Abstract
Background
Nutrient acquisition and allocation integrate foraging and life-history traits in insects. To compensate for the lack of a particular nutrient at different life stages, insects may acquire these through supplementary feeding, for example, on vertebrate secretions, in a process known as puddling. The mosquito Anopheles arabiensis emerges undernourished, and as such, requires nutrients for both metabolism and reproduction. The purpose of this study was to assess whether An. arabiensis engage in puddling on cattle urine to obtain nutrients to improve life history traits.
Methods
To determine whether An. arabiensis are attracted to the odour of fresh, 24 h, 72 h and 168 h aged cattle urine, host-seeking and blood-fed (48 h post-blood meal) females were assayed in a Y-tube olfactometer, and gravid females assessed in an oviposition assay. Combined chemical and electrophysiological analyses were subsequently used to identify the bioactive compounds in all four age classes of cattle urine. Synthetic blends of bioactive compounds were evaluated in both Y-tube and field assays. To investigate the cattle urine, and its main nitrogenous compound, urea, as a potential supplementary diet for malaria vectors, feeding parameters and life history traits were measured. The proportion of female mosquitoes and the amount of cattle urine and urea imbibed, were assessed. Following feeding, females were evaluated for survival, tethered flight and reproduction.
Results
Host-seeking and blood-fed An. arabiensis were attracted to the natural and synthetic odour of fresh and aged cattle urine in both laboratory and field studies. Gravid females were indifferent in their response to cattle urine presence at oviposition sites. Host-seeking and blood-fed females actively imbibed cattle urine and urea, and allocated these resources according to life history trade-offs to flight, survival or reproduction, as a function of physiological state.
Conclusions
Anopheles arabiensis acquire and allocate cattle urine to improve life history traits. Supplementary feeding on cattle urine affects vectorial capacity directly by increasing daily survival and vector density, as well as indirectly by altering flight activity, and thus should be considered in future models.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12936-022-04179-6.
Background
Acquisition and allocation of nutrients integrate foraging and life-history traits in insects [1–3]. Insects are capable of selecting and acquiring diets, and of compensatory feeding, in response to food availability and need for nutrients [1, 3]. Allocation of nutrients is dependent on life-history processes, and may result in different needs of diet quality and quantity at different life stages of the insect [1, 2]. To compensate for the lack of a particular nutrient, insects may acquire these through supplementary feeding, for example on mud, various excrements and secretions of vertebrates, and carrion, in a process referred to as puddling [2]. Although mainly described for various butterfly and moth species, puddling also occurs in other insect orders, where attraction to and feeding on these types of resources has a significant effect on fitness and other life-history traits [2, 4–7]. The malaria mosquito, Anopheles gambiae sensu lato (s.l.), ecloses as an ‘undernourished’ adult [8], and, as such, puddling may play an important role for its life-history traits, but is a behaviour that so far has been overlooked. The inclusion of puddling as a means to enhance nutrient intake in this important vector requires attention, as this may have important epidemiological consequences.
Due to low caloric reserves carried over from the larval stage and a low efficiency of blood meal utilization [9], adult female malaria mosquitoes are limited in their nitrogen intake. Female An. gambiae s.l. often compensate for this by taking additional supplementary blood meals [10, 11], thereby putting more people at risk of contracting disease, and putting the mosquito at increased predation risk. Alternatively, mosquitoes could use supplementary feeding on vertebrate excretions to obtain nitrogenous compounds to enhance fitness and flight mobility, as demonstrated for other insects [2]. In this regard, the strong and differential attraction of one of the sibling species within the An. gambiae s.l. species complex, Anopheles arabiensis, to fresh and aging cattle urine [12–14], is intriguing. Anopheles arabiensis is opportunistic in its host preference and is well-known to associate with and feed on cattle. Cattle urine is a resource rich in nitrogenous compounds, with urea making up 50–95% of the total nitrogen in fresh urine [15, 16]. As cattle urine ages, microbes make use of these resources, thereby reducing the complexity of nitrogen-containing compounds within 24 h [15]. With the rapid increase in ammonia, correlating with the decline in organic nitrogen, alkalophilic microbes, many of which produce compounds toxic to mosquitoes, thrive [15], which may be a lead cause of why female An. arabiensis are preferentially attracted to urine aged for 24 h or less [13, 14].
In this study, host-seeking and blood-fed An. arabiensis, within their first gonotrophic cycle, were assessed as to whether they acquire nitrogenous compounds, including urea, through urine puddling. Next, a series of experiments were conducted to assess how female mosquitoes allocate this potential nutrient resource to enhance survival, reproduction and further foraging. Finally, the odour of fresh and aging cattle urine was assessed to determine whether these provide reliable cues for host-seeking and blood-fed An. arabiensis in their search for this potential nutrient resource, and the chemical correlates underlying the observed differential attraction identified. The synthetic odour blend of volatile organic compounds (VOCs) identified in 24 h aged urine was further evaluated under field conditions, expanding on the results obtained under laboratory conditions, and demonstrating the efficacy of cattle urine odour to attract mosquitoes of different physiological states. The obtained results confirm that An. arabiensis acquire and allocate nitrogenous compounds found in vertebrate urine to affect life history traits. These results are discussed in the context of potential epidemiological consequences and how these may be used towards vector monitoring and control.
Methods
Mosquito rearing
Anopheles arabiensis (Dongola strain) were maintained at 25 ± 2 °C, 65 ± 5% RH and at a 12:12 h light: dark cycle. Larvae were reared in plastic trays (20 cm × 18 cm × 7 cm), filled with distilled water, and fed on Tetramin® fish food (Tetra Werke, Melle, DE). Pupae were collected in 30 ml cups (Nolato Hertila, Åstorp, SE) and transferred to Bugdorm cages (30 cm × 30 cm × 30 cm; MegaView Science, Taichung, TW) for the adults to emerge. Adults were provided with 10% sucrose solution ad libitum until 4 days post-emergence (dpe), at which time host-seeking females were either provided with the diet immediately, or starved overnight with access to distilled water, prior to experiments, as described below. Females used for the flight tube experiments were only starved for 4–6 h with ad libitum access to water. To prepare blood-fed mosquitoes for subsequent bioassays, 4 dpe females were provided defibrinated sheep blood (Håtunalab, Bro, SE) using a membrane feeding system (Hemotek Discovery Workshops, Accrington, UK). Fully engorged females were subsequently transferred to separate cages and provided either a diet directly, as described below, or ad libitum access to 10% sucrose for 3 days, prior to the experiments described below. The latter females were used for the flight tube bioassays and were transferred to the experimental room, then starved with ad libitum access to distilled water 4–6 h prior to the experiments.
Quantification of urine and urea imbibed
Feeding assays were used to quantify the consumption of urine and urea by adult An. arabiensis females. Host-seeking and blood-fed females were provided with diets containing a 1% dilution of fresh and aged cattle urine, various concentrations of urea, as well as two controls, 10% sucrose and water, for 48 h. In addition, a food colourant (1 mg ml−1 xylene cyanole FF; CAS 2650-17-1; Sigma-Aldrich, Stockholm, SE), was added to the diet and provided in a 4 × 4 matrix of 250 µl microfuge tubes (Axygen Scientific, Union City, CA, US; Fig. 1A) filled to the rim (ca. 300 µl). To avoid competition among mosquitoes and the potential influence of the colour of the dye, ten mosquitoes were placed in large Petri dishes (12 cm diameter, 6 cm height; Semadeni, Ostermundigen, CH; Fig. 1A) in complete darkness at 25 ± 2 °C and 65 ± 5% RH. These experiments were replicated from 5 to 10 times. Following exposure to the diets, the mosquitoes were placed at − 20 °C until further analysis.
Fig. 1.
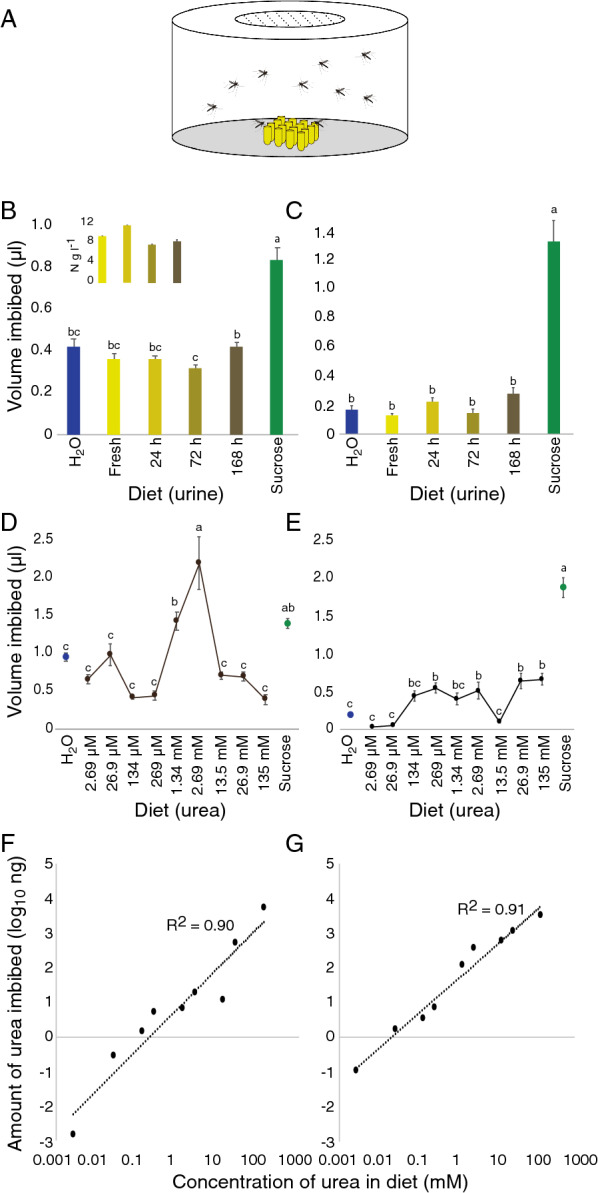
Cattle urine and urea imbibed by host-seeking and blood-fed female Anopheles arabiensis. Female mosquitoes were provided with diets consisting of fresh and aged cattle urine, various concentrations of urea, sucrose (10%) and distilled water (H2O) in a feeding assay (A). Host-seeking (B) and blood-fed (C) females imbibed larger volumes of sucrose than any of the other diets tested. Note that host-seeking females imbibed less 72 h aged cattle urine than 168 h aged cattle urine (B). The average total nitrogen content of the urine (± standard deviation) is represented in the inset. Urea was imbibed by host-seeking (D, F) and blood-fed (E, G) females in a dose-dependent manner. The mean volume imbibed (D, E) with different letter designations are significantly different from one another (one-way analysis of variance with a Tukey’s post hoc analysis; p < 0.05). Error bars represent the standard error of the mean (B–E). The straight dotted lines represent the log-linear regression lines (F, G)
To release the diet imbibed, mosquitoes were placed individually in 1.5 ml microfuge tubes containing 230 µl of distilled water, and the tissues disrupted using a disposable pestle and cordless motor (VWR International, Lund, SE), and then centrifuged at 10 krpm for 10 min. The supernatants (200 µl) were transferred to a 96-well microplate (Sigma-Aldrich) and the absorbance (λ620 nm) determined using a spectrophotometer-based microplate reader (SPECTROStar® Nano, BMG Labtech, Ortenberg, DE). Alternatively, the mosquitoes were ground in 1 ml of distilled water, 900 µl of which was transferred to a cuvette for spectrophotometric analysis (λ620 nm; UV 1800, Shimadzu, Kista, SE). To quantify the diet imbibed, a standard curve was prepared by a serial dilution resulting in a range of 0.2 µl to 2.4 µl of 1 mg ml−1 xylene cyanol. Then, the optical density of known dye concentrations was used to determine the volume of diet imbibed by each mosquito.
