Abstract
The diversity of SARS-CoV-2 continues to lead to the emergence of new SARS-CoV-2 variants. SARS-CoV-2 antibody assays are crucial in managing the COVID-19 pandemic by determining the neutralizing antibody response. This study aims to investigate vaccine-induced antibodies against most common variants of SARS-CoV-2 in Egypt. Sera samples were collected from vaccinated participants and neutralizing activity against the SARS-CoV-2 variants was determined using microneutralization assay. Our results show that the BNT162b2 (Pfizer-BioNTech), ChAdOx1 nCov-19 (AstraZeneca), and Ad26.COV2.S COVID-19 (Janssen) vaccines elicited neutralizing antibody responses more than the BBIBP-CorV vaccine (Sinopharm) against B.1, C.36.3, and AY.32 (Delta) variants. While vaccines remain highly effective in managing the COVID-19 pandemic, ongoing monitoring of vaccine effectiveness is needed.
Keywords: SARS-CoV-2, COVID-19 pandemic, Neutralizing antibodies, Microneutralization assay, SARS-CoV-2 vaccine, Vaccine effectiveness
1. Introduction
Vaccination against the Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) is an important strategy for protection against the coronavirus disease of 2019 (COVID-19). Several platforms of vaccines have been introduced worldwide. Neutralizing antibodies develop after vaccination and are maintained for several months [1], [2]. Till now, more than 12 different types of vaccines have completed phase III clinical trials and acquired emergency use authorization. Yet, the rapid transmission and diversity of SARS-CoV-2 continue to lead to the emergence of SARS-CoV-2 variants.
Since the declaration of the first case of COVID-19 in Egypt on 14 February 2020, over 375,919 cases and 21,361 fatalities (case-fatality 5.6%) have been reported till December 2021 [3]. The Egyptian authority started to apply a comprehensive response plan to control the spread of the virus. Vaccines were rolled out in January 2021 and included four types: ChAdOx1 nCov-19 (Oxford/AstraZeneca), BNT162b2 (Pfizer/BioNTech), BBIBP-CorV (Sinopharm), and Ad26.COV2.S COVID-19 (Janssen/Johnson). The vaccination rate in Egypt remained low. Till 10 November 2021, 33.7 million doses have been administered and 13.3 million people (13% of the total population) have been fully vaccinated [4]. Prior to the emergence of the Omicron variant, the Alpha and Delta variants of concern were detected in Egypt. Based on published sequences, the B.1, C36, and C36.3 were the most dominant strains in Egypt. We conducted a serological study to investigate vaccine-induced antibodies against the most common variants of SARS-CoV-2 in Egypt among a segment of vaccinated participants.
2. Material and methods
2.1. Study design and population
In total, 35 vaccinated adult individuals without evidence of prior SARS-CoV-2 infection were enrolled in this study as part of a larger cross-sectional serosurvey among employees of a major research institution in Egypt located in Greater Cairo and were distributed as follows: 10 persons who received the BBIBP-CorV vaccine (Sinopharm) (samples obtained ∼99 days post second dose), 19 persons who received the AstraZeneca ChAdOx1 nCov-19 vaccine (samples obtained ∼99 days post second dose), three persons who received the Pfizer–BioNTech BNT162b2 vaccine (samples obtained ∼52 days post second dose), and three persons who received the Janssen Ad26.COV2.S COVID-19 vaccine (samples obtained ∼30 days post single dose). The study population was employees of a major research institution in Egypt located in Greater Cairo and sera were collected from all participants between September and November 2021.
2.2. Ethical statement
Written informed consent were obtained from all participants. The study was approved by the Research Ethics Committee of the National Research Centre (Egypt) (protocol number 14 155, dated March 22, 2020).
