Abstract
Background
Few longitudinal studies have explored exploring the relationship between sleep duration and sarcopenia. Evidence concerning the relationship between sleep duration and sarcopenia is limited and inconsistent. The purpose of this 3-year prospective study was to explore whether sleep duration was associated with sarcopenia onset in suburb-dwelling older Chinese individuals.
Methods
This was a prospective study that included 754 Chinese suburb-dwelling men and women aged ≥60 years (men n=327, mean age 65.24± 4.87 years) who were not initially diagnosed with sarcopenia. We defined sarcopenia using the diagnostic algorithm recommended by the Asian Working Group for Sarcopenia. Self-reported sleep duration was a component of the interview measured by trained interviewers. Subjects were categorized into 3 groups at baseline [short: <6 h, medium: 6–8 h, and long: >8 h].
Results
The incidence of sarcopenia during the 3-year follow-up was 12.2%. Multivariate logistic regression analyses showed that after adjustments for potential confounders long sleep duration was independently associated with sarcopenia incidence from baseline through the 3-year follow-up: when using the 6–8 h sleep duration group as a reference, the adjusted ORs for sarcopenia of the groups who slept <6 and >8 hours were 2.74 (95% CI 1.05–7.13) and 1.84 (95% CI 1.07–3.14).
Conclusion
Both short and long sleep durations were associated with a greater incidence of sarcopenia. Thus, sleep duration should be considered when developing prevention and management strategies for sarcopenia.
Keywords: longitudinal study, older, sarcopenia, sleep duration, suburb-dwelling
Introduction
Sarcopenia is a disease that is characterized by a decline in skeletal muscle mass, muscle strength, and physical performance.1 It is associated with the ageing process and is known to be associated with adverse health outcomes, such as physical inactivity, disability, diabetes, metabolic syndrome, poor quality of life, and mortality.2 Sarcopenia contributes to current increased health care costs and is becoming a major public health problem.3 Because of the debilitating symptoms and increased health care cost burden, it is of great importance to determine the factors related to sarcopenia and to prevent it at an early stage.
Furthermore, because sarcopenia is difficult to notice in daily life, few people pay attention to it. It is important to observe its risk factors that can be easily avoided, and sleep duration may be a good factor. Sarcopenia is closely related to increased insulin resistance and proinflammatory markers, such as C-reactive protein and interleukin-6.4,5 It has been reported that long duration sleepers have significantly higher levels of C-reactive protein and interleukin-6, and long sleep duration is closely related to increased insulin resistance.6,7 Therefore, we have reasons to believe that there might be a certain relationship between sleep disorders and sarcopenia. A previous study demonstrated that long sleep duration is related to skeletal muscle loss in postmenopausal women.8 Previous studies9,10 also found that longer sleep duration is associated with a significantly higher prevalence of sarcopenia. A systematic review11 showed a U-shaped relationship between sleep duration and sarcopenia. However, the studies used in this meta-analysis were cross-sectional in nature, not longitudinal. The first longitudinal cohort study of sleep duration and sarcopenia revealed that only long sleep duration was associated with sarcopenia.12 Hence, the relationship between sleep duration and sarcopenia remains uncertain. Furthermore, most previous studies are cross-sectional studies; there are few longitudinal studies exploring the causal relationship between them. Therefore, it is necessary to conduct longitudinal studies to determine the effect of sleep duration on sarcopenia.
The aim of this study was to explore whether sleep duration influences sarcopenia in suburb-dwelling older adults. This is a particularly significant study population since in China, more than 70% of elderly individuals live in suburban areas.13 Those living in suburban areas often have a relatively low level of medical treatment and poor health. Offering them health guidance is particularly meaningful to them. Furthermore, if sleep duration has an effect on sarcopenia, it can be used as a risk factor allowing for early diagnosis of sarcopenia and consequent prompt interventions.
