Abstract
Throughout history, pandemics of infectious diseases caused by emerging viruses have spread worldwide. Evidence from previous outbreaks demonstrated that pregnant women are at high risk of contracting the diseases and suffering from adverse outcomes. However, while some viruses can cause major health complications for the mother and her fetus, others do not appear to affect pregnancy. Viral surface proteins bind to specific receptors on the cellular membrane of host cells and begin therewith the infection process. During pregnancy, the molecular features of these proteins may determine specific target cells in the placenta, which may explain the different outcomes. In this review, we display information on Variola, Influenza, Zika and Corona viruses focused on their surface proteins, effects on pregnancy, and possible target placental cells. This will contribute to understanding viral entry during pregnancy, as well as to develop strategies to decrease the incidence of obstetrical problems in current and future infections.
Keywords: Zika, Variola, Influenza, Coronavirus, Pregnancy
1. Introduction
Defining viruses is surprisingly controversial. Throughout history they have been called pathogenic entities [[1], [2], [3]], microscopic parasites [4], small-sized infectious agents [5], collections of genes surrounded by a protein coat [6] or organic particles [7], but most definitions can agree on something: viruses are not living beings as they rely on other cells to survive and replicate. Despite their common dependency from host cell for replicating, viruses are surprisingly diverse and based on their physical characteristics can be grouped into four classes: virus particles or virions, virus-like particles, endogenous viral elements and temperate viruses [5].
Virions are the particles responsible for infection in humans [5,7]. They consist of at least an inner nucleic acid core containing genetic material such as DNA or RNA, either single- or double-stranded, and a protein shell known as capsid, which encloses and protects their nucleic acid genome. In some cases, virions can be further coated by an envelope composed of a lipid bilayer which is derived from cell membranes. Finally, there are other viral particles such as surface glycoproteins and matrix proteins which can be found on most extracellular enveloped virions [5,8].
Several factors on cell and virion surfaces influence host-cell interaction and intracellular viral tropism which consequently ensure an efficient propagation and viral replication [9]. There are two main mechanisms of viral tropism: receptor-dependent tropism based on binding of the virus to specific molecules on host cells for initiation of invasion, and receptor-independent tropism involving virus entry in the host cell through a vector [9,10]. The majority of viruses rely on receptor-dependent tropism, thus, the sole receptor expression on a specific cell type is indicative for its susceptibility for viral infection, cell entry and viral replication [10,11].
Consequently, the viral structure comprises two major roles, the protective one accomplished by the capsid proteins and the infectious one which drives the recognition of cellular receptors by surface proteins and subsequent viral entry to the host cells [8]. The proteins or protein domains on the virus surface are subject to selective pressure by the host immune system. Such selective pressure leads viruses to mutate constantly, especially on antibody-binding regions or epitopes, giving birth to new viral variants [12,13]. In addition to mutation by selection, some viruses can go under genetic recombination of large nucleic acid regions leading to new viral subtypes [14].
Those viruses with enhanced adaptability are often responsible for spreading diseases across large regions or even worldwide resulting in pandemics. Throughout history, pandemics of diseases such as smallpox or influenza have revealed that pregnant women are a common denominator in the high-risk groups of many human viruses [[15], [16], [17]].
Although substantial progress has been made so far to understand the immune response during pregnancy in the scenario of a viral infection, many questions remain unanswered. Further, the importance of understanding the role of viral infection during pregnancy is becoming more and more relevant as currently, we are confronting one of the biggest pandemics of all times.
During pregnancy, women undergo an immunological adaptation that keeps the immune system functional but allows tolerance of the fetus [18]. Viruses can gain access to the decidua and placenta by ascendant infection or via blood dissemination [19,20]. Viral tropism to the decidua and placenta is dependent on viral entry receptor expression in these tissues as well as on the maternal immune response to the virus; Consequently, infection can cause perinatal outcomes ranging from no effect to pregnancy loss by spontaneous abortion or to fetal infection with resulting congenital viral syndromes [[20], [21], [22]]. Remarkably, less is known about the mechanisms responsible for the infection of placental cells and how this impacts the pregnancy outcome.
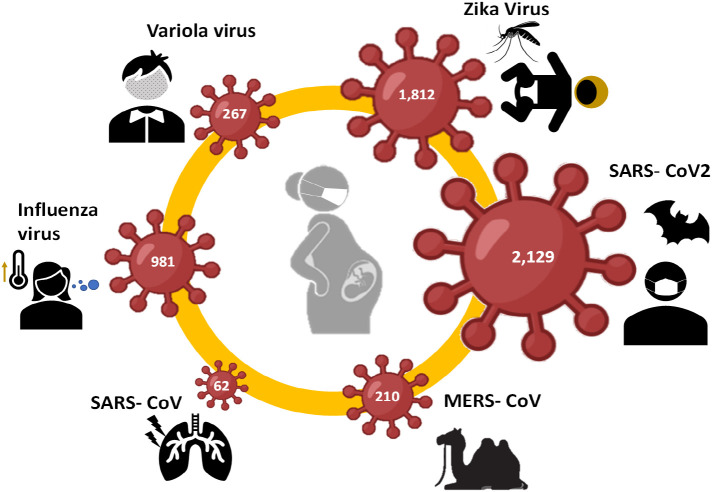
In this Review, we discuss the current understanding on the viral surface proteins of some pandemic viruses and their interaction with receptors expressed in placental cells. Epidemiological and biological data on Variola, Influenza, Zika and Corona viruses was summarized focused on their effects on pregnancy and the reported target placental cells. These viruses have caused major pandemics and are known for having differential effects on the mother and fetus wellbeing, which is reflected in the number of studies identified on PubMed using the respective virus and MeSH terms for “pregnancy” (Fig. 1 ). Understanding placental infection and the differences among pandemic viruses could prove to be useful in the future for the control and perhaps prediction of deleterious effects of virus infection during pregnancy.
Fig. 1.
Selected pandemic viruses for this review. Infographic of number of publications reported in PubMed concerning the respective virus in pregnancy.
2. Variola virus
2.1. General aspects
Among others, the Orthopoxviridae genus includes closely related viral species such as Variola virus (VarV) major and minor, the two variants that cause smallpox in humans; Vaccinia virus (VacV), successfully used in the vaccine against smallpox because of its antigenic similarity; and some animal orthopoxviruses such as Monkeypox (MonV), Camelpox (CamV) and Cowpox (CowV), which infect naturally a wide variety of mammalian species including humans and are responsible for several zoonotic outbreaks [23]. Orthopoxvirus are unique in many ways: For instance, their big size of 200–400 nm makes them visible under a light microscope. Their replication and assembly occur entirely in the cytoplasm, completely independent of their host's nucleus, something untypical for a linear double-stranded DNA virus. No specific cellular receptor or protein is targeted or utilized for entry into its host, and finally, they have high stability under challenging environmental conditions such as heat or dehydration [[24], [25], [26]].
VarV was most frequently transmitted by droplets entering via the respiratory system or less frequently by contact with skin lesions. The infection spread through lymph nodes and blood stream causing a systemic smallpox disease with variable clinical manifestations ranging from mild symptoms such as fever, cough, skin and mucous injuries and rash to severe highly lethal cardiovascular or hemorrhagic forms. Blindness and disfigurations due to skin scabs were common complications in survivors [23,27,28].
Historically, smallpox was the first illness to be prevented by immunization back in the 1700s using a technique called “variolation” consisting on inserting dried smallpox scabs up the nose or throughout a puncture in the skin. This technique was later modified after Edward Jenner's demonstration that inoculation of CowV cross-protected against smallpox in humans [29]. Thanks to a consistent program of compulsory vaccination with VacV strain by the World Health Organization (WHO), since 1979 smallpox is considered the only human disease that has been successfully eradicated [30,31]. However, the reappearance of smallpox or a smallpox-like disease poses a major health concern, due to the emergence or reemergence of VarV-like infections. Some animal Orthopoxviruses could become human-adapted in a common process among the genus, called host-switch. Host switch would cause not only zoonotic outbreaks but also potential epidemics [29,32]. For example, monkeypox is endemic to some regions Africa, and recent zoonotic outbreaks have been reported in distant countries such as USA [27].
Another form of reemergence considered by health organizations is as a form of bioterrorism. Following the eradication of smallpox, scientists and public health officials agreed to reduce the number of laboratories holding stocks of variola virus to only four locations. Nowadays, there are only two locations that officially store and handle variola virus for research under WHO supervision: the Centers for Disease Control and Prevention in Atlanta, Georgia, and the State Research Center of Virology and Biotechnology (VECTOR Institute) in Koltsovo, Russia [27,33]. Although there is no immediate direct threat of a bioterrorist attack using smallpox, if the virus were used in a bioterrorist attack, people who come into contact with the virus would be sick as the vaccine is no longer given routinely [27].
2.2. Epidemiology and pregnancy outcomes
Smallpox was one of the most devastating diseases known to humanity and caused millions of deaths before it was eradicated. It is believed to have existed for at least 3000 years. Although the origin of VarV remains unknown; the first clinical descriptions of smallpox were noted in China (4th century), India (7th century), and in the Mediterranean area (10th century) [34]. By the 15th and 16th centuries, at least 150 countries had reported cases of smallpox [35]. The last natural outbreak of smallpox in the United States happened in 1949, and the last case in the entire world was reported in 1977 [23,27,36].
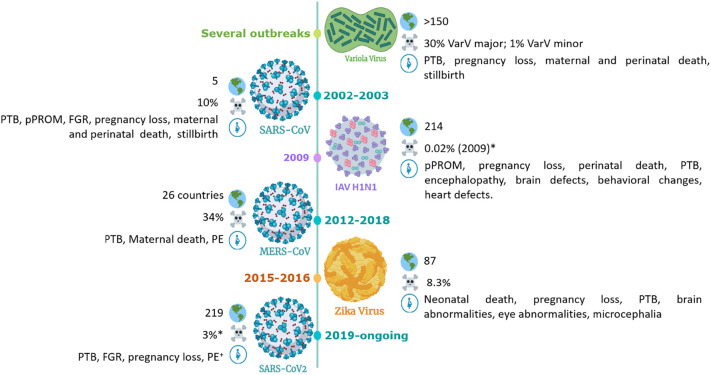
Both vaccinated and unvaccinated women who contract smallpox during pregnancy, suffer much higher mortality rates than non-pregnant ones. Estimate of case fatality rates due to variola major infection among non-vaccinated males and non-pregnant females is approximately 30% (Fig. 2 ). However, 63% of non-vaccinated females suffer lethal infections if they contract the disease while pregnant. Vaccination with VarcV reduces mortality to 2% among non-pregnants, but 26% of pregnant women would still die if they contract the disease [28]. This is partially dependent on the four different types of disease: ordinary, modified, flat and hemorrhagic. Both flat and hemorrhagic types have 100% fatal outcome, but only the hemorrhagic type seems to be not preventable by vaccination [37,38]. This type is also more common in adults, among which two-thirds are women. During pregnancy, 16% of smallpox infections are hemorrhagic, while the rates in non-pregnant women and men are significantly lower (0.9% and 0.8%, respectively) [37]. The higher susceptibility of pregnant women for the hemorrhagic type has been attributed to the imbalance of Th1/Tc cells levels in pregnancy [39].
