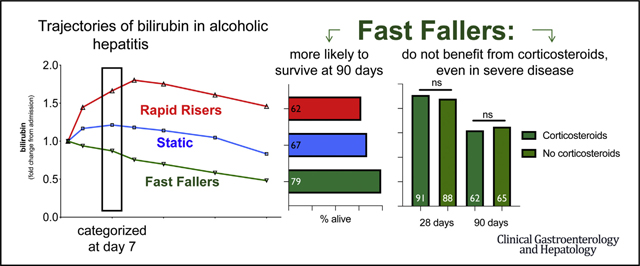
Abstract
BACKGROUND AND AIMS:
Alcoholic hepatitis (AH) is a severe condition with poor short-term prognosis. Specific treatment with corticosteroids slightly improves short-term survival but is associated with infection and is not used in many centers. A reliable method to identify patients who will recover spontaneously will minimise the numbers of patients who experience side effects of available treatments.
METHODS:
We analysed the trajectory of serum bilirubin concentration over the course of hospital admissions in patients with AH to predict spontaneous survival and the need for treatment.
RESULTS:
data from 426 patients were analysed. Based on bilirubin trajectory, patients were categorized into three groups: ‘fast fallers’ (bilirubin <0.8 x admission value at day 7), ‘static’ (bilirubin of >0.9 – <1.2 x admission value) and ‘rapid risers’ (bilirubin of ≥1.2 x admission bilirubin). Fast fallers had significantly better 90-day survival compared to other groups (log rank p < .001), and showed no benefit of corticosteroid therapy (OR for survival at 28 days of treatment, 0.94, 95% CI 0.06 – 8.41). These findings remained even amongst patients with severe disease based on initial DF, GAHS or MELD scores.
CONCLUSIONS:
We present an intuitive method of classifying patients with AH based on the trajectory of bilirubin over the first week of admission. It is complimentary to existing scores that identify candidates for corticosteroid treatment or assess response to treatment. This method identifies a group of patients with AH who recover spontaneously and can avoid corticosteroid therapy.
Keywords: Alcoholic Hepatitis, Outcomes, Corticosteroid
Graphical Abstract
Alcoholic hepatitis (AH) is a severe clinical entity characterized by acute onset of jaundice and coagulopathy in patients with alcohol use disorder.1 The incidence of AH is increasing over recent years.2 Short-term mortality of AH remains very high globally3,4: 15%–40% of patients with AH die within 30 days of admission to hospital.5
The medical management of AH has not evolved substantially in the last 2 decades, with multiple potential therapeutic agents tested without a great deal of success.6 Corticosteroids remain the only established treatment for AH. Although the largest randomised controlled trial of corticosteroids showed only a modest short-term (28-day) survival benefit,5 a recent network meta-analysis showed a survival benefit,7 but this comes at the cost of a greater risk of infection.5 There is considerable heterogeneity regarding corticosteroid use among physicians, with a recent survey of practice showing that a quarter of clinicians did not use corticosteroids in patients with AH.8
In contrast to effective treatments, methods to predict prognosis in AH are legion and all perform with a roughly similar degree of accuracy.9–11 Some of these scores have been used to identify the subgroup of patients who will benefit from corticosteroid therapy, most commonly the discriminant function (DF),12 the Model for End-Stage Liver Disease (MELD) score, and the Glasgow alcoholic hepatitis score (GAHS).13 Baseline bilirubin concentration is an important indicator of severity of AH and prognosis in patients with AH, and is included in all scoring systems. The dynamic change in bilirubin over the first days of admission has been identified as an important prognostic indicator14 and a marker of response to corticosteroid treatment.15 The Lille model uses change in bilirubin after initiation of prednisolone to gauge response to therapy and guide ongoing treatment and is conventionally applied after 7 days of therapy16 but is also useful after shorter duration of treatment.17
We used computer-aided analysis to study how the trajectory of bilirubin concentration during admission might be useful to identify clinical phenotypes, stratify the probability of short-term survival, and guide treatment in a real-world, multinational observational cohort of patients with AH.
Patients and Methods
Study Population, Data Collection and Definitions
Data from consecutive patients were collected prospectively from 3 centers in the United Kingdom (University Hospitals Birmingham NHS Foundation Trust, Birmingham; University Hospitals Bristol NHS Foundation Trust, Bristol; Plymouth Hospital NHS Trust, Plymouth) and from the InTEAM consortium (details of participating centers are included in the Supplementary Appendix). These cohorts were observational only; no patients underwent treatments or procedures outside the standard of care in each institution.