The volumetric data were analysed using a one-way analysis of variance (ANOVA) followed by a Tukey’s post hoc pairwise comparison (JMP Pro, v14.0.0, SAS Institute Inc., Cary, NC, US, 1989–2007). Linear regression analysis described the concentration dependent urea intake and comparisons were made between the responses of host-seeking and blood-fed mosquitoes (GraphPad Prism v8.0.0 for Mac, GraphPad Software, San Diego, CA, US).
Urine and nitrogen analysis
Approximately 20 µl of sample urine from each age category was bound on Chromosorb® W/AW (10 mg 80/100 mesh, Sigma Aldrich), and enclosed in tin capsules (8 mm × 5 mm). The capsule was inserted into a combustion chamber of a CHNS/O analyser (Flash 2000, Thermo Fisher Scientific, Waltham, MA, US) to determine the nitrogen content of fresh and aging urine, according to the manufacturer’s protocol. Total nitrogen (g N l−1) was quantified based on known concentrations of urea used as the standard.
Survival analysis
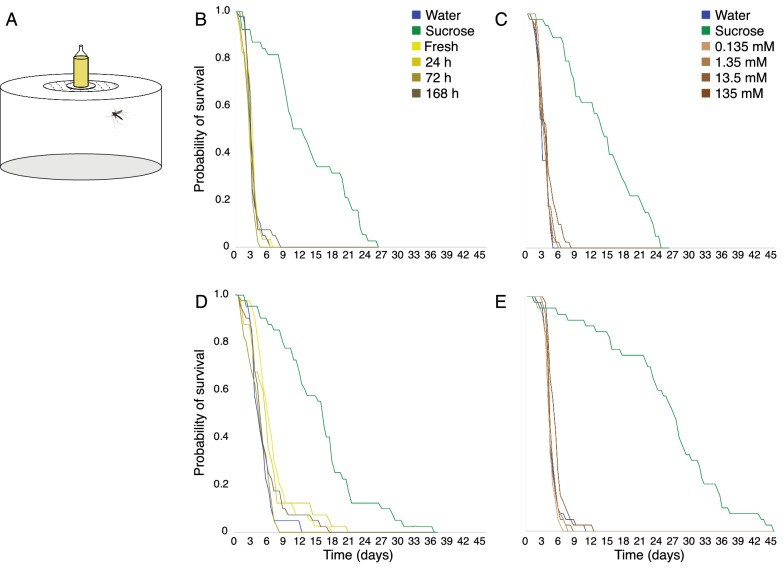
To assess the effect of diet on the survival of host-seeking and blood-fed females, mosquitoes were placed individually into large Petri dishes (diameter 12 cm, height 6 cm; Semadeni), with a mesh covered hole in the lid (3 cm diameter) for ventilation and diet provision. The diets, consisting of a 1% dilution of fresh and aged cattle urine, four concentrations of urea, as well as two controls, 10% sucrose and water, were provided directly after 4 dpe. Each diet was pipetted onto dental cotton rolls (DAB Dental AB, Upplands Väsby, SE) inserted into 5 ml syringes (Thermo Fisher Scientific, Gothenburg, SE), with the plunger removed, and then placed on top of the Petri dishes (Fig. 1A). The diets were replaced daily. The experimental room was maintained as described above. Surviving mosquitoes were counted twice daily, while discarding dead mosquitoes, until the final mosquito died (n = 40 per treatment). The survival of the mosquitoes feeding on the respective diets was analysed using Kaplan-Meyer survival curves and log rank test statistics for survival distribution comparison between diets (IBM SPSS Statistics 24.0.0.0).
Tethered flight assay
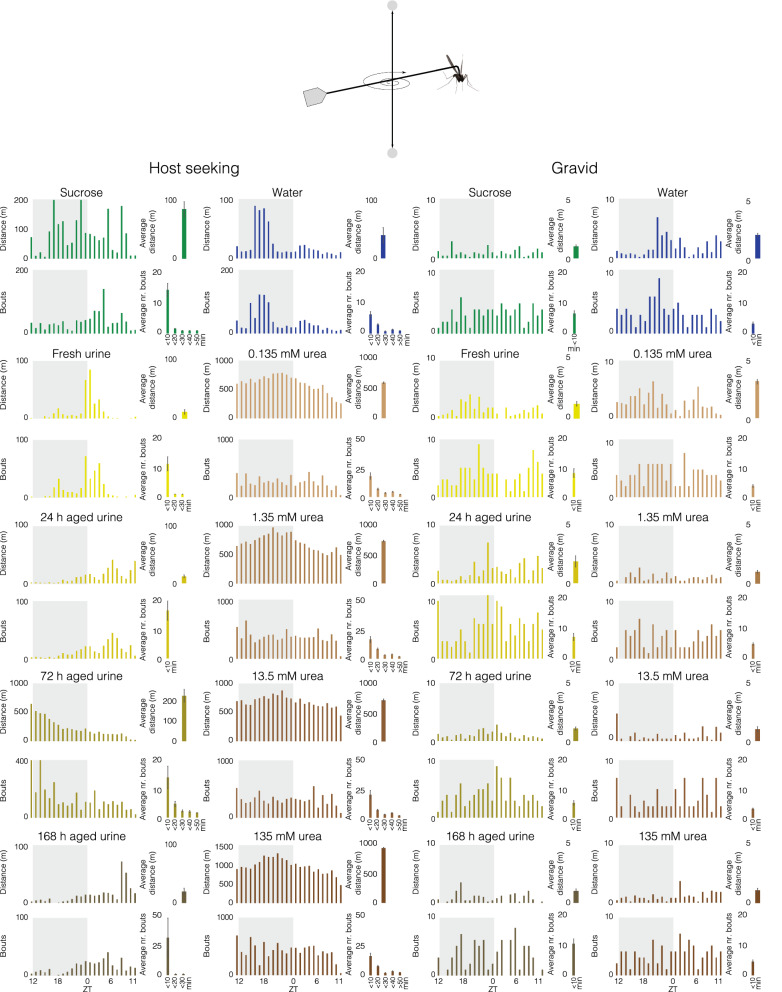
A custom-made mosquito flight-mill, based on Attisano et al. [17], was made from 5 mm thick clear acrylic panels (10 cm W × 10 cm L × 10 cm H) lacking front and back panels (Fig. 3: top). A pivot assembly, with a vertical tube constructed from a gas chromatography column (0.25 mm i.d; 7.5 cm L) glued to insect pins at both ends, was suspended between a pair of neodymium magnets, 9 cm apart. A horizontal tube made of the same material (6.5 cm L) bisected the vertical tube and created a tethering arm and an arm that carried a small piece of aluminium foil as a photo interruption signal.
Fig. 3.
Flight performance of host-seeking and blood-fed female Anopheles arabiensis fed on cattle urine and urea. Female mosquitoes fed on diets consisting of fresh and aged cattle urine, various concentrations of urea, sucrose (10%) and distilled water (H2O) were tethered to a horizontal, free-spinning arm in a flight mill assay (top). The overall distance and number of bouts flown per hour over 24 h (scotophase: grey; photophase: white) were recorded for each diet for both host-seeking (left) and blood-fed (right) females. The average distance and average number of bouts are shown to the right of the diurnal activity plots. Error bars represent the standard error of the mean. See the main text for statistical analysis
Prior to tethering, 24 h starved females, were provided access to the diets described above for 30 min. Fully fed female mosquitoes were then individually anaesthetized on ice for 2–3 min and glued onto an insect pin using bee’s wax (Joel Svenssons Vaxfabrik AB, Munka Ljungby, SE) on their mesothorax, and then tethered onto the arm of the horizontal tube of the flight mill. Each flight revolution was logged by a customized data logger, then stored and displayed using the PC-Lab 2000™ software (v4.01; Velleman, Gavere, BE). The flight mill was placed in a climate-conditioned room (12 h: 12 h, light: dark, 25 ± 2 °C, 65 ± 5% RH).
To visualize the pattern of flight activity, the overall distance flown (m) and the overall number of bouts of continuous flight activity were calculated each hour over the course of a 24 h period. In addition, the average distance flown by an individual female was compared among the various treatments and analysed using a one-way analysis of variance followed by a Tukey post hoc analysis (JMP Pro, v14.0.0, SAS Institute Inc.), in which the average distance was considered a dependent variable, while the treatments were the independent factors. Moreover, the average number of bouts was calculated in 10-min increments.
