Abstract
Purpose
There is variation in the safety profile of antidepressants. Rates of adverse events along with the costs of treating them can be an important factor influencing the choice of depression treatment. This study sought to estimate the comparative safety of commonly prescribed antidepressants, and how the costs of treating these varied across European countries.
Methods
A systematic literature review was conducted (in Medline, Embase, PsycINFO and CENTRAL) to identify placebo-controlled trials reporting rates of at least one type of sexual dysfunction, weight change, insomnia, anxiety, and anhedonia. Eight antidepressants were considered: duloxetine, escitalopram, fluoxetine, paroxetine, sertraline, trazodone, venlafaxine, and vortioxetine. This evidence was synthesised via Bayesian random effects network meta-analyses to provide comparative estimates of safety. A systematic search identified country-specific costs of managing depression and adverse events of antidepressants. Evidence on costs and safety was combined in an economic model to provide country-specific costs for Bulgaria, the Czech Republic, Greece, Hungary, Italy, Romania, Slovakia, Portugal, and Poland.
Results
Trazodone had the lowest rates of both insomnia (odds ratio 0.66, 95% credible interval 0.31 to 1.38) and anxiety (0.13, <0.01 to 1.80). All antidepressants were associated with increased rates of sexual dysfunction relative to placebo. Weight change was largest for fluoxetine (kg change −1.01, −1.40 to −0.60) and sertraline (−1.00, −1.36 to −0.65), although heterogeneity was extreme for this outcome. No evidence was identified for anhedonia. Total costs were lowest for trazodone in all nine of the countries evaluated. This was primarily due to reduced rates of treatment discontinuation.
Conclusion
Trazodone generally had the best safety profile of the antidepressants evaluated. This led to healthcare costs being lowest for trazodone in all nine European countries, emphasising the importance of considering rates of adverse events when choosing a pharmacological treatment to treat symptoms of depression.
Keywords: cost analysis, side-effects, Europe, treatment discontinuation
Plain Language Summary
Antidepressants are a type of medicine that can be used to treat people with depression. Antidepressants can be effective at treating the symptoms of depression but can sometimes cause adverse events. Rates of adverse events vary by antidepressant. Hence, it is important to consider both the safety profile of antidepressants and the costs of treating side effects. The aim of our research was to identify rates of key adverse events for common antidepressants, along with the costs of treating these. Costs were estimated for a variety of European countries. The results of this research will help to guide the choice of medicine for treating the symptoms of depression.
We considered five side effects (sexual dysfunction, weight change, insomnia, anxiety, and loss of pleasure) for eight antidepressants. We obtained cost data for nine European countries: Bulgaria, the Czech Republic, Greece, Hungary, Italy, Romania, Slovakia, Portugal, and Poland. We performed two searches. The first was to identify rates of adverse events for each antidepressant. This information was combined using statistical models. The second search was on country-specific costs. The results of this were combined with the first search in a mathematical model to estimate antidepressants and country-specific costs.
We could not find any evidence for loss of pleasure. For other adverse events, trazodone generally had the best safety profile (lowest rates of adverse events) of the antidepressants. Country-specific costs were always the lowest for trazodone.
Introduction
Major depressive disorder is a common mental disorder. It affects approximately 350 million people globally and is the 13th leading cause of lost health in the 2019 Global Burden of Disease Study.1,2 Antidepressants are a common treatment option for major depressive disorder. Many antidepressants have similar efficacy but can vary with their rates of adverse events, which are a common cause of treatment discontinuation, affecting up to 43% of patients with depression.3,4 Further, the incremental effectiveness of antidepressants is modest when compared with placebo, with only one in nine patients experiencing a benefit.5 This underscores the importance of considering rates of adverse events when choosing an antidepressant. Treatment of adverse events and management of treatment discontinuation can impose large costs on healthcare systems. Hence, contemporary comparative evidence on both rates of adverse events can be a key factor influencing the choice of antidepressant.
Rates of anxiety, insomnia, sexual dysfunction, weight change and anhedonia (loss of pleasure in normal activities) were identified by clinical experts as important outcomes of depression treatment that are relatively common and can be increased by antidepressant treatment, with important differences by drug.3,6–10 For example, the prevalence of sexual dysfunction amongst patients taking a selective serotonin reuptake inhibitor has been estimated to be up to 70%.4,11 The economic implications of these adverse events have only been evaluated for a small number of antidepressants and a small number of countries. This study had four main aims:
Perform a systematic literature review to identify placebo-controlled randomised controlled trials (RCTs), with no geographic restriction, of commonly prescribed antidepressants reporting on the short-term incidence of selected adverse events. This included SSRIs, a serotonin antagonist and reuptake inhibitor, and a norepinephrine reuptake inhibitor.
Synthesise the results of the systematic review to estimate the relative safety of the antidepressants using a network meta-analysis (NMA).
Perform a systematic literature search to identify European country-specific costs and resource use associated with the treatment of depression, including adverse events.
Develop a de novo economic model to combine the results of the NMA with the cost search and provide country-specific estimates of the healthcare cost associated with the use of different antidepressants.
Methods
This study considered five adverse events (sexual dysfunction, weight change (in kg), insomnia, anxiety, and anhedonia), eight antidepressants (duloxetine, escitalopram, fluoxetine, paroxetine, sertraline, trazodone, venlafaxine, and vortioxetine), and nine European countries (Bulgaria, the Czech Republic, Greece, Hungary, Italy, Romania, Slovakia, Portugal, and Poland). It was designed to provide a range of European countries, along with antidepressants that are typically prescribed in these countries. The methods pertaining to each of the study aims are discussed in turn.
