Abstract
Background
Reversible splenial lesion syndrome (RESLES) is characterized by a temporary lesion in the splenium of the corpus callosum. RESLES is one of the most common causes of Mild encephalitis/encephalopathy reversible splenial lesion (MERS) and a rare clinical syndrome for the pediatric population. In a limited number of pediatric case reports, association with SARS-COV-2 in was reported. We aimed to increase the awareness of neurological involvement and treatment options of RESLES in children diagnosed with MIS-C.
Case presentation
We report two cases with a diagnosis of multisystem inflammatory syndrome-children who developed RESLES during the disease course. Fever, blurred vision, ataxia and encephalopathy were the main central nervous system symptoms. In our first case, we observed a rapid recovery in clinical symptoms and complete resolution of the splenial lesion in with intravenous immunoglobulin (IVIG) and methylprednisolone treatment. However, our second case did not respond to IVIG and methylprednisolone treatment. We performed therapeutic plasma exchange therapy and observed a successful recovery both in brain magnetic resonance imaging and echocardiographic findings.
Conclusion
Although IVIG and methylprednisolone are the first choice treatment methods in MIS-C cases progressing with RESLES, therapeutic plasma exchange may be an option for the treatment of unresponsive cases.
Keywords: RESLES, COVID-19, Therapeutic plasma exchange
1. Introduction
The novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), which causes coronavirus disease 2019 (COVID-19) was declared as a pandemic on 1 March 2020, by World Health Organization [1]. Centers for Disease Control and Prevention described the multisystem inflammatory syndrome in children (MIS-C) who was diagnosed after the SARS-CoV-2 pandemic and showed evidence of dysregulated immune response against the virus, causing cardiac involvement and clinical course similar to Kawasaki disease [2]. Various neurological symptoms have been reported both in the clinical course of COVID-19 in adults and in children diagnosed with MIS-C [3], [4], [5]. In some cases of MIS-C, encephalitis/encephalopathy, and splenial involvements are observed in cranial parenchymal imaging [5].
Reversible splenial lesion syndrome (RESLES) is a rare clinical-radiological syndrome involving the splenium of the corpus callosum and is frequently related to infections, epilepsy, and metabolic or electrolyte disturbance [6]. Mild encephalitis/encephalopathy reversible splenial lesion (MERS) is a viral infection-related radiologically isolated syndrome among RESLES [7]. Cases of RESLES have been reported in acute or subacute encephalitis/encephalopathy secondary to antiepileptic drug toxicity, early discontinuation of antiepileptic drugs, high altitude cerebral edema, hypoglycemia, hyponatremia, or hypernatremia. The reversibility of lesions in RESLES cases examined in the literature has been accepted as an indicator of good prognosis [6], [7].
Therapeutic plasma exchange (TPE) is an extracorporeal treatment in which plasma is separated from the cellular components of the blood to remove pathological substances such as autoantibodies, immunocomplexes, cryoglobulins, endotoxins, cytokines, or other substances. and then exchanged with replacement fluids [8].
Herein we aimed to describe the clinical and radiological characteristics of two pediatric patients who developed splenial infarction associated with COVID19 infection and were treated with intravenous immunoglobulin (IVIG), methylprednisolone, and TPE.
