Abstract
Data on clinical outcomes of patients hospitalized with coronavirus disease 2019 (COVID-19) in private health facilities in Uganda is scarce. We conducted a retrospective cohort study of patients hospitalized with COVID-19 at Case Hospital, Kampala, Uganda, between June 2020 and September 2021. Data of 160 participants (median age 45 years (interquartile range [IQR]: 37–57) and 63.5% male) was analyzed. Seventy-seven (48.1%) participants had non-severe, 18 (11.3%) severe, and 83 (51.9%) critical COVID-19 illness. In 62 participants with chest computed tomography findings, 54 (87%) had bilateral disease, with 22 (35%) having ground-glass opacities. The median duration of hospitalization was 5 days (IQR: 3–9 days). Overall, 18 (11.3%) participants died. Survival at 14 and 28 days was 89% and 72%, respectively. Factors strongly associated with all-cause mortality were as follows: age >50 years (odds ratio [OR]: 8.6, 95% confidence interval [CI]: 1.1–69.2, and p=0.042), having at least 1 comorbidity (OR: 3.2, 95% CI: 1.1–8.9, and p=0.029), hypertension (OR: 3.2, 95% CI: 1.2–8.6, and p=0.024), diabetes mellitus (OR: 2.9, 95% CI: 1.0–8.5, andp=0.056), and oxygen saturation <92% (OR: 5.1, 95% CI: 1.8–14.4, and p=0.002). In this private health facility, mortality was about 1 in 10 patients, and more people presented with critical illness in the second wave of the pandemic, and most deaths occurred after 2 weeks of hospitalization.
1. Introduction
The coronavirus disease 2019 (COVID-19), which was first reported as a pneumonia of unknown etiology in Wuhan, Hubei Province, China, at the end of 2019, is an emerging viral illness caused by the novel beta coronavirus known as severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) [1, 2]. COVID-19 is an important global public health concern and is currently the leading cause of death from a single infectious agent, with an estimated 530 million cases resulting in over 6.3 million deaths in about 30 months [3].
COVID-19 is a multisystemic disease with varying clinical manifestations and severity depending on several host factors such as age, immune status, and the presence of co-morbidities [4, 5]. However, recently, some variants of SARS CoV-2 have been shown to be more transmissible and pathogenic and are associated with more symptomatic disease, severe/critical illness, and breakthrough infections in patients who have received the COVID-19 vaccine [6]. Most patients with COVID-19 are asymptomatic [7]. In those who are symptomatic; cough, chest pain, loss of smell, and nasal congestion as well as other extrapulmonary symptoms such as fever, joint paints, and headaches are the most common symptoms [8, 9]. However, about 5 to 15% of patients present with severe and/or critical disease characterized by acute respiratory distress syndrome and/or coagulopathy due to an exaggerated host immune response, also known as, the cytokine storm [10]. Previous studies have identified risk factors for severe/critical COVID-19 illness as the presence of comorbidities such as diabetes mellitus, hypertension, and obesity [11–13]. These are the group of individuals with the highest risk of mortality and morbidity due to COVID-19 [14].
Uganda has experienced 3 waves of the COVID-19 pandemic and recently experienced the third wave, with a rapid increase in the number of cases in mid-December 2021 and confirmation of the omicron variant, which spread very rapidly, globally. In the first wave, the first case of COVID-19 was reported on the 20th of March 2020, and in the middle of April 2021, a second surge in the number of COVID-19 cases was observed. As of 27th May 2022, over 164,000 cases and 3,596 COVID-19-related deaths have been reported as the country battles the third wave of the pandemic, predominantly with the omicron variant [3]. The first 100 cases were predominantly asymptomatic or mild cases with 100% survival [15, 16]. However, in the second wave, mortality was as high as 36% at the National COVID-19 Treatment Unit (CTU) [17].
Our understanding of the clinical presentation, radiological features, and outcomes of patients hospitalized with COVID-19 in Uganda is incompletely understood, with currently published studies based only on data from selected CTUs in public health facilities [16, 17]. However, data regarding the clinical presentation, imaging findings, and outcomes of patients with COVID-19 in private facilities in Uganda is still lacking. Here, we report on the clinical characteristics, treatment, and outcomes of patients with COVID-19 hospitalized at a CTU of a major private facility in Uganda.
2. Methods
2.1. Study Design
This was a descriptive, single-center, retrospective cohort study of patients hospitalized with COVID-19 at Case Hospital, Kampala, Uganda, between June 2020 and September 2021.
2.2. Study Setting
Case Hospital (also known as Case Medical Centre), established in 1995, is an urban, private, upscale, tertiary hospital situated in the heart of Kampala, Uganda. Case Hospital offers specialized, multidisciplinary healthcare in an 80-bed facility with a fully equipped intensive care unit, laboratory facilities, and state-of-the-art imaging modalities. Case Hospital started receiving patients with COVID-19 in early May 2020. The hospital has been accredited by the Ministry of Health of Uganda as a CTU.
2.3. Study Population
We included data of all patients hospitalized with a confirmed diagnosis of COVID-19 during the study period. COVID-19 was diagnosed by polymerase chain reaction and SARS-CoV-2 rapid antigen test, with or without a compatible chest imaging. We imaging. We excluded patients transferred out of the facility for whom outcome records could not be traced.
