Abstract
We report the autopsy findings of a case of disseminated mucormycosis caused by Cunninghamella bertholletiae, a rare pathogenic fungus of the family Mucoraceae. The patient was a 49-year-old woman with B-lymphoblastic leukemia with hyperdiploidy, who died of progressive heart failure 4 months after induction chemotherapy successfully brought about complete remission of the leukemia. Granulocyte colony-stimulating factor (G-CSF) had been administered along with anti-neoplastic drugs, and her blood neutrophil count was markedly elevated. Autopsy revealed disseminated mycotic thromboembolism and abscess formation in the heart, lung, liver, kidney, and spleen. The most marked feature was a large mural thrombus in the left ventricle containing numerous fungal hyphae. In the myocardium and disseminated foci in visceral organs, giant cell-rich, fibrotic reactions to the mycotic infection were observed. Both the formation of a large intra-ventricular mural thrombus and giant cell reactions are rare findings in mucormycosis. We considered that the recovery and marked increase in neutrophil count induced by chemotherapy and G-CSF administration prolonged the clinical course and pathologically elicited an atypical, giant cell reaction to the mycotic infection. The prolonged clinical course also contributed to the formation of an unusually large intra-ventricular mural thrombus.
Keywords: Cunninghamella bertholletiae, disseminated mucormycosis, giant cell reaction, granulocyte colony-stimulating factor, intra-ventricular mural thrombus, leukocytosis
Introduction
Mucormycosis (also known as zygomycosis or phycomycosis) is the third most common opportunistic mycotic infection caused by fungi belonging to the family Mucoraceae (class Zygomycetes, order Mucorales) [1,2]. Mucormycosis predominantly affects patients with hematological malignancies and takes several clinico-pathological forms: rhino-orbito-cerebral, sinopulmonary, cardiovascular, gastrointestinal, cutaneous, and disseminated [2]. Among these, the disseminated form consistently results in a fatal outcome after an acute or fulminant clinical course [2]. Among Mucoraceae family members, Cunninghamella bertholletiae (C. bertholletiae) is rarely the causative agent of this opportunistic mycotic infection [1-7].
We report a case of disseminated mucormycosis that occurred in a middle-aged woman with B-lymphoblastic leukemia with hyperdiploidy [8] and resulted in the patient’s death due to progressive heart failure after a prolonged clinical course. Autopsy demonstrated disseminated mucormycosis that involved the heart, lung, liver, kidney, spleen, and chest wall, and the causative fungus was identified as C. bertholletiae. The most marked feature was the formation of a large left intra-ventricular mural thrombus containing numerous fungal hyphae. Fungal hyphae in the heart and other organs evoked giant cell-rich, fibrotic reactions surrounding necrotic foci.
Clinical history
The patient was a 49-year-old woman without any significant past medical history. Four months before her death, she consulted a local physician complaining of right upper abdominal pain and low-grade fever. Blood analyses demonstrated an erythrocyte count of 282×104/μL, leukocyte count of 1,160/μL with the appearance of abnormal lymphoid cells (58%), and platelet count of 13.1×104/μL. No lymph node swelling was noted. Bone marrow aspiration established a diagnosis of B-lymphoblastic leukemia with hyperdiploidy [8], and induction chemotherapy was started. At that time, no evidence of local or systemic infection was noted. The chemotherapy rapidly led to complete remission of the leukemia. Granulocyte colony-stimulating factor (G-CSF, genetically recombinant filgrastim, 150 μg/m2 of body surface/day, 10 days in 2 months) was also administered to improve leukopenia, and recovery of the neutrophil count was achieved in approximately 2 months. The serum G-CSF value reached 316 pg/mL (normal value: <39.0 pg/mL), and the leukocyte count increased and peaked at 91,800/μL (70% neutrophils).
However, the patient developed drug-induced hepatic dysfunction and diffuse pulmonary edema with bilateral pleural effusion. Despite prophylactic administration of anti-fungal drugs, the pulmonary edema gradually increased and a round, cavitary nodule suggestive of mycotic infection appeared in the right lower lobe 2 months prior to death. She was diagnosed with left-sided heart failure, and echocardiography and computed tomography demonstrated mitral stenosis and a mass lesion measuring 46×29 mm in the left ventricular cavity. The mass was adherent to the posterior wall and involved the papillary muscles. Endomyocardial biopsy was conducted, but the specimen contained only fibrotic endocardium and fragments of necrotic myocardial tissue, and no fungal hyphae were detected. Although the leukemia remained in complete remission, persistent fever and frequent bouts of ventricular arrhythmia developed, and her heart failure rapidly worsened. Although she was transferred to the intensive care unit and placed under artificial respiratory control, she died of severe hemodynamic deterioration approximately 3 months after the appearance of pulmonary edema. Throughout the clinical course, serum 1, 3-β-D-glucan levels remained within the normal range.
