Abstract
Background
Cervical cancer is currently the second-leading cause of cancer death among women in Ethiopia. Vaccination against the human papillomavirus (HPV) is an effective primary prevention strategy for HPV-related illnesses. The knowledge and willingness of parents toward the HPV vaccine are crucial to increasing the uptake of the vaccine. The vaccine's acceptance by children and young adolescents is dependent on parental consent. Therefore, this study aimed to assess knowledge, willingness, and associated factors of the human papillomavirus vaccine among parents of girls aged 9–14 years at Debre Tabor Town.
Method
A community-based cross-sectional study was conducted among participants from December 10, 2020, to January 15, 2021. A simple random sample technique was used to include 638 participants. A structured face-to-face interviewer-administered questionnaire was used to collect data. The data were entered and analyzed using Epi-Data and SPSS software, respectively. Bivariate and multivariable analyses were used to examine the association. The Odds Ratio (OR), 95% CI, and p-values less than 0.05 were used to determine the statistical association.
Results
Thirty-five percent (35.4%, 95% CI = 31.4%, 38.8%) and 44.8% (95% CI = 40.40%, 48.67%) of participants were knowledgeable about HPV vaccination and willing to get it, respectively. Being government employees (AOR = 5.46, 95% CI = 2.42, 9.34), and having a family history of sexually transmitted diseases (STD) (AOR = 1.76, 95% CI = 1.14, 2.72) were significantly associated with knowledge of the human papilloma virus (HPV) vaccine. Participants’ age (AOR = 1.43, 95% CI = 1.16, 2.87), secondary education and above (AOR = 1.70, 95% CI = 1.05, 2.74), fear of HPV infection (AOR = 2.29, 95% CI = 1.21, 4.32), and having good knowledge of the HPV vaccine (AOR = 3.30, 95% CI = 2.21, 4.93) were significantly associated with willingness to receive the HPV vaccine.
Conclusion and recommendation
The knowledge and willingness of parents toward the HPV vaccine were low. Then, health officials should boost HPV vaccination promotion through public media. In schools, churches, mosques, and health facilities, health extension workers and health professionals provide information about the HPV vaccine for the parents. Mixed quantitative and qualitative studies are preferable for future research to address “why” issues.
Keywords: Human papillomavirus vaccine, Knowledge, Attitude, Debre Tabor Town
Plain language summary
Infection with the Human Papillomavirus (HPV) causes nearly 99% of cervical cancer cases and more than 20% of breast, neck, and anogenital cancers. The HPV vaccines protect against high-risk types of HPV (types 16 and 18), which account for approximately 70% of cervical cancers. Global coverage of the HPV vaccine was 39.7%, with high-income countries (68%), middle-income countries (28%), and lower-middle-income countries (2.7%). For different reasons, cervical cancer screening is very poor in Ethiopia (below 2%). Cervical cancer is mostly asymptomatic more than 20 years after infection. Primary prevention (enhancing the HPV vaccine) is the best way to protect women from cervical cancer. Adolescents’ uptake and acceptance of the vaccine depend on parental consent. Assessing parental knowledge and willingness at a community level is very crucial.
A simple random sample technique was used to include 638 participants. A structured and pre-tested face-to-face interviewer-administered questionnaire was used to collect the data. The data were entered and analyzed using Epi-Data, and SPSS software, respectively. Bivariate and multivariable analyses were used to examine the association.
Nearly one-third (35.4%) and less than half (44.8%) of participants were knowledgeable and willing to receive the HPV vaccination. The knowledge and willingness of the parents are significantly lower. Being government employees and having a family history of sexually transmitted diseases (STD) were factors affecting the knowledge of parents about the human papillomavirus vaccine. Participants’ age, secondary education and above, fear of HPV infection, and having good knowledge of the HPV vaccine were significantly associated with their willingness to use the HPV vaccine. The knowledge and willingness of the parents are significantly lower. Health officials and stakeholders should scale up HPV vaccine promotion through public media.
Background
The Centers for Disease Control and Prevention states that almost 99% of cervical cancer cases and more than 20% of breast, neck, and anogenital cancers are caused by infection with human papillomavirus (HPV) [1]. In October 2011, the Advisory Committee on Immunization Services complied with guidelines to vaccinate all children, boys, and girls, to stop the ever-increasing incidence of HPV infection [2]. Currently, available HPV vaccines protect against high-risk types of HPV (types 16 and 18), which account for approximately 70% of cervical cancers and vaginal, oral, and anal cancers [3, 4]. A quadrivalent vaccine also protects against two low-risk types of the virus, which are responsible for 90% of genital warts (HPV types 6 and 11) [5–7].
Cervical cancer is the second most prevalent female cancer worldwide [8] and is the world's leading cause of female cancer mortality [9], especially in Sub-Saharan Africa [10]. Almost all of the girls who have been immunized against the HPV virus can be protected against more than 75% of cervical cancer cases [11]. In developed countries, cervical cancer has been decreasing for many years, largely due to the cervical cytology-screening program, which is now being replaced by HPV screening. However, cervical cancer is increasing in developing countries where nationwide cervical cancer screening is currently unavailable [12, 13].
Until recently, cytology-based screening programs were the main tool to detect and treat precancerous abnormalities and the early stages of cancer, preventing up to 80% of cervical cancers in developed countries. However, effective screening programs have been difficult to implement in low-resource settings. This is one reason why cervical cancer mortality rates are much higher in the developing world [14]. To prevent women from cervical cancer-related illness and mortality, the HPV vaccine is a better alternative than cytology screening or DNA testing, especially in resource-limited nations [15]. Global coverage of the HPV vaccine was 39.7%, with high-income countries (68%), middle-income countries (28%), and lower-middle-income countries (2.7%) [16]. The majority of cervical cancer researchers in Africa have focused on secondary prevention (cervical screening), whereas the number of publications focusing on primary prevention, notably HPV vaccination, is only approximately 23.4% [17].
