Abstract
Purpose
To provide consensus-based current guidelines on optimal dosimetry and patient selection for MicroPulse Transscleral Laser Therapy (TLT) based on a review of the literature and a Delphi method.
Methods
A comprehensive search of Pub Med led to the identification and analysis of 61 studies on MicroPulse TLT that contained information on laser settings and patient selection. To determine consensus in areas where there was not enough available literature, a three-round Delphi method was conducted.
Results
The response rate was 90% in the first round, 90% in the second round, and 80% in the third round of the Delphi technique. Once all responses were aggregated, a live meeting was held with 80% attendance, and consensus was achieved on each of the findings detailed in this manuscript.
Conclusion
Micropulse TLT is a useful addition to the glaucoma armamentarium. When used with proper surgical technique at energy settings within the boundaries described in this manuscript, MicroPulse TLT is a safe and effective treatment for many types and stages of glaucoma. Based on current knowledge and experience, the consensus recommendation of this expert panel is that the standard MicroPulse TLT settings using the revised MicroPulse P3 Probe should be 2500 mW, 31.3% duty cycle, and 4 sweeps at a sweep velocity of 20 seconds each per hemisphere. Both hemispheres avoiding the 3 and 9 clock hours should be treated. The panel also reached consensus on patient selection for MicroPulse TLT providing guidance for the use of the procedure.
Keywords: glaucoma, MicroPulse, transscleral laser therapy
Introduction
MicroPulse Transscleral Laser Therapy (TLT), commonly referred as transscleral cyclophotocoagulation in MicroPulse mode, is a non-incisional laser procedure used to treat multiple types of glaucoma. The Cyclo G6 Laser (Iridex, Mountain View, CA, USA) uses an 810 nm infrared diode laser applied in a transscleral fashion with the MicroPulse P3 Delivery Device, also referred to as the MicroPulse P3 Probe (Iridex, Mountain View, CA, USA). The premise of MicroPulse TLT is that it provides a superior safety profile compared to traditional continuous-wave transscleral laser cyclophotocoagulation while still providing significant intraocular pressure (IOP) lowering, therefore allowing its use in a broader spectrum of patients.
During the last several years, the body of experience and information on MicroPulse TLT has grown significantly. However, there are no published MicroPulse TLT treatment guidelines to determine optimal dosing to balance and maximize safety and efficacy. Because the treatment protocols in the literature have been very heterogeneous and because of the introduction of the revised MicroPulse P3 Probe to the market in 2020, there is a need to refine dosimetry of MicroPulse TLT. The refinement of dosimetry and patient selection, based on current clinical evidence and expert opinion, is the first consensus-based guideline on the topic of MicroPulse TLT.
Background: History, Patient Selection, Efficacy
MicroPulse technology divides a continuous-wave of laser energy into short “ON” pulses separated by longer “OFF” pauses which allows the target tissue to cool, reducing thermal buildup in surrounding non-target tissues and minimizing collateral damage. Transscleral cyclophotocoagulation delivered in MicroPulse treatment mode has been performed across a wide range of primary and secondary glaucomas including primary open-angle (POAG), angle-closure (ACG), normo-tensive, neovascular, pseudo-exfoliative (PXG), congenital, uveitic, pigmentary glaucoma (PG) no pigment disperson, post-keratoplasty, post-vitrectomy, aphakic, iridocorneal endothelial (ICE) syndrome and steroid-induced glaucoma.1–18,29 In addition, studies have reported the use of MicroPulse TLT in patients with ocular hypertension, in addition to mild, moderate, severe, and end-stage glaucoma.4,7,9,29
There are many published papers on the clinical use of MicroPulse TLT. However, there are important weaknesses in the current literature around treatment parameters and laser application. Whereas the traditional continuous-wave transscleral laser cyclophotocoagulation application consists of the application of discrete spots of laser, the MicroPulse TLT surgical technique consists of a sweeping motion around the eye with the MicroPulse P3 Delivery Device. Grippo et al22 recently highlighted the importance of sweep velocity – a parameter that is not consistently described in the literature - and suggested for the first time to consider the calculation of fluence (the amount of energy delivered to a given area) as an important measure of dosimetry for MicroPulse TLT. Contrary to total energy, fluence incorporates sweep velocity into its equation and, when combined with total treatment time, may be more representative than total energy for calculating the energy delivered to target tissues. This may have implications on outcomes as well as patient selection. Considering these important changes, this paper aims to define the current optimal dosimetry for MicroPulse TLT as well as patient selection.
Methods
The authors conducted a search of MicroPulse TLT on PubMed and identified 61 studies, reviews, or published commentaries that contained information on laser settings used to perform MicroPulse TLT and patient selection, and then analyzed the available data. To determine consensus in areas where there was not enough available literature, a three-round Delphi method with a panel of international clinical experts was held between September and October 2021. The first questionnaire included questions on the current treatment parameters that each physician was using, how each manipulated the parameters for different patients, and if each could increase their current level of energy delivery. The second questionnaire sought to look at specific parameters and solicit expert opinion on suggested energy delivery. The third questionnaire further refined the responses to reach specific recommendations. Finally, a virtual meeting was held to present each finding and gain consensus accordingly.
