Summary
Background
Bacterial vaginosis might increase HIV risk by eliciting genital inflammation and epithelial barrier disruption, whereas vaginal Lactobacillus crispatus is associated with immune quiescence and HIV protection. We investigated the effect of a live biotherapeutic containing L crispatus CTV-05 (LACTIN-V) on genital immunology and key vaginal bacteria.
Methods
This substudy included women aged 18–45 years who participated in the randomised, placebo-controlled, phase 2b trial of LACTIN-V to reduce bacterial vaginosis recurrence, conducted at four universities and hospitals in the USA. Women with negative results for sexually transmitted infection, pregnancy, and urinary tract infection were provided a 5-day course of vaginal metronidazole 0·75% gel. Those who met at least three of four clinical Amsel criteria for bacterial vaginosis and had a Nugent score of 4–10 from Gram staining were eligible. Participants in the LACTIN-V trial were randomly assigned (2:1) to receive either LACTIN-V or placebo, applied vaginally once per day for 5 days during the first week and then twice per week for 10 more weeks. Follow-up visits occurred 4, 8, 12, and 24 weeks after enrolment. Soluble immune factors and the absolute abundance of bacterial taxa were assayed by mutliplex ELISA and quantitative PCR. The primary outcomes were vaginal levels of IL-1α and soluble E-cadherin at 24 weeks (ie, 13 weeks after treatment cessation).
Findings
Between Feb 21, 2020 and March 18, 2021, we characterised genital immune parameters and the vaginal microbiota in a subset of 66 highly adherent participants who were randomly selected, with no exclusion criteria, from those who had attended all study follow-up visits (n=166) in the larger LACTIN-V clinical trial (n=288). 32 (48%) participants received LACTIN-V and 34 (52%) received placebo. LACTIN-V treatment was significantly associated with lower concentrations of the proinflammatory cytokine IL-1α (β coefficient 0·310, SE 0·149; p=0·042) and soluble E-cadherin (0·429, 0·199; p=0·035), a biomarker of epithelial barrier disruption.
Interpretation
Vaginal administration of LACTIN-V following standard bacterial vaginosis therapy resulted in a sustained reduction in genital inflammation and a biomarker of epithelial integrity. The potential of LACTIN-V to reduce HIV susceptibility merits further investigation.
Funding
Canadian Institutes of Health Research and the National Institutes of Health National Institute of Allergy and Infectious Diseases.
Introduction
Bacterial vaginosis has been linked to adverse reproductive health outcomes among women and to increased risk of HIV acquisition.1,2 Bacterial vaginosis is characterised by diverse anaerobic bacteria3,4 and might elevate HIV risk by eliciting genital inflammation, which not only recruits HIV-susceptible CD4+ T cells to mucosal tissues but also causes epithelial barrier disruption with the cleavage of E-cadherin, a key component of epithelial cell-to-cell junctions,5,6 thereby increasing viral access to these target cells.7–9 Although standard antibiotic therapy for bacterial vaginosis rapidly reduces vaginal prototypic inflammatory cytokine IL-1α,10 treatment has been shown to increase vaginal concentrations of some chemoattractant immune factors linked to HIV risk, such as IFN-γ-induced protein (IP)-10,9,11 and so the immununological benefits of treatment remain uncertain.
In the absence of bacterial vaginosis, one of several species of Lactobacillus is typically predominant in the vaginal microbiome and has been linked to protection against HIV acquisition.3,12 For example, Lactobacillus crispatus predominance in the female genital tract has been linked to protection against HIV acquisition,7,13 most likely due to direct anti-inflammatory effects6,14 or the competitive exclusion of proinflammatory bacterial vaginosis-associated bacteria, or both.15 The standard of care for clinical bacterial vaginosis treatment involves oral or topical antibiotics targeting bacterial vaginosis-associated anaerobes, but recurrence rates are high16 and treatment does not always result in predominance of the protective L crispatus species.11 Although studies have investigated Lactobacillus spp-based probiotics or live biotherapeutics as alternative treatment strategies for bacterial vaginosis, none have linked probiotic use to reduced genital inflammation, which might be because of the use of probiotics containing Lactobacillus species that are not commonly predominant in the female genital tract.17–19 A phase 2 trial investigating the vaginal administration of a live biotherapeutic containing the L crispatus strain CTV-05 (LACTIN-V) following standard antibiotic treatment for bacterial vaginosis found that participants receiving LACTIN-V had lower rates of bacterial vaginosis recurrence than those receiving placebo, and these benefits were sustained for at least 3 months after treatment cessation.20
We investigated whether LACTIN-V administration has a sustained effect on genital immunology, and the associations between immune alterations and vaginal microbiota.