Reproductive performance
To assess the effect of diet on the reproductive performance of An. arabiensis, six females (4 dpe) were transferred to Bugdorm cages (30 cm × 30 cm × 30 cm) directly after blood feeding, and then provided with experimental diets, as described above, for 48 h. Diets were then removed and oviposition cups (30 ml; Nolato Hertila), filled with 20 ml distilled water, were provided on the third day and made available for 48 h, replacing the cups every 24 h. Each diet regime was replicated 20–50 times. The eggs were counted and recorded for each experimental cage. A subsample of eggs was used to assess the average size and variation among the lengths of individual eggs (n ≥ 200 per diet) using a Dialux-20 microscope (DM1000; Ernst Leitz Wetzlar, Wetzlar, DE) equipped with a Leica camera (DFC 320 R2; Leica Microsystem Ltd, DE). The remaining eggs were maintained in a climate-controlled chamber under standard rearing conditions for 24 h, and a subsample of recently emerged 1st instar larvae (n ≥ 200 per diet) were measured, as above. The number of eggs, as well as the size of both eggs and larvae, were compared among the various treatments and analysed using a one-way analysis of variance followed by a Tukey post hoc analysis (JMP Pro, v14.0.0, SAS Institute Inc.).
Headspace volatile collections from fresh and aged cattle urine
Headspace volatiles from fresh (1 h post-sampling), 24 h, 72 h and 168 h aged urine were collected from samples collected from Zebu cattle, Arsi race. For convenience and availability, the urine sample collections were carried out early in the morning while the cattle were still in the shed. Urine samples were collected from ten individuals, with 100–200 ml of each sample transferred into separate polyamide roasting bags (Toppits Cofresco, Frischhalteprodukte GmbH and Co., Minden, DE) placed inside a 3 l polyvinylchloride plastic bucket with a lid. Headspace volatiles from each individual cattle urine sample were either collected directly (fresh) or following maturation for 24 h, 72 h and 168 h at room temperature, i.e., each urine sample was represented in each of the age groups.
For the headspace volatile collection, a closed loop system was used, by circulating an activated charcoal-filtered airstream (100 ml min−1) through the polyamide bag onto an adsorbent column, using a diaphragm vacuum pump (KNF Neuberger, Freiburg, DE), for 2.5 h. As a control, headspace collection from an empty polyamide bag was performed. The adsorbent column was made of Teflon tubing (5.5 cm × 3 mm i.d.) holding 35 mg Porapak Q (50/80 mesh; Waters Associates, Milford, MA, US) between glass wool plugs. The columns were rinsed with 1 ml re-distilled n-hexane (Merck, Darmstadt, DE) and 1 ml pentane (99.0% pure solvent GC grade, Sigma Aldrich) before use. Adsorbed volatiles were eluted with 400 μl pentane. Headspace collections were pooled and then stored at -20 °C until used for further analyses.
Attraction of Anopheles arabiensis to fresh and aged cattle urine odour
Behavioural responses of host-seeking and blood-fed An. arabiensis mosquitoes to the headspace volatile extracts collected from fresh, 24 h, 72 h and 168 h aged urine were analysed using a straight glass tube olfactometer [18]. The experiments were conducted during the peak host-seeking activity period, ZT 13 -15, of An. arabiensis [19]. The glass tube olfactometer (80 cm × 9.5 cm i.d.) was illuminated with red light from above at 3 ± 1 lx. A charcoal-filtered and humidified air stream (25 ± 2 °C, 65 ± 2% relative humidity) passed through the bioassay at 30 cm s−1. The air passed through a series of stainless-steel mesh screens to generate a laminar flow and a homogenous plume structure. Dental cotton roll dispensers (4 cm × 1 cm; L:D; DAB Dental AB), suspended from a 5 cm wire coil at the upwind end of the olfactometer, were used and the stimulus replaced every 5 min. For the analysis, 10 μl of each headspace extract, at a 1:10 dilution, was used as the stimulus. An equivalent amount of pentane was used as a control. Individual host-seeking or blood-fed mosquitoes were placed in separate release cages 2–3 h before the onset of the experiments. The release cage was placed at the down-wind end of the olfactometer, and mosquitoes were allowed 1 min to acclimatize before the butterfly valve of the cage was opened for their release. Attraction to either treatment or control was analysed as the proportion of mosquitoes that made source contact within 5 min after release. Each headspace volatile extract and control was replicated at least 30 times, and to avoid any day effect, the same number of treatments and controls were tested on each experimental day. Responses of host-seeking and blood-fed An. arabiensis to the headspace collections were analysed using a nominal logistic regression followed by pairwise comparisons of the odd’s ratios (JMP Pro, v14.0.0, SAS Institute Inc.).
Oviposition of Anopheles arabiensis in response to fresh and aged cattle urine odour
The oviposition response of An. arabiensis to the headspace extracts of fresh and aged cattle urine was analysed in Bugdorm cages (30 cm × 30 cm × 30 cm; MegaView Science). Plastic cups (30 ml; Nolato Hertila) filled with 20 ml distilled water provided the oviposition substrate, and were placed in opposite corners of the cage, 24 cm apart. The treatment cup was conditioned with 10 μl of each headspace extract, at a 1:10 dilution. An equivalent amount of pentane was used to condition the control cup. Treatment and control cups were exchanged in between each experiment to control for location effects. Ten blood-fed females were released into the experimental cages at ZT 9–11, and the number of eggs in the cups were counted after 24 h. An oviposition index was calculated by: (number of eggs laid in treatment cups – number of eggs laid in the control cups)/(total number of eggs). Each treatment was replicated 8 times.
Combined gas chromatography and electroantennographic detection (GC-EAD)
Combined gas chromatography and electroantennographic detection (GC-EAD) analyses of female An. arabiensis were performed as previously described [20]. Briefly, an Agilent Technologies 6890 GC (Santa Clara, CA, US), equipped with an HP-5 column (30 m × 0.25 mm i.d., 0.25 μm film thickness, Agilent Technologies), was used to separate the headspace volatile extracts of fresh and aged urine. Hydrogen was used as the mobile phase at an average linear flow rate of 45 cm s−1. Each sample (2 μl) was injected in splitless mode, for 30 s, at an injector temperature of 225 °C. The GC oven temperature was programmed from 35 °C (3 min hold) at 10 °C min−1 to 300 °C (10 min hold). At the GC effluent splitter, 4 psi of nitrogen was added and split 1:1 in a Gerstel 3D/2 low dead volume four-way cross (Gerstel, Mülheim, DE) between the flame ionization detector and the EAD. The GC effluent capillary for the EAD passed through a Gerstel ODP-2 transfer line, which tracked the GC oven temperature plus 5 °C, into a glass tube (10 cm × 8 mm), where it was mixed with charcoal-filtered, humidified air (1.5 l min−1). The antenna was placed 0.5 cm from the outlet of this tube. Each individual mosquito accounted for a single replicate, and at least three replicates were performed for each age of the urine samples, for host-seeking mosquitoes.
Chemical analysis
Bioactive compounds in the headspace collections of fresh and aged cattle urine, eliciting an antennal response in the GC-EAD analyses, were identified using a combined GC- and mass spectrometer (GC–MS; 6890 GC and 5975 MS; Agilent Technologies), operated in the electron impact ionization mode at 70 eV. The GC was equipped with an HP-5MS UI coated fused silica capillary column (60 m × 0.25 mm i.d., 0.25 μm film thickness), and helium was used as the mobile phase at an average linear flow rate of 35 cm s−1. A 2 μl sample was injected using the same injector settings and oven temperatures as for the GC-EAD analysis. Compounds were identified according to their retention times (Kovát’s indices) and mass spectra, in comparison with custom-made and NIST14 libraries (Agilent). Identified compounds were confirmed by the injection of authentic standards (Additional file 1: Table S2). For quantification, heptyl acetate (10 ng, 99.8% chemical purity, Aldrich) was injected as an external standard.
Behavioural assays with synthetic odour blends
To assess the efficacy of the synthetic odour blends, composed of the bioactive compounds identified in fresh and aged urine, to attract host-seeking and blood-fed An. arabiensis, the same olfactometer and protocol were used as described above. The synthetic blends mimicked the composition and ratio of compounds in the pooled headspace volatile extracts of fresh, 24 h, 48 h, 72 h and 168 h aged urine (Fig. 5D–G; Additional file 1: Table S2). For the analysis, 10 μl of a 1:100 dilution of the full synthetic blends, at overall release rates ranging from approximately 140–2400 ng h−1, were used to assess attraction of host-seeking and blood-fed mosquitoes. Thereafter, subtractive blends, in which single compounds of the full blend were removed, were tested against the full blend. Responses of host-seeking and blood-fed An. arabiensis to the synthetic and subtractive blends were analysed using a nominal logistic regression followed by pairwise comparisons of the odd’s ratios (JMP Pro, v14.0.0, SAS Institute Inc.).
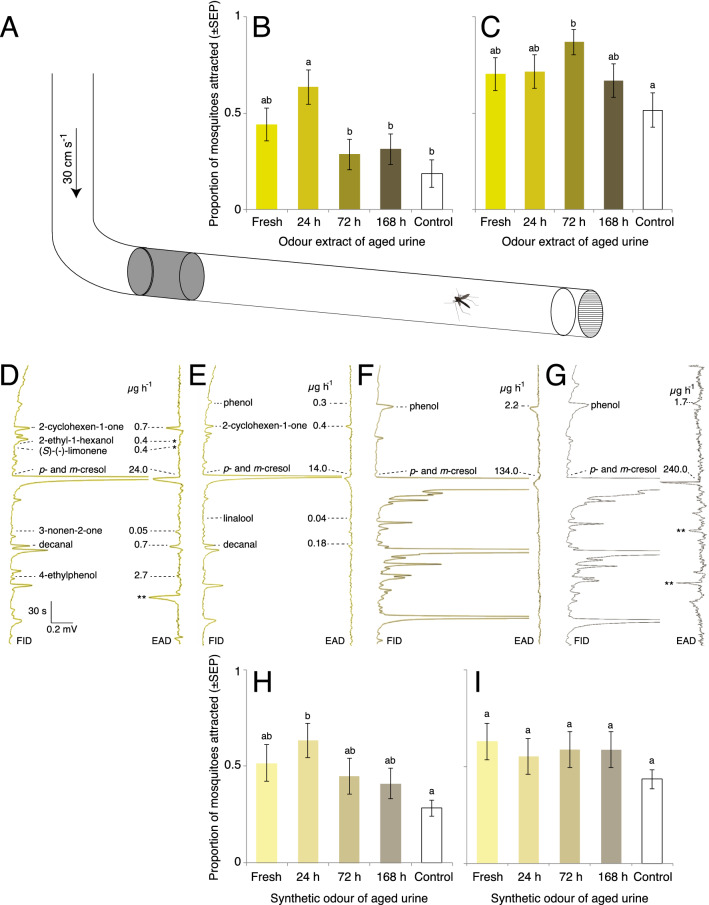
Fig. 5.