Ten glaucoma specialists with extensive experience performing MicroPulse TLT were recruited from the United States, Argentina, the Netherlands, and Switzerland. All responses remained anonymous until the final virtual meeting, and the study sponsor was not privy to the questionnaires, responses, data collection or preparation of the manuscript. This study did not require approval from an ethics committee due to its design.
Results
The first-round Delphi Panel was completed by nine glaucoma experts and the second and third rounds by eight. The virtual meeting was attended by eight physicians. The degree of consensus by the third round and in the virtual meeting was high, with all participants expressing agreement with the findings stated below.
Finding 1: The amount of laser energy applied determines if the IOP-lowering effect is achieved, and if that effect is reversible or irreversible. Whereas low total energy (TE) results in a lack of efficacy and/or sustainability of the IOP-lowering effect, increased TE is associated with a more permanent effect.
Evidence: In 2018, Sanchez et al19 presented a case series demonstrating that a lower amount of total energy application (62.6 J), though safe in terms of side effects, translated into a lack of initial efficacy or a short-lived IOP-lowering effect. Higher amounts of total energy translated into both greater IOP lowering as well as longer duration of IOP-lowering effect. In an experimental study of autopsy eyes, Johnstone et al20 demonstrated that MicroPulse TLT caused contraction of longitudinal ciliary muscle fibers with tightening of the scleral spur. This resulted in the possible prevention or reversal of collapse of Schlemm’s canal with potential increase in trabecular outflow. The authors further demonstrated that this contraction of the muscle fibers could be totally or partially reversible depending on the amount of total energy used during the experiment.
The overlap of subsequent work by Sanchez et al21 and Johnstone’s experimental work allows one to infer that there is a lower threshold of energy delivery to the eye below which there is no significant or durable IOP lowering effect. This could be because the total energy delivered is too low to meaningfully impact the target tissue, or because the effect is reversible and therefore very short-lived. It is important to note that the work by Johnstone was experimental and used discrete spots of applied laser energy, while the clinical review done by Sanchez was based on outcomes from heterogeneous clinical series in which MicroPulse TLT was typically performed with a sweeping technique, often at unknown sweep velocity.
Finding 2: The upper and lower boundaries most commonly used in the published literature where all parameters were described when using the original MicroPulse P3 Probe are 62 to 200 J of total energy per eye and a fluence value between 52.4 and 69.2 J/cm2. Treatment duration per hemisphere ranged between 50 and 160 s. The most frequently used sweep velocity was 10-second sweeps per hemisphere. When using these parameters, the clinician can expect an initial significant IOP reduction, which in most cases is sustained over time with a good safety profile.
Evidence: By converting the multiple available parameter sets reported in the literature into fluence and total energy values, Grippo et al22 showed that fluence and total energy used across various studies actually fell within a limited range. In the published literature, fluence ranged between 52.4 and 69.2 J/cm2 with a mean of 55.6 J/cm2 (SD 5.7) and a median of 54.2 J/cm2. Total energy per eye ranged from 62.6 to 200.4 J with a mean of 112 J (SD 43) and a median of 100.2 J. Treatment duration per hemisphere ranged between 50 and 160 s (mean 86.7 s, SD 35.3). In general, the balance between safety and efficacy was good, with an IOP-lowering effect ranging from 27.8% to 57.2% reduction from baseline with a mean of 40.6% (SD 9.5%).
Finding 3: Based on the literature using the original MicroPulse P3 Probe, a range of energy application from 150 to 200 J of total energy (most frequently applied with 10-second sweeps per hemisphere) appears to balance a sustainable IOP reduction with limited side effects. At some level of energy, a maximal response is achieved and further increases in energy may not lead to increased IOP reduction.
Evidence: A 2018 review of the scientific literature by Sanchez et al proposed a dose response relationship for MicroPulse TLT.21 The authors suggested application of total energy between 112 and 150J as the optimal range that would result in a good balance of IOP-lowering effect (around 30%) and safety with MicroPulse TLT. This work helped to refine the range of the treatment parameters used in clinical practice, though variability in published works persists.
A more recent case series looked at re-treatment with MicroPulse TLT in patients with advanced glaucoma, many of them with neovascular glaucoma.23 This paper suggests that the best balance of efficacy and safety is found with an application of total energy between 150 and 199.9 J. The authors found a limited IOP response if less than 150 J of total energy were used, and no statistically significant difference in IOP reduction if more than 200 J of total energy (P=0.16) was used. This is the first report of a maximal response. Of note, the patients in this study had advanced glaucoma and had received repeated treatments, resulting in a population that may require higher energy levels to obtain IOP-lowering effects.