Methods
Study design and participants
This substudy included women aged 18–45 years who participated in the randomised, placebo-controlled, phase 2b trial20 of LACTIN-V to reduce bacterial vaginosis recurrence, conducted at four universities and hospitals in the USA. The trial evaluated the ability of LACTIN-V to prevent bacterial vaginosis recurrence following standard antibiotic treatment. At entry screening, the participants’ medical history was obtained, and physical and pelvic examinations were performed. Women with negative results for sexually transmitted infection (STI), pregnancy, and urinary tract infection (appendix p 1) were provided a 5-day course of vaginal metronidazole 0·75% gel. Within 48 h of completing treatment, participants returned to the clinic and those who met at least three of four clinical Amsel criteria for bacterial vaginosis and had a Nugent score of 4–10 from Gram staining (done at screening before antibiotic treatment) were eligible for the trial. The LACTIN-V trial (NCT02766023) obtained written consent from all participants before enrolment. Study protocols for the larger trial were approved by the institutional review boards (IRBs) at University of California, San Francisco General Hospital (San Francisco, CA, USA; IRB 15-18143), Stroger Hospital of Cook County (Chicago, IL, USA), University of California San Diego Antiviral Research Center (San Diego, CA, USA; IRB: 160023X), and Washington University Infectious Disease Clinical Research Unit (St Louis, MO, USA). Immunology and microbiology performed at the University of Toronto (Toronto, ON, Canada) were reviewed and approved by the University of Toronto HIV Research Ethics Board (protocol #36947).
Procedures
Participants in the LACTIN-V trial were randomly assigned (2:1) to receive either LACTIN-V or placebo, which were applied vaginally once per day for 5 days during the first week and then twice per week for 10 more weeks. Participants returned for follow-up visits at 4, 8, 12, and 24 weeks after enrolment. Vaginal swabs were collected at all visits (appendix p 1).
For the soluble immune factor measurement, cervico-vaginal fluid obtained from vaginal swabs was thawed and centrifuged at 4500 rpm for 30 min. Supernatant was removed for immune factor analysis and the bacterial pellet was left intact for quantitative PCR (qPCR) analyses. The soluble immune factors IL-1α, IFN-α2A, IL-17A, IL-6, IFN-γ, IP-10, IL-8, macrophage inflammatory protein (MIP)-1β, MIP-3α, monokine induced by IFN-γ (MIG), soluble E-cadherin, and matrix metalloproteinase (MMP)-9 were measured in duplicate with mutliplex ELISA on the Meso Scale Discovery platform (Meso Scale Diagnostics, Rockville, MD, USA).21
At the University of Toronto, DNA was extracted from 175 μL of bacterial pellet using the Qiagen DNEasy PowerSoil Kit (Qiagen, Valencia, CA, USA). Targeted qPCR was used to estimate the absolute abundance of key bacterial species. At the University of California, San Francisco, DNA was extracted from 200 μL of vaginal sample using the EZ1 DNA Tissue Kit (Qiagen, Valencia, CA, USA) and eluted into 50 μL volume. The absolute abundance of L crispatus and L crispatus CTV-05 were estimated with qPCR. Details on microbiome analyses are provided in the appendix (p 1).
Outcomes
The primary outcomes were the vaginal IL-1α and soluble E-cadherin concentrations at 24 weeks (ie, 13 weeks after treatment cessation). Exploratory outcomes included vaginal concentrations of soluble immune factors IP-10, IL-6, IL-8, MIP-1b, MIP-3a, MIG, and MMP-9, and the absolute abundance of key vaginal bacteria taxa including L crispatus, L crispatus strain CTV-05, Lactobacillus iners, Lactobacillus jensenii, Lactobacillus gasseri, Gardnerella vaginalis, Atopobium vaginae, Megasphaera species, and Prevotella species.
Statistical analysis
After visual inspection of distribution, soluble immune factor concentrations and bacteria copy numbers were normalised using log10 transformation. Linear regression was used to evaluate the association between treatment group as an independent variable and both the concentrations of soluble immune factors and abundance of key vaginal bacteria taxa at 24 weeks as dependent variables. Soluble immune factors that were less than the lower limit of detection in more than 50% of participants were dichotomised as detectable and undetectable. Binary logistic regression was used to evaluate the association between treatment group and the detectability of soluble immune factors at 24 weeks. For all regression models that predicted concentrations and the detectability of soluble immune factors or abundance of vaginal bacteria taxa, baseline measurements of the dependent variable were included in the model to control for interindividual variability. To evaluate potential mediating effects of bacterial taxa on the relationship between LACTIN-V and soluble immune factors, we followed the framework by Baron and Kenny22 outlined in the appendix (p 1). Mann-Whitney U tests were performed to compare the change in soluble immune factors from baseline to 24 weeks between women with vaginal predominance of non-CTV-05 L crispatus and those with predominance of L crispatus CTV-05. All statistical tests were performed with SPSS (version 270.0.0) or GraphPad Prism (version 9.0.2).
Role of the funding source
The funder of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
Results
Between Feb 21, 2020 and March 18, 2021, we characterised genital immune parameters and the vaginal microbiota in a subset of 66 highly adherent participants who were randomly selected, with no exclusion criteria, from those who had attended all study follow-up visits (n=166) in the larger LACTIN-V clinical trial (n=288);20 32 (48%) participants received LACTIN-V and 34 (52%) received placebo.