Behavioural response of host-seeking and blood-fed Anopheles arabiensis to natural and synthetic cattle urine odour. Diagram of the glass tube olfactometer (A). Attraction to the headspace volatile extracts of fresh and aged cattle urine of host-seeking (B) and blood-fed (C) mosquitoes. The antennal responses of host-seeking An. arabiensis to fractioned headspace extracts from fresh (D), 24 h (E), 72 h (F) and 168 h (G) aged cattle urine are shown. Electroantennographic detection (EAD) traces show voltage changes in response to the bioactive compounds in the headspace eluting from the gas chromatograph and detected by the flame ionization detector (FID). Scale bar indicates the amplitude of response (mV) versus the retention time (s). The identity and release rate (µg h−1) of the bioactive compounds are indicated. A single asterisk (*) indicates consistent low amplitude responses. Double asterisks (**) indicate irreproducible responses. Host-seeking (H) and blood-fed (I) An. arabiensis are differentially attracted to the synthetic blends of fresh and aged cattle urine odour. The mean proportion of mosquitoes attracted with different letter designations are significantly different from one another (one-way analysis of variance with a Tukey’s post hoc analysis; p < 0.05). Error bars indicate the standard error of the proportion
Assessment of cattle urine odour as a host habitat cue
To assess whether cattle urine serves as a host habitat cue for malaria mosquitoes, fresh and aged cattle urine, collected as above, as well as water, were placed into mesh-covered 3 l buckets (100 ml), with side perforations, and set on top of host decoy traps (BG-HDT version; BioGents, Regensburg, DE). The ten traps were placed 50 m apart in a pasture, separated 400 m away from a village community (Sile, Ethiopia, 5°53´24´´N, 37°29´24´´E) and devoid of cattle, situated between the permanent breeding site and the village. Five traps were heated to simulate the presence of a host, while five traps remained unheated. The position of each treatment was rotated nightly for a total of five nights. Comparisons among the number of mosquitoes caught in traps baited with different ages of the urine were made using logistic regression with a beta binomial distribution (JMP Pro, v14.0.0, SAS Institute Inc.).
Field evaluation of synthetic cattle urine odour
The efficacy of the synthetic 24 h cattle urine odour blend to attract wild mosquitoes in the field was assessed in a malaria-endemic village nearby the town of Meki in the Oromia region of Ethiopia (8° 11′ 08″ N, 38° 81′ 70″ E; Fig. 6A). The study was conducted between mid-August to mid-September prior to the annual indoor residual spraying, in conjunction with the long rainy season. Five pairs of houses (20–50 m apart), located in the periphery of the village were selected for the study (Fig. 6A). The criteria used to select the houses were: no animals were allowed to be kept inside the houses, no cooking (smoking firewood or charcoal) was allowed indoors (at least during the trial period), and houses with a maximum of two inhabitants, sleeping under a non-insecticide treated bed nets. Ethical approval was obtained from the Institutional Research Ethics Review Board, College of Natural Sciences, (CNS-IRB), Addis Ababa University (IRB/022/2016), according to the guidelines set out by the World Medical Association Declaration of Helsinki. Consent from each household head was obtained with assistance of health extension workers. The whole process was endorsed by the local administration at district and ward (´kebele´) level. The experimental design followed a 2 × 2 Latin square design, in which the synthetic blend and control were assigned to paired houses at the first night and exchanged between the houses on the next experimental night. This procedure was replicated ten times. In addition, to estimate the activity of mosquitoes in the selected houses, CDC traps, without synthetic blend dispensers, were set to operate during the same hours of the day, at the beginning, middle and end of the field trials for five nights.
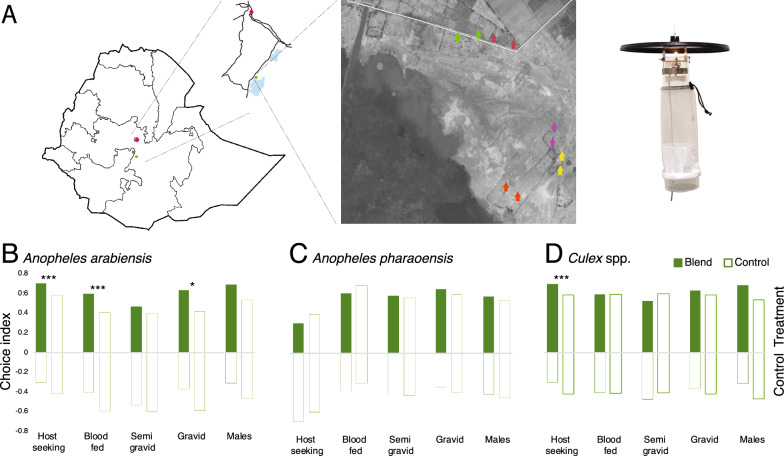
Fig. 6.
Field evaluation of the efficacy of the 24 h synthetic cattle urine odour blend. The field trials were carried out in south central Ethiopia (map), nearby the town of Meki (insert), using Centers of Disease Control (CDC) light traps (right) in paired houses using a Latin square design (aerial map) (A). CDC light traps baited with the synthetic odour differentially attracted and captured female Anopheles arabiensis (B), but not Anopheles pharoensis (C), an affect that was dependent on physiological state. In addition, the traps caught significantly higher numbers of host-seeking Culex spp. (D) compared to the controls. Bars on the left represent the average choice indices of mosquitoes caught in paired odour baited (green) and control (open) traps (N = 10), whereas bars on the right represent the choice indices of mosquitoes caught in paired control traps (open; N = 5). Asterisks denote the level of statistical significance (*p = 0.01 and ***p < 0.0001)
The synthetic blend, containing the six bioactive compounds in their natural ratio (7:9:156:156:1:4; Fig. 5D–G; Additional file 1: Table S2) was dissolved in heptane (97.0% solvent GC grade, Sigma Aldrich), and released at 140 ng h−1 using cotton wick dispensers [20]. The wick dispensers allow for the release of all compounds in constant proportions throughout the 12 h experiment. Heptane was used as a control. The vials were suspended next to the entrance point of a Center for Disease Control and Prevention (CDC) light trap (John W. Hock Company, Gainesville, FL, US; Fig. 6A). The traps were suspended 0.8 – 1 m above the ground next to the foot side of a bed with a volunteer sleeping under an untreated bed net and operated between 18h00 and 06h30. Caught mosquitoes were sorted by sex and physiological state (unfed, fed, semi-gravid and gravid [21]. Subsequently, the mosquitoes were identified morphologically to species [9, 22] and placed in 1.5 ml microfuge tubes with dry silica gel. Five per cent of the mosquitoes that were morphologically identified as An. gambiae s.l. were subsequently screened using polymerase chain reaction (PCR) analysis to identify the member of the species complex [23]. To assess the effect of treatment to that of the control in the field studies, trap captures of the paired houses were analysed using a nominal logistic fit model, in which attraction was the dependent variable and treatment (synthetic blend vs. control) the fixed effect (JMP® 14.0.0. SAS Institute Inc.). Here, we report the χ2 and p-value from the Likelihood Ratio Test.
Results
Host-seeking and blood-fed mosquitoes feed on urine and urea
To assess whether An. arabiensis are able to acquire urine, and its main source of nitrogen, urea, through direct feeding, 4-day post-emergence (dpe) host-seeking and blood-fed females were given access to these diets over a period of 48 h in a feeding assay (Fig. 1A). Both host-seeking and blood-fed females imbibed significantly larger volumes of sucrose than any of the other diets or water (F(5,426) = 20.15, p < 0.0001 and F(5,299) = 56.00, p < 0.0001, respectively; Fig. 1B, C). In addition, host-seeking females fed less on 72 h aged urine compared with 168 h aged urine (Fig. 1B). When provided with diets containing urea, host-seeking females imbibed a significantly larger volume of 2.69 mM urea compared with all other concentrations and water, while not differing from 10% sucrose (F(10,813) = 15.72, p < 0.0001; Fig. 1D). This differed from the response of blood-fed females, which generally imbibed significantly larger volumes of urea-containing diets compared to water, although imbibing significantly smaller volumes compared to 10% sucrose (F(10,557) = 78.35, p < 0.0001; Fig. 1E). Moreover, when comparing between the two physiological states, blood-fed females imbibed more urea at the lowest concentration than their host-seeking counterparts, while these females imbibed similar amounts at higher concentrations (F(1,953) = 78.82, p < 0.0001; Fig. 1F, G). While the volume of intake of the urea-containing diets appeared to have an optimum (Fig. 1D, E), females of both physiological states were able to regulate the amount of urea imbibed over the full range of urea concentrations in a log-linear fashion (Fig. 1F, G). Similarly, mosquitoes appear to control their intake of nitrogen by regulating the volume of urine imbibed, as the amount of nitrogen in the urine is reflected in the volume imbibed (Fig. 1B, C and B inset).