Fig. 2.
Timeline of pandemic virus circulation. Outbreaks of selected viruses are presented including the number of countries affected, total case fatality rate and adverse outcomes on pregnancy. Created with BioRender.com.
Maternal fatality cases increase with gestational age from 22.9% in the first trimester, 29.2% in the second and over 40% in the third semester. The risk of miscarriage and stillbirth is considered high in all trimesters ranging between 39 and 45% [40]. About 55% of the living newborns die within 14 days, mostly during the first 72 h and 9% of these children showed typical dermal signs of smallpox, but it remains unclear if further children suffered from an intrauterine infection [41]. Additionally, evidence of intrauterine transfer of smallpox was additionally presented in a report of primary vaccination [42]. Two cases comprising a revaccination after a long interval and a non-immune gravida infected by her newly vaccinated child were included in this report. Stillborn fetuses showed typical dermal pox signs as well as microscopic visible virus particles in dermal cells and other fetal organs. This was accompanied by morphological changes in the placenta showed yellowish knots composed of necrotic villi and amounts of fibrin, as well as detection by electron microscopy of VacV in the stroma of placenta villi, which confirmed tissue infection [42].
The administration of the live VacV vaccine made possible the global eradication of smallpox. However, vaccination of pregnant women and their close relatives is contraindicated due to the potential vertical transfer of VacV from mother to fetus, known as “Vaccinia Fetalis”. It is a relatively rare but often fatal complication of primary VacV vaccination during pregnancy [41], underlining the need to develop both vaccines and antiviral drugs against VarV that can be safely applied during pregnancy to prevent congenital transmission.
2.3. Surface proteins and cell targets in the placenta
Poxviruses feature two infectious forms: intracellular mature virions (IMVs) and extracellular enveloped virions (EEVs) [[43], [44], [45]]. IMVs develop in the cytoplasm of the host cell and are released by cell lysis. This single-layered form is very stable and can be transferred between hosts. EEVs possess an extra, but fragile membrane supporting cell-cell-transfer within the host and exit from the host cell by exocytosis [24,44,46]. Poxviruses genome encodes at least 17 proteins for cell binding, membrane fusion and penetration [45] which results in diverse host entry mechanisms varying from strain to strain and from host cell type to host cell type [45,47]. Remarkably, poxviruses lack specific host cell receptors making their entry as a complex and multifactorial process involving ubiquitous elements in mammalian cell surfaces such as glycosaminoglycans or components of the extracellular matrix [25].
One proposed mechanism is the entry by cell attachment and fusion of viral and host membranes. EEVs attachment is not inhibited by proteinases and thus, the binding process is still unclear [45]. However, EEVs possess several extra surface proteins at their additional membrane, forming an entry fusion complex able to fuse viral and cell membranes to give the viral core access to the host cell cytoplasm [45,48]. Conversely, surface of IMV contains seven attachment proteins: D8 binds chondroitin sulfate, A27 and H3 proteins bind heparan sulfate and A26 protein binds laminin, glycosaminglycans and glycoproteins at the target cell [47].
A second endocytic route implies the viral induction of macropinocytosis by the host cell activated by exposure of phosphatidylserine at the membrane of IMVs but not EEVs [49,50]. Masquerading apoptotic cells, these viruses are recognized by many cell types and trigger endocytic engulfment and intracellular tropism in a process called “apoptotic mimicry” [51]. This strategy enhances viral tropism and immune evasion to viruses lacking specific receptor-binding proteins [51]. It has been proposed in VacV strain that the soluble serum protein Gas6 mediates binding of viral phosphatidylserine complexes to a TAM tyrosine kinase receptor at target cells to activate cell entry [52,53]. TAM receptors can be found on immune and nervous cells, but also in the placenta [54,55].
The ability of the syncytiotrophoblast (ST) to perform macropinocytosis has been shown in recent studies [56]. It has been reported that about 10% of trophoblast cells from term placentas could be infected in vitro with VacV [57]. VacV infected trophoblast cultures show cytopathic changes like cytoplasmic condensation, cell rounding and a decrease in human chorionic gonadotropin production [58] (Fig. 3, Fig. 4 ).
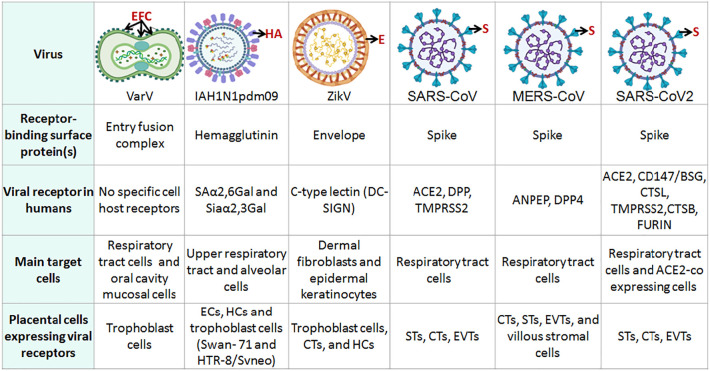
Fig. 3.
Viral surface proteins involved in cell entry and the presence of receptors in placenta cells. ECs: Endothelial cells; HCs: Hofbauer Cells; STs: Syncytiotrophoblast; CTs: Cytotrophoblast cells; EVTs: Extravillious trophoblast cells. Viral structures were created with BioRender.com.
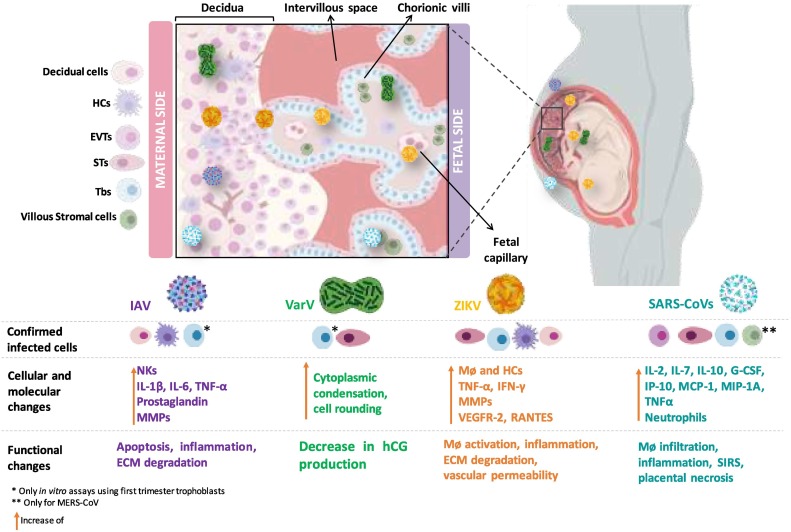
Fig. 4.
Viral infections and main effects in the human placenta. Illustration of placental target structures and cells of pandemic viruses, molecular reactions and consequent functional changes in the human placenta summarized from the current literature. DeCs: Decidual Cells HCs: Hofbauer Cells; EVTs: Extravillious trophoblast cells; STs: Syncytiotrophoblast; CTs: Cytotrophoblast cells; Tbs: Trophoblast cells. Created with BioRender.com.
3. Influenza virus
3.1. General aspects
Influenza is an acute respiratory disease that ranges from mild to severe infection or even death, mainly among high risk groups such as pregnant women, children under 4 years, elderly, individuals with chronic medical or immunosuppressive conditions and health care workers [59].
Influenza is caused by Influenza viruses which belong to the Orthomyxoviridae family and include four genera: influenza A virus, influenza B virus, influenza C virus and influenza D virus [60]. Influenza virions are enveloped and contain the viral genome, a negative, single-stranded RNA divided into segments [61,62]. The integral surface glycoproteins Haemagglutinin (HA) and Neuraminidase (NA) are embedded into the envelope. The HA-NA proportion in the surface of the EEVs is 4:1 where HA is the most abundant glycoprotein [63,64] HA and NA are also the main pathogenic viral determinants and responsible for the antigenic characteristics.
The majority of human influenza infections are caused by viral types A and B but Influenza A virus (IAV) is the most important due to its high pandemic potential: high mutation and transmission rates [60].
Influenza viruses possess two unique antigenic variation mechanisms: antigenic drift and antigenic shift [65,66]. During antigenic drift minor changes are introduced through point mutations in the viral genome during replication, causing mutants that escape the immune response [63,64,67]. Antigenic shift, on the other hand, results in sudden major changes and is possible due to the segmented genome of Influenza viruses. When two different viral subtypes co-infect the same host, a complete exchange of HA and NA genes may occur, in a process called reassortment [[63], [64], [65],68]. Although antigenic shift is a less frequent variation mechanism, it depicts serious problems since it is responsible for the emergence of new potentially pandemic strains of “non-human” origin which have acquired specificity for human receptors and to which no human being has previously developed immunity against [59,61,63,67,68].
3.2. Epidemiology and pregnancy outcomes
According to the WHO, every year between 5 and 10% of the adult population and 20 to 30% of children under the age of 2 years present signs and symptoms of infection by the Influenza virus [69]. It is estimated that between 3 and 5 million individuals suffer from severe influenza annually [69,70].
Pandemic influenza outbreaks are characterized by being unpredictable; however, epidemiological surveillance has demonstrated some regularity. The first major influenza pandemic of the 20th century occurred in 1918. It was caused by an AH1N1 strain and infected about a third of the global population of that time with a death toll of 20 to 40 million people [59,63,69]. In 1957 in Singapore an AH2N2 strain of avian IAV triggered the so-called “Asian Flu” pandemic, causing 1 to 3 million deaths. Later in 1968, an H3N2 pandemic strain emerged in Hong Kong, which is estimated to have caused 4 million deaths, after its complete dissemination [59,69].
