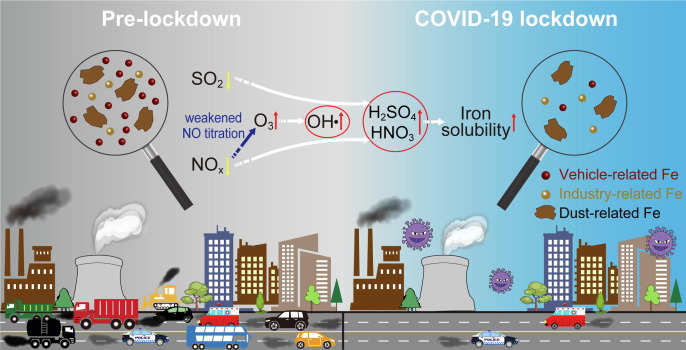
Graphical abstract
Keywords: COVID-19, Iron solubility, PM2.5, Enrichment factor, Aerosol acidification
Abstract
Iron (Fe) in the atmosphere can affect atmospheric chemical processes and human health. When deposited into oceans, it can further influence phytoplankton growth. These roles of Fe fundamentally depend on its concentration and solubility. However, the sources of aerosol Fe and controlling factors of Fe solubility in megacities remain poorly understood. The outbreak of the COVID-19 pandemic causes large changes in human activities, which provides a unique opportunity to answer these key issues. Field observations were conducted before, during, and after the COVID-19 lockdown in Hangzhou, China. Our results show that in the COVID-19 lockdown stage, the concentrations of total Fe (FeT, 75.0 ng m−3) and soluble Fe (FeS, 5.1 ng m−3) in PM2.5 decreased by 78% and 62%, respectively, compared with those (FeT 344.7 ng m−3, FeS 13.5 ng m−3) in the pre-lockdown stage. The sharp reduction (81%) in on-road vehicles was most responsible for the aerosol Fe decrease. Surprisingly, the Fe solubility increased by a factor of 1.9, from 4.2% in the pre-lockdown stage to 7.8% in the COVID-19 lockdown stage. We found that the atmospheric oxidizing capacity was enhanced after lockdown restrictions were implemented, which promoted the formation of more acidic species and further enhanced the dissolution of aerosol Fe.
1. Introduction
Iron (Fe), as one of the most abundant heavy metals in atmospheric fine particles (PM2.5), can significantly affect atmospheric chemical processes such as the transition metal-catalyzed oxidation of S(IV) to S(VI) and iron-driven formation of secondary brown carbon (Al-Abadleh et al., 2020, Harris et al., 2013). After inhalation, Fe can impair human health by inducing the formation of reactive oxygen species, which causes oxidative stress on cells in vivo and thus leads to diseases such as lung inflammation and cardiovascular disorders (Abbaspour et al., 2014, Charrier and Anastasio, 2012). Furthermore, the aerosol Fe is an important external source of bioavailable Fe for marine phytoplankton growth on remote ocean surfaces, which can modulate marine ecosystems and promote atmospheric carbon dioxide (CO2) uptake, thereby indirectly affect the global climate (Matsui et al., 2018). The biogeochemical cycles of Fe and its aforementioned roles significantly depend on the aerosol total Fe (FeT) concentration and Fe solubility (%FeS, i.e., the concentration ratio of water-soluble Fe (FeS) to FeT) (Shi et al., 2012, Zhu et al., 2020).
Natural Fe-containing mineral dust and anthropogenic pyrogenic Fe-containing aerosols from fossil fuel and biomass combustion are the two main sources of aerosol Fe in the atmosphere (Ito et al., 2019, Ito and Shi, 2016, Matsui et al., 2018). The %FeS from different sources can span four orders of magnitude (Mahowald et al., 2005, Schroth et al., 2009). It is reported that the pyrogenic Fe has a higher solubility although smaller global emission flux than crustal Fe (Fu et al., 2012, Ito et al., 2019, Mahowald et al., 2009), and the atmospheric processes such as photochemistry, acid dissolution, organic complexion, and cloud/fog processing can largely alter the %FeS (Chen et al., 2012, Ito, 2015, Shi et al., 2020, Shi et al., 2015, Zhang et al., 2014). In recent years, many observational and modeling studies have shown that the pyrogenic Fe may be an important source of soluble Fe to the oceans (Conway et al., 2019, Hamilton et al., 2020, Ito and Shi, 2016). Due to the fast industrialization and urbanization, high levels of anthropogenic Fe-containing aerosols (e.g., coal and oil fly ashes) has been frequently detected in the ambient air and even the cloud droplets in eastern China (Li et al., 2012, Liu et al., 2018, Yuan et al., 2020). It has been well documented that the acid dissolution of Fe associated with air pollution–aerosol interactions in eastern China substantially increases the solubility of pyrogenic Fe from coal combustion and steel industries (Li et al., 2017). However, due to various emission sources of aerosol Fe and complex atmospheric physicochemical processes in polluted air in megacities of eastern China, the source contributions, the aerosol Fe solubility, and its influencing factors remain less known.
In order to control the spread of the COVID-19 pandemic, the Chinese government raised the national public health response to the first level (i.e., the highest state of emergency) and implemented a series of strict lockdown restrictions on outdoor human activities, such as stay-at-home orders, suspended public transports, and halted construction activities, since 23 January 2020 (Tian et al., 2020). The nationwide lockdown caused an unprecedented modification of human interactions, which provides us a unique opportunity to confirm the sources of aerosol Fe in the atmosphere of megacities and to understand the variations in concentration and solubility of aerosol Fe responding to the changes of anthropogenic emissions. The changes in sources and concentrations of major air pollutants caused by the COVID-19 pandemic have been widely explored all over the world (Cao et al., 2021, Chang et al., 2020, Cui et al., 2020, Lv et al., 2020, Parker et al., 2020, Shi et al., 2021, Sun et al., 2020, Zhang et al., 2020). However, few studies focused on the variation in aerosol Fe solubility. In this study, field observation, covering before, during, and after the COVID-19 lockdown, was carried out at an urban site in the megacity of Hangzhou which is located in the south region of the Yangtze River Delta (YRD). The concentrations of FeT and FeS in PM2.5 were characterized before, during, and after the COVID-19 lockdown. Furthermore, the sources of aerosol Fe and influencing factors of Fe solubility were explored.