2. Case 1
A 14-year-old male patient, who was previously healthy, was admitted to our pediatric emergency service with complaints of fever, chills, ataxia, and hallucinations that started the day before. He had a history of SARS-COV-2 infection and had a fever for 3 days. He was conscious, agitated, and weak on admission to the pediatric emergency department. Following the tachypnea of the patient on physical examination at admission, thorax computerized tomography (CT) imaging was performed and bilateral peripheral patched ground densities in both lung parenchymal areas were observed. The patient was referred to the pediatric intensive care unit (PICU) with the diagnosis of MIS-C. After monitoring, tachypnea and tachycardia were observed and capillary refill time was prolonged (>3s). Elevated C-reactive protein (CRP), procalcitonin, ferritin, fibrinogen, and D-dimer levels were detected in blood sample tests ( Table 1). Due to the chest CT findings and elevated acute phase reactants, a single dose of ceftriaxone was administered to the patient after three intravenous saline loading. During the follow-up, the patient was normotensive, and his tachypnea and tachycardia regressed. Neurological examination showed no signs of meningeal irritation, there was no neurological deficit and cranial nerve examination was normal. The nasopharyngeal swab test that was taken at admission for SARS CoV-2 reverse transcription-polymerase chain reaction (RT-PCR) was negative. The test sample was sent for COVID Immunoglobulin M (IgM) and Immunoglobulin G (IgG) antibodies. He had no need for respiratory and inotropic support. Contrast-enhanced cranial magnetic resonance imaging (MRI) was performed and a nonspecific lesion in the splenium of the corpus callosum which was showing millimetric diffusion restriction was observed. An acute lesion in the splenium of the corpus callosum was observed as hyperintense areas specific for type 1 MERS in the T2 sequence, and hypointense areas in the T1 sequence. Echocardiography was normal. Anti-SARS CoV-2 IgM and IgG tests were positive and supported our MIS-C diagnosis. IVIG (2 gr/kg) and methylprednisolone (2 mg/kg/day) treatment was initiated. We observed complete regression in neurological symptoms and splenium lesion in the control cranial MRI scan, which was performed on the 7th day of his hospitalization. Steroid treatment was gradually ceased and he was discharged on the 10th day of hospitalization without any neurologic sequelae.
Table 1.
Laboratory results of the cases.
| Blood analysis | C1 | C2 | Blood analysis | C1 | C2 |
|---|---|---|---|---|---|
| Hemoglobin (g/dL) | 12 | 12.1 | Phosphorus (mg/dL) | 3.7 | 2.43 |
| Hematocrit (%) | 34.8 | 35.5 | Potassium (mmol/L) | 4.77 | 3.73 |
| White blood cell count (103/uL) | 9.71 | 16.84 | Sodium (mmol/L) | 135 | 128 |
| Neutrophil count (103/uL) | 7.58 | 15.6 | CRP (mg/L) | 250.3 | 80.13 |
| Lymphocyte count (103/uL) | 1.21 | 0.49 | Procalcitonin (µg/L) | 1.07 | 13.24 |
| Thrombocyte count (103/uL) | 173 | 117 | Ferritin (mcg/L) | 357.8 | 1311 |
| ALT (IU/L) | 12 | 13.7 | APTT (sec) | 37.3 | 36.7 |
| AST (IU/L) | 20 | 16 | INR (sec) | 1.87 | 1.36 |
| GGT (IU/L) | 12 | 10 | Fibrinogen (mg/dL) | 724 | 633 |
| Albumin (g/L) | 34.2 | 30.5 | HS-Troponin T (ng/L) | 4 | 71.94 |
| Total protein (g/dL) | 44.8 | 4.48 | Creatinine kinase (IU/L) | 86 | 80 |
| Sedimentation (mm/h) | 11 | 117 | Pro-BNP (ng/L) | 1810 | 7326 |
| Bilirubin, total (mg/dL) | 0.51 | 0.51 | LDH (IU/L) | 425 | 286 |
| Bilirubin, direct (mg/dL) | 0.24 | 0.24 | Blood Gas Analysis | ||
| Creatinine (mg/dL) | 0.91 | 0.75 | pH | 7.56 | 7.56 |
| Urea (mg/dL) | 38 | 50 | pCO2 | 28.1 | 28.1 |
| Triglyceride (mg/dl) | 240.3 | pO2 | 73.4 | 73.4 | |
| Calcium (mg/dL) | 9 | 7.95 | Lactate | 3.49 | 3.49 |
C1: Case 1, C2: Case 2, ALT: Alanine aminotransferase, AST: Aspartate aminotransferase, CRP: C-reactive protein, APTT: Activated partial thromboplastin time, INR: International normalized ratio, BNP: Brain natriuretic peptide, LDH: Lactate dehydrogenase, pCO2: Partial pressure of carbon dioxide, pO2: Partial pressure of oxygen
3. Case 2
