Abstract
COVID-19 has shown a relevant heterogeneity in spread and fatality among countries together with a significant variability in its clinical presentation, indicating that host genetic factors may influence COVID-19 pathogenicity. Indeed, subjects carrying single pathogenic variants of the Cystic Fibrosis (CF) Transmembrane Conductance Regulator (CFTR) gene – i.e. CF carriers – are more susceptible to respiratory tract infections and are more likely to undergo severe COVID-19 with higher risk of 14-day mortality.
Given that CF carrier prevalence varies among ethnicities and nations, an ecological study in 37 countries was conducted, in order to determine to what extent the diverse CF carrier geographical distribution may have affected COVID-19 spread and fatality during the first pandemic wave.
The CF prevalence in countries, as indicator of the geographical distribution of CF carriers, significantly correlated in a direct manner with both COVID-19 prevalence and its Case Fatality Rate (CFR). In a regression study weighted for the number of tests performed, COVID-19 prevalence positively correlated with CF prevalence, while CFR correlated with population percentage older than 65-year, cancer and CF prevalence. Multivariate regression model also confirmed COVID-19 CFR to be associated with CF prevalence, after adjusting for elderly, cancer prevalence, and weighting for the number of tests performed.
This study suggests a putative contribution of population genetics of CFTR in understanding the spatial distribution of COVID-19 spread and fatality.
Keywords: SARS-CoV-2, COVID-19, CFTR, Cystic fibrosis, CF carriers
Introduction
The COVID-19 pandemic has shown a significant variability in spread and fatality among geographical areas.1 While demographic, socioeconomic and environmental factors explain to some extent such geo-epidemiological variability,2, 3, 4 it's conceivable that the genetic profile of populations may also influence COVID-19 spatial spread and fatality.5
At individual level, several genetic variants have been identified in association with severe COVID-19.6, 7, 8, 9, 10 In particular, we have shown with the GEN-COVID consortium,11 that individuals carrying single pathogenic variants of the Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) gene – i.e. CF carriers - are more likely to undergo the severe form of COVID-19 and have higher risk of 14-day mortality.11 In addition, ultra-rare variants of CFTR have been identified in association with COVID-19 severity.9 The CFTR gene encodes for a chloride and bicarbonate channel expressed on the apical membrane of epithelial cell.12 If both copies of CFTR exhibit pathogenic variants, individuals develop Cystic Fibrosis (CF), a severe congenital disease characterized by high viscosity of secreted fluids and by an abnormal inflammatory cascade.12
CF prevalence varies significantly among ethnicities - and therefore nations - being more frequent in Caucasian (1/2500) and Ashkenazi Jews (1/2300) compared to African Americans (1/15,000) and Asians (1/35,000).13 Importantly, given that CF is an autosomal recessive inherited disorder, CF prevalence in a specific country is considered an indicator of the prevalence of CF carriers among those inhabitants.12 CF carriers are more likely to undergo respiratory tract infections14 , 15 and have higher risk of the severe form of COVID-19.11 Therefore, our hypothesis is that the CF prevalence of different populations could be an additional factor that may contribute to explain the high variability of COVID-19 spread and CFR among countries.1
Here we present an ecological study16 in 37 countries on the prevalence of officially registered CF patients12 in relation to COVID-19 prevalence and CFR during the first pandemic wave. Such time frame is chosen in order to avoid possible confounding factors from the immune protection generated by prior exposure, as occurs in subsequent waves.17 The final aim is to investigate a putative scaled impact of CFTR population genetics on COVID-19 geo-epidemiological variability.