Methods
Study Participants
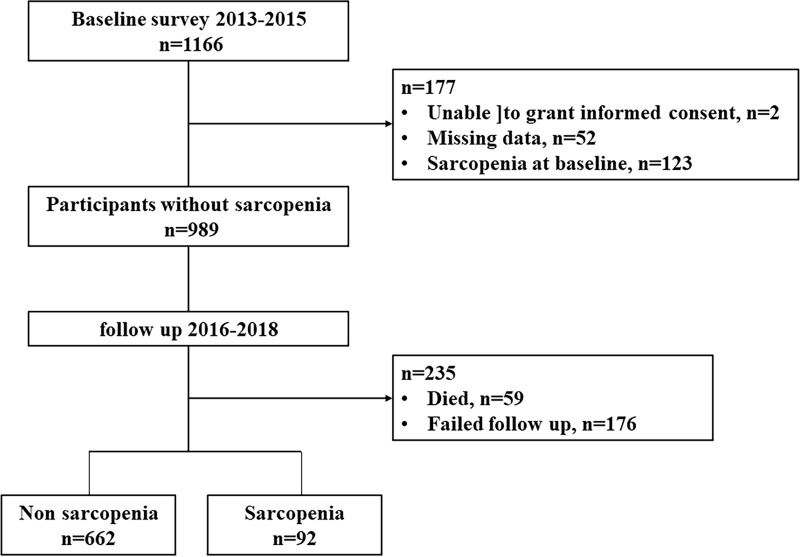
All participants were 60 years of age or older and had enrolled in the National Free Physical Examination Program in the Chadian of Hangu area, Tianjin, China, which is a relatively large sample of suburb-dwelling older people living in a rural area. We enrolled 1166 participants from March 2013 to August 2015 at baseline who were invited to participate in a comprehensive geriatric assessment. Participants with the following conditions were excluded from the study: (1) unable to grant informed consent (n=2); (2) unable to perform anthropometric measurements (n=52); and (3) diagnosed with sarcopenia using the AWGS criteria (n=123). The final study population was comprised of 989 subjects without sarcopenia. The cohort was invited to attend repeat questionnaire interviews and physical measurements after 3 years. During the follow-up years, 59 participants died. A total of 176 participants were excluded because they were lost to follow-up. The incidence and risk factors for sarcopenia were therefore assessed in 754 persons (Figure 1). This study was carried out according to the Declaration of Helsinki. All participants provided informed consent prior to participation. This study is part of the Adult Physical Fitness and Health Cohort Study, which is a prospective dynamic cohort focusing on the relationship between physical fitness and the health status of a population living in Tianjin, China.14–16 This study was approved by the Ethics Committee of Tianjin Medical University.
Figure 1.
Participant flow in this study.
Covariates
Data regarding sociodemographic, and behaviour characteristics, and medical conditions were obtained as previously described (via face-to-face questions).14 Sociodemographic variables, including age, gender, marital status, educational level, and occupation, were assessed. Behaviour characteristics included smoking and drinking habits. The daily physical activity and sitting time were evaluated using the short form of the International Physical Activity Questionnaire (IPAQ).17 Depressive symptoms were assessed using the Geriatric Depression Scale. Participants with a score of ≥11 were considered to have depressive symptoms.18 The prevalence of specific medical conditions was established using standardized criteria that combined information from history of physical illness evaluated on the basis of participants’ response (yes or no) to questions, physician diagnosis, and taking corresponding medication or undergoing other treatment now or in the past.
Assessment of Sarcopenia
Sarcopenia was defined according to the Asian Working Group for Sarcopenia (AWGS) criteria,19 in which a person who has low muscle mass, low muscle strength and/or low physical performance was identified as having sarcopenia. Low muscle mass was classified as relative skeletal muscle mass index (ASM/ht2) less than 7.0 kg/m2 and 5.7 kg/m2 in men and women, respectively; low muscle strength was defined as grip strength <28 kg or <18 kg for males and females, respectively; and low physical performance was defined as walking speed <1.0 m/s for both males and females. Details of the measurement methods have been described in our previous study.15
Assessment of Sleep Behaviour
Self-reported sleep duration was measured by trained interviewers as a component of the interview. Participants were asked the time they usually went to bed and got up during the past month, which is the same measure as in some previous studies20 and our previous study.16 We calculated the time they slept per night. Subjects were categorized into 3 groups: short: < 6 h, medium:6–8 h, and long: > 8 h.