Baseline samples were missing from three participants who received placebo; immune and microbiota analyses (except for L crispatus CTV-05 quantitation, which had been performed earlier) were not performed on these samples. Baseline participant characteristics and sexual behaviours, including recent sex and hormonal contraceptive use did not substantially differ between groups (table 1). All participants were women, with a median age of 33 years (IQR 27–38). As per eligibility criteria, none of the women tested positive for HIV, syphilis, Neisseria gonorrhoea, Chlamydia trachomatis, or Trichomonas vaginalis at baseline. All participants reported the application of at least 75% of LACTIN-V doses, which was confirmed with trypan blue staining of applicators.20
Table 1:
Participant characteristics
| LACTIN-V (n=32) | Placebo (n=34) | |
|---|---|---|
| Sociodemographic factors | ||
|
| ||
| Median age, years | 32 (27–36) | 34 (27–38) |
| Ethnicity | ||
| Asian | 3 (9%) | 2 (6%) |
| Black or African American | 14 (44%) | 15 (44%) |
| Multiracial | 0 | 2 (6%) |
| White | 14 (44%) | 12 (35%) |
| Unknown | 1 (3%) | 3 (9%) |
|
| ||
| Sexual behaviours, intravaginal practices, and contraception | ||
|
| ||
| Vaginal sex within 30 days of enrolment | 24 (75%) | 30 (88%) |
| Vaginal sex between the 24-week visit and the previous visit | 21 (66%) | 27 (79%) |
| Douching or intravaginal practices at any time | 12 (38%) | 8 (24%) |
| Douching or intravaginal practices between the 24-week visit and the previous visit | 3 (9%) | 2 (6%) |
| Hormonal contraception use | 8 (25%) | 10 (29%) |
Data are median (IQR), n (range), or n (%). Data on age, race, and hormonal contraceptive use were collected at baseline, and all the other data were collected at baseline and the 24-week visit.
LACTIN-V treatment was significantly associated with lower concentrations of the proinflammatory cytokine IL-1α (β coefficient 0·310, SE 0·149; p=0·042) and soluble E-cadherin (0·429, 0·199; p=0·035), a biomarker of epithelial barrier disruption (figure 1). Among the six additional immune factors that were included as exploratory endpoints, LACTIN-V was associated with elevated concentrations of IP-10 at 24 weeks (−0·380, 0·185; p=0·044; figure 1). IFN-α2a and IL-17A were undetectable in more than 50% of samples, so were dichotomised as detectable or undetectable. Detectability of IFN-α2a or IL-17A was not significantly associated with the treatment group (appendix p 6).
Figure 1: Association between LACTIN-V treatment and vaginal soluble immune factors at 24 weeks.
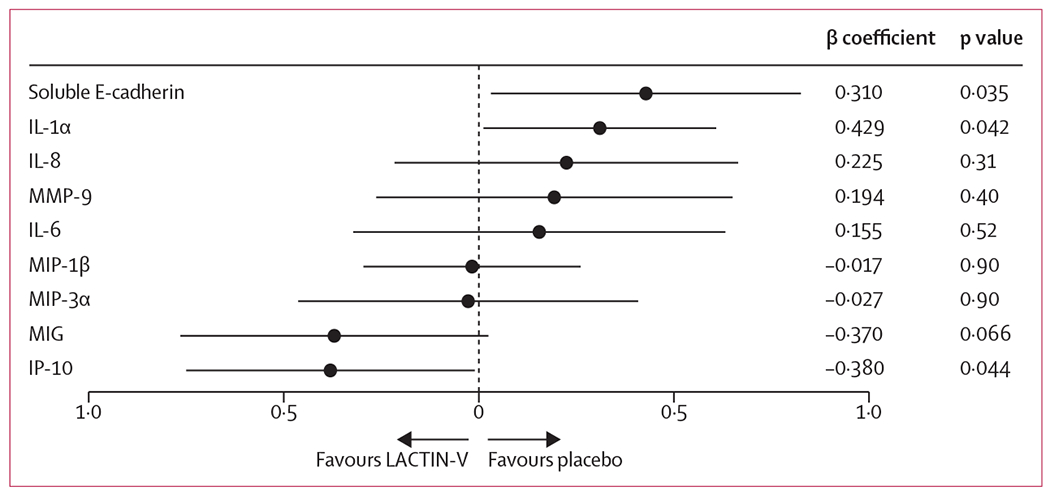
Data are β coefficient. Error bars are 95% CIs.
We hypothesised that an altered abundance of specific vaginal bacteria were causing the sustained differences between treatment groups in genital immune parameters. LACTIN-V treatment was associated with a sustained increase in the abundance of L crispatus (β −2·085, SE 0·649; p=0·0021) and L gasseri 13 weeks after treatment cessation (−0·803, 0·346; p=0·024; table 2). LACTIN-V treatment was also associated with a sustained reduction in the abundance of the bacterial vaginosis-associated genus Prevotella spp (1·033, 0·509; p=0·047) and borderline significance of reduced Megasphaera spp abundance (1·452, 0·731, p=0·052). L crispatus was more likely to be detected 13 weeks after product cessation among participants who received LACTIN-V (68·8% vs placebo 31·2%; p=0·0002) and the L crispatus strain CTV-05 was also present in those who received LACTIN-V (53·1% vs 2·9%; p<0·0001; appendix p 2). Among participants with previous LACTIN-V treatment and a high abundance of L crispatus (>1×106 copies per mL) at 24 weeks, the L crispatus strains present were predominantly non-CTV-05 in seven (41%) of 17 participants and predominantly CTV-05 in ten (59%; appendix p 4).