2. Methodology
2.1. Observation site and sample collection
Field observation was carried out during 1 January–31 March 2020 at the Hangzhou National Reference Climatological Station (NRCS, 30°14′N, 120°10′E), which is located in the center of urban Hangzhou (Fig. 1 ). The NRCS is a typical urban site situated in residential and commercial areas. There are no local industrial emission sources around the site. Therefore, the NRCS is treated as a representative site receiving mixed pollutants from local urban plumes (e.g., vehicular and residential emissions) and regional air masses from other regions in the YRD (Liu et al., 2021). During this field observation, gaseous pollutants, i.e., SO2, NOx, and O3 were monitored by a set of commercial analyzers, i.e., model 43i SO2 analyzer, 42i NOx analyzer, and 49i O3 analyzer (ThermoFisher Scientific, USA). Ambient PM2.5 samples were collected onto 47 mm-diameter quartz filters (Whatman, Middlesex, UK) using a low-volume sampler (BGI PQ200, Mesa Laboratories, Inc., USA) at a flow rate of 16.67 L min−1 for 23 h 50 min (i.e., 10:00 am to 9:50 am the next day). Field blank samples were collected for 15 min without starting the sampler. Before collection, the quartz filters were prebaked at 450 °C for 6 h to remove any possible contaminants. In total, 60 PM2.5 samples were collected. All the PM2.5 samples were sealed individually in aluminum foil bags and stored at −20 °C in a freezer for further analysis.
Fig. 1.
Location of the observation site (NRCS) in Hangzhou. (Map copyright © Google Earth).
2.2. Sample analysis
The FeT and other metal concentrations were acquired through the non-destructive analysis of PM2.5 samples using an energy-dispersive X-ray fluorescence (ED–XRF) spectrometer (Epsilon 4, PANalytical B.V., Netherlands). The fine particulate matter standard reference material (SRM 2786, National Institute of Standards and Technology, USA) was used to calibrate the working curve of ED–XRF spectrometer before analyzing samples. The detection limit of the ED–XRF spectrometer for FeT is 0.4 ng cm−2.
For the analysis of FeS and WSIIs, half of each PM2.5 sample filter was cut into pieces with ceramic scissors and put into acid-cleaned polypropylene vials with 20 mL of ultrapure Milli-Q water (≥18.2 MΩ·cm). After that, the vials were placed in an ultrasonic water bath for 60 min of ultrasonic extraction. The extracts were further filtered using PTFE syringe filters with 0.22 μm pores to remove insoluble components. Then, a 5 mL aliquot of the filtrate was used to analyze the WSIIs directly by the ion chromatography system (Dionex ICS 600, ThermoFisher Scientific, USA). The concentrations of SO4 2− and NO3 − were obtained. The remaining filtrate was further used to analyze FeS. According to the procedures reported by Oakes et al. (2012), a 10 mL aliquot of the remaining filtrate was taken out, then 100 μL hydroxylamine hydrochloride (HA) solution (5.5 mM) was added in to reduce soluble Fe(III) to Fe(II). After 10 min of incubation time, 100 μL ferrozine solution (5.1 mM) was added subsequently for the reaction of Fe(II)–ferrozine complex. After 10 min, the resulting solution was analyzed immediately by a UV–Visible spectrophotometer (USB4000, Ocean Optics, USA). A liquid waveguide capillary cell (LWCC) with 100 cm path length (LPC-100CM, Ocean Optics, USA) was used to enhance the measurement sensitivity. Light absorptions at 562 nm (maximum absorption wavelength of Fe(II)–ferrozine complex) and 700 nm (background) were obtained. Then, the concentration of FeS in each sample was calculated according to the calibration curve plotted using five ammonium Fe(II) sulfate standards with Fe(II) concentrations ranging 0–20 ng mL−1.
2.3. Enrichment factor (EF) calculation
The EF can be used to evaluate the relative contribution of natural and anthropogenic sources of elements in atmospheric aerosols (Chester et al., 1993). The EF of element i (EFi) is calculated as follows:
where represents the ratio of the examined element i concentration (Xi) and the reference element concentration (Xref) in aerosols; represents the ratio of the examined element i concentration (Xi) and the reference element concentration (Xref) in the crust. The elemental concentrations in the crust are based on the Chinese background soil values from Chen et al. (1991). The elements that are stable, spatially homogeneous, and least impacted by anthropogenic pollution can be selected as reference elements (Wei et al., 1999). Conventionally, if EFi < 10, element i is mainly originated from natural crustal sources (e.g., dust and soil); if EFi > 10, element i is significantly contributed by anthropogenic sources (e.g., industries and vehicles) (Zhou et al., 2014). In this study, Al was selected as the reference element and the EF of Fe (EFFe) in PM2.5 samples were calculated.