Sleep quality was assessed by asking the subjects “Do you think you slept enough during the past month?” (The subjects had have 4 choices: 1=very well, 2=good, 3=not enough, 4=very poor). We also asked the subjects whether they took sleep drugs21(including Eszopiclone, Zaleplon, Zolpidem, Melatonin, Ramelteon, Benzodiazepine Hypnotics, Antidepressants, Suvorexant et al) before they went to bed.
Statistical Analysis
Continuous variables (age, ASM/Height2, grip strength, 4-meter walking test, and, BMI) that were normally distributed were expressed as means and standard deviation. Nonnormal distribution parameters (IPAQ, sitting time) are given as medians ± 25–75th percentiles. Additionally, classification variables were reported as percentages. Differences in the characteristics according to sarcopenia status were analysed using t–tests, Chi-square tests, and Kruskal–Wallis rank tests. Risk factors for sarcopenia incidence at the follow-up years were analysed individually using logistic regressions. The models considered were Model 1, crude model with adjusted for no variables; Model 2, including age, sex, BMI, sitting time, and depression; and Model 3, including the use of sleep drugs, sleep quality and medical history (diabetes, hypertension, hyperlipidaemia, heart disease, and stroke) along with the covariates of Model 2. All statistical analyses were performed using SPSS version 19.0, and P values less than 0.05 were considered statistically significant.
Results
Table 1 presents the characteristics of these participants by sarcopenia status. Of the 754 participants included in this study, there were 327 men (43.3%) and 427 women (56.7%). Between baseline and 3-year follow-up, 92 of the participants without sarcopenia at baseline had developed sarcopenia. The incidence of sarcopenia was 12.2% at the 3-year follow up, representing an incidence of 15.9% men and 9.4% women. The mean ages of the participants without sarcopenia and with sarcopenia were 65.75±5.41 and 69.16±6.18 years, respectively. The incidence of sarcopenia in the < 6-, 6-8-, and > 8- hour groups was 19.5%, 7.1%, and 15.7%, respectively. In the group with no sarcopenia at baseline, subjects with new-onset sarcopenia had a significantly lower BMI than subjects without sarcopenia (p<0.05). Compared to subjects without sarcopenia, those with new-onset sarcopenia were more likely to be older, and have short or longer sleep durations.
Table 1.
Baseline Characteristics of Study Participants According to the Presence of Sarcopenia
| Characteristic | Non Sarcopenia (n=662) | Sarcopenia (n=92) | t, Z or χ2 value | P-value |
|---|---|---|---|---|
| Age (y) | 65.75±5.41 | 69.16±6.18 | 5.6a | <0.001 |
| Sex, n (%) | 7.4b | 0.005 | ||
| Male | 275(41.5) | 52(56.5) | ||
| Female | 387(58.5) | 40(43.5) | ||
| ASM/Height2 (kg/m2) | 7.32±2.34 | 7.21±0.97 | 0.3a | 0.729 |
| Grip strength (kg) | 26.62±9.15 | 25.90±8.41 | 0.7a | 0.474 |