Table 2:
Association between the treatment group and absolute abundance of key bacterial taxa at 24 weeks
| β coefficient | SE | p value | |
|---|---|---|---|
| Lactobacillus crispatus * | −2·085 | 0·649 | 0·0021 |
| Lactobacillus iners | 0·383 | 0·449 | 0·397 |
| Lactobacillus gasseri * | −0·803 | 0·346 | 0·024 |
| Lactobacillus jensenii | −0·894 | 0·557 | 0·11 |
| Prevotella spp* | 1·033 | 0·509 | 0·047 |
| Gardnerella vaginalis | 0·996 | 0·543 | 0·072 |
| Megasphaera spp* | 1·452 | 0·731 | 0·052 |
| Atopobium vaginae | 0·633 | 0·727 | 0·39 |
Baseline measurements of bacteria absolute abundance (defined as log10 transformed copy numbers) were included in each model to control for interindividual variation.
Represents discrete models.
LACTIN-V administration was associated with the concentrations of soluble immune factors and abundances of multiple bacterial taxa. To explore potential causal pathways underpinning these relationships, we assessed the mediating effects of key bacterial taxa on the relationship between LACTIN-V treatment and concentrations of IL-1α, soluble E-cadherin, and IP-10 at 24 weeks. Change in IL-1α was positively associated with change in the abundance of Prevotella spp (β 0·179, SE 0·039; p<0·0001) and Megasphaera spp (0·085, 0·026; p=0·0016; table 3). Change in soluble E-cadherin was negatively associated with change in L crispatus (−0·128, 0·036; p=0·0007) and positively associated with change in Prevotella spp (0·338, 0·038; p<0·0001) and Megasphaera spp (0·157, 0·029; p<0·0001). Change in IP-10 was positively associated with change in L crispatus (0·108, 0·035; p=0·0029) and negatively associated with change in Prevotella spp (−0·161, 0·050, p=0·0022) and Megasphaera spp (−0·135, 0·029; p<0·0001).
Table 3:
Associations between the change in soluble immune factors and absolute abundance of key vaginal bacteria from baseline to 24 weeks
| Change in IL-1α | p value | Change in soluble E-cadherin | p value | Change in IP-10 | p value | |
|---|---|---|---|---|---|---|
| Univariable models | ||||||
|
| ||||||
| Change in Lactobacillus crispatus* | −0·050 (0·031) | 0·11 | −0·128 (0·036) | 0·0007 | 0·108 (0·035) | 0·0029 |
| Change in Lactobacillus gasseri | −0·011 (0·059) | 0·85 | −0·106 (0·074) | 0·16 | 0·129 (0·069) | 0·067 |
| Change in Prevotella spp* | 0·179 (0·039) | <0·0001 | 0·338 (0·038) | <0·0001 | −0·161 (0·050) | 0·0022 |
| Change in Megasphaera spp | 0·085 (0·026) | 0·0016 | 0·157 (0·029) | <0·0001 | −0·135 0·029 | <0·0001 |
|
| ||||||
| Multivariable models | ||||||
|
| ||||||
| Change in Lactobacillus crispatus* | −0·033 (0·032) | 0·30 | −0·114 (0·038) | 0·0037 | 0·096 (0·037) | 0·011 |
| LACTIN-V treatment group* | 0·333 (0·204) | 0·11 | 0·271 (0·243) | 0·27 | −0·235 (0·237) | 0·32 |
| Change in Prevotella spp | 0·167 (0·039) | <0·0001 | 0·327 (0·038) | <0·0001 | −0·148 (0·051) | 0·0051 |
| LACTIN-V treatment group | 0·254 (0·175) | 0·15 | 0·218 (0·169) | 0·20 | −0·307 (0·226) | 0·18 |
| Change in Megasphaera spp* | 0·075 (0·026) | 0·0058 | 0·148 (0·030) | <0·0001 | −0·128 (0·029) | <0·0001 |
| LACTIN-V treatment group* | 0·264 (0·189) | 0·17 | 0·235 (0·215) | 0·28 | −0·204 (0·213) | 0·34 |
Data are unstandardised β coefficients (SE). Change in log10 transformed copy number for all bacterial taxa and log10 transformed concentrations of soluble immune factors. Bacterial taxa only included if significantly associated with the treatment group and at least one of the soluble immune factors: IL-1α, E-cadherin, and IP-10.
Represents discrete models.