Pathologic findings
Autopsy demonstrated disseminated mucormycosis that formed many septic thromboemboli and caused multifocal, necrotizing, and inflammatory lesions in the visceral organs. Leukemia was in a state of complete remission, and no residual leukemic cells were found in the bone marrow, lymph nodes, or visceral organs.
On gross examination, the largest lesion was present in the heart (weighing 340 g), where a large mural thrombus of irregular shape (measuring 35×25×20 mm) was adherent to the posterior wall of the left ventricle and involved the papillary muscles and chordae tendineae (Figure 1). The cut surface of the thrombus showed an annular ring-like, laminated appearance. A discrete intra-mural abscess measuring 10 mm in diameter was also found in the left ventricular wall.
Figure 1.

Gross appearance of the bisected left ventricle. A large mural thrombus adhering to the posterior wall and involving the papillary muscles and chordae tendineae was observed (center). A small abscess was noted in the left ventricular wall (arrow).
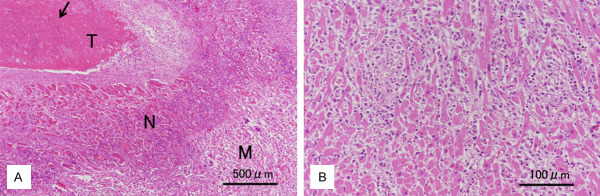
Microscopic examination revealed that the mural thrombus contained a large amount of laminated fibrin and numerous, often entangled, fungal hyphae (Figure 2A). The hyphae invaded deeply into the contiguous papillary muscles and ventricular wall, causing myocardial necrosis. In the boundary area between the necrotic and viable myocardium, a zone of fibrotic granulation tissue had formed. Inflammatory cells within granulation tissue consisted primarily of histiocytes that infiltrated the myocardial interstitium (Figure 2B). Several multinucleated giant cells of the foreign body type were admixed with mononuclear histiocytes. In the ventricular wall abscess, necrotic small arteries were found to be occluded by thromboemboli containing many fungal hyphae; in addition, multiple thrombosed vessels and scattered microscopic foci of an anemic infarct or abscess in the myocardium were observed.
Figure 2.
Histopathologic findings. A. Beneath the mural thrombus (T) containing many, palely basophilic fungal hyphae (arrow), a zone of necrotizing myocardial tissue (N) was observed, which was adjacent to viable myocardial tissue (M) (Left ventricle, H&E stain, scale bar 500 μm). B. Diffuse interstitial infiltration of histiocytes was observed in the myocardium adjacent to the mural thrombus (Left ventricle, H&E stain, scale bar 100 μm).
The fungi formed broad, ribbon-like, and non-septated hyphae exhibiting irregular branching at wide angles. The hyphae had thin walls that were faintly positive for a periodic acid-Schiff reaction and stained pale black with Grocott stain (Figure 3A). Genomic DNA was extracted from the fungi isolated from a portion of the cardiac thrombus taken before fixation and subjected to polymerase chain reaction (PCR) analysis. The fungi were identified as C. bertholletiae.
Figure 3.
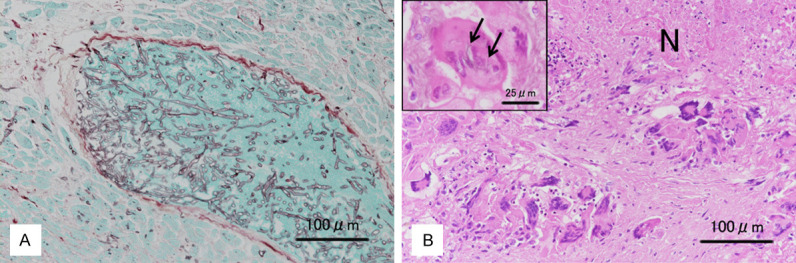
Histopathologic findings. A. A thromboembolus containing numerous hyphae of mucormycosis occluding the lumen of a medium-sized vein within the myocardium (Left ventricle, Grocott stain, scale bar 100 μm). B. Fibrotic tissue containing many multinucleated giant cells surrounding a necrotic focus (N). (Kidney, H&E stain, scale bar 100 μm). Some giant cells phagocytized fungal hyphae, stained pale black with Grocott stain (arrows) (Kidney, H&E-Grocott stain, scale bar 25 μm).
