Abstract
There is evidence suggesting that ghrelin and peptide YY (PYY) modulate stress responses and the rewarding effects of drugs, although no research has examined the impact of exposure to early life stress on these hormones in smokers nor during smoking cessation. This study examined the relationships between early life adversity (ELA) and circulating ghrelin and PYY during ad libitum smoking and early withdrawal in tobacco smokers (N = 98) who were interested in cessation. We also included a comparison group of nonsmokers (N = 36). We prospectively compared levels of hormones between smokers who were successful in quitting within a 2-week period, smokers who relapsed during that period, and nonsmokers. Results showed that ELA was positively associated with elevated ghrelin in nonsmokers. Among those reporting no ELA, successful quitters had higher ghrelin levels than nonsmokers during ad libitum smoking, while relapsers had higher ghrelin levels than nonsmokers during withdrawal. In addition, having no ELA was associated with a decline in ghrelin from the ad libitum to abstinence sessions in successful quitters; this withdrawal-related decline was not found in relapsers. Although effects of ELA, smoking group, and time on PYY were not significant, greater PYY was associated with reduced urges to smoke during withdrawal. These findings suggest the importance of considering changes in appetite-related hormones in individuals who are dependent on tobacco. This research provides additional indications for effects of ELA on appetite-stimulating hormones.
Keywords: Tobacco withdrawal, Relapse, Early life adversity, Appetite hormones, Ghrelin, Peptide YY
1. Introduction
Accumulating research demonstrates that exposure to early life adversity (ELA) increases risk for various behavioral and addictive problems. This increased risk is likely mediated by alterations in reward-related pathways (Duffy et al., 2018; Lovallo et al., 2017; McLaughlin et al., 2019) and stress response systems (Agorastos et al., 2018; al’Absi et al., 2018; Lovallo et al., 2019a, 2019b). Research has also shown links between ELA and distress, depression, smoking withdrawal symptoms, and smoking relapse (al’Absi et al., 2017; Lemieux et al., 2016; Lovallo et al., 2018). Mechanisms mediating the role of ELA in increasing risk for substance use and relapse are not known, and may involve systems that regulate reward and stress responses. Existing research indicates that various appetite regulating hormones are involved in reward-related pathways of the brain (Jerlhag et al.,et al., 2006; Jerlhag et al., 2009; Malik et al., 2008; Schloegl et al., 2011) and in addiction (Aguiar-Nemer et al., 2013; Lee et al., 2006; Leggio et al., 2012). Therefore, in this study, we examined two appetite regulating hormones, ghrelin and peptide YY (PYY), as potential factors associated with ELA and smoking relapse.
2. Appetite hormones, reward-related pathways, and smoking
Appetite regulating hormones interact directly and indirectly with neurobiological pathways that mediate effects of drug use, including reward systems (Kenny, 2011; Menzies et al., 2012; Volkow et al., 2012). For example, ghrelin, an orexigenic hormone which is released primarily by the stomach, plays an important role in reward/reinforcement and behavior-activating properties of drugs of abuse, such as nicotine, alcohol, and cocaine (Gomez et al., 2015; Vengeliene, 2013). Ghrelin is also positively associated with enhanced craving for addictive substances, such as alcohol (Leggio et al., 2012; Zallar et al., 2017). Research shows that smokers tend to have higher levels of ghrelin than nonsmokers (Wittekind et al., 2020); and decreases in ghrelin levels occur after a period of abstinence from tobacco (Lee et al., 2006). Furthermore, a recent study found lower ghrelin levels in abstaining smokers compared to nonsmokers, suggesting a possible rebound effect (Ardeshiripur et al., 2018). Another study found that high levels of ghrelin during early smoking abstinence are associated with increased risk for smoking relapse, independent of withdrawal or craving (al’Absi et al., 2014). These observations suggest a positive association between smoking and ghrelin levels, an association that is reversed during abstinence, particularly among those likely to remain abstinent.
Less research has focused on PYY, an anorexigenic hormone released from cells in the ileum and colon in response to food intake. Studies indicate that PYY interacts directly and indirectly with neurons in brain regions linked to the rewarding effects of drugs, including the mesolimbic dopaminergic pathway (Batterham et al., 2007; Stadlbauer et al., 2014). Although PYY’s involvement in smoking relapse is not yet clear, preliminary results indicate that higher PYY may be linked with reduced tobacco craving and increased positive affect during smoking abstinence (al’Absi et al., 2014). However, one study found that smoking relapse was associated with increases in PYY during early abstinence (Lemieux and al’Absi M, 2018).
3. Appetite hormones and ELA
Relatively little research has investigated the relationship between ELA and appetite hormones. One preclinical study focusing on the link between early life stress and ghrelin showed that exposure to early stress in mice was associated with reduced ghrelin, especially in females (Yam et al., 2017). To date, only one study has examined the association between ELA and ghrelin in humans and it found that early life stress was associated with higher acylated ghrelin (Yousufzai et al., 2018). Though no studies have examined PYY in the context of ELA, a few preclinical studies have examined the link between neuropeptide Y (NPY) and early life stress (Grove et al., 2001; Miragaia et al., 2018). NPY is expressed in multiple sensory, cerebral, and autonomic pathways; and NPY receptors mediate effects of PYY and directly regulate its function. These studies demonstrate that maternal deprivation in rats is associated with decreased NPY mRNA expression in the hypothalamus (Maniam et al., 2014; Mela et al., 2012) and reduced NPY in the hippocampus and the striatum (Husum and Mathé 2002).