Systematic Review of Adverse Events of Antidepressants
This review updates an existing systematic review,3 which analysed adverse effects of antidepressants based on placebo-controlled RCTs in adults published up to May 2018. The updated search was conducted at Medline, Embase, PsycINFO and the Cochrane Central Register of Controlled Trials (CENTRAL) in February 2021. Search terms included terms for the selected antidepressants combined with terms for placebo, a search filter for RCTs, and a date limit for 2018 onwards. The Medline search strategy is provided in Appendix 1. Results from the de novo systematic review were combined with studies from existing reviews.2,3,6–8 In addition, further systematic reviews of antidepressants were also checked for relevant studies.
Studies were retained if they reported on the incidence of at least one adverse event of interest. Intermediate-release formulations are excluded, and full inclusion and exclusion criteria are provided in Appendix 2 (Table A1). Shifting of titles and abstracts was conducted by one reviewer (AC), with a selection of full-texts conducted by one reviewer (KC). A 10% sample was checked by a second reviewer (ME), where there was agreement on all decisions. Any disagreements would have been checked with the project lead (BK). Data were extracted by one reviewer (KC) and all numerical data checked by a second reviewer (ME).
Network Meta-Analysis
Separate NMAs were conducted for each adverse event. Modelling followed published best-practice,12 with details provided in Appendix 3. For dichotomous outcomes (adverse events which are either present or absent in an individual: sexual dysfunction, insomnia, anxiety, and anhedonia), a binomial likelihood (logit link) was used, and treatment effects were reported as odds ratios (ORs). An OR less than one reflects a reduced likelihood relative to placebo. A sensitivity analysis was conducted using a Poisson likelihood (complementary log-log link) to assess the impact of length of follow-up on results. Weight change was analysed as a continuous variable with a normal likelihood (identity link). Given that each study is conducted according to its protocol, heterogeneity is expected in the estimation of the treatment effects between studies. As such, study-specific treatment effects were considered to be exchangeable, and a random effects model was used to allow for heterogeneity in treatment effects. Inconsistency between direct and indirect evidence was not assessed since only placebo-controlled trials were included. All analyses were conducted using OpenBUGS and R13 and the R2OpenBugs interface package.14 Convergence to the target posterior distributions was assessed using the Gelman-Rubin statistic, as modified by Brooks and Gelman, for two chains with different initial values.15 For all outcomes, a burn-in of 100,000 iterations of the Markov chain was used with further 20,000 iterations retained to estimate parameters.
The absolute goodness of fit was checked by comparing the total residual deviance to the total number of data points included in an analysis. The deviance information criterion (DIC) provides a relative measure of goodness-of-fit that penalises complexity and was used to compare different models for the same likelihood and data.16 Lower values of DIC are favourable, suggesting a more parsimonious model. Results are presented using the posterior median treatment effects, 95% credible intervals (CrI) and 95% prediction intervals. The probability of each intervention ranking was computed by counting the proportion of iterations of the Markov chain in which each intervention had each rank, with median treatment rankings also presented. The estimated between-study standard deviation, τ, for each analysis is also presented. Values below 0.05 are considered to indicate low heterogeneity. Values between 0.05 and 0.5 are considered to indicate moderate heterogeneity. Values between 0.5 and 1.0 are considered to indicate high heterogeneity. Values above 1.0 are considered to indicate extremely high heterogeneity.
For each antidepressant, there was variation in the doses used between studies. In addition, some studies used fixed dosing and some flexible dosing. For the NMA, all studies of each antidepressant were grouped together irrespective of dose. For dichotomous outcomes, this involved summing the event counts and number randomised. For weight change, the outcome and standard deviation were pooled according to the Cochrane Handbook for Systematic Reviews of Interventions.17 Missing standard deviations were imputed by treating them as unknown parameters with a weak uniform prior distribution, modelled using a Gamma likelihood.18
Country-Specific Cost Data
A systematic search of the economic literature was undertaken to identify country-specific costs and resource use. This search updated and expanded a previous (2017) systematic review19 of existing economic evaluations of depression treatments. Both model-based and trial-based evaluations were included if they reported either the costs or resources used for treating any aspect of depression (excluding drug costs). Four databases were searched in March 2021 (Medline, Embase, the Cochrane Database of Systematic Reviews, and PsycINFO), with the Medline search strategy provided in Appendix 1.
Economic Model
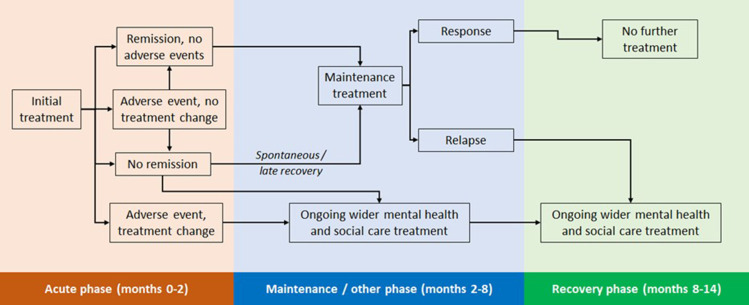
The design of the economic model was informed by existing depression models, which explicitly modelled adverse events,20–23 an existing technology appraisal of an antidepressant,24,25 and the economic models developed to support clinical practice guidelines produced by the UK National Institute for Health and Care Excellence (NICE).26,27 A schematic model is provided in Figure 1. The model was designed to reflect a course of depression treatment and so had a time horizon of 14 months. This was divided into three phases, described in turn below with more details provided in Appendix 4.
Acute phase (months one to two): captures treatment-specific differences in the incidence of adverse events; a proportion of these lead to changes in depression treatment. A proportion of successfully treated patients enter remission.
Maintenance phase (months two to eight): patients in remission continue their initial antidepressant treatment. All other patients discontinue their initial antidepressant; subsequent resource use for these is based on a UK costing study to reflect wider depression treatment.27 At the end of month eight, a proportion of those in remission will respond to treatment, the remainder relapse.