Marchand et al24 conducted a prospective study with 18 months of follow-up with the original MicroPulse P3 probe in predominantly Caucasian eyes with glaucoma. Treatment time was varied while the remaining treatment parameters were maintained. Two watts of power and a 31.3% duty cycle applied in 10-second sweeps was used in all eyes. Twenty-eight eyes received treatment for 320 seconds and 23 eyes received treatment for 240 seconds. The mean baseline IOP of 32.6 mmHg was reduced by 40.1% in the group that received 320 seconds of treatment compared to 30.8% in the group that received 240 seconds of treatment (Table 1). There were no cases of persistent anterior chamber inflammation and no patient developed sympathetic ophthalmia or phthisis bulbi. No cases of mydriasis or loss of accommodation were observed. These findings support the evidence that there is a dose response for MicroPulse TLT.
Table 1.
Comparative Study by Marchand et al Found a Greater IOP Reduction with Greater Total Tretment Time24
| Total Energy | Fluence | IOP Reduction at 18 Months | |
|---|---|---|---|
| 320 sec treatment | 200.4 | 62.6 | − 40.1% |
| 240 sec treatment | 150.2 | 62.6 | − 30.8% |
Note: Data from Marchand et al.24
Finding 4: In many instances we may be undertreating, and the upper range is still to be defined. All panel members agree that, based on mean settings used in the published literature, it is possible to increase dosing (energy delivery to target tissues) and thereby increase IOP reduction and/or duration of effect while still maintaining the safety profile.
Evidence: Some studies in the literature included treatment enhancements over time. Published studies in general have reported few side effects, highlighting that sustainability of efficacy over time is more of an issue than safety with currently used parameters.2,19,24,29 As previously discussed, it has been proposed that within certain boundaries, higher energy delivery to the eye causes more lasting effects.19–22 Therefore, it is important to revisit how the upper energy boundaries of MicroPulse TLT are defined. Studies by Williams et al25 and Emmanuel et al5 are frequently cited as exploring the upper limits of treatment due to suggestions that lower levels of energy be used to prevent the high numbers of complications seen in their studies. However, these studies were not designed to evaluate dosing. The studies had some limitations, and the ability to project their findings onto a broader group is limited.
Williams et al25 reported the initial experience of one surgeon with MicroPulse TLT in 79 eyes of 79 patients with advanced or refractory glaucoma. These patients had multiple ocular co-morbidities and poor baseline visual acuities. A discrete spot application technique was used, where the probe was held in place for 10 seconds before the adjacent section of perilimbal conjunctiva was treated. Settings used were 2000 mW of power, a duty cycle of 31.3% and a mean treatment time of 300 ± 42 seconds with a minimum of 120 seconds and maximum of 360 seconds. It is difficult to compare this modified stop and go technique with the traditional sweeping motion that most other surgeons perform at present. However, the amount of energy delivered to each treatment spot with discrete application spots is tremendously high compared to the amount of energy delivered to the same spot during a sweeping motion.
The authors obtained an average reduction in IOP of 51% at last follow-up with an average of 0.8 fewer glaucoma medications for the cohort. Complications were significant including 7 patients (8.8%) with hypotony, 21 patients (26%) with prolonged anterior chamber inflammation, 13 patients (16.5%) with loss of ≥2 lines of BCVA, 4 patients with macular edema (5%), 2 patients with corneal edema (2.5%) and 2 patients with phthisis bulbi (2.5%). Central retinal vein occlusion with macular edema in one patient and retinal detachment in another patient were noted as ocular comorbidities. Both patients experienced vision loss post MicroPulse TLT due to macular edema. The authors performed a linear mixed effects model predicting the effect of time on IOP and found it insignificant, indicating that post-treatment IOP did not change significantly during the follow-up interval, which had a mean of 7.8 ± 4.5 months. Non-white patients had 3.6 times greater odds of prolonged inflammation compared with whites. In addition, there was no difference in IOP control for those patients whose inflammation had resolved at either 3 months or 6 months. One of the weaknesses of this study was the high rate of patients lost to follow-up, and this could have prejudiced the data towards higher apparent complication rates and lower success rates as patients with uneventful postoperative courses are less likely to return to the tertiary care referral center for long-term follow-up.
Emanuel et al5 reported short-term results (average 4 months of follow-up) in a retrospective analysis of 84 eyes of mostly white patients. Compared to commonly used settings described before, they used much higher fluence values – up to 400 J/cm2 or 8 times the mean fluence in the literature - and showed a significant reduction in IOP and medications. For the few patients who were followed for 12 months, pressure decreased from a mean of 27 mmHg to a mean of 11 mmHg. No phthisis bulbi or sympathetic ophthalmia occurred. Up to 41% of the patients had some degree of vision loss, but study follow-up was short and may not have captured the transient nature of some of the vision decrease seen during the follow-up period. Most patients had serious glaucoma and approximately 70% had undergone prior incisional glaucoma surgery.