4. This study
To our knowledge, no study has investigated the extent to which ELA alters ghrelin and PYY release in smokers who relapse or successfully quit smoking. Therefore, this study addresses that gap. We report novel findings related to the influence of ELA on levels of circulating ghrelin and PYY during ad libitum smoking and early withdrawal periods among tobacco smokers who were successful or unsuccessful at quitting smoking. We also included a comparison group of nonsmokers to enhance the interpretability of our findings. Given the novelty of the study, we did not have specific predictions for the influence of early life adversity on ghrelin and PYY within the context of smoking cessation. Based on earlier findings, however, we expected that ELA would be associated with elevated ghrelin among nonsmokers. This research will help define the potential role of ELA in altering mechanisms of reward, appetite-stimulating hormones, and risk for smoking relapse. We also obtained subjective measures of craving, withdrawal symptoms, and positive and negative affect to assess the impact of tobacco use and nicotine withdrawal.
5. Method
5.1. Protocol/procedures
Flyers and community postings were used to recruit smokers and nonsmokers to participate in a study on stress, pain processing, and smoking cessation. Interested individuals attended a medical screening to provide informed consent and to determine eligibility, which required: weight within 30% of the Metropolitan Life Insurance norms; no self-reported physician care for acute nor chronic physical or mental health in the last year; consuming an average of two or fewer alcoholic beverages per day; and no use of prescription nor recreational drugs (with exception of contraceptives). In addition, smokers had to report smoking a minimum of 10 cigarettes per day for two or more years; they had to express a desire to quit.
Eligible participants completed two lab sessions, the first of which took place when smokers were smoking ad libitum. The second session took place approximately 48 h after smokers initiated a quit attempt; and they were required to remain abstinent from all tobacco and nicotine for the 48 h between quit initiation and start of the second lab. Abstinence was verified via self-report and by expected reductions in exhaled carbon monoxide levels at the abstinent session. Each lab session began with a resting period, after which participants provided a blood sample (for testing ghrelin and PYY) and they completed measures about their subjective states, withdrawal symptoms, and craving. After the second lab, participants completed several follow-up visits at which smoking relapse was assessed. Here, we focus on early relapse data collected during weekly follow-up visits after the second lab (Fig. 1). All recruitment and study procedures were approved by the Institutional Review Board at the University of Minnesota.
Fig. 1.
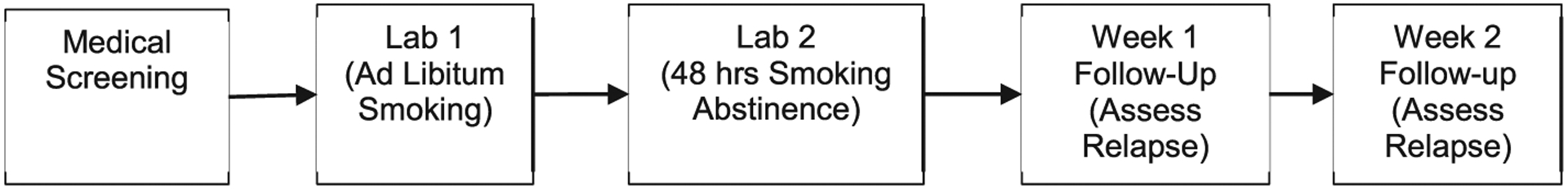
Visit protocol.
6. Measures
Participant Characteristics.
At the medical screening, participants completed several questionnaires regarding their demographics, smoking history, and nicotine dependence (FTND; (Heatherton et al., 1991)). Smokers also rated their desire to quit smoking on a scale that ranged from 1 (no desire) to 7 (very strong desire). In addition, lab staff recorded the height and weight of all participants, which were used to calculate Body Mass Index (BMI).
ELA Groups.
Participants completed a questionnaire based on Anda et al.,’s (Anda et al., 1999) measure of Adverse Childhood Experiences (ACE), which allowed assessment of the presence or absence (scored 1 and 0, respectively) of 8 types of ELAs: verbal abuse, physical abuse, sexual abuse, battered mother, household substance abuse, household mental illness, household member incarceration, parental separation or divorce. Total scores were calculated by summing across the 8 types of ELAs; and participants were binned into three categories for analysis: No ELA (ACE = 0), Low ELA (ACE = 1), and High ELA (ACE >= 2).
Smoking Groups.
At follow-up visits, participants reported daily tobacco use occurring after their quit date. These data were subsequently used to divide smokers into two groups for analysis: Relapsers and Successful Quitters. We defined relapse as 7+ consecutive days of daily tobacco use; and we classified participants as Relapsers if two conditions were met: (a) reported using tobacco daily for 7+ consecutive days and (b) no more than 7 days elapsed between their quit date and their first day of daily tobacco use within the string of 7+ days. Smokers who did not meet these criteria were classified as Successful Quitters; and Nonsmokers recruited in the same study were included as a comparison group.
Ghrelin & PYY.
Blood samples were collected in EDTA tubes after an initial rest period at the beginning of each of the two lab visits. Samples were treated with AEBSF for stabilization (Blatnik and Soderstrom 2011); and samples were tested for plasma total ghrelin and PYY using ELISA kits from EMD Millipore (St. Charles, Missouri; human ghrelin kit # EZGRT-89K; human PYY kit # EZHPYYT66K). All processing and testing followed the ELISA kit manufacturer’s instructions.
Affective measures.
Participants rated the extent to which they were currently experiencing several subjective states and physical symptoms using a response scale that ranged from 0 (Not at all) to 7 (Very Strong). Responses to several items were summed to create indices of positive affect (calmness, cheerful, content, controllability, interest), distress (impatience, irritability, restlessness, anxiety), and smoking withdrawal (irritability, restlessness, anxiety, anger, depressed or sad mood, difficulty concentrating, hunger); and craving was assessed using a single item (desire to smoke; (al’Absi et al., 2007)). Smokers also completed the Questionnaire on Smoking Urges – Brief (QSU-b), which is purported to measure smoking urges associated with positive outcomes of smoking (F1) and smoking urges associated with alleviation of negatives states (F2; (Cox et al., 2001)).