Recovery phases (months eight to 14): Patients who have responded do not require any further treatment. All other patients receive wider depression treatment.
Figure 1.
Model schematic.
As the focus of this analysis was on quantifying the impact of different rates of adverse events, it was assumed that clinical effectiveness outcomes would not vary with antidepressants. This assumption was also supported by existing systematic reviews of effectiveness, which have generally found very little difference in estimates, with large overlap of confidence intervals across antidepressants.2,27 The probabilities of remission, spontaneous remission, and relapse were all taken from the economic modelling performed for the NICE clinical guidelines on depression in adults.27 The values were 62%, 20%, and 55%, respectively. Spontaneous remission was applied to those who did not achieve remission (1–62%) to give a value of 7.6%. It was assumed that 25% of patients experiencing an adverse event would stop treatment with their initial antidepressant. This percentage was based on clinical expert opinion of a previous NICE appraisal of an antidepressant for major depression.24 Patients who experienced an adverse event but did not stop treatment were assumed to have the same rates of remission as patients who did not experience an adverse event.
Results
Systematic Review and NMA
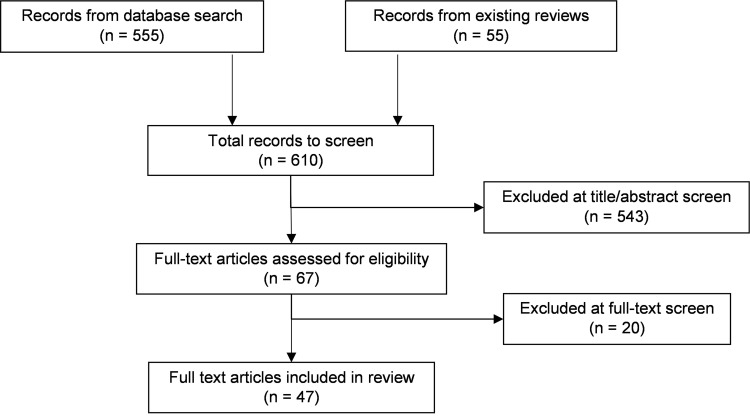
A PRISMA flow chart for the systematic literature review of adverse events is provided in Figure 2. In total, 610 references were identified, after removal of duplication, 555 from the database searches and 55 from existing systematic reviews. Of these 610 references, 67 full texts were examined for eligibility, and 47 were included in the clinical review.
Figure 2.
PRSIMA flow chart for review of adverse events.
The characteristics of the 47 included studies are shown in Appendix 2 (Table A2). All studies were double-blind RCTs, and all included adults with major depressive disorder. Most studies (N = 30) had a treatment duration of 8 weeks, while others had a duration of 6 weeks (in five studies), 9 weeks (in five studies), 10 weeks (in two studies) or 12 weeks (in five studies). The most commonly assessed antidepressant was duloxetine (17 studies) followed by paroxetine (10 studies), vortioxetine (eight studies) and fluoxetine (seven studies). Escitalopram, sertraline and venlafaxine were each assessed in six studies, whilst trazodone was assessed in two studies.
Of the studies used in the NMA, the incidence of insomnia was reported in 36 studies, while anxiety was reported in 10 studies, and anhedonia was not reported in any studies. Sexual dysfunction as an overall measure was reported in 15 studies. Definitions of sexual dysfunction varied widely, but overall heterogeneity (based on the results of the NMA) was only moderate. Weight change (as a continuous measure) was reported quantitatively in 21 studies, while further eight studies noted that there were no statistically significant or clinically relevant changes (but did not report sufficient data to be used in an NMA). Of these eight studies, escitalopram, trazodone, and vortioxetine were each included in two studies, whilst duloxetine and venlafaxine were each included in one study. In addition, the proportion of patients with a change in weight of at least 7% was reported in eight studies (covering all the antidepressants apparent from escitalopram and trazodone). This data could not be combined with the continuous evidence used in the NMA. Study-specific data for each adverse event are reported in Appendix 2 (Tables A3 to A5).
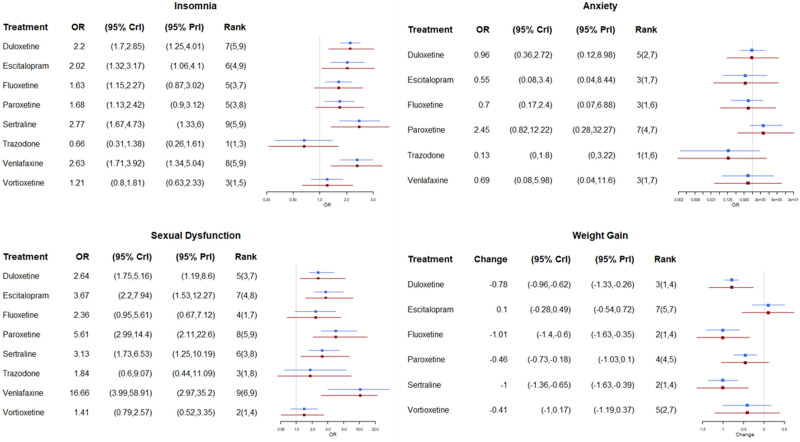
Results from the NMA for the four adverse events with data are provided in Figure 3. For each antidepressant, this shows the risk of a specific adverse event relative to placebo. For insomnia, only trazodone had a reduced risk relative to placebo (OR 0.66, 95% CrI 0.31 to 1.38) and it had the highest probability of having the lowest rates of insomnia (97%), whereas for all the other antidepressants there was an increase in the risk of having insomnia, with the highest risk for sertraline (OR 2.77, 1.67 to 4.73), venlafaxine (OR 2.63, 1.71 to 3.92) and escitalopram (OR 2.02, 1.32 to 3.17). All treatments except paroxetine were associated with lower rates of anxiety than placebo. Trazodone had the lowest rate (OR 0.13, 95% CrI <0.01 to 1.80) with an 89% probability of having the lowest rate. All active treatments had an increased risk of sexual dysfunction compared to placebo, with the lowest rates for vortioxetine (ORs 1.41, 0.79 to 2.57) and trazodone (ORs 1.84, 0.60 to 9.07). Weight change was largest for fluoxetine (kg change −1.01, −1.40 to −0.60) and sertraline (kg change −1.00, −1.36 to −0.65). In this outcome, neither trazodone nor venlafaxine could be included in the NMA due to a lack of evidence in the identified trials, but both were reported to be not significantly different from placebo.
