Abstract
Background
COVID-19 pandemic caused families to stay home and cancel everyday activities. Hospital admissions decreased, affecting changes in diagnoses and management of chronic disease in children.
Aims
We analyzed how the first lockdown influenced clinical presentation and manifestation of children with diabetes mellitus (DM) in a German University Hospital.
Methods
During March 15th and October 11th 2020, data on general patient information, clinical symptoms and on lab results related to diabetic ketoacidosis (DKA) were analyzed in children (0–18 years) who presented with new onset of DM or poor metabolic control of known DM. All data including frequency and severity of DKA were compared to data from patients who presented in 2019.
Results
Data from 125 participants with DM were evaluated (2020: n = 52; 2019: n = 73). In 2020, twelve patients (23.1%) were diagnosed with new onset DM, two of them with type2 diabetes, and 66.7% presented with DKA including both patients T2DM. In 2019, 24.5% of patients had new onset DM, and 50% of them presented with DKA.
In 2020, patients with new onset DM were younger, presented with more severe symptoms of DKA and had to stay longer in hospital compared to 2019. In 2020, six children (50%) with new onset DM were <6 years, whereas in 2019 most children with new onset DM were adolescents (n = 7, 38.9%).
Conclusion
COVID-19 lockdown aggravated complications of diabetes onset and therapy management, including severity and frequency of DKA. It underlines the need of health education for early DKA diagnosis to early identify children at risk.
Keywords: Diabetes mellitus, Children, COVID-19, Diabetic ketoacidosis, Lockdown
1. Introduction
The COVID-19 pandemic has caused a nationwide lockdown in Germany, starting March 15th 2020, which initially ended in May 2020 but still showed ongoing effects on the everyday life of children, especially on those with chronic illnesses such as diabetes mellitus. Schools and kindergartens were closed, fun activities cancelled and the focus of society and health care system laid on coping with the pandemic. In November 2020, the government decided for a second lockdown that is not yet concluded.
Besides influence on family life and psychological health, hospital admissions also decreased [1]. Diabetic ketoacidosis as complication of delayed diagnosis was present in more children with onset type 1 diabetes during lockdown from March to May 2020 than in 2018 and 2019 [2]. The SARS-CoV-2-virus using ACE-2-receptor for cell penetration may also cause diabetic ketoacidosis directly [3]. Other groups have reported cases of children with COVID-19 where the disease might have precipitated the type 1 diabetes mellitus diagnoses [4,5]. Yet the overall incidence of T1DM in 2020 during lockdown in spring increased in the same manner as between 2011 and 2019 without showing short-term effects of the COVID-19 pandemic [6]. Even though children with diabetes seem – to our current understanding- not to be at higher risk for severe COVID-19 illness in contrast to adults with diabetes, a connection between the impacts of the pandemic and diabetes in children has been observed [7].
The aim of this study was to analyze how far lockdown, starting in March, and the remaining lifestyle changes influenced clinical presentation and manifestation of children with diabetes mellitus. Did children and adolescents with newly or already diagnosed diabetes mellitus type 1 and type 2 suffer more frequently from diabetic ketoacidosis than the year before? Lockdown may have caused more severe cases and the need of longer treatment/longer hospitalization than in 2019. We thus aimed to analyze the clinical presentation and severity of DKA in children with newly diagnosed or known diabetes during the first lockdown in 2020, and we retrospectively compared all data with the same period in 2019.
2. Methods
2.1. Study population
This study evaluated data from children and adolescents who were admitted to our hospital during the first lockdown of the COVID-19-pandemic from March 15th to October 2020, with new onset DM or known DM and poor metabolic control. All data were compared to data of diabetic patients within the same time period in 2019. All consecutively enrolled children, who were newly diagnosed or already diagnosed dealing with metabolic decompensation and who were treated at the University Hospital in Halle (Saale), Germany during these months, were included. We collected data from electronic medical records on general patient information, on overall clinical condition and on laboratory parameters related to diabetic ketoacidosis and blood-glucose management. In detail we surveyed age at admission, manifestation age, gender, BMI-percentile, BMI-SDS, current therapeutic method (CSII = continuous subcutaneous insulin infusion or ICT = intensified conventional therapy), days spent in hospital, symptoms at hospital admittance, blood-glucose at admittance, pH-level, base excess (=BE, mmol/l) bicarbonate in serum (mmol/l) and HbA1c (mmol/mol and %). Parameters were classified in different categories. For age, the children were divided in preschool (<6 years), primary school (6–11 years) and adolescents (12–18 years). The BMI-score was calculated as previously described, based on Germany reverence percentiles, and was evaluated with BMI-percentile counting percentile >90 ≤ 97 as overweight, percentile >97 ≤ 99.5 as obese and >99.5 as extremely obese [8]. As definition for diabetic ketoacidosis we used a pH-level < 7.3 and/or bicarbonate level < 15 mmol/l and categorized the severity in mild (pH-level < 7.3 and bicarbonate < 15 mmol/l), moderate (pH-level < 7.2 and bicarbonate < 10 mmol/l) and severe (pH-level < 7.1 and bicarbonate < 5 mmol/l) [9]. The evaluated symptoms included polyuria, polydipsia, stomach pain, fatigue and weight loss. Duration of symptoms was divided in “days”, “one week”, “few weeks” and “months”. Besides interpreting these values for the overall group of children, we separated them into newly (onset) and already diagnosed cases.