The greatest influenza pandemic of this century in 2009 was caused once again by an IAV new pandemic subtype: AH1N1pdm09. It originated in April in Mexico where it caused severe illness in previously healthy adults and spread quickly to over 214 cities on 5 continents [59,69,71]. It infected over 10% of the global population, with a death toll estimated varying from 20,000 to over 500,000 [36]. Unlike other typical outbreaks of seasonal influenza, the new strain caused many infections in summer in the northern hemisphere and even more cases in winter in this same part of the world. Additionally, it demonstrated certain patterns that had not been seen before in influenza infections including a large number of deaths corresponded to young, clinically healthy patients [36,71]. The lessons learned during this pandemic have provided valuable insight into the continued challenges of influenza, in terms of its unpredictability and severity. To date, this strain continues in circulation and is a common constituent in the formulation of the vaccines designed annually [59,62,67,69,71].
Pregnancy is the highest risk factor for increased severe illness and lethality in both pandemic and seasonal influenza [72]. Since the last pandemic, several studies worldwide have described the impact of influenza infection on pregnant women highlighting an increased risk of untoward maternal and pregnancy outcomes that range from morbidity to mortality [21,[73], [74], [75], [76], [77], [78]].
According to the Centers for Disease Control and Prevention (CDC), pregnant women had a disproportionately high mortality rate during the 2009 H1N1 pandemic. Despite the fact that pregnant women account for only about 1% of the general population, they accounted for 5% of all influenza deaths reported [79,80]. Among these deaths, 7.1% occurred in the first trimester, 26.8% in the second, and 64.3% in the third trimester [79].
Over the last years, the effects of influenza infection during pregnancy on the fetus and neonate have also received considerable attention suggesting the potential of maternal influenza infection to predispose to long-term neurological sequelae in the in utero–exposed offspring. First trimester maternal influenza exposure is associated with an increased risk of any congenital anomaly, including neural tube defects, hydrocephaly, congenital heart defects, aortic valve atresia/stenosis, ventricular septal defect, cleft lip, digestive system and limb reduction defects [81]. Influenza AH1N1 infections have been associated with an increase in perinatal mortality, preterm birth (PTB), low Apgar score, increased risk on non-chromosomal congenital anomalies, such as neural tube defects, hydrocephalia, congenital heart defects, cleft lip, digestive system defects, and limb reduction defects [20,72,73]. There are also several studies addressing histopathological changes in the brain and behavioral alterations in the offspring such as schizophrenia and Parkinson's disease [20]. Further, it has been demonstrated that the risk of fetal death following influenza during the pandemic season remained elevated throughout the entire pregnancy [77].
Only few studies suggest placental infection and vertical transmission of influenza viruses in vivo. A few dated case reports disclosed presence of human influenza A H3N2 in placenta and amniotic fluid [[82], [83], [84]]. A more recent study on H1N1 reports singular histological changes and no evidence of viral RNA in any of the seven tested specimens. One case report describes an early newborn who tested positive for IAV by PCR [82]. Viral particles have been detected in lungs, Kupffer cells and circulating mononuclear cells of a 4 months old fetus from a mother who died from H5N1 [85]. Thus, viral replication in the placenta and vertical transmission of IAV may occur, but is extremely rare.
Therefore, induced pathology in the offspring can still not be attributed to a direct cytolytic effect of the virus per se but may implicate other mechanisms [79,86]. One explanation is based on the significant anatomic and physiologic changes during normal pregnancy that increase the risk of respiratory failure, making pregnant women more susceptible to respiratory compromise [87]. On the other hand, it has been suggested that fetal effects could be secondary to the maternal inflammatory response, rather than the result of a direct viral effect [20,21,72,86,87].
On the basis of evidence documenting excessive influenza-related mortality in pregnant women during historical and recent pandemics and higher rates of influenza-related morbidity requiring hospitalization during seasonal epidemics, the WHO has recommended prioritization of women in the influenza vaccination program who are, or will be, pregnant during the influenza season. For these women, an inactivated influenza vaccine should be used [75]. Further, according to WHO suggestions, due to the high mutation rate of HA it is necessary to refresh vaccination every year [71]. Furthermore, pregnant women with symptoms of influenza should be screened and treated immediately, especially those with comorbid medical conditions.
3.3. Surface proteins and cell targets in the placenta
IAV can be divided into subtypes based on the antigenic properties of their surface glycoproteins. As of today, 18 different subtypes of HA (H1-H18) and 11 subtypes of NA (N1−N11) have been reported with variations in their amino acid sequences of at least 30% among each other [65,71]. Although there are a lot of possible HA-NA combinations, only the subtypes H1N1, H2N2 and H3N2 can be transmitted among humans [61,64].
Recognition and binding to viral receptors occur through HA. EEVs enter the body via the upper respiratory tract and bind to molecules of N-acetylneuraminic acid, the predominant sialic acid (SA) on the cell surface, followed by the entrance to the host cell via endocytosis [61,64,88]. SA also defines the particular tropism of Influenza virus strains for specific types of glycosidic linkages to an adjacent galactose such as the α2, 3 or α2, 6 linkage [61,66,[88], [89], [90]]. Upper respiratory tract cells express SA α2, 6, whereas lower respiratory tract cells such as alveolar cells express SA α2, 3, where the most significant pathologies occur [79,90].
Expression of IAV viral receptor α-2,6-linked SA has been found by immunostaining also on other tissues, including placenta, and therein, localized to Hofbauer cells (HCs) and ECs [91]. Likewise, evidence in vitro on the susceptibility of the human first trimester trophoblast cell lines Swan-71 and HTR-8/SVneo to H1N1 [92] and H3N2 [93] IAVs is indicative for the presence of IAV receptors on trophoblast cells. Decidua but not placenta tissue seems to support replication of IAV [94], suggesting the possibility of viral replication in maternal tissues prior to spreading to placental and fetal tissues [79]. In vitro assays on first trimester human trophoblast-derived cell lines [92] and primary cultured chorion and amnion cells from human fetal membranes [95] have shown viral induced apoptosis and changes in gene expression of proinflammatory cytokines IL-1β, IL-6, and TNF-α, which in turn may facilitate premature rupture of membranes by collapsing the amniotic epithelial cell layer (Fig. 2, Fig. 4).
4. ZIKA virus
4.1. General aspects
The ZIKA virus (ZIKV) belongs to the family of Flaviviridae and was first isolated from a rhesus monkey in 1947 [96]. ZIKV is a single-stranded RNA virus that possesses a genome of around 11 kb, which is translated into a big polyprotein encoding 10 viral proteins, including the 3 structural proteins: capsid protein (C), precursor protein (prM) and envelope protein (E); and 7 non-structural proteins (NS) used by the virus to orchestrate viral replication or to avoid host defenses [97]. Other known members of Flaviviridae are Dengue Virus, West Nile Virus and Yellow Fever virus [98] and much of current research on ZIKV is based on knowledge from their previous studies. However, there is a growing interest for ZIKV in recent years as illustrated by the increasing number of publications: While for the term “Dengue” 13,427 search results can be found on Pubmed until 2014, the term “ZIKA” had only 180 results. For the years 2015–2021, there are similar results with 11,965 publications for “Dengue” and 9242 for “ZIKA”.
4.2. Epidemiology and pregnancy outcomes
ZIKV was first identified in the Zika forest area of Uganda, hence its name, but the first human infection was reported in Nigeria in 1954. Over a 50-year period, sporadic ZIKV infections were identified across Africa and Southeast Asia thanks immunologic surveillance [99]. The first outside outbreak occurred in 2007 in the Yap Islands, Micronesia, and it is estimated than 73% of their population got infected [100]. Remarkably, no deaths due to ZIK were reported and thus, these infections were considered as relatively harmless [100]. The first indications of a change in the epidemiology of ZIKV were reported after the outbreaks in 2013–2014 in the French Polynesia that derived in the pandemic spread to other pacific islands and to the Americas [99]. In this outbreak, clusters of the Guillain–Barré syndrome, a neurological disorder that could lead to paralysis and death, caused by ZIKV were described for the first time.
The introduction of ZIKV to Brazil probably attributable to tourist waves of the World Cup soccer competition and the Va'a World Sprint Championship canoe race in 2014 caused the most devastated outbreak of ZIKV in 2015 [99]. In general, ZIKV infection during pregnancy does not appear to influence the risks of prematurity, low birth weight, small-for-gestational-age, or fetal death [101]. However, during the Brazilian outbreak, there was a rise in adverse pregnancy outcomes, including severe birth defects, which was an unprecedented phenomenon for a mosquito-borne virus. In 2016, the WHO lifted the pandemic's emergency status for ZIKV, but it is estimated that due to the infection more than 3700 children were born with malformations including severe microcephaly only in the followed two years [102]. In the USA, evidence of ZIKV infection was reported in more than 3900 pregnancies (live births and pregnancy losses at any gestational age) during the years 2016–2017. 5% of these cases were associated with serious brain abnormalities including microcephaly and other severe birth defects [103]. Among pregnancies with nucleic acid test- confirmed ZIKV infection in the first, second, and third trimester. Potential ZIKV-associated birth defects were reported in 8%, 5%, and 4% of fetuses or infants, respectively [103].
Up to date, 86 countries and territories have reported evidence of ZIKV infections and although the number of congenital abnormalities has remitted, they are still reported in the several outbreaks and infection clusters continuing to occur in regions where the vector is present, including the Americas, India and Southeast Asia [102,104].
4.3. Surface proteins and cell targets in the placenta
The main transmission route for ZIKV infection is through a mosquito vector, especially Aedes aegypti. However, unlike other flaviviruses, ZIKV can be transmitted from human to human without involvement of an insect vector [102]. Due to its thermostability, ZIKV is detectable in diverse human body fluids like semen, saliva, breast milk or urine, even days or weeks after the disappearance of the clinical symptoms [99]. Hence, some cases of ZIKV infection have occurred by sexual contact, blood or platelet transfusion, mother-to child transmission during pregnancy and, very rarely, upon close contact in a single reported case [97]. Other factors such as virus strain [105], targeted cell type [106], pregnancy stage [107] and previous infection with Dengue Virus [108,109] influence ZIKV infection both in vitro and in vivo.