7. Analytic approach
Baseline characteristics were examined and summarized using counts, means, and standard deviations. Differences in baseline characteristics that were relevant to both smokers and nonsmokers were examined using chi-square (and adjusted standardized residuals with Bonferroni adjusted p-values for two-sided tests) and 3 (ELA: No ELA, Low ELA, High ELA) × 3 (Smoking Group: Nonsmokers, Successful Quitters, Relapsers) ANOVA. Baseline characteristics that were only relevant to smokers were examined with 3 (ELA: No ELA, Low ELA, High ELA) × 2 (Relapse Status: Successful Quitters vs. Relapsers) ANOVA.
Previous research indicates significant differences in PYY based on BMI (Cooper 2014); and significant differences in ghrelin based on BMI, age, and sex (e.g., (Lemieux and al’Absi, 2018; Makovey et al., 2007; Rigamonti et al., 2002). We also observed significant relationships between these factors and the hormones in our study; therefore, these factors were included as covariates in our analysis of these hormones. Mixed ANCOVA was used to examine the relationships between ELA (3: No ELA, Low ELA, High ELA), Smoking Groups (3: Nonsmokers, Successful Quitters, Relapsers), and Time (2: Session 1 – ad libitum, Session 2 - withdrawal) on ghrelin and on square-root transformed PYY. Residuals approximated a normal distribution for both analyses. All significant Omnibus tests were followed-up with simple effects tests and pairwise comparisons to which Bonferroni adjustments were applied to account for multiple comparisons.
Mixed ANOVA was used to examine the relationships between ELA (3: No ELA, Low ELA, High ELA), Smoking Groups (3: Nonsmokers, Successful Quitters, Relapsers), and Time (2: Session 1- ad libitum, Session 2 - withdrawal) on positive affect and distress. We also used mixed ANOVA to examine the relationships between ELA (3: No ELA, Low ELA, High ELA), Relapse Status (2: Successful Quitters, Relapsers), and Time (2: Session 1 – ad libitum, Session 2 - withdrawal) on craving, smoking urges, and withdrawal.1
When there was no evidence that residuals deviated substantially from a normal distribution, significant Omnibus tests were followed-up with simple effects tests and pairwise comparisons to which Bonferroni adjustments were applied to account for multiple comparisons. When there was evidence that ANOVA residuals deviated substantially from normal, we employed generalized linear mixed models with a random intercept to test the same sets of predictors. These models utilized a gamma distribution and log link; Sattherwaite approximation and robust covariances were implemented. Significant omnibus tests were followed-up with pairwise comparisons to which sequential Bonferroni adjustment was applied. All analyses were performed using SPSS Version 24.
8. Results
8.1. Baseline characteristics
The final sample consisted of 134 participants (63 female), the majority of whom identified as Caucasian (78%, n = 105). Overall, the study included 36 nonsmokers (17 no ELA, 8 low ELA, 11 high ELA), 54 successful quitters (13 no ELA, 11 low ELA, 30 high ELA), and 44 relapsers (8 no ELA, 13 low ELA, 23 high ELA). There were slightly more Nonsmokers with no ELA than would be expected by chance, χ2 (df 4) =10.49, p < .05, adjusted standardized residual = 2.9, but no other group sizes deviated substantially. All participants were between 18 and 64 years of age (M = 33.80, SD = 12.04); however, there was a significant difference in age between ELA groups, F(2, 125) = 4.99, p < .01, partial η2 = 0.07, such that the No ELA group (EMM = 29.24, SE = 1.93) was substantially younger than the High ELA group (EMM = 37.02, SE = 1.55). There were no significant group differences in BMI, which was an average of 25.51 (SD = 4.04) in this sample.
On average, smokers began using cigarettes at 16.16 (SD = 3.53) years of age; they were moderately dependent on nicotine (FTND, M = 5.44, SD = 2.08); they reported an average of 18.67 (SD = 6.64) cigarettes smoked per day; and their desire to quit smoking was an average of 5.78 (SD = 1.02). Smokers’ mean expired CO was 24.51 (SD = 11.18) at Session 1 (ad libitum) and 4.40 (SD = 3.28) at Session 2 (withdrawal), a difference that was statistically significant, F(1, 91) = 261.45, p < .001, partial η2 = 0.74. There were no differences based on ELA nor relapse status for any of these smoking characteristics (Fs ≤ 3.78, Ps > 0.05).
9. Appetite hormones
Ghrelin.
Omnibus results provided evidence of a three-way interaction between ELA × Smoking Group × Time, F(4, 91) = 2.97, p < .05, partial η2 = 0.12. A significant simple effect of ELA was observed for Nonsmokers at Session 2, F(2, 91) = 6.64, p < .01; pairwise comparisons indicated that Nonsmokers with High ELA had significantly higher ghrelin than their No ELA and Low ELA counterparts. Though the effect was not statistically significant at Session 1, a similar pattern of estimated means was obtained for Nonsmokers at Session 1 (see Table 1), suggesting that ELA may be associated with elevated ghrelin.
Table 1.