It is important to highlight that visual acuity loss may not always be related to the procedure itself. Varikuti et al16 used average energy settings and looked specifically at changes in vision following MicroPulse TLT. In their series, 10 eyes out of 49 had vision loss >2 lines at 12 months post-operative. Among these 10 eyes with vision loss, 5 were due to cataract progression. One eye had a history of CME, which recurred post MicroPulse TLT. Two eyes had unexplained vision loss that was attributed to glaucoma progression, and the remaining two eyes had a decrease in vision due to recurrent iritis that resolved at subsequent follow-up visits after the study completion. In this study, a significant percentage of vision loss may not have been directly related to the MicroPulse TLT procedure.
Comparing the energies used in the Williams and in the Emanuel studies to settings extrapolated from Grippo’s review22 (median total energy of 100 J per eye, median fluence of 54.2 j/cm2 with 2000 mW, 10-second sweeps, mean treatment duration per hemisphere of 87 seconds), the panel consensus is that there is room to increase energy delivery and thereby improve success without reaching the side effects profile reported by Williams25 and Emanuel.5
Finding 5: Fluence is a parameter that takes into account power, sweep velocity and duty cycle and shows better correlation with efficacy compared to total energy. Fluence and total treatment time (number of sweeps × duration of sweeps) are both components to be considered when performing MicroPulse TLT as they incorporate all treatment variables relevant to therapy.
Evidence: Grippo et al22 analyzed the available literature through July 2020 and concluded that sweep velocity was an important parameter not fully recognized in the published literature nor in clinical practice. Slower sweep velocity equates to higher fluence with corresponding higher tissue effect.
An analogy is a finger that is passed back-and-forth over a candle quickly will feel significantly less heat than one that is passed slowly over the same candle. For MicroPulse TLT, the treatment “dose” or fluence is the combination of average power, duty cycle, area of the laser probe in direct contact with the scleral surface treated and scanning or “sweep” rate.
The authors concluded that fluence, which includes sweep velocity, compared to total energy, showed a closer relationship with IOP-lowering effect than total energy. A relationship between fluence and number of sweeps (which generates total treatment time) may be the optimal combination of variables to measure overall energy delivery to the eye. This resembles the traditional medical concept for pharmaceutical treatment of dosage (fluence) + dose frequency (number of sweeps). Accurately measuring overall energy delivery to the eye may allow for proper dosimetry to achieve a sustainable IOP lowering effect while maximizing the safety profile.
Finding 6: Most panel members agree that sweep velocity is a significant factor to consider with MicroPulse TLT.
Evidence: There are three main treatment parameters that can be augmented to increase the amount of energy delivery to the eye. Total treatment time, power and sweep velocity all factor into the success of the procedure. As demonstrated by Sarrafpour et al,13 increasing power is associated with better IOP reduction. Sanchez et al21 showed that increasing treatment time is associated with a higher success rate over time. Marchand et al24 showed that increasing total treatment time increases the percentage of IOP reduction. Until Grippo et al22 analyzed fluence, there were no published papers analyzing sweep velocity as a factor that contributes to the success of MicroPulse TLT. In an attempt to analyze both total energy and fluence, Grippo et al performed a meta-analysis to determine a comparison between fluence, total energy and the outcomes of MicroPulse TLT in the same cohorts of patients. Fluence is the energy delivered per unit area (F = Energy used × duty cycle × dwell time/area). A direct comparison between all three parameters (power, sweep velocity and total treatment time) has not been performed to date. However, the authors found that fluence was better correlated to IOP reduction than total energy.
Finding 7: The panel members find that they are able to use higher energy with the revised MicroPulse P3 Probe (higher power and slower sweep velocity) with more effective and longer-lasting IOP lowering without increasing the side effect profile.
Evidence: The revised MicroPulse P3 Probe allows for a more consistent treatment and reduces the side effects associated with anterior treatment (Figures 1 and 2). The original probe consisted of a bulkier tip that in some eyes was difficult to maintain in a perpendicular position to the globe. In addition, the plate surface was convex, which contributed to a tendency for the probe to slide anteriorly at times, delivering the energy in an inconsistent and suboptimal manner. The laser tip also protruded and sometimes traumatized the conjunctiva with movement. The resulting subconjunctival hemorrhages may reduce efficacy as well as cause patient concern post-operatively.
Figure 1.

Original MicroPulse P3 Delivery Device.
Note: Reprinted with permission from IRIDEX. Available from: https://www.iridex.com/Products/GlaucomaDevices/micropulsep3.aspx.30
Figure 2.