Cellular targets of ZIKV are mostly neural-type cells as demonstrated by the presence of ZIKV RNA in postmortem fetal brain tissue, even after becoming undetectable in maternofetal circulation, placental biopsy, and amniotic fluid [110]. Although ZIKV RNA has been consistently detected in placenta tissue across studies, the detection of viral antigens in placenta cell populations has been heterogeneous. In a first study, placentas from pregnant women with PCR-confirmed ZIKV infection were stained using anti-flavivirus and anti-ZIKV monoclonal antibodies resulting in positive signals exclusively in HCs, but not in ST or other trophoblast cells regardless of the gestational period at which infection occurred [111]. It has been confirmed by immunohistochemistry for the E and NS1 antigens of ZIKV, as well as by situ hybridization for the ZIKV genome, that HCs are the principal targets for ZIKV in the placenta, but other cell types including decidual cells, ST, trophoblast, endothelial and mesenchymal cells of the chorionic villi may be also permissive to ZIKV infection [112]. As HCs play an important role in pregnancy homeostasis, their infection causes an imbalance in the production of MMPs that degrade collagen, as well as TNF that activates and attracts other immune cells [111]. This correlates with an increase in CD8+ T lymphocytes, responsible for the production of IFN-γ, which in turn, activates macrophages. Further, infected endothelium exhibits large expression of VEGFR-2 and RANTES, which induce increased vascular permeability and macrophage migration [112]. Altogether, infection of placenta cells and the concurrent morphological changes allow ZIKV to traverse the placental barrier and cause infection in the fetal brain, resulting in the observed teratogenicity or fetal demise (Fig. 4).
Viral protein E is the most abundant in the ZIKV surface and mediates binding to host cell receptors mostly through C-type lectin receptors, followed by entry by endocytosis. Other cellular phosphatidylserine receptors, such as T cell immunoglobulin mucin (TIM) and several tyrosine kinase receptor (TAM) families (TYRO3, AXL and MER) or Glycosaminoglycans have been proposed, but further studies are needed [97,98].
In Cytotrophoblast (CTs) and ST derived from placental villi at term, expression of TAM receptors and their natural ligands PROS1 and GAS6 was significantly lower than in their corresponding models for first trimester trophoblast cells derived from embryonic stem cells [107]. This indicates attachment of the virus capsid surface to phosphatidylserine on the cell membrane as basis for viral entry in early pregnancy using the “viral apoptotic mimicry” strategy described above. The low expression of these factors in term trophoblast cells, in combination with their high expression of viral defense genes and the primate-specific microRNA cluster on chromosome 19 (C19MC) with potential anti-viral properties [113] might serve as basis for resistance of human third trimester ST to ZIKV viral infection. Further potential ways of attachment consider TIM receptors involved in interaction with phosphatidylserine, C-type lectin receptors (e.g., DC-SIGN) and ZIKV E protein [98].
As a second entry mechanism, endocytosis of ZIKV by activation of several pathways has been discussed. ZIKV particles were observed to co-localize with caveolin-1, suggesting a potential implication of caveola endocytosis [114]. Likewise, dynamin and clathrin are required for ZIKV entry to host cells indicating a clathrin-mediated endocytosis with subsequent transport in acidic endosomes and RNA release into the cytoplasm after membrane fusion [114]. The latest may be the main mechanism by which ZIKV infiltrate human host cells [98], but further research is needed to establish specific infection of placental cells.
After completion of the first trimester, ZIKV may still infect placental villi by cross-reactive, non-neutralizing antibodies originally targeting E protein of DENV, which is similar to that of ZIKV [109] in a process called antibody-dependent enhancement (ADE). In a previous work we were able to demonstrate that presence of DENV antibodies led to increased virus production in placental villous tissue explants which indicates antibody-mediated transport into Fcγ receptor bearing non-resistant target cells such as HCs or CTs [108] (Fig. 3).
Additionally, alterations of tight junction protein expression in ZIKV infected placentas have been identified and suggested an additional paracellular pathway for ZIKV [115]. ZIKV transcytosis has also been described on the basis of cell culture experiments [116].
5. Coronaviruses: SARS-CoV, MERS-CoV and SARS-CoV2
5.1. General aspects
Coronaviruses (CoVs) belong to the Coronaviridae family, a group of single-stranded RNA viruses divided into four genera: alpha-, beta-, gamma-, and delta-CoVs. The alpha- and beta-CoV are known to infect mammals and among them, humans [117]. Coronaviruses genome codes for two large polyproteins, spike protein (S), envelope protein (E), membrane protein (M), and nucleocapsid proteins (N) [118]. S proteins consist of an N-terminal S1 subunit that recognizes and binds to the host cell and a C-terminal S2 subunit in charge of the membrane fusion. SARS-CoV2 shares with the SARS-CoV about 80% of similarity of the genome sequence, but its S protein receptor affinity is ~10–20-fold higher than in SARS-CoV, which can be the reason why it spreads faster than the other coronaviruses [119,120].
5.2. Epidemiology and pregnancy outcomes
Coronaviruses have been studied for decades, but this interest increased since the severe acute respiratory syndrome (SARS) epidemic outbreak in 2002–2003, in South China. It affected more than 8000 people in 29 countries and had a death toll of 916 people [121]. Ten years later, another epidemic by CoVs appeared, named Middle East Respiratory Syndrome (MERS). With over 2200 laboratory-confirmed cases, 83% of them were reported in Saudi Arabia. It caused 791 deaths in 27 countries until the 1st of August 2018 [122]. From 2019, the latest coronavirus pandemic COVID-19, caused by SARS-CoV2, has reached the entire world: until June 2021, more than 173 million cases have been confirmed and over 3.7 million deaths reported to the WHO [123]. These three outbreaks were caused by beta-CoVs that share bats as their reservoir hosts, which transmit the virus to intermediary hosts such as palm civets (SARS-CoV) and dromedary camels (MERS-CoV) prior dissemination to humans.
Despite the number of outbreaks caused by CoVs, data on their effect on human pregnancy was limited until 2020 when the COVID-19 pandemic attracted the spotlight. SARS-CoV infection is associated with critical maternal illness, PTB and preterm premature rupture of membranes (pPROM), FGR, spontaneous abortion, or maternal death [124]. MERS-CoV infection during pregnancy has been associated with onset of preeclampsia in over 19% of cases as well as to perinatal adverse outcomes such as stillbirth and neonatal death, but no FGR or pPROM has been reported [124]. In the several SARS-CoV2 case reports and systematic reviews on cohorts with more than 100 cases [[124], [125], [126]], maternal deaths were almost absent, and the disease had a mild course in the majority of pregnancies (> 90%) independently of the gestational age at which infection occurs [[127], [128], [129]]. This implies that pregnant women are at similar risk of SARS-CoV2 infections as the general population and commonly, they do not suffer a more severe course of infection than non-pregnant women [127,130,131].
The majority of pregnant women with SARS-CoV2 infection reported in literature delivered by caesarean section, although there is currently no confirmed evidence that a vaginal birth or delivery by Caesarean section would be detrimental or safer if COVID-19 is suspected or confirmed [132]. Moreover, infection with SARS-CoV2 may be associated with preterm birth, stillbirth and preeclampsia but the data on the latest two remains heterogeneous [[133], [134], [135]]. These adverse pregnancy outcomes can be caused by COVID-19 complications rather than by the infection per se. It has been demonstrated that a severe SARS-CoV2 infection leads to maternal hypoxia followed by placental ischemia. This can exacerbate the production of inflammatory elements [136]. The uncontrolled release of inflammatory cytokines could amplify the maternal immune response and increase the risk of placental damage, FGR, abortion, or preterm labor [137].
Few reports disclosed isolated cases of neonatal death [[125], [126], [127]], but no teratogenic effects of SARS-CoV2 infection have been described in the neonates to date. In a recent study 24 (~8%) out of 313 neonates born to mothers with COVID-19 tested positive for SARS-CoV2 [138]. In a meta-analysis, vertical transmission assessed by positive PCR test occurred in only 1.5% of the cases [135]. Further evidence is needed to provide definite evidence for or against vertical transmission in SARS-CoV2 infected pregnant patients, but this raises an important question about the placental mechanism preventing or allowing the intrauterine transmission of SARS-CoV2 to the fetus.
Evidence shows that SARS-CoV2 viral plasma load in COVID-19 cases positively correlates with disease severity [139]. Infants born from mothers with severe COVID-19 disease show high rates of SARS-CoV2 positivity in the placenta and nasopharyngeal swab immediately after birth [140], supporting that maternal viremia is a prerequisite for transplacental transmission [[141], [142], [143]]. Based on the current reports, it may be expected that a SARS-CoV2 infection in the 2nd and 3rd trimester of pregnancy has a moderate course and the majority of newborns stay healthy.
Therefore, the innate immune system, the placental barrier, as well as the interaction between decidual immune cells may play a role in the placental protective mechanisms against SARS-CoV2 infection and transmission [143].
Finally, the safety and efficacy of maternal vaccination against viral infections have been largely studied. One of the main aims in terms of protection is the immune transmission to the newborn by placental passage of antibodies. A comparable newborn protection would be expected after maternal vaccination against SARS-CoV-2. So far, only a preprint case report associates an infant with SARS-CoV-2 IgG antibodies against viral S protein. Those were detected in cord blood after maternal vaccination with a first Moderna mRNA COVID-19 vaccine dose at gestational age of 36 weeks 3 days [144]. Further studies are needed to clarify how long is the duration of the antibody protection and whether the passive transmission can be done through breast milk.
5.3. Surface proteins and cell targets in the placenta
Accumulating evidence supports that SARS-CoVs are not merely respiratory viruses and can affect other organ systems including the placenta. However, there is no definitive evidence for SARS-CoV and MERS-CoV placental or fetal infection. Specimens such as maternal or cord blood from infected pregnant patients tested negative for SARS-CoV and MERS-CoV [145]. Thus, the adverse fetal outcomes reported for these viruses may be related to the severe acute respiratory illness and the impact on the entire maternal organism rather than to the viral infection per se [[145], [146], [147], [148], [149], [150]].
Conversely, SARS-CoV2 viral N and S protein mRNAs were successfully identified in placentas and are expressed in STs, low expressed in CTs, stromal cells, chorionic villi endothelial cells, and intervillous mononuclear cells [151,152]. Viral mRNA has been detected also by in situ hybridization in decidual endothelial cells and endometrial glands [[153], [154], [155]]. Several case reports of pregnant women with COVID-19 identified virions in placental tissue. Using transmission electron microscopy, virions have been found within different cells of the placenta such as STs, maternal macrophages and HCs. They have been localized in microvilli and extensions of fibroblasts in terminal villi as well as in fetal capillary endothelial cells close to villous surfaces and close to intravascular mononuclear cells [141,156]. A case report showed that placenta was positive for both E and S genes of SARS-CoV2 by using RT-PCR. Intense cytoplasmic positivity of EVTs has been observed by immunostaining using an antibody against SARS-CoV-2 N-protein [157]. Further, the viral load was proved to be higher in placental tissue than in amniotic fluid or maternal blood, indicating viral replication in the placenta cells [158].
