Abstract
Background:
Increased rates of respiratory adverse events have been observed in people ≥12 years of age with cystic fibrosis homozygous for the Phe508del-CFTR mutation treated with lumacaftor/ivacaftor, particularly in those with percent predicted forced expiratory volume in 1 s (ppFEV1) of <40%. We evaluated the safety, tolerability, and efficacy of tezacaftor/ivacaftor in people with cystic fibrosis homozygous for Phe508del-CFTR who discontinued lumacaftor/ivacaftor due to treatment-related respiratory signs or symptoms.
Methods:
Participants ≥12 years of age with cystic fibrosis homozygous for Phe508del-CFTR with ppFEV1 of ≥25% and ≤90% were randomized 1:1 and treated with tezacaftor/ivacaftor or placebo for 56 days.
Results:
Of 97 participants, 94 (96.9%) completed the study. The primary endpoint was incidence of predefined respiratory adverse events of special interest (chest discomfort, dyspnea, respiration abnormal, asthma, bronchial hyperreactivity, bronchospasm, and wheezing): tezacaftor/ivacaftor, 14.0%; placebo, 21.3%. The adverse events were mild or moderate in severity. None were serious or led to treatment interruption or discontinuation. Overall, the discontinuation rate was similar between groups. The mean (SD) ppFEV1 at baseline was 44.6% (16.1%) with tezacaftor/ivacaftor and 48.0% (18.1%) with placebo. The posterior mean difference in absolute change in ppFEV1 from baseline to the average value of days 28 and 56 was 2.7 percentage points with tezacaftor/ivacaftor vs placebo.
Conclusions:
Tezacaftor/ivacaftor was generally safe, well tolerated, and efficacious in people ≥12 years of age with cystic fibrosis homozygous for Phe508del-CFTR with ppFEV1 of ≥25% and ≤90% who previously discontinued lumacaftor/ivacaftor due to treatment-related respiratory signs or symptoms.
Keywords: Cystic fibrosis, Clinical trial, Phase 3, Phe508del-CFTR
1. Introduction
Cystic fibrosis (CF)–a rare, autosomal recessive, life-shortening disease–affects more than 90,000 people worldwide [1]. CF is caused by mutations in the CF transmembrane conductance regulator (CFTR) gene that lead to decreased quantity and/or defective function of epithelial cell–surface CFTR proteins, resulting in reduced ion transport and dysfunction in numerous organ systems [2, 3]. Phe508del is the most prevalent CFTR mutation worldwide; approximately 45% of people with CF (pwCF) in the United States [4] and 38% with CF worldwide are homozygous for the Phe508del-CFTR mutation [5]. Addressing the underlying CFTR protein defect in pwCF homozygous for the Phe508del-CFTR mutation has required the combination of a CFTR corrector to increase pro- cessing and trafficking of CFTR to the cell surface and a CFTR potentiator to increase channel open probability [6,7]. The corrector lumacaftor combined with the potentiator ivacaftor was the first CFTR modulator combination therapy approved to treat pwCF homozygous for the Phe508del-CFTR mutation [8,9]. Tezacaftor, an alternative CFTR corrector, in combination with ivacaftor, was later approved to treat pwCF ≥6 years old in the United States and ≥12 years old in other regions with tezacaftor/ivacaftor-responsive mutations.
Lumacaftor/ivacaftor was generally well tolerated and efficacious across phase 3 clinical studies. However, pwCF ≥12 years of age homozygous for Phe508del-CFTR who received lumacaftor/ivacaftor (400 mg/250 mg every 12 hours) reported a higher incidence of certain respiratory adverse events (AEs) than pwCF who received placebo, including dyspnea (13.0% vs 7.8%) and chest tightness (8.7% vs 5.9%) [10]. Additional studies revealed that these respiratory AEs occurred more frequently in pwCF with more severe lung disease (percent predicted forced expiratory volume in 1 s [ppFEV1] of <40%) [11,12]. A subgroup analysis of pooled data from phase 3 studies of lumacaftor/ivacaftor therapy in pwCF ≥12 years of age homozygous for the Phe508del-CFTR mutation showed an increased incidence of respiratory AEs, most notably in pwCF with a ppFEV1 of <40% at baseline [13]. In an observational study, all 12 pwCF with a ppFEV1 of < 40% at screening treated with lumacaftor/ivacaftor experienced an acute decline in ppFEV1 from baseline to 2 hours that persisted at 24 hours but resolved in most pwCF after 1 month [14]. Because the respiratory AE profile may limit the use of lumacaftor/ivacaftor in pwCF homozygous for the Phe508del-CFTR mutation–particularly pwCF ≥12 years of age with a ppFEV1 of < 40%–alternative CFTR modulator therapies have been evaluated.