2.3. Measurement of neutralizing antibodies’ activity using microneutralization assay
Collected sera underwent microneutralization assay (MN) to detect the presence of vaccine-induced SARS-CoV-2 neutralizing antibodies and viral titer was determined using median tissue culture infectious dose (TCID50). The MN assay was performed as previously described [5]. Briefly, sera samples were inactivated at 37 °C for 30 min and diluted at 2-fold in serial dilutions using Dulbecco's Modified Eagle Medium (DMEM) starting with a dilution of 1:10 and mixed with equal volumes of 100 TCID50/ml of hCoV-19/Egypt/NRC-03/2020 (B.1 lineage), hCoV-19/Egypt/NRC-369/2021 (C.36.3 lineage), or hCoV-19/Egypt/NRC-1483/2021 (AY.32 delta variant) SARS-CoV-2 isolates. After 1 h incubation at 37 °C, the virus-sample mixture was added in duplicate to Vero-E6 cell monolayers in 96-well culture plates. After 1 h of adsorption, the inoculums were removed and 100 µl of pre-warmed infection medium were added to each well. The plates were then incubated for three more days at 37 °C under 5% CO2 in a humidified incubator. A virus back-titration was performed without immune serum to assess input virus dose and positive and negative hamster sera were included in assays as control. Cytopathic effect (CPE) was read at 3 days post infection. The highest serum dilution that completely protected the cells from CPE was recorded as the neutralizing antibody titer.
2.4. Statistical analysis
Data analysis was performed using SPSS v23 (IBM, Armonk, NY). Student’s t-test was used to compare means and chi-square used to compare categories. A p-value ≤ 0.05 was considered statistically significant. For multiple comparisons, the ANOVA test was used with Tukey’s post hoc test using the GraphPad Prism 9 (GraphPad Software, La Jolla, CA, USA).
3. Results
To investigate the cross-reactivity of neutralizing antibodies induced by the vaccination against circulating SARS-CoV-2 strains in Egypt, we used sera samples obtained from 35 vaccinated participants to assess neutralizing activity against the SARS-CoV-2 variants in a live-virus microneutralization assay as previously described [5].
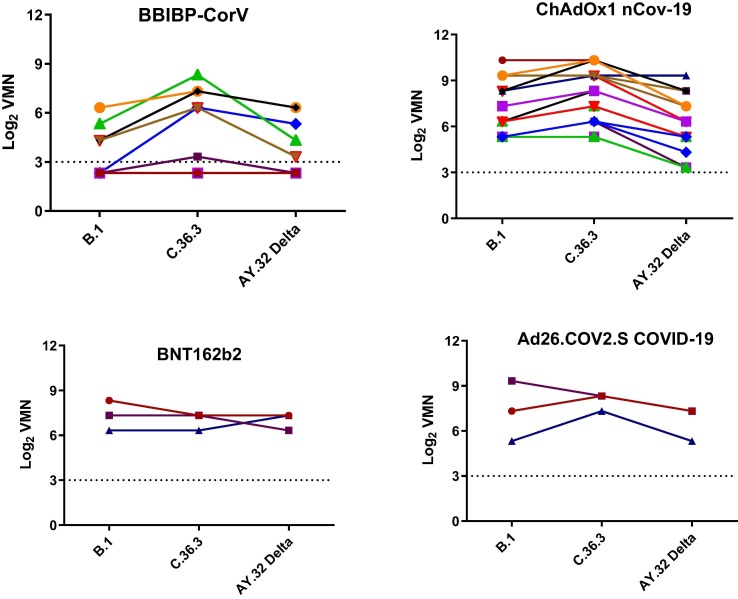
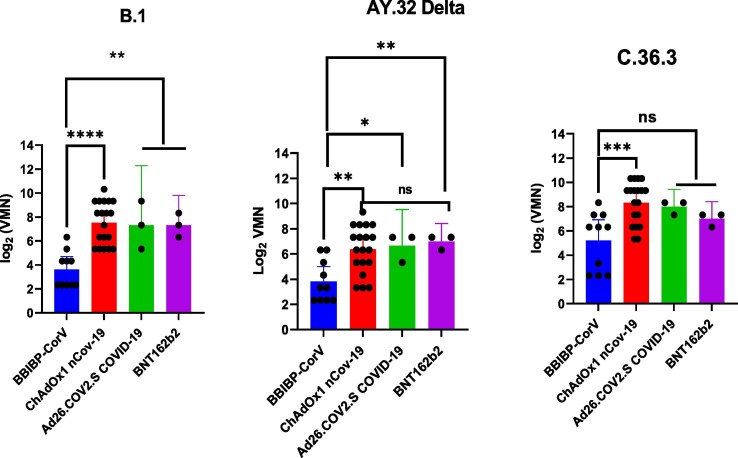
All samples from vaccinated persons with BBIBP-CorV vaccine showed less neutralizing activity against both the B.1 (with a D614G mutation isolated during the first wave) and AY.32 Delta variants than against C.36.3 (most common in Egypt with L452R substitutions and 69–70 del) but the difference was not statistically significant. No significant differences were observed in the neutralizing activity of persons vaccinated with ChAdOx1 nCov-19 when comparing B.1 to C.36.3, but a significant difference was noted when comparing the AY.32 Delta variant