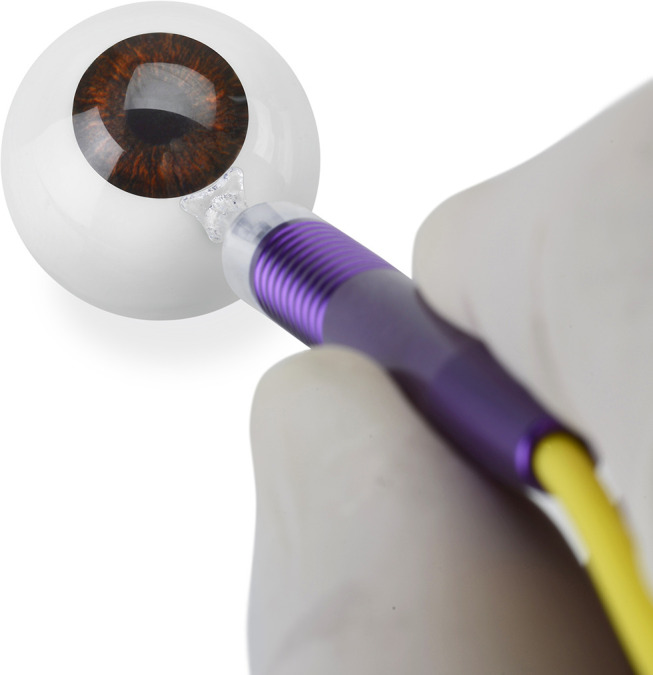
Revised MicroPulse P3 Delivery Device.
Note: Reprinted with permission from IRIDEX. Available from: https://www.iridex.com/Products/GlaucomaDevices/micropulsep3.aspx.30
The revised probe has resolved many of the ergonomic issues with the original MicroPulse P3 probe. It has a thinner profile and therefore is easier to use in eyes with less access to the post-limbal area. There is a flange that slides under the eyelids instead of abutting the eyelid margin thus helping to position the probe correctly. The footplate surface is concave which improves stability and makes it easier to keep the probe angled to the scleral surface during treatment. The angulation of the probe facilitates energy delivery away from the limbal tissues and the tip is recessed to reduce conjunctival trauma with movement. The distance of the laser fiber from the leading edge of the probe also increased to 3 mm, locating the treatment area more posterior to the limbus. These refinements have helped to produce more consistent laser delivery and thus more consistent treatments. Due to the improved ergonomic features, this consensus panel finds that they can increase energy delivery with the revised MicroPulse P3 Delivery Device without negatively impacting the side effect profile, thereby increasing the efficacy and duration of the IOP lowering effect.
Finding 8: The consensus of this panel is that the standard MicroPulse TLT settings using the revised MicroPulse P3 Probe should be 2500 mW, 31.3% duty cycle, and 4 sweeps at a sweep velocity of 20 seconds each per hemisphere (Table 2). These parameters can be used in the majority of cases unless there is a compelling reason to adjust the energy delivery up or down. There is not enough evidence or experience to provide definite minimum and maximum settings as there is too much variation in patient characteristics and clinical scenarios. When an adjustment in energy delivery is needed, consensus is that a 20–25% change in energy delivery is appropriate. This can be achieved by increasing or decreasing either the power, the sweep velocity, or the number of sweeps.
Table 2.
Consensus Standard Settings for MicroPulse TLT
| Power | Duty Cycle | No. of Sweeps | Sweep Velocity |
|---|---|---|---|
| 2500 mW | 31.3% | 4 | 20 seconds per hemisphere |
Evidence: This finding was concluded based on a Delphi process approach that started with a questionnaire about what settings were used by panel members in general, a debate about experience with the original probe and an interpretation of the literature, consensus findings and expert clinical experience to fine-tune the settings.
Finding 9: Although it is reasonable to reduce the energy in highly pigmented eyes, there is a lack of evidence in the literature for adjusting settings based on race or pigmentation. The consensus of this panel is that, with currently recommended settings, it is not necessary to significantly adjust them based on race or pigmentation. More evidence is needed.
Evidence: There is some evidence that clinical success is achieved with less energy in more pigmented populations. For example, Awoyesuku et al26 conducted a study in Nigeria with 12 patients over a six-month period with a total energy of 225.4 J and saw a mean IOP reduction of 38% at 6 months and a mean decrease in medications from 3 to 1. This can be compared to the study by Marchand et al24 on a primarily Caucasian population that used a similar total energy (200.4 J) and saw a mean decrease in IOP of 40% with no significant reduction in number of medications at 18 months.
Evidence exists that the melanin in light and brown irises reacts in the same manner to treatment with radiation. Menon et al showed that the electron spin resonance of the melanin of the irises of both light and dark pigmented eyes was the same.27
Finding 10. Patient Selection for MicroPulse TLT
The literature shows that MicroPulse TLT has been performed with a good safety profile in patients with POAG and ACG.1–7,9–12,14,16,17,29 The literature also documents the use of MicroPulse TLT to treat various types of secondary glaucoma (neovascular,1–10,12–15,17,18 post keratoplasty,1,3,4,6,8,10,15,17–20 traumatic,1,2,4–6,8–10,12 post vitrectomy,2–6,10,14,15 congenital,1,6,8–11,14,18,21 PXG,5,7,9,11–13,16,18 PDG,5,6,9,10 aphakic,1,2,4,10,16,21 pseudophakic,15,21,22 ICE syndrome1,9,10,17,21 and steroid-induced glaucoma15,21,22), with a good safety profile, although efficacy may vary. Attention should be paid to the treatment of any underlying causes and, if necessary, additional postoperative care.