All patients included had alcoholic hepatitis, defined as (1) alcohol use of at least 60 g/d in men and >40 g/ d in women for >5 years, (2) last drink within 4 weeks of admission, (C) serum bilirubin >3 mg/dL, (4) elevated transaminases >50 and <400 IU/L, (5) aspartate transaminase-to-alanine transaminase ratio >2:1, and (6) exclusion of other concomitant liver disease.18 Liver biopsy was performed only in cases of diagnostic uncertainty, consistent with recent National Institute on Alcohol Abuse and Alcoholism guidance for trialists.18 When biopsy was performed, the histological diagnosis of AH was defined by the presence of hepatocellular damage (hepatocellular ballooning and presence of Mallory bodies), inflammatory infiltrate (predominantly polymorphonuclear cells), and pericellular fibrosis. These criteria are consistent with the InTEAM project inclusion and exclusion criteria (detailed in Supplementary Appendix). Baseline (ie, earliest recorded data after admission to first admitting hospital) clinical and biochemical characteristics, specific treatments for AH, and presence of complications during admission (infection or acute kidney injury) were collected. Clinical complications during admission such as ascites, spontaneous bacterial peritonitis, renal dysfunction, hepatic encephalopathy, or gastrointestinal bleeding were treated according to current international guidelines in effect at the time of admission.19–21
Bilirubin trajectory across 28 days of admission was analyzed in patients who did not receive corticosteroid therapy, using Traj software (http://www.andrew.cmu.edu/user/bjones/) in Stata version 15 (StataCorp, College Station, TX). Distinct trajectories were identified that categorized patients into 3 groups. All other statistical analyses were done in SPSS version 24 (IBM, Armon, NY).
Statistical Analysis
The accuracy of predicting bilirubin trajectory at various points within an individual’s hospital admission was tested with k values. Groups were compared with t tests (2 group comparisons) or 1-way analysis of variance (for multiple groups). The odds of spontaneous survival at 90 days after admission (ie, without corticosteroid therapy) were calculated for each group and odds ratios calculated based on fast fallers as a reference group. Survival was analyzed with Kaplan-Meier univariate analysis, and multivariate Cox Proportional hazard analysis to control for multiple factors. Scoring systems (DF, MELD, GAHS, Lille) were calculated as per published data.
To examine the efficacy of corticosteroid use22 in each group, patients were included if they received corticosteroids after 7 or more days of admission to allow for bilirubin trajectory early in admission to be analyzed, or if they did not receive corticosteroid therapy. As differences existed between treated and nontreated patients, propensity score matching was used to assemble a comparable cohort. Patients were matched for age, bilirubin concentration, creatinine concentration, and albumin concentration using the SPSS propensity score–matching function (Supplementary Table 1). To analyze the value of using bilirubin trajectory to predict patient who may not require treatment, risk ratios for survival at 28 days (as per standard outcomes in therapeutic studies) were calculated with or without corticosteroid therapy.
Results
In total, data from 426 patients with AH were collected, of whom 324 had a DF above 32 at admission to hospital and 105 received corticosteroid therapy. The demographic and biochemical characteristics of included patients are depicted in Table 1.
Table 1.