A 15-year-old previously healthy male patient was admitted to our pediatric emergency service with complaints of fever, headache, dizziness, blurred vision, agitation, vomiting, and diarrhea for five days. On admission to the pediatric emergency department, he was conscious and the vital signs monitoring showed tachycardia without any other disturbances. Physical examination was significant for cervical lymphadenopathy, diffuse maculopapular rash, and non-purulent conjunctivitis. Laboratory examinations revealed lymphopenia, elevated troponin, elevated D-dimer, elevated pro-BNP, elevated CRP, and procalcitonin levels (Table 1). Other biochemical tests and blood gas analyses were normal. He was diagnosed as MIS-C according to the CDC criteria and hospitalized with positive SARS-CoV-2 antibody test results. Due to the presence of high acute phase reactants, antibiotherapy with cefotaxime and teicoplanin in conjunction with the intravenous immunoglobulin (IVIG,2 g/kg) and methylprednisolone (2 mg/kg/day) was administered. During the clinical course, he had worsening of blurred vision and later became unconscious. He was admitted to the PICU due to his poor general condition. Neurological examination showed no signs of meningeal irritation. Nasopharyngeal SARS CoV-2 RT-PCR was negative. There was no requirement for supplemental oxygen, mechanical ventilation, and inotropic support. Contrast-enhanced cranial MRI showed a nonspecific focus in the corpus callosum splenium showing millimetric diffusion restriction. Additionally, an acute lesion in the corpus callosum splenium was observed in hyperintense areas specific to type 1 MERS in the T2 sequence, and hypointense areas in the T1 sequence. Echocardiography was performed and dilatation in the left coronary artery (5 mm, z score: +3,2) was detected. Although 48 h have passed since the patient's IVIG and steroid treatments that were given in the emergency department, his neurological findings worsened and coronary dilatation was observed on echocardiography. Due to the patient's high erythrocyte sedimentation rate and procalcitonin levels, it was decided to apply TPE treatment instead of pulse steroid treatment in the next step. TPE was performed with the Prismaflex® (Baxter, USA) system, and TPE 2000 sets were used. The amount of plasma (2500 ml) was calculated as follows: estimated plasma volume (L) = 0.065 × weight (kg) × (1 − hematocrit). In our country, since albumin can be obtained with a drug report, it is more difficult to obtain and more expensive, so we used fresh frozen plasma as the replacement fluid. Saline 0.9 % was used to prime the TPE circuit. During the TPE procedures, blood flow was adjusted to 140 ml/min (2 ml//kg/min). Vital signs were thoroughly monitored during the TPE procedure. Control blood samples were taken immediately before and after TPE. Neither blood products nor electrolyte replacement was needed. Five sessions of TPE treatment were carried out every other day. We observed that the laboratory parameters improved and abnormal findings resolved in the control cranial MRI and echocardiography, performed on hospital day 10. Steroid treatment was tapered and stopped. He was discharged on hospital day 14 with complete recovery in neurological symptoms.
4. Discussion
RESLES is a rare clinical syndrome for the pediatric population and one of the most common causes of MERS. It frequently affects children and young adults. Viral infections have often been blamed for disease pathogenesis in the literature [6], [9]. Previously it was reported that RESLES was the most common neuroradiological finding among 45 patients who were diagnosed with MIS-C [10]. In this report, the diagnosis of MIS-C-associated RESLES secondary to SARS CoV-2 infection was established with anti-SARS-CoV-2 IgG and IgM antibody positivity, clinical and radiological findings. In a limited number of pediatric case reports encephalopathy was reported as the most common clinical symptom in patients diagnosed with SARS-COV-2 related RESLES [5]. In accordance with the literature, the main neurological clinical symptoms indicating encephalopathy were agitation and hallucinations in our cases.
While lesions in MERS may be limited to the splenium of corpus callosum (type I) in cranial MRI, they may extend to the frontotemporal area, white matter, and even the cerebellum (type II). As complete recovery without sequelae usually occurs rapidly in type 1 involvement, both radiological and neurological findings persist in type II involvement [9].
A previous study reported a complete recovery of neurological disturbances and resolution of the splenial lesion in an adult patient with MERS related to SARS CoV-2 infection without any immunomodulatory therapy [11]. The recovery time in the clinical course was reported as approximately 1 week without any treatment in adult patients [4]. On the other hand, children who developed MERS as a neurological complication of Kawasaki disease have been reported in the literature, and it is thought that the pathophysiological mechanisms are similar in cases with MERS in the background of MIS-C [12]. In an article from our country, it was reported that clinical and radiological complete recovery was achieved with IVIG and steroid treatments in 2 cases of RESLES developing on the basis of MIS-C [13].