2.4. Data Collection
Three trained research assistants collected data regarding demographics (age, sex, occupation, level of education, etc.), comorbidities, HIV status, clinical symptoms and duration, clinical findings, severity of the disease, radiological findings, laboratory findings, prescription information concerning drugs (dose and duration), interventions (oxygen and mechanical ventilation), duration of hospitalization, and outcomes at discharge (alive or dead).
2.5. Definition of COVID-19 Disease Severity
Disease severity was defined according to the World Health Organization (WHO) Severity definitions [18].
2.5.1. Critical COVID-19
Defined by the criteria for acute respiratory distress syndrome (ARDS), sepsis, septic shock, or other conditions that would normally require the provision of life-sustaining therapies such as mechanical ventilation (invasive or noninvasive) or vasopressor therapy.
2.5.2. Severe COVID-19
Defined by any of (1) oxygen saturation <90% on room air, (2) respiratory rate >30 breaths/min in adults, (3) signs of severe respiratory distress (accessory muscle use, inability to complete full sentences, and, in children, very severe chest wall indrawing, grunting, central cyanosis, or the presence of any other general danger signs).
2.5.3. Non-Severe COVID-19
Defined as the absence of any criteria for severe or critical COVID-19.
2.6. Data Analysis
Anonymized data was analyzed using STATA version 16.0 (StataCorp LLC, College Station, Texas, USA). We summarized continuous variables using means with standard deviations or medians with interquartile range (IQR) and categorical variables using frequencies and percentages. Chi-square test was performed to compare categorical variables, and Mann-Whitney U and t-tests for nonparametric and parametric continuous variables, respectively. A multivariable logistic regression model was constructed for the analysis of treatment outcomes accounting for all important confounders. The results of the logistic regression models were presented as odds ratios (ORs) with 95% confidence intervals (95% CIs). Kaplan–Meier survival analysis was performed to determine 14- and 28-day in-hospital mortality and time-to-event. For all analyses, p < 0.05 was considered statistically significant.
2.7. Ethical Considerations
The Mulago Hospital Research and Ethics Committee (MHREC) approved the study protocol and provided a waiver of informed consent of the study participants (approval reference: MHREC 2065). All ethical principles outlined in the Declaration of Helsinki were observed.
3. Results
3.1. Baseline Characteristics
Data of a total of 160 participants was eligible for analysis (Table 1). The median age was 45 years (IQR: 37–57) and 61 (38.9%) participants were 50 years of age or older. Sixty-seven (41.9%) participants had at least one comorbidity, with the majority having hypertension (26.9%, n = 43) or diabetes mellitus (16.9%, n = 27). There were surges in admissions between October–December 2020 and April–July 2021, coinciding with the first and second waves of COVID-19 in Uganda (Figure 1).
Table 1.
Demographic and clinical characteristics at admission among participants with confirmed COVID-19 at case hospital.
| Demographics | Frequency/median | Percentage/interquertile range |
|---|---|---|
| Age: (median, interquertile range) years | 45 | 37–57 |
| Age category | ||
| 18–35 | 30 | 19.1 |
| 36–40 | 62 | 39.5 |
| <18 | 4 | 2.6 |
| ≥50 | 61 | 38.9 |
|
| ||
| Sex (n = 159) | ||
| Female | 58 | 36.5 |
| Male | 101 | 63.5 |
|
| ||
| Occupation | ||
| Formal | 10 | 6.3 |
| Informal | 2 | 1.3 |
| Unknown | 148 | 92.5 |
|
| ||
| Comorbidities | ||
| None | 93 | 58.1 |
| At least one comorbidity | 67 | 41.9 |
|
| ||
| Types of comorbidities | ||
| Hypertension | 43 | 26.9 |
| Diabetes | 27 | 16.9 |
| HIV | 3 | 1.9 |
| Others | 13 | 8.1 |
|
| ||
| Symptoms at admission | ||
| Dry cough | 115 | 71.9 |
| General body weakness | 115 | 71.9 |
| Dyspnoea | 102 | 63.8 |
| Fever | 94 | 58.8 |
| Chest pain | 71 | 44.4 |
| Chest discomfort | 41 | 25.6 |
| Sore throat | 24 | 15 |
| Headache | 24 | 15 |
| Loss of smell | 18 | 11.3 |
| Productive cough | 18 | 11.3 |
| Anorexia | 7 | 4.4 |
| Others∗ | 7 | 4.4 |
| Chills | 6 | 3.8 |
| Diarrhea | 5 | 3.1 |
| Haemoptysis | 3 | 1.9 |
| Weight loss | 1 | 0.6 |
|
| ||
| Vital measurements | Median | Interquartile range |
| Systolic blood pressure (mmHg, n = 156) | 131 | 117–144 |
| Diastolic blood pressure (mmHg, n = 156) | 80 | 73–90 |
| Heart rate (beats per minute, n = 158)∗∗ | 90 | 80–104 |
| Respiratory rate (breaths per minute, n = 95)∗∗∗ | 20 | 20–22 |
| Temperature (°C, n = 151)∗∗∗∗ | 36 | 36–37 |
| Random blood sugar (mmol/L, n = 40)∗∗∗∗∗ | 9 | 6–14 |
| SPO2 (%, n = 158)∗∗∗∗∗∗ | 95 | 91–97 |
| Length of hospitalization (days) | 5 | 3–9 |
∗ Other symptoms included vomiting, lower back pain, joint pains, convulsion, abdominal swelling, left-sided weakness, and altered mental state, in one patient each. ∗∗Tachycardia (≥100 beats per minute) was present in 48 (30.4%) patients. ∗∗∗Tachypnea (>20 breaths per minute) was present in 39 (41.1%) patients. ∗∗∗∗Fever (≥38.0°C) was present in 11 (7.3%) patients. ∗∗∗∗∗Hyperglycemia (≥11.1 mmol/L) was present in 18 (45%) patients. ∗∗∗∗∗∗Hypoxia (<92%) was present in 41 (25.9%).