The efficacy, safety, and tolerability of tezacaftor/ivacaftor were previously established in a randomized controlled clinical trial in pwCF ≥12 years of age homozygous for the Phe508del-CFTR mutation with a ppFEV1 between 40% and 90% [15]. The primary objective of this phase 3b study was to evaluate the respiratory safety of tezacaftor/ivacaftor in pwCF ≥12 years of age homozygous for the Phe508del-CFTR mutation with a ppFEV1 of ≥25% and ≤90% who previously discontinued lumacaftor/ivacaftor due to respiratory signs or symptoms considered related to treatment with lumacaftor/ivacaftor. Some of the results of this study have been previously reported in the form of a poster [16].
2. Methods
2.1. Study design
This was a phase 3b, randomized, double-blind, placebo-controlled, parallel-group, multicenter study (study VX16–661-114; ClinicalTrials.gov identifier, NCT03150719; EudraCT number, 2017–000540-18). The study included a screening period (days –28 through −1), a treatment period (days 1 through 56 ± 5 days), and a safety follow-up period (28 ± 7 days after the last dose of study drug). The treatment-emergent period included the time from the first dose of the study drug to the safety follow-up contact. Participants were stratified by age (<18 vs ≥18 years), sex, and ppFEV1 severity (<40% vs ≥40%) at screening and then randomized 1:1 to receive either placebo or the fixed-dose combination tablet of tezacaftor 100 mg/ivacaftor 150 mg in the morning and an ivacaftor 150-mg tablet in the evening for 56 days ( Supplementary Fig. 1 ). European participants who completed the day 56 visit were able to enroll in a long-term, open-label safety study of tezacaftor/ivacaftor if they met eligibility criteria, and participants in the United States were given the opportunity to receive tezacaftor/ivacaftor through an expanded access program.
2.2. Study oversight
The protocol was approved by the institutional review board or ethics committee at each site. Written informed consent was obtained from each participant or caregiver before screening. Safety data were reviewed by an independent data monitoring committee.
2.3. Study participants
People ≥12 years of age with CF homozygous for the Phe508del-CFTR mutation and a ppFEV1 of ≥25% and ≤90% who previously discontinued treatment with lumacaftor/ivacaftor due to ≥1 respiratory sign or symptom considered related to treatment were eligible. Participants were required to have resolution or stabilization of respiratory signs and symptoms >28 days prior to screening. Additionally, any person with a history of any comorbidity (eg, liver cirrhosis with portal hypertension, cardiovascular or cerebrovascular disease) that, in the opinion of the investigator, might confound the results of the study or pose an additional risk in administering study drug to the participant was excluded. See the online supplement for full inclusion and exclusion criteria.
2.4. Endpoints
The primary endpoint was the incidence of 7 predefined respiratory AEs of special interest (RAESIs) throughout the treatment-emergent period. RAESIs were chest discomfort, dyspnea, respiration abnormal, asthma, bronchial hyperreactivity, bronchospasm, and wheezing. The key secondary endpoint was the absolute change in ppFEV1 from baseline to the average value of days 28 and 56. Secondary endpoints included tolerability based on drug discontinuation through day 56 and additional safety assessments based on AEs, clinical laboratory values (hematology, serum chemistry, coagulation studies, and urinalysis), vital signs, pulse oximetry, and postdose spirometry on day 1.
2.5. Statistical analyses
Safety and efficacy were assessed in all participants who received ≥1 dose of study drug. Descriptive summary statistics were provided for safety endpoints, including the primary endpoint.
The key secondary endpoint of absolute change in ppFEV1 from baseline to the average value of days 28 and 56 measurements was considered successfully met if the Bayesian posterior probability of the treatment difference being >0 was ≥80%. Assuming a mean treatment difference of 3.0 percentage points between tezacaftor/ivacaftor and placebo, an SD of 6.0 percentage points, and a dropout rate of 5%, a sample size of 90 participants would provide approximately 93% probability to demonstrate a positive treatment effect of tezacaftor/ivacaftor over placebo using a noninformative prior distribution. For analysis, the posterior mean for the treatment difference and its associated 95% credible intervals were provided.