Studies showed that in Hong Kong, 47.3%; Uganda, 56%; Southwest Nigeria, 79%; medical students in Southwest Ethiopia, 56.2% did not know about the HPV vaccine [18–21]. Socio-demographic factors like gender and educational level [22, 23], parents’ occupation [21, 24], participants’ family members’ history of cervical cancer, and participants who had information about the HPV vaccine (from school, newspaper, and internet) [24] and fear of HPV infection [25] were determinant factors of knowledge of HPV vaccine. In Southwest Nigeria, less than (40%); of Jima University medical students (36.8%); the United States (52%); in Morocco (27%) of the participant were willing to get the HPV vaccine for their adolescents [20–23], respectively. Parental age < 40 years [26], ethnicity [27], gender [28], parents who were worried about the potential risk of cervical cancer [29], parents’ adolescents who did not receive clinician recommendation to be vaccinated for HPV [30] were associated factors of the willingness of HPV vaccine.
Ethiopia is one of the resource-limited countries, access to cervical cancer screening is very less (below 2% among cervical screening eligible women) and human papillomavirus infection is mostly asymptomatic and causes cervical cancer mainly after 20 years. Because of quite challenging the natural history of HPV infection: (1) most sexually active individuals will acquire an HPV infection at some point in their lives. (2) A majority of HPV infections are asymptomatic and resolve spontaneously within a year or two. (3) HPV-related disease may not develop for years to decades following infection. Therefore, due to these challenges primary prevention (enhancing the HPV vaccine) is better than secondary prevention (HPV detection) to protect women from cervical cancer and genital wart.
Female adolescents’ uptake and acceptance of the vaccine are depending on parental consent. Ethiopia launched the HPV vaccine school-based approach implementation in December 2018. However, studies of knowledge and willingness toward the HPV vaccine have involved few Ethiopian parents, particularly in the study area, making it difficult to implement possible intervention strategies among this population. Therefore, assessing knowledge and willingness of human papillomavirus vaccine among parents of female adolescents and identifying factors affecting their children’s vaccine utilization is very vital in designing, implementing, and monitoring effective HPV vaccine immunization programs.
Methods
Study area and study period
A community-based cross-sectional study was conducted in Debre Tabor Town from January 1, 2021, to February 28, 2021. The town is located 665 km northwest of Addis Ababa (Ethiopia's capital city) and 97 km east of Bahir Dar City. The town is divided into six small administrative units called kebeles with a total population of 96,973 people, of whom 49,753 were men and 47,220 women, based on a population projection of Ethiopia for all regions at the Wereda level in 2017. All parents of girls aged 9–14 years who lived in Debre Tabor Town during the data collection period were included in the study.
Sample size determination: Epi-Info version 7 statistical software was used to calculate the sample size for objective one prevalence of knowledge (58.4%) and objective two prevalence of willingness to receive the HVP vaccine (59.9%) with the assumption of a 4% margin of error with a 95% confidence interval. Based on the assumptions, the final sample size was 641 and 634 with a 10% non-response rate for the prevalence of knowledge and prevalence of willingness, respectively.
Sampling procedure
A simple random sampling technique was applied to select 641 parents for the study. Four thousand two hundred seventy households were in the town. Then, a census was conducted in all selected kebeles to identify parents who fulfilled the inclusion criteria (parents of girls aged 9–14). An identification number was given after a house-to-house visit. Then, a proportional-to-size allocation technique was employed to determine the study participants from each kebele. Finally, sample units were selected using a simple random sampling technique, and one mother and one father, or either of the mother or father per household, were interviewed. Participants in the selected household were not present at the time of data collection; three revisits were made to interview the mothers or fathers.
Variables of the study
Dependent variables Parents’ knowledge and willingness to receive the HPV vaccine. Independent variables: socio-demographic variables (age, religion, marital status, educational status, occupation), reproductive health-related factors (family history of cervical cancer, fear of HPV infection, history of sexually transmitted diseases), sources of information (newspapers, radio, TV, schools, health professionals, health extension workers), understanding of the HPV vaccine, as well as HPV infection and cervical cancer).
Operational definitions
Knowledge of the HPV vaccine Adolescents’ level of knowledge was measured based on correct responses to HPV vaccine knowledge questions. Each correct and incorrect response scores one (yes = 1) and zero (no and I do not know = 0) points, respectively. Using knowledge question items, participants who scored above or equal to 50% were considered to have knowledge (Yes), whereas those who scored less than the 50% score were measured as having no knowledge (No). Willingness is considered a participant’s score of 50% or above among the questions.
Data collection procedure and techniques
A structured face-to-face interviewer-administered questionnaire was used to collect data. First, the questionnaires were developed in English and translated to the local language (Amharic) and then back to English by language experts to maintain consistency. Health extension workers and two Bachelors of Science (BSc.) midwives who are familiar with the local language and customs were recruited as data collectors and supervisors, respectively. The training was given to data collectors and supervisors for two days about data collection procedures, the content of the questionnaire, interview techniques, and confidentiality of the information obtained from the respondents.
Data quality assurance
Data quality was ensured during collection, entry, and analysis. Before conducting the main study, a pretest was carried out on 32 (5%) of the sample. The principal investigator and supervisors conducted day-to-day on-site supervision during the whole period of data collection. At the end of each day, the questionnaires were reviewed and checked for completeness and accuracy by all the research team members, who undertook corrective discussion.
Data processing and analysis
Epi-Data version 4.2 was used to code and enter the data, which was then exported to SPSS 23 for analysis. Descriptive analyses were conducted to summarize the data, and the results of the study were presented in the form of text, figures, and tables. Binary logistic regression analysis was performed by computing the odds ratio (OR) with a 95% confidence interval to see the crude association between each independent and dependent variable. Model fitness was checked by using Hosmer and Lemeshow goodness of fit. Finally, all independent variables by binary logistic regression p ≤ 0.2 were entered into multivariable logistic regression for further analysis, and significant associations were identified based on p < 0.05 and adjusted odds ratio (AOR) with 95% CI.
Ethical clearance
Ethical clearance for this study was obtained from the ethical review committee of Debre Tabor University College of Medicine and Health Sciences. A supporting letter was obtained from Debre Tabor Town’s head office. Informed consent was obtained from participants after explaining the purpose of the study. Participants were informed that all the data obtained from them would be kept confidential and anonymous, and they had the right to withdraw at any point during data collection.