Urine and urea affect the survival of malaria mosquitoes
To assess the role of urine and urea on the survival of host-seeking and blood-fed mosquitoes, females were fed on all four ages of urine (fresh, 24 h, 72 h and 168 h post-deposition) and a range of urea concentrations, as well as on distilled water and 10% sucrose as controls (Fig. 2A). This survival analysis revealed that diet had a significant impact on the overall survival rate of host-seeking females (urine: χ2 = 108.5, df = 5, p < 0.0001; urea: χ2 = 122.8, df = 5, p < 0.0001; Fig. 2B, C) and blood-fed females (urine: χ2 = 93.0, df = 5, p < 0.0001; urea: χ2 = 137.9, df = 5, p < 0.0001; Fig. 2D, E). In all experiments, females feeding on urine, urea and water had a significantly reduced survival compared to those provided with sucrose as a diet (Fig. 2B–E). Host-seeking females feeding on fresh and aged urine exhibited differential survival, with those feeding on 72 h aged urine (p = 0.016) having the lowest probability of survival (Fig. 2B). Moreover, host-seeking females fed on 135 mM urea survived longer than on the water control (p < 0.04) (Fig. 2C). Blood-fed females survived longer when fed on fresh and 24 h aged urine compared with water (p = 0.001 and p = 0.012, respectively; Fig. 2D), while those fed on 72 h aged urine survived for a shorter time than those fed on fresh and 24 h aged urine (p < 0.0001 and p = 0.013, respectively; Fig. 2D). When fed on 135 mM urea, blood-fed females survived longer than all other concentrations of urea and water (p < 0.013; Fig. 2E).
Fig. 2.
Survival of host-seeking and blood-fed female Anopheles arabiensis fed on cattle urine and urea. Female mosquitoes were provided with diets consisting of fresh and aged cattle urine, various concentrations of urea, sucrose (10%) and distilled water (H2O) in a bioassay (A). The survival of individual host-seeking (B, C) and blood-fed (D, E) mosquitoes was recorded every 12 h, until all females fed on urine (B, D) and urea (C, E), as well as the controls, sucrose and water, had died
Flight behaviour is affected by urine and urea diet
The overall distance and number of bouts, as determined in a flight mill assay over a 24 h period, differed between host-seeking and blood-fed mosquitoes, with blood-fed mosquitoes displaying less flight activity overall (Fig. 3). Host-seeking mosquitoes provided with fresh and aged urine, or sucrose and water, displayed varying flight patterns (Fig. 3), with females fed on fresh urine being more active at dawn, while those fed on 24 h and 168 h aged urine displaying predominantly daytime activity. Female mosquitoes provided with either sucrose or 72 h aged urine demonstrated activity throughout the 24 h period, whereas those provided with water were more active during mid-scotophase. Mosquitoes fed on sucrose demonstrated the highest levels of activity during the late night and early morning, while those that imbibed 72 h aged urine decreased activity steadily over the 24 h (Fig. 3).
In general, the overall bouts of flight activity by host-seeking females followed a similar pattern to that of the distance flown over the 24 h period. The diet imbibed significantly affected the average distance flown (F(5, 138) = 28.27, p < 0.0001), with host-seeking females having imbibed 72 h aged urine flying significantly longer distances than all other diets (p < 0.0001), and sucrose-fed mosquitoes flying longer distances than those fed on fresh (p = 0.022) and 24 h aged urine (p = 0.022). In contrast to the flight activity patterns described for the urine diets, host-seeking females fed on urea demonstrated continuous flight activity over the course of the 24 h period with a peak of activity during the second half of scotophase (Fig. 3). While the pattern of activity was similar, host-seeking females fed on urea significantly increased the average distance flown depending on the concentration imbibed (F(5, 138) = 1310.91, p < 0.0001). Host-seeking females feeding on any concentration of urea tested flew longer distances than those fed on water or sucrose (p < 0.03).
The overall flight activity of blood-fed mosquitoes was stable and continuous over the 24 h period for all diets, with an increase in activity in the latter half of the scotophase for females fed on water as well as those fed on fresh and 24 h aged urine (Fig. 3). While the urine diet significantly affected the average distance flown by blood-fed females (F(5, 138) = 4.83, p = 0.0004), urea diets had no discernible effect (F(5, 138) = 1.36, p = 0.24). Only blood-fed females fed on 24 h aged urine displayed an increased average flight distance compared to the other urine and control diets (fresh, p = 0.0091; 72 h, p = 0.0022; 168 h, p = 0.001; sucrose, p = 0.0017; dH2O, p = 0.036).
Urine and urea affect reproductive parameters
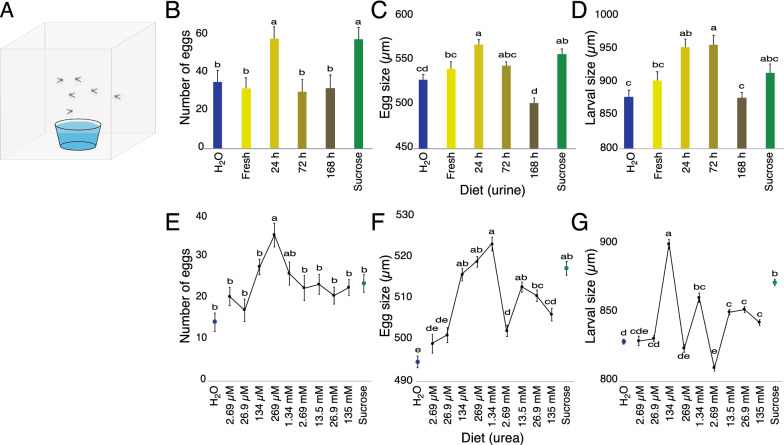
The effect of urine and urea feeding on reproductive parameters were assessed in an oviposition bioassay (Fig. 4A) and investigated in terms of the number of eggs laid per female, as well as the size of the eggs and the newly hatched first instar larvae. The number of eggs laid by An. arabiensis females fed on urine varied with diet (F(5, 222) = 4.38, p = 0.0008; Fig. 4B). Females fed on 24 h aged urine, post-blood meal, laid significantly more eggs than when fed on other urine diets, and similar to that laid by those fed on sucrose (Fig. 4B). Similarly, the size of eggs laid by females fed on urine differed based on diet (F(5, 209) = 12.85, p < 0.0001), with females fed on 24 h aged urine and sucrose laying significantly larger eggs than those fed on water, while eggs from females fed on 168 h aged urine were significantly smaller (Fig. 4C). Moreover, the urine diets significantly affected larval size (F(5, 187) = 7.86, p < 0.0001), with significantly larger larvae emerging from eggs laid by females that fed on 24 h and 72 h aged urine than those from the eggs of water-fed and 168 h aged urine-fed females (Fig. 4D).
Fig. 4.
Reproductive performance of female Anopheles arabiensis fed on cattle urine and urea. Blood-fed female mosquitoes fed on diets consisting of fresh and aged cattle urine, various concentrations of urea, sucrose (10%) and distilled water (H2O) over a period of 48 h, and then placed in a bioassay with access to an oviposition substrate for 48 h (A). The number of eggs (B, E), size of eggs (C, F) and size of larvae (D, G) were significantly affected by the diet provided (cattle urine: B–D; urea: E–G). The mean for each parameter measured with different letter designations are significantly different from one another (one-way analysis of variance with a Tukey’s post hoc analysis; p < 0.05). Error bars represent the standard error of the mean
As the primary nitrogenous component of urine, urea, when offered as a diet to blood-fed females, differentially and significantly affected all of the reproductive parameters studied. The number of eggs laid by females fed on urea, post-blood meal, differed depending on the concentration of urea (F(11, 360) = 4.69; p < 0.0001), with females fed on urea concentrations between 134 µM and 1.34 mM laying more eggs (Fig. 4E). Females fed on concentrations of urea at or above 134 µM laid larger eggs than those fed on water (F(10, 4245) = 36.7; p < 0.0001; Fig. 4F), whereas larval size, while affected by similar concentrations of urea imbibed by the mother (F(10, 3305) = 37.9; p < 0.0001), was more variable (Fig. 4G).
Attraction of Anopheles arabiensis to cattle urine odour
The overall attraction to the headspace volatile extracts of cattle urine of host-seeking An. arabiensis, as assessed in a glass tube olfactometer (Fig. 5A), was significantly affected by the age of the urine (χ2 = 15.9, df = 4, p = 0.0032; Fig. 5B). Post hoc analysis revealed that 24 h aged urine odour elicited a significantly higher level of attraction compared to all other treatments (72 h: p = 0.0060, 168 h: p = 0.012, pentane: p = 0.00070), except fresh urine odour (p = 0.13; Fig. 5B). While there was no significant difference in the overall attraction to urine odour by blood-fed mosquitoes (χ2 = 8.78, df = 4, p = 0.067; Fig. 5C), these females were found to be significantly more attracted to the headspace volatile extract of 72 h aged urine compared to the control (p = 0.0066; Fig. 5C).
Cattle urine odour does not affect egg laying
Female An. arabiensis, 72 h and 120 h post-blood meal, did not demonstrate a preference for the headspace volatile extracts of fresh and aged cattle urine over that of the pentane control during oviposition (χ2 = 3.07, p > 0.05; Additional file 1: Fig. S1).
Age affects the bioactive compounds present in cattle urine odour
For female An. arabiensis, the GC-EAD and GC–MS analyses identified eight, six, three and three bioactive compounds in the headspace volatile extracts of fresh, 24 h, 72 h and 168 h aged cattle urine, respectively (Fig. 5D–G). Despite the observed difference in the number of compounds eliciting an electrophysiological response, the majority of these compounds were present in each of the headspace volatile extracts collected from fresh and aged urine. Thus, only compounds that produced above-threshold physiological responses from the female antennae, for each extract, were included in further analyses.
The total volatile release rate of the bioactive compounds in the headspace collections increased from 29 µg h−1 in fresh urine to 242 µg h−1 in 168 h aged urine, predominantly due to the increase of p- and m-cresol, as well as phenol. In contrast, the release rate of other compounds, for example, 2-cyclohexen-1-one and decanal, decreased with an increasing age of the urine, correlating with the observed decrease in signal intensity (abundance) in the chromatogram (Fig. 5D–G left panel) and in the physiological response to these compounds (Fig. 5D-G right panel).
