Abstract
Severe acute respiratory syndrome coronavirus 2 is a worldwide public health issue. Almost 2 years into the pandemic, the persistence of symptoms after the acute phase is a well-recognized phenomenon.
We conducted a scoping review to map cognitive domain impairments, their frequency, and associated psycho-affective disorders in people with a previous COVID-19 infection. We searched PubMed/MEDLINE, Scopus, and PsycInfo to identify relevant reports published between December 1, 2019 and February 21, 2022. We followed the PRISMA (Preferred-Reporting-Items-for-Systematic-Reviews-and-Meta-Analyses) extension for scoping review guidelines.
Three independent reviewers selected and charted 25 records out of 922. Memory, attention, and executive functions appeared to be the most affected domains. Delayed recall and learning were the most impaired domains of memory. Among the executive functions, abstraction, inhibition, set shifting, and sustained and selective attention were most commonly impaired. Language and visuo-spatial abilities were rarely affected, although this finding might be biased by the scarcity of reports. Neurological and respiratory conditions were often reported in association with cognitive deficits. Results on psycho-affective conditions were inconclusive due to the low frequency of reported data. Admission to an intensive care unit is not related to cognitive deficits.
This review highlighted a potential effect of a previous post-COVID-19 infection on a pattern of memory, attention, and executive functions impairments. These findings need to be confirmed on larger cohorts with comprehensive neuropsychological batteries and correlated to neurophysiological and neurobiological substrates.
Keywords: SARS-CoV-2, Coronavirus, Neuropsychological abnormalities, Cognitive impairment, Post-COVID-19 syndrome, Long-COVID-19 syndrome
1. Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has been a major public health issue since December 2019 (Zhou et al., 2020b). The scientific community has so far focused on pathophysiological mechanisms and symptoms related to the acute phase of the infection. More than 2 years into the pandemic, attention is shifting to the post-acute symptoms (Augustin et al., 2021).
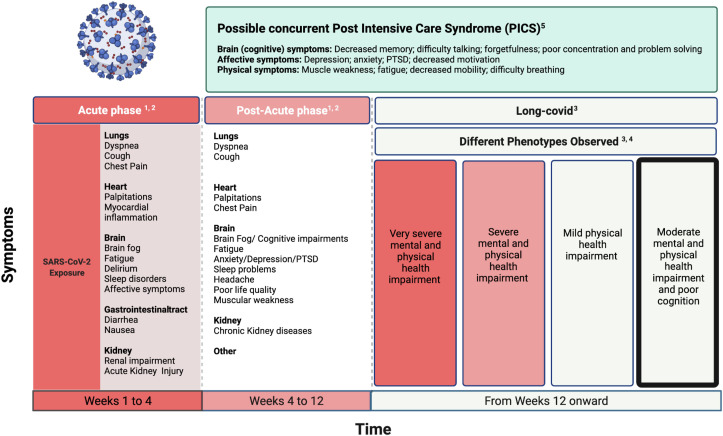
Long-COVID-19 or post-COVID-19 syndrome has not yet been fully defined: fatigue, headache, dyspnoea, and cognitive deficits are among the symptoms more frequently reported (Cares-Marambio et al., 2021; Lopez-Leon et al., 2021; Shah et al., 2021; Yong, 2021). A clear temporal definition of what should be considered as “long-COVID” has been similarly debated. A recent review (Yong, 2021) referred to long-COVID-19 as symptoms lasting longer than 3 months from acute infection. A central nervous system (CNS) involvement has been postulated, considering the reported neurotropism of the virus for the CNS (Alomari et al., 2020). In fact, an inflammatory reaction and microvascular damage (Cosentino et al., 2021) seem to be the major determinants in the genesis of neuropsychiatric symptoms. As a further confounding factor, cognitive and psychiatric abnormalities are frequently described in post-intensive care syndrome (PICS), challenging a correct etiological diagnosis (Needham et al., 2012). We refer the reader to Fig. 1 for a summary of long-term symptoms occurring after the COVID-19 infection, their different temporal definitions, and the overlap with PICS.
Fig. 1.
Long-Covid symptoms. 1: Davis et al., 2021; 2: Lopez-Leon et al., 2021; 3: Yong, S. J., 2021; 4: Evans et al., 2021; 5: Neetham et al., 2012.
Few studies have systematically assessed post-COVID-19 cognitive deficits (see for example, Hampshire et al., 2021). A recent systematic review (Ceban et al., 2021) highlighted the presence of fatigue and cognitive impairment as the most common and debilitating symptoms experienced 12 weeks following a COVID-19 diagnosis. In particular, 22% of patients experienced cognitive impairment, assessed by a brief cognitive screening (e.g., Montreal Cognitive Assessment, MoCA; Pilotto et al., 2021), telephone interviews (Miyazato et al., 2020), or an online survey (Orrù et al., 2021). However, as most studies did not ascertain the presence of cognitive impairment prior to the COVID-19 diagnosis, it is unclear to what extent outcomes are linked to the COVID-19 infection itself. Another review (Badenoch et al., 2022) highlighted cognitive impairments persisting at 6 months from COVID-19 diagnosis in 20.2% of the patients as well as anxiety (19.1%) and posttraumatic stress (15.7%).
Despite rising evidence, a clear picture of the cognitive alterations after COVID-19 infection is missing. Characterizing the post-COVID-19 pattern of cognitive impairments and their frequency will contribute to a better understanding of its pathophysiology. The final aim is to set up appropriate care and rehabilitation pathways.
Toward this goal, we aimed to answer the following research questions:
-
1.
Which are the most frequent and severe cognitive sequelae of post-COVID-19 infections?
-
2.
Are there any clinical/psychological factors more often associated with post-COVID-19 cognitive impairments?
-
3.
Have different admission settings (e.g., intensive care unit [ICU], general ward, home recovery) led to different cognitive outcomes?
Considering the high heterogeneity of the studies’ designs, the assessment tools adopted, and the cognitive domains evaluated, we deemed a scoping review the best-suited tool to answer our research questions.
2. Method
We followed the guidelines by Levac and colleagues (Levac et al., 2010) and the Preferred Reporting Items for Systematic reviews and Meta-Analysis Extension for Scoping Reviews checklist (PRISMA-ScR; Tricco et al., 2018).
2.1. Eligibility criteria
Inclusion criteria included the following:
-
i)
Full-length, peer-reviewed, original research articles written in English, published between December 1, 2019 and February 21, 2022.
-
ii)
Studies enrolling people with a confirmed molecular diagnosis of COVID-19 and assessed with standardized neuropsychological assessment tools
We excluded single case studies, animal studies, studies including people with preexisting neurological, cognitive, and respiratory conditions not related to COVID-19, and those not meeting the inclusion criteria (see Appendix).
2.2. Data sources and search strategy
Two authors (MB, LC) independently conducted a systematic search on post-COVID-19 cognitive sequelae on Scopus, PubMed/MEDLINE, and PsycInfo. Databases were consulted by using the following agreed keywords, combined with the following boolean operators (AND/OR): (COVID-19 OR SARS-CoV-2) AND (Cognitive impairment OR Neuropsychological impairment OR Cognitive assessment OR Neuropsychological assessment OR Cognitive dysfunctions ).
Records were then filtered based on text availability, publication date (December 1, 2019–February 21, 2022), human species, and English language.
Detailed search queries for PubMed are provided in the Appendix.
The electronic database search was supplemented by screening the reference lists of the included studies and relevant reviews. Results were exported in the MENDELEY bibliographic software package, where duplicates were removed.
2.3. Study selection
To increase consistency, two reviewers (MB and LC) independently screened the same initially identified studies and made a first selection based on titles and abstracts. After removing the duplicates, three reviewers (MB, LC, and MR) carried out a full-text examination of the remaining studies. A fourth reviewer (ADF) revised studies in case of disagreement on data extraction or inclusion.
2.4. Quality assessment
We jointly established six ad hoc quality criteria by adapting the already published criteria for systematic reviews (Taborri et al., 2018) and scoping reviews (Rubega et al., 2021):
-
1.
The aim(s) of the study was/were clearly stated.
-
2.
Selection bias: Participants' inclusion and exclusion criteria were clearly described.
-
3.
Performance bias: (1) The data collection process was precisely detailed and reliable; (2) neuropsychological test scores were corrected by age and education; and (3) a control group was present.
-
4.
Detection bias: The outcomes reported were topic relevant.
-
5.
Results presentation: The neuropsychological test scores were correctly and completely reported (i.e., mean, median, and standard deviation [SD]).
-
6.
Statistical approach: (1) Appropriate statistics were performed; (2) a clear description of the adopted statistics was provided; and (3) a sufficient number of subjects were included.
We assigned each item a score ranging from 0 to 2 (0 = not meeting the criteria, 1 = partially meeting the criteria, 2 = fully meeting the criteria). We summed the single item scores for each study, resulting in a final quality score from 0 to 12. We excluded studies receiving a score lower than 60% (<7) of the maximum possible score (12; see Table 1 ).
Table 1.