Results
Socio-economic characteristics of participants
Six hundred thirty-eight (638) parents were interviewed with a response rate of 99.5%. The mean age of the respondents was 36.41 (SD ± 5.8). Three hundred seventy-four (58.6%) of the parents were in the age group of 31–40. The majority of study participants, 574 (90.0%), were married, and 600 (94.0%) were Orthodox religious followers. Regarding educational status, more than half of the participants were in secondary school and above 387 (51.6%) (Table 1).
Table 1.
Sociodemographic characteristics versus human papillomavirus vaccine among parents of children aged 9–14 Years in Debre Tabor Town, 2021
| Variables | Frequency | Percent |
|---|---|---|
| Age | ||
| 23–30 | 51 | 8.0 |
| 31–40 | 374 | 58.6 |
| 41–46 | 213 | 33.4 |
| Sex | ||
| Male (fathers) | 317 | 49.7 |
| Female (mothers) | 321 | 50.3 |
| Marital status | ||
| Single | 21 | 3.3 |
| Married | 574 | 90.0 |
| Widowed | 15 | 2.4 |
| Divorced | 28 | 4.4 |
| Religion | ||
| Orthodox | 600 | 94.0 |
| Protestant /Muslim | 38 | 6.0 |
| Educational level | ||
| Unable to read and write | 52 | 8.2 |
| Able to read and write | 124 | 19.4 |
| 1–8th class | 75 | 11.8 |
| 9–12th class | 154 | 24.1 |
| Diploma and above | 233 | 36.5 |
| Occupation | ||
| Housewife | 99 | 15.5 |
| Self-employees | 122 | 19.1 |
| Government employees | 227 | 35.6 |
| Health professional | 7 | 1.1 |
| Merchants | 183 | 28.7 |
Reproductive related characteristics
Most of the study participants had no family history of cervical cancer 636 (99.7%). The majority of participants were afraid of sexually transmitted infections 586 (84.0%), and 25 (3.9%) participants had a history of sexually transmitted diseases (Table 2).
Table 2.
Reproductive health versus human papillomavirus vaccine among parents of children aged 9–14 Years in Debre Tabor Town, 2021
| Variables | Frequency | Percent |
|---|---|---|
| Heard about cervical cancer | ||
| Yes | 122 | 19.1 |
| No | 516 | 80.9 |
| Family history of cervical cancer | ||
| Yes | 2 | 0.3 |
| No | 636 | 99.7 |
| History of STD | ||
| Yes | 102 | 18.0 |
| No | 536 | 82.0 |
| Fear of sexually transmitted infection | ||
| Yes | 536 | 84.0 |
| No | 102 | 16.0 |
| Did your girl/s take the HPV vaccine | ||
| Yes | 87 | 13.6 |
| No | 551 | 86.4 |
Sources of information about the HPV vaccine
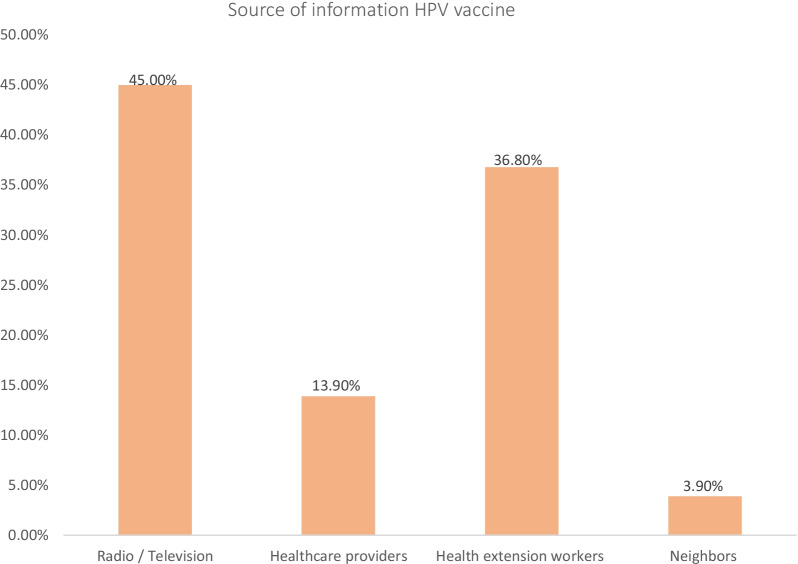
Nearly half of the participants 48.7% had heard of the HPV vaccine. Among these, below the half of the respondents said the main sources of information were radio/television 140 (45.0%) and health extension workers (36.8%), respectively (Fig. 1).
Fig. 1.
Source of information about human papillomavirus vaccine among parents of children aged 9–14 Years in Debre Tabor Town, 2021 (n = 311)
Parents’ knowledge of the HPV vaccine and cervical cancer
Nearly one-third of participants (35.1%, 95% CI = 31.4%, 38.8%) had good knowledge about the human papillomavirus vaccine and cervical cancer. Of the 205 (32.1%) responded that the main cause of cervical cancer is HPV infection (Table 3).
Table 3.