The study protocol was approved by the Ethics Committee of the Medical Faculty of University Halle/S.
2.2. Statistical analyses
Statistical analyses were performed using IBM SPSS Statistics. We created descriptive statistics for categorial values regarding frequencies and metric values regarding mean value with 95%-confidence interval. Mean values were compared by means of t-test for independent samples and illustrated on forest plot-diagrams to underline the relevance of the differences between groups in 2019 and 2020. A two-sided p-value < 0.05 was considered as statistically significant. We calculated the binary logistic regression for presentation with or without DKA regardless of age, gender and BMI, and equally pointed out the effect via 95%-confidence interval (p-value < 0.05 statistically significant). Frequency of DKA and its clinical classification was compared using Fisher's exact test with two-sided p-value and Mann-Whitney-U test with exact p-value, considering p < 0.05 as statistically significant.
3. Results
3.1. Study population with regard to gender and age distribution
Data from a total of 125 consecutively enrolled children and adolescents were included in the analyses (2020: 52; 2019: 73). In 2020, twelve children (23.1%) had new onset DM, two of them were diagnosed for Type2 DM (T2DM). In 2019, eighteen children (24.65%) had new onset DM, all of them with type1 DM. Descriptive statistics of the entire study population is presented in Table 1 .
Table 1.
Descriptive statistics of study population in 2020 and 2019: absolute numbers or mean values (95%-CI), respectively, as well as percentages are given.
| Overall |
New onset DM |
Already diagnosed |
|||||
|---|---|---|---|---|---|---|---|
| 2020 | 2019 | 2020 | 2019 | 2020 | 2019 | ||
| n | 52 | 73 | 12 (23.1%) | 18 (24.65%) | 40 (76.9%) | 55 (75.34%) | |
| Sex | Male | 21 (40.4%) | 36 (49.3%) | 6 (50%) | 11 (61.1%) | 15 (37.5%) | 25 (46.3%) |
| Female | 31 (59.6%) | 37 (50.7%) | 6 (50%) | 7 (38.9%) | 25 (62.5%) | 29 (53.7%) | |
| Age | <6 years | 16 (30.8%) | 13 (17.8%) | 6 (50%) | 5 (27.8%) | 10 (25%) | 8 (14.8%) |
| 6–11 years | 11 (21.2%) | 19 (26%) | 3 (25%) | 6 (33.3%) | 8 (20%) | 12 (22.2%) | |
| 12–18 | 25 (48%) | 41 (56,2%) | 3 (25%) | 7 (38.9%) | 22 (55%) | 34 (63%) | |
| Duration of symptoms | Days | 6 (50%) | 8 (40%) | 2 (28.6%) | 3 (23.1%) | 4 (80%) | 5 (71.4%) |
| 1 week | 2 (16.7%) | 4 (20%) | 2 (28.6%) | 4 (30.8%) | 0 | 0 | |
| Few weeks | 2 (16.7%) | 6 (30%) | 2 (28.6%) | 4 (30.8%) | 0 | 2 (28.6%) | |
| Few months | 2 (16.7%) | 2 (10%) | 1 (14.3%) | 2 (15.4%) | 1 (20%) | 0 | |
| Variables | |||||||
| Age (years) | 9.48 (8.12–10.84) | 10.64 (9.61–11.67) | 7.50 (4.38–10.62) | 9.24 (6.77–11.7) | 10.07 (8.55–11.6) | 11.11 (9.95–12.27) | |
| Age at manifestation (years) | 6.4 (5.2–7.6) | 7.13 (6.02–8.23) | 7.50 (4.38–10.62) | 9.24 (6.77–11.7) | 6.08 (4.75–7.4) | 6.58 (5.36–7.81) | |
| BMI-SDS | 0.68 (0.23–1.13) | 0.62 (0.27–0.97) | 0.19 (−1.08–1.45) | −0.38 (−1.07–0.31) | 0.83 (0.35–1.30) | 0.95 (0.57–1.33) | |
| Blood-glucose at admittance (mmol/l) | 16.69 (14.46–18.92) | 17.80 (15.51–20.09) | 22.27 (17.94–26.60) | 24.89 (20.29–29.51) | 15.02 (12.58–17.46) | 15.39 (13.01–17.77) | |
| pH-level | 7.2702 (7.2074–7.3330) | 7.3074 (7.2571–7.3578) | 7.1668 (7.0394–7.2941) | 7.2661 (7.1619–7.3704) | 7.3241 (7.2590–7.3893) | 7.3344 (7.2822–7.3867) | |
| Base excess (mmol/l) | −8,01 (−11,56–(−4,47)) | −6,23 (−8,82–(−3,64)) | −15,08 (−22,62–(−7,55)) | −6,46 (−10,89–(−2,04)) | −4,75 (−8,25–(−1,25)) | −6,06 (−9,47–(−2,64)) | |
| HbA1c (mmol/mol) | 88.98 (82.34–95.61) | 93.82 (83.47–104.16) | 98.73 (85.67–111.79) | 100.66 (89.65–111.66) | 82.82 (76.28–89.35) | 86.98 (68.71–105.24) | |
| HbA1c (%) | 10.27 (9.68–10.86) | 10.9 (10.25–11.55) | 11.18 (9.98–12.38) | 11.45 (10.55–12.35) | 9.72 (9.15–10.28) | 10.51 (9.53–11.49) | |
| Days in hospital | 10.13 (8.62–11.65) | 9.86 (8.71–11.02) | 17.33 (15.06–19.61) | 14.56 (13.32–15.79) | 7.98 (6.75–9.20) | 8.41 (7.15–9.66) | |
In 2020, more girls were admitted to hospital due to diabetes diagnosis (new onset or already known disease) compared to 2019. However, in 2019 gender was nearly evenly distributed whereas in 2020, twenty-five (62.5%) of the patients with DM were females.