Corona virus infection relays on the interaction with receptors on the susceptible cell types. The S1 subunit of protein S binds to angiotensin-converting enzyme 2 (ACE2), the viral receptor of SARS-CoV and SARS-CoV2, or to dipeptidyl peptidase 4 (DPP4), the viral receptor of MERS-CoV [159]. ACE2 is found on endothelial cells, intestine, and respiratory tract epithelia, where ciliated cells and alveoli type II are the primary target cells [160]. DPP4, also known as CD26, is expressed on epithelial cells in the kidney, small intestine, liver, and lung alveoli. DPP4 is a key factor in the activation of T cells and immune response costimulatory signals in T cells, which could suggest a possible manipulation of the host immune system by the virus [159]. MERS-CoV receptors ANPEP and DPP4 can be found expressed in first and second trimester placental cells: ANPEP was detected in the EVTs, CTs and STs, but not in the STs of the first trimester placenta; DPP4 was detected in CTs, STs, EVTs, and villous stromal cells. Expression levels of ANPEP significantly decreased, while those of DPP4 significantly increased in second trimester EVTs [161].
The classical SARS-CoV2 access mode to human cells is mainly via ACE2 but it can also bind to CD147/basigin (BSG) on an alternate entry pathway [162]. Other host entry factors have been identified, including neuropilin-1 and TMPRSS2, a transmembrane serine protease involved in S protein maturation (LEE 2020). Human proteases such as endosomal protease cathepsin L (CTSL), cathepsin B (CTSB), and furin also promote viral entry [163].
ACE2 was found expressed in ST and CTs, decidual stromal cells, decidual perivascular cells and in endothelial and vascular smooth muscle cells. TMPRSS2 has been detected in ST and CTs in first trimester placenta and increased in second trimester placenta. Trophoblast subtypes such as STs, CTs and extravillous trophoblasts (EVTs) were found to co-express ACE2, TMPRSS2, BSG, and CTSL in different combinations. Trophoblast cells co-expressing ACE2 and TMPRSS2 also express FURIN and CTSB and endosomal sorting complexes required for transport (ESCRT) genes, involved in viral budding and replication [161]. This abundant expression of ACE2 and ACE2/TMPRSS2 co-expression in the human placenta may increase the vulnerability of the placenta and fetus to SARS-CoV2 infection and contribute to its vertical transplacental transmission [161,164,165] (Fig. 2).
It is suggested that the pregnancy complications due to SARS-CoV2 placental infection could be triggered by ACE2 downregulation via formation of viral-ACE2 complexes. This causes downregulation of plasma angiotensin-(1–7) levels, which in turn potentiates vasoconstriction and pro-coaulopathic state, potentially leading to early onset of PE [166]. The SARS-CoV2 tropism for endothelium via the ACE2 receptor increases susceptibility to vascular endothelial dysfunction, leading to a coagulopathy state in infected patients and susceptibility to microthrombi formation [167]. SARS-CoV2 infection increases intervillous macrophage infiltration in infected placenta. Such macrophage infiltration may serve as a trigger for a strong immune response leading to tissue destruction and placental disfunction triggering pregnancy complications. The presence of virions within the cytoplasm of fetal vascular monocytes may also play a role in the transmission and propagation of SARS-CoV2 to the fetus [168,169]. In silico studies suggest that SARS-CoV2 might affect placental functions influencing other proteins such as those involved in trophoblast invasion, migration, syncytialization, and implantation [170]. Finally, the data published to date found no signs of SARS-CoV2 in breastmilk [171], vaginal secretions or cervical exfoliated cells samples of infected mothers [172].
ACE2 and TMPRSS2 entail a negative correlation with gestational age. They are expressed in the placenta with a decreasing trend towards delivery [173]. Thus, pregnant women may be more vulnerable to SARS-CoV2 transplacental transmission during the first trimester than in later stages of pregnancy [165].
Thus far, CoVs have jumped from animals to humans in at least 3 epidemics now, causing a high number of morbidity and mortality. It is foreseeable that further outbreaks and epidemics of CoVs are inevitable in the future. Therefore, it is important to research and develop improved CoV prevention and therapeutic approaches.
6. Conclusions
The epidemiology of the pandemic viruses revised in this work differs greatly among them in terms of fatality rates, but they all share a risk for worse outcomes in pregnancy (Fig. 1). This may be caused by the general clinical manifestations and symptoms of each viral disease in combination with the special adaptations of the organism in pregnancy, but also by a potential infection of placental and fetal cells and tissues. As virus-receptor interactions are the key for viruses to invade host cells, presence of viral receptors on placental cells may infer their susceptibility for infection (Fig. 3). However, further evidence is required to confirm pandemic virus infection at cellular level in placenta and the consequent effects.
Apoptotic mimicry and clathrin-mediated endocytosis were found common mechanisms responsible for the infection of placenta cells, but it becomes necessary to address other viral entry mechanisms to host cells during pregnancy. Understanding the different mechanisms of viral recognition and entry to host cell as the first step in a misfortunate cascade of events can help develop better management and prevention strategies to decrease the incidence of adverse pregnancy outcomes. Only then, obstetrical problems induced by viral infections will be controlled and neonatal outcomes improved.
Funding sources
DMMP and URM have been supported by the German Research Foundation (DFG, grant Mo2017/2 and Mo2017/3 to DMMP and Ma1550/12-1 to URM) and the Interdisciplinary Center for Clinical Research (IZKF, DMMP FF05) at the Jena University Hospital. URM, AnS and AsS are supported by a grant from the German Ministry of Education and Research (03VP08692). JMMC has received a postgraduate grant from CONACyT (CVU: 446429). PFZ receives a Ph.D. scholarship (Personal Reference No. 91771964) from the German Academic Exchange Service (DAAD).
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Lwoff A. The concept of virus. J. Gen. Microbiol. 1957;17(2):239–253. doi: 10.1099/00221287-17-2-239. [DOI] [PubMed] [Google Scholar]
- 2.Tajouri L. What is a virus? How do they spread? How do they make us sick? 2020. https://theconversation.com/what-is-a-virus-how-do-they-spread-how-do-they-make-us-sick-133437 Marc 13 [cited 2021 07.01]; Available from.
- 3.Greene S.E., .R.A. American Society for Microbiology; 2013; Washington (DC: 2013, July. Viruses throughout Life & Time: Friends, Foes, Change Agents: A Report on an American Academy of Microbiology Colloquium San Francisco. [PubMed] [Google Scholar]
- 4.Vidyasagar A. What are viruses? 2016. https://www.livescience.com/53272-what-is-a-virus.html January 2016 [cited 2021 Jan 07]; Available from.
- 5.Krug R.M.a.W., Robert R. Virus. 2020, November 12. https://www.britannica.com/science/virus Available from.
- 6.Graham B.J. Virus. Glossary of genetic terms. 2020, January 10. https://www.genome.gov/genetics-glossary/Virus [cited 2021 January 9]; Available from.
- 7.Harvey Lodish A.B., Zipursky S. Lawrence, Matsudaira Paul, Baltimore David, Darnell James. In: Viruses: Structure, Function, and Uses., in Molecular Cell Biology. Freeman W.H., editor. 2000. New York. [Google Scholar]
- 8.Ryu W.-S. In: Chapter 2 - Virus Structure. Ryu W.-S., editor. Academic Press; 2017. Molecular virology of human pathogenic viruses; p. 440. [Google Scholar]
- 9.Nomaguchi M., et al. Viral tropism. Front. Microbiol. 2012;3:281. doi: 10.3389/fmicb.2012.00281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maginnis M.S. Virus-receptor interactions: the key to cellular invasion. J. Mol. Biol. 2018;430(17):2590–2611. doi: 10.1016/j.jmb.2018.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Murgolo N., et al. SARS-CoV-2 tropism, entry, replication, and propagation: considerations for drug discovery and development. PLoS Pathog. 2021;17(2) doi: 10.1371/journal.ppat.1009225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gelderblom H.R. In: Medical Microbiology. th, Baron S., editors. 1996. Structure and classification of viruses. Galveston (TX) [PubMed] [Google Scholar]
- 13.Modrow S., Falke D., Truyen U., Schätzl H. Viruses: definition, structure, classification. Mol. Virol. 2013, Aug 12:17–30. [Google Scholar]
- 14.Simmonds P., Aiewsakun P. Virus classification - where do you draw the line? Arch. Virol. 2018;163(8):2037–2046. doi: 10.1007/s00705-018-3938-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Favre G., et al. 2019-nCoV epidemic: what about pregnancies? Lancet. 2020;395(10224) doi: 10.1016/S0140-6736(20)30311-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schwartz D.A., Graham A.L. Potential maternal and infant outcomes from (Wuhan) coronavirus 2019-nCoV infecting pregnant women: lessons from SARS, MERS, and other human coronavirus infections. Viruses. 2020;12(2) doi: 10.3390/v12020194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shorey S., Chan V. Lessons from past epidemics and pandemics and a way forward for pregnant women, midwives and nurses during COVID-19 and beyond: a meta-synthesis. Midwifery. 2020;90 doi: 10.1016/j.midw.2020.102821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mor G., Cardenas I. The immune system in pregnancy: a unique complexity. Am. J. Reprod. Immunol. 2010;63(6):425–433. doi: 10.1111/j.1600-0897.2010.00836.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kwon J.Y., Romero R., Mor G. New insights into the relationship between viral infection and pregnancy complications. Am. J. Reprod. Immunol. 2014;71(5):387–390. doi: 10.1111/aji.12243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Racicot K., Mor G. Risks associated with viral infections during pregnancy. J. Clin. Invest. 2017;127(5):1591–1599. doi: 10.1172/JCI87490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Silasi M., et al. Viral infections during pregnancy. Am. J. Reprod. Immunol. 2015;73(3):199–213. doi: 10.1111/aji.12355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cardenas I., et al. Viral infection of the placenta leads to fetal inflammation and sensitization to bacterial products predisposing to preterm labor. J. Immunol. 2010;185(2):1248–1257. doi: 10.4049/jimmunol.1000289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Haller S.L., et al. Poxviruses and the evolution of host range and virulence. Infect. Genet. Evol. 2014;21:15–40. doi: 10.1016/j.meegid.2013.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schmidt F.I., Bleck C.K., Mercer J. Poxvirus host cell entry. Curr. Opin. Virol. 2012;2(1):20–27. doi: 10.1016/j.coviro.2011.11.007. [DOI] [PubMed] [Google Scholar]
- 25.Massung R.F., et al. Analysis of the complete genome of smallpox variola major virus strain Bangladesh-1975. Virology. 1994;201(2):215–240. doi: 10.1006/viro.1994.1288. [DOI] [PubMed] [Google Scholar]
- 26.Faridi W., Lappin S.L. StatPearls. 2021. Poxviruses. Treasure Island (FL) [Google Scholar]
- 27.CDC, U Smallpox virus. 2017. https://www.cdc.gov/smallpox/index.html July 2012 [cited 2021 Feb 23]; Available from.