Estimated means (standard errors) for ghrelin & PYY by ELA group & smoking group.
| Ghrelin | PYY | |||||
|---|---|---|---|---|---|---|
| Successful Quitters | Relapsers | Nonsmokers | Successful Quitters | Relapsers | Nonsmokers | |
| M (SE) | M (SE) | M (SE) | M (SE) | M (SE) | M (SE) | |
| No ELA | ||||||
| Lab 1 (ad libitum) | 685.87 (78.60) | 592.31 (87.40) | 349.37 (60.04) | 8.00 (0.67) | 8.96 (0.84) | 8.14 (0.54) |
| Lab 2 (withdrawal) | 550.56 (65.04) | 633.39 (72.32) | 376.29 (49.68) | 9.45 (0.58) | 9.20 (0.73) | 8.10 (0.47) |
| Low ELA | ||||||
| Lab 1 (ad libitum) | 425.72 (73.29) | 565.00 (69.23) | 318.66 (86.59) | 9.84 (0.70) | 8.30 (0.67) | 8.88 (0.78) |
| Lab 2 (withdrawal) | 433.14 (60.64) | 479.57 (57.29) | 298.69 (71.65) | 8.89 (0.61) | 9.84 (0.58) | 8.66 (0.68) |
| High ELA | ||||||
| Lab 1 (ad libitum) | 491.67 (52.84) | 593.20 (58.36) | 481.76 (86.45) | 8.45 (0.47) | 7.93 (0.52) | 7.95 (0.78) |
| Lab 2 (withdrawal) | 476.54 (43.72) | 516.72 (48.29) | 641.85 (71.54) | 8.77 (0.41) | 8.68 (0.46) | 7.12 (0.68) |
The simple effects for Smoking Group provide evidence of differences in how Successful Quitters and Relapsers compared to Nonsmokers, but only among those who had not experienced ELA. Specifically, the simple effect of Smoking Group was significant among No ELA participants at Session 1 (ad libitum smoking), F(2, 91) = 6.97, p < .01, and at Session 2 (withdrawal), F(2, 91) = 5.23, p < .01. Pairwise comparisons indicated that Successful Quitters had substantially higher ghrelin than Nonsmokers at Session 1 (ad libitum); and Relapsers had substantially higher ghrelin than Nonsmokers at Session 2 (withdrawal). Thus, although No ELA Successful Quitters tended to have elevated ghrelin during ad libitum smoking, their ghrelin levels were no longer significantly different than nonsmokers’ ghrelin when the Successful Quitters were experiencing withdrawal. Moreover, a simple effect of Time emerged for these No ELA Successful Quitters, indicating that they experienced a significant reduction in ghrelin during withdrawal compared to during ad libitum smoking, F(1, 91) = 6.58, p < .05. There was also a significant simple effect of Time for High ELA Nonsmokers, F (1, 91) = 7.62, p < .01, who had higher ghrelin at Session 2 relative to Session 1. No other effects were significant.
PYY.
After accounting for BMI, there were no significant effects of Time, ELA, Smoking Group, nor their interactions on PYY (within-subjects Fs < 3.51, Ps > 0.06; between-subjects Fs < 1.87, Ps ≥ 0.16).
10. Subjective measures
Distress and Positive Affect.
A significant Time by Smoking Group interaction was found for distress, F(2, 32) = 7.27, p < .01. There was a significant Time effect for Nonsmokers, F(1, 22) = 14.39, p < .01, who reported lower distress at Session 2 (2.89, SE = 0.48) relative to Session 1 (4.10, SE = 0.44), t(22) = 3.79, p < .01. There was also a significant Smoking Group effect at Session 2 (withdrawal), F(2, 79) = 5.40, p < .01, wherein Nonsmokers reported lower distress compared to both Successful Quitters (5.13, SE = 0.65), t(64) = −2.77, p < .05, and Relapsers (5.10, SE = 0.69), t(101) = −2.61, p < .05. For positive affect, there was a main effect of Time, F(1, 125) = 28.50, p < .01, partial η2 = 0.19); positive affect was lower at Session 2 (withdrawal; EMM = 14.18, SE = 0.68) compared to Session 1 (ad libitum smoking; EMM = 17.10, SE = 0.67).
Smoking Urges, Craving, & Withdrawal.
For QSU-F1, there was a main effect of Time, F(1, 92) = 16.14, p < .01, partial η2 = 0.15; QSU-F1 was substantially lower during withdrawal at Session 2 (EMM = 21.61, SE = 1.20) relative to during ad libitum smoking at Session 1 (EMM = 27.50, SE = 1.33). There was also a between-subjects effect for Relapse Status, F(2, 92) = 5.08, p < .05, partial η2 = 0.05, with Relapsers reporting greater QSU-F1 (EMM = 26.89, SE = 1.53) than Successful Quitters (EMM = 22.23, SE = 1.39). No other main effects nor interactions, including those involving ELA, reached significance for QSU-F1, QSU-F2, the single-item measure of craving, nor withdrawal (Fs <= 2.69, Ps > 0.05). In addition, examination of correlations between subjective and hormonal measures among smokers showed significant correlations between ghrelin and craving during ad libitum smoking (r = 0.21, p < .05) and between PYY and urges to smoke to alleviate negative states during withdrawal (r = −0.26, p < .05).
11. Discussion
In this study, we observed the following: 1) Nonsmokers who experienced high ELA tended to have higher ghrelin. 2) Compared to non-smokers, successful quitters experienced higher levels of ghrelin during ad libitum smoking, but only if they did not experience early life adversities. 3) Successful quitters with no ELA also experienced lower levels of ghrelin during withdrawal compared to their ghrelin levels during ad libitum smoking. 4) Among those with no ELA, relapsers experiencing withdrawal had higher ghrelin than nonsmokers. 5) Although effects of ELA, smoking group, and time on PYY were not significant, greater PYY was inversely associated with smoking urges during withdrawal.
The moderating effects of tobacco use in the association between ELA and ghrelin may reflect chronic effects of tobacco use leading to disrupted regulation of this hormone, similar to tobacco’s dysregulating effects on the HPA stress response (al’Absi, 2018) that contributes to maintenance and relapse (al’Absi et al., 2005, 2015). To interpret this observation in context, it is important to consider the appetitive nature of ghrelin, which contributes to the rewarding effects of drugs (Addolorato et al., 2009; Gomez et al., 2015; Jerlhag et al., 2010; Wellman et al., 2012). Ghrelin also activates neural responses in brain regions regulating appetitive behaviors, including the amygdala, orbitofrontal cortex, anterior insula, and striatum (Malik et al., 2008). The present findings suggest a potential advantage drawn from elevated ghrelin during ad libitum smoking, which allows for a subsequent drop in ghrelin during smoking withdrawal. It is worth noting that this potentially protective pattern of ghrelin levels was observed among successful quitters despite these individuals reporting relatively comparable levels of craving, distress, withdrawal, and positive affect compared to smokers who relapsed. Given that smokers with high ELA did not experience significant decreases in ghrelin during withdrawal, these smokers may be at a significant disadvantage during cessation. We note, however, that it is premature to infer specific conclusions from these findings at this time.