Characteristics of Included Patients. Data are presented as mean and standard error of mean except for ˇmedian and interquartile rang
| Entire cohort (N = 426) | Patients without Corticosteroid Use | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
||||||||||
| Modelling cohort (n = 321) | Fast fallers (n = 150) | Static (n = 99) | Rapid risers (n = 72) | P value (ANOVA) | |||||||
|
|
|
|
|
||||||||
| Average | Variance | Average | Variance | Average | Variance | Average | Variance | Average | Variance | ||
| Age, y | 49.3 (43–57) | 10.0 | 49 (42–56) | 10.1 | 48 (42–55.6) | 1.0 | 50 (42–56) | 1.0 | 51 (41.8–58) | 1.6 | .161 |
| Bilirubin, μmol/L | 271 | 143 | 269 | 139 | 271 | 14 | 296 | 16 | 240 | 19 | .098 |
| Prothrombin time, s | 24.2 | 8.8 | 23.4 | 8.38 | 21.1 | 0.7 | 26.1 | 0.9 | 25.1 | 1.4 | <.001a |
| White cell count (×109/L) | 10.9 | 6.1 | 10.9 | 6.0 | 10.6 | 0.6 | 12.0 | 0.9 | 10.3 | 0.9 | .300 |
| Creatinine, mmol/L | 91.7 | 78.5 | 90.2 | 80.0 | 88.2 | 6.3 | 91.4 | 8.3 | 109 | 0.9 | .378 |
| Sodium, mmol/L | 131 | 7.1 | 131 | 7.6 | 131 | 1 | 131 | 1 | 132 | 1 | .723 |
| Urea, mmol/L | 5.56 | 13.4 | 5.56 | 13.3 | 7.56 | 2.30 | 4.66 | 0.50 | 5.20 | 0.98 | .400 |
| Albumin, g/L | 26.7 | 6.2 | 26.9 | 6.4 | 26.1 | 0.8 | 27.3 | 0.6 | 27.5 | 1.0 | .318 |
| Platelets (×109/L) | 132 | 82 | 131 | 81 | 149 | 13 | 129 | 8 | 115 | 12 | .128 |
| DF >32 | 324 (75) | 243 (75) | 103 | 85 | 53 | ||||||
| MELD >20 | 317 (73) | 191 (59) | 86 | 61 | 42 | ||||||
| GAHS ≥9 | 238 (56) | 187 (58) | 84 | 59 | 43 | ||||||
NOTE. Values are median and interquartile range; mean and SE; or n (%), unless otherwise indicated.
ANOVA, analysis of variance; DF, discriminant function; GAHS, Glasgow alcoholic hepatitis score; MELD, Model for End-Stage Liver Disease.
Identification of Bilirubin Trajectories
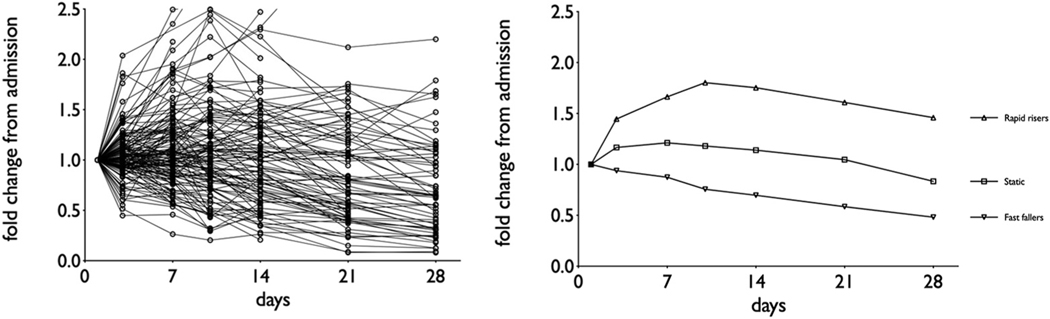
Patients who did not receive corticosteroids (n = 321) were used as a modeling group to identify and analyze bilirubin trajectories. Sequential bilirubin values up to 28 days after admission revealed 3 groups with distinct trajectories of bilirubin concentration over time (Figure 1): patients with a rapid decrease in bilirubin concentrations (fast fallers), patients with bilirubin concentration that remained elevated but without obvious improvement or deterioration (static), and patients that showed a bilirubin concentration rising inexorably after admission (rapid risers).
Figure 1.
Differing trajectories of change in serum bilirubin concentration over time after admission with AH.
To allow for clinical application of this classification system, the accuracy of using bilirubin observed trajectory at 3 or 7 days after admission to predict subsequent bilirubin trajectory was examined using k values. Groups could not be identified with accuracy at day 3 (k= 0.39) but by day 7 bilirubin trajectory over the course of 28 days could be accurately predicted (k = 0.92). Accordingly, bilirubin trajectory as assessed at 7 days after admission was used for all analyses of clinical utility. Fast fallers had a bilirubin of less than 0.9× admission value at day 7, the static group had a bilirubin of ≥0.9× to ≤1.2 admission value and rapid risers had a bilirubin >1.3 × admission value. Importantly, in published trials of corticosteroid therapy in AH, the median delay from admission to treatment is 8 days with no association between treatment delay and survival (Supplementary Figure 1), suggesting that a 7-day evaluation period is acceptable in hospitalized cases.
The characteristics of each trajectory group are shown in Table 1. A statistically nonsignificant difference in bilirubin concentration between groups was observed (1-way analysis of variance, P = .098), in which the highest bilirubin concentration was observed in the static group, with lower bilirubin concentrations in the fast-faller and rapid-risers groups.