Quality assessment.
| Study | Aim of the work |
Selection Bias Inclusion/Exclusion Criteria |
Performance Bias/Data collection/processing |
Detection Bias Outcomes |
Results presentation |
Statistical approach |
Quality score (Max.12) |
|---|---|---|---|---|---|---|---|
| The research question is clearly stated | Participants' inclusion and exclusion criteria are clearly defined | 1. Data collection is clearly described and reliable 2. NPSY scores are corrected 3. Control group |
Outcomes are topic-relevant | NPSY-test scores are clearly reported | 1. Statistical procedures performed are clearly described 2. Sufficient number of subjects |
||
| Aiello et al. (2022) | 2 | 0 | 1 (lack of control group) | 2 | 2 | 2 | 9 |
| Albu et al. (2021) | 2 | 2 | 1 (lack of control group) | 2 | 2 | 2 | 11 |
| Alemanno et al. (2021) | 2 | 2 | 1 (lack of control group) | 2 | 2 | 2 | 11 |
| Almeria et al. (2020) | 2 | 2 | 1 (lack of control group) | 2 | 2 | 2 | 11 |
| Bonizzato et al. (2022) | 2 | 2 | 1 (lack of control group) | 2 | 2 | 2 | 11 |
| De Lorenzo et al. (2020) | 2 | 2 | 1 (lack of control group) | 1 | 0 | 2 | 8 |
| Del Brutto et al. (2021) | 2 | 2 | 2 | 2 | 1 | 2 | 11 |
| Di Pietro et al. (2021) | 2 | 2 | 1 (lack of control group) | 2 | 2 | 1 | 10 |
| Frontera et al. (2021) | 2 | 2 | 1 (lack of control group) | 1 | 2 | 2 | 10 |
| Graham et al. (2021) | 2 | 1 (exclusion criteria not defined) | 1 (lack of control group) | 2 | 2 | 2 | 10 |
| Groiss (2020) | 1 | 1 (exclusion criteria not defined) | 1 (lack of control group) | 1 | 2 | 0 | 6 EXCLUDED |
| Heyns et al. (2021) | 2 | 2 | 1 (lack of control group) | 2 | 2 | 2 | 11 |
| Hosp et al. (2021) | 2 | 2 | 1 (lack of control group) | 2 | 2 | 2 | 11 |
| Jaywant et al. (2021) | 2 | 2 | 1 (lack of control group) | 2 | 1 | 2 | 10 |
| Matos et al. (2021) | 2 | 2 | 1 (lack of control group) | 2 | 1 | 1 | 9 |
| Mattioli et al. (2021) | 2 | 1 (exclusion criteria not defined) | 2 | 2 | 2 | 2 | 11 |
| Mattioli et al. (2022) | 2 | 1 (exclusion criteria not defined) | 1 (lack of control group) | 2 | 2 | 2 | 10 |
| Mazza et al. (2021) | 2 | 2 | 1 (lack of control group) | 2 | 2 | 2 | 11 |
| Miskowiak et al. (2021) | 2 | 2 | 2 | 2 | 2 | 2 | 12 |
| Morin (2021) | 2 | 2 | 1 (lack of control group) | 1 | 0 | 1 | 7 EXCLUDED |
| Negrini et al. (2021) | 2 | 2 | 1 (lack of control group) | 2 | 2 | 0 | 9 |
| Ortelli et al. (2021) | 2 | 1 (exclusion criteria not defined) | 2 | 2 | 2 | 1 | 10 |
| Pirker-Kees et al. (2021) | 2 | 2 | 2 | 2 | 2 | 1 | 11 |
| Pistarini et al. (2021) | 2 | 2 | 1 (lack of control group) | 2 | 2 | 2 | 11 |
| Raman et al. (2021) | 2 | 2 | 2 | 1 | 1 | 2 | 10 |
| Versace et al. (2021) | 2 | 1 (exclusion criteria not defined) | 2 | 2 | 2 | 1 | 10 |
| Zhou et al. (2020a) | 2 | 2 | 2 | 2 | 2 | 2 | 12 |
2.5. Charting of data
All of the reviewers jointly developed an ad hoc form by adapting the one proposed in the Cochrane Handbook for Systematic Reviews (see Appendix) to extract variables relevant to the research question.
In the first part of the extraction form, we reported the general identifying information of each study (journal, first author, year, title, publication type, and a short description of each study's content).
The second part includes the eligibility form based on participants, methodology characteristics, and the assessment of variables of interest.
We eventually charted eligible studies, filling out the third part of the extraction form, which comprises the following:
-
1.
Study characteristics: Diagnosis/ Treatment/ Recovery setting/ Clinical characteristics related to COVID-19/ Studies' inclusion and exclusion criteria/ Neuropsychological assessment tests/ Reported results of interests/ Confounders
-
2.
Trial characteristics: Sample size/ Number of excluded participants/ Recruitment method/ Setting of the assessment/ Trial design/ Number of groups
-
3.
Participant characteristics: Number of participants/ Mean or median age/ Ethnicity (%)/ Gender (%)
3. Results
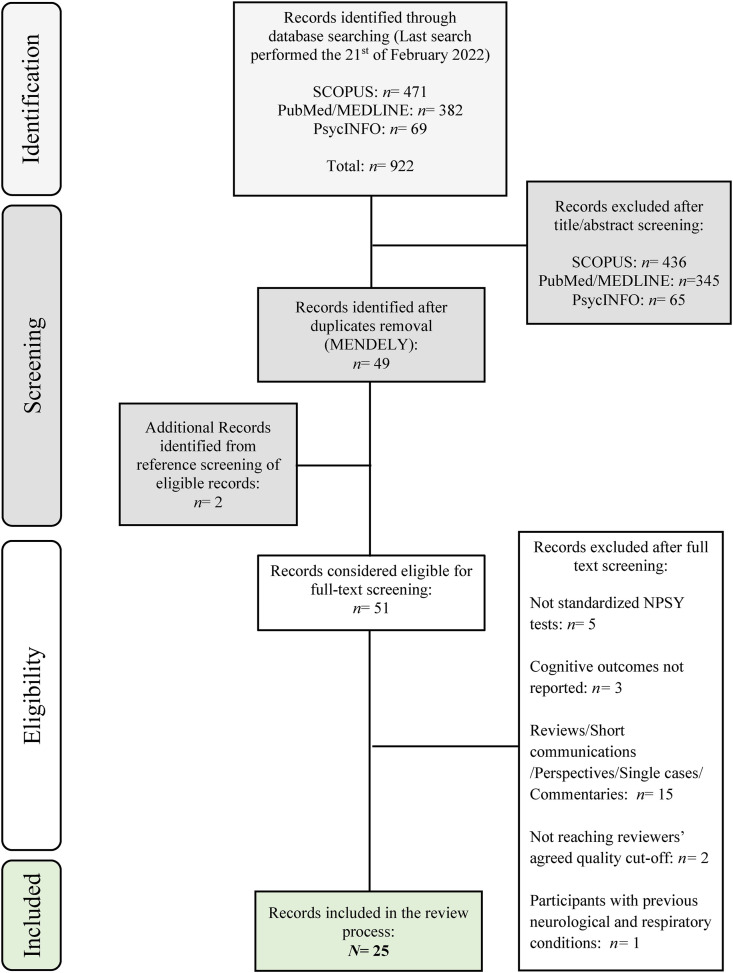
The search retrieved 922 studies; the first selection based on titles and abstracts led to 76 record inclusions. After removing duplicates, 51 studies were screened. We excluded a total of 26 studies for the following reasons: 15 were not full-text but peer-reviewed studies; 5 did not use standardized neuropsychological assessment tools; 3 did not report cognitive outcomes; 2 did not reach the agreed quality cutoff; and 1 included participants with previous history of neurological and respiratory conditions.
Finally, 25 studies were included. Fig. 2 reports the full selection procedure.
Fig. 2.
Flow Chart of the selection procedure.
We grouped the outcomes of the 25 included studies according to the cognitive domains assessed: general measures of cognitive impairment, memory, attention, executive functions, language, and visuo-spatial abilities. Working memory was classified as a memory function by most of the included articles; thus, we reported data on working memory tests in the memory section.
For each domain, we summarized the global number of studies assessing each cognitive domain, the assessment methods, the number of studies finding impairments, and comparisons between the cognitive scores of differently treated COVID-19 groups (i.e., ICU admitted, non-ICU admitted, and home recovery).
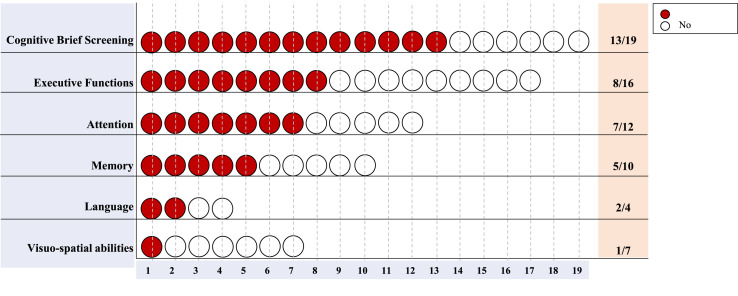
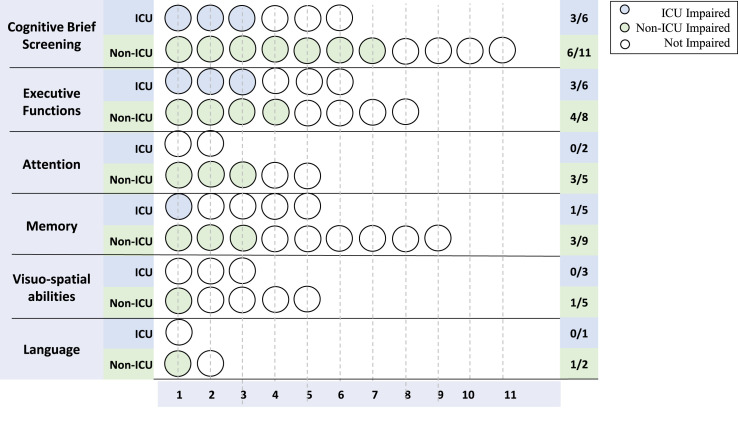
Not all studies clearly differentiated cognitive scores related to each recovery setting. Thus, we summarized the cognitive patterns without recovery settings (Fig. 3 ) and with subgroups’ specific cognitive scores, whenever data were provided (Fig. 4 ).
Fig. 3.
Frequencies of COVID-19-related cognitive impairments. Red dots represent the number of studies reporting cognitive impairments for each cognitive domain over the total number of studies assessing the given domain.
Fig. 4.
Frequencies of COVID-19-related cognitive impairments according to recovery setting. Blue dots represent the number of studies reporting cognitive impairments in ICU-admitted persons, while green dots represent the number of studies reporting cognitive impairments in non-ICU-admitted persons.
We considered cognitive domains to be impaired when the mean or median scores of the tests resulted under the normative values or if the overall percentage of cognitively impaired subjects was at least 50% of the evaluated sample.
The cognitive scores for each study, divided by cognitive domains, are summarized in the Supplementary Material (Table S1).
3.1. Study characteristics
Included studies (n = 25) were heterogeneous in terms of research designs (i.e., 9 cross-sectional, 9 longitudinal, 3 case series, 2 retrospective, 1 prospective observational, and 1 cohort study), assessment tools, and assessed cognitive functions (Table 2 ).
Table 2.
NPSY test.