Consistent with expected changes, reported positive affect was lower during the withdrawal session compared to ad libitum smoking. There was also a significant negative association between PYY and urges to smoke to alleviate negative states during withdrawal. The latter correlation is consistent with our earlier findings linking high levels of PYY with reduced craving during withdrawal (al’Absi et al., 2014). While we did not replicate previous research that found relapse was associated with elevated PYY (Lemieux and al’Absi, 2018), our study examined relapse over a shorter period of time (7 days vs. 28 days in previous research).
While it is not clear what mechanisms mediate the effects of ELA on ghrelin, it is worth relating these findings to other observations involving stress hormonal systems. ELA is associated with enhanced basal HPA activity and blunted adrenocortical responses to stress (al’Absi et al., 2018). This may reflect chronic effects on stress systems triggered by exposure to high levels of adversity early in life (Doom et al., 2014; Heim et al., 2000; McEwen, 2007). One additional factor for the current population is the possibility that these observations reflect long-term effects caused by ongoing stimulation by chronic tobacco use (Bruijnzeel and Gold, 2005; Coplan et al., 2011). Nicotine increases CRF release, contributing to elevated basal HPA activity and leading to subsequent adaptive processes manifested by long-term dysregulation of HPA activity (al’Absi, 2006). Therefore, in the context of ELA and tobacco use, it is possible that chronic effects of stress and tobacco trigger counter regulation leading to dysregulation of stress and appetite related hormones (Fries et al.,et al., 2005; Heim et al., 2000; McEwen, 2007; Tyrka et al., 2016), including ghrelin release.
Considering the potential role of ghrelin in facilitating dopamine release in reward and emotional processing pathways (Jerlhag, Janson, Waters, & Engel, 2012; Palotai et al., 2013a,b), it is possible that the impact of ELA on these hormones involves dopaminergic and emotion regulation systems. Exposure to high levels of ELA produces neurobiological alterations (Sheridan et al., 2017; Teicher et al., 2012; Teicher and Khan, 2019) that may also be mediated by changes in availability and sensitivity of ghrelin receptors, although no studies have directly addressed this hypothesis. It is worth noting that there is evidence that the reward nexus is involved in linking appetite regulation with addictive reward circuitry, potentially implicating this nexus in the reinforcement and learning processes that promote maintenance of tobacco addiction in vulnerable individuals (al’Absi, 2018; Berridge et al., 2010; Volkow and Wise, 2005). The role of ELA in altering epigenetic modifications, such as methylation of various ghrelin-related genes should also be considered. To that end, research has found similar influences of ELA on cortisol-related expression of different genes (Lovallo et al., 2016; McEwen et al., 2015; Tyrka et al., 2016) that, in turn, mediate effects of ELA on HPA responses to stress and risk for substance use.
While the results of this study are novel, we note some methodological limitations, including subjective assessment of early life adversity, inclusion of a single reading of appetite hormones during each assessment session, and relatively small cell sizes. In this study, we only recruited individuals free from active and significant history of psychiatric problems and free of other substance use problems. These exclusionary criteria may limit the generalizability of our findings. It is important to explore the roles of appetite hormones in mediating the effects of stress on tobacco use and relapse and to examine these associations over longer follow-up periods. Finally, we examined total ghrelin instead of acetylated, active, ghrelin because total ghrelin better captures the effects of nicotine (Pilhatsch et al., 2014) and has been associated with other substance use problems (Koopmann et al., 2012; Leggio et al., 2012). Future research may need to specifically account for total versus active ghrelin in the effects of ELA and tobacco withdrawal. Strengths of this study include repeated measures and integration of biological, psychological, and behavioral measures related to early life adversity and tobacco addiction.
In conclusion, the findings of the current study are novel because ghrelin and PYY had not been investigated in the context of ELA with tobacco addiction or relapse. Findings indicate that decreases in ghrelin during withdrawal were associated with successful smoking cessation among those with no history of ELA. These findings suggest the importance of considering changes in appetite-related hormones in individuals who are dependent on tobacco. This research provides additional indications for potential effects of ELA in altering or shaping appetite-stimulating hormones. The absence of reductions in these hormones among smokers with high ELA may help explain increased risk for addictive behaviors and relapse in these individuals.
Acknowledgements
We would like to thank the following individuals for their help with collecting (Barbara Gay, Elizabeth Ford, Dayna Schleppenbach, Soni Rraklli, Angie Forsberg) and managing (Jie Gooder) the data for this study. Nikki Neumann, Christopher Schweiger, and Dan Vuicich helped with conducting the assays.
Role of funding sources
This research was supported in part by National Institutes of Health grants R01DA016351 and R01DA027232. NIH was not involved in data collection, analysis, writing, nor submission of this manuscript.
Footnotes
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
We examined correlations between sex, ethnicity (Caucasian: yes, no), age, and FTND with smoking urges, craving, withdrawal symptoms, and affect during acute withdrawal. Only FTND was significantly correlated with smoking urges (QSF1, QSF2) and with craving, rs > 0.27, ps < 0.007. Including FTND as a covariate in the analyses did not change any main effects nor interactions.