3. Results and discussion
3.1. Changes in concentrations and solubilities of aerosol Fe in PM2.5
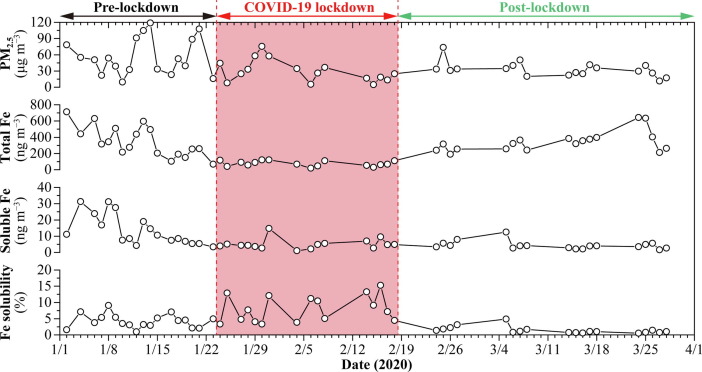
The Chinese government implemented a nationwide lockdown since 23 January 2020 due to the outbreak of the COVID-19 pandemic (Tian et al., 2020). As the COVID-19 pandemic was gradually under control, the lockdown in Hangzhou was loosened by the Hangzhou municipal government after 18 February 2020 (Liu et al., 2021). Therefore, according to the timeline of the governments’ responses to control the spread of the COVID-19 pandemic, we divided the whole observation period into three stages: pre-lockdown (1–23 January 2020), COVID-19 lockdown (24 January–18 February 2020), and post-lockdown (19 February–31 March 2020). Fig. 2 shows the variations of daily PM2.5, FeT, and FeS concentrations and %FeS during the whole observation period in urban Hangzhou. It should be noted that the analysis results of the PM2.5 samples collected on rainy days were excluded in this study. Daily PM2.5 concentration ranged 4.8–118.8 µg m−3, with its average concentration (±standard deviation) being 40.2 ± 26.7 µg m−3 during the whole observation period. Daily FeT concentration shows a similar variation trend to that of PM2.5 and ranged 18.5–712.3 ng m−3, with its average concentration being 261.2 ± 181.9 ng m−3. Daily FeS concentration ranged 1.1–31.3 ng m−3 and its average concentration (7.7 ± 7.2 ng m−3) was much lower than that of FeT (261.2 ± 181.9 ng m−3). Based on the variations of FeT and FeS concentrations, we calculated that %FeS ranged 0.5%–15.3% and its average value was 4.4%±3.8%.
Fig. 2.
Time series of daily PM2.5, total Fe (FeT), and water-soluble Fe (FeS) concentrations and Fe solubility (%FeS) during the whole observation period in urban Hangzhou. The divisions of the pre-lockdown, COVID-19 lockdown, and post-lockdown stages are marked on the top of this figure.
The average PM2.5 concentration decreased to 30.1 ± 20.4 µg m−3 in the COVID-19 lockdown stage, a decline of 47% compared with that (56.4 ± 33.8 µg m−3) in the pre-lockdown stage, and then increased slightly (9%) to 32.9 ± 13.9 µg m−3 in the post-lockdown stage (Fig. 3 a). The decrease in average FeT concentration (78%) from the pre-lockdown stage (344.7 ± 187.8 ng m−3) to the COVID-19 lockdown stage (75.0 ± 34.1 ng m−3) was more significant than that (47%) of PM2.5. In the post-lockdown stage, the average FeT concentration (343.1 ± 125.7 ng m−3) gradually returned to the level in the pre-lockdown stage (Fig. 3b). The FeS concentration decreased by 62% from the pre-lockdown stage (13.5 ± 9.3 ng m−3) to the COVID-19 lockdown stage (5.1 ± 3.3 ng m−3), but unlike the increasing trends of PM2.5 and FeT concentrations in the post-lockdown stage, the FeS concentration still decreased slightly (16%) to 4.3 ± 2.6 ng m−3 in the post-lockdown stage (Fig. 3c). Surprisingly, we found that the %FeS presented the highest value (7.8%±4.3%) in the COVID-19 lockdown stage, which increased significantly by a factor of 1.9 compared with that (4.2%±2.1%) in the pre-lockdown stage, despite sharp decreases in the concentrations of FeT and FeS. In the post-lockdown stage, the %FeS decreased to 1.4%±1.1% which is even much lower than that (4.2%±2.1%) in the pre-lockdown stage (Fig. 3d). We noticed that the observed FeT concentration (75.0 ± 34.1 ng m−3) in the COVID-19 lockdown stage is much lower than most of the values in different regions and circumstances reported by previous papers, whereas the observed %FeS (7.8%±4.3%) presents a higher value (Table 1 ). In order to understand the causes of high %FeS in the COVID-19 lockdown stage in Hangzhou, we further analyze the source changes of aerosol Fe after the outbreak of the COVID-19 pandemic and explore the main factors contributing to the increase of %FeS in the following sections.
Fig. 3.
Comparisons of (a) PM2.5, (b) total Fe (FeT), and (c) water-soluble Fe (FeS) concentrations and (d) Fe solubility (%FeS) among the pre-lockdown, COVID-19 lockdown, and post-lockdown stages. The box represents the 25th (lower line) and 75th (upper line) percentiles; the solid line and circle in the box represent the median and mean values, respectively; and the lower and upper whiskers represent the 10th and 90th percentiles, respectively.
Table 1.
The aerosol total Fe (FeT) concentration and Fe solubility (%FeS) in this study and in other regions under different circumstances reported by previous papers.