MoCA: Montreal Cognitive Assessment; MMSE: Mini Mental State Examination; SCIP-D: Screen for cognitive impairment in psychiatry Danish version; BACS: Brief Assessment of Cognition in Schizophrenia; RAVLT: Ray Auditory Verbal Learning Test; HVLT-R: Hopkins Verbal Learning Test- Revised; TAVEC: Test de Aprendizaje Verbal España-Complutense; TMT: Trail Making Test; CDT: Clock Drawing Task; SDMT: Symbol Digit Modality Test; CPT: Continuous Performance Test; NIH: National Institutes of Health Toolbox v2.1 instrument;CVLT: California Verbal Learning Test; OVLT: Oral Verbal Learning Test; SPART: Spatial Recall Test; TEA:Test for Examination of Attention; FAB:Frontal Assessment Battery; COWA: Controlled Oral Word Association by categories; BNT: Boston Naming Test; BMET: Brief Memory and Executive Test.
Participants’ cognitive evaluations were conducted at different times since symptoms onset; that is, most of the studies (17/25) reported data related to the first 3 months, whereas the other 8 studies reported data within the first 4 (Mattioli et al., 2021, 2022; Miskowiak et al., 2021), 6 (Aiello et al., 2022; Albu et al., 2021; Graham et al., 2021) and 6 + months (Del Brutto et al., 2021; Frontera et al., 2021) from acute infection.
3.2. Demographic variables (sex and age)
Males were generally more affected (n males = 1067; 55%). Considering admission settings, males were more frequently admitted to the ICU (n males = 100; 79.36%), and females were more frequently admitted to other medical wards (non-ICU, n females = 352; 55, 26%; Supplementary Material, Fig. F1). Six studies did not specify age in relation to the recovery setting (Almeria et al., 2020; Frontera et al., 2021; Jaywant et al., 2021; Ortelli et al., 2021; Raman et al., 2021; Zhou et al., 2020a).
The mean age of participants ranged from 42.57 ± 7.23 (Matos et al., 2021) to 79 ± 8 (Pirker-Kees et al., 2021), yet in the majority of studies the mean age was 60 (Supplementary Material, Fig. F2). Five studies reported only median age, respectively: 47.86 (26–65; Mattioli et al., 2021), 54 (43.8–62; Albu et al., 2021), 57 (48–67; De Lorenzo et al., 2020), 68 (55–77; Frontera et al., 2021), and 72 (58–86; Heyns et al., 2021).
3.3. COVID-19-related cognitive impairment
3.3.1. Brief cognitive screening
Brief cognitive screening was administered in 19 of the 25 studies through the Montreal Cognitive Assessment (MoCA), Mini Mental State Examination (MMSE), Screen for Cognitive Impairment in Psychiatry - Danish version (SCIP-D), the Brief Assessment of Cognition in Schizophrenia (BACS), and the Brief Memory and Executive Test (BMET). Thirteen studies detected significant cognitive impairments compared to normative scores. When performed, subitem analysis highlighted the cognitive deficits of executive functions (Alemanno et al., 2021; Hosp et al., 2021; Mazza et al., 2021; Pistarini et al., 2021), immediate recall, working memory (Alemanno et al., 2021; Hosp et al., 2021; Miskowiak et al., 2021), and attention (Hosp et al., 2021; Negrini et al., 2020).
Participants admitted to ICU performed on average under the normality cutoff in 3 of the 6 studies (mean and SD of the MoCA score of 31 participants: 21.65 ± 5.23 [Alemanno et al. (2021) reported normality cutoff = 26]; mean and SD of the MMSE global score of three ICU admitted participants: 23.06 ± .53 [Negrini et al. (2020) reported normality cutoff = 24]; one study reported only the percentage of patients under cutoff [Jaywant et al., 2021]).
Non-ICU-admitted subjects were assessed in a higher proportion of studies (11/19), showing cognitive impairment in 7 studies (Alemanno et al., 2021; Bonizzato et al., 2022; Heyns et al., 2021; Hosp et al., 2021; Jaywant et al., 2021; Miskowiak et al., 2021; Pirker-Kees et al., 2021).
Few studies compared cognitive scores between COVID-19 subgroups or contrasted with healthy controls (HC). Non-ICU-admitted participants treated with a Venturi mask performed worse than those receiving mechanical ventilation (p = .024; Alemanno et al., 2021). In another study, COVID-19 subjects with associated neurological symptoms performed worse at cognitive assessment than those without (p = .03; Frontera et al., 2021). No significant differences were found between persons who recovered in a non-ICU ward or at home (p = .26; De Lorenzo et al., 2020), as well as between a non-ICU COVID-positive group and a post-COVID-19 group (MMSE p = .22, MoCA and p = .35; Pistarini et al., 2021).
When compared to HC groups matched by age, sex, and education, COVID-19 groups scored significantly worse regardless of the treatment settings: non-ICU vs HC, p = .01 (Miskowiak et al., 2021) and p = .042 (Pirker-Kees et al., 2021). This also included participants admitted to a general ward vs HC, p < .001 (Ortelli et al., 2021).
3.3.2. Memory
Memory was assessed in 10 studies as immediate recall, delayed recall (Aiello et al., 2022; Albu et al., 2021; Almeria et al., 2020; Bonizzato et al., 2022; Di Pietro et al., 2021; Hosp et al., 2021; Mattioli et al., 2021; Mattioli et al., 2022; Zhou et al., 2020a), and working memory (Albu et al., 2021; Bonizzato et al., 2022; Almeria et al., 2020; Di Pietro et al., 2021; Graham et al., 2021; Hosp et al., 2021; Zhou et al., 2020a).
Short-term memory, assessed with the Digit Span Forward test (WAIS-III), Rey Auditory Verbal Learning Test (RAVLT), Test de Aprendizaje Verbal España-Complutense (TAVEC), Hopkins Verbal Learning Test- Revised (HVLT-R), and brief story test (immediate recall) revealed no impairments, neither as global scores (Almeria et al., 2020; Hosp et al., 2021; Zhou et al., 2020a) nor dividing groups according to the setting (Albu et al., 2021; Bonizzato et al., 2022; Mattioli et al., 2021, 2022), with the exception of finding early recall deficits in the brief story test (Di Pietro et al., 2021; mean and SD z-scores: −.58 ± 1.1; normality cutoff < −2).
Long-term memory, assessed with the RAVLT, TAVEC, HVLT-R, the Babcock Memory Test, SPART, CVLT-delay, OVMT-delay, and brief story test delayed recall, resulted to be impaired in 2 of the 8 studies (Aiello et al., 2022; Albu et al., 2021; Almeria et al., 2020; Bonizzato et al., 2022; Di Pietro et al., 2021; Hosp et al., 2021; Mattioli et al., 2021, 2022). Specifically, one study (Di Pietro et al., 2021) found a delayed recall impairment in the brief story test (mean and SD z-scores: .24 ± 1.60; normality cutoff < −2), and the other reported 7/14 non-ICU participants performing below 1.5 SD from normality in the HVLT-R delayed recall test (Hosp et al., 2021).
Working memory was evaluated with the Digit Span Backward test and the NIH list sorting working memory test (Albu et al., 2021; Almeria et al., 2020; Di Pietro et al., 2021; Graham et al., 2021; Hosp et al., 2021; Zhou et al., 2020a). Two of the 7 studies found impaired working memory: 1 reported that 6 of the 15 participants performed below 1.5 SD from normality in the Digit Span Backward test (Hosp et al., 2021), and 1 found significantly impaired scores in the list sorting memory test (from the National Institutes of Health [NIH] Toolbox v2.1 instrument) of the COVID-19 group, as compared to a normal population sample (median value: 43 [37.5–48.75], p = .007; Graham et al., 2021).
Memory subitem scores of the MoCA, MMSE, and SCIP-D revealed impairment mainly in the late recall of previously presented words: one study reported a significantly impaired sub-score of the MoCA (mean and SD: 1.92 ± 1.70; max score: 5; Hosp et al., 2021), another reported that the ICU-admitted group performed better than non-ICU-admitted group (MoCA p = .005; MMSE p = .017; Alemanno et al., 2021), and a third reported that 3/3 ICU participants demonstrated impairment in immediate recall based on the MMSE score.
In the only study adopting the SCIP-D (Miskowiak et al., 2021), severe memory impairments emerged in the subitems of the verbal learning test (VLT mean ± SD: 19.9 ± 4.2; expected score based on age, sex and education: 22.1 ± 1.2) and working memory test (WM mean ± SD: 18.2 ± 4.2; expected score based on age, sex and education: 19.9 ± .7) in a sample of non-ICU-admitted subjects.
Differences between ICU- and non-ICU-admitted participants did not have a significant result, with the only exception of Alemanno et al. (2021), who found that ICU-admitted participants scored significantly better than non-ICU-admitted ones in the delayed (p = .005) and immediate recall (p = .001) subitems of the MoCA and words recall subitem of the MMSE (p = .017). The few studies comparing COVID-19 groups to HC led to contradictory evidence, with one paper reporting significantly worse scores in the COVID-19 group for both verbal learning memory (p = .003) and working memory (p = .04; Miskowiak et al., 2021) and 2 studies reporting no difference (p = .843, Zhou et al., 2020a; p < .05, Mattioli et al., 2021).
3.3.3. Attention
Attention was evaluated in 12 studies, of which 7 reported impairments (Alemanno et al., 2021; Bonizzato et al., 2022; Graham et al., 2021; Hosp et al., 2021; Negrini et al., 2021; Ortelli et al., 2021; Zhou et al., 2020a).
The 4 studies using attention-specific neuropsychological tests found significant impairments, specifically the following: one reported the median scores of the attention subitem of the NIH Toolbox in a group of COVID-19 participants to be significantly different from the test's normative values (median: 41.5 (37–48.25), p < .001; Graham et al., 2021). Another study using the Navon task (NT) to test divided and selective attention found lower reaction times in the COVID-19 group (mean ± SD in COVID-19 group: 1327.1 ± 525.3 vs mean ± SD in HC group: 850.3 ± 144.2, p < .046) and higher error rates (mean ± SD in COVID-19 group: 3.8 ± 1.2% vs mean ± SD in HC group 1.2 ± .3, p < .00; Ortelli et al., 2021). The same was found with the percentage of errors of a vigilance task (mean ± SD in COVID-19 group: 3.2 ± 1 vs mean ± SD in HC group: .9 ± .2, p > .001; Ortelli et al., 2021). A third study, assessing sustained and selective attention (the continuous performance test, CPT) reported a higher rate of missing trials compared to HC (CPT part 2, COVID-19 group's mean and SD of missing number: 41.54 ± 2.90 vs HC: 39.59 ± 2.31, p = .006; CPT part 3, COVID-19 group's mean and SD of missing number: 40.38 ± 3.10 vs HC: 38.45 ± 2.13, p = .002) and a lower number of correct trials (CPT part 3, COVID-19 group's mean and SD of missing number: 6.34 ± 2.50 vs HC: 8.21 ± 1.90, p = .008; Zhou et al., 2020a). Lastly, one study reported an impairment in 5/8 patients in the Symbol Digit Modality Test (SDMT; Bonizzato et al., 2022). None of these studies reported results by COVID-19 recovery setting.