Figure 1.

Trends in admission of patients with COVID-19 at case hospital.
3.2. Clinical Presentation
Dry cough and general body weakness were the most frequent presenting complaints, present in 115 (71.9%) participants each (Table 1). More than half of the participants also had dyspnea (63.8%, n = 102) and fever (58.8%, n = 94). Eighteen (11.3%) participants had loss of smell. Three (1.9%) participants had hemoptysis and 5 (3.1%) had diarrhea. The median durations for all symptoms at admission (Figure 2) were ≤7 days, highest for sore throat (7 days, IQR: 3–7) and productive cough (6 days, IQR: 4–8), and the least for dyspnea (3 days, IQR: 2–7) and anorexia (3 days, IQR: 3–7).
Figure 2.
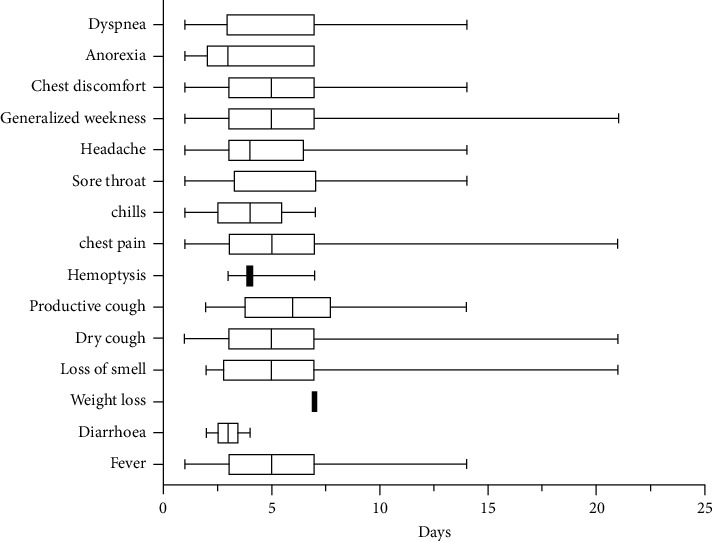
Duration of symptoms among COVID-19 patients at baseline.
3.3. Vital Status at Admission
Table 1 also summarizes the vital measurements at admission. The median systolic and diastolic blood pressure was 131/80 mmHg. About 9 (5.6%) participants had hypotension at the time of admission. Forty-eight (30.4%) participants had tachycardia (heart rate ≥100) and only 4 (2.5%) had bradycardia (heart rate <60 beats per minute). Some 39 (41.1%) participants had tachypnoea, and none had bradypnea. Up to 25.9% (n = 41) of the participant had hypoxia (SpO2 <92%). Median vital measurements remained within normal limits over two weeks of monitoring (Figure 3).
Figure 3.

Vital status of the patients during the first two weeks of admission.
3.4. Chest Imaging
Chest CT and/or X-ray scans were done in 88 (55%) patients and 77 (87.5%) had abnormal findings. The pathologies were reported in 62 chest CT findings, bilateral in 87% (n = 54) and unilateral in 8% (n = 5) participants. Figure 4 shows the chest CT abnormalities recorded in the patient's files. More than one-third (35%, n = 22) noted ground-glass opacities, whereas 21% (n = 13) had findings suggestive of pneumonia. Patchy lung opacities were seen in 8% (n = 5) of the participants' chest CT scans. Bilateral lung fibrosis and interstitial lung disease were observed in one patient each (1.4%). Chest X-ray scans were done in four (2.5%) participants and were abnormal in three participants, which showed interstitial lung disease with fibrosis, basal pneumonia with fibrosis, and atypical pneumonia, each.
Figure 4.

Chest CT findings among patients admitted with COVID-19 at case hospital, Uganda (n = 71). Data label presented as frequency; percentage.
3.5. Laboratory Investigations
The median white blood cell count at admission was 7.2 × 106 cells/mm3 with 34 (21.3%) and 17 (10.6%) having leukocytosis and leukopenia, respectively. Twenty-three (14.4%) participants had thrombocytopenia and 25 (15.6%) had anemia. The median values for renal function tests, electrolytes, and liver function tests were otherwise within normal ranges (Table 2).
Table 2.