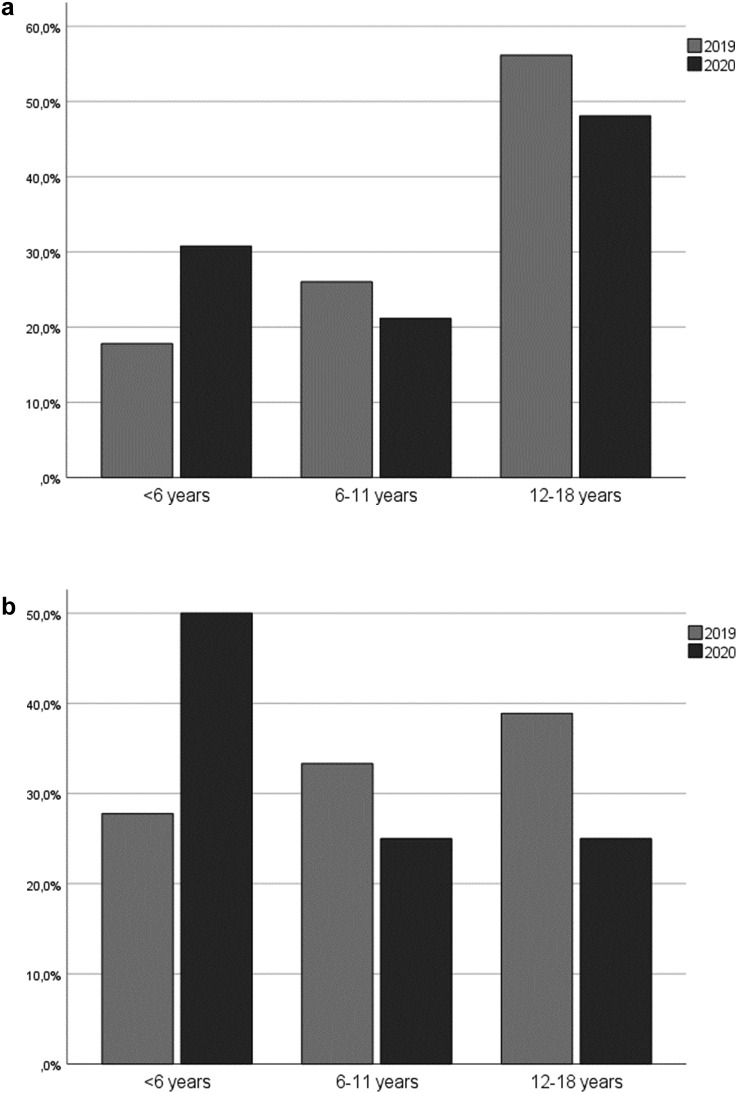
Comparing the age, children with new onset DM were 1.74 years younger in 2020 compared to 2019. In both years, the most frequently admitted age group of the entire study population were adolescents with twenty-five patients (48%) in 2020 and forty-one patients (56.2%) in 2019. With regard to already diagnosed cases, adolescents were more frequently admitted to hospital in both years due to poor metabolic control compared to younger age groups, constituting twenty-two of patients (55%) in 2020 and thirty-four (63%) in 2019 (Fig. 1a). In 2020, ten (25%) of these patients were under the age of 6 years whereas in 2019, only eight patients (14.8%) were preschool children (Fig. 1a). In 2020, 50% of the patients with new onset DM (6 children) were under the age of 6 years. Between 6–11 years and 12–18 years, each group contributed three (25%) of the newly diagnosed patients in 2020. In 2019, five (27.8%) patients with new onset DM were under the age of 6 years, and most patients were adolescents (7 patients, 38.9%; Fig. 1b; Table 1).
Fig. 1.
a: Distribution of age groups in patients with known DM who had to be hospitalized in 2020 compared to 2019. b: Distribution of age groups in patients with new onset DM in 2020 compared to 2019.