| Location | Site type | Sample type | Period (weather condition) | FeT (ng m−3) | %FeS (%) | Reference |
|---|---|---|---|---|---|---|
| Hangzhou | Urban | PM2.5 | Pre-lockdown (haze) | 345 | 4.2 | This study |
| PM2.5 | COVID-19 lockdown (clear) | 75 | 7.8 | This study | ||
| PM2.5 | Post-lockdown (clear) | 343 | 1.4 | This study | ||
| Beijing | Urban | PM2.5 | Winter (haze) | 1490 | 5.0 | Zhu et al. (2020) |
| Handan | Urban | PM2.5 | Winter (haze) | 1310 | 4.5 | Zhu et al. (2020) |
| Zhengzhou | Urban | PM2.5 | Winter (haze) | 1132 | 2.7 | Zhu et al. (2020) |
| Hangzhou | Urban | PM2.5 | Winter (haze) | 869 | 3.0 | Zhu et al. (2020) |
| Qingdao | Coastal | TSP | Winter and Spring (clear) | 3180 | 1.1 | Shi et al. (2020) |
| TSP | Winter and Spring (fog) | 2720 | 5.8 | Shi et al. (2020) | ||
| TSP | Winter and Spring (dust) | 15,000 | 0.3 | Shi et al. (2020) | ||
| TSP | Winter and Spring (haze) | 5130 | 1.7 | Shi et al. (2020) | ||
| East China Sea | Ocean | TSP | Winter and Spring (dust) | 2224 | 1.5 | Hsu et al. (2010) |
| TSP | Winter and Spring (non-dust) | 268 | 7.7 | Hsu et al. (2010) | ||
| TSP | Summer (non-dust) | 187 | 11.5 | Hsu et al. (2010) | ||
| Mt. Abu (Western India) | Mountain | PM2.5 | Summer (dust) | 161–915 | 1.6 | Kumar and Sarin (2010) |
| PM2.5 | Winter (haze) | 50–397 | 8.1 | Kumar and Sarin (2010) |
3.2. Changes in sources of aerosol Fe in PM2.5
Aerosol Fe in megacities has complex mixed sources, including crustal sources (e.g., resuspended dust and construction dust) and anthropogenic sources (e.g., motor vehicles, industries, and coal/biomass burning) (Ohata et al., 2018, Zhang et al., 2014). The EF has been widely used as the first step to identify the relative contribution of crustal or anthropogenic sources to certain elements in aerosols (Gao et al., 2020, Xia and Gao, 2010, Zhou et al., 2014). Table 2 shows the changes of EFFe in PM2.5 before, during, and after the COVID-lockdown. The EFFe was 13.3 in the pre-lockdown stage, indicating a dominant contribution of anthropogenic sources to FeT in PM2.5 under normal conditions in Hangzhou before the outbreak of the COVID-19 pandemic. The EFFe decreased sharply to 1.6 in the COVID-19 lockdown stage, which reflects that the FeT in PM2.5 mainly originated from crustal sources. It is reasonable because outdoor human activities were strictly prohibited after the implementation of lockdown measures, which caused sharp reductions in anthropogenic emissions in Hangzhou (Liu et al., 2021, Xu et al., 2020, Yuan et al., 2021). In the post-lockdown stage, with the gradual lifting of restrictions, the anthropogenic emissions began to recover. Therefore, the EFFe increased to 6.6 in the post-lockdown stage but was still lower than that (13.3) in the pre-lockdown stage. This result indicates that both the crustal and anthropogenic sources contributed significantly to FeT in PM2.5 in the post-lockdown stage.
Table 2.
Variations in average enrichment factors of Fe (EFFe), concentrations of NOx, SO2, and O3, and numbers of on-road vehicles among the three stages in Hangzhou.
| Stage | EFFe | NOx (ppb) | SO2 (ppb) | O3 (ppb) | Vehicle numbers (Normalized) |
|---|---|---|---|---|---|
| Pre-lockdown | 13.3 | 37.5 ± 17.5 | 1.6 ± 0.7 | 10.1 ± 6.9 | 1.00 |
| COVID-19 lockdown | 1.6 | 6.8 ± 2.7 | 1.3 ± 0.4 | 29.3 ± 10.5 | 0.19 |
| Post-lockdown | 6.6 | 23.4 ± 9.2 | 1.7 ± 0.5 | 23.4 ± 9.2 | 0.72 |
The COVID-19 lockdown also provides us a good opportunity to further identify the anthropogenic sources of FeT in PM2.5 in Hangzhou, according to the reduced levels of human activities and the concentration changes of anthropogenic pollutants. As shown in Table 2, the NOx concentration (6.8 ± 2.7 ppb) decreased sharply by 82% in the COVID-19 lockdown stage compared with that (37.5 ± 17.5 ppb) in the pre-lockdown stage, whereas the SO2 concentration only decreased by 19% (1.6 ± 0.7 ppb to 1.3 ± 0.4 ppb). The quite low SO2 concentration during the whole observation period and its small decrease in the COVID-19 lockdown stage suggest that industrial emissions contributed a small fraction of air pollutants in Hangzhou. Besides, the domestic coal combustion for heating can be ruled out because it does not exist in southern China. We noticed that the on-road vehicle numbers (normalized) decreased by 81% in the COVID-19 lockdown stage compared with that in the pre-lockdown stage, which is in good consistency with the declines of NO3 − and FeT (Table 2 and Fig. 3). It has been reported that the traffic-related tire abrasion, brake linings, lubricants, and melting of engines can release large amounts of iron oxides (Huang et al., 2018, Liati et al., 2015, Ohata et al., 2018). Thus, we conclude that vehicular emissions are the major sources of aerosol Fe in Hangzhou under normal conditions.
3.3. Factors controlling the Fe solubility
It has been known that the %FeS mainly depends on the sources and atmospheric processes of aerosol Fe (Gao et al., 2020, Ito et al., 2019, Mahowald et al., 2018). As we have mentioned in Section 3.2, the anthropogenic sources, especially vehicular emissions, significantly contributed to aerosol Fe in the pre-lockdown stage (Table 2). After the outbreak of the COVID-19 pandemic, the concentrations of air pollutants (e.g., PM2.5, NOx, and FeT) decreased sharply due to the lockdown controls and the FeT in the COVID-19 lockdown stage mainly originated from crustal sources (Fig. 3 and Table 2). Many studies have reported that the %FeS in soil dust is commonly less than 1% (Hsu et al., 2010, Schroth et al., 2009, Shi et al., 2020). Therefore, the source changes cannot explain the observed high %FeS in the COVID-19 lockdown stage, which suggests that the %FeS must have been influenced by chemical processing such as acid dissolution.