3. Results
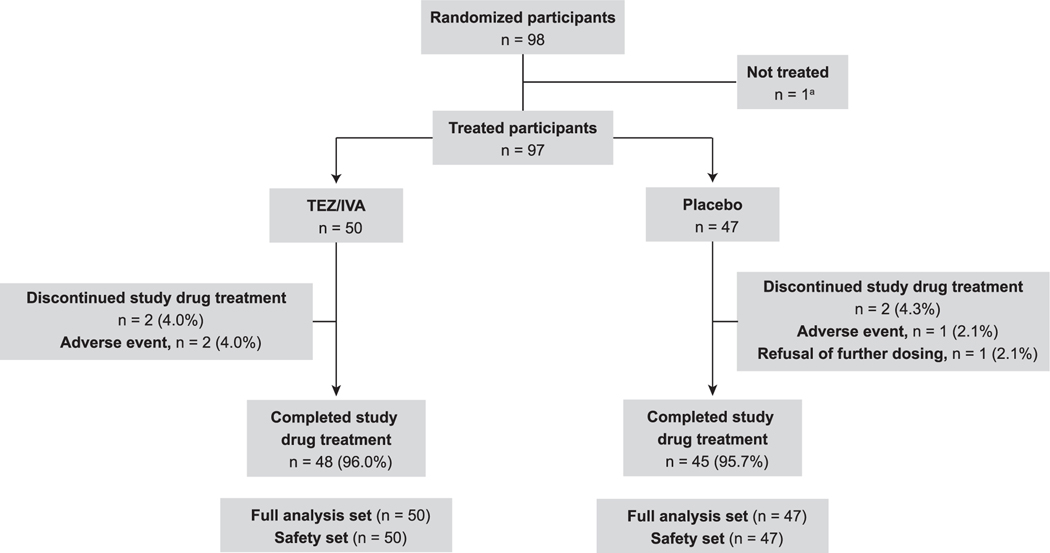
Ninety-eight participants were randomized, and 97 received ≥1 dose of tezacaftor/ivacaftor (n = 50) or placebo (n = 47) (Fig. 1). One participant who was randomized to the tezacaftor/ivacaftor group was judged not clinically stable by the investigator at the Day 1 visit and did not receive tezacaftor/ivacaftor. Ninety-four participants completed the study, and 93 completed study treatment, 48 (96.0%) in the tezacaftor/ivacaftor group and 45 (95.7%) in the placebo group). Baseline characteristics were generally similar between the treatment groups ( Table 1 ). The mean (SD) ppFEV1 at baseline was 44.6% (16.1%) in the tezacaftor/ivacaftor group and 48.0% (18.1%) in the placebo group; overall, 49.5% of participants had a ppFEV1 of <40% at baseline.
Fig. 1.
Participant disposition. a Participant was randomized but never received treatment because the participant was judged not clinically stable at the day 1 visit by the investigator. IVA, ivacaftor; TEZ, tezacaftor.
Table 1.
Demographics and baseline characteristics.
| Tezacaftor/ivacaftor (n = 50) | Placebo (n = 47) | |
|---|---|---|
| Age, mean (SD), years | 34.3 (8.7) | 33.3 (10.0) |
| ≥18 years of age at screening, n (%) | 50 (100.0) | 46 (97.9) |
| Female, n (%) | 31 (62.0) | 30 (63.8) |
| ppFEV1, mean (SD), % | 44.6 (16.1) | 48.0 (18.1) |
| ppFEV1 category at baseline, n (%) | ||
| < 40% | 27 (54.0) | 21 (44.7) |
| ≥40% and < 70% | 19 (38.0) | 17 (36.2) |
| ≥70% | 4 (8.0) | 9 (19.1) |
| Country of enrollment, n (%) | ||
| United States | 24 (48.0) | 24 (51.1) |
| Germany | 18 (36.0) | 19 (40.4) |
| France | 8 (16.0) | 4 (8.5) |
| Use of inhaled bronchodilator, n (%)a | ||
| Yes | 48 (96.0) | 46 (97.9) |
| No | 2 (4.0) | 1 (2.1) |
ppFEV1, percent predicted forced expiratory volume in 1 s.
Included medications started before the first dose of study drug.
3.1. Respiratory safety
Seventeen participants (17.5%) experienced ≥1 RAESI (Table 2): 7 participants (14.0%) in the tezacaftor/ivacaftor group and 10 (21.3%) in the placebo group. RAESIs in both arms were mild or moderate in severity; no severe or life-threatening RAESIs occurred, and none were considered to be serious AEs (SAEs) or led to treatment interruption or discontinuation. The most common RAESI was dyspnea (tezacaftor/ivacaftor: 5 [10.0%]; placebo: 5 [10.6%]). The only RAESI that occurred more frequently in the tezacaftor/ivacaftor group compared with the placebo group was respiration abnormal (reported as respiratory chest tightness; tezacaftor/ivacaftor: 3 [6.0%]; placebo: 1 [2.1%]). Five participants (5.2%) experienced RAESIs considered related or possibly related to treatment by the investigator, including 1 (2.0%) in the tezacaftor/ivacaftor group (respiration abnormal) and 4 (8.5%) in the placebo group (dyspnea [n=2], asthma [n=1], and chest discomfort [n=1]). RAESIs occurred most frequently within the first 2 weeks of treatment.
Table 2.