- 28.Hassett D.E. Smallpox infections during pregnancy, lessons on pathogenesis from nonpregnant animal models of infection. J. Reprod. Immunol. 2003;60(1):13–24. doi: 10.1016/s0165-0378(03)00038-x. [DOI] [PubMed] [Google Scholar]
- 29.Kisalu N.K., Mokili J.L. Toward understanding the outcomes of monkeypox infection in human pregnancy. J. Infect. Dis. 2017;216(7):795–797. doi: 10.1093/infdis/jix342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McFadden G. Smallpox: an ancient disease enters the modern era of virogenomics. Proc. Natl. Acad. Sci. U. S. A. 2004;101(42):14994–14995. doi: 10.1073/pnas.0406207101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Arita I. Smallpox. 2021 https://www.who.int/health-topics/smallpox#tab=tab_1 2021 [cited 2021 Feb, 12]; Available from. [Google Scholar]
- 32.Gelderblom H.R., Madeley D. Rapid viral diagnosis of orthopoxviruses by electron microscopy: optional or a must? Viruses. 2018;10(4) doi: 10.3390/v10040142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Singh R.K., et al. Emergence and reemergence of vaccinia-like viruses: global scenario and perspectives. Indian J. Virol. 2012;23(1):1–11. doi: 10.1007/s13337-012-0068-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Meyer H., Ehmann R., Smith G.L. Smallpox in the post-eradication era. Viruses. 2020;12(2) doi: 10.3390/v12020138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ochmann S.A.M.R. Smallpox. 2018. https://ourworldindata.org/smallpox [cited 2021 Feb, 12]; Available from.
- 36.Huremović D. Nature Public Health Emergency Collection. 2019, May 16. Brief history of pandemics (pandemics throughout history) pp. 7–35. [Google Scholar]
- 37.Rao A.R., O. World Health . Rao. World Health Organization World Health Organization: Geneva; Geneva, Switzerland: 1972. Clinical smallpox classification and frequency of type of variola major/by A.R. [Google Scholar]
- 38.Administration, U.S.F.a.D Smallpox. Vaccines 2018 23.03. 2018. https://www.fda.gov/vaccines-blood-biologics/vaccines/smallpox [cited 2021 11.05.2021]; Available from.
- 39.Lane J.M. Remaining questions about clinical variola major. Emerg. Infect. Dis. J. 2011;17(4):676. doi: 10.3201/eid1704.101960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nishiura H. Smallpox during pregnancy and maternal outcomes. Emerg. Infect. Dis. 2006;12(7):1119–1121. doi: 10.3201/eid1207.051531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Benning N., Hassett D.E. Vaccinia virus infection during murine pregnancy: a new pathogenesis model for vaccinia fetalis. J. Virol. 2004;78(6):3133–3139. doi: 10.1128/JVI.78.6.3133-3139.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Töndury G., Kistler G. Die Gefährdung des Ungeborenen bei Pockenschutzimpfung in graviditate. Z. Praventivmed. 1973;18(1):45–50. [Google Scholar]
- 43.US, I.o.M . National Academies Press (US); Washington, DC: 1999. Smallpox and its Control, in Assessment of Future Scientific Needs for Live Variola Virus. [PubMed] [Google Scholar]
- 44.Doms R.W., Blumenthal R., Moss B. Fusion of intra- and extracellular forms of vaccinia virus with the cell membrane. J. Virol. 1990;64(10):4884–4892. doi: 10.1128/jvi.64.10.4884-4892.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Moss B. Poxvirus cell entry: how many proteins does it take? Viruses. 2012;4(5):688–707. doi: 10.3390/v4050688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Moss B. Vol. 60. Semin Cell Dev Biol; 2016. Membrane fusion during poxvirus entry; pp. 89–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Laliberte J.P., Weisberg A.S., Moss B. The membrane fusion step of vaccinia virus entry is cooperatively mediated by multiple viral proteins and host cell components. PLoS Pathog. 2011;7(12) doi: 10.1371/journal.ppat.1002446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Louten J. In: Poxviruses, in Essential Human Virology. Press A., editor. 2016. pp. 276–290. USA. [Google Scholar]
- 49.Schmidt F.I., et al. Vaccinia extracellular virions enter cells by macropinocytosis and acid-activated membrane rupture. EMBO J. 2011;30(17):3647–3661. doi: 10.1038/emboj.2011.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Moser T.S., et al. A kinome RNAi screen identified AMPK as promoting poxvirus entry through the control of actin dynamics. PLoS Pathog. 2010;6(6) doi: 10.1371/journal.ppat.1000954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Amara A., Mercer J. Viral apoptotic mimicry. Nat. Rev. Microbiol. 2015;13(8):461–469. doi: 10.1038/nrmicro3469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Morizono K., et al. The soluble serum protein Gas6 bridges virion envelope phosphatidylserine to the TAM receptor tyrosine kinase Axl to mediate viral entry. Cell Host Microbe. 2011;9(4):286–298. doi: 10.1016/j.chom.2011.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Moller-Tank S., Maury W. Phosphatidylserine receptors: enhancers of enveloped virus entry and infection. Virology. 2014;468-470:565–580. doi: 10.1016/j.virol.2014.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ja-Young Kwon Y.P., Kwon Ha-Yan, Maeng Yong-Sun, Aldo Paulomi Bole, Kim Se Hoon. In: Reproductive Immunology. Mor G., editor. Academic Press; 2021. AM receptors in pregnancy; pp. 349–363. [Google Scholar]
- 55.Roy S.G. In: International Review of Cell and Molecular Biology. Davra L.G. Viralkumar., editor. Academic Press; 2020. TAM receptors: a phosphatidylserine receptor family and its implications in viral infections; pp. 81–122. [DOI] [PubMed] [Google Scholar]
- 56.Shao X., et al. Placental trophoblast syncytialization potentiates macropinocytosis via mTOR signaling to adapt to reduced amino acid supply. Proc. Natl. Acad. Sci. U. S. A. 2021;118(3) doi: 10.1073/pnas.2017092118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Delorme-Axford E., et al. Human placental trophoblasts confer viral resistance to recipient cells. Proc. Natl. Acad. Sci. U. S. A. 2013;110(29):12048–12053. doi: 10.1073/pnas.1304718110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Norskov-Lauritsen N., et al. In vitro infection of human placental trophoblast by wild-type vaccinia virus and recombinant virus expressing HIV envelope glycoprotein. Res. Virol. 1992;143(5):321–328. doi: 10.1016/s0923-2516(06)80120-2. [DOI] [PubMed] [Google Scholar]
- 59.WHO . 2018. Influenza Fact Sheet. [Google Scholar]
- 60.Fernandes-Matano L., et al. Analysis of influenza data generated by four epidemiological surveillance laboratories in Mexico, 2010-2016. Epidemiol. Infect. 2019;147 doi: 10.1017/S0950268819000694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bouneb R., et al. Characteristics and outcome of ill critical patients with influenza A infection. Pan Afr. Med. J. 2018;29:174. doi: 10.11604/pamj.2018.29.174.13098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Arbeitskreis Blut U. Influenza virus. Transfus. Med. Hemother. 2009;36(1):32–39. doi: 10.1159/000197314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Louten J. In: Essential Human Virology. Press A., editor. 2016. Influenza virus; pp. 171–191. USA. [Google Scholar]
- 64.Dubovi E.J. In: Orthomixoviridae, in Fenner’s Veterinary Virology. MacLachlan E.J.D.N. James., editor. 2017. pp. 389–410. [Google Scholar]
- 65.Kim J.I., et al. Reassortment compatibility between PB1, PB2, and HA genes of the two influenza B virus lineages in mammalian cells. Sci. Rep. 2016;6:27480. doi: 10.1038/srep27480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yoo E. Conformation and linkage studies of specific oligosaccharides related to H1N1, H5N1, and human flu for developing the second Tamiflu. Biomol. Ther. (Seoul) 2014;22(2):93–99. doi: 10.4062/biomolther.2014.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Webster R.G., Govorkova E.A. Continuing challenges in influenza. Ann. N. Y. Acad. Sci. 2014;1323:115–139. doi: 10.1111/nyas.12462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Salazar M.I., et al. The origin of the genetic variability of influenza viruses. Gac. Med. Mex. 2010;146(3):199–206. [PubMed] [Google Scholar]
- 69.Garcia-Garcia J., Ramos C. Influenza, an existing public health problem. Salud Publica Mex. 2006;48(3):244–267. doi: 10.1590/s0036-36342006000300009. [DOI] [PubMed] [Google Scholar]
- 70.Puig-Barbera J., et al. Influenza epidemiology and influenza vaccine effectiveness during the 2014–2015 season: annual report from the global influenza hospital surveillance network. BMC Public Health. 2016;16(Suppl. 1):757. doi: 10.1186/s12889-016-3378-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Munoz-Medina J.E., et al. In Silico identification of highly conserved epitopes of influenza A H1N1, H2N2, H3N2, and H5N1 with diagnostic and vaccination potential. Biomed. Res. Int. 2015;2015:813047. doi: 10.1155/2015/813047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.van Riel D., et al. Influenza pathogenicity during pregnancy in women and animal models. Semin. Immunopathol. 2016;38(6):719–726. doi: 10.1007/s00281-016-0580-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Beigi R.H. Influenza during pregnancy: a cause of serious infection in obstetrics. Clin. Obstet. Gynecol. 2012;55(4):914–926. doi: 10.1097/GRF.0b013e31827146bd. [DOI] [PubMed] [Google Scholar]
- 74.Meijer W.J., et al. Influenza virus infection in pregnancy: a review. Acta Obstet. Gynecol. Scand. 2015;94(8):797–819. doi: 10.1111/aogs.12680. [DOI] [PubMed] [Google Scholar]