References
- Addolorato G, Leggio L, Hillemacher T, Kraus T, Jerlhag E, & Bleich S (2009). Hormones and drinking behaviour: New findings on ghrelin, insulin, leptin and volume-regulating hormones. An ESBRA Symposium report. Drug and Alcohol Review, 28, 160–165. [DOI] [PubMed] [Google Scholar]
- Agorastos A, Pervanidou P, Chrousos GP, & Kolaitis G (2018). Early life stress and trauma: Developmental neuroendocrine aspects of prolonged stress system dysregulation. Hormones (Athens), 17(4), 507–520. [DOI] [PubMed] [Google Scholar]
- Aguiar-Nemer AS, Toffolo MCF, Silva C. J.d., Laranjeira R, & Silva-Fonseca VA (2013). Leptin influence in craving and relapse of alcoholics and smokers. Journal of Clinical Medicine Research, 5, 164–167–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- al’Absi M (2006). Hypothalamic-pituitary-adrenocortical responses to psychological stress and risk for smoking relapse. International Journal of Psychophysiology, 59(3), 218–227. [DOI] [PubMed] [Google Scholar]
- al’Absi M (2018). Stress response pathways, appetite regulation, and drug addiction. Biological Psychology, 131, 1–4. [DOI] [PubMed] [Google Scholar]
- al’Absi M, et al. (2005). Attenuated adrenocorticotropic responses to psychological stress are associated with early smoking relapse. Psychopharmacology, 181(1), 107–117. 10.1007/s00213-005-2225-3. [DOI] [PubMed] [Google Scholar]
- al’Absi M, Carr SB, & Bongard S (2007). Anger and psychobiological changes during smoking abstinence and in response to acute stress: Prediction of smoking relapse. International Journal of Psychophysiology, 66(2), 109–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- al’Absi M, Lemieux A, & Nakajima M (2014). Peptide YY and ghrelin predict craving and risk for relapse in abstinent smokers. Psychoneuroendocrinology, 49, 253–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- al’Absi M, et al. (2015). Sex differences in hormonal responses to stress and smoking relapse: A prospective examination. Nicotine & Tobacco Research, 17(4), 382–389. 10.1093/ntr/ntu340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- al’Absi M, Lemieux A, Westra R, & Allen S (2017). Early life adversity influences stress response association with smoking relapse. Psychopharmacology (Berl), 234 (22), 3375–3384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- al’Absi M, Nakajima M, & Lemieux A (2018). Impact of early life adversity on the stress biobehavioral response during nicotine withdrawal. Psychoneuroendocrinology, 98, 108–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anda RF, Croft JB, Felitti VJ, Nordenberg D, Giles WH, Williamson DF, & Giovino GA (1999). Adverse childhood experiences and smoking during adolescence and adulthood. JAMA, 282, 1652–1658. [DOI] [PubMed] [Google Scholar]
- Ardeshiripur M, Rhein M, Frieling H, Bleich S, Hillemacher T, Muschler M, et al. (2018). Desacylghrelin but not acylghrelin is reduced during smoking cessation. Journal of Neural Transmission (Vienna), 125(12), 1885–1889. [DOI] [PubMed] [Google Scholar]
- Batterham RL, Ffytche DH, Rosenthal JM, Zelaya FO, Barker GJ, Withers DJ, et al. (2007). PYY modulation of cortical and hypothalamic brain areas predicts feeding behaviour in humans. Nature, 450(7166), 106–109. [DOI] [PubMed] [Google Scholar]
- Berridge KC, Ho CY, Richard JM, & DiFeliceantonio AG (2010). The tempted brain eats: Pleasure and desire circuits in obesity and eating disorders. Brain Research, 1350, 43–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blatnik M, & Soderstrom CI (2011). A practical guide for the stabilization of acylghrelin in human blood collections. Clinical Endocrinology, 74, 325–331. [DOI] [PubMed] [Google Scholar]
- Bruijnzeel AW, & Gold MS (2005). The role of corticotropin-releasing factor-like peptides in cannabis, nicotine, and alcohol dependence. Brain Research. Brain Research Reviews, 49(3), 505–528. [DOI] [PubMed] [Google Scholar]
- Cooper JA (2014). Factors affecting circulating levels of peptide YY in humans: A comprehensive review. Nutrition Research Reviews, 27(1), 186–197. [DOI] [PubMed] [Google Scholar]
- Coplan JD, Abdallah CG, Kaufman J, Gelernter J, Smith EL, Perera TD, et al. (2011). Early-life stress, corticotropin-releasing factor, and serotonin transporter gene: A pilot study. Psychoneuroendocrinology, 36(2), 289–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox LS, Tiffany ST, & Christen AG (2001). Evaluation of the brief questionnaire of smoking urges (QSU-brief) in laboratory and clinical settings. Nicotine & Tobacco Research, 3(1), 7–16. [DOI] [PubMed] [Google Scholar]
- Doom JR, Cicchetti D, & Rogosch FA (2014). Longitudinal patterns of cortisol regulation differ in maltreated and nonmaltreated children. Journal of the American Academy of Child and Adolescent Psychiatry, 53(11), 1206–1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffy KA, McLaughlin KA, & Green PA (2018). Early life adversity and health-risk behaviors: Proposed psychological and neural mechanisms. Annals of the New York Academy of Sciences, 1428, 151–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fries E, Hesse J, Hellhammer J, & Hellhammer DH (2005). A new view on hypocortisolism. Psychoneuroendocrinology, 30(10), 1010–1016. [DOI] [PubMed] [Google Scholar]