In pediatric patients, as there is no sufficient evidence, some studies suggested that TPE may take a part in the treatment algorithm of the multisystem inflammatory syndrome in children (MIS-C), which may present with Kawasaki-like symptoms, toxic shock syndrome symptoms, and signs of secondary hemophagocytic lymphohistiocytosis (HLH)/macrophage activation syndrome (MAS) [14]. In another study from Turkey, it was reported that TPE was applied in 10 of the 21 patients with MIS-C diagnosis in the pediatric intensive care unit, who were resistant to other treatments and an improvement both in clinical and laboratory findings was observed with TPE treatment [15]. Despite all these articles, CDC does not have a definite recommendation regarding the use of TPE in the treatment of MIS-C.
In the literature, we did not find any case in which TPE treatment was used in patients with MIS-C-related RESLES. In addition, a single case of RESLES treated with TPE has been reported in the literature, which is associated with TTP and was discharged without sequelae after TPE treatment [16]. In our first case, both clinical and radiological findings and rapid response to treatment were similar to RESLES cases seen in the course of Kawasaki disease. However, in our second case with coronary dilatation, when there was no clinical improvement despite steroid and IVIG treatments, TPE treatment was used and both clinical and laboratory improvement was achieved with the TPE treatment. In our opinion, our second case is the first case report in the literature with a splenial lesion associated with MIS-C and successfully treated with TPE. ( Fig. 1, Fig. 2).
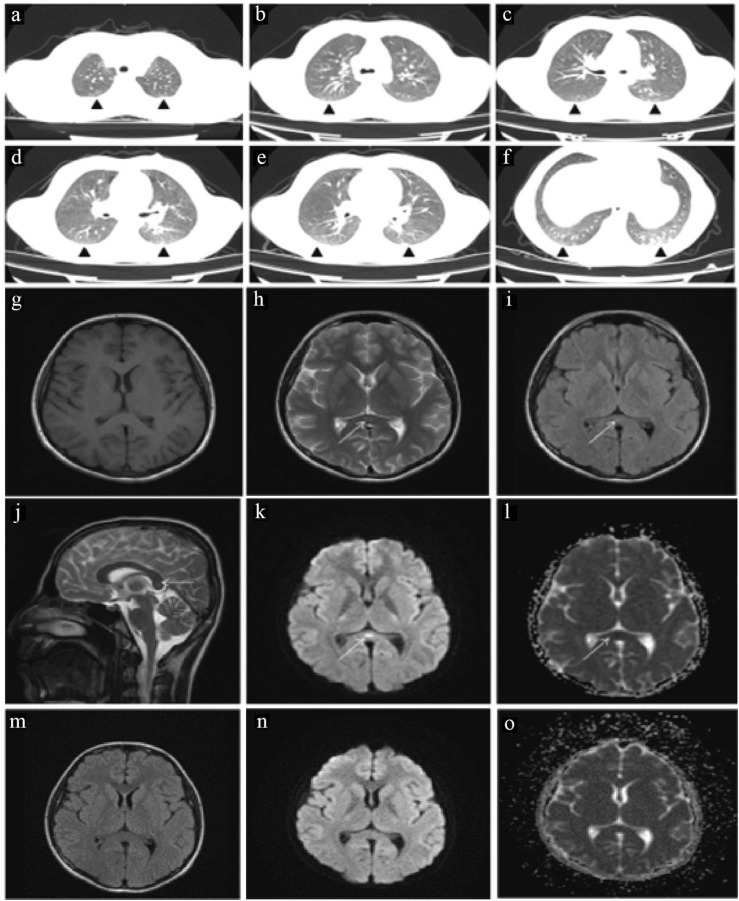
Fig. 1.
: Thorax CT and Cranial MRI images of the first case. (a-f) Bilateral peripheral patched ground glass densities in both lung parenchymal areas and subpleural striations in the left lung lower lobe superior and posterobasal segments. (g) In the axial plane not selectable on T1W images (h) T2W (I) FLAIR (J) Sagittal T2W images slightly hyperintense, shown with an arrow in the corpus callosum splenium, the same in axial (k) b1000 weighted series and (l) DAC maps. Focus causing diffusion restriction indicated by arrow sign in localization. (m,n,o) In the axial plane obtained after one week of treatment, it was observed that the previously described diffusion-restricting lesion in (m) FLAIR (n) b1000 weighted imaging and o) ADC maps could not be selected in the present study.