Median laboratory parameters among COVID-19 patients admitted at case hospital.
| Laboratory variables | n | Median | Interquartile range |
|---|---|---|---|
| Complete blood count | |||
| White blood cells | 150 | 7.2 | 5.4–10.6 |
| Basophils | 149 | 0.02 | 0.01–0.02 |
| Lymphocytes | 151 | 1.2 | 0.8–1.9 |
| Eosinophils | 148 | 0.01 | 0.0–0.06 |
| Monocytes | 149 | 0.5 | 0.3–0.7 |
| Platelets | 152 | 215 | 176–276 |
| Haemoglobin | 149 | 14.4 | 12.7–15.9 |
| MCV | 149 | 85.2 | 81.1–89.5 |
|
| |||
| Renal function test | |||
| Urea | 129 | 4.1 | 3.1–6.1 |
| Creatinine | 137 | 75.7 | 61.7–96.1 |
| Sodium | 139 | 138 | 134–140 |
| Potassium | 138 | 4.1 | 3.7–6.4 |
| Magnesium | 16 | 0.8 | 0.7–1.1 |
| Calcium | 14 | 1.9 | 1.8–2.1 |
| Chloride | 138 | 102 | 99–110 |
|
| |||
| Liver function test | |||
| ALT | 127 | 34 | 23–59 |
| AST | 121 | 35 | 24–60 |
| ALP | 63 | 63 | 50–87 |
| GGT | 122 | 56 | 33–111 |
| Albumin | 131 | 38.3 | 33.6–41.5 |
| International normalized ratio | 37 | 1.35 | 1.16–1.66 |
| Total bilirubin | 129 | 10.3 | 7.4–14.4 |
| Direct bilirubin | 121 | 5.1 | 3.5–7.0 |
| Vitamin D | 18 | 24.6 | 16.1–138.4 |
3.6. COVID-19 Severity
Seventy-seven (48.1%) participants had non-severe disease, whereas 18 (11.3%) had critical disease (Figure 5). More than half were categorized as severe critical COVID-19 disease (51.9%, n = 83).
Figure 5.

COVID-19 severity among the participants.
3.7. Treatment and Patient Outcomes
Majority of the participants received azithromycin (76.9%), dexamethasone (75.0%), vitamin C supplementation (71.3%), vitamin D supplementation (71.3%), and enoxaparin (70.6%). Only 13 patients (8.1%) received remdesivir, whereas hydroxychloroquine was not given to any patient (Table 3). The dose of azithromycin given to patients was 500 mg once a day. The median duration on treatment was 3 days (interquartile range: 2–5 days). For dexamethasone, 41.2% received 4 mg and 39.5% received 8 mg daily, with a median duration of 3 (IQR: 2.0–4.5) days. About 50% of the patients on enoxaparin were treated with 40 mg daily, 45.5% received 60 mg daily, and 4.5% received 80 mg daily.
Table 3.
Treatment modalities administered to patients admitted at case hospital.
| Medication (N = 160) | Received: frequency (%) | Modal dose (%) | Duration (median days, IQR) |
|---|---|---|---|
| Azithromycin | 123 (76.9) | 500 mg | 3 (2–5) |
| Dexamethasone | 120 (75) | 4 mg (41.2%) | 3 (2–4.5) |
| Vitamin C | 114 (71.3) | 500 mg | 4 (2–5) |
| Vitamin D | 114 (71.3) | 1000 IU | 4 (2–5) |
| Enoxaparin | 113 (70.6) | 40 mg (50%) | 3 (2–5) |
| Zinc | 106 (66.3) | 20 mg | 3 (2–5) |
| Ulinastatin | 96 (60) | 100000 IU (60.2%) | 3 (2–5) |
| Ivermectin | 18 (11.3) | 12 mg | 3.5 (2–5) |
| Remdesivir | 13 (8.1) | 100 mg | 4 (2–4) |
| Warfarin | 1 (0.6) | 2.5 mg | 2 (NA) |
Overall, a total of 18 patients (11.3%) with COVID-19 disease died during the study period. Survival was 99% at 7 days, 89% at 14 days, and 72% at both 21 days and 28 days (Figure 6). At bivariate analysis (Table 4), participants who died were significantly older than those who survived (median age: 58 (IQR: 55–61) versus 42 (IQR: 36–56) years, p < 0.001). Of the 18 participants who died, 14 (78%) were older than 50 years of age. Stratified age was also significantly associated with death (p=0.005). Participants with at least one comorbidity also significantly had a high mortality compared to those who did not (21.8% versus 6.9%, p=0.024). Having hypertension (p=0.026) and diabetes (p=0.048) were significantly associated with mortality. However, mortality did not differ by gender (p=0.805). Participants who died significantly had a higher respiratory rate (24 (20–36) versus 20 (20–22), p=0.002) and a lower SpO2 (87 (75–98) versus 95 (92–97), p=0.031 at admission compared to those who survived. A significantly smaller proportion of participants who received ivermectin (p=0.002) and remdesivir died (p=0.042).
Figure 6.

Survival among the participants.
Table 4.
Distribution of mortality across patient demographics, admission vitals and medications administered to patients with COVID-19 at case hospital.