Methods
Data on the crude CF cases where collected from 37 countries through the official registry reports, available online, of the Cystic Fibrosis Foundation (https://www.cff.org), the European Cystic Fibrosis Society (www.ecfs.eu), and the Cystic Fibrosis Canada (www.cysticfibrosis.ca), all recording CF patients for over 20 years. The prevalence of COVID-19 cases was obtained from the World Health Organization at the end of the first pandemic wave, i.e. on June 1, 2020. Population data were taken from the Population Reference Bureau (PRB) (www.prb.org). Data on population density, age, and cancer prevalence were obtained from the datasets of the World Bank (www.data.worldbank.org). Data on the tests performed and the stringency index (SI) – a composite measure of 9 indicators of national responses, including school/workplaces closure, travel bans - were taken from the datasets of Our World in Data (https://ourworldindata.org/). The SI of the first pandemic wave for each country was considered as the average of the daily index from January 21 to June 1, 2020. The relationship between variables was explored using Pearson's correlation methods after logarithmic transformation. Multiple linear regression method was used to study the relationship between COVID-19 prevalence/CFR and explanatory factors like the population age. When indicated, data were weighted for the number of tests performed (per 1000 inhabitants) or by the number of inhabitants in each country. Statistical analysis was performed with the aid of STATA/IC 15.0 software on a Mac workstation.
Results
COVID-19 prevalence correlates with CF prevalence
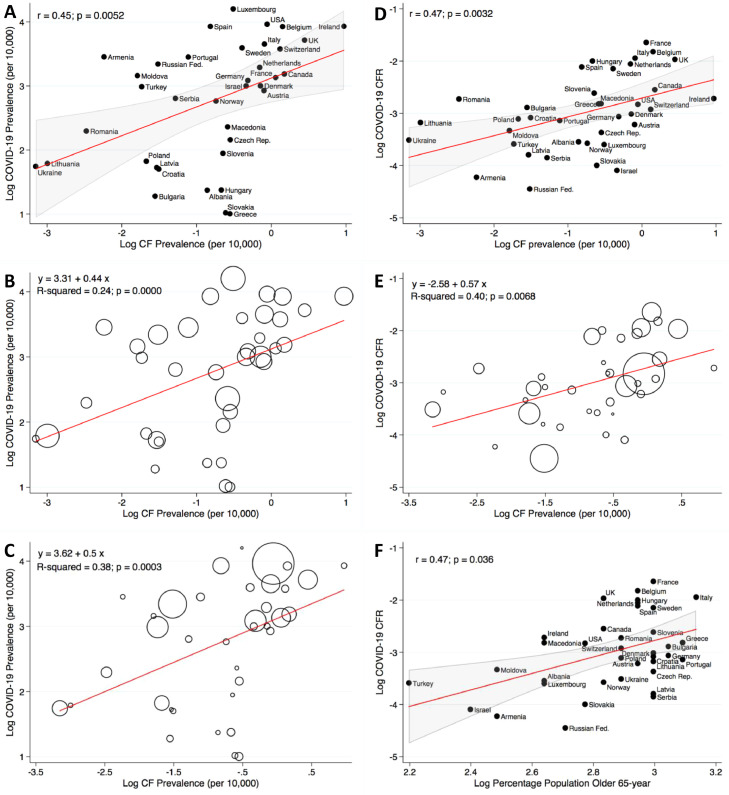
In the 37 studied countries COVID-19 prevalence directly and significantly correlated with the prevalence of CF in each country (Fig. 1 A; r = 0.45; p = 0.0052). Ireland, United Kingdom, Belgium were the countries with the highest prevalence of both CF patient (2.64, 1.56, 1.29 /10,000 inhabitants respectively), and COVID-19 cases.
Fig. 1.
CF Prevalence Correlation with COVID-19 Prevalence and CFR. Panel A shows a scatterplot with line of best fit (red) and the 95% confidence interval (gray), illustrating a significant direct Pearson's correlation between the prevalence of CF and COVID-19 prevalence in 37 countries. r = 0.45, p = 0.0052. In panel B the liner regression model is weighted for the number of tests performed per 1000 inhabitants in each country. A positive linear correlation between CF and COVID-19 prevalences is confirmed (R2=0.24; p = 0.0000). The size of the marker represents the number of tests performed per 1000 inhabitants. Panel C illustrates a linear regression model weighted for the countries’ inhabitants. A positive correlation between CF and COVID-19 prevalences is seen (R2 = 0.38; p = 0.0003). The size of the marker represents the number of inhabitants in each country. Panel D shows a scatterplot with line of best fit (red) and the 95% confidence interval (gray), illustrating a significant direct Pearson's correlation between the prevalence of CF and the COVID-19 CFR. r = 0.47, p = 0.0032. Panel E illustrates a liner regression model weighted for the countries’ inhabitants. A positive correlation between CF prevalence and COVID-19 CFR is seen (R2 = 0.40; p = 0.0068). The size of the marker represents the number of inhabitants in each country. In panel F is a scatterplot with line of best fit (red) and the 95% confidence interval (gray), illustrating a significant direct Pearson's correlation between COVID-19 CFR and the percentage of inhabitants older than 65-year. r = 0.47, p = 0.036. Prevalence is expressed per 10,000 inhabitants and all data are in logarithmic scale.