| 4-meter walking test (m/s) | 1.02±0.19 | 0.97±0.23 | 2.6a | 0.011 |
| BMI (kg/m2) | 26.04±3.11 | 24.0±3.38 | 5.9a | <0.001 |
| IPAQ (Met/wk) | 2099.5(939.8,4991.3) | 2079.0(1386.0,4452.0) | 1.1c | 0.261 |
| Sitting time (h) | 4.0(2.0–6.0) | 3.0(2.0–5.0) | 1.6c | 0.119 |
| Depression, n (%) | 1.6b | 0.139 | ||
| No | 597(90.2) | 79(85.9) | ||
| Yes | 65(9.8) | 13(14.1) | ||
| Sleep duration (h/d), n (%) | 14.5b | 0.001 | ||
| ≤6 | 33(5.0) | 8(8.7) | ||
| 6–8 | 302(45.6) | 23(25.0) | ||
| >8 | 327(49.4) | 61(66.3) | ||
| Use of sleep drug, n (%) | 0b | 0.380 | ||
| No | 600(90.6) | 82(89.1) | ||
| Yes | 62(9.4) | 10(10.9) | ||
| Sleep quality, n (%) | 3.3b | 0.349 | ||
| Very well | 304(45.9) | 35(38.0) | ||
| Good | 205(31.0) | 29(31.5) | ||
| Not enough | 68(10.3) | 14(15.2) | ||
| Very poor | 85(12.8) | 14(15.2) | ||
| Living alone, n (%) | 0.8b | 0.230 | ||
| No | 586(88.5) | 78(84.8) | ||
| Yes | 76(11.5) | 14(15.2) | ||
| Illiteracy, n (%) | 0.2b | 0.378 | ||
| No | 421(63.6) | 60(65.2) | ||
| Yes | 241(36.4) | 32(34.8) | ||
| Farming, n (%) | 0.3b | 0.369 | ||
| No | 109(16.5) | 13(14.1) | ||
| Yes | 553(83.5) | 79(85.9) | ||
| Drinking, n (%) | 0.8b | 0.226 | ||
| No | 482(72.8) | 63(68.5) | ||
| Yes | 180(27.2) | 29(31.5) | ||
| Smoking, n (%) | 2.3b | 0.083 | ||
| No | 459(69.3) | 57(62.0) | ||
| Yes | 203(30.7) | 35(38.0) | ||
| Diseases | ||||
| Diabetes, n (%) | <0.1b | 0.450 | ||
| No | 582(87.9) | 80(87.0) | ||
| Yes | 80(12.1) | 12(13.0) | ||
| Hypertension, n (%) | 0.2b | 0.366 | ||
| No | 343(51.8) | 50(54.3) | ||
| Yes | 319(48.2) | 42(45.7) | ||
| Hyperlipidemia, n (%) | 2.1b | 0.097 | ||
| No | 486(73.4) | 61(66.3) | ||
| Yes | 176(26.6) | 31(33.7) | ||
| Heart disease, n (%) | 0.1b | 0.401 | ||
| No | 515(77.8) | 70(76.1) | ||
| Yes | 147(22.2) | 22(23.9) | ||
| Stroke, n (%) | <0.1b | 0.545 | ||
| No | 620(93.7) | 86(93.5) | ||
| Yes | 42(6.3) | 6(6.5) |
Note: at value, bχ2 value, cZ value.
Abbreviations: ASM, appendicular skeletal muscle mass; BMI, body mass index; IPAQ, international physical activity questionnaire; Met/wk, metabolic equivalent task minutes per week.
The outcomes of the applications of the models that tested the effects of sleep duration on sarcopenia incidence are illustrated in Table 2. Compared to the reference group (6–8 h sleep duration), the ORs of the slept < 6 h and > 8 h groups for Model 1 were 3.18 (95% CI 1.32–7.69) and 2.45 (95% CI 1.48–4.06), respectively, with statistical significance; the results were also statistically significant for Model 2 and Model 3 with added control variables. After adjustments for potential confounders, shorter and longer sleep durations were independently associated with sarcopenia incidence from baseline to the 3-year follow-up in Model 3: the adjusted ORs of the groups who slept < 6 h and > 8 h for sarcopenia were 2.74 (95% CI 1.05–7.13) and 1.84 (95% CI 1.07–3.14), respectively.
Table 2.