| Demographics | Died: n (%) N = 18 |
Alive: n (%) N = 142 |
p-value |
|---|---|---|---|
| Age in years | 58 (55–61) | 42 (36–56) | 0.001 |
|
| |||
| Age categories | |||
| 18–35 | 1 (3.3) | 29 (96.7) | 0.005 |
| 36–40 | 3 (4.6) | 62 (95.4) | |
| <18 | 14 (23) | 47 (77) | |
| ≥50 | 0 (0) | 4 (100) | |
|
| |||
| Sex | |||
| Female | 7 (12.1) | 51 (87.9) | 0.805 |
| Male | 11 (10.8) | 91 (89.2) | |
|
| |||
| Occupation | |||
| Formal | 2 (20) | 8 (80) | 0.470 |
| Informal | 0 (0) | 2 (100) | |
| Unknown | 16 (11) | 130 (89) | |
|
| |||
| Comorbidities | |||
| None | 6 (6.5) | 87 (93.5) | 0.024 |
| At least one | 12 (17.9) | 55 (82.1) | |
|
| |||
| Hypertension | |||
| No | 9 (7.7) | 108 (92.3) | 0.026 |
| Yes | 9 (20.9) | 34 (79.1) | |
|
| |||
| Diabetes mellitus | |||
| No | 12 (9) | 121 (91) | 0.048 |
| Yes | 6 (22.2) | 21 (77.8) | |
|
| |||
| HIV | |||
| No | 17 (10.8) | 140 (89.2) | 0.303 |
| Yes | 1 (33.3) | 2 (66.7) | |
|
| |||
| Others | |||
| No | 16 (10.9) | 131 (89.1) | 0.643 |
| Yes | 2 (15.4) | 11 (84.6) | |
|
| |||
| Symptoms at admission | |||
| Asymptomatic | 1 (33.3) | 2 (66.7) | 0.303 |
| Symptomatic | 17 (10.8) | 140 (89.2) | |
|
| |||
| Vitals at admission | |||
| Systolic blood pressure | 131 (116–154) | 131 (117–141) | 0.772 |
| Diastolic blood pressure | 76 (64–82) | 80 (73–90) | 0.151 |
| Heart rate | 96 (83–124) | 89 (80–102) | 0.253 |
| Respiratory rate | 24 (20–36) | 20 (20–22) | 0.002 |
| Temperature | 36.4 (35.9–37.0) | 36.4 (35.4–36.9) | 0.852 |
| Glasgow comma scale | 15 (15–15) | 15 (15–15) | >0.999 |
| Random blood sugar | 10.5 (4.7–16.3) | 8.5 (5.8–14.1) | 0.955 |
| SpO2 | 87 (75–98) | 95 (92–97) | 0.031 |
| ≥92% | 7 (6.0) | 110 (94.0) | 0.001 |
| <92% | 10 (24.4) | 31 (75.6) | |
|
| |||
| Medications | |||
| Azithromycin | 14 (11.4) | 109 (88.6) | >0.999 |
| Dexamethasone | 13 (10.8) | 107 (89.2) | 0.773 |
| Vitamin C | 14 (12.3) | 100 (87.7) | 0.593 |
| Vitamin D | 14 (12.3) | 100 (87.7) | 0.593 |
| Enoxaparin | 15 (13.3) | 98 (86.7) | 0.277 |
| Zinc | 12 (11.3) | 94 (88.7) | 0.968 |
| Ulinastatin | 11 (11.5) | 85 (88.5) | 0.919 |
| Ivermectin | 6 (33.3) | 12 (66.7) | 0.002 |
| Remdesivir | 4 (30.8) | 9 (69.2) | 0.042 |
| Warfarin | 0 (0) | 1 (100) | >0.999 |
3.8. Factors Associated with Mortality
Table 5 shows the factors that were associated with mortality in patients with COVID-19 in this study. Participants aged 50 years and above were 8.6 times (OR: 8.6, 95% CI: 1.1–69.2, p=0.042) more likely to die compared to their younger counterparts (18–35 years). Similarly, patients with at least one comorbidity were three times more likely to die (OR: 3.2, 95% CI: 1.1–8.9, p=0.029). Participants with hypertension (OR: 3.2, 95% CI: 1.2–8.6, p=0.024) and diabetes mellitus (OR: 2.9, 95% CI: 1.0–8.5, p=0.056) were more likely to die than their counterparts. An increase in respiratory rate was associated with a 10% increase in the likelihood to die (OR: 1.1, 95% CI: 1.0–1.2, p=0.002). A SpO2 <92% was associated with 5-fold high odds of dying (OR: 5.1, 95% CI: 1.8–14.4, p=0.002). Patients treated with either ivermectin or remdesivir were 80% less likely to die (Table 5).
Table 5.
Factors associated with mortality due to COVID-19 among the study participants.
| Variables | Odds ratio (95% CI) | p |
|---|---|---|
| Age in years | ||
| 18–35 | 1.0 | |
| 36–40 | 1.4 (0.1–14.1) | 0.773 |
| ≥50 | 8.6 (1.1–69.2) | 0.042 |
|
| ||
| Sex | ||
| Female | 1.0 | |
| Male | 0.9 (0.3–2.4) | 0.805 |
|
| ||
| Occupation | ||
| Formal | 1.0 | |
| Unknown | 0.5 (0.1–2.5) | 0.395 |
|
| ||
| Comorbidities | ||
| None | 1.0 | |
| At least one | 3.2 (1.1–8.9) | 0.029 |
|
| ||
| Hypertension | ||
| No | 1.0 | |
| Yes | 3.2 (1.2–8.6) | 0.024 |
|
| ||
| Diabetes mellitus | ||
| No | 1.0 | |
| Yes | 2.9 (1.0–8.5) | 0.056 |
|
| ||
| HIV | ||
| No | 1.0 | |
| Yes | 4.1 (0.4–47.8) | 0.258 |
|
| ||
| Others | ||
| No | 1.0 | |
| Yes | 1.5 (0.3–7.3) | 0.625 |
|
| ||
| Symptoms at admission | ||
| Asymptomatic | 1.0 | |
| Symptomatic | 0.2 (0–2.8) | 0.258 |
|
| ||
| Vitals at admission | ||
| Systolic blood pressure | 1.0 (1.0–1.0) | 0.879 |
| Diastolic blood pressure | 1.0 (0.9–1.0) | 0.123 |
| Heart rate | 1.0 (1.0–1.0) | 0.207 |
| Respiratory rate | 1.1 (1.0–1.2) | 0.002 |
| Temperature | 1.2 (0.7–2.2) | 0.511 |
| Random blood sugar | 1.0 (0.8–1.2) | 0.945 |
| SpO2 <92 | 5.1 (1.8–14.4) | 0.002 |
|
| ||
| Medications | ||
| Azithromycin | 0.9 (0.3–3.1) | 0.923 |
| Dexamethasone | 1.2 (0.4–3.5) | 0.773 |
| Vitamin C | 0.7 (0.2–2.2) | 0.518 |
| Vitamin D | 0.7 (0.2–2.2) | 0.518 |
| Enoxaparin | 0.4 (0.1–1.6) | 0.219 |
| Zinc | 1.0 (0.3–2.8) | 0.968 |
| Ulinastatin | 0.9 (0.3–2.6) | 0.919 |
| Ivermectin | 0.2 (0.1–0.6) | 0.004 |
| Remdesivir | 0.2 (0.1–0.9) | 0.030 |
4. Discussion
In the beginning of the COVID-19 pandemic in Uganda, all patients diagnosed with COVID-19 were hospitalized only in major public health facilities. Private health facilities were only cleared to manage patients with COVID-19 when the number of cases had significantly increased, overwhelming the public health facilities. This is the first study to describe the clinical outcomes of patients with COVID-19 hospitalized in a private facility in Uganda. In this study, we report several important findings.