Next, we assessed whether genital immune changes associated with LACTIN-V were mediated through treatment-elicited alterations in vaginal bacteria. When change in IL-1α was the dependent variable, there was a significant association with change in Prevotella spp (β 0·167, SE 0·039; p<0·0001; table 3) and Megasphaera spp (0·075, 0·026, p=0·0058), but not with LACTIN-V treatment. Using change in soluble E-cadherin as the dependent variable, there were associations with change in L crispatus (−0·114, 0·038, p=0·0037), Prevotella spp (0·327, 0·038; p<0·0001), and Megasphaera spp (0·148, 0·030; p<0·0001), but not with LACTIN-V treatment. Similarly, change in IP-10 was not significantly associated with the treatment group, but was positively associated with change in L crispatus (0·096, 0·037; p=0·011) and negatively associated with change in Prevotella spp (−0·148, 0·051, p=0·0051) and Megasphaera spp (−0·128, 0·029; p<0·0001).
Finally, we investigated whether there was a differential immune effect of post-treatment vaginal colonisation with the CTV-05 strain of L crispatus. Of 21 selected participants with high vaginal abundance of L crispatus at 24 weeks, 14 (67%) showed CTV-05 strain predominance and seven (33%) showed non-CTV-05 strain predominance. Although predominance by CTV-05 or non-CTV-05 strains of L crispatus was non-significantly associated with similar reductions in IL-1α and soluble E-cadherin (p=0·74 for both), increases in vaginal IP-10 concentrations were significantly greater among participants with predominance of non-CTV-05 strains compared with predominance of the CTV-05 strain after LACTIN-V treatment (p=0·010; figure 2).
Figure 2: Change in soluble immune factors from baseline to 24 weeks.
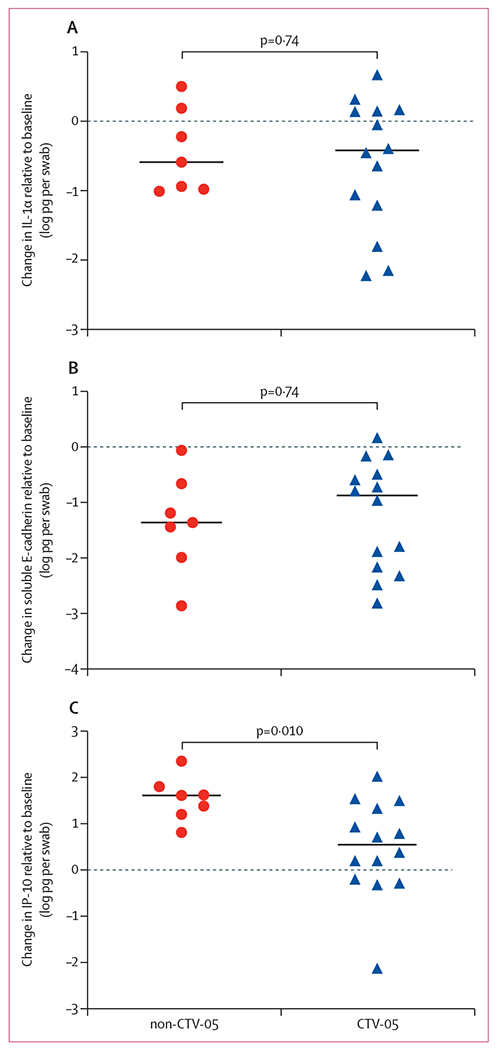
Horizonal line indicates median. For participants with high Lactobacillus crispatus abundance (>1 × 106 copies per mL), the change in vaginal IL-1α (A), soluble E-cadherin (B), and IP-10 (C) was compared between groups with sustained predominance by the L crispatus non-CTV-05 strain versus the LACTIN-V L crispatus CTV-05 strain.
Discussion
Vaginal application of the novel L crispatus-based live biotherapeutic LACTIN-V after bacterial vaginosis treatment with topical metronidazole reduces recurrence, with effects sustained for 3 months after the last dose of LACTIN-V.20 In this study, we show that LACTIN-V treatment was associated with similarly sustained reductions in genital mucosal inflammation and a biomarker of epithelial barrier disruption. Echoing findings from a study examining the immune effect of standard bacterial vaginosis therapy,11 secondary analysis showed an increase in vaginal concentrations of IP-10 (a chemoattractant chemokine linked to increased risk of HIV acquisition in women) after successful bacterial vaginosis treatment.9,23 These sustained genital immune effects were primarily mediated by a reduced abundance of bacterial vaginosis-associated taxa, particularly Prevotella spp and Megasphaera spp, and to a lesser extent by an elevated abundance of L crispatus. Increases in vaginal IP-10 were specifically linked to abundance of non-CTV-05 L crispatus strains, rather than to the therapeutic CTV-05 strain.