- 75.Fell D.B., et al. Maternal influenza and birth outcomes: systematic review of comparative studies. BJOG. 2017;124(1):48–59. doi: 10.1111/1471-0528.14143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mertz D., et al. Pregnancy as a risk factor for severe outcomes from influenza virus infection: a systematic review and meta-analysis of observational studies. Vaccine. 2017;35(4):521–528. doi: 10.1016/j.vaccine.2016.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gunnes N., et al. Seasonal and pandemic influenza during pregnancy and risk of fetal death: a Norwegian registry-based cohort study. Eur. J. Epidemiol. 2020;35(4):371–379. doi: 10.1007/s10654-020-00600-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bowkalow S., et al. Severe H1N1-infection during pregnancy. Arch. Gynecol. Obstet. 2011;284(5):1133–1135. doi: 10.1007/s00404-011-1967-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Raj R.S., Bonney E.A., Phillippe M. Influenza, immune system, and pregnancy. Reprod. Sci. 2014;21(12):1434–1451. doi: 10.1177/1933719114537720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Rasmussen I.S., et al. The association between seasonal influenza-like illness cases and foetal death: a time series analysis. Epidemiol. Infect. 2018:1–7. doi: 10.1017/S0950268818003254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Luteijn J.M., Brown M.J., Dolk H. Influenza and congenital anomalies: a systematic review and meta-analysis. Hum. Reprod. 2014;29(4):809–823. doi: 10.1093/humrep/det455. [DOI] [PubMed] [Google Scholar]
- 82.Yawn D.H., et al. Transplacental transfer of influenza virus. JAMA. 1971;216(6):1022–1023. [PubMed] [Google Scholar]
- 83.Jewett J.F. Influenza pneumonia at term. N. Engl. J. Med. 1974;291(5):256–257. doi: 10.1056/NEJM197408012910513. [DOI] [PubMed] [Google Scholar]
- 84.McGregor J.A., et al. Transplacental passage of influenza A/Bangkok (H3N2) mimicking amniotic fluid infection syndrome. Am. J. Obstet. Gynecol. 1984;149(8):856–859. doi: 10.1016/0002-9378(84)90604-5. [DOI] [PubMed] [Google Scholar]
- 85.Gu J., et al. H5N1 infection of the respiratory tract and beyond: a molecular pathology study. Lancet. 2007;370(9593):1137–1145. doi: 10.1016/S0140-6736(07)61515-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Liong S., et al. Influenza A virus causes maternal and fetal pathology via innate and adaptive vascular inflammation in mice. Proc. Natl. Acad. Sci. U. S. A. 2020;117(40):24964–24973. doi: 10.1073/pnas.2006905117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Memoli M.J., et al. Influenza in pregnancy. Influenza Other Respir. Viruses. 2013;7(6):1033–1039. doi: 10.1111/irv.12055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Neumann G., Kawaoka Y. Reverse genetics of influenza virus. Virology. 2001;287(2):243–250. doi: 10.1006/viro.2001.1008. [DOI] [PubMed] [Google Scholar]
- 89.Suzuki Y., et al. Sialic acid species as a determinant of the host range of influenza A viruses. J. Virol. 2000;74(24):11825–11831. doi: 10.1128/jvi.74.24.11825-11831.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Matrosovich M., et al. Early alterations of the receptor-binding properties of H1, H2, and H3 avian influenza virus hemagglutinins after their introduction into mammals. J. Virol. 2000;74(18):8502–8512. doi: 10.1128/jvi.74.18.8502-8512.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Yao L., et al. Avian influenza receptor expression in H5N1-infected and noninfected human tissues. FASEB J. 2008;22(3):733–740. doi: 10.1096/fj.06-7880com. [DOI] [PubMed] [Google Scholar]
- 92.Komine-Aizawa S., et al. H1N1/09 influenza A virus infection of immortalized first trimester human trophoblast cell lines. Am. J. Reprod. Immunol. 2012;68(3):226–232. doi: 10.1111/j.1600-0897.2012.01172.x. [DOI] [PubMed] [Google Scholar]
- 93.Trinh Q.D., et al. H3N2 influenza A virus replicates in immortalized human first trimester trophoblast cell lines and induces their rapid apoptosis. Am. J. Reprod. Immunol. 2009;62(3):139–146. doi: 10.1111/j.1600-0897.2009.00723.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Rosztoczy I., et al. Replication of influenza virus in organ cultures of human and simian urogenital tissues and human foetal tissues. Br. J. Exp. Pathol. 1975;56(4):322–328. [PMC free article] [PubMed] [Google Scholar]
- 95.Uchide N., et al. Differential mRNA expression of inflammatory cytokines in cultured human fetal membrane cells responding to influenza virus infection. Biol. Pharm. Bull. 2002;25(2):239–243. doi: 10.1248/bpb.25.239. [DOI] [PubMed] [Google Scholar]
- 96.Dick G.W., Kitchen S.F., Haddow A.J. Zika virus. I. Isolations and serological specificity. Trans. R. Soc. Trop. Med. Hyg. 1952;46(5):509–520. doi: 10.1016/0035-9203(52)90042-4. [DOI] [PubMed] [Google Scholar]
- 97.Gasco S., Munoz-Fernandez M.A. A review on the current knowledge on ZIKV infection and the interest of organoids and nanotechnology on development of effective therapies against Zika infection. Int. J. Mol. Sci. 2020;22(1) doi: 10.3390/ijms22010035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Agrelli A., et al. ZIKA virus entry mechanisms in human cells. Infect. Genet. Evol. 2019;69:22–29. doi: 10.1016/j.meegid.2019.01.018. [DOI] [PubMed] [Google Scholar]
- 99.Musso D. Zika virus transmission from French Polynesia to Brazil. Emerg. Infect. Dis. 2015;21(10):1887. doi: 10.3201/eid2110.151125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Duffy M.R., et al. Zika virus outbreak on Yap Island, Federated States of Micronesia. N. Engl. J. Med. 2009;360(24):2536–2543. doi: 10.1056/NEJMoa0805715. [DOI] [PubMed] [Google Scholar]
- 101.Sanchez Clemente N., et al. Zika virus infection in pregnancy and adverse fetal outcomes in Sao Paulo State, Brazil: a prospective cohort study. Sci. Rep. 2020;10(1):12673. doi: 10.1038/s41598-020-69235-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Musso D., Ko A.I., Baud D. Zika virus infection - after the pandemic. N. Engl. J. Med. 2019;381(15):1444–1457. doi: 10.1056/NEJMra1808246. [DOI] [PubMed] [Google Scholar]
- 103.Shapiro-Mendoza C.K., et al. Pregnancy outcomes after maternal Zika virus infection during pregnancy - U.S. territories, January 1, 2016-April 25, 2017. MMWR Morb Mortal Wkly Rep. 2017;66(23):615–621. doi: 10.15585/mmwr.mm6623e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.WHO Zika virus. 2018. 2018, July, 20. https://www.who.int/news-room/fact-sheets/detail/zika-virus [cited 2021 February, 17]; Available from.
- 105.Sheridan M.A., et al. African and Asian strains of Zika virus differ in their ability to infect and lyse primitive human placental trophoblast. PLoS One. 2018;13(7) doi: 10.1371/journal.pone.0200086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Quicke K.M., et al. Zika virus infects human placental macrophages. Cell Host Microbe. 2016;20(1):83–90. doi: 10.1016/j.chom.2016.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Sheridan M.A., et al. Vulnerability of primitive human placental trophoblast to Zika virus. Proc. Natl. Acad. Sci. U. S. A. 2017;114(9):E1587–E1596. doi: 10.1073/pnas.1616097114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Hermanns K., et al. Zika virus infection in human placental tissue explants is enhanced in the presence of dengue virus antibodies in-vitro. Emerg. Microbes Infect. 2018;7(1):198. doi: 10.1038/s41426-018-0199-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Bardina S.V., et al. Enhancement of Zika virus pathogenesis by preexisting antiflavivirus immunity. Science. 2017;356(6334):175–180. doi: 10.1126/science.aal4365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Schaub B., et al. Analysis of blood from Zika virus-infected fetuses: a prospective case series. Lancet Infect. Dis. 2017;17(5):520–527. doi: 10.1016/S1473-3099(17)30102-0. [DOI] [PubMed] [Google Scholar]
- 111.de Noronha L., et al. Zika virus infection at different pregnancy stages: anatomopathological findings, target cells and viral persistence in placental tissues. Front. Microbiol. 2018;9:2266. doi: 10.3389/fmicb.2018.02266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Rabelo K., et al. Zika induces human placental damage and inflammation. Front. Immunol. 2020;11:2146. doi: 10.3389/fimmu.2020.02146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Bayer A., et al. Chromosome 19 microRNAs exert antiviral activity independent from type III interferon signaling. Placenta. 2018;61:33–38. doi: 10.1016/j.placenta.2017.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Li M., et al. Characterization of Zika virus endocytic pathways in human glioblastoma cells. Front. Microbiol. 2020;11:242. doi: 10.3389/fmicb.2020.00242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Miranda J., et al. Syncytiotrophoblast of placentae from women with Zika virus infection has altered tight junction protein expression and increased Paracellular permeability. Cells. 2019;8(10) doi: 10.3390/cells8101174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Chiu C.F., et al. The mechanism of the Zika virus crossing the placental barrier and the blood-brain barrier. Front. Microbiol. 2020;11:214. doi: 10.3389/fmicb.2020.00214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Elrashdy F., Redwan E.M., Uversky V.N. Why COVID-19 transmission is more efficient and aggressive than viral transmission in previous coronavirus epidemics? Biomolecules. 2020;10(9) doi: 10.3390/biom10091312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Liu D.X., et al. Accessory proteins of SARS-CoV and other coronaviruses. Antivir. Res. 2014;109:97–109. doi: 10.1016/j.antiviral.2014.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Zhou P., et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579(7798):270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Wrapp D., et al. Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science. 2020;367(6483):1260–1263. doi: 10.1126/science.abb2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.WHO issues consensus document on the epidemiology of SARS. Wkly Epidemiol. Rec. 2003;78(43):373–375. [PubMed] [Google Scholar]
- 122.WHO MERS global summary and assessment of risk. 2018. https://www.who.int/publications/i/item/10665-326126 2019 [cited 2021 January, 04]; Available from.