First, over half of the patients in our study presented with severe or critical illness, with about 11% of the patients requiring ICU admission and invasive mechanical ventilation. In the study by Bongomin and colleagues at Mulago National Referral Hospital (MNRH) CTU, a public facility and the largest CTU in Uganda, over 80% of patients presented with severe/critical COVID-19 illness, with up to 20% of patients requiring ICU admissions [17]. The observed difference could be because the majority of patients admitted to MNRH CTU are referral cases from across the country. These findings agree with the published data showing a high proportion of patients presenting with severe or critical COVID-19 illness during the second wave attributed to the emergence of delta and other variants of the SARS CoV-2 [19, 20].
Chest radiographs may be normal in early or mild disease. We noted that about 12% of our participants had normal chest CT images. However, of the 80% of the patients who had abnormal chest imaging findings, over 90% had bilateral disease. Chest imaging is a key in the diagnosis of COVID-19, grading its severity, as well as the investigation of complications such as acute respiratory distress syndrome (ARDS), embolism, and pulmonary infections [21, 22]. Although chest CT may be more sensitive than chest radiograph and some chest CT findings may be characteristic of COVID-19, no finding can completely rule in or rule out the possibility of COVID-19. In the United States, the American College of Radiology (ACR) does not recommend using chest CT for screening or diagnosing of COVID-19 and recommends reserving it for hospitalized patients when needed for management. Chest CT in patients with COVID-19 most commonly demonstrates ground-glass opacification with or without consolidative abnormalities, consistent with viral pneumonia [22–24].
Secondly, we found an overall in-hospital mortality of approximately 11%, with a significantly higher mortality of 26% during the second wave compared to 5% during the first wave of the COVID-19 pandemic in Uganda. Our findings are consistent with previous reports from Uganda showing a higher mortality during the second wave of the COVID-19 pandemic in Uganda [16, 17]. However, the mortality reported in this study is much lower than that reported at MNRH CTU [17]. A higher proportion of patients presenting to MNRH CTU had severe/critical illness compared to those in our setting (>80% versus 52%), and challenges related to late presentation and shortage of oxygen and healthcare workers were also reported to have contributed to the high mortality in public health facilities across Uganda. Mortality reported in our study is also much lower than the 32% reported in Cameroon [25], and 48·2% in a cohort study of over 3,000 critically ill patients with COVID-19 pneumonia enrolled in 64 hospitals in ten African countries [26]. In the latter study, in addition to the traditional risk factors for adverse COVID-19 outcome, persons living with HIV/AIDS and those who experienced delayed access to high-care units and ICU had higher mortality rates.
Thirdly, we showed that advanced age, diabetes mellitus, hypertension, and hypoxemia were significantly associated with mortality. These findings are consistent with studies from Uganda [17] and several meta-analyses which showed increased severity and mortality in patients with co-morbidities [27, 28]. The relationship between COVID-19 and comorbidities such as diabetes mellitus and hypertension are bilateral. With COVID-19 increasing the incidence and exacerbating the control of these conditions, and yet these conditions also worsen COVID-19 outcomes [4, 29, 30].
There are currently several drugs recommended by the WHO for the treatment of COVID-19 [31]. These include the Pfizer combination nirmatrelvir and ritonavir, molnupiravir, sotrovimab, and remdesivir for non-severe disease [31]. Molnupiravir is an oral antiviral agent with clinical utility in the treatment of mild moderate disease, shortening the duration of hospitalization, and halting disease progression to severe or critical illness [31]. Corticosteroids like dexamethasone, tocilizumab (IL-6 receptor blocker), and baricitinib (JAK 1 and JAK 2 inhibitor) are indicated in the management of patients with severe to critical COVID-19 [31]. In this study, we found that remdesivir and ivermectin were each associated with an 80% reduction in mortality. Previous studies have shown that remdesivir may be used as an add-on therapy for patients with severe/critical illness [32, 33]. However, recent studies have failed to show any benefit of ivermectin for the treatment of both moderate and severe/critical illness [34].