The remaining three studies reported subitem scores related to the attention subdomains of theMoCA, MMSE, and BACS, as well as highlighted all attention deficits (Alemanno et al., 2021; Hosp et al., 2021; Negrini et al., 2020).
Alemanno et al. (2021) were the only researchers to perform a comparison between ICU- and non-ICU-admitted subjects, reporting better scores in the attention subitems of the MMSE and MoCA for the ICU group compared to the non-ICU group, respectively (MMSE p = .03 and MoCA p = .016).
3.3.4. Executive functions
Executive functions (EFs) were frequently assessed, with 8 of the 16 studies reporting cognitive impairments.
Most studies assessed EFs with specific neuropsychological tests (see Table 2). Among these, 4 studies revealed significant deficits: one found the time score of the Trail Making Test Part B (TMT-B) of non-ICU-admitted groups to be higher than a matched HC group (mean time and SD of COVID-19 group: 116.2 ± 65.0 sec vs HC: 80.6 ± 18.7 sec; p = .002; Miskowiak et al., 2021). Another study reported impaired performance of the non-ICU-admitted COVID-19 subjects compared to HC in the Stroop test (mean percentage of errors and SD in COVID-19 group: 4.6 ± .8 vs HC: 1.2 ± .3; p < .001; mean time and SD in COVID-19 group: 969.4 ± 152.1 vs HC: 802.1 ± 122.0; p = .015), as well as in the Frontal Assessment Battery (FAB; mean total score and SD in COVID-19 group: 12.3 ± 2.3 vs HC: 16.7 ± 1.2; p = .001; Ortelli et al., 2021). The FAB global score was also impaired in the third study (Versace et al., 2021), reporting that 12/12 non-ICU-admitted subjects performed under the normality cutoff. Lastly, one study reported 5/7 subjects with mild COVID-19 performing under the normality cutoff (mean and SD: 5 ± 2.94; max score: 10; cutoff: 9) during the clock drawing test (Matos et al., 2021).
Three studies compared ICU- and non-ICU-admitted groups, reporting confounding results: one found the non-ICU-admitted group scoring to be worse than the ICU-admitted group in the abstraction subdomain of the MoCA (p = .024; Alemanno et al., 2021); another reported worse performance of the ICU group in the Tower of London test compared to the non-ICU group (p = .003; Mattioli et al., 2022); and the last did not report differences in a verbal fluency task (Albu et al., 2021).
3.3.5. Language and visuo-spatial abilities
Language and visuo-spatial abilities were less tested, with just one study specifically assessing language (Almeria et al., 2020) and 5 specifically assessing visuo-spatial abilities (Almeria et al., 2020; Bonizzato et al., 2022; Di Pietro et al., 2021; Mattioli et al., 2021, 2022). The other 3 studies indirectly assessed these functions with the language and visuo-spatial subitems of general assessment screening tests (the MMSE and MoCA; Alemanno et al., 2021; Hosp et al., 2021; Pistarini et al., 2021).
Language was found to be impaired in the subitem scores of the MMSE in the COVID-19 group treated with a Venturi mask, as compared with the ICU group (p = .024; Alemanno et al., 2021). Another study found lower language sub-scores of the MMSE and MoCA for the COVID-19 group compared with the group of post-COVID-19 subjects (respective means, SD and p-values of the COVID-19 group and controls in the MMSE: 6.83 ± 1.50 vs 7.80 ± .52, p = .02; MoCA: 1.55 ± .99 vs 2.40 ± .68, p = .01; Pistarini et al., 2021). The only study specifically assessing language by means of the Boston Naming Test failed to find significant impairments, reporting that only 2.9% of the COVID-19 group performed under the normality cutoff (Almeria et al., 2020).
Five studies specifically assessed the visuo-spatial abilities by administering the visual reproduction of the Wechsler Memory Scale, the Rey–Osterrieth Complex Figure (copy), and the Corsi Test, and none of which found relevant deficits (Almeria et al., 2020; Bonizzato et al., 2022; Di Pietro et al., 2021; Mattioli et al., 2021, 2022). In a study analyzing MoCA visuo-spatial subitems, the mean score of a sample of non-ICU-admitted subjects revealed impaired function (mean, SD: 2.50 ± 1.34//max score: 4; Hosp et al., 2021).
3.4. COVID-19-related clinical and psychiatric symptoms
Clinical conditions associated with a post-COVID-19 infection were detected in all of the included studies. Most of the observed symptoms were respiratory and neurological ones. Specifically, 12 studies reported respiratory conditions following COVID-19 and 17 neurological disorders persisting over 1 month from acute infection. In Table 3, Table 4 , we summarized for each study the number of subjects affected by each clinical condition.
Table 3.
Respiratory conditions COVID-19-related.
| Respiratory conditions COVID-related | Papers | N° affected subjects/ Tot |
N° affected subjects_ICU/ Tot_ICU |
N° affected subjects non_ICU/ Tot_non_ICU |
|---|---|---|---|---|
| ARDS | Albu et al. (2021) | 6/30 | 6/16 | 0/14 |
| Negrini et al. (2021) | 9/9 | 5/5 | 4/4 | |
| Mattioli et al. (2022) | 43/215 | 43/52 | 0/163 | |
| Pneumonia | Albu et al. (2021) | 24/30 | 16/16 | 8/14 |
| Bonizzato et al. (2022) | 6/12 | 0/0 | 6/12 | |
| Di Pietro et al. (2021) | 9/12 | 4/7 | 5/6 | |
| Pulmonary embolism | Albu et al. (2021) | 4/30 | 4/16 | 0/14 |
| Mattioli et al. (2022) | 7/215 | 7/52 | 0/163 | |
| Raman et al. (2021) | 7/58 | NA | NA | |
| Cough | Almeria et al. (2020) | 31/35 | NA | NA |
| Mattioli et al. (2021) | 1/120 | 0/0 | 1/120 | |
| Raman et al. (2021) | 35/58 | NA | NA | |
| Breathing difficulties/shortness of breath | Raman et al. (2021) | 51/58 | NA | NA |
| Almeria et al. (2020) | 33/35 | NA | NA | |
| Graham et al. (2021) | 17/50 | 0/0 | 0/0 | |
| COPD | De Lorenzo et al. (2020) | 2/185 | NA | 2/126 |
| Dyspnea | De Lorenzo et al. (2020) | 58/185 | NA | 40/126 |
| Mattioli et al. (2021) | 13/102 | 0/0 | 13/102 | |
| Tachypnoea | De Lorenzo et al. (2020) | 41/185 | NA | 33/126 |
| Asthma | Miskowiak et al. (2021) | 10/29 | 0/0 | 10/29 |
| Hypoxia/hypoxemic respiratory failure | Jaywant et al. (2021) | 50/56 | NA | NA |
ARDS: Acute Respiratory Distress Syndrome; COPD: Chronic Obstructive Pulmonary Disease.
Table 4.
Neurological conditions COVID-19-related.
| Neurological conditions COVID-related |
Papers | N° affected subjects/ Tot |
N° affected subjects_ICU/ Tot_ICU |
N° affected subjects_ non_ICU/ Tot_non_ICU |
|---|---|---|---|---|
| CIP/CIM | Albu et al. (2021) | 3/30 | 3/16 | 0/14 |
| Frontera et al. (2021) | 7/196 | NA | NA | |
|
Ortelli et al., 2021.a Versace et al., 2021a |
6/12 | NA | 6/12 | |
| CIP/CIM + Spinal epidural hematoma | Albu et al. (2021) | 1/30 | 1/30 | 0/14 |
| Brain Stroke | Albu et al. (2021) | 4/30 | 4/30 | 0/14 |
| Bonizzato et al. (2022) | 4/12 | 0/0 | 4/12 | |
| Frontera et al. (2021) | 21/196 | NA | NA | |
|
Ortelli et al., 2021.a Versace et al., 2021.a |
1/12 | 0/0 | 1/12 | |
| Encephalopathy | Albu et al. (2021) | 2/30 | 2/30 | 0/14 |
| Frontera et al. (2021) | 102/196 | NA | NA | |
|
Ortelli et al., 2021.a Versace et al., 2021.a |
3/12 | 0/0 | 3/12 | |
| Anosmia | Alemanno et al. (2021) | 18/87 | Not specified | Not specified |
| Almeria et al. (2020) | 20/35 | NA | NA | |
| Mattioli et al. (2021) | 23/120 | 0/0 | 23/120 | |
|
Ortelli et al., 2021.a Versace et al., 2021.a |
6/12 | 0/0 | 6/12 | |
| Pirker-Kees et al. (2021) | 6/7 | 0/0 | 6/7 | |
| Raman et al. (2021) | 26/58 | NA | NA | |
| Graham et al. (2021) | 27/50 | 0/0 | 0/0 | |
| Hosp et al. (2021) | 25/29 | NA | 25/29 | |
| Myalgias | Almeria et al. (2020) | 30/35 | NA | NA |
| Mattioli et al. (2021) | 11/120 | 0/0 | 11/120 | |
|
Ortelli et al., 2021.a Versace et al., 2021.a |
3/12 | 0/0 | 3/12 | |
| Graham et al. (2021) | 30/50 | 0/0 | 0/0 | |
| Dysgeusia | Almeria et al. (2020) | 20/35 | NA | NA |
| Mattioli et al. (2021) | 13/120 | 0/0 | 13/120 | |
|
Ortelli et al., 2021.a Versace et al., 2021.a |
1/12 | 0/0 | 1/12 | |
| Raman et al. (2021) | 29/58 | NA | NA | |
| Hosp et al. (2021) | 29/29 | 0/0 | 29/29 | |
| Graham et al. (2021) | 32/50 | 0/0 | 0/0 | |
| Myasthenia | Di Pietro et al. (2021) | 1/12 | 1/7 | 0/5 |
| Delirium | Negrini et al. (2021) | 1/9 | 0/4 | 1/5 |
| Di Pietro et al. (2021) | 2/12 | 2/7 | 0/5 | |
| Neuropathy | Frontera et al. (2021) | 15/196 | NA | NA |
| Di Pietro et al. (2021) | 1/12 | 1/7 | 0/5 | |
| Seizures | Frontera et al. (2021) | 21/196 | NA | NA |
| Matos et al. (2021) | 1/7 | NA | NA | |
| Negrini et al. (2021) | 2/9 | 0/4 | 2/5 | |
| Movement disordersb | Frontera et al. (2021) | 15/196 | NA | NA |
| Graham et al. (2021) | 3/50 | 0/0 | 0/0 | |
| Heyns et al. (2021) | 71/86 | 22/25 | 49/61 | |
| Mattioli et al. (2021) | 1/120 | 0/0 | 1/120 | |
| Mattioli et al. (2022) | 2/215 | 0/52 | 2/163 | |
| Guillain Barre Syndrome | Frontera et al. (2021) | 3/196 | NA | NA |
| Mattioli et al. (2022) | 2/215 | 2/52 | 0/163 | |
|
Ortelli et al., 2021.a Versace et al., 2021.a |
2/12 | 0/0 | 2/12 | |
| Neglect |
Ortelli et al., 2021.a Versace et al., 2021.a |
1/12 | 0/0 | 1/12 |
| Hosp et al. (2021) | 1/29 | 0/0 | 1/29 | |
| Cranial nerve dysfunctions | Hosp et al. (2021) | 3/29 | 0/0 | 3/29 |
| Numbness | Graham et al. (2021) | 29/50 | 0/0 | 0/0 |
| Dizziness | Graham et al. (2021) | 29/50 | 0/0 | 0/0 |
| Mattioli et al. (2021) | 1/120 | 0/0 | 1/120 | |
| Blurred vision | Graham et al. (2021) | 9/50 | 0/0 | 0/0 |
| Hearing Loss | Mattioli et al. (2021) | 2/120 | 0/0 | 2/120 |
| Tinnitus | Graham et al. (2021) | 12/50 | 0/0 | 0/0 |
| Dysarthria | Graham et al. (2021) | 2/50 | 0/0 | 0/0 |
| Aphasia | Graham et al. (2021) | 1/50 | 0/0 | 0/0 |
| Memory difficultiesc | Mattioli et al. (2021) | 8/120 | 0/0 | 8/120 |
| Attention difficultiesc | Mattioli et al. (2021) | 14/120 | 0/0 | 14/120 |
| EEG abnormalities | Del Brutto et al. (2021) | 2/52 | NA | NA |
| Temporal/Spatial Confusion | Matos et al. (2021) | 5/7 | NA | NA |
CIP/CIM: Critical Illness Polyneuropathy/Critical Illness Myopathy.