The Fe-containing particles have been frequently found to be coated with sulfate and nitrate aerosols in the atmosphere, which could enhance the dissolution of aerosol Fe through the presence of acidic components (Li et al., 2017, Zhu et al., 2020). The molar ratio of (2SO4 2−+NO3 −)/FeT has been widely used to indicate the relative acidification degree of aerosols with respect to FeT (Shi et al., 2020, Zhu et al., 2020). Fig. 4 shows that the %FeS has a good positive correlation with (2SO4 2−+NO3 −)/FeT (Pearson’s r = 0.77, p < 0.01), which indicates that the acid processing is a main factor influencing the solubility of aerosol Fe. In particular, we found that the value of (2SO4 2−+NO3 −)/FeT (222 ± 70 nmol nmol−1) in the COVID-19 stage was much higher than those in the pre- (85 ± 41 nmol nmol−1) and post-lockdown (46 ± 26 nmol nmol−1) stages (Fig. 4), which suggests the highest aerosol acidification degree in the COVID-19 lockdown stage compared with the pre- and post-lockdown stages. Consequently, the highest %FeS was observed in the COVID-19 lockdown stage due to the highest aerosol acidification degree (Fig. 3d and 4). Furthermore, we found that the (2SO4 2−+NO3 −)/FeT is closely related to the O3 concentration (Fig. 4). The O3 concentration (29.3 ± 10.5 ppb) increased significantly by a factor of 2.9 in the COVID-19 lockdown stage compared with that (10.1 ± 6.9 ppb) in the pre-lockdown stage (Table 2), which indicates an enhanced oxidizing capacity after the implementation of lockdown measures in Hangzhou. This phenomenon was mainly attributed to the weakened NO-titration effect on O3 due to the sharp reduction of vehicular emissions, which has been analyzed in detail in a related paper we have published (Liu et al., 2021). The enhanced oxidizing capacity can significantly promote the oxidation of SO2 and NOx through the gas-phase and multi-phase reactions (Huang et al., 2021, Liu et al., 2021). Both the model simulation and field observation have confirmed the significant increase (~30%) in the concentration of sulfuric acid in the atmosphere of the YRD region due to the COVID-19 lockdown (Huang et al., 2021). Therefore, we conclude that the enhanced oxidizing capacity due to the COVID-19 lockdown produced more acidic species in the atmosphere, which further enhanced the dissolution of aerosol Fe. Based on the above results and discussion, we drew a conceptual model (Fig. 5 ) to clearly illustrate the changes in concentrations and sources of aerosol Fe and the corresponding enhanced oxidizing capacity that increases the %FeS after the implementation of COVID-19 lockdown in Hangzhou.
Fig. 4.
Correlations between the Fe solubility (%FeS) and the molar ratio of (2SO42−+NO3−)/total Fe (FeT), colored by O3 concentration. The black line represents the linear regression for all the samples collected during pre-lockdown (solid squares), COVID-19 lockdown (solid circles), and post-lockdown (solid triangles) stages.
Fig. 5.
A conceptual model illustrating the changes in concentrations and sources of aerosol Fe after the implementation of COVID-19 lockdown in Hangzhou and the corresponding enhanced oxidizing capacity that increases the Fe solubility.
4. Conclusions
The outbreak of the COVID-19 pandemic provides a unique opportunity to understand the sources of aerosol Fe and influencing factors of %FeS in megacities. In this study, we conducted field observations from 1 January to 31 March 2020, which covers the before, during, and after the COVID-19 lockdown in urban Hangzhou. Our results show that aerosol Fe in PM2.5 was dominated by anthropogenic sources, especially by the vehicular emissions in Hangzhou under normal conditions. After the outbreak of the COVID-19 pandemic, the concentrations of FeT and FeS in PM2.5 decreased significantly by 78% and 62%, respectively, which consists well with the sharp decline (81%) of on-road vehicles, and the aerosol Fe in PM2.5 was dominated by the crustal sources. However, it is surprising that the highest %FeS solubility was observed in the COVID-19 lockdown stage compared with those in the pre- and post-lockdown stages. We found that an enhanced atmospheric oxidizing capacity was presented in the COVID-19 lockdown stage, which could promote the formation of more acidic species and further enhance the dissolution of aerosol Fe through the increased aerosol acidification degree.
CRediT authorship contribution statement
Lei Liu: Conceptualization, Methodology, Formal analysis, Data curation, Writing - original draft, Funding acquisition. Qiuhan Lin: Investigation, Formal analysis, Data curation, Writing - original draft. Zhuoran Liang: Funding acquisition, Investigation. Rongguang Du: Investigation, Funding acquisition. Guizhen Zhang: Investigation. Yanhong Zhu: Writing - review & editing. Bing Qi: Resources, Investigation, Funding acquisition. Shengzhen Zhou: Methodology, Data curation. Weijun Li: Supervision, Funding acquisition, Writing - review & editing.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work was supported by the China Postdoctoral Science Foundation (2020M681823), National Natural Science Foundation of China (42075096), Zhejiang Provincial Natural Science Foundation of China (LZ19D050001), LAC/CMA (2020B02), Scientific Research Projects of Zhejiang Administration for Market Regulation (20200103), and Key Laboratory of Tropical Atmosphere-Ocean System (Sun Yat-sen University), Ministry of Education.