It is also important to note that only a small proportion of patients in our facility received ivermectin and remdesivir, and the wide confidence interval indicates a lack of precision. Therefore, these positive findings should be cautiously interpreted. The massive use of azithromycin and vitamins in our study is attributed to guidance from the first edition of Uganda national guidelines for COVID-19 management recommending their use even for mild-moderate disease [35]. The frequent use of ulinastatin in our study could be supported by studies that reported some studies that reported some benefits, especially for COVID-19 patients with sepsis, or moderate-severe disease [36, 37]. Our agreement is that clinicians should follow updated guidelines by their respective health authorities/organization while accounting for the evolving data on the safety and efficacy of various therapeutic agents.
Our study has some important limitations. First, it was a retrospective review of medical records and not all relevant data could be obtained for all participants. For example, no data was extracted on COVID-19 vaccination status of the participants. Secondly, this was a single-center study, recruiting mainly participants from higher socioeconomic status around the capital city, and may not be generalized to other private facilities, particularly in upcountry settings. Despite this, we had a robust dataset including clinical, laboratory, and radiological variables, which provides a better understanding of the complete picture of COVID-19 manifestation in Ugandans. Our findings inform clinical practice and future studies to optimize the clinical outcomes of patients with COVID-19 in Uganda and similar settings.
5. Conclusions
In conclusion, in a private clinical practice where about half of the patients presented with severe or critical COVID-19 illness, about 1 in 10 died, with a significantly higher proportion of patients dying in the second wave compared to the first wave of the COVID-19 pandemic in Uganda. Most deaths, however, occurred after 7 days of hospitalization.
Acknowledgments
The patients, clinicians/nurses, and administration of the case hospital. This study was funded by the administration of Case Hospital, Kampala.
Data Availability
The data that support the findings of this study is available on request from the corresponding author and approval from the administration of Case Hospital, Uganda.
Disclosure
Mirriam Apiyo and Ronald Olum are the co-first authors.
Conflicts of Interest
The authors declare no conflicts of interests.
Authors' Contributions
All authors contributed significantly to data collection, analysis & interpretation, drafting the manuscript, and final review of the manuscript prior to submission.
References
- 1.Zhou P., Yang X. L., Wang X.-G., et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature . 2020;579(7798):270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization. Coronavirus Disease (COVID-19) Outbreak . Geneva, Switzerland: World Health Organisation; 2020. [Google Scholar]
- 3.Woldometer. COVID-19 Coronavirus Pandemic . Worldometers.info, Dover, DE, U.S.A; 2021. [Google Scholar]
- 4.Richardson S., Gitlin J., Kozel Z., et al. In-hospital 30-day survival among young adults with coronavirus disease 2019: a cohort study. Open Forum Infectious Diseases . 2021;8(6) doi: 10.1093/ofid/ofab233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Meisenberg B. R., Qureshi S., Monika Thandalam S., Ali Q., Karpman M., Rhule J. COVID-19 mortality in an acute care hospital: association of patient factors with decision to forego the intensive care unit. American Journal of Hospice and Palliative Medicine . 2021;39(4):481–486. doi: 10.1177/10499091211028849. [DOI] [PubMed] [Google Scholar]
- 6.Bergwerk M., Gonen T., Lustig Y., et al. COVID-19 breakthrough infections in vaccinated health care workers. New England Journal of Medicine . 2021;385(16):1474–1484. doi: 10.1056/nejmoa2109072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. WHO. COVID-19 COVID-19. WHO J Covid 19, Reg Situational Updat Africa 2020.
- 8.Dhama K., Khan S., Tiwari R., et al. Coronavirus disease 2019–COVID-19. Clinical Microbiology Reviews . 2020;33(4) doi: 10.1128/CMR.00028-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Atrah H. I. Alternative management of COVID-19 infection. Scottish Medical Journal . 2020;65(3):72–75. doi: 10.1177/0036933020941497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fajgenbaum D. C. Cytokine storm. New England Journal of Medicine . 2020;383(23):2255–2273. doi: 10.1056/nejmra2026131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shaikh K., Shrestha C., Dutta D. Treatment options in people with COVID-19: selecting the best armamentarium against the novel virus. Journal of Pakistan Medical Association . 2020;70(0):1–73. doi: 10.5455/JPMA.22. [DOI] [PubMed] [Google Scholar]
- 12.Ji Y., Ma Z., Peppelenbosch M. P., Pan Q. Potential association between COVID-19 mortality and health-care resource availability. Lancet Global Health . 2020;8(4):p. e480. doi: 10.1016/S2214-109X(20)30068-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhou F., Yu T., Du R., et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. The Lancet . 2020;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grasselli G., Greco M., Zanella A., et al. Risk factors associated with mortality among patients with COVID-19 in intensive care units in lombardy, Italy. JAMA Internal Medicine . 2020;180(10):p. 1345. doi: 10.1001/jamainternmed.2020.3539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Olum R., Bongomin F. Uganda’s first 100 COVID-19 cases: trends and lessons. International Journal of Infectious Diseases . 2020;96:517–518. doi: 10.1016/j.ijid.2020.05.