- Gomez JL, Cunningham CL, Finn DA, Young EA, Helpenstell LK, Schuette LM, et al. (2015). Differential effects of ghrelin antagonists on alcohol drinking and reinforcement in mouse and rat models of alcohol dependence. Neuropharmacology, 97, 182–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grove KL, Brogan RS, & Smith MS (2001). Novel expression of neuropeptide Y (NPY) mRNA in hypothalamic regions during development: Region-specific effects of maternal deprivation on NPY and Agouti-related protein mRNA. Endocrinology, 142, 4771–4776. [DOI] [PubMed] [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, & Fagerstrom KO (1991). The Fagerstrom test for nicotine dependence: A revision of the Fagerstrom Tolerance Questionnaire. British Journal of Addiction, 86(9), 1119–1127. [DOI] [PubMed] [Google Scholar]
- Heim C, Ehlert U, & Hellhammer DH (2000). The potential role of hypocortisolism in the pathophysiology of stress-related bodily disorders. Psychoneuroendocrinology, 25(1), 1–35. [DOI] [PubMed] [Google Scholar]
- Husum H, & Mathé AA (2002). Early life stress changes concentrations of neuropeptide Y and corticotropin-releasing hormone in adult rat brain. Lithium treatment modifies these changes. Neuropsychopharmacology, 27, 756–764. [DOI] [PubMed] [Google Scholar]
- Jerlhag E, Egecioglu E, Dickson SL, Andersson M, Svensson L, & Engel JA (2006). Ghrelin stimulates locomotor activity and accumbal dopamine-overflow via central cholinergic systems in mice: Implications for its involvement in brain reward. Addiction Biology, 11(1), 45–54. [DOI] [PubMed] [Google Scholar]
- Jerlhag E, Egecioglu E, Dickson SL, & Engel JA (2010). Ghrelin receptor antagonism attenuates cocaine- and amphetamine-induced locomotor stimulation, accumbal dopamine release, and conditioned place preference. Psychopharmacology (Berl), 211(4), 415–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jerlhag E, Egecioglu E, Landgren S, Salome N, Heilig M, Moechars D, et al. (2009). Requirement of central ghrelin signaling for alcohol reward. ProcNatlAcadSciUSA, 106(27), 11318–11323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jerlhag E, Janson AC, Waters S, & Engel JA (2012). Concomitant release of ventral tegmental acetylcholine and accumbal dopamine by ghrelin in rats. PLoS One, 7(11), Article e49557. 10.1371/journal.pone.0049557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenny PJ (2011). Common cellular and molecular mechanisms in obesity and drug addiction. Nature Reviews Neuroscience, 12(11), 638–651. [DOI] [PubMed] [Google Scholar]
- Koopmann A, et al. (2012). The association of the appetitive peptide acetylated ghrelin with alcohol craving in early abstinent alcohol dependent individuals. Psychoneuroendocrinology, 37(7), 980–986. 10.1016/j.psyneuen.2011.11.005. [DOI] [PubMed] [Google Scholar]
- Lee H, Joe K-H, Kim W, Park J, Lee D-H, Sung K-W, & Kim D-J (2006). Increased leptin and decreased ghrelin level after smoking cessation. Neuroscience Letters, 409(1), 47–51. [DOI] [PubMed] [Google Scholar]
- Leggio L, Ferrulli A, Cardone S, Nesci A, Miceli A, Malandrino N, et al. (2012). Ghrelin system in alcohol-dependent subjects: role of plasma ghrelin levels in alcohol drinking and craving. Addiction Biology, 17, 452–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemieux A, Olson L, Nakajima M, Schulberg L, & al’Absi M (2016). Life adversity is associated with smoking relapse after a quit attempt. Addictive Behaviors, 60, 71–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemieux AM, & al’Absi M (2018). Changes in circulating peptide YY and ghrelin are associated with early smoking relapse. Biological Psychology, 131, 43–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovallo WR, Acheson A, Vincent AS, Sorocco KH, & Cohoon AJ (2018). Early life adversity diminishes the cortisol response to opioid blockade in women: Studies from the Family Health Patterns project. PLoS ONE, 13(10), e0205723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovallo WR, Cohoon AJ, Acheson A, Sorocco KH, & Vincent AS (2019). Blunted stress reactivity reveals vulnerability to early life adversity in young adults with a family history of alcoholism. Addiction, 114(5), 798–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovallo WR, Cohoon AJ, Sorocco KH, Vincent AS, Acheson A, Hodgkinson CA, et al. (2019). Early-life adversity and blunted stress reactivity as predictors of alcohol and drug use in persons with COMT (rs4680) Val158Met genotypes. Alcoholism, Clinical and Experimental Research, 43, 1519–1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovallo WR, Enoch MA, Acheson A, Cohoon AJ, Sorocco KH, Hodgkinson CA, et al. (2016). Early-life adversity interacts with FKBP5 genotypes: Altered working memory and cardiac stress reactivity in the Oklahoma family health patterns project. Neuropsychopharmacology, 41(7), 1724–1732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovallo WR, Enoch MA, Sorocco KH, Vincent AS, Acheson A, Cohoon AJ, et al. (2017). Joint impact of early life adversity and COMT Val158Met (rs4680) genotypes on the adult cortisol response to psychological stress. Psychosomatic Medicine, 79(6), 631–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makovey J, Naganathan V, Seibel M, & Sambrook P (2007). Gender differences in plasma ghrelin and its relations to body composition and bone – an opposite-sex twin study. Clinical Endocrinology (Oxf), 66, 530–537. [DOI] [PubMed] [Google Scholar]
- Malik S, McGlone F, Bedrossian D, & Dagher A (2008). Ghrelin modulates brain activity in areas that control appetitive behavior. Cell Metabolism, 7(5), 400–409. [DOI] [PubMed] [Google Scholar]
- Maniam J, Antoniadis C, & Morris MJ (2014). Early-life stress, HPA axis adaptation, and mechanisms contributing to later health outcomes. Frontiers in Endocrinology (Lausanne), 5, 73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS (2007). Physiology and neurobiology of stress and adaptation: Central role of the brain. Physiological Reviews, 87(3), 873–904. [DOI] [PubMed] [Google Scholar]