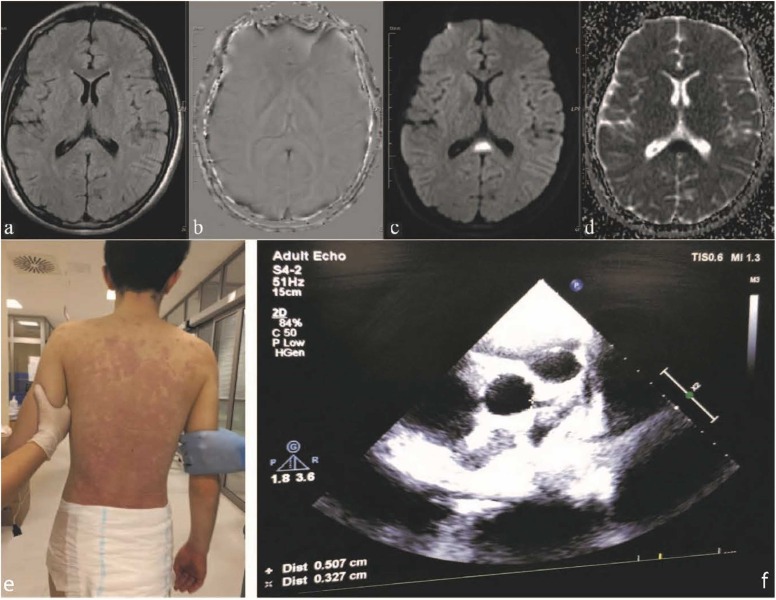
Fig. 2.
: MRI, Clinical and Echocardiographic findings of the second case. (a) T2W is slightly increased signal intensity in the Corpus Callosum Splenium. (b) SWI, no significant sensitivity artifact in the same localization. (c) DWI and (d) ADC images. There is an increase in signal intensity consistent with restricted diffusion in diffusion weighted and signal loss in ADC imaging sections passing through the same level. (e) Generalized maculopapular rash and (f) Coronary artery dilatation.
5. Conclusion
As a result, we describe the clinical course of two RESLES cases associated with SARS CoV-2 infection in the MIS-C spectrum. In this report, we aimed to increase the awareness of neurological involvement and RESLES in children diagnosed with MIS-C. On the other hand, we aimed to emphasize that plasma exchange can be used effectively in the treatment of these patients when there is no response to IVIG and steroid treatment.
Funding
No external funding was used for this study.
Authors’ contribution
FV, NE, and YYC take responsibility for all aspects of the reliability and freedom from bias of the data presented and their discussed interpretation. FV, NE, EGS, and UE designed the case, planned the concept, and prepared and edited the manuscript. FV, SG, and HC had a role in the literature overview and data analysis.
Compliance with ethical standards
Detailed informed consent was obtained from our patient’s parents. The photographs of the children are being used with the permission of the parents/guardians of the children.
Conflict of interest statement
None of the authors of this paper has a conflict of interest, including specific financial interests, relationships, and/or affiliations relevant to the subject matter or materials included. Financial disclosure: The authors have no financial relationships relevant to this article to disclose.
References
- 1.World Health Organization (WHO) Coronavirus disease (COV-ID-19) Situation Report 2020–104 [Internet]. [cited 2020 May 7]. Available at 〈https://www.who.int/docs/default-source/coron-aviruse/situation-reports/20200503-covid-19-sitrep-104.pdf?sfvrsn=53328f46〉.