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kirenga B., Muttamba W., Kayongo A., et al. Characteristics and outcomes of admitted patients infected with SARS-CoV-2 in Uganda. BMJ Open Respiratory Research . 2020;7(1) doi: 10.1136/bmjresp-2020-000646.e000646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bongomin F., Fleischer B., Olum R., et al. High mortality during the second wave of the coronavirus disease 2019 (COVID-19) pandemic in Uganda: experience from a national referral COVID-19 treatment unit. Open Forum Infectious Diseases . 2021;8(11) doi: 10.1093/ofid/ofab530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marshall J. C., Murthy S., Diaz J., et al. A minimal common outcome measure set for COVID-19 clinical research. The Lancet Infectious Diseases . 2020;20(8):e192–e197. doi: 10.1016/S1473-3099(20)30483-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Planas D., Veyer D., Baidaliuk A., et al. Reduced sensitivity of SARS-CoV-2 variant delta to antibody neutralization. Nature . 2021;596(7871):276–280. doi: 10.1038/s41586-021-03777-9. [DOI] [PubMed] [Google Scholar]
- 20.Lustig Y., Zuckerman N., Nemet I., et al. Neutralising capacity against Delta (B.1.617.2) and other variants of concern following Comirnaty (BNT162b2, BioNTech/Pfizer) vaccination in health care workers, Israel. Euro Surveillance . 2021;26(26) doi: 10.2807/1560-7917.ES.2021.26.26.2100557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zheng C., Wang J., Guo H., et al. Risk-adapted treatment strategy for COVID-19 patients. International Journal of Infectious Diseases . 2020;94:74–77. doi: 10.1016/j.ijid.2020.03.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.George V., John R., Manesh A., Karthik R., Abraham O. Clinical management of COVID-19. Indian Journal of Medical Research . 2020;151(5):p. 401. doi: 10.4103/ijmr.IJMR_957_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li Y., Xia L. Coronavirus disease 2019 (COVID-19): role of chest CT in diagnosis and management. American Journal of Roentgenology . 2020;214(6):1280–1286. doi: 10.2214/AJR.20.22954. [DOI] [PubMed] [Google Scholar]
- 24.Odedra D., Chahal B. S., Patlas M. N. Impact of COVID-19 on Canadian Radiology residency training programs. Canadian Association of Radiologists Journal . 2020;71(4):482–489. doi: 10.1177/0846537120933215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mekolo D., Bokalli F. A., Fru Mcwright C., et al. Clinical and epidemiological characteristics and outcomes of patients hospitalized for COVID-19 in douala, Cameroon. Pan African Medical Journal . 2021;38 doi: 10.11604/pamj.2021.38.246.28169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Biccard B. M., Pragasan Dean G., Miller M., et al. Patient care and clinical outcomes for patients with COVID-19 infection admitted to African high-care or intensive care units (ACCCOS): a multicentre, prospective, observational cohort study. The Lancet . 2021;397(10288):1885–1894. doi: 10.1016/S0140-6736(21)00441-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moon S. J., Rhee E. J., Jung J. H., et al. Independent impact of diabetes on the severity of coronavirus disease 2019 in 5,307 patients in South Korea: a nationwide cohort study. Diabetes & Metabolism Journal . 2020;44(5):737–746. doi: 10.4093/dmj.2020.0141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Silverio A., Maio Marco D., Citro R., et al. Cardiovascular risk factors and mortality in hospitalized patients with COVID-19: systematic review and meta-analysis of 45 studies and 18,300 patients. BMC Cardiovascular Disorders . 2021;21(1):p. 23. doi: 10.1186/s12872-020-01816-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Matsushita K., Ding N., Kou M., et al. The relationship of COVID-19 severity with cardiovascular disease and its traditional risk factors: a systematic review and meta-analysis. Global Heart . 2020;15(1):p. 64. doi: 10.5334/gh.814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zheng Y. Y., Ma Y. T., Zhang J. Y., Xie X. COVID-19 and the cardiovascular system. Nature Reviews Cardiology . 2020;17(5):259–260. doi: 10.1038/s41569-020-0360-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.World Health Organization. Therapeutics and COVID-19 Living Guideline . Geneva, Switzerland: WHO; 2022. [PubMed] [Google Scholar]
- 32.Beigel J. H., Tomashek K. M., Dodd L. E., et al. Remdesivir for the treatment of COVID-19-final report. New England Journal of Medicine . 2020;383(19):1813–1826. doi: 10.1056/nejmoa2007764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wilt T. J., Kaka A. S., MacDonald R., Greer N., Obley A., Duan-Porter W. Remdesivir for adults with COVID-19: a living systematic review for an American College of physicians practice points. Annals of Internal Medicine . 2020;174 doi: 10.7326/M20-5752.M20-M5752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cruciani M., Pati I., Masiello F., Malena M., Pupella S., De Angelis V. Ivermectin for prophylaxis and treatment of COVID-19: a systematic review and meta-analysis. Diagnostics . 2021;11(9):p. 1645. doi: 10.3390/diagnostics11091645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ministry of Health. National Guidelines for Management of COVID-19 . 1st. Kampala, Uganda: Ministry of Health; 2020. [Google Scholar]
- 36.Huang H., Hu P.-F., Sun L. L., et al. Treatment of patients with COVID-19 with a high dose of ulinastatin. Experimental and Therapeutic Medicine . 2021;23(2):p. 121. doi: 10.3892/etm.2021.11044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ansari A. S. Cytokine storm in novel coronavirus disease (COVID-19): expert management considerations. Indian Journal of Critical Care Medicine . 2020;24(6):429–434. doi: 10.5005/jp-journals-10071-23415. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study is available on request from the corresponding author and approval from the administration of Case Hospital, Uganda.