3.2. Study population with regard to weight status and type of diabetes diagnosis
In both years, the majority of the patients were of normal weight (33 patients, 63.5% in 2020 and 50 patients, 68.5% in 2019). In 2020 and following lockdown, extreme obesity occurred more often than in 2019. Three of the 12 children with new onset DM (25%) had a BMI > 99.5 percentile. Two of them were newly diagnosed with T2DM: Both patients were boys, 13 and 16 years old, with a body weight of 104 kg (99.8 percentile; BMI-SDS 2.88) and 115.5 kg (99.9 percentile; BMI-SDS 3.04), respectively. Both patients initially presented with severe DKA: pH-level 6.91 and 7.07; base excess −29.4 mmol/l and − 21.7 mmol/l; bicarbonate 2.5 mmol/l and 6.8 mmol/l, respectively. HbA1c-levels at admission were 129.1 mmol/mol (14%) and 93.8 mmol/mol (10.7%), respectively. Both had severe symptoms of polyuria and polydipsia, the younger boy since a few days and the older one, who also was increasingly somnolent at admittance, since a few weeks.
In 2020, four (10%) of the patients with already known DM had severe obesity, three of them were previously diagnosed with T2DM. In 2019, five patients (6.8%) had presented with severe obesity, however, none of them in the group of new onset DM. However, four of these five patients were diagnosed with T2DM already in 2019.
3.3. Frequency and severity of diabetic ketoacidosis at admission
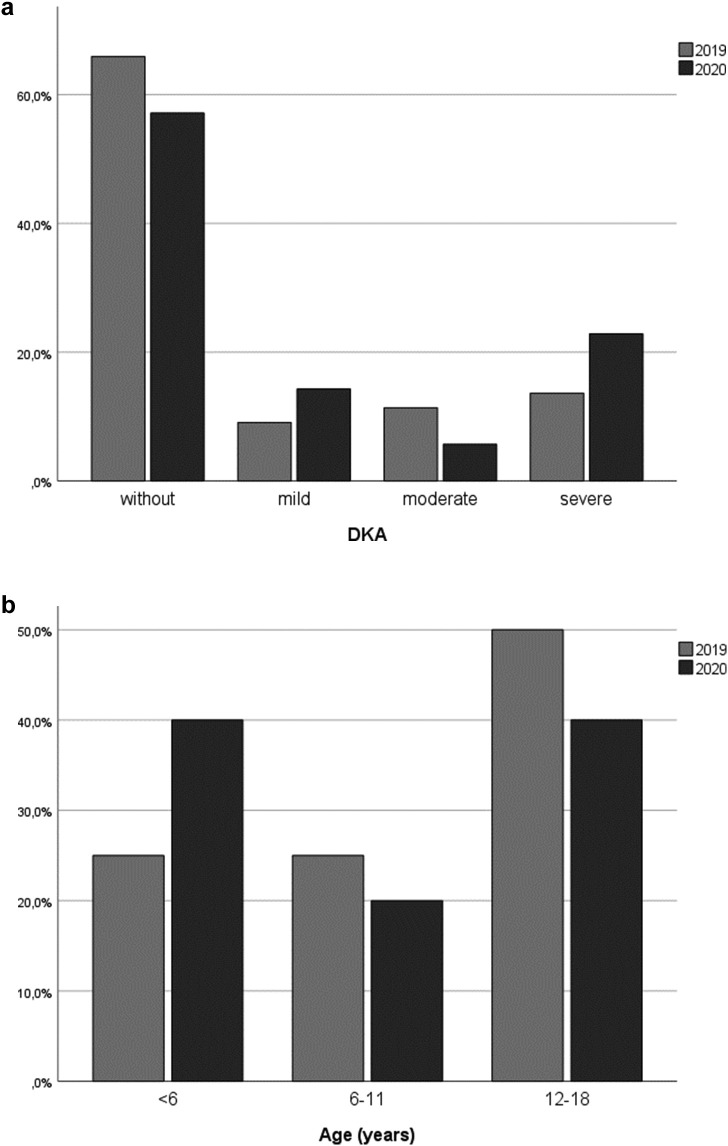
Regarding the frequency and severity of DKA, more patients were diagnosed with DKA in 2020 compared to 2019: In 2020, fifteen (28.8%) of all diabetic patients were admitted with DKA, eight (53.3%) of them with severe DKA, compared to fifteen (20.5%) of patients with DKA in 2019 and among them only six patients (40%) with severe DKA (Fig. 2a). Comparing the patients with new onset DM, even a higher difference was noticeable: In 2020, eight children (66.7%) with new onset DM presented with DKA, and five of them (62.5%) with severe DKA. The base excess at admission to hospital was on average significantly lower in 2020 (−15.08 mmol/l) compared to 2019 (−6.46 mmol/l; p = 0.03). In 2019, nine children (50%) with new onset DM were admitted with DKA, four (44.4%) of them with severe DKA (Fig. 2a). In 2020, two patients (40%) with new onset DM and severe DKA were under the age of 6 years, whereas in 2019 only one child (25%) was younger than 6 years (Fig. 2b). In addition, children that had already previously been diagnosed with DM had to be treated for DKA more frequently during lockdown in 2020 compared to 2019. With regard to all patients with known DM in 2020, seven children (17.4%) were admitted with DKA, three (13%) of them with severe DKA. During the same period of time in 2019, only six children (10.9%) presented with DKA, and only two of them (7%) with severe DKA.
Fig. 2.
a: Frequency and severity of DKA: patients with new onset DM in 2020 compared to 2019. b: Severe DKA in patients with new onset DM: Distribution of age groups in 2020 compared to 2019.