Fast-Falling Bilirubin Trajectory Is Associated With Spontaneous Recovery
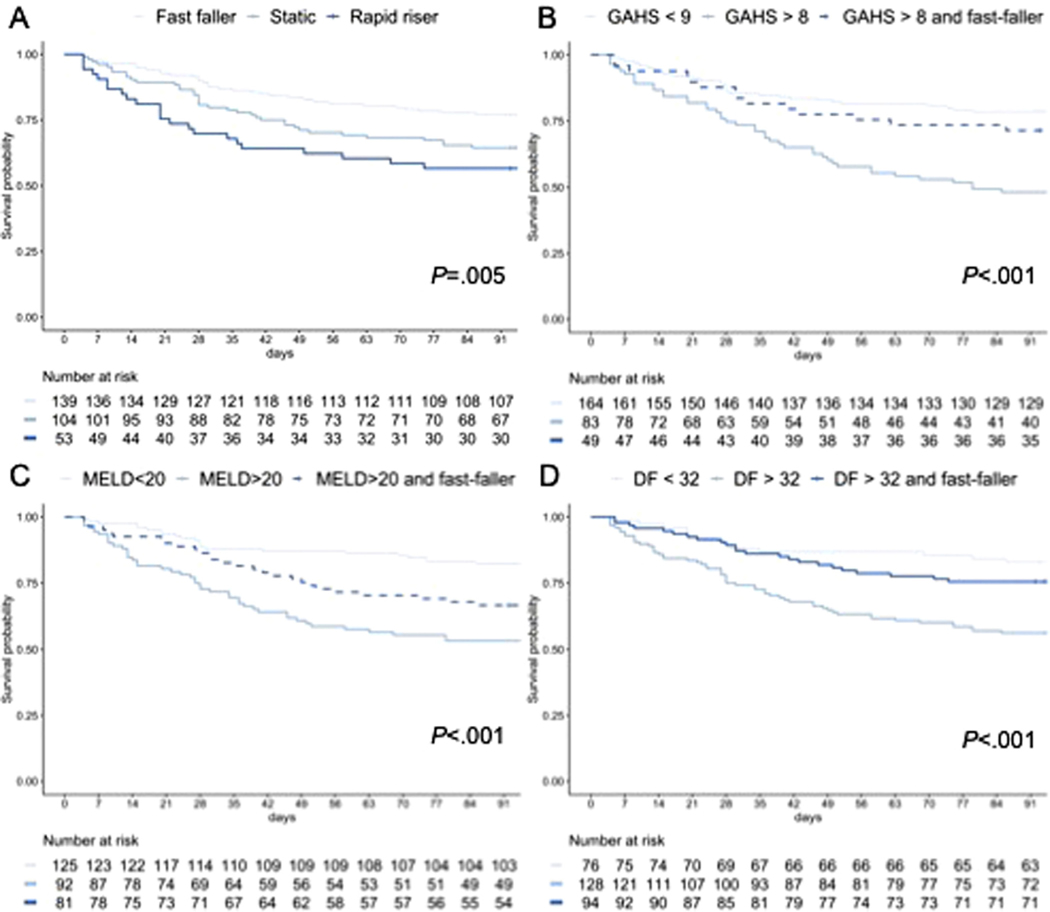
Spontaneous recovery (ie, survival without corticosteroid therapy at 90 days) varied between groups: fast fallers were more likely to recover than patients with static or rising bilirubin concentration (odds ratio [OR], 0.54; 95% confidence interval [CI], 0.31–0.94; and OR, 0.39; 95% CI, 0.19–0.76, respectively) (Table 2, Figure 2A). This remained when severe AH was considered defined by the GAHS, MELD, or DF: in each case, patients with severe disease who had a fast fall in bilirubin had rates of spontaneous recovery comparable to those without severe disease (Figure 2B–D). After controlling for baseline differences in bilirubin and prothrombin time between groups with Cox proportional hazard analysis, bilirubin trajectory was independently associated with mortality at 90 days (P = .042). Dynamic changes in MELD score between days 1 and 7 and the Lille score were also predictors of spontaneous recovery.
Table 2.