Overview of RAESIs.
| RAESI (preferred term), n (%) | Tezacaftor/ivacaftor (n = 50) | Placebo (n = 47) |
|---|---|---|
| Participants with any RAESI | 7 (14.0) | 10 (21.3) |
| Dyspnea | 5 (10.0) | 5 (10.6) |
| Respiration abnormal a | 3 (6.0) | 1 (2.1) |
| Bronchospasm | 0 | 2 (4.3) |
| Wheezing | 0 | 2 (4.3) |
| Chest discomfort | 0 | 1 (2.1) |
| Asthma | 0 | 1 (2.1) |
| Bronchial hyperreactivity | 0 | 0 |
| RAESI related or possibly related to study drug | 1 (2.0) | 4 (8.5) |
| Serious RAESI | 0 | 0 |
| RAESI leading to discontinuation | 0 | 0 |
| Time of onset of RAESI by time interval, n (%) b | ||
| > 0 to ≤2 weeks | 5 (10.0) | 8 (17.0) |
| > 2 to ≤4 weeks | 0 (0.0) | 3 (6.4) |
| > 4 to ≤8 weeks | 1 (2.0) | 0 |
| > 8 weeks | 1 (2.0) | 0 |
RAESI, respiratory adverse event of special interest.
Includes the verbatim term “respiratory chest tightness.”
One participant in the placebo group had 2 RAESIs at different time points.
On day 1, the mean (SD) absolute change in ppFEV1 from predose to 2 and 4 hours postdose was −0.6 (2.1) and −0.8 (4.3) percentage points in the tezacaftor/ivacaftor group (n = 45) and 0.3 (1.9) and 0.0 (1.9) percentage points in the placebo group (n = 43), respectively. No participant in either treatment group had a decline in ppFEV1 of ≥10 percentage points at 2 hours postdose. One participant treated with tezacaftor/ivacaftor had a 21.3 percentage point absolute decline in ppFEV1 4 hours postdose on day 1. This participant’s other spirometric parameters–including forced vital capacity, FEV1 to forced vital capacity ratio, and flow-volume loop–did not demonstrate an obstructive pattern but rather a sub-optimal inspiratory effort. On the same day, the participant had a mild AE of respiratory chest tightness that resolved by day 6 without treatment. The participant subsequently completed the study with an improvement in ppFEV1 from baseline.
3.2. Other safety assessments
Treatment-emergent AEs, including RAESIs, occurred in 37 participants (74.0%) receiving tezacaftor/ivacaftor and 39 (83.0%) receiving placebo (Table 3). The most commonly observed AEs (≥10% incidence in either group) were cough, pulmonary exacerbation of CF, headache, dyspnea, nasopharyngitis, constipation, abdominal upper pain, and sputum increased. Fourteen participants (14.4%) had an SAE: 5 (10.0%) in the tezacaftor/ivacaftor group and 9 (19.1%) in the placebo group. No SAE was considered related to tezacaftor/ivacaftor. The only SAE that occurred in >1 participant was pulmonary exacerbation (tezacaftor/ivacaftor group, n = 3 [6.0%]; placebo group, n = 7 [14.9%]). One participant in the tezacaftor/ivacaftor group had 2 SAEs considered unrelated to study drug that led to treatment discontinuation and death (multiple organ dysfunction syndrome and sepsis in the setting of influenza infection). Treatment discontinuation due to AEs occurred in 2 participants (4.0%) in the tezacaftor/ivacaftor group (1 with malaise, 1 with the 2 SAEs mentioned above) and 1 participant (2.1%) in the placebo group (pleuritic pain). Treatment interruptions due to AEs occurred in 1 participant in the TEZ/IVA group (distal intestinal obstruction syndrome and abdominal pain) and 1 participant in the placebo group (nausea and fatigue).
Table 3.