- McEwen BS, Bowles NP, Gray JD, Hill MN, Hunter RG, Karatsoreos IN, et al. (2015). Mechanisms of stress in the brain. Nature Neuroscience, 18(10), 1353–1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin KA, DeCross SN, Jovanovic T, & Tottenham N (2019). Mechanisms linking childhood adversity with psychopathology: Learning as an intervention target. Behaviour Research and Therapy, 118, 101–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mela V, Llorente-Berzal Á, Díaz F, Argente J, Viveros M-P, & Chowen JA (2012). Maternal deprivation exacerbates the response to a high fat diet in a sexually dimorphic manner. PLoS ONE, 7(11), e48915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menzies JR, Skibicka KP, Dickson SL, Leng G (2012) Neural Substrates Underlying Interactions between Appetite Stress and Reward Obes Facts, pp. 208–220. [DOI] [PubMed] [Google Scholar]
- Miragaia AS, de Oliveira Wertheimer GS, Consoli AC, Cabbia R, Longo BM, Girardi CEN, et al. (2018). Maternal deprivation increases anxiety- and depressive-like behaviors in an age-dependent fashion and reduces neuropeptide Y expression in the amygdala and hippocampus of male and female young adult rats. Frontiers in Behavioral Neuroscience, 12, 159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palotai M, et al. (2013a). Ghrelin and nicotine stimulate equally the dopamine release in the rat amygdala. Neurochemical Research, 38, 1989–1995. 10.1007/s11064-013-1105-1. [DOI] [PubMed] [Google Scholar]
- Palotai, et al. (2013b). Ghrelin amplifies the nicotine-induced dopamine release in the rat striatum. Neurochemistry International, 63(4), 239–243. 10.1016/j.neuint.2013.06.014. [DOI] [PubMed] [Google Scholar]
- Pilhatsch M, et al. (2014). Nicotine administration in healthy non-smokers reduces appetite but does not alter plasma ghrelin. Human Psychopharmacology: Clinical and Experimental, 29(4), 384–387. 10.1002/hup.2405. [DOI] [PubMed] [Google Scholar]
- Rigamonti AE, Pincelli AI, Corra B, Viarengo R, Bonomo SM, Galimberti D, et al. (2002). Plasma ghrelin concentrations in elderly subjects: comparison with anorexic and obese patients. Journal of Endocrinology, 175, R1–5. [DOI] [PubMed] [Google Scholar]
- Schloegl H, Percik R, Horstmann A, Villringer A, & Stumvoll M (2011). Peptide hormones regulating appetite–focus on neuroimaging studies in humans. Diabetes/Metabolism Research and Reviews, 27(2), 104–112. [DOI] [PubMed] [Google Scholar]
- Sheridan MA, Peverill M, Finn AS, & McLaughlin KA (2017). Dimensions of childhood adversity have distinct associations with neural systems underlying executive functioning. Development and Psychopathology, 29(5), 1777–1794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stadlbauer U, Weber E, Langhans W, & Meyer U (2014). The Y2 receptor agonist PYY(3–36) increases the behavioural response to novelty and acute dopaminergic drug challenge in mice. International Journal of Neuropsychopharmacology, 17(03), 407–419. [DOI] [PubMed] [Google Scholar]
- Teicher MH, Anderson CM, Polcari A (2012) Childhood maltreatment is associated with reduced volume in the hippocampal subfields CA3, dentate gyrus, and subiculum. [DOI] [PMC free article] [PubMed]
- Teicher MH, & Khan A (2019). Childhood maltreatment, cortical and amygdala morphometry, functional connectivity, laterality, and psychopathology. Child Maltreatment, 24(4), 458–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyrka AR, Parade SH, Welch ES, Ridout KK, Price LH, Marsit C, et al. (2016). Methylation of the leukocyte glucocorticoid receptor gene promoter in adults: associations with early adversity and depressive, anxiety and substance-use disorders. Translational Psychiatry, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vengeliene V (2013) The role of ghrelin in drug and natural reward. Addiction Biology 18: 897–900. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Tomasi D, & Baler R (2012). Food and drug reward: Overlapping circuits in human obesity and addiction. In Carter CS, & Dalley JW (Eds.), Brain imaging in behavioral neuroscience (Current Topics in Behavioral Neurosciences) (pp. 1–24). Berlin, Heidelberg: Springer. [DOI] [PubMed] [Google Scholar]
- Volkow ND, & Wise RA (2005). How can drug addiction help us understand obesity? NatNeurosci, 8(5), 555–560. [DOI] [PubMed] [Google Scholar]
- Wellman PJ, Clifford PS, Rodriguez JA, Hughes S, Di Francesco C, Melotto S, et al. (2012). Brain reinforcement system function is ghrelin dependent: studies in the rat using pharmacological fMRI and intracranial self-stimulation. Addiction Biology, 17, 908–919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittekind DA, Kratzsch J, Mergl R, Enzenbach C, Witte V, Villringer A, et al. (2020). Higher fasting ghrelin serum levels in active smokers than in former and never-smokers. The World Journal of Biological Psychiatry, 21(10), 748–756. [DOI] [PubMed] [Google Scholar]
- Yam KY, Ruigrok SR, Ziko I, De Luca SN, Lucassen PJ, Spencer SJ, et al. (2017). Ghrelin and hypothalamic NPY/AgRP expression in mice are affected by chronic early-life stress exposure in a sex-specific manner. Psychoneuroendocrinology, 86, 73–77. [DOI] [PubMed] [Google Scholar]
- Yousufzai M. Iu. A., Harmatz ES, Shah M, Malik MO, & Goosens KA. (2018). Ghrelin is a persistent biomarker for chronic stress exposure in adolescent rats and humans. Translational Psychiatry, 8, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zallar LJ, Farokhnia M, Tunstall BJ, Vendruscolo LF, & Leggio L (2017). The Role of the Ghrelin System in Drug Addiction. International Review of Neurobiology, 136, 89–119. [DOI] [PubMed] [Google Scholar]