Rapid bacterial vaginosis recurrence following standard antibiotic treatment has generated substantial interest in alternative treatment strategies and antibiotic adjuncts.16 Among these strategies is the use of non-crispatus Lactobacillus probiotics or live biotherapeutics, either alone or in combination with standard antibiotics. However, not all probiotics have been shown to reduce rates of bacterial vaginosis recurrence,24 and the three studies that have evaluated genital immunology showed little or no effect of Lactobacillus probiotics,17,18 or an increase in proinflammatory cytokines following probiotic use.19 The present study is the first to show that application of an L crispatus-based live biotherapeutic after standard topical metronidazole therapy reduces concentrations of a proinflammatory genital cytokine and a marker of epithelial disruption. An important distinction between the present study and previous studies was the use of a live biotherapeutic comprised entirely of L crispatus, a species of Lactobacillus known to naturally predominate in the vagina,3 which has been associated with genital immune benefits and HIV protection among women.7,14,25
In a longitudinal cohort study,7 vaginal predominance by L crispatus, but not L iners, was associated with reduced risk of HIV acquisition compared with a bacterial vaginosis-type microbiota. Whether the HIV-protective effects of L crispatus are due to direct anti-inflammatory effects, the exclusion of bacterial vaginosis-associated bacteria, or other mechanisms is unclear. Vaginal predominance by L crispatus is associated with reduced vaginal concentrations of proinflammatory cytokines, including IL-1α,14 and our group has linked an increased L crispatus proportional abundance to reduced concentrations of genital proinflammatory cytokines and chemokines.25 Women with L crispatus predominance also exhibit fewer transitions to a bacterial vaginosis-type microbiota than women with L iners predominance, suggesting that L crispatus can competitively exclude inflammatory bacterial vaginosis-associated bacteria,26 mediated partly by the production of lactic acid and antimicrobial metabolites.27 In vitro models show that L crispatus is a key producer of lactic acid in the female genital tract,27 which might directly dampen the production of proinflammatory cytokines.28,29 Furthermore, addition of supernatant with L crispatus or L crispatus alone, or both, to epithelial cells decreased the production of proinflammatory cytokines relative to bacterial vaginosis-associated bacteria,6,14 and protected against epithelial barrier disruption in response to proinflammatory stimuli.6 Reduced genital inflammation following LACTIN-V could be mediated by the exclusion of bacterial vaginosis-associated bacteria, although our study showed that L crispatus might have direct but minor effects on concentrations of soluble E-cadherin and IP-10.
Observational studies have linked bacterial vaginosis with reduced vaginal concentrations of IP-10,10,25 suggesting that either the absence of bacterial vaginosis-associated bacteria or presence of Lactobacillus spp might elicit IP-10.10 Joag and colleagues11 showed that successful antibiotic bacterial vaginosis treatment resulting in an L iners predominant vaginal microbiome increased vaginal concentrations of several chemokines at 1 month, including IP-10 and MIG. In this study, we provide evidence that the reduced concentrations of soluble E-cadherin and elevated concentrations of IP-10 observed among participants receiving LACTIN-V were mediated partly by an elevated abundance of non-CTV-05 L crispatus strains. This finding suggests that L crispatus CTV-05 (LACTIN-V) might reduce genital inflammation and enhance epithelial barrier integrity in the same way as non-CTV-05 L crispatus, without eliciting an elevation in proinflammatory chemokines. Importantly, CTV-05 was detected in one participant who received placebo, probably because the CTV-05 strain found in LACTIN-V is derived from a naturally occurring vaginal strain of L crispatus.
There are several limitations of our study. First, STIs were routinely assessed at screening, but afterwards were only tested if women presented with symptoms. Therefore, we were unable to control for the presence of asymptomatic STIs during the study, which might affect genital immunology at follow-up visits. However, we would expect a low incidence of STIs because all participants were negative for STIs at trial screening, diagnostics were performed in the context of any genital symptoms, and the overall 6-month incidence of STIs is low in sexually active women in the USA.30 Second, the stage of the menstrual cycle was not recorded at each visit, except for menstruation. Although no study visits occurred during menstruation, this limitation reduced our ability to control for fluctuations in sex hormones, a potential determinant of genital immunology. Third, targeted qPCR rather than 16S rRNA gene sequencing or metagenomic sequencing was used to show the association of cervicovaginal IP-10 concentrations with non-CTV-05 L crispatus strains, limiting our ability to evaluate the vaginal microbiota and identify contributions by other microbes. Fourth, the study population consisted only of US American women, albeit a heterogeneous cohort in terms of ethnicity. Finally, the immunology analyses involved a subset of participants who had attended all trial visits in a larger clinical trial, which had strict eligibility criteria.20 Therefore, although we believe that these results reflect a causal relationship between LACTIN-V and genital immunology, they should be viewed as hypothesis generating. To ensure generalisability and definitively show causation, future studies are needed.
Strong links between the vaginal microbiota and HIV risk suggest that promoting vaginal predominance by L crispatus might reduce HIV risk. Treatment with LACTIN-V following standard antibiotics has been shown to reduce bacterial vaginosis recurrence compared with placebo, even at 3 months after the last dose.20 In this study, we show that LACTIN-V treatment was also associated with sustained reductions in biomarkers of genital inflammation and epithelial barrier damage, through the promotion of increased L crispatus abundance and decreased abundance of Prevotella species and Megasphaera species. Additionally, LACTIN-V was associated with elevated concentrations of the proinflammatory chemokine IP-10, although this finding appeared to be driven by non-CTV-05 L crispatus rather than L crispatus CTV-05. Future work is needed to determine whether LACTIN-V administration following standard antibiotics can reduce HIV acquisition among women at high risk.
Supplementary Material
Research in context.