Overview of TEAEs
| Tezacaftor/Ivacaftor (n = 50) | Placebo (n = 47) | |
|---|---|---|
| Total no. of TEAEs | 124 | 155 |
| Participants with any TEAEs, n (%) | 37 (74.0) | 39 (83.0) |
| Participants with TEAEs related or possibly related to study druga | 10 (20.0) | 16 (34.0) |
| Participants with serious TEAEs, n (%) | 5 (10.0) | 9 (19.1) |
| Participants with serious TEAEs related to study druga | 0 | 1 (2.1) |
| Participants with TEAEs leading to treatment discontinuation, n (%) b | 2 (4.0) | 1 (2.1) |
| Participants with TEAEs leading to treatment interruption, n (%) | 1 (2.0) | 1 (2.1) |
| Participants with TEAEs leading to death, n (%) b | 1 (2.0) | 0 |
| Participants with TEAEs related to study drug leading to death | 0 | 0 |
| Participants with TEAEs by maximum severity, n (%) | ||
| Mild | 16 (32.0) | 20 (42.6) |
| Moderate | 18 (36.0) | 16 (34.0) |
| Severe | 2 (4.0) | 3 (6.4) |
| Life-threateningb | 1 (2.0) | 0 |
| Most frequent TEAEs (≥10% in any group) by preferred term, n (%) | ||
| Cough | 9 (18.0) | 8 (17.0) |
| Infective pulmonary exacerbation of cystic fibrosis | 7 (14.0) | 13 (27.7) |
| Headache | 6 (12.0) | 7 (14.9) |
| Nasopharyngitis | 6 (12.0) | 0 |
| Constipation | 5 (10.0) | 0 |
| Dyspnea | 5 (10.0) | 5 (10.6) |
| Abdominal pain upper | 4 (8.0) | 5 (10.6) |
| Sputum increased | 2 (4.0) | 5 (10.6) |
| SAEs by preferred term, n (%) | ||
| Infective pulmonary exacerbation of cystic fibrosis | 3 (6.0) | 7 (14.9) |
| Constipation | 1 (2.0) | 0 |
| Multiple organ dysfunction syndrome | 1 (2.0) | 0 |
| Sepsis | 1 (2.0) | 0 |
| Suicidal ideation | 1 (2.0) | 0 |
| Lower respiratory tract infection | 0 | 1 (2.1) |
| Musculoskeletal chest pain | 0 | 1 (2.1) |
| Pericardial effusion | 0 | 1 (2.1) |
| Pleuritic pain | 0 | 1 (2.1) |
SAE, serious adverse event; TEAE, treatment-emergent adverse event.
As deemed related or possibly related by the investigator.
Due to postinfluenza sepsis and multiple organ dysfunction syndrome, resulting in a fatal outcome deemed not related to study drug by the investigator.
There were no clinically meaningful trends in laboratory values (hematology, serum chemistry, coagulation studies, and urinalysis), vital signs, or pulse oximetry that were attributable to treatment with tezacaftor/ivacaftor. No participant had alanine aminotransferase or aspartate aminotransferase levels of >3 times the upper limit of normal during the treatment-emergent period.
3.3. Efficacy
The Bayesian posterior probability that tezacaftor/ivacaftor resulted in a larger absolute change in ppFEV1 from baseline to the average value of days 28 and 56 compared with placebo was >99%, exceeding the 80% probability threshold and therefore demonstrating a positive treatment effect on ppFEV1 with tezacaftor/ivacaftor treatment. The posterior mean difference with tezacaftor/ivacaftor vs placebo in the absolute change in ppFEV1 from baseline to the average value of days 28 and 56 was 2.7 percentage points, and its associated 95% credible interval was 1.0 to 4.4 percentage points. The mean absolute within-group change in ppFEV1 from baseline to the average value of days 28 and 56 measurements was 2.2 percentage points in participants receiving tezacaftor/ivacaftor and −0.6 percentage points in those receiving placebo.
4. Discussion
In this phase 3b, randomized, double blind, placebo-controlled clinical study, tezacaftor/ivacaftor did not result in an in- creased incidence of RAESIs compared with placebo in pwCF ≥12 years of age homozygous for the Phe508del -CFTR mutation with a ppFEV1 of ≥25% and ≤90% who previously discontinued lumacaftor/ivacaftor due to treatment-related respiratory signs or symptoms. Among the RAESIs observed in the tezacaftor/ivacaftor group, most were considered not related to treatment, and none were serious or led to treatment interruption or discontinuation. The respiratory safety profile of tezacaftor/ivacaftor observed in this lumacaftor/ivacaftor–intolerant population of pwCF is consistent with that seen in previous clinical studies of tezacaftor/ivacaftor in pwCF homozygous for Phe508del-CFTR [15]. Additionally, the rate of discontinuation due to treatment-emergent AEs was low (4%), and the incidence of SAEs was lower in the tezacaftor/ivacaftor group than in the placebo group. No new safety concerns were identified, and AEs seen were consistent with CF disease manifestations and the known safety profile of tezacaftor/ivacaftor. Tezacaftor/ivacaftor treatment also led to improvements in lung function compared with placebo. Thus, the results support tezacaftor/ivacaftor as a generally safe, well-tolerated, and efficacious treatment in the study population at risk for respiratory AEs due to prior occurrence of these events.