Odds of Spontaneous Recovery in Each Trajectory Group, Analyzed With Fisher’s Exact Test
| Odds of spontaneous Recovery | Odds ratio | 95% CI | P value | |
|---|---|---|---|---|
| Fast faller | 3.16 | 1 (reference) | ||
| Static | 1.68 | 0.54 | 0.30–0.93 | .028 |
| Rapid riser | 1.32 | 0.39 | 0.19–0.76 | .005 |
| All non–fast faller | 1.61 | 0.48 | 0.29–0.80 | .005 |
| Improved MELD | 3.22 | 1 (reference) | ||
| Stable MELD | 2.20 | 0.68 | 0.37–1.27 | .2 |
| Deteriorating MELD | 1.21 | 0.38 | 0.20–0.69 | .002 |
| Lille response | 9.29 | 1 (reference) | ||
| Lille partial | 2.12 | 0.23 | 0.09–0.53 | .001 |
| Lille null response | 1.02 | 0.10 | 0.04–0.25 | <.001 |
CI, confidence interval; MELD, Model for End-Stage Liver Disease.
Figure 2.
Kaplan-Meier analysis of survival after admission to hospital with AH. (A) Survival differs between bilirubin trajectory categories (log-rank P < .01). Patients with fast-falling bilirubin concentration but with severe disease are defined as (B) GAHS above 8, (C) MELD score above 20, or (D) DF above 32 and have outcomes comparable to nonsevere disease.
Use of Bilirubin Trajectory to Stratify Treatment in AH
To investigate the utility of using bilirubin trajectory to guide treatment, we examined patients who received corticosteroid treatment after 7 days of admission, allowing for bilirubin trajectory to be assessed at day 7 before therapy. Patients who received corticosteroid therapy after 7 days differed from those who did not (Table 1), so propensity score matching was used to identify comparable groups. After removing patients who received corticosteroid therapy before 7 days of admission and doing propensity score matching, 180 patients were analyzed, of whom 90 received corticosteroids and 90 did not.
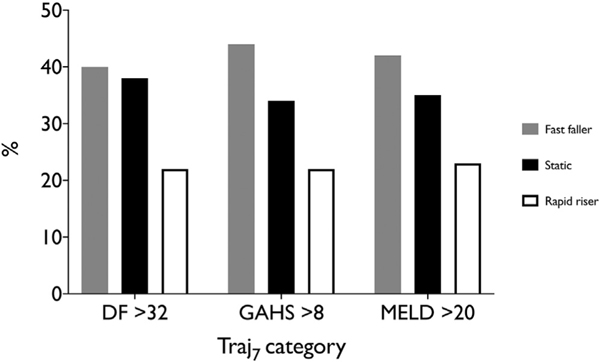
Corticosteroid therapy had no survival benefit in fast fallers (OR for survival at 28 days of treatment, 0.94; 95% CI, 0.06–8.41; P = .879). When analysis was limited to patients with severe disease (defined as DF >32, GAHS >8, or MELD score >20) this remained the case, with no benefit of corticosteroids observed in each case (Table 3, Supplementary Figure 2). Corticosteroid therapy in rapid risers showed a trend toward benefit without reaching statistical significance (OR, 4.82; 95% CI, 0.85–24.4; P= .079). The use of bilirubin trajectory to allow patients with severe disease but a fast-falling bilirubin to be managed without corticosteroids would have excluded many patients from treatment: 43% of patients with a DF >32, 45% of patients with a MELD score >20, and 45% of patients with a GAHS >8 (Table 1, Figure 3).
Table 3.
Propensity-Matched Analysis of Patients Treated With or Without Corticosteroids
| Corticosteroids (%) | No corticosteroids (%) | OR | 95% CI | P value (Fisher’s exact test) | |
|---|---|---|---|---|---|
| 28-d survival | |||||
| Fast faller | 96 | 96 | 0.94 | 0.060–8.41 | .999 |
| GAHS >8 and fast faller | 95 | 83 | 3.60 | 0.411–31.6 | .416 |
| MELD >20 and fast faller | 91 | 88 | 1.37 | 0.208–9.02 | .999 |
| DF >32 and fast faller | 96 | 96 | 1.00 | 0.086–11.6 | .999 |
| 90-d survival | |||||
| Fast faller | 79 | 79 | 0.99 | 0.317–3.10 | .988 |
| GAHS >8 and fast faller | 56 | 50 | 0.80 | 0.173–3.71 | .776 |
| MELD >20 and fast faller | 62 | 65 | 1.16 | 0.352–3.84 | .805 |
| DF >32 and fast faller | 76 | 75 | 0.94 | 0.292–3.01 | .914 |
CI, confidence interval; DF, discriminant function; GAHS, Glasgow alcoholic hepatitis score; MELD, Model for End-Stage Liver Disease; OR, odds ratio.