The lungs (each weighing 370 g) showed edema and bronchopneumonia, and a round nodule measuring 14 mm in diameter with a central cavitation was found in the right lower lobe. The cavity contained a small mass of necrotic lung tissue, within which many fungal hyphae had invaded the walls of small arteries. In the kidney and spleen, multiple wedge-shaped, anemic infarcts caused by mycotic thromboembolism were observed. The kidney, spleen, and liver also contained multiple microscopic abscesses with thin pyogenic membranes and containing many multinucleated giant cells (Figure 3B); however, the formation of true discrete granulomas was not observed. Some giant cells had phagocytized fungal hyphae (Figure 3B). Hematopoiesis in the bone marrow was hyperactive, and extramedullary hematopoiesis was observed in sinusoids of the liver and spleen.
Discussion
Mucormycosis predominantly affects immunologically compromised patients, particularly patients who are neutropenic, patients with diabetic keto-acidosis, and patients receiving iron chelation therapy with deferoxamine for iron overload [1,2,5,9]. Mucormycosis exhibits characteristic angio-invasive or angio-destructive pathologic features [9,10]. In most cases, mucormycosis initially affects the lower respiratory tract, and fungal hyphae readily disseminate to systemic organs by a hematogenous route, where they form multiple septic thromboemboli that produce anemic or hemorrhagic infarcts or mycotic abscesses in the involved organs [1,2,9]. Although the heart is one of the most commonly involved organs in disseminated mucormycosis [2,10,11], most cardiac lesions in mucormycosis are microabscesses [10], endocarditis [6], or myocarditis [12], and the formation of intra-ventricular or intra-atrial thrombi sufficiently large to be discerned by imaging studies has only rarely been documented [3,4,10,11].
The pathogenic fungus in the present case was C. bertholletiae. The majority of cases of mucormycosis are caused by fungi of the genera Rhizopus and Absidia, and infection by fungi of the genus Cunninghamella is rare [2-7,9]. Five species of Cunninghamella have been described, but only C. bertholletiae is considered pathogenic to humans [1,2]. Although C. bertholletiae is considered more virulent than Rhizopus species [2], C. bertholletiae infections comprise only 3 to 4% of mucormycosis cases [9], and reports of its pathologic features are limited [3-7]. In contrast to infection by members of the genus Rhizopus, in which the rhino-orbito-cerebral form is the most common, rhino-orbito-cerebral infection is less frequent in C. bertholletiae infection (4.7%) in comparison with the disseminated (48.8%) and pulmonary (30.2%) forms [2].
Mucormycosis complicating hematologic malignancies usually pursues a rapidly progressive clinical course ending in a fatal outcome [1,2,5,7,9]. The disease is pathologically characterized by septic thromboembolic lesions associated with extensive necrosis due to vascular occlusion by the angio-invasive fungi [4,5,9-11]. In the present case, however, the clinical course was protracted, and an intra-ventricular mass lesion (representing a mural thrombus) was detected approximately 1.5 months prior to death. McGinnis et al. reported a similar case of C. bertholletiae infection with a protracted clinical course (Case 1 of their series) [3]. Interestingly, that case was not iatrogenically immunocompromised and thought to have had chronic fungal infection that may have persisted for many months.
In the present case, the blood neutrophil count recovered after induction of leukemia remission following the commencement of chemotherapy and administration of G-CSF and was even markedly elevated during the subsequent clinical course. Pagano et al. conducted a univariate analysis of the prognostic factors in numerous patients with mucormycosis and found that recovery of the neutrophil count after chemotherapy was positively correlated with improved outcome [9]. Neutrophils play a critical role in protecting tissue against mucormycosis [9,13]. Gonzalez et al. reported that administration of G-CSF in addition to liposomal amphotericin B led to recovery of blood neutrophil count and markedly improved the clinical outcome of mucormycosis [13]. In the present case, a marked elevation in blood neutrophil count was brought about by the administration of G-CSF and also presumably by the response to mycotic infection. We consider that restoration of the neutrophil count prolonged the clinical course and led to a pathologic feature unusual for mucormycosis: the formation of giant cell-rich, fibrotic reaction surrounding necrotic foci. A giant cell reaction to fungi was also observed in the above-mentioned case of McGinnis et al. [3]. In our case, prolongation of the clinical course also probably gave the mural thrombus sufficient time to grow to an unusually large size.
The present case demonstrated that C. bertholletiae infection can take a protracted or chronic clinical course following restoration of the neutrophil count, leading to the formation of a large mural thrombus in the heart. This case also demonstrated that the pathologic features of chronic lesions include giant cell-rich, fibrotic reactions. The paucity of lymphocytic infiltration within the lesions in this case probably reflected the post-chemotherapeutic state of leukemia in the patient.
Cardiac involvement in mucormycosis occasionally appears as myocardial infarction because of vascular invasion and occlusion by the fungi, but ante-mortem clinical diagnosis is usually very difficult [4,14]. In the acute development of an intraventricular mass lesion in immunocompromised patients, the possibility of a septic mural thrombus forming due to disseminated mycosis should always be considered.