Knowledge of human papillomavirus vaccine and cervical cancer among parents of children aged 9–14 Years in Debre Tabor Town, 2021
| Variables | Frequency | Percent |
|---|---|---|
| Having multiple sexual partners is the risk factor for HPV infection | ||
| Yes | 262 | 41.1 |
| No | 195 | 30.6 |
| I don’t know | 181 | 28.4 |
| Sex at an early age increases the risk of transmission of HPV infection | ||
| Yes | 124 | 19.4 |
| No | 90 | 14.1 |
| I don’t know | 424 | 66.5 |
| Being smokers increase the risk of HPV infection | ||
| Yes | 114 | 17.9 |
| No | 200 | 31.3 |
| I don’t know | 324 | 50.8 |
| Sexual contact is the main transmitting route of HPV infection | ||
| Yes | 237 | 37.2 |
| No | 136 | 21.3 |
| I don’t know | 265 | 41.5 |
| The main cause of cervical cancer is HPV infection | ||
| Yes | 205 | 32.1 |
| No | 174 | 27.3 |
| I don’t know | 259 | 40.6 |
| People can transmit HPV to their partner even if they have no symptoms of infection | ||
| Yes | 118 | 18.5 |
| No | 246 | 38.6 |
| I don’t know | 274 | 42.9 |
| Cervical cancer can be prevented by taking the HPV vaccine before sexual intercourse | ||
| Yes | 379 | 59.4 |
| No | 116 | 18.2 |
| I don’t know | 143 | 22.4 |
| The recommended age for taking the HPV vaccine is 9–14-year-olds | ||
| Yes | 168 | 26.3 |
| No | 249 | 39.1 |
| I don’t know | 221 | 34.6 |
| Knowledge | ||
| Have knowledge (yes) | 222 | 35.1 |
| Have no knowledge (no) | 414 | 64.9 |
The willingness for the HPV vaccine
Two hundred eighty-six (44.8%, 95% CI = 40.40%, 48.67%) participants were willing to receive the HPV vaccination (Table 4).
Table 4.
Willingness towards cervical cancer prevention and human papillomavirus vaccine among parents of children aged 9–14 Years in Debre Tabor Town, 2021
| Variables | Frequency | Percent |
|---|---|---|
| A person who has only one sex partner can protect from HPV infection | ||
| Strongly agree | 221 | 34.6 |
| Agree | 197 | 30.9 |
| Disagree | 199 | 31.2 |
| Strongly disagree | 21 | 3.3 |
| HPV vaccine education should be given for school adolescents | ||
| Strongly agree | 205 | 32.1 |
| Agree | 252 | 39.5 |
| Disagree | 116 | 18.2 |
| Strongly disagree | 21 | 3.3 |
| Indifferent | 44 | 6.9 |
| Cervical cancer is a big problem for women | ||
| Strongly agree | 205 | 32.1 |
| Agree | 200 | 31.3 |
| Disagree | 170 | 26.6 |
| Strongly disagree | 42 | 6.6 |
| Indifferent | 21 | 3.3 |
| Cervical cancer causes death in women | ||
| Strongly agree | 298 | 46.7 |
| Agree | 180 | 28.2 |
| Disagree | 118 | 18.5 |
| Strongly disagree | 21 | 3.3 |
| Indifferent | 21 | 3.3 |
| Men involvement is important to prevent cervical cancer | ||
| Strongly agree | 103 | 16.1 |
| Agree | 241 | 37.8 |
| Disagree | 271 | 42.5 |
| Strongly disagree | 21 | 3.3 |
| Indifferent | 2 | .3 |
| Getting a Pap test examination is not an embarrassment | ||
| Strongly agree | 298 | 46.7 |
| Agree | 90 | 14.1 |
| Disagree | 208 | 32.6 |
| Strongly disagree | 21 | 3.3 |
| Indifferent | 21 | 3.3 |
| Girls should get HPV vaccine before first sexual intercourse | ||
| Strongly agree | 336 | 52.7 |
| Agree | 183 | 28.7 |
| Disagree | 97 | 15.2 |
| Strongly disagree | 22 | 3.4 |
| Health information about the HPV vaccine is needed for adolescents | ||
| Strongly agree | 218 | 34.2 |
| Agree | 197 | 30.9 |
| Disagree | 139 | 21.8 |
| Strongly disagree | 62 | 9.7 |
| Indifferent | 22 | 3.4 |
| The HPV vaccine is effective to prevent cervical cancer | ||
| Strongly agree | 454 | 71.2 |
| Agree | 69 | 10.8 |
| Disagree | 115 | 18.0 |
| Parents willingness | ||
| Yes | 286 | 44.8 |
| No | 352 | 55.2 |
Why not have parents’ adolescent children receive the HPV vaccine?
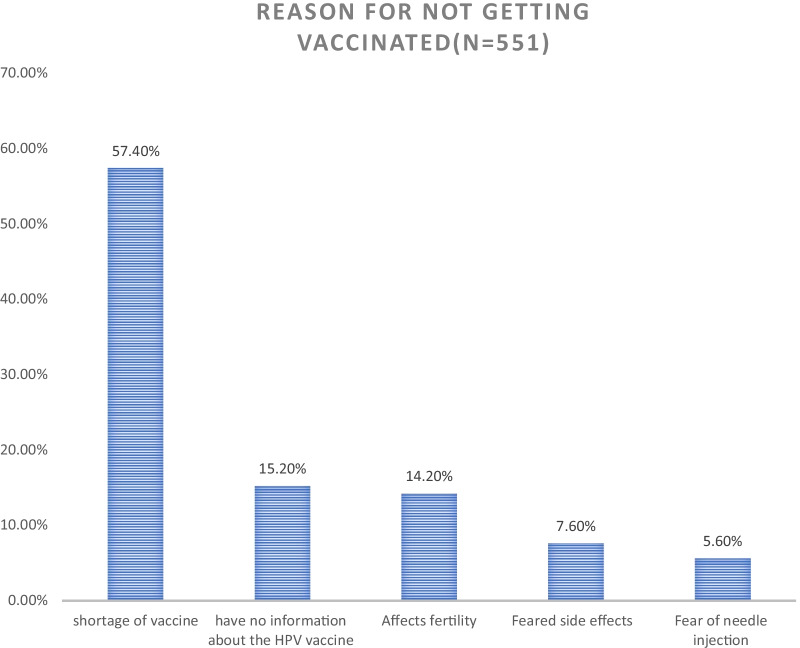
The majority of parents’ girls (9–14 years old), 551 (86.4%), were not taking the HPV vaccine. Parents list various reasons for not taking the HPV vaccine (Fig. 2).
Fig. 2.