Figure 3.
Identification of patients for with severe disease: bilirubin trajectory analysis identifies a different group of patients with compared with the DF, GAHS, or MELD.
Discussion
This large multicenter, multinational study of patients with AH used computer-assisted analysis of bilirubin trajectory to identify 3 groups of patients, with distinct clinical phenotypes. A fast falling bilirubin trajectory indicated a group of patients who had a greater chance of spontaneous survival and who did not benefit from corticosteroid therapy, even among patients with severe disease. Identification of this group of fast fallers with a better outcome can allow clinicians confidence to avoid treatment with prednisolone and may facilitate earlier discharge from hospital.
Bilirubin has long been identified as important dynamic measure in AH in terms of prognostication14 and assessing response to treatment.15 Our data confirm the value of observing this indicator in AH both as a guide to outcome and a guide to therapy. The use of more complex scores—MELD or Lille—over the same 7-day period also gave prognostic information with accuracy comparable to bilirubin alone. The Lille score in particular, if below 0.16 (responders when used in the context of corticosteroid use) is a strong predictor of spontaneous survival. These scores may have advantages over the simple measure of bilirubin—although in a direct comparison, the area under the receiver-operating characteristic values are similar (0.58 [95% CI, 0.52–0.64] vs 0.63 [95% CI, 0.59–0.68] for spontaneous survival). Assessment of bilirubin has the advantage of being intuitive and simple to calculate at the bedside. Considering of how to predict spontaneous survival should not detract from the simple biological observation that if patients are getting better, they are likely to continue to improve, and liver-specific treatment might be avoided, beyond standard supportive measures.
Importantly, the trajectory system is different to the Lille score, as trajectory identifies individuals likely to do well before starting treatment with corticosteroids, whereas the Lille score is designed to assess response after starting treatment. Indeed, the 2 scores could be used sequentially in the same patient before and after starting therapy to further minimize futile exposure to corticosteroids. Several scores exist that predict mortality in patients with AH. Bilirubin trajectory does not replace these as a means of prognostication, but it can serve as an additional means of identifying patients who may not require corticosteroid therapy despite severe disease. Liver transplantation for patients who fail to respond to corticosteroid therapy has been shown to be effective in AH.23,24 Fast fallers who are likely to improve without specific treatment are less likely to require liver transplantation, and this may influence decision making around patients with severe disease at admission but who display a favorable trajectory of bilirubin.
The observed trajectories of bilirubin concentration that we describe are from the point of hospital admission, rather than from the onset of jaundice. While there are few data to illustrate this, anecdotally there is significant variation in the delay between the onset of jaundice and admission to hospital. The impact of this on observed trajectories of bilirubin can only be speculated about, but it is conceivable that it is more individuals who become sicker outside of hospital eventually present to medical services, whereas those who improve do not. This variation in presentation may be further complicated by transfer of patients with AH between centers that is common in some health systems, principally in the United States. The data used for this study were the earliest available values. The observed trajectories therefore are from the time of admission or very close to it. This may introduce a bias to the observed trajectories when considering AH as a whole, but our findings remain relevant to patients who are admitted to hospital. Further research will illustrate the impact of jaundice to door time and its impact on outcome and observed bilirubin trajectory.
The study has limitations. The research cohort is observational, such that the question of the clinical utility of using bilirubin trajectory to guide treatment was not tested specifically. This is a similar technique to derivation of existing scoring systems in AH, and also represents a real-world sample in which there is known to be marked heterogeneity regarding use of corticosteroids. There may be reasons why certain patients received corticosteroid therapy that were not have been captured and thus introduced bias to the dataset despite propensity score matching. Clinicians eager to start specific therapies may baulk at the requirement to observe patients for 7 days before patients can be allocated to a group. However, in practice, a period of 7 days allows for time exclusion of infection, other liver diseases, and biopsy if this is felt necessary. We note that the median delay from admission to treatment in the Steroids or Pentoxifylline in Alcoholic Hepatitis (STOPAH) trial was 6.7 days, and overall delay in various trials of corticosteroids over the years is more than a week (Supplementary Figure 1). Validation of these observations is key, and it is obvious that propensity score matching resulted in a much smaller group than the initial, large cohort.