This study was conducted because previous clinical and real-world studies demonstrated an increased incidence of certain respiratory AEs, such as dyspnea and abnormal respiration, with lumacaftor/ivacaftor compared with placebo in pwCF ≥12 years of age homozygous for Phe508del-CFTR, especially in pwCF with more severe lung disease (ppFEV1 <40%). [10–13]. In addition, lumacaftor/ivacaftor has been associated with an acute postdose decline in ppFEV 1 in these pwCF with more severe lung damage [14]. A phase 3 study of tezacaftor/ivacaftor in pwCF ≥12 years of age homozygous for the Phe508del-CFTR mutation with a ppFEV1 of ≥40% and ≤90% demonstrated that the combination was not associated with an increased incidence of RAESIs compared with placebo or with an acute postdose decline in ppFEV1 [15]. In the current study, in which 48 of 97 participants (49.5%) had a baseline ppFEV1 of <40%, there was no increased incidence of RAESIs in participants receiving tezacaftor/ivacaftor compared with participants receiving placebo. Furthermore, this study did not demonstrate an acute postdose decline in ppFEV1 considered to be related to tezacaftor/ivacaftor treatment.
The rate of treatment discontinuation due to treatment-emergent AEs was low in both the tezacaftor/ivacaftor and placebo arms in this study. The most commonly reported AEs across both groups were cough, infective pulmonary exacerbation of CF, headache, and dyspnea, which were generally consistent with AEs reported in the pivotal phase 3 studies [15,17]. The overall safety profile was consistent with that in the previous tezacaftor/ivacaftor studies [ 15,17], although the participants in this study had a lower mean ppFEV1 at baseline. In addition, treatment with tezacaftor/ivacaftor improved lung function in this study, as demonstrated by the positive effect on ppFEV1.
It should be noted that tezacaftor/ivacaftor is the foundation of a triple combination CFTR therapy (elexacaftor/tezacaftor/ivacaftor). A recent Phase 3 study confirmed that the combination of elexacaftor, tezacaftor, and ivacaftor resulted in significant and clinically meaningful improvements in ppFEV1, sweat chloride levels, CF Questionnaire-Revised respiratory domain scores, and nutritional parameters compared with tezacaftor/ivacaftor dual combination therapy in participants ≥12 years of age with CF who were homozygous for the Phe508del-CFTR mutation [18]. Elexacaftor/tezacaftor/ivacaftor combination therapy was recently approved in the United States to treat pwCF ≥12 years of age with ≥1 copy of the Phe508del-CFTR mutation. The present data provide an important basis for the future use of elexacaftor/tezacaftor/ivacaftor in pwCF who have had respiratory adverse events with LUM/IVA.
One limitation of this study was that analyses of subgroups such as participants with a ppFEV1 of <40% vs ≥40% or participants <18 years vs ≥18 years could not be performed due to sample size. In addition, the 8-week duration of the study limited the ability to further assess long-term safety and efficacy in this population. However, respiratory AEs with lumacaftor/ivacaftor predominantly occurred within the first few weeks of treatment initiation in clinical studies and real-world settings [19]; therefore, the study was considered to be of sufficient duration to evaluate the primary objective of respiratory safety with tezacaftor/ivacaftor in pwCF who discontinued lumacaftor/ivacaftor due to respiratory signs or symptoms. Future studies will provide additional information on the longer-term safety, tolerability, and efficacy of tezacaftor/ivacaftor in pwCF.
In conclusion, tezacaftor/ivacaftor was not associated with an increased incidence of RAESIs in this study, and discontinuations in this population were low and not associated with RAESIs. In addition, tezacaftor/ivacaftor improved lung function compared with placebo. These data support the use of tezacaftor/ivacaftor in pwCF ≥12 years of age homozygous for the Phe508del-CFTR mutation with a ppFEV1 of ≥25% and ≤90% who were unable to tolerate lumacaftor/ivacaftor due to treatment-related respiratory signs or symptoms.
Supplementary Material
Acknowledgments
We thank the patients and their families for participating in this trial and the trial investigators and coordinators for their contributions to the trial. A list of investigators and trial sites is included in the supplement. Editorial coordination and support were provided by Thomas Pickette, PharmD, MBA, of Vertex Pharmaceuticals Incorporated, who may own stock or stock options in that company. Writing and editorial support was provided under the direction of the authors by Apurva Davé, PhD; Christopher Edwards, PhD; and Michelle Yochum, PhD, with support from Vertex Pharmaceuticals Incorporated. Data quality control was provided by Robyn Neitzschman of Vertex Pharmaceuticals Incorporated, who may own stock or stock options in that company.
Sources of support
This study was designed by Vertex Pharmaceuticals Incorporated in collaboration with the authors. Data gathering and analysis were conducted by Vertex Pharmaceuticals Incorporated in collaboration with the authors. This study was supported by Vertex Pharmaceuticals Incorporated, supported in part by the National Institutes of Health (grant numbers UL1RR024153 and UL1TR000005 to the University of Pittsburgh U54TR001368 and P30DK072482 to the University of Alabama at Birmingham), and supported in part by the UK National Institute for Health Research (NIHR) Respiratory Disease Biomedical Research Unit at the Royal Brompton and Harefield National Health Service (NHS) Foundation Trust and Imperial College London. The views expressed in this publication are those of the authors and not necessarily those of the NHS, NIHR, or the Department of Health.