Reasons for not getting the human papillomavirus vaccine among parents of children aged 9–14 years in Debre Tabor Town, 2021 (n = 551)
Factors associated with knowledge of human papillomavirus vaccine and HPV vaccine
Two models were fitted to assess knowledge and attitude towards the HPV vaccine. The first model was fitted to assess the knowledge of the HPV vaccine. Variables such as occupation and family history of STDs were significantly associated with the knowledge of the HPV vaccine. Being government employees, they were 5.46 times more likely to know about the HPV vaccine (AOR = 5.46, 95% CI = 2.42, 9.34) as compared to those participants whose occupation was a housewife. Participants who had a family history of STDs were 1.76 times (AOR = 1.76, 95% CI = 1.14, 2.72) more likely to know about the HPV vaccine than those who had no history of STDs (Table 5).
Table 5.
Factors associated with knowledge of human papillomavirus vaccine among parents of children aged 9–14 Years in Debre Tabor Town, 2021
| Variables | Knowledge of HPV vaccine | COR (95% CI) | AOR (95% CI) | p-value | |
|---|---|---|---|---|---|
| Yes (N, %) | No (N, %) | ||||
| Sex | |||||
| Male | 104 (46.4) | 213 (51.5) | 0.81 (0.59, 1.13) | 0.80 (0.58, 1.12) | |
| Female | 120 (53.6) | 201 (48.5) | 1 | ||
| Educational status | |||||
| No formal education | 72 (32.1) | 104 (25.1) | 1 | ||
| Primary education | 77 (34.4) | 150 (36.3) | 0.74 (0.49, 1.11) | 0.76 (0.50, 1.15) | |
| Secondary and above | 75 (33.5) | 160 (38.6) | 0.67 (0.45, 1.01) | 0.70 (0.46, 1.05) | |
| Occupation | |||||
| Housewife | 18 (8.1) | 92 (22.2) | 1 | 0.001 | |
| Government employees | 60 (26.8) | 63 (15.2) | 4.86 (2.62, 9.01) | 5.46 (2.42, 9.34** | |
| Self-employees | 31 (13.8) | 61 (14.7) | 2.59 (1.33, 5.05) | 2.57 (1.25, 5.27) | |
| Merchants | 69 (30.8) | 114 (27.5) | 3.09 (1.72, 5.56) | 3.10 (1.62, 5.93) | |
| Family history of STD | |||||
| Yes | 48 (21.4) | 54 (13.0) | 1.81 (1.18, 2.79) | 1.76 (1.14, 2.72)** | 0.002 |
| No | 176 (78.6) | 360 (89.0) | 1 | ||
**Variables were significant at p-values of < 0.05
Factors associated with willingness of human papillomavirus vaccine and cervical cancer
The second model was fitted to assess factors associated with the attitude toward the HPV vaccine among parents of children aged 9–14 years. Variables such as the age of participants, educational status, fear of HPV infection, and knowledge of the HPV vaccine were significantly associated with the attitude of parents of children aged 9–14 years towards the HPV vaccine. Participants whose age was 31–40 years old were 1.43 times (AOR = 1.43, 95% CI = 1.16, 2.87) more likely to have willingness towards HPV vaccine utilization as compared to participants whose age was greater than or equal to 41 years old.
Participants who had secondary education and above were 1.7 times (AOR = 1.70, 95% CI = 1.05, 2.74) more likely to have willingness toward the HPV vaccine, as compared to those participants who had no formal education (unable to read and write plus able to read and write). Participants who fear HPV infection were 2.29 times (AOR = 2.29, 95% CI = 1.21, 4.32) more likely to have willingness toward the HPV vaccine as compared to participants who did not fear HPV infection. Participants who knew about the HPV vaccine and cervical cancer were 3.30 times (AOR = 3.30, 95% CI = 2.21, 4.93) more likely to have willingness toward the HPV vaccine as compared to those participants who did not know (Table 6).
Table 6.
Factors associated with the attitude of human papillomavirus vaccine and cervical cancer among parents of children aged 9–14 Years in Debre Tabor Town, 2021
| Variables | Willingness towards HPV vaccine | COR (95% CI) | AOR (95% CI) | p-values | |
|---|---|---|---|---|---|
| Yes (N, %) | No (N, %) | ||||
| Age | |||||
| 23–30 | 12 (6.7) | 39 (8.5) | 1.08 (0.52, 2.24) | 0.78 (0.33, 1.85) | 0.003 |
| 31–40 | 119 (66.9) | 255(55.4) | 1.64 (1.11, 2.43) | 1.43 (1.16, 2.87)** | |
| 41–46 | 47 (26.4) | 166(36.1) | 1 | 1 | |
| Sex | |||||
| Male | 103 (57.9) | 214 (46.5) | 1.57 (1.11, 2.23) | 1.49 (1.00, 2.22) | |
| Female | 75 (42.1) | 246 (53.5) | 1 | 1 | |
| Educational status | |||||
| No formal education | 33 (18.5) | 143 (31.1) | 1 | 0.001 | |
| Primary education | 77 (43.3) | 150 (32.6) | 2.22 (1.39, 3.55) | 1.15 (0.34, 3.44) | |
| Secondary and above | 68 (38.2) | 167 (36.3) | 1.76 (1.10, 2.82) | 1.70 (1.05, 2.74)** | |
| Occupation | |||||
| Government employees | 64 (36.0) | 163 (35.4) | 1 | ||
| Others* | 114 (64.0) | 297 (64.6) | 1.02 (0.71, 1.46) | 1.02 (0.51, 2.01) | |
| Fear of HPV infection | |||||
| Yes | 162 (91.0) | 374 (81.3) | 2.32 (1.32, 4.09) | 2.29 (1.21, 4.32)** | 0.001 |
| No | 16 (9.0) | 86 (18.7) | 1 | ||
| Did your child took HPV vaccine? | |||||
| Yes | 118 (66.3) | 320 (70.0) | 1 | ||
| No | 60 (33.7) | 140 (30.0) | 0.86 (0.59, 1.24) | 0.65 (0.43, 1.00) | |
| Knowledge on cervical cancer and HPV vaccine | |||||
| No | 115 (49.4) | 301 (82.8) | 1 | 0.001 | |
| Yes | 153(50.6) | 69 (17.2) | 5.80 (3.37, 7.61) | 3.30 (2.21, 4.93)** | |
*Self-employ, farmer, merchant, daily labourer
**Variables were significant at p-values of < 0.05
Discussion
This study was conducted to assess knowledge and willingness of the HPV vaccine and associated factors among parents of children aged 9–14 years in Debre Tabor Town, Ethiopia. The involvement of parents in the decision to take the HPV vaccine for their children is very crucial for the acceptability and utilization of the vaccine. However, different factors such as participant age, educational status, occupation, family history of STD, and fear of HPV infection were significantly associated with knowledge and willingness to use the HPV vaccine.