Figure 3.
Results from the network meta-analysis. Weight change is in kg. All outcomes are relative to placebo, ORs < 1 indicate lower rates.
Abbreviations: CrI, credible interval; OR, odds ratio; PrI, predictive interval.
For all outcomes, the model fitted the data well (Appendix 3, Table A6). The use of time-dependent models for the dichotomous outcomes provided very similar results and did not improve model fit based on DIC. Heterogeneity was found to be moderate for insomnia and sexual dysfunction, high for anxiety, and extreme for weight change (Appendix 3, Table A7).
Cost Data and Economic Model
The PRISMA flow chart for the economic evidence review is shown in Appendix 4 (Figure A1). In total, 3777 references were identified, 3636 from the database searches and 141 from existing reviews.19,28 Of these, 187 full texts were examined for eligibility and 132 studies were identified as reporting cost information for any country. For the nine European countries of interest, evidence was identified for Italy (eight studies),29–36 the Czech Republic (four),37–40 Greece (three),41–43 Portugal (two),44,45 Hungary, and Poland (one each).46,47 No literature was identified for Bulgaria, Romania, or Slovakia, so cost estimates were obtained via local expert opinion. Of these studies, only one reported evidence on the treatment of adverse events (sexual dysfunction and insomnia).43 Hence, evidence on the costs of treating adverse events was sought for other countries. The cost of treating insomnia was reported in six studies,20–23,43 sexual dysfunction in five studies,20,22,23,43 12), weight change in two studies22,23 and anxiety in one study.48 An overview of the resource use associated with each adverse event is provided in Appendix 4 (Table A8). Each study assumed that the occurrence of an adverse event would require one or more visits to a general practitioner, with most also requiring additional healthcare visits, typically to a psychiatrist. The use of additional medication was included for insomnia in five of the six studies, in two of the five sexual dysfunction studies, and in a single anxiety study.
For use in the economic model, evidence from the most recent study was used for each adverse event. Hence for weight change, sexual dysfunction, and insomnia an average of 2.8 general practitioner visits, 1.0 psychiatrist visit and 2.1 visits for psychotherapy or counselling were included. As country-specific costs for psychotherapy or counselling were often not available, it was assumed that each visit to these would cost the same as a psychiatrist's visit. Anxiety required two general practitioner visits to treat. The costs of additional medication were also included for insomnia; these were country-specific costs for zolpidem where available, otherwise country-specific costs for amitriptyline were included. Additional medication costs for anxiety were not included due to a lack of country-specific evidence. For weight change, it was assumed that a change of more than 7% from baseline weight would incur costs.28
Country-specific costs of antidepressants were provided by Angelini and are confidential. For consistency with the NMA approach, immediate-release formulations were excluded from the cost model. This was used to derive costs-per-mg, which were converted into daily drug costs based on the recommended dosing for each antidepressant on its licence. This evidence was obtained from the British National Formulary.49 As previously noted, the costs of additional mental health and social care treatment were taken from a UK costing study, with conversion to country-specific costs using purchasing power parities.50 It was assumed that treatment switching would incur one visit to a general practitioner, whilst patients with stable disease would not incur any costs. An overview of the country-specific costs used in the model is provided in Table 1, with total incurred healthcare costs associated with each initial antidepressant provided in Table 2. Corresponding costs in a common currency are provided in the Table A9. There were some country-specific drug prices, which were not available, so could not be included in subsequent results.
Table 1.
Costs Used in the Economic Model (2020)
| Country | Sexual Dysfunction | Weight Change | Insomnia | Anxiety | Discontinue Treatment | No Remission /Relapse |
|---|---|---|---|---|---|---|
| Bulgaria (BGN) | 38.4 | 38.4 | 41.8 | 13.0 | 6.5 | 231.8 |
| Czech Republic (CZK) | 1656.2 | 1656.2 | 1682.1 | 468.3 | 234.1 | 4175.4 |
| Greece (€) | 163.7 | 163.7 | 165.1 | 20.3 | 10.1 | 220.4 |
| Hungary (HUF) | 20,074.3 | 20,074.3 | 20,365.9 | 3362.0 | 1681.0 | 48,823.6 |
| Italy (€) | 261.0 | 261.0 | 269.3 | 96.8 | 48.4 | 220.4 |
| Romania (RON) | 93.5 | 93.5 | 97.8 | 31.7 | 15.8 | 565.1 |
| Slovakia (€) | 39.4 | 39.4 | 40.8 | 6.0 | 3.0 | 220.4 |
| Poland (PLN) | 363.0 | 363.0 | 368.3 | 0.0 | 0.0 | 587.8 |
| Portugal (€) | 181.8 | 181.8 | 183.8 | 69.3 | 34.6 | 220.4 |
Table 2.