3.4. Symptoms of diabetes manifestation and duration of hospitalization
Common symptoms of diabetes manifestation were present in 73% of the children during lockdown in 2020 while in 2019, only 68.4% of the children presented with typical symptoms. More detailed, in both years, polydipsia was the most frequent symptom with 23.1% in 2020 and 19.2% in 2019. Most patients reported that they had dealt with these symptoms for a few days (2020: 50%; 2019: 40% of children).
It was of special interest, if children with new onset DM who were not yet familiar with typical symptoms of DKA, waited longer until they reported their complaints to a physician. In both years, most patients went to see a doctor after one week or a few weeks of symptoms. In 2020, 28% of the children with new onset DM and common symptoms indicated a duration of days; the same number of children reported a duration of one week or a few weeks, respectively. Only 14.3% of children indicated that they had observed a duration of clinical symptoms for several months. In 2019, 23% of children with new onset DM showed symptoms for a duration of days, 30.8% for one week, the same percentage for a few weeks and 15.4% for a duration of months (Table 1).
To evaluate the duration of hospitalization, we compared days spent in hospital. This analysis showed more hospital days during lockdown than in 2019. On average, the children spent 10.13 days in hospital in 2020 compared to 9.86 days on average in 2019. Considering only patients with newly diagnosed DM, children were treated almost three days longer in 2020 than in 2019.
3.5. HbA1c levels and applied therapy as well as therapy compliance
To evaluate the blood-glucose management, the mean HbA1c and blood glucose levels of all patients with diabetes diagnoses were compared: both measures showed no differences between 2019 and 2020. Concerning therapy compliance, the HbA1c levels of the patients with already known DM were of special interest. These patients showed surprisingly lower HbA1c levels in 2020 compared to 2019.
In comparison of the applied therapy in patients with already known diabetes, the distribution of therapy methods showed differences between both years: In 2020, 42% of all admitted patients with known DM were treated with CSII therapy whereas 58,3% had ICT. In 2019, 56% of the cases used CSII therapy compared to 44% using ICT.
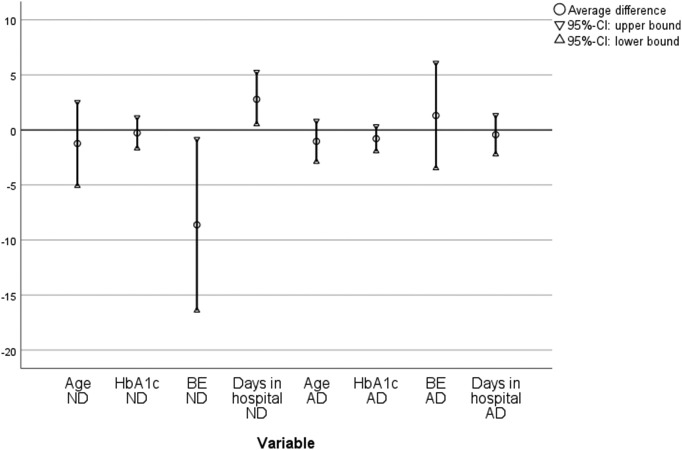
t-Test for independent variables provided no significant differences for all data included between 2020 and 2019 except for the average difference of base excess as measure for severity of DKA at time of admission, with lower values in the group of the newly diagnosed cases. By using the 95%-CI lower bound and upper bound, the above-mentioned difference trends could be visualized, however. For the overall group, lower pH-levels were a marker for severity of DKA during lockdown. In the group of the newly diagnosed patients, the trend for younger age, lower pH-levels, significantly lower base excess can be seen for the patients admitted in 2020 compared to 2019. The variable “days spent in hospital” shows a significant trend for longer hospital stays for patients with new onset DM in 2020. Consistent to the differences mentioned above in the group of patients with already known disease, there is a trend for younger age during lockdown (Fig. 3 ).
Fig. 3.
Average difference and 95%-CI in 2020 compared to 2019 (ND = new onset DM; AD = already diagnosed DM; base excess in mmol/l; HbA1c in %; age = age at admittance to hospital).
Binary logistic regression calculated a 1.65 times higher odds-ratio (95%-CI: 0.58–4.74) for being admitted with a DKA in 2020 compared to 2019 adjusted for age, gender and BMI. Regardless of the observed differences in frequency and severity of DKA between 2020 and 2019, Fisher's exact test and Mann-Whitney-U test could not underline these results with statistical significance (p-values shown in Table 2 ).
Table 2.
p-Values Fisher's exact test and Mann-Whitney-U test for frequency and severity of DKA (mild, moderate, severe).
| Fisher's exact test (DKA yes/no) Two-sided exact p-value |
Mann-Whitney-U test (DKA clinical classification) Exact p-value |
|
|---|---|---|
| Overall | 0,488 | 0,4 |
| New onset DM | 0,465 | 0,346 |
| Already diagnosed | 0,747 | 0,570 |
4. Discussion
Children and adolescents with new onset or known DM who were admitted to our hospital during the first lockdown of the Corona Pandemic in 2020 were analyzed with regard to clinical presentation, severity of DKA and therapy compliance and were compared to a group of children with DM who presented during the same time in 2019.