Synthetic urine odour attracts female mosquitoes
Overall, synthetic blends approximating the natural ratio of bioactive compounds identified in the headspace volatile extracts of fresh and aged urine (Fig. 5D–G), did not appear to elicit significant attraction in host-seeking (χ2 = 8.15, df = 4, p = 0.083; Fig. 5H) or in blood-fed mosquitoes (χ2 = 4.91, df = 4, p = 0.30; Fig. 5I). However, a post hoc pairwise comparison among the treatments revealed a significant attraction of host-seeking mosquitoes to the synthetic blend of 24 h aged urine, as compared to the pentane control (p = 0.0086; Fig. 5H).
To assess the role of individual components in the synthetic blend of 24 h aged urine, six subtractive blends, from which individual compounds were removed, were evaluated against the full blend in a Y-tube assay. For host-seeking mosquitoes, subtraction of individual compounds from the full blend had a significant effect on the behavioural response (χ2 = 19.63, df = 6, p = 0.0032; Additional file 1: Fig S2A), with all subtractive blends being less attractive than the full blend. In contrast, the removal of individual compounds from the full synthetic blend did not affect the behavioural response of blood-fed mosquitoes (χ2 = 11.38, df = 6, p = 0.077), with the exception of decanal, which resulted in a reduced level of attraction compared with the full blend (p = 0.022; Additional file 1: Fig. S2B).
Synthetic cattle urine odour attracts mosquitoes under field conditions
The efficacy of the synthetic blend of 24 h aged cattle urine to attract mosquitoes under field conditions was evaluated over ten nights in a malaria endemic rural village in Ethiopia (Fig. 6A). A total of 4861 mosquitoes were captured and identified, of which 45.7% were An. gambiae s.l., 18.9% were Anopheles pharoensis and 35.4% were Culex spp. (Additional file 1: Table S1). Anopheles arabiensis was the only member of the An. gambiae species complex to be identified by PCR analysis. On average, 320 mosquitoes were caught per night, during which time the traps baited with the synthetic blend caught more mosquitoes than the paired traps without the blend (χ2(0, 3196) = 170.0, p < 0.0001). During each of the five control nights at the beginning, middle and end of the trial, non-baited traps were set. Similar numbers of mosquitoes were caught in each paired trap, demonstrating that there was no bias between houses (χ2(0, 1665) = 9 × 10–13, p > 0.05) with no decline in the population over the study period. There were significantly higher numbers of mosquitoes caught in traps containing the synthetic blend compared to the control traps: host-seeking (χ2(0, 2107) = 138.7, p < 0.0001), recently blood fed (χ2(0, 650) = 32.2, p < 0.0001) and gravid (χ2(0, 228) = 6.27, p = 0.0123; Additional file 1: Table S1). This was also reflected in the total number of mosquitoes caught: host-seeking > blood-fed > gravid > semi-gravid > males.
The three species were differentially caught in the traps containing the synthetic blend. A significantly higher number of host-seeking (χ2(1, 1345) = 71.7, p < 0.0001), blood-fed (χ2(1, 517) = 16.7, p < 0.0001) and gravid (χ2(1, 180) = 6.11, p = 0.0134) An. arabiensis were caught in traps releasing the synthetic blend (Fig. 6B), whereas no difference in the number of An. pharoensis, at different physiological states, was found (Fig. 6C). For the Culex spp., only the number of host-seeking mosquitoes was found to be significantly higher in the traps baited with the synthetic blend (χ2(1, 1319) = 12.6, p = 0.0004; Fig. 6D), compared to the control trap.
Cattle urine odour is not a host habitat cue for mosquitoes
Host decoy traps, situated away from potential hosts between the breeding site and a rural village community in Ethiopia, were used to assess whether malaria mosquitoes use cattle urine odour as a host habitat cue. In absence of the host cue, heat, no mosquitoes were caught, with or without the presence of cattle urine odour (Additional file 1: Fig. S3). However, in the presence of both heat and cattle urine odour, female malaria mosquitoes were attracted and caught, although in low numbers, irrespective of the age of the urine (χ2(5, 25) = 2.29, p = 0.13; Additional file 1: Fig. S3). In contrast, the water control caught no malaria mosquitoes in the presence of heat (Additional file 1: Fig. S3).
Discussion
Malaria mosquitoes acquire and allocate nitrogenous compounds through compensatory feeding on cattle urine, i.e., puddling, to enhance life-history traits, similar to that of other insects [2, 4, 24–26]. Cattle urine is a readily available and renewable resource found in close association with resting sites, e.g., cattle sheds and tall vegetation close to rural households and oviposition sites, for malaria vectors. Female mosquitoes locate this resource through olfaction, and are able to regulate the uptake of nitrogenous compounds in urine, including the main nitrogenous constituent of urine, urea [15, 16]. Depending on the physiological state of the female mosquito, the nutrients within urine are allocated to enhance flight activity and survival in host-seeking females, and survival and reproductive traits in blood-fed individuals during their first gonotrophic cycle. As such, urine puddling plays an important nutritive role for malaria vectors that eclose as undernourished adults [8], by providing female mosquitoes with the ability to obtain vital nitrogenous compounds through engaging in low-risk feeding. This finding has significant epidemiological consequences, as females increase their life expectancy, activity and reproductive output, all of which affect vectorial capacity. Moreover, this behaviour may be targeted in future vector management programmes.
The VOC profile of urine changes with age as a result of microbial activity [15, 27–29]. Host-seeking female An. arabiensis are attracted to the VOCs of fresh and 24 h aged urine (this study, [12, 13]), which is different from that found for other dipterans, including tsetse and tabanids, which prefer VOCs of older aged urine [27, 30, 31]. The overall complexity of VOCs increases as the urine ages, with phenol and phenolic derivatives as the predominant VOCs (this study, [27, 32]). While blends of phenolic VOCs are sufficient to elicit attraction in tsetse and tabanids [30–33], these fail to do so in An. arabiensis, as corroborated by Mahande et al. [13] and Kweka et al. [12]. In contrast, the blends of antennally-detected VOCs that attract female An. arabiensis are more complex. These blends also contain phenol, p- and m-cresol, although at lower release rates compared to that found in the older urine. A synthetic blend of these phenolic compounds, along with three additional antennally active VOCs is required to recapitulate the behavioural response of host-seeking females to cattle urine under laboratory conditions. This suggests an evolutionarily conserved function of phenolic compounds among dipterans, however the context in which these phenolics are presented is adaptive for different species. When assessed under field conditions, the same blend elicited attraction of host-seeking An. arabiensis and Culex spp. females, but not of An. pharoensis, emphasizing a conserved, yet species-dependent, response. Cattle urine VOCs have been proposed to act as host habitat cues, i.e., long-range attractants that indicate the presence of a potential host within a particular area, for tsetse, tabanids and other non-Culicidae flies [34]. Mosquitoes, however, do not appear to use cattle urine as a host habitat cue, emphasizing a different ecological function in Culicidae (mosquitoes) and non-Culicidae flies.
While the synthetic odour of 24 h aged urine attracted recently blood-fed and gravid An. arabiensis in the field, this was not observed under laboratory conditions. In contrast, blood fed females exhibited a strong attraction to the background humid air, with little to no effect of the cattle urine VOCs. These behavioural results in the laboratory are likely confounded by the fact that humidity itself is a strong preoviposition attractant [35], but is a prerequisite for the bioassay. Cattle urine has been proposed to regulate oviposition by gravid mosquitoes [14], however this was not supported in this study. While cattle urine may not stimulate oviposition, it cannot be ruled out that gravid mosquitoes will imbibe urine deposited in or next to oviposition sites. As oviposition was not affected by cattle urine, other plausible explanations for the attraction of mosquitoes to cattle urine were assessed.
Fresh urine mainly contains salts and nitrogenous compounds, two nutrient classes frequently sought for by insects using supplemental feeding to increase fitness [2]. Host-seeking and blood-fed An. arabiensis actively imbibe cattle urine, at a similar level as water intake, irrespective of the age of the urine, which may be due to similar overall levels of nitrogen in fresh and aged urine. As cattle urine ages, microbes make use of the nitrogenous compounds in urine, particularly hydrolysing urea to ammonia, resulting in a changing complexity of the microbial communities [15]. While females do not display any obvious feeding preference for fresh or aged urine, mosquitoes demonstrate a dose-dependent response to urea, revealing that both host-seeking and blood-fed mosquitoes regulate their intake of nitrogenous compounds. Host-seeking mosquitoes imbibe a wide range of urea concentrations. However, these females display optimal intake volumes of urea at concentrations similar to those present in fresh and 24 h aged cattle urine [15, 16], and which does not differ from the sucrose control. While blood-fed mosquitoes imbibe lower volumes of urea and water than host-seeking females, as recently blood-fed females are constrained by the previous meal, these females display a lower threshold of response to urea. Mosquitoes are unable to metabolize urea, and likely use gut bacteria that possess ureases to hydrolyse urea to ammonia [36, 37]. Midgut tissues and fat bodies in mosquitoes are able to convert ammonia into the amino acids, glutamate, glutamine, alanine and proline [38, 39], which are important components of yolk proteins and, in the case of proline, can be used as an energy source for flight [40].
Allocation patterns of assimilated nitrogenous nutrients from urine, including urea, are not independent, as these nutrients are allocated as a function of physiological state to survival, flight and reproduction in order to provide the life history traits demonstrated in host-seeking and blood-fed mosquitoes. This conforms with the general aspect of nutrient allocation found for other insects, in which life history traits are constrained by one or more limiting nutrients [1, 2]. The need to allocate nutrients to more than one trait at the same time can generate physiological trade-offs among those traits [1], as demonstrated in a pair-wise manner for host-seeking (flight vs. survival) and blood-fed (survival vs. reproduction) mosquitoes. The need to increase acquisition of a food type containing a limiting nutrient has been shown to result in excess consumption of nutrients that are deleterious to another allocation target [1, 41]. Such trade-offs may explain why host-seeking female mosquitoes are attracted to and imbibe 72 h aged urine, which contains toxic microbiota [15], increasing the distance flown but resulting in a significantly reduced life span. On the other hand, the acquisition, by blood-fed females, of excess nutrients in 24 h aged urine, allow allocation of these resources to more than one trait, i.e., survival, flight and reproduction. This demonstrates that compensatory feeding on urine can be used for similar purposes as multiple blood meals within one gonotrophic cycle [42]. This allocation framework [1, 3] provides a mechanistic understanding of life history patterns and how resources are allocated to survival, dispersal and reproduction.