Results of the Economic Model (2020 Currencies)
| Antidepressant | Bulgaria (BGN) | Czech Republic (CZK) | Greece (€) | Hungary (HUF) | Italy (€) | Romania (RON) | Slovakia (€) | Portugal (€) | Poland (PLN) |
|---|---|---|---|---|---|---|---|---|---|
| Trazodone | 1536 | 27,580 | 1465 | 323,815 | 1501 | 3742 | 1432 | 1456 | 3943 |
| Duloxetine | 1585 | 30,039 | 1550 | 346,437 | 1602 | 4034 | N/A | 1571 | 4126 |
| Escitalopram | 1601 | 31,426 | 1539 | 338,352 | 1567 | 3883 | 1533 | 1559 | N/A |
| Fluoxetine | N/A | N/A | 1506 | N/A | 1531 | 3802 | N/A | 1490 | 3998 |
| Paroxetine | 1600 | 30,292 | 1571 | 343,054 | 1622 | N/A | 1504 | 1586 | N/A |
| Sertraline | 1575 | 31,388 | 1547 | N/A | 1594 | 3822 | 1492 | 1551 | 4085 |
| Venlafaxine | 1698 | 33,099 | 1696 | N/A | 1785 | 4129 | N/A | 1703 | 4494 |
| Vortioxetine | 1722 | 30,102 | 1535 | 353,505 | 1581 | 4082 | 1527 | 1556 | 4393 |
Abbreviation: N/A, Not available.
Total costs were lowest for trazodone in all nine of the countries evaluated. This was primarily due to reduced rates of treatment discontinuation (due to a lower incidence of adverse events, primarily insomnia and anxiety – see Table A10), which led to reduced rates of subsequent mental health treatments compared with most other antidepressants. The cost of fluoxetine was only available for five countries; for these it always provided the second lowest total costs. For the remaining four countries, the second lowest costs were observed for sertraline (Bulgaria, Slovakia), duloxetine (the Czech Republic), and escitalopram (Hungary). Subsequent treatment costs were very similar for vortioxetine and trazodone, reflecting their similar safety profile in the NMA. The costs of vortioxetine treatment were, however, the highest for eight of the countries (being the second highest in the Czech Republic, after escitalopram). The ranking of total costs for vortioxetine ranged from being the third lowest (the Czech Republic and Greece) to the most expensive (Bulgaria, Hungary). Cost data were available for venlafaxine in seven countries; of these it was associated with the largest overall costs in six countries, and the second largest in Bulgaria.
Discussion
Evidence was synthesised for four adverse events and eight antidepressants. Adverse event rates were observed to vary with antidepressants. Trazodone had the lowest rates for both anxiety and insomnia and, along with vortioxetine, had the lowest rates of sexual dysfunction. Trazodone also had similar outcomes to placebo for weight change. The largest odds ratios were observed for sertraline (insomnia), paroxetine (anxiety), and venlafaxine (sexual dysfunction). Based on the results of the economic model, use of trazodone was associated with the lowest overall healthcare costs for all nine countries. The beneficial outcomes for trazodone compared with the other antidepressants may reflect the differing pharmacological properties of these treatments.51,52 For example, trazodone is the only serotonin antagonist and reuptake inhibitor considered (all other treatments were selective serotonin reuptake inhibitor or serotonin and norepinephrine reuptake inhibitor). The second lowest total costs were observed for fluoxetine, where data were available. Healthcare costs were typically the highest for vortioxetine.
Existing analyses of the impact of adverse events only considered a small number of antidepressants for a single country, with rates of adverse events typically taken from a single trial. This limits the comparability of their results in this study. A previous study compared escitalopram, duloxetine, and venlafaxine in Sweden.21 Escitalopram had the most favourable adverse-event profile and resulted in lower costs than the other two antidepressants. Based on the NMA in this study, escitalopram had the lowest ORs of the three antidepressants for insomnia and anxiety, whilst duloxetine had the lowest ORs for sexual dysfunction. Of the six countries with cost data for all three antidepressants, escitalopram had the lowest costs for four and duloxetine the lowest for two. This illustrates the importance of conducting local evaluations, as results for one country may not generalise to other countries.
The strength of this study is the extensive inclusion of eight antidepressants and nine countries, along with the de novo NMA for four adverse events. This comprehensive analysis demonstrates differences by antidepressant for rates of adverse events, and country-specific differences in the ranking of some of the antidepressants. Both the clinical and cost evidence used within the economic model were based on systematic searches in the literature, with the clinical evidence synthesised in the NMA. This will ensure that the most relevant evidence is identified and avoids the limitations of relying on single evidence sources.
A limitation of the systematic review of adverse event studies was that no evidence was identified for anhedonia, and we were only able to consider a selection of short-term adverse events. Future work could consider additional adverse events (including longer-term outcomes) and search for other study designs where this is no evidence from clinical trials (as occurred for anhedonia). In addition, this review updated an existing review, which was restricted to placebo-controlled studies. This ensured that there was a common comparator for the NMA but raises the risk that some relevant evidence has been omitted. A previous review concluded that use of placebo-controlled studies led to smaller between-drug differences in outcomes.2 Hence, the results reported here may be conservative for the most effective antidepressants, particularly trazodone and vortioxetine. A further limitation is that adverse events may have been assessed differently between studies. For example, definitions used to report sexual dysfunction varied markedly. Sometimes specific cut-offs on questionnaires including the Arizona Sexual Experiences Scale and the Changes in Sexual Functioning Questionnaire were used. Some studies used any report of adverse events due to sexual dysfunction, whilst other studies used a specified range of sub-categories. These differences will create heterogeneity in the results. The results of the NMA indicted extreme heterogeneity for weight change, suggesting that the results may not be robust to this outcome. However, modelled rates of clinically significant weight changes were all very small; removing this adverse event did not materially affect conclusions. The NMA results also indicated that differing reporting periods did not affect the results. Evidence for trazodone was based on two studies, which created uncertainties in the results. However, a head-to-head trial comparing trazodone with fluoxetine and sertraline found that rates of sexual dysfunction were lowest for trazodone.53 Due to the paucity of country-specific evidence, information from the UK was used by all countries for both the proportion of adverse events leading to treatment discontinuation, and resource use for these discontinuations. It is unclear if this will lead to an under-estimate or over-estimate of country-specific costs of treating adverse events. This study only considered the costs due to adverse events. These will also impact patient outcomes. As trazodone has the lowest rates of insomnia and anxiety (as well as the second lowest for sexual dysfunction), it is likely that it will also provide the greatest benefit to patient quality-of-life.