One of our main findings is that the general incidence and severity of DKA was higher in 2020 compared to 2019, independent of the fact whether children presented with new onset DM or were admitted to hospital with known DM and poor metabolic control. However, and consistent with previous studies [2,7] more cases of (severe) DKA were related to patients with new onset DM compared to children with already known diabetes. In addition, our study showed that the number of already diagnosed patients in need for therapy adjustment and who presented with DKA, especially severe DKA, increased in 2020 compared to 2019. However, when interpreting these results it has to be mentioned that, although odds ratio was 1.65 times higher for being admitted with a DKA in 2020 compared to 2019 adjusted for age, gender and BMI, the observed differences in frequency and severity of DKA between 2020 and 2019 did not reach statistical significance, which may be attributable to the small sample size.
The information available on the symptoms and duration of symptoms did not point out if parents or children waited longer until they came to the emergency department. In 2020, less children were treated with DM in hospital, and a greater severity of DKA was found in 2020, which may be explained by a lower tendency for seeking for medical advice during the COVID-19 related lockdown, which in turn may have led to lower admission rate to hospital in 2020. Fear of exposure to SARS-CoV-2, the commitment to not overload the health system or unavailability of professional help may have influenced the delayed diagnosis and furthermore the severity of DKA [10]. As far as a delayed diagnosis is concerned, factors such as a younger age < 6 years and migration background seem to be major risk factors for severity of DKA, which underlines the need to support awareness of the typical symptoms. In the “Stuttgart Diabetes Awareness Campaign” the incidence of DKA decreased significantly by informing and encouraging parents to see a physician if their child shows polydipsia, polyuria, weight loss and lack of energy [11]. More specifically, patients with new onset DM were younger in this study compared to the year before.
The influence of lockdown on handling health care might have been stronger for smaller children. Lack of preschool teachers who notice symptoms and missed diagnosis because of unspecific symptoms as stomach pain could have worsened the clinical presentation [12]. This underlines the necessity of parents to be informed about the common symptoms. The U1-J1 examinations that are regularly performed within Germany, might be a good time slot for health education, and the model may be transferred to other countries. In addition, the German Working Group of Pediatric Diabetology (AGPD) of the German Diabetes Association has initiated a campaign to early detect and diagnose type 1 diabetes in children: Pediatricians shall explain the early “cardinal” symptoms of new onset of DM to parents at routine checkups and will hand out a flyer to parents with important information [13]. This project may serve as a model, too, to be adapted from other Associations such as the ISPAD.
In the group of already diagnosed cases, adolescents in particular were facing problems with DKA. It is necessary, especially in times of lockdown, to support compliance and understanding of the disease. Adolescents might have had less physical activity, less structure in daily life and more psychological stress. This could cause less motivation and sensibility to be aware of and react on blood-glucose changes. In this age group the supervision of parents for disease management decreases. Staying at home due to COVID-19 lockdown may have supported the desire in adolescents to act independently in diabetes therapy. Even though no higher HbA1c levels were found in this study or other studies on continuous glucose measurement have shown no changes of TIR (time in range) during lockdown [14], ways to prevent DKA in adolescents have to be developed. The ISPAD (International Society for Pediatric and Adolescent Diabetes) underlines insulin omission as the cause of DKA. Children with pump therapy, who fail to take extra insulin when necessary or renounce insulin therapy e.g. to lose weight, need psychological support and further education. ISPAD suggests measurement of blood BOHB (β-Hydroxybutyrate) in case of emergency in children with pump therapy to identify ketosis faster [9]. The pediatric department in Wisconsin developed further steps to reduce complications for DKA through diagnosing DKA faster. POC tests (point of care), order panels, provider guidelines, and nursing guidelines reduced the time to determine DKA from 86 to 30 minutes [15]. Investing in structured education and guidelines for primary health care staff is an important step, particularly in times of COVID-19, to prevent complications in DKA diagnosis and therapy. In addition, it is important to know risk factors and to identify children at risk. In our study more adolescent girls were admitted to hospital in 2020 as well as in 2019. Besides female gender and adolescent age, risk factors for DKA can be socioeconomic disadvantage, high HbA1c levels i.e. therapy incompliance, previous DKA, and psychiatric comorbidities (e.g. eating disorders and depression) [16].
As an intervention in times of COVID-19, one could introduce patients to telemedicine and check in with them on a more regular basis without asking them to come to the hospital [17]. This could help adolescents to stay motivated. Furthermore, pediatricians might recognize adolescents, especially girls, struggling with their therapy and potential eating disorders.
In 2020, two cases of newly diagnosed Type 2 diabetes were treated in our hospital, and both patients presented with severe ketoacidosis and in poor clinical condition. Less physical activity, disordered eating and more screen time during lockdown may have worsened insulin resistance and caused diabetes manifestation [17]. The development of creative ways to use screen time for example to promote physical activity has to be supported [18].
Our results are in line with a recently published study from a Canadian group, who have also analyzed DKA in children with T1DM during the COVID-19 pandemic: The authors did not find a difference in the amount newly diagnosed patients with DM1 between 2019 and 2020. However, they have also and in concordance with our results seen a higher frequency of DKA at DM1 onset in 2020 compared to 2019 (68.2% vs 45.6%; p < 0.001). In addition, the incidence of severe DKA was also higher (27.1% in 2020 vs 13.2% in 2019; p = 0.01) [19].