Same cohort.
Frontera: not specified movement disorders; Graham: self-reported abnormal movements; Mattioli: Tremor; Heyns: Five time sit to stand test below cut-off or impossible to perform.
Self-reported cognitive difficulties.
Renal and cardiovascular symptoms were reported as well but less consistently (Supplementary Material, Table S2).
Fourteen of the 25 included studies assessed the presence of psychiatric conditions related to post-COVID-19 syndrome.
Eleven studies assessed the presence of depression (Albu et al., 2021; Alemanno et al., 2021; Almeria et al., 2020; Bonizzato et al., 2022; Del Brutto et al., 2021; Frontera et al., 2021; Heyns et al., 2021; Mattioli et al., 2021; Mazza et al., 2021; Ortelli et al., 2021; Pistarini et al., 2021) by means of the Hospital Anxiety and Depression scale (HADS), Anxiety and Depression short scale (AD-R), Hamilton Rating Scale for Depression (HRSD), Neuropsychiatric Inventory (NPI), and Beck Depression Inventory (BDI). Three of these reported mild symptoms of depression (BDI score: 3.05 ± 4.55 in Mazza et al., 2021; 3.8 ± 2.9 in Ortelli et al., 2021; HRSD score: 8.05 ± 5.60 in Pistarini et al., 2021).
Anxiety was assessed with the HASD, AD-R, State-Trait Anxiety Inventory (STAI-Y), and General Anxiety Disorder (GAD-7) in 9 studies (Albu et al., 2021; Bonizzato et al., 2022; De Lorenzo et al., 2020; Frontera et al., 2021; Heyns et al., 2021; Mattioli et al., 2021; Mazza et al., 2021; Raman et al., 2021; Zhou et al., 2020a), of which 2 detected significant symptoms (HASD score: median: 6 (4–10) for Albu et al., 2021; GAD score: 4.28 ± 4.05 for Zhou et al., 2020a).
Of the 3 studies evaluating the presence of posttraumatic stress disorder (PTSD) respectively with the Davidson Trauma Scale (DTS), the PTSD checklist for DSM-5 (PCL-5), and the Impact of Event Scale (IES-R; De Lorenzo et al., 2020; Mazza et al., 2021; Pistarini et al., 2021), only one found significant impairment (IES-R score: 15.90 ± 14.43 for Pistarini et al., 2021).
The single study evaluating the presence of obsessive-compulsive disorders due to COVID-19 failed to find significant results (OCI score: 14.04 ± 11.71 for Mazza et al., 2021). Psychiatric symptoms were reported only in the non-ICU admitted group.
4. Discussion
This review highlights the existence of cognitive impairment in people with a previous COVID-19 infection. The small number of studies on this topic, marred by clinical and methodological heterogeneity, prevented the definition of a clear-cut cognitive impairment pattern. However, based on the available evidence, we could answer our three research questions.
First, we aimed to characterize the cognitive sequelae of a post-COVID-19 infection. Most of the included studies assessed cognitive functions by means of brief cognitive screens (i.e., the MMSE and MoCA): subitem scores analyses highlighted a major contribution of memory, attention, and executive functions in determining the under-normality threshold global scores. This general trend is confirmed by studies adopting domain-specific assessment tools. Delayed recall and learning tasks were more affected among memory functions, whereas impairments in immediate recall and working memory were less consistent. Sustained and divided attention, as well as abstraction and inhibition, were frequently impaired. Language and visuo-spatial abilities seemed to be less affected, but this finding may be biased by the few studies focusing on these domains and by inaccurate testing. Overall, the predominant emerging profile is compatible with a mild executive dysfunction syndrome (Ardila & Lahiri, 2020). Executive function disturbances have been documented in association with a diversity of psychiatric and neurological pathologies, including dementia, traumatic head injury, depression, and other conditions characterized by diffused brain abnormalities (Ardila & Lahiri, 2020). Seventeen of our 26 included studies reported neurological symptoms persisting after COVID-19, suggesting a CNS involvement. The wide range of neurological manifestations associated with a COVID-19 infection (e.g., anosmia, stroke, paralysis, cranial nerve deficits, encephalopathy, seizures) and the associated cognitive decline might be related to brain inflammatory reaction, hypoxic-ischemic damage or microbleeding/microvascular damage; a direct neurotropism of the virus seems less likely but cannot be excluded (Alomari et al., 2020; Cosentino et al., 2021; Karnik et al., 2021). A similar excessive inflammatory response (the so-called “cytokine storm”) is reportedly associated with other systemic perduring symptoms. Additionally, brain imaging studies in people with COVID-19 revealed structural and functional abnormalities in the olfactory cortices, amygdala, hypothalamus, insula, entorhinal cortex, anterior cingulate cortex, hippocampus, and prefrontal cortex (Najt et al., 2021), all anatomical regions related with memory and executive alterations as well as with the reported affective symptoms.
Given this evidence on COVID-19-related CNS alterations and their prevalence, some authors specifically referred to neuro-COVID-19 instead of using the expression “long-COVID,” which encloses a broader set of symptoms (Akbarialiabad et al., 2021; Chiappelli, 2020; Greenhalgh et al., 2020).
Our second aim was to identify possible psychological/psychiatric factors associated with post-COVID-19 cognitive impairments. We found depression and anxiety to be the most prevalent psycho-affective conditions. Both ICU and non-ICU COVID-19 survivors are likely to experience psychological distress (Cai et al., 2020; Egede et al., 2022), which in most severe cases may lead to PTSD (e.g., Kaseda & Levine, 2020; Nagarajan et al., 2022), depression, and anxiety (Parker et al., 2021). This raises the question about whether the observed cognitive alterations can be related to psychiatric or psycho-affective conditions rather than substantial cognitive impairments. We cannot a priori exclude psychiatric factors as determinants of the reported cognitive sequelae in post-COVID-19. Also, the limited duration of follow-up in the retrieved studies prevents the definition of the natural history of this disturbance: PTSD may persist up to 12 months from hospital discharge (Parker et al., 2015), and this also includes depression (Jackson et al., 2014), which may be observed in some cases up to 5 years (Adhikari et al., 2011), and anxiety (Hopkins et al., 2010; Mikkelsen et al., 2012; Myhren et al., 2010). Long-term follow-up studies are needed to monitor the eventual evolution pyscho-affective symptoms after a COVID-19 infection.
Lastly, we wondered if different admission settings (e.g., intensive care unit, general ward, home recovery) could have led to different cognitive outcomes. We could not derive any conclusion on home-recovered people, as only one study reported cognitive data in this subgroup. We gathered scarce evidence of differences between ICU- and non-ICU-admitted groups in cognitive and clinical post-COVID-19 symptoms because of the paucity of studies comparing the two groups. Apparently, non-ICU-admitted groups reported more frequent impairments compared to ICU-admitted groups, although this finding may be biased by the higher number of studies enrolling from non-ICU medical wards.
ICU admission is a relevant variable when considering cognitive dysfunctions. Indeed, according to some authors, what is currently termed “long-COVID” can rather be referred to as PICS (Mahase, 2020). PICS is a common condition affecting people after a prolonged stay in ICU, characterized by persisting physical, cognitive, and emotional difficulties (LaBuzetta et al., 2019). Major risk factors for PICS include the duration of delirium in ICU, hypoxia, respiratory failure requiring prolonged mechanical ventilation, acute respiratory distress syndrome (ARDS), and preexisting cognitive impairment (Rawal et al., 2017). These conditions can eventually manifest in people with a COVID-19 infection.
Attention, executive functions, visual and working memory, and visuo-spatial skills are the main affected domains that highly overlap with post-COVID-19 ones (Jackson et al., 2003; 2007; Mikkelsen et al., 2012). Thus, the well-documented contribution of PICS to cognitive deficits (Rawal et al., 2017) can mask an eventual independent contribution of a COVID-19 infection in determining the cognitive deficits observed in those admitted to the ICU.