to B.1. No differences were noted for BNT162b2 or Ad26.COV2.S COVID-19 vaccines (Fig. 1 ). The mean titers in those vaccinated with BNT162b2, ChAdOx1 nCov-19, Ad26.COV2.S COVID-19, and BBIBP-CorV vaccines against B.1 were 7.32, 7.53, 7.32, and 3.95, while the mean titers against C.36.3 were 6.99, 8.32, 7.99, and 5.44, and the mean titers against AY.32 Delta were 6.99, 6.37, 6.66, and 3.82, respectively. All vaccines produced higher titers against B.1 and AY.32 Delta variants than BBIBP-CorV. The ChAdOx1 nCov-19 vaccine produced a significant higher titer than BBIBP-CorV vaccine against the C.36.3 variant (Fig. 2 ).
Fig. 1.
Neutralizing antibody responses against the B.1, C36.3, and AY.32 Delta variants.
Fig. 2.
Antibody response elicited by SARS-CoV-2 vaccines.
4. Discussion
Consistent with previous studies [6], our results show that the mRNA vaccine (BNT162b2) and adenovirus vector-based vaccines (ChAdOx1 nCov-19 and Ad26.COV2.S COVID-19) induce considerable levels of neutralizing antibodies against most common strains of SARS-CoV-2 in Egypt, while the BBIBP-CorV inactivated vaccine (Sinopharm) is much less efficient. This difference in neutralizing antibody titers could translate in differences in vaccine effectiveness. Previous studies reported higher antibody levels in those vaccinated with BNT162b2 compared to BBIBP-CorV with estimated efficacies of 95% and 72.8% respectively [7], [8]. Seroconversion rates were lower in the group vaccinated with BBIBP-CorV compared with other platforms e.g vaccinated with BNT162b2 and Gam-COVID-Vac [9]. Recent study showed varying degrees of neutralization potency of BBIBP-CorV vaccine against multiple variants [10].
Some limitations of this study include the limited number of participants and variation of the number of subjects receiving each vaccine, lack of a control group, lack of consecutive samples from the same subject, difference in sampling schedule post vaccination between vaccine types, and not examining the cell-mediated immunity. While vaccination is considered as a highly effective control strategy, ongoing monitoring of vaccine effectiveness against emerging variants is crucial.
5. Conclusions
In conclusion, all vaccines appear to be able to neutralize the three studied variants, yet antibody titers induced by BBIBP-CorV were significantly lower than the other vaccines. Large-scale cross-national population studies are needed to further determine vaccine effectiveness against variants of concern and/or common clades circulating in Egypt, in particular after the spread of Omicron variant. Finally, our results strongly support vaccination against SARS-CoV-2 and support maximizing vaccination coverage with different platforms of vaccines in moderately vaccinated populations such as in Egypt.