Considering that substantial variation in national responses to the pandemic occurred and that the reported numbers of COVID-19 cases were influenced by the number of diagnostic tests executed and by the population size, further liner regression studies, weighted for each of those values, and adjusted for the SI, were performed. A significant direct correlation was confirmed between COVID-19 and CF prevalences when data were weighted for the number of diagnostic tests performed (Fig. 1B; p = 0.0000) also after adjusting for the SI (R2 = 0.32; beta = 0.52; CI = 0.39–0.65; p = 0.000). Weighting for the number of inhabitants (Fig. 1C; p = 0.0003), and additionally adjusting for the SI confirmed the finding (R2 = 0.39; beta = 0.51; CI = 0.26–0.75; p = 0.000).
In addition, in a regression model weighted for the number of tests performed, no significant correlation was found between COVID-19 prevalence and the percentage of population older than 65 years (beta = −0.9; CI: −2.38–0.58; p = 0.225).
COVID-19 CFR correlates with CF prevalence
The CFR of COVID-19 significantly correlated in a direct manner with CF prevalence (Fig. 1D; r = 0.47, p = 0.0032) also in a regression model weighted for the number of inhabitants (Fig. 1E; p = 0.0068) or performed tests (beta = 0.33; CI: 0.05–0.6; p = 0.022). In addition, COVID-19 CFR correlated with the percentage of population older than 65 years (Fig. 1F; r = 0.47; p = 0.036) and the total cancer prevalence (beta = 1.1; CI: 0.35–1.85 p = 0.005) in a regression weighted for the number of tests performed.
In a multiple linear regression model the correlation between CF prevalence and COVID-19 CFR was confirmed to be significant after adjusting for the prevalence of people older that 65 years, the cancer prevalence and weighting the analysis for the number of tests performed (R2 = 0.40; beta = 0.32; CI = 0.01–0.64; p = 0.043).
Discussion
This report shows that the country-specific CFR and prevalence of COVID-19 during the first pandemic wave directly correlated with the CF carrier prevalence in 37 countries.
In our previous report on a cohort of 874 COVID-19 patients from Italy, CF carriers had a significant Hazard Ratio for 14-day mortality of 3.1 and deaths of CF carriers accounted for 7.27% of the deaths of the entire cohort.11 Considering that Italy reported 33,415 COVID-19 deaths during the first pandemic wave1 about 2429 of them would be potentially related to CF carriers. In addition, the prevalence of CF carriers in Italy is estimated to be around 1 out of 31 inhabitants,18 resulting in a projected population of about 2 million Italian and, theoretically, up to 24 million European CF carriers. As they are more likely to undergo respiratory tract infections14 , 15 and severe COVID-19,11 the burden of COVID-19 morbidity and mortality attributable to the CF-carrier status may be relevant.