Declaration of Competing Interests
All authors received nonfinancial support (assistance with manuscript preparation) from ArticulateScience LLC, which received funding from Vertex. Additional disclosures are as follows. BGB, MED, and NA are employees of Vertex and may own stock or stock options in Vertex. PKA, TJF and XY are former employees of Vertex and may own stock or stock options in Vertex. CS received a grant from Vertex and personal fees from Vertex, PTI, Chiesi, and Teva outside the submitted work. RF received an honorarium from Vertex during the conduct of the study. SMR received grants, personal fees, and nonfinancial support from Vertex during the conduct of the study; grants from Bayer, Forest Research Institute, AstraZeneca, Nivalis, Novartis, Galapagos/AbbVie, Proteostasis, Eloxx, Celtaxsys, PTC, and Vertex; personal fees from Bayer, Celtaxsys, Novartis, and Vertex; and nonfinancial support from Vertex outside the submitted work. SS received personal fees from Proteostasis, Novartis and Vertex outside the submitted work; has served as an investigator in clinical trials for Galapagos, Proteostasis, Celtaxsys, Flatley, Novartis, and Vertex; and has consulted for Proteostasis and Vertex. RCK and RE have no additional disclosures.
Abbreviations:
- AE
adverse event
- CF
cystic fibrosis
- CFTR
cystic fibrosis transmembrane conductance regulator
- IVA
ivacaftor
- ppFEV1
percent predicted forced expiratory volume in 1 s
- pwCF
people with cystic fibrosis
- RAESI
respiratory adverse event of special interest
- SAE
serious adverse event
- TEZ
tezacaftor
Footnotes
Data from this trial have been presented as a poster presentation at the Deutsche Mukoviszidose Tagung, 22–24 November 2018 in Wurzburg, Germany.
CRediT authorship contribution statement
Carsten Schwarz: Conceptualization, Investigation, Writing - original draft, Writing - review & editing. Sivagurunathan Sutharsan: Investigation, Resources, Writing - review & editing. Ralph Epaud: Investigation, Data curation, Writing - original draft, Writing - review & editing, Supervision, Project administration. Ross C. Klingsberg: Methodology, Investigation, Resources, Writing - original draft, Writing - review & editing. Rainald Fischer: Investigation, Resources, Data curation, Writing - review & editing. Steven M. Rowe: Conceptualization, Investigation, Writing - review & editing. Paul K. Audhya: Conceptualization, Methodology, Formal analysis, Resources, Writing - original draft, Writing - review & editing, Visualization, Supervision, Project administration, Funding acquisition. Neil Ahluwalia: Formal analysis, Investigation, Writing - original draft, Writing - review & editing. Xiaojun You: Conceptualization, Methodology, Software, Validation, Formal analysis, Resources, Data curation, Writing - original draft, Writing - review & editing, Visualization, Project administration, Funding acquisition. Thomas J. Ferro: Validation, Formal analysis, Investigation, Resources, Data curation, Writing - original draft, Writing - review & editing, Visualization, Supervision, Project administration. Margaret E. Duncan: Methodology, Resources, Writing - original draft, Writing - review & editing, Visualization, Supervision, Funding acquisition. Bote G. Bruinsma: Methodology, Investigation, Writing - original draft, Writing - review & editing, Visualization, Supervision, Project administration.
Supplementary materials
Supplementary material associated with this article can be found, in the online version, at doi: 10.1016/j.jcf.2020.06.001.
References
- [1].Bell SC, Mall MA, Gutierrez H, Macek M, Madge S, Davies JC, et al. The future of cystic fibrosis care: a global perspective. Lancet Respir Med 2020;8:65–124. 10.1016/S2213-2600(19)30337-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Riordan J, Rommens J, Kerem B, Alon N, Rozmahel R, Grzelczak Z, et al. Identification of the cystic fibrosis gene: cloning and characterization of complementary DNA. Science 1989;245:1066–73. 10.1126/science.2475911. [DOI] [PubMed] [Google Scholar]
- [3].Dalemans W, Barbry P, Champigny G, Jallat S, Dott K, Dreyer D, et al. Altered chloride ion channel kinetics associated with the delta F508 cystic fibrosis mutation. Nature 1991;354:526–8. 10.1038/354526a0. [DOI] [PubMed] [Google Scholar]
- [4].Cystic Fibrosis Foundation 2017 Patient registry: annual data report. Bethesda, Maryland: Cystic Fibrosis Foundation; 2018. [Google Scholar]
- [5].Clinical and functional translation of CFTR (CFTR2). Available from: https://www.cftr2.org. Accessed 5 February 2020.