Evidence before this study
We performed a PubMed search using the search terms “(“immun*” OR “inflammat*” OR HIV) AND (“bacterial vaginosis” AND “treat*” AND (probiotic OR “live biotherap*”)) NOT (review[Publication Type])” from database inception to July 16, 2021, which returned 56 results. Only studies written in English that addressed our hypothesis that bacterial vaginosis (BV) treatment with a Lactobacillus-based probiotic or live biotherapeutic might reduce genital inflammation, and by extension HIV risk, were included. Although many studies investigated the ability of Lactobacillus-based probiotics to treat BV, none used Lactobacillus crispatus and only three studies included analyses of the effect on genital immune factors. None of the studies found significant differences in proinflammatory cytokines or chemokines linked to HIV acquisition between women receiving probiotic and those receiving placebo. One study showed elevated vaginal IL-5 and upregulation of genes related to proinflammatory responses and epithelial barrier function among women who received the probiotic.
Added value of this study
Previous studies investigating the genital immune impact of BV treatment with a Lactobacillus-based probiotic or live biotherapeutic have only evaluated probiotics containing Lactobacillus species that are not typically predominant in the female genital tract and have not been linked to HIV protection in vivo. To our knowledge, this study is the first to examine the genital immune impact of a topical L crispatus-based live biotherapeutic (LACTIN-V) that has been shown to reduce BV recurrence compared with placebo in a large randomised controlled trial. By measuring the abundance of key vaginal bacterial taxa, we were able to explore potential mechanisms by which LACTIN-V modulates genital immunology. We showed a significant association between probiotic or live biotherapeutic use following standard BV treatment and genital immune correlates of HIV acquisition. Exploratory analyses suggest that these associations might be driven by elevated L crispatus abundance and reduced BV-associated anaerobe abundance.
Implications of all the available evidence
We showed that BV treatment with a Lactobacillus-based live biotherapeutic might confer lower genital immune correlates of HIV risk among women. Our results differ from previous studies, although these studies did not evaluate probiotics containing Lactobacillus species that are common in the female genital tract, such as L crispatus. BV treatment with LACTIN-V following standard antibiotic treatment might represent a novel HIV prevention strategy among women.
Acknowledgments
This study is funded by the Canadian Institutes of Health Research and the NIH National Institute of Allergy and Infectious Diseases. We thank all study participants for involvement in this study. We thank Marie-Christine Perry and Rachel Liu for technical assistance with qPCR assays. We also thank Emily Crawford and Gloria Castañeda for organising sample shipping and logistics.
Declaration of interests
CRC is chair of the scientific advisory board for Osel. BC reports grants from Western Foundation, Canadian Institutes of Health Research, Canadian Cancer Society, McLaughlin Foundation, National Cancer Institute, and Cystic Fibrosis Foundation; and material receipts for research from Nubiyota. CRC reports grants from the National Institutes of Health (NIH) National Institute of Allergy and Infectious Diseases and NIH National Institute of Child Health and Human Development (HHSN2722013000141 and HHSN27200007); and holds stock options in Osel and Evvy. RK reports a grant from Canadian Institutes of Health Research (#PJT-156123). All other authors declare no competing interests.
Footnotes
Data sharing
Data requests should be sent to the corresponding author. The full clinical trial protocol is available at https://www.clinicaltrials.gov/ct2/show/NCT02766023.
References
- 1.Low N, Chersich MF, Schmidlin K, et al. Intravaginal practices, bacterial vaginosis, and HIV infection in women: individual participant data meta-analysis. PLoS Med 2011; 8: e1000416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Atashili J, Poole C, Ndumbe PM, Adimora AA, Smith JS. Bacterial vaginosis and HIV acquisition: a meta-analysis of published studies. AIDS 2008; 22: 1493–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ravel J, Gajer P, Abdo Z, et al. Vaginal microbiome of reproductive-age women. Proc Natl Acad Sci USA 2011; 108 (suppl 1): 4680–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McKinnon LR, Achilles SL, Bradshaw CS, et al. The evolving facets of bacterial vaginosis: implications for HIV transmission. AIDS Res Hum Retroviruses 2019; 35: 219–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nold C, Anton L, Brown A, Elovitz M. Inflammation promotes a cytokine response and disrupts the cervical epithelial barrier: a possible mechanism of premature cervical remodeling and preterm birth. Am J Obstet Gynecol 2012; 206: 208.e1–7. [DOI] [PubMed] [Google Scholar]
- 6.Anton L, Sierra LJ, DeVine A, et al. Common cervicovaginal microbial supernatants alter cervical epithelial function: mechanisms by which Lactobacillus crispatus contributes to cervical health. Front Microbiol 2018; 9: 2181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gosmann C, Anahtar MN, Handley SA, et al. Lactobacillus-deficient cervicovaginal bacterial communities are associated with increased HIV acquisition in young South African women. Immunity 2017; 46: 29–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arnold KB, Burgener A, Birse K, et al. Increased levels of inflammatory cytokines in the female reproductive tract are associated with altered expression of proteases, mucosal barrier proteins, and an influx of HIV-susceptible target cells. Mucosal Immunol 2016; 9: 194–205. [DOI] [PubMed] [Google Scholar]