Of the participants who had had information about the HPV infection and vaccine (48.7%), the two most important sources were radio/television (45.00%) and health extension workers (36.8%), followed by health care providers. More than half of the study participants (51.3%) had no information about the vaccine. Parents had more information about the immunization as their daughters had received the HPV vaccine. Fathers have access to more information than mothers do. This might be because parents gain access to information about the vaccine from their children after vaccination, and mothers, mostly because they are homemakers.
The prevalence of knowledge of parents about the HPV vaccine was 35.1%, which was in line with the systematic review and meta-analysis study (37%) [29], Nigeria (36.5%) [31], but lower than studies conducted in Romania (85.8%) [32], the United Kingdom (54.8%) [33], Kenya (48%) [34], and Thailand (60%) [28]. The explanation might be a lack of HPV vaccination advertising in the media or on social media. In contrast to these findings, other studies conducted among health professionals in Lagos in Nigeria [35], Enugu in Nigeria [36], and South Africa [37] revealed comparatively high levels of knowledge of 85.0%, 74.0%, and 96.0%, respectively. The variations in awareness in these earlier studies are most probably related to the health personnel's greater exposure to information regarding HPV infection in the health facility. On the other hand, this finding was higher than a study conducted in Iran (24%) [26]. This variation might be due to differences in the study setting, study population, and time of the study.
Forty-four percent (44.4%) of participants had a willingness to the HPV vaccine and cervical cancer prevention. This study was lower than the study conducted on Danish parents (80%) [38], Canada (70%) [39], Nigeria (81.8%) [31], Kenya (89%) [34], Tanzania (93.0%) [40], Honduras (91.0%) [41], and Birmingham (88.1%) [27]. This might be explained by a lack of understanding about the advantage of the HPV vaccination, fear of adverse effects, and concern about infertility as a result of the vaccination.
The reasons were given by those respondents why their children were not getting the human papillomavirus vaccine. The major reasons for not immunizing the HPV vaccine were the scarcity/cost of the HPV vaccine 57.4%. The second most reason was poor information about the HPV vaccine 15.2%, having doubts about HPV vaccination (undesirable impact on fertility) 14.2%, feared side effects 7.6%, and fear of needle injection 5.6%. This was similar to a study conducted in Nigeria were high cost (55.6%), worries about the side effects (48.1%), and poor availability (25.9%) of the vaccines were the main factors [31].
In this study, the odds of knowing the HPV vaccine were higher among parents of girls who worked as government employees as compared to participants whose occupations were housewives. This finding was in line with studies conducted in Saudi Arabia [24]. Participants who had relatively high educational status, agree to take the vaccine [32] and government employees are educated. Participants that are more educated may have better access to media (print, social, and mass media) exposure to HPV vaccination information. Participants who had family exposure to sexually transmitted diseases were more likely to know about the vaccine than those who did not have exposure to sexually transmitted diseases (STDs). This might be explained by the exposure to STDs in the family, health facility visits, and the gaining of important information, including the HPV vaccine.
Participants whose age was 31–40 years old were more likely to have a willingness as compared to those participants whose age was greater than or equal to 40 years old. This finding was similar to a study conducted in Iran [26]. The reason is unclear, but it is claimed that vaccines are our country’s most recent innovation, and young adults may have more information than older adults may have. Educational status was also significantly associated with a willingness to the HPV vaccine. Participants who had secondary education or above were more likely to have a willingness as compared to participants with no formal education. This might be because parents who have secondary and above-secondary educational levels are more likely to have information from school, mass media, newspapers, and the internet.
Parents who fear the infection of the human papillomavirus vaccine were more likely to experience a willingness to take the HPV vaccine than parents who did not fear the infection of HPV. These might be parents who have a fear of acquiring an HPV infection; they might have an intention to understand the prevention of the HPV infection.
Parents’ knowledge about cervical cancer and the HPV vaccine was significantly associated with their willingness to receive the HPV vaccine. Parents who knew were three times more likely to have a willingness to have the HPV vaccine as compared to those parents who did not know. This was similar with others studies in North Gondar [42], Malaysia [43, 44], Honduras [41], United Arab Emirate [45], Europe [46], and Kenya [47].This might be explained by the parents who have evidence about the route of transmission, a consequence of infection, and complications of cervical cancer that forced them to agree to take the HPV vaccine.
Strength and limitation
Community-based study design and using a large sample size can be taken as the strength of the study. The limitation would be due to recall bias that might be faced. Another limitation of this study focused on the quantitative approach, which could not address the “why” questions in detail.
Conclusion and recommendation
In this study, the knowledge and willingness of parents toward the HPV vaccine were low. Then, health authorities through mass media should strengthen HPV vaccine promotion. Health extension workers and health professionals provide pertinent information about the vaccine in schools, and health facilities by delivering leaflets and brochures. Mixed quantitative and qualitative studies are better for further investigations to answer “why” questions.
Acknowledgements
We are high thanks the College of Medicine and Health Sciences, Debre Tabor University, for giving a supportive letter for conducting this study. We would like to extend our thanks to teachers and school directors for providing the necessary preliminary information. We would also like to extend our appreciation to the study participants, supervisors, and data collectors for their active participation.
Abbreviations
- AOR
Adjusted odds ratio
- COR
Crude odds ratio
- HPV
Human papillomavirus
- STD
Sexually transmitted disease
- WHO
World health organization
Author contributions
GNM conceived the idea, involved in data analyses and interpretation. GNM, TML, ADA, HGB, TSY, and ADM were involved in the data collection, interpretation, and manuscript writing. All authors read and approved the final manuscript.