We could also not rule out the contribution of depression and anxiety on cognitive performance itself. Indeed, the association between depression and anxiety with cognitive deficits, especially on tasks of episodic memory, working memory, attention, and executive functioning, is well known (Dotson et al., 2014). We found a higher incidence of depression and anxiety in the non-ICU-admitted group, which included those who also emerged as more cognitively affected after COVID-19. These affective symptoms could have impacted the non-ICU-admitted group's cognitive performances, masking again the eventual contribution of COVID-19 on the resulting cognitive outcome.
In light of the many uncertainties still surrounding cognitive impairments in a long-COVID-19 syndrome, clinicians must take into account the multifaceted genesis of these deficits and build upon this knowledge to provide the best care and limit these impairments.
5. Limitations
This review has several limitations. First, literature on this topic is still restricted and characterized by methodological heterogeneity, which prevents a precise extraction of a COVID-19 cognitive profile. The majority of studies enrolled small and scarcely selected samples. Second, most of the included studies did not report cognitive test scores but percentages of impaired subjects, not always distinguishing between COVID-19 subgroups. Thus, we could not adequately analyze differences in cognitive consequences between ICU and non-ICU hospitalized people.
Moreover, the use of brief cognitive screeners, such as the MoCA and MMSE, may provide only general information of domain-specific impairments that require further investigation through domain-specific tasks. In addition, 2 of the included studies adopted brief cognitive screening tests originally developed and validated to assess patients with psychiatric disorders, and one study employed a telephone-administered version of the MoCA.
Lastly, the included studies did not equally assess cognitive and psycho-affective domains, hampering the disentangling of their mutual relationship in determining the final cognitive outcome.
Our review also has methodological limitations: we used an arbitrary cutoff of 50% of participants with an impairment domain to consider the function affected. This may have created a reporting bias, thus increasing the frequency of impairments.
6. Conclusion
We reviewed the existing evidence on long-term cognitive sequelae after a COVID-19 infection, characterized by a pattern of memory, attention, and executive function impairments. Whether these cognitive symptoms are a direct consequence of CNS involvement during the acute infection or a reactive distress syndrome due to hospitalization and/or pandemic isolation remains to be clarified. Further studies, with bigger samples and comprehensive neuropsychological and psychiatric assessment batteries, are needed to disclose long-term COVID-19 consequences and link them to their neurophysiological and neurobiological substrate.
Credit author statement
Margherita Bertuccelli: Conceptualization, Methodology, Investigation, Writing - Original Draft, Review & Editing; Luciana Ciringione: Conceptualization, Methodology, Investigation, Writing - Original Draft, Review & Editing; Maria Rubega: Conceptualization, Methodology, Writing - Review & Editing; Patrizia Bisiacchi: Conceptualization, Supervision; Stefano Masiero: Supervision; Alessandra Del Felice: Conceptualization, Writing - Review & Editing, Supervision, Project Administration.
Funding
This work was supported by the “Department of Excellence 2018-2022” initiative of the Italian Ministry of Education (MUR) awarded to the Department of Neuroscience - University of Padova, and by the fellowship “Clinical-instrumental screening for the assessment of psychopathological, cognitive, and sleep disorders in COVID-19 survivors,” crowdfunded by the University of Padova (LC). MR is also supported by REACT EU - PON “Ricerca e Innovazione 2014–2020”, DM 1062/202. ADF is also supported by an EU-funded H2020 Research and Innovation Staff Exchange grant: PRO GAIT grant agreement No. 778043 (PRO GAIT project, www.progait.eu), and from the Italian Ministry for foreign Affairs and International Cooperation under grant No. PGR-01045 (SoftAct project).
Declaration of competing interest
The authors have no relevant financial or nonfinancial interests to disclose.
Action editor Brad Dickerson
Reviewed 17 February 2022
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.cortex.2022.06.002.
Appendix.
1. PubMed search strategy.
PubMed queries
| Search number | Query | Results |
|---|---|---|
| 1 | (COVID-19[MeSH Terms]) OR (SARS-CoV-2[MeSH Terms]) | 145,442 |
| 2 | ((((cognitive impairment [MeSH Terms]) OR (neuropsychological impairment [MeSH Terms])) OR (cognitive assessment [MeSH Terms])) OR (neuropsychological assessment [MeSH Terms])) OR (cognitive dysfunction [MeSH Terms]) |
208,66 |
| 3 | #1 AND #2 | 382 |
2. Data extraction form.
Data extraction form
| ORGANISATIONAL ASPECTS | EX | IN | |
|---|---|---|---|
| REF ID | Reviewer, Date | Checked by | |
| Author, Year | |||
| Journal/Source | Study ID | NR/ | |
| Country of origin | |||
| Publication type | Full text/Abstract/Book chapter/internal progress report other (please specify) | ||
| Fate | Decision pending/Check references/Use for discussion/EX without listing/EX with listing/Other (please specify) | ||
| Notes/Short description | |||
| CURRENT STATUS:(NAME OF REVIEWER + DATE) | |||
| Question to author | |||
| Status verified with study investigators or sponsors: Yes/No | |||
| Enter name of the source (e.g., PI, sponsor, etc.)________________ | |||
| Contact address: | |||
Eligibility Form.
Eligibility form: Inclusion criteria
| Factors | Assessment | Comments |
|---|---|---|
| Article characteristics | ||
| 1. Did the study undergo a full peer review? | YES NO UNCLEAR | If NO → exclude |
| 2. Is it a single case study? | YES NO UNCLEAR | If YES → exclude |
| 3. Is it an animal study? | YES NO UNCLEAR | If YES → exclude |
| 4. Is it written in English? | YES NO UNCLEAR | If NO → exclude |
| Participants | ||
| Were participants diagnosed with COVID-19 infection? | YES NO UNCLEAR | If NO → exclude |
| Were participants diagnosed with other neurological or respiratory disorders in comorbidity? | YES NO UNCLEAR | If YES → exclude |
| Methodology | ||
| Were participants tested with standardized neuropsychological tests? | YES NO UNCLEAR | If NO → exclude |
| Outcomes | ||
| Did the study report cognitive outcomes? | YES NO UNCLEAR | If NO → exclude |
|
FINAL DECISION |
YES NO |
|
|
REASONS FOR EXCLUSION FROM REVIEW |
||
| Article type | No full-length article/Animal study/Single case study/Review/No English language | |
| Methods | Observational evaluations/Questionnaires/Not standardized neuropsychological tests | |
| Population | Infection other than COVID-19 and comorbidities | |
| Outcomes | No cognitive dysfunctions assessed | |
| Other | ||
| None | INCLUDED | |
Eligibility form: Study characteristics
| STUDY CHARACTERISTICS | ||
|---|---|---|
| Diagnosis | ||
| Treatment/Recovery | ICU recovery No ICU recovery Home recovery Other: |
|
| Clinical characteristics related to COVID-19 | ARDS (assisted ventilation) peripheral neurological deficits due to COVID-19 delirium myocardial dysfunction renal failure other |
|
| Inclusion criteria | ||
| Exclusion criteria | ||
| Neuropsychological test used |
MoCA MMSE FAB Stroop Symbol Digit Trail Making Test Digit span 15 Rey Other |
|
| Reported results |
||
| Confounders |
Medications |
|
|
TRIAL CHARACTERISTICS | ||
| Sample size | ||
| Number of excluded subjects | ||
| Recruitment method | ||
| Setting of assessment | hospital/home/unclear/NR | |
| Trial Design | longitudinal cross-sectional retrospective case control (?) |
|
| Length of follow-up | From_________till_ | |
| Time of cognitive assessment from symptoms' onset | 0–3 months/3–6 months/from 6 months/unclear/unspecified | |
| Number of groups |
||
| BASELINE CHARACTERISTICS OF PATIENTS | ||
| Number of subjects | ||
| Age | ||
| mean/± | ± | |
| median/± | ± | |
| Ethnicity No. % |
||
| Gender No. % |
||
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- Adhikari N.K.J., Tansey C.M., McAndrews M.P., Matté A., Pinto R., Cheung A.M., Diaz-Granados N., Herridge M.S. Self-reported depressive symptoms and memory complaints in survivors five years after ARDS. Chest. 2011;140(6):1484–1493. doi: 10.1378/chest.11-1667. [DOI] [PubMed] [Google Scholar]
- Aiello E.N., Fiabane E., Manera M.R., Radici A., Grossi F., Ottonello M.…Pistarini C. Episodic long-term memory in post-infectious SARS-CoV-2 patients. Neurological Sciences. 2022;43(2):785–788. doi: 10.1007/s10072-021-05752-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akbarialiabad H., Taghrir M.H., Abdollahi A., Ghahramani N., Kumar M., Paydar S., Razani B., Mwangi J., Asadi-Pooya A.A., Malekmakan L., Bastani B. Long COVID, a comprehensive systematic scoping review. Infection. 2021 doi: 10.1007/s15010-021-01666-x. 0123456789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albu S., Zozaya N.R., Murillo N., García-Molina A., Chacón C.A.F., Kumru H. What's going on following acute COVID-19? Clinical characteristics of patients in an out-patient rehabilitation program. Neurorehabilitation. 2021;48(4):469–480. doi: 10.3233/nre-210025. [DOI] [PubMed] [Google Scholar]