We used the National Institute on Alcohol Abuse and Alcoholism criteria to identify a coherent group of patients to study. These criteria use a bilirubin threshold of 3 mg/dL, whereas clinical criteria and histological concordance has only previously been established for higher levels of bilirubin. However, in this cohort, only a few patients had a bilirubin below 5 mg/dL (10 of 321 in the group not treated with corticosteroids and used for model building). Removing this group from the analysis did not result in any meaningful differences in the observations regarding likelihood of survival (data not shown). Lower bilirubin concentrations would make it difficult to understand what small fluctuations may mean, but this group of patients are unlikely to be considered for corticosteroid therapy in practice.
We present a novel, intuitive method of classifying patients with AH based on the trajectory of bilirubin over the first week of an admission. This system aids in prognostication but most importantly identifies a group of patients who are likely to have better outcomes and do not benefit from corticosteroid therapy.
Supplementary Material
What You Need to Know.
Background
Corticosteroid treatment of alcoholic hepatitis is difficult: some patients will respond, but there are concerns about side effects. This study examined if patients with severe disease could avoid corticosteroids if they showed a spontaneous reduction in bilirubin concentration.
Findings
Patients with a significant fall in bilirubin concentration over the first 7 days after admission showed high rates of spontaneous recovery without corticosteroid treatment, even in patients with severe disease.
Implications for patient care
This observation allows patients to avoid corticosteroid therapy if they are improving without treatment. Corticosteroids can be reserved for cases without spontaneous improvement.
Acknowledgments
Jose Altamirano wishes to express his gratitude to the Mexican National Council of Science and Technology (CONACyT, Mexico City, Mexico) for partially supporting his predoctoral stay in Barcelona, Spain.
Funding
No specific financial support was received for this work. The INTEAM consortium is supported by funding from the National Institute on Alcohol Abuse and Alcoholism (1U01AA021908-01). Juan Pablo Arab and Marco Arrese were supported by the Chilean government through the Fondo Nacional de Desarrollo Científico y Tecnológico (1200227 to JPA and 1191145 to MA) and the Comisión Nacional de Investigación Científica y Tecnológica (AFB170005, CARE Chile UC). Joaquin Cabezas was supported by an AEEH (Spanish Association for the Study of the Liver) grant (“Juan Rodés 2015”).
Abbreviations used in this paper:
- AH
alcoholic hepatitis
- CI
confidence interval
- DF
discriminant function
- GAHS
Glasgow alcoholic hepatitis score
- MELD
Model for End-Stage Liver Disease
- OR
odds ratio
Footnotes
Supplementary Material
Note: To access the supplementary material accompanying this article, please click here.
Conflicts of interest
These authors disclose the following: Richard Parker has received advisory board fees from Sandoz. Ramon Bataller has received consulting fees from EchoSens (none of these grants have relationship with the current manuscript). The remaining authors disclose no conflicts.
References
- 1.Lucey MR, Mathurin P, Morgan TR. Alcoholic hepatitis. N Engl J Med 2009;360:2758–2769. [DOI] [PubMed] [Google Scholar]
- 2.Sandahl TD, Jepsen P, Thomsen KL, Vilstrup H. Incidence and mortality of alcoholic hepatitis in Denmark 1999–2008: a nationwide population based cohort study. Hepatol 2011; 54:760–764. [DOI] [PubMed] [Google Scholar]
- 3.Gao B, Bataller R. Alcoholic liver disease: pathogenesis and new therapeutic targets. Gastroenterology 2011;141: 1572–1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Altamirano J, Fagundes C, Dominguez M, et al. Acute kidney injury is an early predictor of mortality for patients with alcoholic hepatitis. Clin Gastroenterol Hepatol 2012;10:65–71.e3. [DOI] [PubMed] [Google Scholar]
- 5.Thursz MR, Richardson P, Allison M, et al. Prednisolone or pentoxifylline for alcoholic hepatitis. N Engl J Med 2015; 372:1619–1628. [DOI] [PubMed] [Google Scholar]