Although the majority of children with new onset DM are diagnosed as T1DM, there is increasing evidence that although T2DM is more frequently seen in the pediatric population: A recent study that has investigated 835 children with newly diagnosed DM, and 84% of these patients were diagnosed as T1D, 5.7% as T2D, 5.3% as clinical MODY and 5% as being cases of other types of diabetes. More interestingly and in line with our results, fourteen of these patients (29.2%) with T2D presented with ketosis and two of them (4.2%) had DKA at initial diagnosis. The authors could clearly show that a significant increase in the frequency of T2D is to be found in recent years and that a quite increasing number of patients present with clinical symptoms of DKA at diagnosis [20].
Regarding therapy management in the group of patients with known DM, lower HbA1c and blood-glucose levels were found in 2020 compared to 2019. Chowdhury and Goswami have published a review based on PubMed search from December 2019 to May 2020 and found either no changes in blood-glucose management or better measures during lockdown. They suggest that parents could have had a positive influence on insulin therapy. Other explanations could be more regular meals at home and less stress in school during lockdown [21].
In 2020, more children with ICT compared to CSII needed treatment. It is known that pump therapy reduces the rate of hypoglycemia and ketoacidosis [22]. Also, the use of CGM-systems decreases complications in diabetes therapy [23]. Lockdown may have aggravated the difficulties in autonomous ICT therapy and blood-glucose measurements. Children and adolescents at risk for complications could benefit from pump therapy and should be adjusted for CSII.
The strengths of this study is that we have analyzed a well-defined and characterized cohort of patients who presented during the COVID-19 Pandemic Lockdown in 2020 to a Pediatric University Hospital and have compared them to identical data of patients from 2019. New information is provided on two cases with new-onset Type2 Diabetes, and both patients presented with severe DKA, which is very rare in the pediatric population.
Limitations of this work include the small sample size, exclusive use of electronic medical records without asking parents and children on their handling with lockdown and lack of information on the socioeconomic status and migration background of the cases.
5. Conclusions
In conclusion, the results of our study show that the Lockdown during the COVID19-pandemic has led to more severe DKA in patients with newly diagnosed or already known DM, and that patients were younger at first presentation compared to a year before and had to stay longer in hospital. We thus confirm and extend previous findings by showing that different age groups are differently affected and that there are also differences between genders. In addition, we show for the first time that two adolescent patients who presented with severe DKA and in bad clinical condition during the 2020 Pandemic were diagnosed with Type 2 DM. Thus, although the most frequent cause of newly diagnosed DM is type 1 diabetes in childhood, there is a clear trend towards an increase in the frequency of type 2 diabetes, and some of these patients present with severe DKA.
In summary, COVID-19 lockdown aggravated common complications of diabetes onset and therapy management, including severity and frequency of DKA and new manifestation of DM2. Health education for early DKA diagnosis to early identify children at risk is urgently needed and should be routinely implemented in pediatric routine visits, especially in times of lockdown.
CRediT authorship contribution statement
Charlotte Loh: Conceptualization, Methodology, Formal analysis, Writing – original draft. Paul Weihe: Investigation, Validation, Writing – review & editing. Nicole Kuplin: Validation, Writing – review & editing. Kerstin Placzek: Investigation, Validation, Writing – review & editing. Susann Weihrauch-Blüher: Conceptualization, Validation, Resources, Writing – review & editing, Supervision.
Declaration of competing interest
The authors disclose any potential conflict of interest.
Footnotes
No funding was received for the work related to this manuscript.
References
- 1.Kapsner L.A., Kampf M.O., Seuchter S.A., et al. Reduced rate of inpatient hospital admissions in 18 German university hospitals during the COVID-19 lockdown. Front Public Health. 2020;8 doi: 10.3389/fpubh.2020.594117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kamrath C., Mönkemöller K., Biester T., et al. Ketoacidosis in children and adolescents with newly diagnosed type 1 diabetes during the COVID-19 pandemic in Germany. JAMA. 2020;324(8) doi: 10.1001/jama.2020.13445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goldman N., Fink D., Cai J., Lee Y.-N., Davies Z. High prevalence of COVID-19-associated diabetic ketoacidosis in UK secondary care. Diabetes Res Clin Pract. 2020 doi: 10.1016/j.diabres.2020.108291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Soliman A.T., Al-Amri M., Alleethy K., Alaaraj N., Hamed N., de Sanctis V. Newly-onset type 1 diabetes mellitus precipitated by COVID-19 in an 8-month-old infant. Acta Biomed. 2020;91(3) doi: 10.23750/abm.v91i3.10074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rabizadeh S., Hajmiri M., Rajab A., Emadi Kouchak H., Nakhjavani M. Severe diabetic ketoacidosis and coronavirus disease 2019 (COVID-19) infection in a teenage patient with newly diagnosed diabetes. J Pediatr Endocrinol Metab. 2020;33(9) doi: 10.1515/jpem-2020-0296. [DOI] [PubMed] [Google Scholar]