Compensatory feeding for nitrogenous compounds, in the form of multiple blood meals, has been shown in An. gambiae s.l. to either be required for egg development in females with low teneral reserves, or to enhance the number and condition of eggs developing in a single gonotrophic cycle [43–46]. However, blood feeding is risky and presents a trade-off for the female between reproduction and survival [47], making another, low risk, source for nitrogenous compounds, e.g., cattle urine, an adaptive alternative. The increased survival, as well increased numbers and sizes of eggs laid following a compensatory urine or urea meal by a blood fed female reflects that which is observed following multiple blood meals [43, 45, 46]. This suggests that An. arabiensis may minimize the trade-off between the need for nitrogen resources to enhance reproductive traits and survival by making use cattle urine.
Blood-fed mosquitoes allocate the bulk of the nitrogenous compounds from compensatory feeding to reproduction, and in part to survival, while host-seeking mosquitoes predominantly use these to fuel flight [48], analogous to that described for other insects [49, 50]. The immediate and sustained increase in flight activity by host-seeking females following a urea meal suggests that this resource can be used directly to fuel flight activity, potentially through the use of the combination of the previously described conversion of ammonia to proline [39] and its further oxidation of proline to fuel flight muscles [40, 48]. The dawn activity pattern demonstrated by host-seeking females fed on fresh urine reflects that observed for An. gambiae females engaging in compensatory blood feeding [10]. Host-seeking females fed on 24 h and 168 h aged urine, on the other hand, demonstrate an abnormal activity throughout photophase, suggesting that these females may be nutrient seeking during this time. Feeding on 72 h aged urine resulted in activity patterns similar to those demonstrated post-urea feeding, reflecting the high levels of ammonia present in the diet at this time, as a result of microbial activity [15]. Thus, mosquitoes have the capacity to use cattle urine, and its main nitrogenous component urea, as fuel for flight.
Malaria mosquitoes demonstrate complex behavioural and physiological strategies to adapt to their environment. Feeding on cattle urine compensates for the need to take multiple blood meals by enhancing life history traits in a state dependent manner. This is likely to affect vectorial capacity by increasing the probability of daily survival and vector density, while decreasing the interaction between the vector and the host, by reducing the need for multiple blood meals. Whether insecticides and antiparasitic drugs administered to cattle are present in the urine of the cattle and thus negatively affect the life history trait gain to the vector, or hasten the development of resistance to these treatments, requires further investigation. Urine meals provide an alternate, non-blood, nitrogen source and reduce the number of undernourished females requiring a pre-blood meal for metabolic energy prior to egg development. Further studies are needed to assess whether the propensity to urine feed, and its effects, are differentially affected by age, particularly for older females, which can transmit the parasite to humans. Moreover, there is a need to analyse the effect of this diet on malaria parasite survival and development. Compensatory feeding on cattle urine, or other nitrogen-rich resources, should be taken into consideration in future models of vectorial capacity. To this end, further studies are required to establish the natural role of cattle urine feeding in malaria vectors, and how this behaviour may be manipulated in future vector management strategies. As such, the synthetic cattle urine odour developed here may be used in e.g., mass trapping interventions, at local or regional levels, for malaria vector control.
Supplementary Information
Additional file 1: Table S1. Species, sex and gonotrophic state of the mosquitoes captured in CDC light traps baited with the synthetic odour blend of 24 h aged cattle urine or heptane control. Table S2. Synthetic compounds used for electrophysiological and behavioural analyses. Fig S1. Blood-fed Anopheles arabiensis display no oviposition preference for the headspace volatile extracts of fresh and aged cattle urine. Letter designations indicate no significant difference from one another (one-way analysis of variance with a Tukey’s post hoc analysis; p > 0.05). Error bars indicate the standard error of the proportion. Fig. S2. Behavioural responses of host-seeking (A) and blood-fed (B) Anopheles arabiensis to the full and subtractive synthetic blends of 24 h aged cattle urine. The removal of single components from the synthetic blend (open circles) differentially and significantly affected the response of the females from both physiological states. Different lowercase letters indicate significant differences as determined by a one-way analysis of variance followed by a Dunnett’s post hoc analysis (p < 0.05). Error bars represent the standard error of proportion. Fig. S3. Cattle urine enhances host decoy trap catches only in the presence of the host cue, heat. Host decoy traps only caught malaria mosquitoes in a deserted pasture between the breeding site and the village in the presence of both heat and cattle urine (fresh or aged), but not either alone. Error bars indicate the standard error of the mean.
Acknowledgements
The authors also thank Dr. Elsa Quillery for assisting in the PCR analysis of field collected mosquitoes, and Yared Debebe for his assistance with the HDT experiments.
Author contributions
RI and SRH conceived the study. MDB collected data. MDB and SRH conducted the statistical analyses. GB, HT, SRH and RI supervised the study. MDB, SRH and RI drafted the manuscript. All authors read and approved the final manuscript.
Funding
Open access funding provided by Swedish University of Agricultural Sciences. We thank the Swedish Research Council (VR/2014-3331) for funding this study.
Availability of data and materials
All data supporting the findings of this study are available within the article and its Additional files.
Declarations
Ethics approval and consent to participate
Ethical approval was obtained from the Institutional Research Ethics Review Board, College of Natural Sciences, (CNS-IRB), Addis Ababa University (IRB/022/2016), according to the guidelines set out by the World Medical Association Declaration of Helsinki. Consent from each household head was obtained with assistance of health extension workers, and endorsed by the local administration at district and ´Kebele´ level.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Boggs CL. Understanding insect life histories and senescence through a resource allocation lens. Funct Ecol. 2009;23:27–37. [Google Scholar]
- 2.Molleman F. Puddling: from natural history to understanding how it affects fitness. Entomol Exp Appl. 2010;134:107–113. [Google Scholar]
- 3.Raubenheimer D, Simpson SJ, Mayntz D. Nutrition, ecology and nutritional ecology: toward an integrated framework. Funct Ecol. 2009;23:4–16. [Google Scholar]
- 4.Shen K, Wang HJ, Shao L, Xiao K, Shu JP, Xu TS, et al. Mud-puddling in the yellow-spined bamboo locust, Ceracris kiangsu (Oedipodidae: Orthoptera): does it detect and prefer salts or nitrogenous compounds from human urine? J Insect Physiol. 2009;55:78–84. doi: 10.1016/j.jinsphys.2008.10.011. [DOI] [PubMed] [Google Scholar]
- 5.Hendrichs J, Lauzon CR, Cooley SS, Prokopy RJ. Contribution of natural food sources to adult longevity and fecundity of Rhagoletis pomonella (Diptera: Tephritidae) Ann Entomol Soc Am. 1993;86:250–264. [Google Scholar]
- 6.Bänziger H, Boongird S, Sukumalanand P, Bänziger S. Bees (Hymenoptera: Apidae) that drink human tears. J Kansas Entomol Soc. 2009;82:135–150. [Google Scholar]
- 7.Plotkin D, Goddard J. Blood, sweat, and tears: a review of the hematophagous, sudophagous, and lachryphagous Lepidoptera. J Vector Ecol. 2013;38:289–294. doi: 10.1111/j.1948-7134.2013.12042.x. [DOI] [PubMed] [Google Scholar]
- 8.Van Handel E. The obese mosquito. J Physiol. 1965;181:478–486. doi: 10.1113/jphysiol.1965.sp007776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gillies MT, Coetzee M. A supplement to the Anophelinae of Africa South of the Sahara. Publ S Afr Inst Med Res. 1987;55:1–143. [Google Scholar]
- 10.Klowden MJ, Briegel H. Mosquito gonotrophic cycle and multiple feeding potential: contrasts between. J Med Entomol. 1994;31:618–622. doi: 10.1093/jmedent/31.4.618. [DOI] [PubMed] [Google Scholar]
- 11.Norris LC, Fornadel CM, Hung WC, Pineda FJ, Norris DE. Frequency of multiple blood meals taken in a single gonotrophic cycle by Anopheles arabiensis mosquitoes in Macha, Zambia. Am J Trop Med Hyg. 2010;83:33. doi: 10.4269/ajtmh.2010.09-0296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kweka EJ, Mwang'onde BJ, Kimaro E, Msangi S, Massenga CP, Mahande AM. A resting box for outdoor sampling of adult Anopheles arabiensis in rice irrigation schemes of lower Moshi, northern Tanzania. Malar J. 2009;8:82. doi: 10.1186/1475-2875-8-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mahande AM, Mwang'onde BJ, Msangi S, Kimaro E, Mnyone LL, Mazigo HD, et al. Is aging raw cattle urine efficient for sampling Anopheles arabiensis Patton? BMC Infect Dis. 2010;10:172. doi: 10.1186/1471-2334-10-172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kweka EJ, Owino EA, Mwang'onde BJ, Mahande AM, Nyindo M, Mosha F. The role of cow urine in the oviposition site preference of culicine and Anopheles mosquitoes. Parasit Vectors. 2011;4:184. doi: 10.1186/1756-3305-4-184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kilande G, Tenywa JS, Rwakaikara-Silver MC, Katushabe AA. Cattle urine as a fertiliser: micro-biochemical changes in fermenting cattle urine and implications on plant nutrient conservation. Br Microbiol Res J. 2016;11:1–10. [Google Scholar]