- Alemanno F., Houdayer E., Parma A., Spina A., Del Forno A., Scatolini A., Angelone S., Brugliera L., Tettamanti A., Beretta L., Iannaccone S. COVID-19 cognitive deficits after respiratory assistance in the subacute phase: A COVID-rehabilitation unit experience. Plos One. 2021;16(2) doi: 10.1371/journal.pone.0246590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almeria M., Cejudo J.C., Sotoca J., Deus J., Krupinski J. Cognitive profile following COVID-19 infection: Clinical predictors leading to neuropsychological impairment. Brain, Behavior, & Immunity - Health. 2020;9(October) doi: 10.1016/j.bbih.2020.100163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alomari S.O., Abou-mrad Z., Bydon A. Clinical Neurology and Neurosurgery; January: 2020. COVID-19 and the central nervous system. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ardila A., Lahiri D. Executive dysfunction in COVID-19 patients. Diabetes & Metabolic Syndrome. 2020;14(5):1377. doi: 10.1016/j.dsx.2020.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Augustin M., Schommers P., Stecher M., Dewald F., Gieselmann L., Gruell H., Horn C., Vanshylla K., Cristanziano V. Di, Osebold L., Roventa M., Riaz T., Tschernoster N., Altmueller J., Rose L., Salomon S., Priesner V., Luers J.C., Albus C.…Lehmann C. Post-COVID syndrome in non-hospitalised patients with COVID-19: A longitudinal prospective cohort study. The Lancet Regional Health - Europe. 2021;6:1–8. doi: 10.1016/j.lanepe.2021.100122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badenoch J.B., Rengasamy E.R., Watson C., Jansen K., Chakraborty S., Sundaram R.D.…Rooney A.G. Persistent neuropsychiatric symptoms after COVID-19: A systematic review and meta-analysis. Brain communications. 2022;4(1):fcab297. doi: 10.1093/braincomms/fcab297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonizzato S., Ghiggia A., Ferraro F., Galante E. Cognitive, behavioral, and psychological manifestations of COVID-19 in post-acute rehabilitation setting: Preliminary data of an observational study. Neurological Sciences. 2022;43(1):51–58. doi: 10.1007/s10072-021-05653-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai X., Hu X., Ekumi I.O., Wang J., An Y., Li Z., Yuan B. Psychological distress and its correlates among COVID-19 survivors during early convalescence across age groups. The American Journal of Geriatric Psychiatry. 2020;28(10):1030–1039. doi: 10.1016/j.jagp.2020.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cares-Marambio K., Montenegro-Jiménez Y., Torres-Castro R., Vera-Uribe R., Torralba Y., Alsina-Restoy X., Vasconcello-Castillo L., Vilaró J. Prevalence of potential respiratory symptoms in survivors of hospital admission after coronavirus disease 2019 (COVID-19): A systematic review and meta-analysis. Chronic Respiratory Disease. 2021;18 doi: 10.1177/14799731211002240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceban F., Ling S., Lui L.M., Lee Y., Gill H., Teopiz K.M.…McIntyre R.S. 2021. Fatigue and cognitive impairment in post-COVID-19 syndrome: A systematic review and meta-analysis. Brain, behavior, and immunity. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiappelli F. Towards neuro-CoViD-19. Bioinformation. 2020;16(4):288–292. doi: 10.6026/97320630016288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosentino G., Todisco M., Hota N., Della Porta G., Morbini P., Tassorelli C., Pisani A. Neuropathological findings from COVID-19 patients with neurological symptoms argue against a direct brain invasion of SARS-CoV-2: A critical systematic review. European Journal of Neurology, June. 2021:3856–3865. doi: 10.1111/ene.15045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis H.E., Assaf G.S., McCorkell L., Wei H., Low R.J., Re’em Y., Redfield S., Austin J.P., Akrami A. Characterizing long COVID in an international cohort: 7 months of symptoms and their impact. EClinicalMedicine. 2021;38 doi: 10.1016/j.eclinm.2021.101019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Lorenzo R., Conte C., Lanzani C., Benedetti F., Roveri L., Mazza M.G., Brioni E., Giacalone G., Canti V., Sofia V., D'Amico M., Di Napoli D., Ambrosio A., Scarpellini P., Castagna A., Landoni G., Zangrillo A., Bosi E., Tresoldi M.…Rovere-Querini P. Residual clinical damage after COVID-19: A retrospective and prospective observational cohort study. Plos One. 2020;15(10 October):1–16. doi: 10.1371/journal.pone.0239570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Brutto O.H., Wu S., Mera R.M., Costa A.F., Recalde B.Y., Issa N.P. Cognitive decline among individuals with history of mild symptomatic SARS-CoV-2 infection: A longitudinal prospective study nested to a population cohort. European journal of neurology. 2021;28(10):3245–3253. doi: 10.1111/ene.14775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Pietro D.A., Comini L., Gazzi L., Luisa A., Vitacca M. Neuropsychological pattern in a series of post-acute COVID-19 patients in a rehabilitation unit: Retrospective analysis and correlation with functional outcomes. International Journal of Environmental Research and Public Health. 2021;18(11):3–12. doi: 10.3390/ijerph18115917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dotson V.M., Szymkowicz S.M., Kirton J.W., McLaren M.E., Green M.L., Rohani J.Y. Unique and interactive effect of anxiety and depressive symptoms on cognitive and brain function in young and older adults. Journal of depression & anxiety. 2014;(Suppl 1) doi: 10.4172/2167-1044.S1-003. 22565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egede L.E., Walker R.J., Dawson A.Z., Zosel A., Bhandari S., Nagavally S.…Frank M. Quality of Life Research; 2022. Short-term impact of COVID-19 on quality of life, perceived stress, and serious psychological distress in an adult population in the midwest United States. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frontera J.A., Yang D., Lewis A., Patel P., Medicherla C., Arena V., Fang T., Andino A., Snyder T., Madhavan M., Gratch D., Fuchs B., Dessy A., Canizares M., Jauregui R., Thomas B., Bauman K., Olivera A., Bhagat D.…Galetta S. A prospective study of long-term outcomes among hospitalized COVID-19 patients with and without neurological complications. Journal of the Neurological Sciences. 2021;426(April) doi: 10.1016/j.jns.2021.117486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham E.L., Clark J.R., Orban Z.S., Lim P.H., Szymanski A.L., Taylor C., DiBiase R.M., Jia D.T., Balabanov R., Ho S.U., Batra A., Liotta E.M., Koralnik I.J. Persistent neurologic symptoms and cognitive dysfunction in non-hospitalized Covid-19 “long haulers”. Annals of Clinical and Translational Neurology. 2021;8(5):1073–1085. doi: 10.1002/acn3.51350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenhalgh T., Knight M., A'Court C., Buxton M., Husain L. Management of post-acute covid-19 in primary care. Bmj: British Medical Journal. 2020;370 doi: 10.1136/bmj.m3026. [DOI] [PubMed] [Google Scholar]
- Groiss S.J., et al. Prolonged neuropsychological deficits, central nervous system involvement, and brain stem affection after COVID-19—A case series. Frontiers in Neurology. 2020;11:574004. doi: 10.3389/fneur.2020.574004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampshire A., Trender W., Chamberlain S.R., Jolly A., Grant J.E., Patrick F.…Mehta M.A. Cognitive deficits in people who have recovered from COVID-19. eClinicalMedicine. 2021;39:101044. doi: 10.1016/j.eclinm.2021.101044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heyns A., Dupont J., Gielen E., Flamaing J., Peers K., Gosselink R.…Tournoy J. Impact of COVID-19: Urging a need for multi-domain assessment of COVID-19 inpatients. European geriatric medicine. 2021;12(4):741–748. doi: 10.1007/s41999-021-00486-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins R.O., Key C.W., Suchyta M.R., Weaver L.K., Orme J.F. Risk factors for depression and anxiety in survivors of acute respiratory distress syndrome. General Hospital Psychiatry. 2010;32(2):147–155. doi: 10.1016/j.genhosppsych.2009.11.003. [DOI] [PubMed] [Google Scholar]
- Hosp J.A., Dressing A., Blazhenets G., Bormann T., Rau A., Schwabenland M., Thurow J., Wagner D., Waller C., Niesen W., Frings L., Urbach H., Prinz M., Weiller C., Schroeter N., Meyer P. Cognitive impairment and altered cerebral glucose metabolism in the subacute stage of COVID-19. Brain: a Journal of Neurology. 2021;144(4):1263–1276. doi: 10.1093/brain/awab009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson J.C., Hart R.P., Gordon S.M., Shintani A., Truman B., May L., Ely E.W. Six-month neuropsychological outcome of medical intensive care unit patients. Critical Care Medicine. 2003;31(4):1226–1234. doi: 10.1097/01.ccm.0000059996.30263.94. [DOI] [PubMed] [Google Scholar]
- Jackson J.C., Pandharipande P.P., Girard T.D., Brummel N.E., Thompson J.L., Hughes C.G., Pun B.T., Vasilevskis E.E., Morandi A., Shintani A.K., Hopkins R.O., Bernard G.R., Dittus R.S., Ely E.W. Depression, post-traumatic stress disorder, and functional disability in survivors of critical illness in the BRAIN-ICU study: A longitudinal cohort study. The Lancet Respiratory Medicine. 2014;2(5):369–379. doi: 10.1016/S2213-2600(14)70051-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaywant A., Vanderlind W.M., Alexopoulos G.S., Fridman C.B., Perlis R.H., Gunning F.M. Frequency and profile of objective cognitive deficits in hospitalized patients recovering from COVID-19. Neuropsychopharmacology: Official Publication of the American College of Neuropsychopharmacology. 2021;46(13):2235–2240. doi: 10.1038/s41386-021-00978-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karnik M., Beeraka N.M., Uthaiah C.A., Nataraj S.M., Bettadapura A.D.S., Aliev G., Madhunapantula S.V. A review on SARS-CoV-2-induced neuroinflammation, neurodevelopmental complications, and recent updates on the vaccine development. Molecular Neurobiology. 2021;58(9):4535–4563. doi: 10.1007/s12035-021-02399-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaseda E.T., Levine A.J. Post-traumatic stress disorder: A differential diagnostic consideration for COVID-19 survivors. The Clinical Neuropsychologist. 2020;34(7–8):1498–1514. doi: 10.1080/13854046.2020.1811894. [DOI] [PubMed] [Google Scholar]
- LaBuzetta J.N., Rosand J., Vranceanu A.-M. Review: Post-Intensive care syndrome: Unique challenges in the neurointensive care unit. Neurocritical Care. 2019;31(3):534–545. doi: 10.1007/s12028-019-00826-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levac D., Colquhoun H., O'Brien K.K. Scoping studies: Advancing the methodology. Implementation Science. 2010;5(1):1–9. doi: 10.1186/1748-5908-5-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Leon S., Wegman-Ostrosky T., Perelman C., Sepulveda R., Rebolledo P.A., Cuapio A., Villapol S. More than 50 long-term effects of COVID-19: A systematic review and meta-analysis. Scientific Reports. 2021;11(1):1–12. doi: 10.1038/s41598-021-95565-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahase E. Long covid could be four different syndromes, review suggests. BMJ (Clinical research ed.) 2020;371(October):m3981. doi: 10.1136/bmj.m3981. [DOI] [PubMed] [Google Scholar]