Treatment with MicroPulse TLT has been performed with a good safety profile in patients across a wide spectrum of stages of glaucoma and IOPs.4,7,9,29
MicroPulse TLT has been performed with a good safety profile in adult patients with glaucoma. In pediatric patients with glaucoma, MicroPulse TLT appears to be safe, but there are limited data and more retreatments and failures should be expected.8,21,28 Further clinical investigation to the effect of MicroPulse TLT in pediatric patients is recommended.
MicroPulse TLT has been performed with a good safety profile in patients with glaucoma after trabeculectomy and/or glaucoma drainage device surgery.1,3–8,12,16,18,21,28 MicroPulse TLT also appears to be safe prior to incisional glaucoma surgery although the effect on subsequent incisional surgery has not been widely studied.1,4–6,18
Caution should be exercised in patients with scleral thinning, keeping in mind the underlying pathophysiological mechanism (eg, rheumatoid arthritis versus areas of thinned sclera secondary to prior glaucoma surgery). While avoiding the areas of thin sclera from prior trabeculectomies would be enough in such cases; scleras of patients with underlying rheumatoid arthritis may respond less predictably and may be more prone to potential side effects. When MicroPulse TLT treatment is still indicated in cases of a thinner sclera, one should avoid treatment in those areas and consider reducing the energy delivered to the eye given that there will be more transmission of energy secondary to the thinned sclera. In the literature, scleral thinning of more than one clock hour was often an exclusion criterion.4,14,18,24,25,28
Caution should be exercised in the postoperative care of patients with a higher risk of postoperative inflammation, such as active or history of recurrent uveitis.
Finding 11. Indications of MicroPulse TLT
The literature summary above shows that MicroPulse TLT has been performed with a good safety profile in various types and severities of glaucoma. It is important to look at which therapy is most suitable for a given patient in relation to the entire glaucoma treatment spectrum. In clinical practice, treatment with MicroPulse TLT can be considered in the following patient categories:
1. Patients who are not candidates for incisional glaucoma surgery or who do not wish to undergo incisional surgery. Examples include scarred or unhealthy conjunctival tissue, increased risk of surgical complications (use of blood thinners, aphakia), chance of visual “snuff out” in patients with end-stage glaucoma or patients’ inability to attend frequent postoperative visits (eg, in elderly patients or those with intellectual disability).
2. Patients who have had prior incisional glaucoma surgery. This includes patients after trabeculectomy or a glaucoma drainage device with suboptimal IOP control and/or the desire to use less glaucoma medication.
3. Patients with uncontrolled IOP.
4. Patients with stable glaucoma on maximal tolerated drug therapy with a desire for a reduction in glaucoma medication.
5. Due to the favorable safety profile, it is possible to use MicroPulse TLT in any part of the treatment algorithm. Expert consensus is to consider MicroPulse TLT use after medications and laser trabeculoplasty have already been attempted.
Conclusion
Micropulse TLT is a useful addition to the glaucoma armamentarium. When used with proper surgical technique at energy settings within the boundaries described above, MicroPulse TLT is a safe and effective treatment for many types and stages of glaucoma. Design improvements in ergonomics and laser delivery have enhanced the procedure and made it more user friendly. The extensive physician experience and published literature now available have made it possible to re-evaluate and revise laser settings to optimize results. Based on this current knowledge and experience, the consensus recommendation of this expert panel is that the standard MicroPulse TLT settings using the revised MicroPulse P3 Probe should be 2500 mW, 31.3% duty cycle, and 4 sweeps at a sweep velocity of 20 seconds each per hemisphere. This work by the Delphi panel summarizes and highlights the optimal dosimetry and patient selection for MicroPulse TLT. Future studies will expand on best practices regarding anesthesia, surgical techniques, pre- and post-op care, outcomes, and retreatment.
Acknowledgments
Writing and editing support was provided by Adrianne Resek, MA. Ms Resek participated in the development and analysis of the Delphi method questionnaires in addition to preparation of the manuscript.
Funding Statement
Funding for research on this paper was provided by Iridex Corporation. Funding for writing support for this paper was provided by Iridex Corporation to Adrianne Resek, MA.
Ethics Approval
This paper did not require any original research and thus no ethics approval was needed.
Consent to Publication
Iridex consents to publish the images of the MicroPulse P3 Delivery Devices.