Many factors are certainly involved in the etiology of severe COVID-19, but preclinical evidences regarding coronavirus attacking the cell enlighten a putative pathogenic role of CFTR. In-vitro studies have shown that the Spike protein of SARS-CoV-2 binds to CFTR itself inhibiting its activity19 and cleavage sites of the coronavirus 3CLpro proteinase have been predicted in the intracellular region of CFTR by computational models.20 Accordingly, when WT animals are infected by SARS-CoV-2, a reduced mRNA expression of CFTR is found in lungs,21 together with an increased expression of the epithelial sodium channel (ENaC) that facilitates sodium absorption,21 a pattern similar to the drastic CFTR impairment seen in CF.22
In this context it's important to note that while CF carriers have been reported to have a COVID-19 related mortality of 10%,11 individuals affected by CF do not undergo severe COVID-19 and their highest reported mortality is 3.87%23 raising the hypothesis that CF patients may have intrinsic protective factors.23
Our study has some limitations. First, being based on country-level data, the described associations don't demonstrate a causal link between outcomes and predictors. However, the previous report on severe COVID-19 and higher death risk in CF carriers11 together with the described preclinical evidences,19, 20, 21 enlighten a possible contribution of CFTR in COVID-19 pathogenesis. Detailed laboratory studies in cells expressing single pathogenic CFTR variants are required to understand the eventual molecular mechanism behind our findings. Second, testing and data reporting practices might differ substantially among countries – especially during the first pandemic wave and an underestimation of COVID-19 deaths may have occurred. However, COVID-19 data were taken from the WHO1 and other official registries, ensuring the highest reliability and uniformity possible.
In conclusion, this report suggests a possible contribution of the CFTR genetic profile of populations in understanding the country variability of COVID-19 spread and fatality.
Declaration of Competing Interest
Authors declare no conflict of interest.
Acknowledgments
This work was partially supported by the Swedish Cystic Fibrosis Association (CG). Authors are grateful to the European Cystic Fibrosis Society, the Cystic Fibrosis Foundation and the Cystic Fibrosis Canada for their work in registring CF patients.
References
- 1.World Health Organization . WHO; 2020. Coronavirus disease (COVID-19) Weekly Epidemiological Update and Weekly Operational Update. www.who.int/emergencies/diseases/novel-coronavirus-2019/situation-reports. [Google Scholar]
- 2.Gaudart J., Landier J., Huiart L., Legendre E., Lehot L., Bendiane M.C., et al. Factors associated with the spatial heterogeneity of the first wave of COVID-19 in France: a nationwide geo-epidemiological study. Lancet Public Health. 2021;6(4):e222–e231. doi: 10.1016/S2468-2667(21)00006-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cao Y., Hiyoshi A., Montgomery S. COVID-19 case-fatality rate and demographic and socioeconomic influencers: worldwide spatial regression analysis based on country-level data. BMJ Open. 2020;10(11):43560. doi: 10.1136/bmjopen-2020-043560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Piovani D., Christodoulou M.N., Hadjidemetriou A., Pantavou K., Zaza P., Bagos B.P., et al. Effect of early application of social distancing interventions on COVID-19 mortality over the first pandemic wave: an analysis of longitudinal data from 37 countries. J Infect. 2021;82(1):133–142. doi: 10.1016/j.jinf.2020.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aung A.K., Aitken T., Teh B.M., Yu C., Ofori-Asenso R., Chin K.L., et al. Angiotensin converting enzyme genotypes and mortality from COVID-19: an ecological study. J Infect. 2020;81(6):961–965. doi: 10.1016/j.jinf.2020.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pairo-Castineira E., Clohisey S., Klaric L., Bretherick A.D., Rawlik K., Pasko D., et al. Genetic mechanisms of critical illness in COVID-19. Nature. 2020 doi: 10.1038/s41586-020-03065-y. [DOI] [PubMed] [Google Scholar]