Funding
The authors have no support or funding information to report.
Availability of data and materials
All data were available within the manuscript.
Declarations
Ethics approval and consent to participate
Ethical clearance was obtained from the Ethical review committee of Debre Tabor University and a formal permission letter was written from the Debre Tabor Town education office. Written consent was obtained from each study participant.
Consent for publication
Not applicable.
Competing interests
The authors declare that there is no competing interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Gedefaye Nibret Mihretie, Email: gedefayen@gmail.com.
Tewachew Muche Liyeh, Email: tewye2006@gmail.com.
Alemu Degu Ayele, Email: degualemu53@gmail.com.
Habtamu Gebrehana Belay, Email: hgebrehana@yahoo.com.
Tigist Seid Yimer, Email: tigistseid@yahoo.com.
Agernesh Dereje Miskr, Email: agerdereje119@gmail.com.
References
- 1.Control, C.f.D., and Prevention. Vaccines for Children program (VFC). Atlanta, 2012.
- 2.Elbasha E, Dasbach E. An integrated economic evaluation and HPV disease transmission models-technical report accompanying the manuscript impact of vaccinating boys and men against HPV in the United States. Vaccine. 2010;28(42):6858–6867. doi: 10.1016/j.vaccine.2010.08.030. [DOI] [PubMed] [Google Scholar]
- 3.Eggertson L. Provinces weighing HPV vaccination of boys. Can Med Assoc. 2012. [DOI] [PMC free article] [PubMed]
- 4.Dawar M, Harris MT, McNeil S. Update on Human Papillomavirus (HPV) Vaccines: an Advisory Committee Statement (ACS) National Advisory Committee on Immunization (NACI) Can Commun Dis Rep. 2012;38(1):1. doi: 10.14745/ccdr.v38i00a01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ball SL, et al. Analyses of human papillomavirus genotypes and viral loads in anogenital warts. J Med Virol. 2011;83(8):1345–1350. doi: 10.1002/jmv.22111. [DOI] [PubMed] [Google Scholar]
- 6.Shearer BD. HPV vaccination: understanding the impact on HPV disease. Natl Collab Centre Infect Dis. 2012.
- 7.Shearer B. HPV vaccination: understanding the impact of HPV disease. Purple Paper Natl Collab Centre Infect Dis. 2011;34:1–18. [Google Scholar]
- 8.Hutubessy R, et al. A case study using the United Republic of Tanzania: costing nationwide HPV vaccine delivery using the WHO Cervical Cancer Prevention and Control Costing Tool. BMC Med. 2012;10(1):1–10. doi: 10.1186/1741-7015-10-136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Organization W.H., P.o.C. Control, and W.H.O.R. Health. Cervical cancer screening in developing countries: report of a WHO consultation. 2002: World Health Organization.
- 10.Francis SA, et al. Examining attitudes and knowledge about HPV and cervical cancer risk among female clinic attendees in Johannesburg, South Africa. Vaccine. 2010;28(50):8026–8032. doi: 10.1016/j.vaccine.2010.08.090. [DOI] [PubMed] [Google Scholar]
- 11.Low EL, et al. What do British women know about cervical cancer symptoms and risk factors? Eur J Cancer. 2012;48(16):3001–3008. doi: 10.1016/j.ejca.2012.05.004. [DOI] [PubMed] [Google Scholar]
- 12.Arbyn M, et al. Estimates of incidence and mortality of cervical cancer in 2018: a worldwide analysis. Lancet Glob Health. 2020;8(2):e191–e203. doi: 10.1016/S2214-109X(19)30482-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Paz-Zulueta M, et al. Prevalence of high-risk HPV genotypes, categorized by their quadrivalent and nine-valent HPV vaccination coverage, and the genotype association with high-grade lesions. BMC Cancer. 2018;18(1):1–9. doi: 10.1186/s12885-018-4033-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Organization WH. Cervical cancer, human papillomavirus (HPV) and HPV vaccines: Key points for policy-makers and health professionals. 2008, World Health Organization.
- 15.Poole DN, et al. A cross-sectional study to assess HPV knowledge and HPV vaccine acceptability in Mali. PLoS ONE. 2013;8(2):e56402. doi: 10.1371/journal.pone.0056402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bruni L, et al. Global estimates of human papillomavirus vaccination coverage by region and income level: a pooled analysis. Lancet Glob Health. 2016;4(7):e453–e463. doi: 10.1016/S2214-109X(16)30099-7. [DOI] [PubMed] [Google Scholar]
- 17.Finocchario-Kessler S, et al. Cervical cancer prevention and treatment research in Africa: a systematic review from a public health perspective. BMC Womens Health. 2016;16(1):1–25. doi: 10.1186/s12905-016-0306-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chiang VCL, et al. Attitude, acceptability and knowledge of HPV vaccination among local university students in Hong Kong. Int J Environ Res Public Health. 2016;13(5):486. doi: 10.3390/ijerph13050486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kisaakye E et al. Level and factors associated with uptake of human papillomavirus infection vaccine among female adolescents in Lira District, Uganda. Pan Afr Med J. 2018; 31(1). [DOI] [PMC free article] [PubMed]
- 20.Adejuyigbe FF, et al. Cervical cancer and human papillomavirus knowledge and acceptance of vaccination among medical students in Southwest Nigeria. Afr J Reprod Health. 2015;19(1):140–148. [PubMed] [Google Scholar]
- 21.Geneti HB, Hailu DA, Muleta G. Assessment of the knowledge, attitude and acceptability towards human papillomavirus and its vaccine among undergraduate female medical students. Gynecol Obstet (Sunnyvale) 2016;6(11):1–9. doi: 10.4172/2161-0932.1000410. [DOI] [Google Scholar]
- 22.Blumenthal J et al. Adolescent understanding and acceptance of the HPV vaccination in an underserved population in New York City. J Oncol, 2012. 2012. [DOI] [PMC free article] [PubMed]
- 23.Zouheir Y, et al. Knowledge of human papillomavirus and acceptability to vaccinate in adolescents and young adults of the Moroccan population. J Pediatr Adolesc Gynecol. 2016;29(3):292–298. doi: 10.1016/j.jpag.2015.11.002. [DOI] [PubMed] [Google Scholar]