Disclosure
Iridex was not privy to questionnaires, responses or data collection during the Delphi Consensus process, nor were they privy to the manuscript during its preparation, writing and submission. Dr Tomas M Grippo received personal fees from Iridex, MST, Alcon, during the conduct of the study. Dr Ronald MPC de Crom reports personal fees from Iridex, during the conduct of the study. Dr Michael Giovingo reports personal fees, non-financial support from Iridex Corporation, during the conduct of the study; grants from Santen, outside the submitted work. Dr Marc Töteberg-Harms reports grants, personal fees from Iridex, during the conduct of the study; personal fees from Allergan, personal fees from ELT Sight, personal fees from MLase AG, personal fees from Novartis/Alcon, personal fees from Reichert, grants, personal fees from Santen, personal fees from Heidelberg Engineering, personal fees from Glaukos, outside the submitted work. Dr Brian A Francis reports grants from Iridex, outside the submitted work. Dr Brian Jerkins reports personal fees from Iridex, during the conduct of the study. Dr Jacob Brubaker reports personal fees from Iridex, during the conduct of the study. Dr Nathan Radcliffe reports personal fees from Iridex, during the conduct of the study. Dr Jella An reports personal fees from Iridex, during the conduct of the study. Dr Robert Noecker reports personal fees from Iridex, during the conduct of the study. The authors report no other conflicts of interest in this work.
References
- 1.Al Habash A, Al Ahmadi AS. Outcome of micropulse transscleral photocoagulation in different types of glaucoma. Clin Ophthalmol. 2019;13:2353–2360. doi: 10.2147/OPTH.S226554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aquino MC, Barton K, Tan AM, et al. Micropulse versus continuous wave transscleral diode cyclophotocoagulation in refractory glaucoma: a randomized exploratory study. Clin Exp Ophthalmol. 2015;43(1):40–46. doi: 10.1111/ceo.12360 [DOI] [PubMed] [Google Scholar]
- 3.Ariga M, Nivean PD, Nivean PD, et al. Micropulse trans-scleral diode laser cyclophotocoagulation in refractory glaucoma: an initial experience in Indian eyes. Int Ophthalmol. 2021;41(8):2639–2645. doi: 10.1007/s10792-021-01697-1 [DOI] [PubMed] [Google Scholar]
- 4.de Crom R, Slangen C, Kujovic-Aleksov S, et al. Micropulse trans-scleral cyclophotocoagulation in patients with glaucoma: 1- and 2-year treatment outcomes. J Glaucoma. 2020;29(9):794–798. doi: 10.1097/IJG.0000000000001552 [DOI] [PubMed] [Google Scholar]
- 5.Emanuel ME, Grover DS, Fellman RL, et al. Micropulse cyclophotocoagulation: initial results in refractory glaucoma. J Glaucoma. 2017;26(8):726–729. [DOI] [PubMed] [Google Scholar]
- 6.Garcia GA, Nguyen CV, Yelenskiy A, et al. Micropulse transscleral diode laser cyclophotocoagulation in refractory glaucoma: short-term efficacy, safety, and impact of surgical history on outcomes. Ophthalmol Glaucoma. 2019;2(6):402–412. doi: 10.1016/j.ogla.2019.08.009 [DOI] [PubMed] [Google Scholar]
- 7.Kaba Q, Somani S, Tam E, Yuen D. The effectiveness and safety of micropulse cyclophotocoagulation in the treatment of Ocular hypertension and glaucoma. Ophthalmol Glaucoma. 2020;3(3):181–189. doi: 10.1016/j.ogla.2020.02.005 [DOI] [PubMed] [Google Scholar]
- 8.Lee JH, Shi Y, Amoozgar B, et al. Outcome of micropulse laser transscleral cyclophotocoagulation on pediatric versus adult glaucoma patients. J Glaucoma. 2017;26(10):936–939. doi: 10.1097/IJG.0000000000000757 [DOI] [PubMed] [Google Scholar]
- 9.Logioco C, Perrone LD, Caruso D, et al. Assessment of efficacy and safety of micropulse diode laser treatment in glaucoma: one year follow-up. Arch Soc Esp Oftalmol. 2020;95(7):327–333. doi: 10.1016/j.oftal.2020.03.002 [DOI] [PubMed] [Google Scholar]
- 10.Magacho L, Lima FE, Avila MP. Double-session micropulse transscleral laser (CYCLO G6) for the treatment of glaucoma. Lasers Med Sci. 2020;35:1469–1475. doi: 10.1007/s10103-019-02922-1 [DOI] [PubMed] [Google Scholar]
- 11.Nguyen AT, Maslin J, Noecker RJ. Early results of micropulse transscleral cyclophotocoagulation for the treatment of glaucoma. Eur J Ophthalmol. 2019;30:1120672119839303. [DOI] [PubMed] [Google Scholar]
- 12.Radhakrishnan S, Wan J, Tran B, et al. Micropulse cyclophotocoagulation: a multicenter study of efficacy, safety, and factors associated with increased risk of complications. J Glaucoma. 2020;29(12):1126–1131. doi: 10.1097/IJG.0000000000001644 [DOI] [PubMed] [Google Scholar]