This work raises important avenues for future research. Only a select number of adverse events were considered. These were chosen as those which are clinically significant for the antidepressants considered. It would be beneficial to expand this work to consider additional adverse events in future work. It would also be of use to establish if the results observed in clinical trials are replicated in practice. This is of particular importance as rates of adverse events may be underreported in the literature.54 This underreporting would not be identified via quality assessment tools. Hence, as with the existing review that we updated,3 we did not perform a quality assessment. This may be viewed as a limitation of this study. The analyses presented here did not distinguish between treatment-lines for antidepressants; this may impact outcomes and could be explored in future work. Future work could also consider the incidence of adverse events over a longer time-period.
In conclusion, differing rates of key adverse events can influence both patient outcomes and subsequent costs to the healthcare system. This study provides results of an NMA of four adverse events, along with an economic model to estimate associated healthcare costs. Trazodone often had the lowest rates of adverse events and resulted in the lowest costs for all nine countries. The results suggest that trazodone may be an appropriate treatment choice when the goal is to provide an efficacious treatment, which has low rates of these adverse events.
Funding Statement
Funding for this work was provided by Angelini (which markets Trazodone).
Data Sharing Statement
The data used in this manuscript are provided as supplementary material.
Ethics Approval and Informed Consent
Neither ethical approval nor informed consent was relevant to this research.
Disclosure
Dr Jean Hamilton reports personal fees from Angelini for consultancy, during the conduct of the study. The authors report no other conflicts of interest in this work.
References
- 1.Vos T, Lim SS, Abbafati C, et al. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet. 2020;396(10258):1204–1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cipriani A, Furukawa TA, Salanti G, et al. Comparative efficacy and acceptability of 21 antidepressant drugs for the acute treatment of adults with major depressive disorder: a systematic review and network meta-analysis. Lancet. 2018;391(10128):1357–1366. doi: 10.1016/S0140-6736(17)32802-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sinyor M, Cheung CP, Abraha HY, et al. Antidepressant-placebo differences for specific adverse events in major depressive disorder: a systematic review. J Affect Disord. 2020;267:185–190. doi: 10.1016/j.jad.2020.02.013 [DOI] [PubMed] [Google Scholar]
- 4.Carvalho AF, Sharma MS, Brunoni AR, Vieta E, Fava GA. The safety, tolerability and risks associated with the use of newer generation antidepressant drugs: a critical review of the literature. Psychother Psychosom. 2016;85(5):270–288. doi: 10.1159/000447034 [DOI] [PubMed] [Google Scholar]
- 5.Hengartner MP, Plöderl M. Statistically significant antidepressant-placebo differences on subjective symptom-rating scales do not prove that the drugs work: effect size and method bias matter! Front Psychiatry. 2018;9:517. doi: 10.3389/fpsyt.2018.00517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Serretti A, Chiesa A. Treatment-emergent sexual dysfunction related to antidepressants: a meta-analysis. J Clin Psychopharmacol. 2009;29(3):259–266. doi: 10.1097/JCP.0b013e3181a5233f [DOI] [PubMed] [Google Scholar]
- 7.Serretti A, Mandelli L, Laura M. Antidepressants and body weight: a comprehensive review and meta-analysis. J Clin Psychiatry. 2010;71(10):648. doi: 10.4088/JCP.09r05346blu [DOI] [PubMed] [Google Scholar]
- 8.Reichenpfader U, Gartlehner G, Morgan LC, et al. Sexual dysfunction associated with second-generation antidepressants in patients with major depressive disorder: results from a systematic review with network meta-analysis. Drug Safety. 2014;37(1):19–31. doi: 10.1007/s40264-013-0129-4 [DOI] [PubMed] [Google Scholar]
- 9.Alonso‐Pedrero L, Bes‐Rastrollo M, Marti A. Effects of antidepressant and antipsychotic use on weight gain: a systematic review. Obesity Rev. 2019;20(12):1680–1690. doi: 10.1111/obr.12934 [DOI] [PubMed] [Google Scholar]
- 10.Wang S-M, Han C, Bahk W-M, et al. Addressing the side effects of contemporary antidepressant drugs: a comprehensive review. Chonnam Med J. 2018;54(2):101–112. doi: 10.4068/cmj.2018.54.2.101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reichenpfader U, Gartlehner G, Morgan LC, et al. Sexual dysfunction associated with second-generation antidepressants in patients with major depressive disorder: results from a systematic review with network meta-analysis. Drug Saf. 2014;37(1):19–31. [DOI] [PubMed] [Google Scholar]
- 12.Dias S, Welton NJ, Sutton AJ, Ades A. A Generalised Linear Modelling Framework for Pairwise and Network Meta-Analysis of Randomised Controlled Trials. London: National Institute for Health and Care Excellence (NICE); 2014. [PubMed] [Google Scholar]