- 9.Masson L, Passmore JAS, Liebenberg LJ, et al. Genital inflammation and the risk of HIV acquisition in women. Clin Infect Dis 2015; 61: 260–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Masson L, Mlisana K, Little F, et al. Defining genital tract cytokine signatures of sexually transmitted infections and bacterial vaginosis in women at high risk of HIV infection: a cross-sectional study. Sex Transm Infect 2014; 90: 580–87. [DOI] [PubMed] [Google Scholar]
- 11.Joag V, Obila O, Gajer P, et al. Impact of standard bacterial vaginosis treatment on the genital microbiota, immune milieu, and ex vivo human immunodeficiency virus susceptibility. Clin Infect Dis 2019; 68: 1675–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McClelland RS, Lingappa JR, Srinivasan S, et al. Evaluation of the association between the concentrations of key vaginal bacteria and the increased risk of HIV acquisition in African women from five cohorts: a nested case-control study. Lancet Infect Dis 2018; 18: 554–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Srinivasan S, Richardson BA, Wallis J, et al. Vaginal microbiota and HIV acquisition risk among African women. Conference on Retroviruses and Opportunistic Infections; March 4–7, 2018. (abstr 268). [Google Scholar]
- 14.Anahtar MN, Byrne EH, Doherty KE, et al. Cervicovaginal bacteria are a major modulator of host inflammatory responses in the female genital tract. Immunity 2015; 42: 965–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ojala T, Kankainen M, Castro J, et al. Comparative genomics of Lactobacillus crispatus suggests novel mechanisms for the competitive exclusion of Gardnerella vaginalis. BMC Genomics 2014; 15: 1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bradshaw CS, Morton AN, Hocking J, et al. High recurrence rates of bacterial vaginosis over the course of 12 months after oral metronidazole therapy and factors associated with recurrence. J Infect Dis 2006; 193: 1478–86. [DOI] [PubMed] [Google Scholar]
- 17.Yang S, Reid G, Challis JRG, et al. Effect of oral probiotic Lactobacillus rhamnosus GR-1 and Lactobacillus reuteri RC-14 on the vaginal microbiota, cytokines and chemokines in pregnant women. Nutrients 2020; 12: 368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Happel AU, Singh R, Mitchev N, et al. Testing the regulatory framework in South Africa - a single-blind randomized pilot trial of commercial probiotic supplementation to standard therapy in women with bacterial vaginosis. BMC Infect Dis 2020; 20: 491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bisanz JE, Seney S, McMillan A, et al. A systems biology approach investigating the effect of probiotics on the vaginal microbiome and host responses in a double blind, placebo-controlled clinical trial of post-menopausal women. PLoS One 2014; 9: e104511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cohen CR, Wierzbicki MR, French AL, et al. Randomized trial of LACTIN-V to prevent recurrence of bacterial vaginosis. N Engl J Med 2020; 382: 1906–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mohammadi A, Bagherichimeh S, Perry MC, et al. The impact of cervical cytobrush sampling on cervico-vaginal immune parameters and microbiota relevant to HIV susceptibility. Sci Rep 2020; 10: 8514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Baron RM, Kenny DA. The moderator-mediator variable distinction in social psychological research: conceptual, strategic, and statistical considerations. J Pers Soc Psychol 1986; 51: 1173–82. [DOI] [PubMed] [Google Scholar]
- 23.Sabo MC, Lehman DA, Pintye JC, et al. Elevation of cervical C-etif chemokine ligand 10 levels is associated with HIV-1 acquisition in pregnant and postpartum women. AIDS 2020; 34: 1725–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang Z, He Y, Zheng Y. Probiotics for the treatment of bacterial vaginosis: a meta-analysis. Int J Environ Res Public Health 2019; 16: e3859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shannon B, Gajer P, Yi TJ, et al. Distinct effects of the cervicovaginal microbiota and herpes simplex type 2 infection on female genital tract immunology. J Infect Dis 2017; 215: 1366–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gajer P, Brotman RM, Bai G, et al. Temporal dynamics of the human vaginal microbiota. Sci Transl Med 2012; 4: 132ra52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Happel AU, Kullin B, Gamieldien H, et al. Exploring potential of vaginal Lactobacillus isolates from South African women for enhancing treatment for bacterial vaginosis. PLoS Pathog 2020; 16: e1008559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hearps AC, Tyssen D, Srbinovski D, et al. Vaginal lactic acid elicits an anti-inflammatory response from human cervicovaginal epithelial cells and inhibits production of pro-inflammatory mediators associated with HIV acquisition. Mucosal Immunol 2017; 10: 1480–90. [DOI] [PubMed] [Google Scholar]
- 29.Delgado-Diaz DJ, Tyssen D, Hayward JA, Gugasyan R, Hearps AC, Tachedjian G. Distinct immune responses elicited from cervicovaginal epithelial cells by lactic acid and short chain fatty acids associated with optimal and non-optimal vaginal microbiota. Front Cell Infect Microbiol 2020; 9: 446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kreisel KM, Spicknall IH, Gargano JW, et al. Sexually transmitted infections among US women and men: prevalence and incidence estimates, 2018. Sex Transm Dis 2021; 48: 208–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