- 13.Sarrafpour S, Ayoub S, Radcliffe NM, Radcliffe NM. Micropulse transscleral cyclophotocoagulation A look at long-term effectiveness and outcomes. Ophthalmol Glaucoma. 2019;2:167–171. doi: 10.1016/j.ogla.2019.02.002 [DOI] [PubMed] [Google Scholar]
- 14.Tan AM, Chockalingam M, Aquino MC, et al. Micropulse transscleral diode laser cyclophotocoagulation in the treatment of refractory glaucoma. Clin Exp Ophthalmol. 2010;38(3):266–272. doi: 10.1111/j.1442-9071.2010.02238.x [DOI] [PubMed] [Google Scholar]
- 15.Tekeli O, Kose HC. Outcomes of micropulse transscleral cyclophotocoagulation in primary open-angle glaucoma, pseudoexfoliation glaucoma, and secondary glaucoma. Eur J Ophthalmol. 2021;31(3):1113–1121. doi: 10.1177/1120672120914231 [DOI] [PubMed] [Google Scholar]
- 16.Varikuti VNV, Shah P, Rai O, et al. Outcomes of micropulse transscleral cyclophotocoagulation in eyes with good central vision. J Glaucoma. 2019;28(10):901–905. doi: 10.1097/IJG.0000000000001339 [DOI] [PubMed] [Google Scholar]
- 17.Yelenskiy A, Gillette TB, Arosemena A, et al. Patient outcomes following micropulse transscleral cyclophotocoagulation: intermediate-term results. J Glaucoma. 2018;27(10):920–925. doi: 10.1097/IJG.0000000000001023 [DOI] [PubMed] [Google Scholar]
- 18.Zaarour K, Abdelmassih Y, Arej N, et al. Outcomes of micropulse transscleral cyclophotocoagulation in uncontrolled glaucoma patients. J Glaucoma. 2019;28(3):270–275. doi: 10.1097/IJG.0000000000001174 [DOI] [PubMed] [Google Scholar]
- 19.Sanchez FG, Lerner F, Sampaolei J, et al. and safety of micropulse transscleral cyclophotocoagulation in glaucoma. Archivos de Oftalmología. 2018;93(12):573–579. doi: 10.1016/j.oftal.2018.08.003 [DOI] [PubMed] [Google Scholar]
- 20.Johnstone M, Padilla S, Wen K. Transcleral laser, ciliary muscle shortening & outflow pathway reorganization. Invest Ophthalmol Vis Sci. 2017;58:3468. [Google Scholar]
- 21.Sanchez F, Peirano-Bonomi JC, Grippo TM. Micropulse transscleral cyclophotocoagulation: a hypothesis for the ideal parameters. Med Hypothesis Discov Innov Ophthalmol. 2018;7(3):94–100. [PMC free article] [PubMed] [Google Scholar]
- 22.Grippo TM, Sanchez FG, Stauffer J, Marcellino G. MicroPulse transscleral laser therapy – fluence may explain variability in clinical outcomes; a literature review. Clin Ophthalmol. 2021;15:2411–2419. doi: 10.2147/OPTH.S313875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lim EJY, Aquino CM, Lun KWX, et al. Efficacy and safety of repeated micropulse transscleral diode cyclophotocoagulation in advanced glaucoma. J Glaucoma. 2021;30(7):566–574. doi: 10.1097/IJG.0000000000001729 [DOI] [PubMed] [Google Scholar]
- 24.Marchand M, Singh H, Agoumi Y. Micropulse trans-scleral laser therapy outcomes for uncontrolled glaucoma: a prospective 18-month study. Can J Ophthalmol. 2021;56:371–378. doi: 10.1016/j.jcjo.2021.01.015 [DOI] [PubMed] [Google Scholar]
- 25.Williams AL, Moster MR, Rahmatnejad K, et al. Clinical efficacy and safety profile of MicroPulse cycophotocoagulation in refractory glaucoma. J Glaucoma. 2018;27(5):445–449. doi: 10.1097/IJG.0000000000000934 [DOI] [PubMed] [Google Scholar]
- 26.Awoyesuku EA, Fiebai B. Outcome of micropulse laser in treatment of open angle glaucoma in a peripheral hospital in rivers state, Nigeria: our initial experience. J Advances in Medicine and Medical Research. 2019;29(2):1–7. [Google Scholar]
- 27.Menon IA, Basu PK, Persad S, Avaria M, Felix CC, Kalyanaraman B. Is there any difference in the photobiological properties of melanins isolated from human blue and brown eyes? BJO. 1987;71(549–552):549–552. doi: 10.1136/bjo.71.7.549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Elhefney EM, Mokbel TH, Hagras SM, et al. Micropulsed diode laser cyclophotocoagulation in recurrent pediatric glaucoma. Eur J Ophthalmol. 2020;30(5):1149–1155. doi: 10.1177/1120672119858226 [DOI] [PubMed] [Google Scholar]
- 29.Hooshmand S, Voss J, Hirabayashi M, McDaniel L, An J. Outcomes of initial and repeat micro-pulse transscleral cyclophotocoagulation in adult glaucoma patients. Ther Adv Ophthalmol. 2022;14:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.IRIDEX. Available from: https://www.iridex.com/Products/GlaucomaDevices/micropulsep3.aspx. Accessed may 30, 2022. [Google Scholar]