Acknowledgements
The authors would like to thank Dr. Kiyofumi Okusu, Associate Professor of Microbiology, Gifu University School of Medicine, for performing the PCR assays.
Disclosure of conflict of interest
None.
References
- 1.Ribes JA, Vanover-Sams CL, Baker DJ. Zygomycetes in human disease. Clin Microbiol Rev. 2000;13:236–301. doi: 10.1128/cmr.13.2.236-301.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gomes MZR, Lewis RE, Kontoyiannis DP. Mucormycosis caused by unusual Mucormycetes, non-Rhizopus, -Mucor, and -Lichtheimia species. Clin Microbiol Rev. 2011;24:411–445. doi: 10.1128/CMR.00056-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McGinnis MR, Walker DH, Dominy IE, Kaplan W. Zygomycosis caused by Cunninghamella bertholletiae: clinical and pathologic aspects. Arch Pathol Lab Med. 1982;106:282–286. [PubMed] [Google Scholar]
- 4.Kolbeck PC, Makhoul RG, Bollinger RR, Sanfillippo F. Widely disseminated Cunninghamella mucormycosis in an adult renal transplant patient: case report and review of the literature. Am J Clin Pathol. 1985;83:747–753. doi: 10.1093/ajcp/83.6.747. [DOI] [PubMed] [Google Scholar]
- 5.Rex JH, Ginsberg AM, Fries LF, Pass HI, Kwon-Chung KJ. Cunninghamella bertholletiae infection associated with deferoxamine therapy. Rev Infect Dis. 1988;10:1187–1194. doi: 10.1093/clinids/10.6.1187. [DOI] [PubMed] [Google Scholar]
- 6.Zhang R, Zhang JW, Szerlip HM. Endocarditis and hemorrhagic stroke caused by Cunninghamella bertholletiae infection after kidney transplantation. Am J Kidney Dis. 2002;40:842–846. doi: 10.1053/ajkd.2002.35698. [DOI] [PubMed] [Google Scholar]
- 7.Hsieh TT, Tseng HK, Sun PL, Wu YH, Chen GS. Disseminated zygomycosis caused by Cunninghamella bertholletiae in patient with hematological malignancy and review of published case reports. Mycopathologia. 2013;175:99–106. doi: 10.1007/s11046-012-9595-y. [DOI] [PubMed] [Google Scholar]
- 8.Borowitz MJ, Chan JKC, Downing JR, Le Beau MM, Arber DA. B-lymphoblastic leukaemia/lymphoma with recurrent genetic abnormalities. In: Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H, Thiele J, editors. WHO classification of tumours of haematopoietic and lymphoid tissues, revised 4th edn. Lyon, France: IARC; 2017. pp. 203–209. [Google Scholar]
- 9.Pagano L, Offidani M, Fianchi L, Nosari A, Candoni A, Picardi M, Corvatta L, D’Antonio D, Girmenia C, Martino P, Del Favero A GIMEMA (Gruppo Italiano Malattie EMatologiche dell’Adulto) Infection Program. Mucormycosis in hematologic patients. Haematologica. 2004;89:207–214. [PubMed] [Google Scholar]
- 10.Virmani R, Connor DH, McAllister HA. Cardiac mucormycosis. A report of five patients and review of 14 previously reported cases. Am J Clin Pathol. 1982;78:42–47. doi: 10.1093/ajcp/78.1.42. [DOI] [PubMed] [Google Scholar]
- 11.Van de Glind GJ, Gidding CE, Verlaat CM, Duthoi K, Backx AP, Verweij PE, Warris A. Acute cardiac failure due to intra-atrial mass caused by Zygomycetes in an immunocompromised paediatric patient. Case Rep Med. 2010;2010:241791. doi: 10.1155/2010/241791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fujisawa Y, Hara S, Zoshima T, Maekawa N, Inoue D, Sasaki M, Gamou T, Nagata Y, Hayashi K, Takeji A, Ito K, Mizushima I, Fujii H, Kawano M. Fulminant myocarditis and pulmonary cavity lesion induced by disseminated mucormycosis in a chronic hemodialysis patient: report of an autopsied case. Pathol Int. 2020;70:557–562. doi: 10.1111/pin.12943. [DOI] [PubMed] [Google Scholar]
- 13.Gonzalez CE, Couriel DR, Walsh TJ. Disseminated zygomycosis in a neutropenic patient: successful treatment with amphotericin B lipid complex and granulocyte colony-stimulating factor. Clin Infect Dis. 1997;24:192–196. doi: 10.1093/clinids/24.2.192. [DOI] [PubMed] [Google Scholar]
- 14.Jackman JD Jr, Simonsen RL. The clinical manifestations of cardiac mucormycosis. Chest. 1992;101:1733–1736. doi: 10.1378/chest.101.6.1733. [DOI] [PubMed] [Google Scholar]