- [6].Van Goor F, Hadida S, Grootenhuis PD, Burton B, Cao D, Neuberger T, et al. Rescue of CF airway epithelial cell function in vitro by a CFTR potentiator, VX-770. Proc Natl Acad Sci U S A 2009;106:18825–30. 10.1073/pnas.0904709106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Van Goor F, Hadida S, Grootenhuis PDJ, Burton B, Stack JH, Straley KS, et al. Correction of the F508del-CFTR protein processing defect in vitro by the investigational drug VX-809. Proc Natl Acad Sci U S A 2011;108:18843–8. 10.1073/pnas.1105787108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Orkambi (lumacaftor/ivacaftor) [package insert]. Boston, MA: Vertex Pharmaceuticals Incorporated; 2018. [Google Scholar]
- [9].Orkambi (lumacaftor/ivacaftor) [summary of product characteristics]. Dublin, Ireland: Vertex Pharmaceuticals (Ireland) Limited; 2018. [Google Scholar]
- [10].Wainwright CE, Elborn JS, Ramsey BW, Marigowda G, Huang X, Cipolli M, et al. Lumacaftor-ivacaftor in patients with cystic fibrosis homozygous for Phe508del CFTR. N Engl J Med 2015;373:220–31. 10.1056/NEJMoa1409547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Taylor-Cousar JL, Jain M, Barto TL, Haddad T, Atkinson J, Tian S, et al. Lumacaftor/ivacaftor in patients with cystic fibrosis and advanced lung disease homozygous for F508del-CFTR. J Cyst Fibros 2018;17:228–35. 10.1016/j.jcf.2017.09.012. [DOI] [PubMed] [Google Scholar]
- [12].Hubert D, Chiron R, Camara B, Grenet D, Prévotat A, Bassinet L, et al. Real-life initiation of lumacaftor/ivacaftor combination in adults with cystic fibrosis homozygous for the Phe508del CFTR mutation and severe lung disease. J Cyst Fibros 2017;16:388–91. 10.1016/j.jcf.2017.03.003. [DOI] [PubMed] [Google Scholar]
- [13].Elborn JS, Ramsey BW, Boyle MP, Konstan MW, Huang X, Marigowda G, et al. Efficacy and safety of lumacaftor/ivacaftor combination therapy in patients with cystic fibrosis homozygous for Phe508del CFTR by pulmonary function subgroup: a pooled analysis. Lancet Respir Med 2016;4:617–26. 10.1016/S2213-2600(16)30121-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Popowicz N, Wood J, Tai A, Morey S, Mulrennan S. Immediate effects of lumacaftor/ivacaftor administration on lung function in patients with severe cystic fibrosis lung disease. J Cyst Fibros 2017;16:392–4. 10.1016/j.jcf.2017.02.009. [DOI] [PubMed] [Google Scholar]
- [15].Taylor-Cousar JL, Munck A, McKone EF, van der Ent CK, Moeller A, Simard C, et al. Tezacaftor-ivacaftor in patients with cystic fibrosis homozygous for Phe508del. N Engl J Med 2017;377:2013–23. 10.1056/NEJMoa1709846. [DOI] [PubMed] [Google Scholar]
- [16].Schwarz C, Sutharsan S, Epaud R, Klingsberg R, Fischer R, Rowe SM, et al. Safety, efficacy, and tolerability of tezacaftor/ivacaftor in patients 12 years and older with cystic fibrosis homozygous for F508del-CFTR who have previously discontinued lumacaftor/ivacaftor: a randomized, double-blinded, placebo controlled phase 3B study Poster PW1.11 presented at: Deutsche Mukoviszidose Tagung; November 22–24 Wurzburg, Germany; 2018. [Google Scholar]
- [17].Rowe SM, Daines C, Ringshausen FC, Kerem E, Wilson J, Tullis E, et al. Tezacaftor-ivacaftor in residual-function heterozygotes with cystic fibrosis. N Engl J Med 2017;377:2024–35. 10.1056/NEJMoa1709847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Heijerman HGM, McKone EF, Downey DG, Van Braeckel E, Rowe SM, Tullis E, et al. Efficacy and safety of the elexacaftor plus tezacaftor plus ivacaftor combination regimen in people with cystic fibrosis homozygous for the F508del mutation: a double-blind, randomised, phase 3 trial. Lancet 2019;394:1940–8. 10.1016/S0140-6736(19)32597-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Konstan MW, McKone EF, Moss RB, Marigowda G, Tian S, Waltz D, et al. Assessment of safety and efficacy of long-term treatment with combination lumacaftor and ivacaftor therapy in patients with cystic fibrosis homozygous for the F508del-CFTR mutation (PROGRESS): a phase 3, extension study. Lancet Respir Med 2017;5:107–18. 10.1016/S2213-2600(16)30427-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.