- 6.Hughes E, Hopkins L, Parker R. Survival from alcoholic hepatitis has not improved over time. PLoS One 2018;13: e0192393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Singh S, Murad MH, Chandar AK, et al. Comparative effectiveness of pharmacological interventions for severe alcoholic hepatitis: a systematic review and network meta-analysis. Gastroenterology 2015;149:958–970.e12. [DOI] [PubMed] [Google Scholar]
- 8.Dhanda A, Atkinson S, Thursz M. Variation in the use of corticosteroids for the treatment of acute severe alcoholic hepatitis in the post-STOPAH era: results of a UK national survey. J Hepatol 2017;66:S345. [Google Scholar]
- 9.Ali S, Hussain S, Hair M, Shah AA. Comparison of Maddrey Discriminant Function, Child-Pugh Score and Glasgow Alcoholic Hepatitis Score in predicting 28-day mortality on admission in patients with acute hepatitis. Ir J Med Sci 2013; 182:63–68. [DOI] [PubMed] [Google Scholar]
- 10.Palaniyappan N, Subramanian V, Ramappa V, et al. The utility of scoring systems in predicting early and late mortality in alcoholic hepatitis: whose score is it anyway? Int J Hepatol 2012; 2012:624675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sandahl TD, Jepsen P, Ott P, Vilstrup H. Validation of prognostic scores for clinical use in patients with alcoholic hepatitis. Scand J Gastroenterol 2011;46:1127–1132. [DOI] [PubMed] [Google Scholar]
- 12.Carithers RL Jr, Herlong HF, Diehl AM, et al. Methylprednisolone therapy in patients with severe alcoholic hepatitis. A randomized multicenter trial. Ann Intern Med 1989;110:685. [DOI] [PubMed] [Google Scholar]
- 13.Forrest EH, Morris AJ, Stewart S, et al. The Glasgow alcoholic hepatitis score identifies patients who may benefit from corticosteroids. Gut 2007;56:1743–1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee M, Kim W, Choi Y, et al. Spontaneous evolution in bilirubin levels predicts liver-related mortality in patients with alcoholic hepatitis. PLoS One 2014;9:e100870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mathurin P, Abdelnour M, Ramond M-J, et al. Early change in bilirubin levels is an important prognostic factor in severe alcoholic hepatitis treated with prednisolone. Hepatology 2003; 38:1363–1369. [DOI] [PubMed] [Google Scholar]
- 16.Louvet A, Naveau S, Abdelnour M, et al. The Lille model: a new tool for therapeutic strategy in patients with severe alcoholic hepatitis treated with steroids. Hepatology 2007; 45:1348–1354. [DOI] [PubMed] [Google Scholar]
- 17.Garcia-Saenz-de-Sicilia M, Duvoor C, Altamirano J, et al. A day4 Lille model predicts response to corticosteroids and mortality in severe alcoholic hepatitis. Am J Gastroenterol 2017;112:306. [DOI] [PubMed] [Google Scholar]
- 18.Crabb DW, Bataller R, Chalasani NP, et al. Standard definitions and common data elements for clinical trials in patients with alcoholic hepatitis: recommendation from the NIAAA Alcoholic Hepatitis Consortia. Gastroenterology 2016;150:785–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Garcia-Tsao G, Sanyal AJ, Grace ND, et al. Prevention and management of gastroesophageal varices and variceal hemorrhage in cirrhosis. Hepatology 2007;46:922–938. [DOI] [PubMed] [Google Scholar]
- 20.Moore KP, Wong F, Gines P, et al. The management of ascites in cirrhosis: report on the consensus conference of the International Ascites Club. Hepatology 2003;38:258–266. [DOI] [PubMed] [Google Scholar]
- 21.Ferenci P, Lockwood A, Mullen K, et al. Hepatic encephalopathy– definition, nomenclature, diagnosis, and quantification: final report of the working party at the 11th World Congresses of Gastroenterology, Vienna, 1998. Hepatology 2002;35:716–721. [DOI] [PubMed] [Google Scholar]
- 22.Louvet A, Thursz MR, Kim DJ, et al. Corticosteroids reduce risk of death within 28 days for patients with severe alcoholic hepatitis, compared with pentoxifylline or placebo—a meta-analysis of individual data. Gastroenterology 2018;155:458–468.e8. [DOI] [PubMed] [Google Scholar]
- 23.Mathurin P, Moreno C, Samuel D, et al. Early liver transplantation for severe alcoholic hepatitis. N Engl J Med 2011; 365:1790–1800. [DOI] [PubMed] [Google Scholar]
- 24.Im GY, Kim-Schluger L, Shenoy A, et al. Early liver transplantation for severe alcoholic hepatitis in the United States–a single-center experience. Am J Transplant 2016;16:841–849. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.