Author Contributions: Conceptualization, R.E., A.K., R.J.W. G.K., and M.A.A.; methodology, A.E., M.R.G, and W.H.R.; software, R.E., A.K., and G.K.; validation, R.E., A.K., R.J.W., G.K., and M.A.A.; formal analysis, R.E., A.K., and G.K.; investigation, R.E., A.K., R.J.W., G.K., and M.A.A.; resources, R.J.W., G.K., and M.A.A.; data curation, R.E., and A.K.; writing—original draft preparation, R.E., A.K., and G.K.; writing—review and editing, R.E., A.K., R.J.W., G.K., and M.A.A.; visualization, R.E., A.K.; supervision, G.K. and M.A.A.; project administration, G.K. and M.A.A.; funding acquisition, R.J.W., G.K., and M.A.A. All authors have read and agreed to the published version of the manuscript.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgement
This research was funded by the National Institute of Allergy and Infectious Diseases, National Institutes of Health, and US Department of Health and Human Services (under contract numbers HHSN272201400006C and 75N93021C00016).
Institutional Review Board Statement: The study was conducted in accordance with the Declaration of Helsinki, and approved by the Research Ethics Committee of the National Research Centre (Egypt) (protocol number 14 155, dated March 22, 2020).
Informed Consent Statement: Informed written consent was obtained from all subjects involved in the study.
Data Availability Statement: All data are available within the manuscript.
References
- 1.Edara V.V., Hudson W.H., Xie X., Ahmed R., Suthar M.S. Neutralizing antibodies against SARS-CoV-2 variants after infection and vaccination. JAMA. 2021;325(18):1896. doi: 10.1001/jama.2021.4388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Naaber P., Tserel L., Kangro K., Sepp E., Jürjenson V., Adamson A., et al. Dynamics of antibody response to BNT162b2 vaccine after six months: a longitudinal prospective study. Lancet Reg Health Eur. 2021;10:100208. doi: 10.1016/j.lanepe.2021.100208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.WHO. WHO Coronavirus (COVID-19) Dashboard; 2022. <https://covid19.who.int/>.
- 4.McGill, COVID19 Vaccine Tracker Team. Egypt-COVID19 Vaccine Tracker; 2022. <https://covid19.trackvaccines.org/country/egypt/>.
- 5.Gomaa M.R., El Rifay A.S., Shehata M., Kandeil A., Nabil Kamel M., Marouf M.A., et al. Incidence, household transmission, and neutralizing antibody seroprevalence of Coronavirus Disease 2019 in Egypt: results of a community-based cohort. PLoS Pathog. 2021;17(3):e1009413. doi: 10.1371/journal.ppat.1009413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lim W.W., Mak L., Leung G.M., Cowling B.J., Peiris M. Comparative immunogenicity of mRNA and inactivated vaccines against COVID-19. Lancet Microbe. 2021;2(9):e423. doi: 10.1016/S2666-5247(21)00177-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Klasse P.J., Nixon D.F., Moore J.P. Immunogenicity of clinically relevant SARS-CoV-2 vaccines in nonhuman primates and humans. Sci Adv. 2021;7(12) doi: 10.1126/sciadv.abe8065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Al Kaabi N., Zhang Y., Xia S., Yang Y., Al Qahtani M.M., Abdulrazzaq N., et al. Effect of 2 inactivated SARS-CoV-2 vaccines on symptomatic covid-19 infection in adults: a randomized clinical trial. JAMA. 2021;326(1):35. doi: 10.1001/jama.2021.8565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Petrović V., Vuković V., Patić A., Marković M., Ristić M., Das J. Immunogenicity of BNT162b2, BBIBP-CorV and Gam-COVID-Vac vaccines and immunity after natural SARS-CoV-2 infection-a comparative study from Novi Sad, Serbia. PloS One. 2022;17(2):e0263468. doi: 10.1371/journal.pone.0263468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yu X., Wei D., Xu W., Liu C., Guo W., Li X., et al. Neutralizing activity of BBIBP-CorV vaccine-elicited sera against Beta, Delta and other SARS-CoV-2 variants of concern. Nat Commun. 2022;13(1) doi: 10.1038/s41467-022-29477-0. [DOI] [PMC free article] [PubMed] [Google Scholar]