- 2.Belhadjer Z., Méot M., Bajolle F., Khraiche D., Legendre A., Abakka S., et al. Acute heart failure in multisystem inflammatory syndrome in children (MIS-C) in the context of global SARS-CoV-2 pandemic. Circulation. 2020;142(5):429–436. doi: 10.1161/CIRCULATIONAHA.120. 048360 [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 3.Bridwell R., Long B., Gottlieb M. Neurologic complications of COVID-19. Am J Emerg Med. 2020;38(1549):e3–e7. doi: 10.1016/j.ajem.2020.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hayashi M., Sahashi Y., Baba Y., Okura H., Shimohata T. COVID-19-associated mild encephalitis/encephalopathy with a reversible splenial lesion. J Neurol Sci. 2020;415 doi: 10.1016/j.jns.2020.116941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Abdel-Mannan O, Eyre M, Löbel U, Bamford A, Eltze C, Hameed B, et al. Neurologic and Radiographic Findings Associated With COVID-19 Infection in Children. JAMA Neurol. 2020 Nov 1;77(11):1440-1445. doi: 10.1001/jamaneurol.2020.2687. Erratum in: JAMA Neurol. 2020 Dec 1;77(12):1582. PMID: 32609336; PMCID: PMC7330822. [DOI] [PMC free article] [PubMed]
- 6.Garcia-Monco J.C., Cortina I.E., Ferreira E., Martínez A., Ruiz L., Cabrera A., et al. Reversible splenial lesion syndrome (RESLES): what’s in a name? J Neuroimaging. 2011;21(2):e1–e14. doi: 10.1111/j.1552-6569.2008.00279.x. [DOI] [PubMed] [Google Scholar]
- 7.Chen W.X., Liu H.S., Yang S.D., Zeng S.H., Gao Y.Y., Du Z.H., et al. Reversible splenial lesion syndrome in children: retrospective study and summary of case series. Brain Dev. 2016;38:915–927. doi: 10.1016/j.braindev.2016.04.011. [DOI] [PubMed] [Google Scholar]
- 8.J.T., Kielstein, C., Hafer, E., Zimbudzi, S., Hawes, A change for better exchange-from membrane therapeutic plasma exchange to centrifugal therapeutic plasma exchange. 2020.
- 9.Yıldız A.E., Maraş Genç H., Gürkaş E., Akmaz Ünlü H., Öncel İ.H., Güven A. Mild encephalitis/encephalopathy with a reversible splenial lesion in children. Diagn Inter Radiol. 2018;24(2):108–112. doi: 10.5152/dir.2018.17319. PMID: 29757148; PMCID: PMC5873501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Palabiyik F., Akcay N., Sevketoglu E., Hatipoglu N., Sari E.E., Inci E. Imaging of multisystem inflammatory disease in children (MIS-C) associated with COVID-19. Acad Radiol. 2021;28(9):1200–1208. doi: 10.1016/j.acra.2021.05.030. Epub 2021 Jun 5. PMID: 34284918; PMCID: PMC8179194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.El Aoud S., Sorial D., Selmaoui A., Menif I., Lazard M., Si Hocine M., et al. A first case of mild encephalitis with reversible splenial lesion (MERS) as a presenting feature of SARS-CoV-2. Rev Neurol. 2020;20:30606–30608. doi: 10.1016/j.neurol.2020.06.001. (S0035–3787) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sato T., Ushiroda Y., Oyama T., Nakatomi A., Motomura H., Moriuchi H. Kawasaki disease-associated MERS: pathological insights from SPECT findings. Brain Dev. 2012;34:605–608. doi: 10.1016/j.braindev.2011.09.015. [DOI] [PubMed] [Google Scholar]
- 13.Bektaş G., Akçay N., Boydağ K., Şevketoğlu E. Reversible splenial lesion syndrome associated with SARS-CoV-2 infection in two children. Brain Dev. 2021;43(2):230–233. doi: 10.1016/j.braindev.2020.10.002. Epub 2020 Oct 13. PMID: 33082059; PMCID: PMC7553133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Emeksiz S., Çelikel Acar B., Kibar A.E., Özkaya Parlakay A., Perk O., Bayhan G.İ., et al. Algorithm for the diagnosis and management of the multisystem inflammatory syndrome in children associated with COVID-19. Int J Clin Pract. 2021;75(9) doi: 10.1111/ijcp.14471. Epub 2021 Jun 28. PMID: 34107136; PMCID: PMC8237077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Atay G., Hasbal C., Türk M., Erdoğan S., Sözeri B. Vol. 8. 2021. The role of therapeutic plasma exchange (TPE) in multisystem inflammatory syndrome in children (MIS-C) p. 498. (Children). PMID: 34208141; PMCID: PMC8230791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hu S., Hou X., Liu S., Fei C., Zhou L., Xing A., et al. Thrombotic thrombocytopenic purpura with reversible splenial lesion syndrome: a case report. BMC Neurol. 2020;20(1):122. doi: 10.1186/s12883-020-01696-2. PMID: 32252673; PMCID: PMC7133000. [DOI] [PMC free article] [PubMed] [Google Scholar]