- Matos A.D.M.B., Dahy F.E., de Moura J.V.L., Marcusso R.M.N., Gomes A.B.F., Carvalho F.M.M.…Study Group N.C.B.R. Subacute cognitive impairment in individuals with mild and moderate COVID-19: A case series. Frontiers in Neurology. 2021;12 doi: 10.3389/fneur.2021.678924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattioli F., Piva S., Stampatori C., Righetti F., Mega I., Peli E.…De Palma G. Neurologic and cognitive sequelae after SARS-CoV2 infection: Different impairment for ICU patients. Journal of the neurological sciences. 2022;432 doi: 10.1016/j.jns.2021.120061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattioli F., Stampatori C., Righetti F., Sala E., Tomasi C., De Palma G. Neurological and cognitive sequelae of covid-19: A four month follow-up. Journal of Neurology. 2021;268(12):4422–4428. doi: 10.1007/s00415-021-10579-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazza M.G., Palladini M., De Lorenzo R., Magnaghi C., Poletti S., Furlan R., Ciceri F., Rovere-Querini P., Benedetti F. Persistent psychopathology and neurocognitive impairment in COVID-19 survivors: Effect of inflammatory biomarkers at three-month follow-up. Brain, Behavior, and Immunity. 2021;94(January):138–147. doi: 10.1016/j.bbi.2021.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikkelsen M.E., Christie J.D., Lanken P.N., Biester R.C., Thompson B.T., Bellamy S.L., Localio A.R., Demissie E., Hopkins R.O., Angus D.C. The adult respiratory distress syndrome cognitive outcomes study: Long-term neuropsychological function in survivors of acute lung injury. American Journal of Respiratory and Critical Care Medicine. 2012;185(12):1307–1315. doi: 10.1164/rccm.201111-2025OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miskowiak K.W., Johnsen S., Sattler S.M., Nielsen S., Kunalan K., Rungby J., Lapperre T., Porsberg C.M. Cognitive impairments four months after COVID-19 hospital discharge: Pattern, severity and association with illness variables. European Neuropsychopharmacology. 2021;46:39–48. doi: 10.1016/J.EURONEURO.2021.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazato Y., Morioka S., Tsuzuki S., Akashi M., Osanai Y., Tanaka K.…Ohmagari N. ) Vol. 7. Oxford University Press; US: 2020, November. Prolonged and late-onset symptoms of coronavirus disease 2019; p. ofaa507. (Open forum infectious diseases). No. 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morin L., et al. Four-month clinical status of a cohort of patients after hospitalization for COVID-19. Jama. 2021;325(15):1525–1534. doi: 10.1001/jama.2021.3331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myhren H., Ekeberg Ø., Tøien K., Karlsson S., Stokland O. Posttraumatic stress, anxiety and depression symptoms in patients during the first year post intensive care unit discharge. Critical Care. 2010;14(1):R14. doi: 10.1186/cc8870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagarajan R., Krishnamoorthy Y., Basavarachar V., Dakshinamoorthy R. Prevalence of post-traumatic stress disorder among survivors of severe COVID-19 infections: A systematic review and meta-analysis. Journal of Affective Disorders. 2022;299:52–59. doi: 10.1016/j.jad.2021.11.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Najt P., Richards H.L., Fortune D.G. Brain imaging in patients with COVID-19: A systematic review. Brain, Behavior, & Immunity - Health. 2021;16(June) doi: 10.1016/j.bbih.2021.100290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Needham D.M., Davidson J., Cohen H., Hopkins R.O., Weinert C., Wunsch H., Zawistowski C., Bemis-Dougherty A., Berney S.C., Bienvenu O.J., Brady S.L., Brodsky M.B., Denehy L., Elliott D., Flatley C., Harabin A.L., Jones C., Louis D., Meltzer W.…Harvey M.A. Improving long-term outcomes after discharge from intensive care unit: Report from a stakeholders' conference. Critical Care Medicine. 2012;40(2):502–509. doi: 10.1097/CCM.0b013e318232da75. [DOI] [PubMed] [Google Scholar]
- Negrini F., Ferrario I., Mazziotti D., Berchicci M., Bonazzi M., Sire A. De. the company ’ s public news and information . January; 2020. Since January 2020 Elsevier has created a COVID-19 resource centre with free information in English and Mandarin on the novel coronavirus COVID- 19. The COVID-19 resource centre is hosted on Elsevier Connect. [Google Scholar]
- Negrini F., Ferrario I., Mazziotti D., Berchicci M., Bonazzi M., Sire A.…Koch G. Neuropsychological features of severe hospitalized coronavirus disease 2019 patients at clinical stability and clues for postacute rehabilitation. Archives of Physical Medicine and Rehabilitation. 2021;102(1):155–158. doi: 10.1016/j.apmr.2020.09.376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orrù G., Bertelloni D., Diolaiuti F., Mucci F., Di Giuseppe M., Biella M.…Conversano C. Vol. 9. Multidisciplinary Digital Publishing Institute; 2021, May. Long-COVID syndrome? A study on the persistence of neurological, psychological and physiological symptoms; p. 575. (Healthcare). No. 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortelli P., Ferrazzoli D., Sebastianelli L., Engl M., Romanello R., Nardone R., Bonini I., Koch G., Saltuari L., Quartarone A., Oliviero A., Kofler M., Versace V. Neuropsychological and neurophysiological correlates of fatigue in post-acute patients with neurological manifestations of COVID-19: Insights into a challenging symptom. Journal of the Neurological Sciences. 2021;420(November 2020) doi: 10.1016/j.jns.2020.117271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker C., Shalev D., Hsu I., Shenoy A., Cheung S., Nash S.…Shapiro P.A. Depression, anxiety, and acute stress disorder among patients hospitalized with COVID-19: A prospective cohort study. Journal of the Academy of Consultation-Liaison Psychiatry. 2021;62(2):211–219. doi: 10.1016/j.psym.2020.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker A.M., Sricharoenchai T., Raparla S., Schneck K.W., Bienvenu O.J., Needham D.M. Posttraumatic stress disorder in critical illness survivors: A metaanalysis. Critical Care Medicine. 2015;43(5):1121–1129. doi: 10.1097/CCM.0000000000000882. [DOI] [PubMed] [Google Scholar]
- Pilotto A., Cristillo V., Cotti Piccinelli S., Zoppi N., Bonzi G., Sattin D.…Padovani A. Long-term neurological manifestations of COVID-19: Prevalence and predictive factors. Neurological Sciences. 2021;42(12):4903–4907. doi: 10.1007/s10072-021-05586-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirker-Kees A., Platho-Elwischger K., Hafner S., Redlich K., Baumgartner C. Hyposmia is associated with reduced cognitive function in COVID-19: First preliminary results. Dementia and Geriatric Cognitive Disorders. 2021;50(1):68–73. doi: 10.1159/000515575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pistarini C., Fiabane E., Houdayer E., Vassallo C., Manera M.R., Alemanno F. Cognitive and emotional disturbances due to COVID-19: An exploratory study in the rehabilitation setting. Frontiers in Neurology. 2021;12(May):1–8. doi: 10.3389/fneur.2021.643646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raman B., Cassar M.P., Tunnicliffe E.M., Filippini N., Griffanti L., Alfaro-Almagro F., Okell T., Sheerin F., Xie C., Mahmod M., Mózes F.E., Lewandowski A.J., Ohuma E.O., Holdsworth D., Lamlum H., Woodman M.J., Krasopoulos C., Mills R., McConnell F.A.K.…Neubauer S. Medium-term effects of SARS-CoV-2 infection on multiple vital organs, exercise capacity, cognition, quality of life and mental health, post-hospital discharge. EClinicalMedicine. 2021;31 doi: 10.1016/j.eclinm.2020.100683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawal G., Yadav S., Kumar R. Post-intensive care syndrome: An overview. Journal of Translational Internal Medicine. 2017;5(2):90–92. doi: 10.1515/jtim-2016-0016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubega M., Di Marco R., Zampini M., Formaggio E., Menegatti E., Bonato P., Masiero S., Del Felice A. Muscular and cortical activation during dynamic and static balance in the elderly: A scoping review. Aging Brain. 2021;1 doi: 10.1016/j.nbas.2021.100013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah W., Hillman T., Playford E.D., Hishmeh L. Managing the long term effects of covid-19: Summary of NICE, SIGN, and RCGP rapid guideline. Bmj: British Medical Journal. 2021;372:10–13. doi: 10.1136/bmj.n136. [DOI] [PubMed] [Google Scholar]
- Taborri J., Agostini V., Artemiadis P.K., Ghislieri M., Jacobs D.A., Roh J., Rossi S. Feasibility of muscle synergy outcomes in clinics, robotics, and sports: A systematic review. Applied Bionics and Biomechanics. 2018;2018 doi: 10.1155/2018/3934698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tricco A.C., Lillie E., Zarin W., O'Brien K.K., Colquhoun H., Levac D., Moher D., Peters M.D.J., Horsley T., Weeks L., Hempel S., Akl E.A., Chang C., McGowan J., Stewart L., Hartling L., Aldcroft A., Wilson M.G., Garritty C.…Straus S.E. PRISMA extension for scoping reviews (PRISMA-ScR): Checklist and explanation. Annals of Internal Medicine. 2018;169(7):467–473. doi: 10.7326/M18-0850. [DOI] [PubMed] [Google Scholar]
- Versace V., Sebastianelli L., Ferrazzoli D., Romanello R., Ortelli P., Saltuari L., D'Acunto A., Porrazzini F., Ajello V., Oliviero A., Kofler M., Koch G. Intracortical GABAergic dysfunction in patients with fatigue and dysexecutive syndrome after COVID-19. Clinical Neurophysiology. 2021;132(5):1138–1143. doi: 10.1016/j.clinph.2021.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yong S.J. Long COVID or post-COVID-19 syndrome: Putative pathophysiology, risk factors, and treatments. The Lancet Infectious Diseases. 2021;53(10):737–754. doi: 10.1080/23744235.2021.1924397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou H., Lu S., Chen J., Wei N., Wang D., Lyu H., Shi C., Hu S. The landscape of cognitive function in recovered COVID-19 patients. Journal of Psychiatric Research. 2020;129:98–102. doi: 10.1016/j.jpsychires.2020.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou P., Yang X.L., Wang X.G., Hu B., Zhang L., Zhang W., Si H.R., Zhu Y., Li B., Huang C.L., Chen H.D., Chen J., Luo Y., Guo H., Jiang R. Di, Liu M.Q., Chen Y., Shen X.R., Wang X.…Shi Z.L. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579(7798):270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.