- 24.Al-Shaikh GK, et al. Knowledge of Saudi female university students regarding cervical cancer and acceptance of the human papillomavirus vaccine. Saudi Med J. 2014;35(10):1223. [PMC free article] [PubMed] [Google Scholar]
- 25.Leung JTC, Law C-K. Revisiting knowledge, attitudes, and practice (KAP) on human papillomavirus (HPV) vaccination among female university students in Hong Kong. Hum Vaccin Immunother. 2018;14(4):924–930. doi: 10.1080/21645515.2017.1415685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ghojazadeh M, et al. Parental knowledge and attitudes about human papillomavirus in Iran. Asian Pac J Cancer Prev. 2012;13(12):6169–6173. doi: 10.7314/APJCP.2012.13.12.6169. [DOI] [PubMed] [Google Scholar]
- 27.Walsh CD, et al. Public knowledge and attitudes towards Human Papilloma Virus (HPV) vaccination. BMC Public Health. 2008;8(1):1–9. doi: 10.1186/1471-2458-8-368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Songthap A, et al. Knowledge, attitudes, and acceptability of a human papillomavirus vaccine among students, parents and teachers in Thailand. Southeast Asian J Trop Med Public Health. 2012;43(2):340–353. [PubMed] [Google Scholar]
- 29.Trim K et al. Parental knowledge, attitudes, and behaviours towards human papillomavirus vaccination for their children: a systematic review from 2001 to 2011. Obstet Gynecol Int. 2012. 2012. [DOI] [PMC free article] [PubMed]
- 30.Lindley MC, et al. Comparing human papillomavirus vaccine knowledge and intentions among parents of boys and girls. Hum Vaccin Immunother. 2016;12(6):1519–1527. doi: 10.1080/21645515.2016.1157673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Okunade KS et al. Knowledge and acceptability of human papillomavirus vaccination among women attending the gynecological outpatient clinics of a university teaching hospital in Lagos, Nigeria. J Trop Med. 2017. 2017. [DOI] [PMC free article] [PubMed]
- 32.Voidăzan S, et al. Human Papillomavirus vaccine-knowledge and attitudes among parents of children aged 10–14 years: a cross-sectional study, Tîrgu Mureş, Romania. Cent Eur J Public Health. 2016;24(1):29–38. doi: 10.21101/cejph.a4287. [DOI] [PubMed] [Google Scholar]
- 33.Sherman SM, Nailer E. Attitudes towards and knowledge about Human Papillomavirus (HPV) and the HPV vaccination in parents of teenage boys in the UK. PLoS ONE. 2018;13(4):e0195801. doi: 10.1371/journal.pone.0195801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Masika MM, et al. Knowledge on the HPV vaccine and cervical cancer facilitates vaccine acceptability among school teachers in Kitui County, Kenya. PLoS ONE. 2015;10(8):e0135563. doi: 10.1371/journal.pone.0135563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Makwe CC, Anorlu RI. Knowledge of and attitude toward human papillomavirus infection and vaccines among female nurses at a tertiary hospital in Nigeria. Int J Women’s Health. 2011;3:313. doi: 10.2147/IJWH.S22792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ojiyi CE, et al. Human papillomavirus vaccine: awareness and acceptability amongst female medical students and health workers in a University Teaching Hospital in Eastern Nigeria. Niger J Surg Sci. 2013;23(1):14. [Google Scholar]
- 37.Hoque ME. Acceptability of human papillomavirus vaccination among academics at the University of KwaZulu-Natal, South Africa. South Afr Fam Pract. 2015;57(5):318–321. doi: 10.1080/20786190.2015.1078157. [DOI] [Google Scholar]
- 38.Mortensen GL. Parental attitudes towards vaccinating sons with human papillomavirus vaccine. Dan Med Bull. 2010;57(12):A4230. [PubMed] [Google Scholar]
- 39.Ogilvie GS, et al. Parental intention to have daughters receive the human papillomavirus vaccine. CMAJ. 2007;177(12):1506–1512. doi: 10.1503/cmaj.071022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cunningham MS, et al. Cervical cancer screening and HPV vaccine acceptability among rural and urban women in Kilimanjaro Region, Tanzania. BMJ Open. 2015;5(3):e005828. doi: 10.1136/bmjopen-2014-005828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Perkins RB, et al. Maternal support for human papillomavirus vaccination in Honduras. J Womens Health. 2011;20(1):85–90. doi: 10.1089/jwh.2009.1919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Alene T, et al. Acceptance of human papillomavirus vaccination and associated factors among parents of daughters in Gondar Town, Northwest Ethiopia. Cancer Manag Res. 2020;12:8519. doi: 10.2147/CMAR.S275038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ezat SWP, et al. National HPV immunization program: knowledge and acceptance of mothers attending an obstetrics clinic at a teaching hospital, Kuala Lumpur. Asian Pac J Cancer Prev. 2013;14(5):2991–2999. doi: 10.7314/APJCP.2013.14.5.2991. [DOI] [PubMed] [Google Scholar]
- 44.Jalani FFM et al. Knowledge, attitude and practice of human papillomavirus (HPV) vaccination among secondary school students in rural areas of Negeri Sembilan, Malaysia. Int J Collabo Res Internal Med Public Health. 2016.
- 45.Saqer A, et al. Knowledge and awareness about cervical cancer vaccine (HPV) among parents in Sharjah. Asian Pac J Cancer Prev APJCP. 2017;18(5):1237. doi: 10.22034/APJCP.2017.18.5.1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Smith-Palmer J et al. Estimating the clinical benefits of vaccinating boys and girls against HPV-related diseases in Europe. 2013. [DOI] [PMC free article] [PubMed]
- 47.Vermandere H, et al. Determinants of acceptance and subsequent uptake of the HPV vaccine in a cohort in Eldoret, Kenya. PLoS ONE. 2014;9(10):e109353. doi: 10.1371/journal.pone.0109353. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data were available within the manuscript.