Handling Editor: M. Santosh
References
- Abbaspour N., Hurrell R., Kelishadi R. Review on iron and its importance for human health. J. Res. Med. Sci. 2014;19:164–174. [PMC free article] [PubMed] [Google Scholar]
- Al-Abadleh H.A., Rana M.S., Mohammed W., Guzman M.I. Dark iron-catalyzed reactions in acidic and viscous aerosol systems efficiently form secondary brown carbon. Environ. Sci. Technol. 2020 doi: 10.1021/acs.est.0c05678. [DOI] [PubMed] [Google Scholar]
- Cao Y., Shao L., Jones T., Oliveira M.L.S., Ge S., Feng X., Silva L.F.O., BeruBe K. Multiple relationships between aerosol and COVID-19: A framework for global studies. Gondwana Res. 2021;93:243–251. doi: 10.1016/j.gr.2021.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang, Y., Huang, R.-J., Ge, X., Huang, X., Hu, J., Duan, Y., Zou, Z., Liu, X., Lehmann, M.F., 2020. Puzzling haze events in China during the coronavirus (COVID-19) shutdown, Geophys. Res. Lett., 47, e2020GL088533, 10.1029/2020gl088533, 2020. [DOI] [PMC free article] [PubMed]
- Charrier J.G., Anastasio C. On dithiothreitol (DTT) as a measure of oxidative potential for ambient particles: evidence for the importance of soluble transition metals. Atmos. Chem. Phys. 2012;12:9321–9333. doi: 10.5194/acp-12-9321-2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H., Laskin A., Baltrusaitis J., Gorski C.A., Scherer M.M., Grassian V.H. Coal fly ash as a source of iron in atmospheric dust. Environ. Sci. Technol. 2012;46:2112–2120. doi: 10.1021/es204102f. [DOI] [PubMed] [Google Scholar]
- Chen J., Wei F., Zheng C., Wu Y., Adriano D.C. Background concentrations of elements in soils of China. Water Air Soil Poll. 1991;57:699–712. doi: 10.1007/BF00282934. [DOI] [Google Scholar]
- Chester R., Murphy K., Lin F.-J., Berry A.S., Bradshaw G., Corcoran P. Factors controlling the solubilities of trace metals from non-remote aerosols deposited to the sea surface by the ‘dry’deposition mode. Mar. Chem. 1993;42:107–126. doi: 10.1016/0304-4203(93)90241-F. [DOI] [Google Scholar]
- Conway T.M., Hamilton D.S., Shelley R.U., Aguilar-Islas A.M., Landing W.M., Mahowald N.M., John S.G. Tracing and constraining anthropogenic aerosol iron fluxes to the North Atlantic Ocean using iron isotopes. Nat. Commun. 2019;10:2628. doi: 10.1038/s41467-019-10457-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui Y., Ji D., Maenhaut W., Gao W., Zhang R., Wang Y. Levels and sources of hourly PM2.5-related elements during the control period of the COVID-19 pandemic at a rural site between Beijing and Tianjin. Sci. Total Environ. 2020;744 doi: 10.1016/j.scitotenv.2020.140840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu H., Lin J., Shang G., Dong W., Grassian V.H., Carmichael G.R., Li Y., Chen J. Solubility of iron from combustion source particles in acidic media linked to iron speciation. Environ. Sci. Technol. 2012;46:11119–11127. doi: 10.1021/es302558m. [DOI] [PubMed] [Google Scholar]
- Gao, Y., Yu, S., Sherrell, R.M., Fan, S., Bu, K., Anderson, J.R., 2020. Particle-size distributions and solubility of aerosol iron over the Antarctic Peninsula during austral summer. J. Geophys. Res.-Atmos. 125, e2019JD032082, 10.1029/2019jd032082.
- Hamilton D.S., Moore J.K., Arneth A., Bond T.C., Carslaw K.S., Hantson S., Ito A., Kaplan J.O., Lindsay K., Nieradzik L., Rathod S.D., Scanza R.A., Mahowald N.M. Impact of changes to the atmospheric soluble iron deposition flux on ocean biogeochemical cycles in the anthropocene. Glob. Biogeochem. Cycle. 2020;34:22. doi: 10.1029/2019gb006448. [DOI] [Google Scholar]
- Harris E., Sinha B., van Pinxteren D., Tilgner A., Fomba K.W., Schneider J., Roth A., Gnauk T., Fahlbusch B., Mertes S. Enhanced role of transition metal ion catalysis during in-cloud oxidation of SO2. Science. 2013;340:727–730. doi: 10.1126/science.1230911. [DOI] [PubMed] [Google Scholar]
- Hsu S.-C., Wong G.T.F., Gong G.-C., Shiah F.-K., Huang Y.-T., Kao S.-J., Tsai F., Candice Lung S.-C., Lin F.-J., Lin I.I., Hung C.-C., Tseng C.-M. Sources, solubility, and dry deposition of aerosol trace elements over the East China Sea. Mar. Chem. 2010;120:116–127. doi: 10.1016/j.marchem.2008.10.003. [DOI] [Google Scholar]
- Huang R.-J., Cheng R., Jing M., Yang L., Li Y., Chen Q., Chen Y., Yan J., Lin C., Wu Y., Zhang R., El Haddad I., Prevot A.S.H., O’Dowd C.D., Cao J. Source-specific health risk analysis on particulate trace elements: coal combustion and traffic emission as major contributors in wintertime Beijing. Environ. Sci. Technol. 2018;52:10967–10974. doi: 10.1021/acs.est.8b02091. [DOI] [PubMed] [Google Scholar]
- Huang X., Ding A., Gao J., Zheng B., Zhou D., Qi X., Tang R., Wang J., Ren C., Nie W., Chi X., Xu Z., Chen L., Li Y., Che F., Pang N., Wang H., Tong D., Qin W., Cheng W., Liu W., Fu Q., Liu B., Chai F., Davis S.J., Zhang Q., He K. Enhanced secondary pollution offset reduction of primary emissions during COVID-19 lockdown in China. Natl. Sci. Rev. 2021;8:nwaa137. doi: 10.1093/nsr/nwaa137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito A. Atmospheric processing of combustion aerosols as a source of bioavailable iron. Environ. Sci. Technol. Lett. 2015;2:70–75. doi: 10.1021/acs.estlett.5b00007. [DOI] [Google Scholar]
- Ito A., Shi Z. Delivery of anthropogenic bioavailable iron from mineral dust and combustion aerosols to the ocean. Atmos. Chem. Phys. 2016;16:85–99. doi: 10.5194/acp-16-85-2016. [DOI] [Google Scholar]
- Ito A., Myriokefalitakis S., Kanakidou M., Mahowald N.M., Scanza R.A., Hamilton D.S., Baker A.R., Jickells T., Sarin M., Bikkina S., Gao Y., Shelley R.U., Buck C.S., Landing W.M., Bowie A.R., Perron M.M.G., Guieu C., Meskhidze N., Johnson M.S., Feng Y., Kok J.F., Nenes A., Duce R.A. Pyrogenic iron: The missing link to high iron solubility in aerosols. Sci. Adv. 2019;5:10. doi: 10.1126/sciadv.aau7671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A., Sarin M.M. Aerosol iron solubility in a semi-arid region: temporal trend and impact of anthropogenic sources. Tellus Ser. B-Chem. Phys. Meteorol. 2010;62:125–132. doi: 10.1111/j.1600-0889.2009.00448.x. [DOI] [Google Scholar]
- Li, W., Shi, Z., Zhang, D., Zhang, X., Li.Peiren, J, F. Q., Yuan, Q., and Wang, W., 2012. Haze particles over a coal-burning region in the China Loess Plateau in winter: Three flight missions in December 2010. J. Geophys. Res.-Atmos., 117, D12306, 10.1029/2012jd017720.