- 13.Spiegelhalter D, Thomas A, Best N, Lunn D. OpenBUGS user manual. Version. 2007;3(2):2007. [Google Scholar]
- 14.Sturtz S, Ligges U, Gelman A. R2OpenBUGS: a package for running OpenBUGS from R. J Stat Softw. 2019;1:98. [Google Scholar]
- 15.Brooks SP, Gelman A. General methods for monitoring convergence of iterative simulations. J Computational Graphical Statistics. 1998;7(4):434–455. [Google Scholar]
- 16.Spiegelhalter DJ, Best NG, Carlin BP, Van der linde A. The deviance information criterion: 12 years on. J R Stat Soc Series B Stat Methodol. 2014;76(3):485–493. doi: 10.1111/rssb.12062 [DOI] [Google Scholar]
- 17.Higgins JP, Li T, Deeks JJ. Choosing effect measures and computing estimates of effect. Cochrane Handbook Sys Rev Interventions. 2019;1:143–176. [Google Scholar]
- 18.Stevens JW. A note on dealing with missing standard errors in meta‐analyses of continuous outcome measures in WinBUGS. Pharm Stat. 2011;10(4):374–378. doi: 10.1002/pst.491 [DOI] [PubMed] [Google Scholar]
- 19.Kolovos S, Bosmans JE, Riper H, Chevreul K, Coupé VM, van Tulder MW. Model-based economic evaluation of treatments for depression: a systematic literature review. Pharmacoeconomics-Open. 2017;1(3):149–165. doi: 10.1007/s41669-017-0014-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nuijten MJ, Brignone M, Marteau F, den Boer JA, Hoencamp E. Cost-effectiveness of escitalopram in major depressive disorder in the Dutch health care setting. Clin Ther. 2012;34(6):1364–1378. doi: 10.1016/j.clinthera.2012.04.006 [DOI] [PubMed] [Google Scholar]
- 21.Nordström G, Despiegel N, Marteau F, Danchenko N, Maman K. Cost effectiveness of escitalopram versus SNRIs in second-step treatment of major depressive disorder in Sweden. J Med Econ. 2010;13(3):516–526. doi: 10.3111/13696998.2010.506371 [DOI] [PubMed] [Google Scholar]
- 22.Young AH, Evitt L, Brignone M, et al. Cost-utility evaluation of vortioxetine in patients with Major Depressive Disorder experiencing inadequate response to alternative antidepressants in the United Kingdom. J Affect Disord. 2017;218:291–298. doi: 10.1016/j.jad.2017.04.019 [DOI] [PubMed] [Google Scholar]
- 23.Soini E, Hallinen T, Brignone M, et al. Cost-utility analysis of vortioxetine versus agomelatine, bupropion SR, sertraline and venlafaxine XR after treatment switch in major depressive disorder in Finland. Expert Rev. 2017;17(3):293–302. [DOI] [PubMed] [Google Scholar]
- 24.Excellence NIfHaC. Vortioxetine for treating major depressive episodes. Technology appraisal guidance [TA367]. Available from: https://www.nice.org.uk/guidance/ta367. Accessed June 1, 2022.
- 25.Lomas J, Llewellyn A, Soares M, et al. The clinical and cost effectiveness of vortioxetine for the treatment of a major depressive episode in patients with failed prior antidepressant therapy: a critique of the evidence. Pharmacoeconomics. 2016;34(9):901–912. doi: 10.1007/s40273-016-0417-9 [DOI] [PubMed] [Google Scholar]
- 26.Excellence NIfHaC, Health NCCfM. Depression in Adults with a Chronic Physical Health Problem: Recognition and Management. Excellence NIfHaC, Health NCCfM; 2010. [Google Scholar]
- 27.Excellence NIfHaC, Health NCCfM. Depression in Adults: Recognition and Management (Updated Edition). Excellence NIfHaC, Health NCCfM; 2017. [Google Scholar]
- 28.Kearns B, Cooper K, Cantrell A, Thomas C. Schizophrenia Treatment with Second-Generation Antipsychotics: a Multi-Country Comparison of the Costs of Cardiovascular and Metabolic Adverse Events and Weight Gain. <![CDATA[Neuropsychiatric Disease and Treatment]]>. 2021;17:125. doi: 10.2147/NDT.S282856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cortesi PA, Mencacci C, Luigi F, et al. Compliance, persistence, costs and quality of life in young patients treated with antipsychotic drugs: results from the COMETA study. BMC Psychiatry. 2013;13(1):1–16. doi: 10.1186/1471-244X-13-98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Percudani M, Fattore G, Belloni GC, Gerzeli S, Contini A. Service utilisation and costs of first-contact patients in a community psychiatric service in Italy. Eur Psychiatry. 2002;17(8):434–442. doi: 10.1016/S0924-9338(02)00704-6 [DOI] [PubMed] [Google Scholar]
- 31.Garattini L, Rossi C, Tediosi F, et al. Direct costs of schizophrenia in Italian community psychiatric services. Pharmacoeconomics. 2001;19(12):1217–1225. doi: 10.2165/00019053-200119120-00004 [DOI] [PubMed] [Google Scholar]
- 32.Degli Esposti L, Sangiorgi D, Mencacci C, et al. Pharmaco-utilisation and related costs of drugs used to treat schizophrenia and bipolar disorder in Italy: the IBIS study. BMC Psychiatry. 2014;14(1):1–9. doi: 10.1186/s12888-014-0282-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Degli Esposti L, Sangiorgi D, Ferrannini L, et al. Cost-consequences analysis of switching from oral antipsychotics to long-acting risperidone in the treatment of schizophrenia. J Psychopathol. 2012;18(2):170–176. [Google Scholar]