- 16.Dijkstra J, Oenema O, Van Groenigen JW, Spek JW, Van Vuuren AM, Bannink A. Diet effects on urine composition of cattle and N2O emissions. Animal. 2013;7:292–302. doi: 10.1017/S1751731113000578. [DOI] [PubMed] [Google Scholar]
- 17.Attisano A, Murphy JT, Vickers A, Moore PJ. A simple flight mill for the study of tethered flight in insects. J Vis Exp. 2015;106:e53377. doi: 10.3791/53377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Majeed S, Hill SR, Ignell R. Impact of elevated CO2 background levels on the host-seeking behaviour of Aedes aegypti. J Exp Biol. 2014;217:598–604. doi: 10.1242/jeb.092718. [DOI] [PubMed] [Google Scholar]
- 19.Jones MD, Hill M, Hope AM. The circadian flight activity of the mosquito, Anopheles gambiae: phase setting by the light regime. J Exp Biol. 1967;47:503–511. doi: 10.1242/jeb.47.3.503. [DOI] [PubMed] [Google Scholar]
- 20.Wondwosen B, Birgersson G, Seyoum E, Tekie H, Torto B, Fillinger U, et al. Rice volatiles lure gravid malaria mosquitoes, Anopheles arabiensis. Sci Rep. 2016;6:37930. doi: 10.1038/srep37930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.WHO . Manual on practical entomology in Malaria. Part II. Methods and techniques. Geneva: World Health Organization; 1975. [Google Scholar]
- 22.Verrone GA. Outline for the determination of malarial mosquitoes in Ethiopia. Part I: adult female anophelines. Mosq News. 1962;22:37–49. [Google Scholar]
- 23.Wilkins EE, Howell PI, Benedict MQ. IMP PCR primers detect single nucleotide polymorphisms for Anopheles gambiae species identification, Mopti and Savanna rDNA types, and resistance to dieldrin in Anopheles arabiensis. Malar J. 2006;5:125. doi: 10.1186/1475-2875-5-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Honda K, Takase H, Ômura H, Honda H. Procurement of exogenous ammonia by the swallowtail butterfly, Papilio polytes, for protein biosynthesis and sperm production. Naturwissenschaften. 2012;99:695–703. doi: 10.1007/s00114-012-0951-z. [DOI] [PubMed] [Google Scholar]
- 25.Bodri MS. Puddling behavior of temperate butterflies: preference for urine of specific mammals? J Lepid Soc. 2018;72:116–120. [Google Scholar]
- 26.Petit S, Stonor MB, Weyland JJ, Gibbs J, Amato B. Camponotus ants mine sand for vertebrate urine to extract nitrogen. Austral Ecol. 2020;45:168–176. [Google Scholar]
- 27.Okech M, Hassanali A. The origin of phenolic tsetse attractants from host urine: studies on the pro-attractants and microbes involved. Int J Trop Insect Sci. 1990;11:363–368. [Google Scholar]
- 28.Storer MK, Hibbard-Melles K, Davis B, Scotter J. Detection of volatile compounds produced by microbial growth in urine by selected ion flow tube mass spectrometry (SIFT-MS) J Microbiol Methods. 2011;87:111–113. doi: 10.1016/j.mimet.2011.06.012. [DOI] [PubMed] [Google Scholar]
- 29.Troccaz M, Niclass Y, Anziani P, Starkenmann C. The influence of thermal reaction and microbial transformation on the odour of human urine. Flavour Frag J. 2013;28:200–211. [Google Scholar]
- 30.Vale GA, Hall DR, Gough AJ. The olfactory responses of tsetse flies, Glossina spp. (Diptera: Glossinidae), to phenols and urine in the field. Bull Entomol Res. 1988;78:293–300. [Google Scholar]
- 31.Mihok S, Mulye H. Responses of tabanids to Nzi traps baited with octenol, cow urine and phenols in Canada. Med Vet Entomol. 2010;24:266–272. doi: 10.1111/j.1365-2915.2010.00889.x. [DOI] [PubMed] [Google Scholar]
- 32.Baldacchino F, Cadier J, Porciani A, Buatois B, Dormont L, Jay-Robert P. Behavioural and electrophysiological responses of females of two species of tabanid to volatiles in urine of different mammals. Med Vet Entomol. 2013;27:77–85. doi: 10.1111/j.1365-2915.2012.01022.x. [DOI] [PubMed] [Google Scholar]
- 33.Madubunyi LC, Hassanali A, Ouma W, Nyarango D, Kabii J. Chemoecological role of mammalian urine in host location by tsetse, Glossina spp. (Diptera: Glossinidae) J Chem Ecol. 1996;22:1187–1199. doi: 10.1007/BF02027954. [DOI] [PubMed] [Google Scholar]
- 34.Webster B, Cardé RT. Use of habitat odour by host-seeking insects. Biol Rev. 2017;92:1241–1249. doi: 10.1111/brv.12281. [DOI] [PubMed] [Google Scholar]
- 35.Okal MN, Francis B, Herrera-Varela M, Fillinger U, Lindsay SW. Water vapour is a pre-oviposition attractant for the malaria vector Anopheles gambiae sensu stricto. Malar J. 2013;12:365. doi: 10.1186/1475-2875-12-365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen S, Blom J, Walker ED. Genomic, physiologic, and symbiotic characterization of Serratia marcescens strains isolated from the mosquito Anopheles stephensi. Front Microbiol. 2017;8:1483. doi: 10.3389/fmicb.2017.01483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kämpfer P, Matthews H, Glaeser SP, Martin K, Lodders N, Faye I. Elizabethkingia anophelis sp. nov., isolated from the midgut of the mosquito Anopheles gambiae. Int J Syst Evol Microbiol. 2011;61:2670–2675. doi: 10.1099/ijs.0.026393-0. [DOI] [PubMed] [Google Scholar]
- 38.Scaraffia PY, Isoe J, Murillo A, Wells MA. Ammonia metabolism in Aedes aegypti. Ins Biochem Mol Biol. 2005;35:491–503. doi: 10.1016/j.ibmb.2005.01.012. [DOI] [PubMed] [Google Scholar]
- 39.Scaraffia PY, Zhang Q, Thorson K, Wysocki VH, Miesfeld RL. Differential ammonia metabolism in Aedes aegypti fat body and midgut tissues. J Insect Physiol. 2010;56:1040–1049. doi: 10.1016/j.jinsphys.2010.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Scaraffia PY, Wells MA. Proline can be utilized as an energy substrate during flight of Aedes aegypti females. J Ins Physiol. 2003;49:591–601. doi: 10.1016/s0022-1910(03)00031-3. [DOI] [PubMed] [Google Scholar]
- 41.Lee KP, Simpson SJ, Clissold FJ, Brooks R, Ballard JW, Taylor PW, et al. Lifespan and reproduction in Drosophila: new insights from nutritional geometry. Proc Nat Acad Sci USA. 2008;105:2498–2503. doi: 10.1073/pnas.0710787105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Briegel H, Horler E. Multiple blood meals as a reproductive strategy in Anopheles (Diptera: Culicidae) J Med Entomol. 1993;30:975–985. doi: 10.1093/jmedent/30.6.975. [DOI] [PubMed] [Google Scholar]
- 43.Gillies MT. The recognition of age-groups within populations of Anopheles gambiae by the pre-gravid rate and the sporozoite rate. Ann Trop Med Parasit. 1954;48:58–74. doi: 10.1080/00034983.1954.11685599. [DOI] [PubMed] [Google Scholar]
- 44.Beier JC. Frequent blood-feeding and restrictive sugar-feeding behavior enhance the malaria vector potential of Anopheles gambiae sl and An. funestus (Diptera: Culicidae) in western Kenya. J Med Entomol. 1996;33:613–618. doi: 10.1093/jmedent/33.4.613. [DOI] [PubMed] [Google Scholar]
- 45.Takken W, Klowden MJ, Chambers GM. Effect of body size on host seeking and blood meal utilization in Anopheles gambiae sensu stricto (Diptera: Culicidae): the disadvantage of being small. J Med Entomol. 1998;35:639–645. doi: 10.1093/jmedent/35.5.639. [DOI] [PubMed] [Google Scholar]
- 46.Scott TW, Takken W. Feeding strategies of anthropophilic mosquitoes result in increased risk of pathogen transmission. Trends Parasitol. 2012;28:114–121. doi: 10.1016/j.pt.2012.01.001. [DOI] [PubMed] [Google Scholar]
- 47.Anderson RA, Roitberg BD. Modelling trade-offs between mortality and fitness associated with persistent blood feeding by mosquitoes. Ecol Lett. 1999;2:98–105. [Google Scholar]
- 48.Gaviraghi A, Soares JB, Mignaco JA, Fontes CF, Oliveira MF. Mitochondrial glycerol phosphate oxidation is modulated by adenylates through allosteric regulation of cytochrome c oxidase activity in mosquito flight muscle. Insect Biochem Mol Biol. 2019;114:103226. doi: 10.1016/j.ibmb.2019.103226. [DOI] [PubMed] [Google Scholar]
- 49.Teulier L, Weber JM, Crevier J, Darveau CA. Proline as a fuel for insect flight: enhancing carbohydrate oxidation in hymenopterans. Proc R Soc B. 2016;283:20160333. doi: 10.1098/rspb.2016.0333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tigreros N, Davidowitz G. Flight-fecundity tradeoffs in wing-monomorphic insects. Adv Insect Physiol. 2019;56:1–41. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Table S1. Species, sex and gonotrophic state of the mosquitoes captured in CDC light traps baited with the synthetic odour blend of 24 h aged cattle urine or heptane control. Table S2. Synthetic compounds used for electrophysiological and behavioural analyses. Fig S1. Blood-fed Anopheles arabiensis display no oviposition preference for the headspace volatile extracts of fresh and aged cattle urine. Letter designations indicate no significant difference from one another (one-way analysis of variance with a Tukey’s post hoc analysis; p > 0.05). Error bars indicate the standard error of the proportion. Fig. S2. Behavioural responses of host-seeking (A) and blood-fed (B) Anopheles arabiensis to the full and subtractive synthetic blends of 24 h aged cattle urine. The removal of single components from the synthetic blend (open circles) differentially and significantly affected the response of the females from both physiological states. Different lowercase letters indicate significant differences as determined by a one-way analysis of variance followed by a Dunnett’s post hoc analysis (p < 0.05). Error bars represent the standard error of proportion. Fig. S3. Cattle urine enhances host decoy trap catches only in the presence of the host cue, heat. Host decoy traps only caught malaria mosquitoes in a deserted pasture between the breeding site and the village in the presence of both heat and cattle urine (fresh or aged), but not either alone. Error bars indicate the standard error of the mean.
Data Availability Statement
All data supporting the findings of this study are available within the article and its Additional files.