- Li W., Xu L., Liu X., Zhang J., Lin Y., Yao X., Gao H., Zhang D., Chen J., Wang W., Harrison R.M., Zhang X., Shao L., Fu P., Nenes A., Shi Z. Air pollution–aerosol interactions produce more bioavailable iron for ocean ecosystems. Sci. Adv. 2017;3 doi: 10.1126/sciadv.1601749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liati A., Pandurangi S.S., Boulouchos K., Schreiber D., Dasilva Y.A.R. Metal nanoparticles in diesel exhaust derived by in-cylinder melting of detached engine fragments. Atmos. Environ. 2015;101:34–40. doi: 10.1016/j.atmosenv.2014.11.014. [DOI] [Google Scholar]
- Liu L., Zhang J., Xu L., Yuan Q., Huang D., Chen J., Shi Z., Sun Y., Fu P., Wang Z.F., Zhang D., Li W. Cloud scavenging of anthropogenic refractory particles at a mountain site in North China. Atmos. Chem. Phys. 2018;18:14681–14693. doi: 10.5194/acp-18-14681-2018. [DOI] [Google Scholar]
- Liu, L., Zhang, J., Du, R., Teng, X., Hu, R., Yuan, Q., Tang, S., Ren, C., Huang, X., Xu, L., Zhang, Y., Zhang, X., Song, C., Liu, B., Lu, G., Shi, Z., Li, W., 2021. Chemistry of atmospheric fine particles during the COVID-19 pandemic in a megacity of Eastern China, Geophys. Res. Lett., 48, e2020GL091611, 10.1029/2020GL091611. [DOI] [PMC free article] [PubMed]
- Lv Z., Wang X., Deng F., Ying Q., Archibald A.T., Jones R.L., Ding Y., Cheng Y., Fu M., Liu Y., Man H., Xue Z., He K., Hao J., Liu H. Source-receptor relationship revealed by the halted traffic and aggravated haze in Beijing during the COVID-19 Lockdown. Environ. Sci. Technol. 2020;54:15660–15670. doi: 10.1021/acs.est.0c04941. [DOI] [PubMed] [Google Scholar]
- Mahowald N.M., Baker A.R., Bergametti G., Brooks N., Duce R.A., Jickells T.D., Kubilay N., Prospero J.M., Tegen I. Atmospheric global dust cycle and iron inputs to the ocean. Glob. Biogeochem. Cycle. 2005;19:17. doi: 10.1029/2004gb002402. [DOI] [Google Scholar]
- Mahowald N.M., Engelstaedter S., Luo C., Sealy A., Artaxo P., Benitez-Nelson C., Bonnet S., Chen Y., Chuang P.Y., Cohen D.D., Dulac F., Herut B., Johansen A.M., Kubilay N., Losno R., Maenhaut W., Paytan A., Prospero J.A., Shank L.M., Siefert R.L. Atmospheric iron deposition: global distribution, variability, and human perturbations. Annu. Rev. Mar. Sci. 2009;1:245–278. doi: 10.1146/annurev.marine.010908.163727. [DOI] [PubMed] [Google Scholar]
- Mahowald N.M., Hamilton D.S., Mackey K.R.M., Moore J.K., Baker A.R., Scanza R.A., Zhang Y. Aerosol trace metal leaching and impacts on marine microorganisms. Nat. Commun. 2018;9:15. doi: 10.1038/s41467-018-04970-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsui H., Mahowald N.M., Moteki N., Hamilton D.S., Ohata S., Yoshida A., Koike M., Scanza R.A., Flanner M.G. Anthropogenic combustion iron as a complex climate forcer. Nat. Commun. 2018;9:10. doi: 10.1038/s41467-018-03997-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oakes M., Weber R.J., Lai B., Russell A., Ingall E.D. Characterization of iron speciation in urban and rural single particles using XANES spectroscopy and micro X-ray fluorescence measurements: investigating the relationship between speciation and fractional iron solubility. Atmos. Chem. Phys. 2012;12:745–756. doi: 10.5194/acp-12-745-2012. [DOI] [Google Scholar]
- Ohata S., Yoshida A., Moteki N., Adachi K., Takahashi Y., Kurisu M., Koike M. Abundance of light-absorbing anthropogenic iron oxide aerosols in the urban atmosphere and their emission sources. J. Geophys. Res.-Atmos. 2018;123:8115–8134. doi: 10.1029/2018JD028363. [DOI] [Google Scholar]
- Parker H.A., Hasheminassab S., Crounse J.D., Roehl C.M., Wennberg P.O. Impacts of traffic reductions associated with COVID-19 on Southern California air quality. Geophys. Res. Lett. 2020 doi: 10.1029/2020gl090164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroth A.W., Crusius J., Sholkovitz E.R., Bostick B.C. Iron solubility driven by speciation in dust sources to the ocean. Nat. Geosci. 2009;2:337–340. doi: 10.1038/ngeo501. [DOI] [Google Scholar]
- Shi, J., Guan, Y., Ito, A., Gao, H., Yao, X., Baker, A. R., and Zhang, D., 2020. High production of soluble iron promoted by aerosol acidification in Fog. Geophys. Res. Lett., 47, e2019GL086124, 10.1029/2019GL086124.