- 6.Tittel S.R., Rosenbauer J., Kamrath C., et al. Did the COVID-19 lockdown affect the incidence of pediatric type 1 diabetes in Germany? Diabetes Care. 2020;11 doi: 10.2337/dc20-1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Elbarbary N.S., Dos Santos T.J., de Beaufort C., Agwu J.C., Calliari L.E., Scaramuzza A.E. COVID-19 outbreak and pediatric diabetes: perceptions of health care professionals worldwide. Pediatr Diabetes. 2020 doi: 10.1111/pedi.13084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kromeyer-Hauschild K., Wabitsch M., Kunze D., et al. Perzentile für den Body-mass-Index für das Kindes- und Jugendalter unter Heranziehung verschiedener deutscher Stichproben. Monatsschr Kinderheilkd. 2001;149(8) doi: 10.1007/s001120170107. [DOI] [Google Scholar]
- 9.Wolfsdorf J.I., Glaser N., Agus M., et al. ISPAD Clinical Practice Consensus Guidelines 2018: diabetic ketoacidosis and the hyperglycemic hyperosmolar state. Pediatr Diabetes. 2018:19. doi: 10.1111/pedi.12701. [DOI] [PubMed] [Google Scholar]
- 10.Lazzerini M., Barbi E., Apicella A., Marchetti F., Cardinale F., Trobia G. Delayed access or provision of care in Italy resulting from fear of COVID-19. Lancet Child Adolesc Health. 2020;4(5) doi: 10.1016/S2352-4642(20)30108-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Holder M., Ehehalt S. Significant reduction of ketoacidosis at diabetes onset in children and adolescents with type 1 diabetes-the Stuttgart Diabetes Awareness Campaign, Germany. Pediatr Diabetes. 2020;21(7) doi: 10.1111/pedi.13064. [DOI] [PubMed] [Google Scholar]
- 12.Bui H., To T, Stein R., Fung K., Daneman D. Is diabetic ketoacidosis at disease onset a result of missed diagnosis? J Pediatr. 2010;156(3) doi: 10.1016/j.jpeds.2009.10.001. [DOI] [PubMed] [Google Scholar]
- 13.AGPD Kampagne zur Früherkennung des Diabetes und zur Vermeidung einer diabetischen Ketoazidose bei Kleinkindern. 2021. https://diabetes-kinder.de/news/Diabetische-Ketoazidose-bei-Kleinkindern.html Accessed March 5.
- 14.Ceconi V., Barbi E., Tornese G. Glycemic control in type 1 diabetes mellitus and COVID-19 lockdown: what comes after a “quarantine”? J Diabetes. 2020 doi: 10.1111/1753-0407.13110. [DOI] [PubMed] [Google Scholar]
- 15.Baumer-Mouradian S.H., Gray M.P., Wolfgram P.M., et al. Improving emergency department management of diabetic ketoacidosis in children. Pediatrics. 2019;144(4) doi: 10.1542/peds.2018-2984. [DOI] [PubMed] [Google Scholar]
- 16.Ehrmann D., Kulzer B., Roos T., Haak T., Al-Khatib M., Hermanns N. Risk factors and prevention strategies for diabetic ketoacidosis in people with established type 1 diabetes. Lancet Diabetes Endocrinol. 2020;8(5) doi: 10.1016/S2213-8587(20)30042-5. [DOI] [PubMed] [Google Scholar]
- 17.Whooten R., Kerem L., Stanley T. Physical activity in adolescents and children and relationship to metabolic health. Curr Opin Endocrinol Diabetes Obes. 2019;26(1):25–31. doi: 10.1097/MED.0000000000000455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nagata J.M., Abdel Magid H.S., Pettee Gabriel K. Screen time for children and adolescents during the coronavirus disease 2019 pandemic. Obesity (Silver Spring) 2020;28(9):1582–1583. doi: 10.1002/oby.22917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ho J., Rosolowsky E., Pacaud D., et al. Diabetic ketoacidosis at type 1 diabetes diagnosis in children during the COVID-19 pandemic. Pediatr Diabetes. 2021 doi: 10.1111/pedi.13205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Haliloğlu B., Abali S., Buğrul F., et al. The distribution of different types of diabetes in childhood: a single center experience. J Clin Res Pediatr Endocrinol. 2018;10(2):125–130. doi: 10.4274/jcrpe.5204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chowdhury S., Goswami S. COVID-19 and type 1 diabetes: dealing with the difficult duo. Int J Diabetes Dev Ctries. 2020;40(3) doi: 10.1007/s13410-020-00846-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Karges B., Schwandt A., Heidtmann B., et al. Association of insulin pump therapy vs insulin injection therapy with severe hypoglycemia, ketoacidosis, and glycemic control among children, adolescents, and young adults with type 1 diabetes. JAMA. 2017;318(14):1358–1366. doi: 10.1001/jama.2017.13994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tauschmann M., Hermann J.M., Freiberg C., et al. Reduction in diabetic ketoacidosis and severe hypoglycemia in pediatric type 1 diabetes during the first year of continuous glucose monitoring: a multicenter analysis of 3,553 subjects from the DPV registry. Diabetes Care. 2020;43(3):e40–e42. doi: 10.2337/dc19-1358. [DOI] [PubMed] [Google Scholar]