- 7.Benetti E., Tita R., Spiga O., Ciolfi A., Birolo G., Bruselles A., et al. ACE2 gene variants may underlie interindividual variability and susceptibility to COVID-19 in the Italian population. Eur J Hum Genet. 2020;28(11):1602–1614. doi: 10.1038/s41431-020-0691-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baldassarri M., Picchiotti N., Fava F., Fallerini C., Benetti E., Daga S., et al. Shorter androgen receptor polyQ alleles protect against life-threatening COVID-19 disease in European males. EBioMedicine. 2021;65 doi: 10.1016/j.ebiom.2021.103246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fallerini C., Picchiotti N., Baldassarri M., Zguro K., Daga S., Fava F., et al. Common, low-frequency, rare, and ultra-rare coding variants contribute to COVID-19 severity. Hum Genet. 2022;141(1):147–173. doi: 10.1007/s00439-021-02397-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Croci S., Venneri M.A., Mantovani S., Fallerini C., Benettti E., et al. Gen-Covid Multicenter Study. The polymorphism L412F in TLR3 inhibits autophagy and is a marker of severe COVID-19 in males. Autophagy. 2021:1–11. doi: 10.1080/15548627.2021.1995152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Baldassarri M., Fava F., Fallerini C., Daga S., Benetti E., Zguro K., et al. Severe COVID-19 in hospitalized carriers of single CFTR pathogenic variants. J Pers Med. 2021;11(6):558. doi: 10.3390/jpm11060558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Elborn J.S. Cystic fibrosis. Lancet. 2016:2519–2531. doi: 10.1016/S0140-6736(16)00576-6. [DOI] [PubMed] [Google Scholar]
- 13.Palomaki G.E., Fitzsimmons S.C., Haddow J.E. Clinical sensitivity of prenatal screening for cystic fibrosis via CFTR carrier testing in a United States panethnic population. Genet Med. 2004;6(5):405–414. doi: 10.1097/01.GIM.0000139505.06194.39. [DOI] [PubMed] [Google Scholar]
- 14.Miller A.C., Comellas A.P., Hornick D.B., Stoltz D.A., Cavanaugh J.E., Gerke A.K., et al. Cystic fibrosis carriers are at increased risk for a wide range of cystic fibrosis-related conditions. Proc Natl Acad Sci U S A. 2020;117(3):1621–1627. doi: 10.1073/pnas.1914912117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Polgreen P.M., Brown G.D., Hornick D.B., Ahmad F., London B., Stoltz D.A., et al. CFTR heterozygotes are at increased risk of respiratory infections: a population-based study. Open Forum Infect Dis. 2018;5(11):1–7. doi: 10.1093/ofid/ofy219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Morgenstern H. Ecologic studies in epidemiology: concepts, principles, and methods. Rev Public Health. 1995;16:61–81. doi: 10.1146/annurev.pu.16.050195.000425. [DOI] [PubMed] [Google Scholar]
- 17.Mathews J.D., McBryde E.S., McVernon J., Pallaghy P.K., McCaw J.M. Prior immunity helps to explain wave-like behaviour of pandemic influenza in 1918–9. BMC Infect Dis. 2010;10(1):128. doi: 10.1186/1471-2334-10-128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Picci L., Cameran M., Marangon O., Marzenta D., Ferrari S., Frigo A.C., et al. A 10-year large-scale cystic fibrosis carrier screening in the Italian population. J Cyst Fibros. 2010;9(1):29–35. doi: 10.1016/j.jcf.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 19.Caohuy H., Eidelman O., Chen T., Yang Q., Walton N.W., Pollard H.B. Inflammation in the COVID-19 airway is due to inhibition of CFTR signaling by the SARS-CoV-2 Spike protein. bioRxiv. 2022 doi: 10.1101/2022.01.18.476803v2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lars K., Kiemer L., Lund O., Brunak S., Blom N. Coronavirus 3CLpro proteinase cleavage sites: possible relevance to SARS virus pathology. BMC Bioinform. 2004;5:1–9. doi: 10.1186/1471-2105-5-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Morales L., Oliveros J.C., Enjuanes L., Sola I. Contribution of host miRNA-223-3p to SARS-CoV-induced lung inflammatory pathology. mBio. 2022 doi: 10.1128/mbio.03135-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Strandvik B. Is the ENaC dysregulation in CF an effect of protein-lipid interaction in the membranes? Int J Mol Sci. 2021;22(5):2739. doi: 10.3390/ijms22052739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mathew H.R., Choi M.Y., Parkins M.D., Fritzler M.J. Systematic review: cystic fibrosis in the SARS-CoV-2/COVID-19 pandemic. BMC Pulm Med. 2021;21(1):173. doi: 10.1186/s12890-021-01528-0. [DOI] [PMC free article] [PubMed] [Google Scholar]