- Shi Z., Krom M.D., Jickells T.D., Bonneville S., Carslaw K.S., Mihalopoulos N., Baker A.R., Benning L.G. Impacts on iron solubility in the mineral dust by processes in the source region and the atmosphere: A review. Aeolian Res. 2012;5:21–42. doi: 10.1016/j.aeolia.2012.03.001. [DOI] [Google Scholar]
- Shi Z., Krom M.D., Bonneville S., Benning L.G. Atmospheric processing outside clouds increases soluble iron in mineral dust. Environ. Sci. Technol. 2015;49:1472–1477. doi: 10.1021/es504623x. [DOI] [PubMed] [Google Scholar]
- Shi, Z., Song, C., Liu, B., Lu, G., Xu, J., Van Vu, T., Elliott, R. J. R., Li, W., Bloss, W.J., Harrison, R.M., 2021. Abrupt but smaller than expected changes in surface air quality attributable to COVID-19 lockdowns. Sci. Adv., 7, eabd6696, 10.1126/sciadv.abd6696. [DOI] [PMC free article] [PubMed]
- Sun Y., Lei L., Zhou W., Chen C., He Y., Sun J., Li Z., Xu W., Wang Q., Ji D., Fu P., Wang Z., Worsnop D.R. A chemical cocktail during the COVID-19 outbreak in Beijing, China: Insights from six-year aerosol particle composition measurements during the Chinese New Year holiday. Sci. Total Environ. 2020;742 doi: 10.1016/j.scitotenv.2020.140739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian H., Liu Y., Li Y., Wu C.-H., Chen B., Kraemer M.U.G., Li B., Cai J., Xu B., Yang Q., Wang B., Yang P., Cui Y., Song Y., Zheng P., Wang Q., Bjornstad O.N., Yang R., Grenfell B.T., Pybus O.G., Dye C. An investigation of transmission control measures during the first 50 days of the COVID-19 epidemic in China. Science. 2020;368:638–642. doi: 10.1126/science.abb6105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei F., Teng E., Wu G., Hu W., Wilson W.E., Chapman R.S., Pau J.C., Zhang J. Ambient concentrations and elemental compositions of PM10 and PM2.5 in Four Chinese Cities. Environ. Sci. Technol. 1999;33:4188–4193. doi: 10.1021/es9904944. [DOI] [Google Scholar]
- Xia L., Gao Y. Chemical composition and size distributions of coastal aerosols observed on the US East Coast. Mar. Chem. 2010;119:77–90. doi: 10.1016/j.marchem.2010.01.002. [DOI] [Google Scholar]
- Xu, L., Zhang, J., Sun, X., Xu, S., Shan, M., Yuan, Q., Liu, L., Du, Z., Liu, D., Xu, D., Song, C., Liu, B., Lu, G., Shi, Z., Li, W., 2020. Variation in concentration and sources of black carbon in a megacity of China during the COVID-19 Pandemic. Geophys. Res. Lett., 47, e2020GL090444, 10.1029/2020GL090444. [DOI] [PMC free article] [PubMed]
- Yuan Q., Teng X., Tu S., Feng B., Wu Z., Xiao H., Cai Q., Zhang Y., Lin Q., Liu Z., He M., Ding X., Li W. Atmospheric fine particles in a typical coastal port of Yangtze River Delta. J. Environ. Sci. 2020;98:62–70. doi: 10.1016/j.jes.2020.05.026. [DOI] [PubMed] [Google Scholar]
- Yuan Q., Qi B., Hu D., Wang J., Zhang J., Yang H., Zhang S., Liu L., Xu L., Li W. Spatiotemporal variations and reduction of air pollutants during the COVID-19 pandemic in a megacity of Yangtze River Delta in China. Sci. Total Environ. 2021;751 doi: 10.1016/j.scitotenv.2020.141820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang G., Bi X., Lou S., Li L., Wang H., Wang X., Zhou Z., Sheng G., Fu J., Chen C. Source and mixing state of iron-containing particles in Shanghai by individual particle analysis. Chemosphere. 2014;95:9–16. doi: 10.1016/j.chemosphere.2013.04.046. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Liu X., Fang Y., Liu D., Tang A., Collett J.L. Atmospheric ammonia in Beijing during the COVID-19 outbreak: concentrations, sources, and implications. Environ. Sci. Technol. Lett. 2020 doi: 10.1021/acs.estlett.0c00756. [DOI] [PubMed] [Google Scholar]
- Zhou S., Yuan Q., Li W., Lu Y., Zhang Y., Wang W. Trace metals in atmospheric fine particles in one industrial urban city: Spatial variations, sources, and health implications. J. Environ. Sci. 2014;26:205–213. doi: 10.1016/s1001-0742(13)60399-x. [DOI] [PubMed] [Google Scholar]
- Zhu Y., Li W., Lin Q., Yuan Q., Liu L., Zhang J., Zhang Y., Shao L., Niu H., Yang S., Shi Z. Iron solubility in fine particles associated with secondary acidic aerosols in east China. Environ. Pollut. 2020;264 doi: 10.1016/j.envpol.2020.114769. [DOI] [PubMed] [Google Scholar]