Multiple Logistics Regression on Incidence of Sarcopenia During Baseline to 3-Years
| Sleep Duration | n | Model 1 | Model 2 | Model 3 |
|---|---|---|---|---|
| OR (95% CI) | OR (95% CI) | OR (95% CI) | ||
| <6 h | 41 | 3.18(1.32–7.69) | 2.98(1.17–7.55) | 2.74(1.05–7.13) |
| 6–8 h | 325 | Reference | Reference | Reference |
| >8 h | 388 | 2.45(1.48–4.06) | 1.89(1.12–3.20) | 1.84(1.07–3.14) |
Notes: Model 1: unadjusted; Model 2: adjustment for age, gender, BMI, sitting time, and depression; Model 3: Model 2+ adjustment for use of sleep drugs and sleep quality, and medical history of diabetes, hypertension, hyperlipidemia, heart disease, and stroke.
Discussion
In this study, our results showed that both short and long sleep durations were associated with a greater incidence of sarcopenia during the 3-year study period in older adults. To the best of our knowledge, this study is the first longitudinal study to examine the relationship between sleep duration and sarcopenia.
Incidence of Sarcopenia
The incidence of sarcopenia during the period from baseline to the 3-year follow-up was 12.2% (15.9% in men and 9.4% in women). This is consistent with earlier findings,22,23 which showed that the annual and 4-year incidences of sarcopenia were 4.0%22 and 11.2%,23 respectively. In our study, the incidence of sarcopenia in men was higher than that in women, which is in line with one earlier study that showed that males were more commonly affected by sarcopenia than females,24 while another study showed a higher prevalence of sarcopenia and severe sarcopenia in women than in men.25 However, most studies to date have reported that there are no significant differences in sarcopenia prevalence between men and women.26
The Relationship Between Sleep Duration and Sarcopenia
Our results showed that the risk of developing sarcopenia in older adults who sleep less than 6 hours per night and more than 8 hours was 2.74 times and 1.84 times the risk in older adults who sleep between 6 and 8 hours per night, respectively. The results showed that both short and long sleep durations were associated with sarcopenia onset. Our results were in line with those of a cross-sectional previous study.27 The impact of short sleep duration on sarcopenia may be attributed to the following mechanisms. Regarding sleep-related hormonal pathways, many hormones for growth are released during sleep, such as protein synthesis, tissue growth, and muscle repair.11 Thus, reduced duration of sleep potentially interferes by inhibiting protein synthesis pathways and inducing degradation in skeletal muscle pathways. In addition, short sleep duration caused by chronic insomnia might dysregulate the hypothalamopituitary adrenal axis resulting in endocrine changes, which also induce proteolysis, change body composition and lead to sarcopenia.28 The biological mechanisms underlying the relationship between sleep and sarcopenia remain unclear. Further research with larger sample sizes and longer follow-up durations is needed to better understand the relationship between sleep deprivation and sarcopenia.
Several mechanisms may contribute to the understanding of this longitudinal association between long sleep duration and sarcopenia. First, sleep duration is closely linked to physical inactivity and sedentary lifestyle.29 Our results also showed that participants with longer sleep durations (>8 h) had significantly longer sitting times (obtained from the IPAQ) than those who sleep between 6 and 8 hours (3.92±2.34 vs 4.57±2.56, p=0.038). Lifestyle factors including low physical activity, sedentary behaviour, and smoking have been reported to accelerate the progression of sarcopenia.30 Second, insulin resistance could be one possible link between sleep duration and sarcopenia. Long sleep duration is closely related to increased insulin resistance.7 Resistance to insulin has been reported to contribute a decline in the synthesis of skeletal mass protein and finally ultimately result sarcopenia in older people.4 Our cross-sectional study16 and a previous study20 also found that long duration sleepers had the lowest muscle strength and walking speed of all the groups and experienced the greatest decline in physical performance. Another link between sleep duration and sarcopenia could be chronic inflammation. It was reported that long- duration sleepers have significantly higher levels of proinflammatory markers, such as C-reactive protein and interleukin-6.6 These inflammatory mediators play an important role in sarcopenia by promoting muscle proteolysis.5 Our cohort study also showed that participants with longer sleep durations had significantly more muscle loss, based on the percentage change in muscle at the three-year follow-up appointment relative to the baseline, than those with normal sleep durations (−6.34±1.94% vs −4.15±1.76%, p<0.05).31 In addition, circadian rhythm disruptions and hormonal changes associated with long sleep durations might also be a possible underlying mechanism explaining the relationship between sleep duration and sarcopenia.32
Strengths and Limitations of the Study
This study has a few strengths. First, this study was the first longitudinal study using AWGS criteria to examine the relationship between sleep duration and sarcopenia in Asia. Second, this study was also the first to examine a uniquely defined group of suburban older people living in a discrete geographical area. Our participants were recruited from a suburban area, and leading a more physically active lifestyle, which might be different from subjects in other geographical areas. Another strength of this study was its 3-year longitudinal cohort study design.