- 123.WHO . 2021. WHO Coronavirus Disease (COVID-19) Dashboard. [Google Scholar]
- 124.Di Mascio D., et al. Outcome of coronavirus spectrum infections (SARS, MERS, COVID-19) during pregnancy: a systematic review and meta-analysis. Am. J. Obstet. Gynecol. MFM. 2020;2(2) doi: 10.1016/j.ajogmf.2020.100107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Chen L., et al. Clinical characteristics of pregnant women with Covid-19 in Wuhan, China. N. Engl. J. Med. 2020;382(25) doi: 10.1056/NEJMc2009226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Zaigham M., Andersson O. Maternal and perinatal outcomes with COVID-19: a systematic review of 108 pregnancies. Acta Obstet. Gynecol. Scand. 2020;99(7):823–829. doi: 10.1111/aogs.13867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Yan J., et al. Coronavirus disease 2019 in pregnant women: a report based on 116 cases. Am. J. Obstet. Gynecol. 2020;223(1):111 e1–111 e14. doi: 10.1016/j.ajog.2020.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Schwartz D.A. Arch Pathol Lab Med; 2020. An Analysis of 38 Pregnant Women with COVID-19, their Newborn Infants, and Maternal-Fetal Transmission of SARS-CoV-2: Maternal Coronavirus Infections and Pregnancy Outcomes. [DOI] [PubMed] [Google Scholar]
- 129.Yu N., et al. Clinical features and obstetric and neonatal outcomes of pregnant patients with COVID-19 in Wuhan, China: a retrospective, single-centre, descriptive study. Lancet Infect. Dis. 2020;20(5):559–564. doi: 10.1016/S1473-3099(20)30176-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Li N., et al. Maternal and neonatal outcomes of pregnant women with coronavirus disease 2019 (COVID-19) pneumonia: a case-control study. Clin. Infect. Dis. 2020;71(16):2035–2041. doi: 10.1093/cid/ciaa352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Zhang L., et al. Analysis of the pregnancy outcomes in pregnant women with COVID-19 in Hubei Province. Zhonghua Fu Chan Ke Za Zhi. 2020;55(3):166–171. doi: 10.3760/cma.j.cn112141-20200218-00111. [DOI] [PubMed] [Google Scholar]
- 132.Favre G., et al. Guidelines for pregnant women with suspected SARS-CoV-2 infection. Lancet Infect. Dis. 2020;20(6):652–653. doi: 10.1016/S1473-3099(20)30157-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Mullins E., et al. Pregnancy and neonatal outcomes of COVID-19: coreporting of common outcomes from PAN-COVID and AAP-SONPM registries. Ultrasound Obstet. Gynecol. 2021;57(4):573–581. doi: 10.1002/uog.23619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Wei S.Q., et al. The impact of COVID-19 on pregnancy outcomes: a systematic review and meta-analysis. CMAJ. 2021;193(16):E540–E548. doi: 10.1503/cmaj.202604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Yee J., et al. Clinical manifestations and perinatal outcomes of pregnant women with COVID-19: a systematic review and meta-analysis. Sci. Rep. 2020;10(1):18126. doi: 10.1038/s41598-020-75096-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Ksiezakowska K., et al. SARS-CoV infection and pregnancy. Ginekol. Pol. 2008;79(1):47–50. [PubMed] [Google Scholar]
- 137.Wong S.F., et al. Pregnancy and perinatal outcomes of women with severe acute respiratory syndrome. Am. J. Obstet. Gynecol. 2004;191(1):292–297. doi: 10.1016/j.ajog.2003.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Gajbhiye R.K., Modi D.N., Mahale S.D. Pregnancy outcomes, newborn complications and maternal-fetal transmission of SARS-CoV-2 in women with COVID-19: a systematic review of 441 cases. medRxiv. 2020 doi: 10.1002/ijgo.13793. p. 2020.04.11.20062356. [DOI] [Google Scholar]
- 139.Fajnzylber J., et al. SARS-CoV-2 viral load is associated with increased disease severity and mortality. Nat. Commun. 2020;11(1):5493. doi: 10.1038/s41467-020-19057-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Kotlyar A.M., et al. Vertical transmission of coronavirus disease 2019: a systematic review and meta-analysis. Am. J. Obstet. Gynecol. 2021;224(1):35–53. doi: 10.1016/j.ajog.2020.07.049. e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Hosier H., et al. SARS-CoV-2 infection of the placenta. J. Clin. Invest. 2020;130(9):4947–4953. doi: 10.1172/JCI139569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Tan C., et al. SARS-CoV-2 viremia may predict rapid deterioration of COVID-19 patients. Braz. J. Infect. Dis. 2020;24(6):565–569. doi: 10.1016/j.bjid.2020.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Penfield C.A., et al. Detection of severe acute respiratory syndrome coronavirus 2 in placental and fetal membrane samples. Am. J. Obstet. Gynecol. MFM. 2020;2(3) doi: 10.1016/j.ajogmf.2020.100133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Gilbert P., Rudnick C. Newborn antibodies to SARS-CoV-2 detected in cord blood after maternal vaccination. medRxiv. 2021;21:138. doi: 10.1186/s12887-021-02618-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Robertson C.A., et al. SARS and pregnancy: a case report. Emerg. Infect. Dis. 2004;10(2):345–348. doi: 10.3201/eid1002.030736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Jeong S.Y., et al. MERS-CoV infection in a pregnant woman in Korea. J. Korean Med. Sci. 2017;32(10):1717–1720. doi: 10.3346/jkms.2017.32.10.1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Alserehi H., et al. Impact of Middle East respiratory syndrome coronavirus (MERS-CoV) on pregnancy and perinatal outcome. BMC Infect. Dis. 2016;16:105. doi: 10.1186/s12879-016-1437-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Assiri A., et al. Middle East respiratory syndrome coronavirus infection during pregnancy: a report of 5 cases from Saudi Arabia. Clin. Infect. Dis. 2016;63(7):951–953. doi: 10.1093/cid/ciw412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Malik A., et al. Middle East respiratory syndrome coronavirus during pregnancy, Abu Dhabi, United Arab Emirates, 2013. Emerg. Infect. Dis. 2016;22(3):515–517. doi: 10.3201/eid2203.151049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Payne D.C., et al. Stillbirth during infection with Middle East respiratory syndrome coronavirus. J. Infect. Dis. 2014;209(12):1870–1872. doi: 10.1093/infdis/jiu068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Hsu A.L., et al. Placental SARS-CoV-2 in a pregnant woman with mild COVID-19 disease. J. Med. Virol. 2021;93(2):1038–1044. doi: 10.1002/jmv.26386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Facchetti F., et al. SARS-CoV2 vertical transmission with adverse effects on the newborn revealed through integrated immunohistochemical, electron microscopy and molecular analyses of placenta. EBioMedicine. 2020;59 doi: 10.1016/j.ebiom.2020.102951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Menter T., et al. Placental pathology findings during and after SARS-CoV-2 infection: features of Villitis and Malperfusion. Pathobiology. 2021;88(1):69–77. doi: 10.1159/000511324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Hecht J.L., et al. SARS-CoV-2 can infect the placenta and is not associated with specific placental histopathology: a series of 19 placentas from COVID-19-positive mothers. Mod. Pathol. 2020;33(11):2092–2103. doi: 10.1038/s41379-020-0639-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Zhang P., et al. Detection of severe acute respiratory syndrome coronavirus 2 in placentas with pathology and vertical transmission. Am. J. Obstet. Gynecol. MFM. 2020;2(4) doi: 10.1016/j.ajogmf.2020.100197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Algarroba G.N., et al. Visualization of severe acute respiratory syndrome coronavirus 2 invading the human placenta using electron microscopy. Am. J. Obstet. Gynecol. 2020;223(2):275–278. doi: 10.1016/j.ajog.2020.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Control, E.C.f.D.P.a Diagnostic testing and screening for SARS-CoV-2. COVID-19 2021 21.05. 2021. https://www.ecdc.europa.eu/en/covid-19/latest-evidence/diagnostic-testing [Cited 2021 23.05]; Available from.
- 158.Vivanti A.J., et al. Transplacental transmission of SARS-CoV-2 infection. Nat. Commun. 2020;11(1):3572. doi: 10.1038/s41467-020-17436-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Yin Y., Wunderink R.G. MERS, SARS and other coronaviruses as causes of pneumonia. Respirology. 2018;23(2):130–137. doi: 10.1111/resp.13196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Totura A.L., Baric R.S. SARS coronavirus pathogenesis: host innate immune responses and viral antagonism of interferon. Curr. Opin. Virol. 2012;2(3):264–275. doi: 10.1016/j.coviro.2012.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Ashary N., et al. Single-cell RNA-seq identifies cell subsets in human placenta that highly expresses factors driving pathogenesis of SARS-CoV-2. Front. Cell Dev. Biol. 2020;8:783. doi: 10.3389/fcell.2020.00783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Wang K., et al. SARS-CoV-2 invades host cells via a novel route: CD147-spike protein. bioRxiv. 2020;2020.03.14.988345 doi: 10.1038/s41392-020-00426-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Hoffmann M., et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181(2):271–280. doi: 10.1016/j.cell.2020.02.052. e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Patel V.B., et al. Role of the ACE2/angiotensin 1-7 axis of the renin-angiotensin system in heart failure. Circ. Res. 2016;118(8):1313–1326. doi: 10.1161/CIRCRESAHA.116.307708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Bloise E., et al. Expression of severe acute respiratory syndrome coronavirus 2 cell entry genes, angiotensin-converting enzyme 2 and transmembrane protease serine 2, in the placenta across gestation and at the maternal-fetal interface in pregnancies complicated by preterm birth or preeclampsia. Am. J. Obstet. Gynecol. 2020;224(3):298.e1–298.e8. doi: 10.1016/j.ajog.2020.08.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Narang K., et al. SARS-CoV-2 infection and COVID-19 during pregnancy: a multidisciplinary review. Mayo Clin. Proc. 2020;95(8):1750–1765. doi: 10.1016/j.mayocp.2020.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Connors J.M., Levy J.H. COVID-19 and its implications for thrombosis and anticoagulation. Blood. 2020;135(23):2033–2040. doi: 10.1182/blood.2020006000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Zhou J., et al. Active replication of Middle East respiratory syndrome coronavirus and aberrant induction of inflammatory cytokines and chemokines in human macrophages: implications for pathogenesis. J. Infect. Dis. 2014;209(9):1331–1342. doi: 10.1093/infdis/jit504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Gomez-Rial J., et al. Role of monocytes/macrophages in Covid-19 pathogenesis: implications for therapy. Infect. Drug Resist. 2020;13:2485–2493. doi: 10.2147/IDR.S258639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Seethy A.A., et al. Potential SARS-CoV-2 interactions with proteins involved in trophoblast functions - an in-silico study. Placenta. 2020;103:141–151. doi: 10.1016/j.placenta.2020.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Qiu L., et al. SARS-CoV-2 is not detectable in the vaginal fluid of women with severe COVID-19 infection. Clin. Infect. Dis. 2020;71(15):813–817. doi: 10.1093/cid/ciaa375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Zhu H., et al. Clinical analysis of 10 neonates born to mothers with 2019-nCoV pneumonia. Transl. Pediatr. 2020;9(1):51–60. doi: 10.21037/tp.2020.02.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Lu M., et al. Single-cell expression profiles of ACE2 and TMPRSS2 reveals potential vertical transmission and fetus infection of SARS-CoV-2. Aging (Albany NY) 2020;12(20):19880–19897. doi: 10.18632/aging.104015. [DOI] [PMC free article] [PubMed] [Google Scholar]