.2021.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bütikofer S., Lenggenhager D., Wendel Garcia P.D., et al. Secondary sclerosing cholangitis as cause of persistent jaundice in patients with severe COVID-19. Liver Int. 2021;41(10):2404–2417. doi: 10.1111/liv.14971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sadeghi A., Dooghaie Moghadam A., Eslami P., Pirsalehi A., Salari S., Roshandel E. Vasculopathy-related cutaneous lesions and intrahepatic cholestasis as synchronous manifestations in a COVID-19 patient; a case report. Gastroenterol Hepatol Bed Bench. 2020;13:400–404. [PMC free article] [PubMed] [Google Scholar]
- 20.Kumar P., Kulkarni A., Sharma M., Rao P.N., Reddy D.N. Favipiravir-induced liver injury in patients with coronavirus disease 2019. J Clin Transl Hepatol. 2021;9:276–278. doi: 10.14218/JCTH.2021.00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ambrus C., É Bakos, Sarkadi B., Özvegy-Laczka C., Telbisz Á. Interactions of anti-COVID-19 drug candidates with hepatic transporters may cause liver toxicity and affect pharmacokinetics. Sci Rep. 2021;11 doi: 10.1038/s41598-021-97160-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Satiya J., Lenik J.M., Bergasa N.V. S2382 COVID-19 and cholestasis: potential role of interleukin-6. Am J Gastroenterol. 2020;115:S1267. [Google Scholar]
- 23.Kulkarni A.V., Parthasarathy K., Kumar P., et al. Early liver transplantation after COVID-19 infection: the first report. Am J Transplant. 2021;21:2279–2284. doi: 10.1111/ajt.16509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kumar P., Sharma M., Sulthana S.F., Kulkarni A., Rao P.N., Reddy D.N. Severe acute respiratory syndrome coronavirus 2-related acute-on-chronic liver failure. J Clin Exp Hepatol. 2021;11:404–406. doi: 10.1016/j.jceh.2020.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kulkarni A.V., Vasireddy S., Sharma M., Reddy N.D., Padaki N.R. COVID-19 masquerading as autoimmune hepatitis (AIH) flare-the first report. J Clin Exp Hepatol. 2022;12:241–243. doi: 10.1016/j.jceh.2021.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Satapathy S.K., Roth N.C., Kvasnovsky C., et al. Risk factors and outcomes for acute-on-chronic liver failure in COVID-19: a large multi-center observational cohort study. Hepatol Int. 2021;15:766–779. doi: 10.1007/s12072-021-10181-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Padmanabhan A., Connelly-Smith L., Aqui N., et al. Guidelines on the use of therapeutic apheresis in clinical practice - evidence-based approach from the writing committee of the American society for apheresis: the eighth special issue. J Clin Apher. 2019;34:171–354. doi: 10.1002/jca.21705. [DOI] [PubMed] [Google Scholar]
- 28.Keklik M., Sivgin S., Kaynar L., et al. Treatment with plasma exchange may serve benefical effect in patients with severe hyperbilirubinemia: a single center experience. Transfus Apher Sci. 2013;48:323–326. doi: 10.1016/j.transci.2013.04.009. [DOI] [PubMed] [Google Scholar]
- 29.Krawczyk M., Grünhage F., Langhirt M., Bohle R.M., Lammert F. Prolonged cholestasis triggered by hepatitis A virus infection and variants of the hepatocanalicular phospholipid and bile salt transporters. Ann Hepatol. 2012;11:710–714. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.