Our study has several limitations. First, participants in this study were independent in their activities of daily living and relatively healthy, as we did not include participants who were unable to participate in the free annual national physical examination (eg, those who were bedridden or those with serious diseases). This may lead to an underestimation of the prevalence of sarcopenia and its associated health impact. Second, our study’s follow-up was short; as a result, the change in elderly health status over the short observation time was small. In future research we will increase the sample size and extend follow-up to increase power. In addition, we relied on self-reported estimates of sleep duration. This is a convenient way to estimate sleep duration, and was therefore appropriate for the large sample in our study. Self-reporting is not as accurate as actigraph-measured sleep duration. Previous studies have shown that self-reported sleep durations are moderately correlated with actigraph-measured sleep duration.33 In addition, we have published two articles16,31 using the same method to assess sleep duration before. Therefore, self-reporting could be an effective way to assess sleep behaviour.
In conclusion, we have shown that both short and long sleep durations were associated with a greater incidence of sarcopenia. Although further research is needed to confirm these findings, sleep duration should be considered in developing prevention and management strategies for sarcopenia.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (grant numbers 82172552), Shanghai Sailing Program (20YF1418200), Shanghai Municipal Health Commission (20214Y0329) and Scientific Research Foundation of SUMHS (SSF-21-03-007). The funders had an important role in study design, data collection and analysis, and decision to publish. Peipei Han and Lin Hou are co-first authors for this study.
Disclosure
Peipei Han, Lin Hou, Zhenwen Liang, Wuxiong Chen, Junxue Li, Yazhou Cheng, Wenjing Zhou, Siya Zeng, Jiangtao Pan, Lanshan Xu, Yi Wang, Yangyi Chen, and Qi Guo declare that they have no conflicts of interest in this work.
References
- 1.Delmonico MJ, Harris TB, Visser M, et al. Longitudinal study of muscle strength, quality, and adipose tissue infiltration. Am J Clin Nutr. 2009;90:1579–1585. doi: 10.3945/ajcn.2009.28047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fielding RA, Vellas B, Evans WJ, et al. Sarcopenia: an undiagnosed condition in older adults. Current consensus definition: prevalence, etiology, and consequences. International working group on sarcopenia. J Am Med Dir Assoc. 2011;12:249–256. doi: 10.1016/j.jamda.2011.01.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Curcio F, Ferro G, Basile C, et al. Biomarkers in sarcopenia: a multifactorial approach. Exp Gerontol. 2016;85:1–8. doi: 10.1016/j.exger.2016.09.007 [DOI] [PubMed] [Google Scholar]
- 4.Rasmussen BB, Fujita S, Wolfe RR, et al. Insulin resistance of muscle protein metabolism in aging. FASEB J. 2006;20:768–769. doi: 10.1096/fj.05-4607fje [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cesari M, Kritchevsky SB, Nicklas B, et al. Oxidative damage, platelet activation, and inflammation to predict mobility disability and mortality in older persons: results from the health aging and body composition study. J Gerontol a Biol Sci Med Sci. 2012;67:671–676. doi: 10.1093/gerona/glr246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dowd JB, Goldman N, Weinstein M. Sleep duration, sleep quality, and biomarkers of inflammation in a Taiwanese population. Ann Epidemiol. 2011;21:799–806. doi: 10.1016/j.annepidem.2011.07.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pyykkonen AJ, Isomaa B, Pesonen AK, et al. Sleep duration and insulin resistance in individuals without type 2 diabetes: the PPP-Botnia study. Ann Med. 2014;46:324–329. doi: 10.3109/07853890.2014.902226 [DOI] [PubMed] [Google Scholar]