- 34.Marcellusi A, Fabiano G, Viti R, et al. Economic burden of schizophrenia in Italy: a probabilistic cost of illness analysis. BMJ Open. 2018;8(2):e018359. doi: 10.1136/bmjopen-2017-018359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Colombo GL, Caruggi M, Di Matteo S, Rossi A. An economic evaluation of aripiprazole vs olanzapine adapted to the Italian setting using outcomes of metabolic syndrome and risk for diabetes in patients with schizophrenia. Neuropsychiatr Dis Treat. 2008;4(5):967. doi: 10.2147/NDT.S3701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Senese F, Rucci P, Fantini M, et al. Measuring costs of community mental health care in Italy: a prevalence-based study. Eur Psychiatry. 2018;51:34–41. doi: 10.1016/j.eurpsy.2018.02.001 [DOI] [PubMed] [Google Scholar]
- 37.Einarson TR, Zilbershtein R, Skoupá J, Veselá Š, Garg M, Hemels ME. Economic and clinical comparison of atypical depot antipsychotic drugs for treatment of chronic schizophrenia in the Czech Republic. J Med Econ. 2013;16(9):1089–1095. doi: 10.3111/13696998.2013.820193 [DOI] [PubMed] [Google Scholar]
- 38.Winkler P, Broulíková HM, Kondrátová L, et al. Value of schizophrenia treatment II: decision modelling for developing early detection and early intervention services in the Czech Republic. Eur Psychiatry. 2018;53:116–122. doi: 10.1016/j.eurpsy.2018.06.008 [DOI] [PubMed] [Google Scholar]
- 39.Přibylová L, Kolek M, Veselá Š, Duba J, Šlesinger J, Dolečková J. De novo cost-utility analysis of oral paliperidone in the treatment of schizoaffective disorder. J Psychiatr Res. 2015;70:33–37. doi: 10.1016/j.jpsychires.2015.08.013 [DOI] [PubMed] [Google Scholar]
- 40.Hosak L, Bahbouh R. Costs and outcomes of risperidone treatment in schizophrenia in the Czech Republic. Eur Psychiatry. 2002;17(4):213–221. doi: 10.1016/S0924-9338(02)00665-X [DOI] [PubMed] [Google Scholar]
- 41.Einarson TR, Geitona M, Chaidemenos A, et al. Pharmacoeconomic analysis of paliperidone palmitate for treating schizophrenia in Greece. Ann Gen Psychiatry. 2012;11(1):1–8. doi: 10.1186/1744-859X-11-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Geitona M, Kousoulakou H, Ollandezos M, Athanasakis K, Papanicolaou S, Kyriopoulos I. Costs and effects of paliperidone extended release compared with alternative oral antipsychotic agents in patients with schizophrenia in Greece: a cost effectiveness study. Ann Gen Psychiatry. 2008;7(1):1–12. doi: 10.1186/1744-859X-7-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Maniadakis N, Kourlaba G, Mougiakos T, Chatzimanolis I, Jonsson L. Economic evaluation of agomelatine relative to other antidepressants for treatment of major depressive disorders in Greece. BMC Health Serv Res. 2013;13(1):1–10. doi: 10.1186/1472-6963-13-173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Einarson TR, Maia-Lopes S, Goswami P, Bereza BG, Van Impe K. Economic analysis of paliperidone long-acting injectable for chronic schizophrenia in Portugal. J Med Econ. 2016;19(9):913–921. doi: 10.1080/13696998.2016.1184156 [DOI] [PubMed] [Google Scholar]
- 45.Heeg B, Antunes J, Figueira M, et al. Cost-effectiveness and budget impact of long-acting risperidone in Portugal: a modeling exercise. Curr Med Res Opin. 2008;24(2):349–358. doi: 10.1185/030079907X253834 [DOI] [PubMed] [Google Scholar]
- 46.Németh B, Bendes R, Nagy B, et al. Cost-utility analysis of cariprazine compared to risperidone among patients with negative symptoms of schizophrenia. Health Policy Technol. 2019;8(1):84–91. doi: 10.1016/j.hlpt.2019.01.004 [DOI] [Google Scholar]
- 47.Zaprutko T, Göder R, Kus K, Pałys W, Rybakowski F, Nowakowska E. The economic burden of inpatient care of depression in Poznan (Poland) and Kiel (Germany) in 2016. PLoS One. 2018;13(6):e0198890. doi: 10.1371/journal.pone.0198890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sullivan PW, Valuck R, Saseen J, MacFall HM. A comparison of the direct costs and cost effectiveness of serotonin reuptake inhibitors and associated adverse drug reactions. CNS Drugs. 2004;18(13):911–932. doi: 10.2165/00023210-200418130-00006 [DOI] [PubMed] [Google Scholar]
- 49.Committee JF. British National Formulary (BNF). Vol. 66. Pharmaceutical Press; 2021. [Google Scholar]
- 50.OECD. Purchasing power parities. Available from: https://data.oecd.org/conversion/purchasing-power-parities-ppp.htm. Accessed June 1, 2022. 2020.
- 51.Fagiolini A, Comandini A, Dell’Osso MC, Kasper S. Rediscovering trazodone for the treatment of major depressive disorder. CNS Drugs. 2012;26(12):1033–1049. doi: 10.1007/s40263-012-0010-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Brogden R, Heel R, Speight T, Avery G. Trazodone: a review of its pharmacological properties and therapeutic use in depression and anxiety. Drugs. 1981;21(6):401–429. doi: 10.2165/00003495-198121060-00001 [DOI] [PubMed] [Google Scholar]
- 53.Khazaie H, Rezaie L, Rezaei Payam N, Najafi F. Antidepressant-induced sexual dysfunction during treatment with fluoxetine, sertraline and trazodone; a randomized controlled trial. Gen Hosp Psychiatry. 2015;37(1):40–45. doi: 10.1016/j.genhosppsych.2014.10.010 [DOI] [PubMed] [Google Scholar]
- 54.Golder S, Loke YK, Wright K, Norman G. Reporting of adverse events in published and unpublished studies of health care interventions: a systematic review. PLoS Med. 2016;13(9):e1002127. doi: 10.1371/journal.pmed.1002127 [DOI] [PMC free article] [PubMed] [Google Scholar]