- 8.Fex A, Barbat-Artigas S, Dupontgand S, Filion ME, Karelis AD, Aubertin-Leheudre M. Relationship between long sleep duration and functional capacities in postmenopausal women. J Clin Sleep Med. 2012;8:309–313. doi: 10.5664/jcsm.1922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim RH, Kim KI, Kim JH, Park YS. Association between sleep duration and body composition measures in Korean adults: the Korea National Health and Nutrition Examination Survey 2010. Korean J Fam Med. 2018;39:219–224. doi: 10.4082/kjfm.17.0030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kwon YJ, Jang SY, Park EC, Cho AR, Shim JY, Linton JA. Long sleep duration is associated with sarcopenia in Korean adults based on data from the 2008-2011 KNHANES. J Clin Sleep Med. 2017;13:1097–1104. doi: 10.5664/jcsm.6732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pourmotabbed A, Ghaedi E, Babaei A, et al. Sleep duration and sarcopenia risk: a systematic review and dose-response meta-analysis. Sleep Breath. 2020;24:1267–1278. doi: 10.1007/s11325-019-01965-6 [DOI] [PubMed] [Google Scholar]
- 12.Nakakubo S, Doi T, Tsutsumimoto K, Kurita S, Ishii H, Shimada H. Sleep duration and progression to sarcopenia in Japanese community-dwelling older adults: a 4 year longitudinal study. J Cachexia Sarcopeni. 2021;12:1034–1041. doi: 10.1002/jcsm.12735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.NBoSo C. Statistical Communiqué on the 2012 National Economy and Social Development of People’s Republic of China. China Stat Press; 2013. [Google Scholar]
- 14.Zhang W, Shen SX, Wang W, et al. Poor lower extremity function was associated with pre-diabetes and diabetes in older Chinese people. PLoS One. 2014;9:e115883. doi: 10.1371/journal.pone.0115883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Han PP, Kang L, Guo Q, et al. Prevalence and factors associated with sarcopenia in suburb-dwelling older Chinese Using the Asian working group for sarcopenia definition. J Gerontol a-Biol. 2016;71:529–535. doi: 10.1093/gerona/glv108 [DOI] [PubMed] [Google Scholar]
- 16.Fu LY, Jia LY, Zhang W, et al. The association between sleep duration and physical performance in Chinese community dwelling elderly. PLoS One. 2017;12:e0174832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jiang CQ, Xu L, Lam TH, et al. Effect of physical activity strength on the diabetes mellitus prevalence in the elderly under the influence of international physical activity questionnaire. Zhonghua Liu Xing Bing Xue Za Zhi. 2009;30:462–465. [PubMed] [Google Scholar]
- 18.Mui AC. Geriatric depression scale as a community screening instrument for elderly Chinese immigrants. Int Psychogeriatr. 1996;8:445–458. doi: 10.1017/S1041610296002803 [DOI] [PubMed] [Google Scholar]
- 19.Chen LK, Woo J, Assantachai P, et al. Asian Working Group For Sarcopenia: 2019 consensus update on sarcopenia diagnosis and treatment. J Am Med Dir Assoc. 2020;21(300–307):e302. doi: 10.1016/j.jamda.2019.12.012 [DOI] [PubMed] [Google Scholar]
- 20.Stenholm S, Kronholm E, Bandinelli S, Guralnik JM, Ferrucci L. Self-reported sleep duration and time in bed as predictors of physical function decline: results from the InCHIANTI study. Sleep. 2011;34:1583–1593. doi: 10.5665/sleep.1402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brasure M, MacDonald R, Fuchs E, et al. Management of Insomnia Disorder. AHRQ Comparative Effectiveness Reviews. Rockville (MD): Agency for Healthcare Research and Quality; 2015. [PubMed] [Google Scholar]
- 22.Han P, Zhao J, Guo Q, et al. Incidence, risk factors, and the protective effect of high body mass index against sarcopenia in suburb-dwelling elderly Chinese Populations. J Nutr Health Aging. 2016;20:1056–1060. doi: 10.1007/s12603-016-0704-3 [DOI] [PubMed] [Google Scholar]
- 23.Kim H, Suzuki T, Kim M, et al. Incidence and predictors of sarcopenia onset in community-dwelling elderly Japanese women: 4-year follow-up study. J Am Med Dir Assoc. 2015;16(85):e81–88. doi: 10.1016/j.jamda.2014.10.006 [DOI] [PubMed] [Google Scholar]
- 24.Landi F, Liperoti R, Fusco D, et al. Prevalence and risk factors of sarcopenia among nursing home older residents. J Gerontol a-Biol. 2012;67:48–55. doi: 10.1093/gerona/glr035 [DOI] [PubMed] [Google Scholar]
- 25.Patel HP, Syddall HE, Jameson K, et al. Prevalence of sarcopenia in community-dwelling older people in the UK using the European Working Group on Sarcopenia in Older People (EWGSOP) definition: findings from the Hertfordshire Cohort Study (HCS). Age Ageing. 2013;42:378–384. doi: 10.1093/ageing/afs197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gariballa S, Alessa A. Sarcopenia: prevalence and prognostic significance in hospitalized patients. Clin Nutr. 2013;32:772–776. doi: 10.1016/j.clnu.2013.01.010 [DOI] [PubMed] [Google Scholar]
- 27.Hu XY, Jiang JJ, Wang HZ, Zhang L, Dong BR, Yang M. Association between sleep duration and sarcopenia among community-dwelling older adults A cross-sectional study. Medicine. 2017;96:e6268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Seelig E, Keller U, Klarhofer M, et al. Neuroendocrine regulation and metabolism of glucose and lipids in primary chronic insomnia: a prospective case-control study. PLoS One. 2013;8:e61780. doi: 10.1371/journal.pone.0061780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Al Lawati NM, Patel SR, Ayas NT. Epidemiology, risk factors, and consequences of obstructive sleep apnea and short sleep duration. Prog Cardiovasc Dis. 2009;51:285–293. doi: 10.1016/j.pcad.2008.08.001 [DOI] [PubMed] [Google Scholar]
- 30.Rom O, Kaisari S, Aizenbud D, Reznick AZ. Lifestyle and sarcopenia-etiology, prevention, and treatment. Rambam Maimonides Med J. 2012;3:e0024. doi: 10.5041/RMMJ.10091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fu LY, Yu X, Zhang W, et al. The relationship between sleep duration, falls, and muscle mass: a cohort study in an elderly Chinese population. Rejuv Res. 2019;22:390–398. doi: 10.1089/rej.2018.2102 [DOI] [PubMed] [Google Scholar]
- 32.Piovezan RD, Abucham J, Dos Santos RV, Mello MT, Tufik S, Poyares D. The impact of sleep on age-related sarcopenia: possible connections and clinical implications. Ageing Res Rev. 2015;23:210–220. doi: 10.1016/j.arr.2015.07.003 [DOI] [PubMed] [Google Scholar]
- 33.Lauderdale DS, Knutson KL, Yan LL, Liu K, Rathouz PJ. Self-reported and measured sleep duration: how similar are they? Epidemiology. 2008;19:838–845. doi: 10.1097/EDE.0b013e318187a7b0 [DOI] [PMC free article] [PubMed] [Google Scholar]