Abstract
Recent investigations of oil reservoirs in a variety of locales have indicated that these habitats may harbor active thermophilic prokaryotic assemblages. In this study, we used both molecular and culture-based methods to characterize prokaryotic consortia associated with high-temperature, sulfur-rich oil reservoirs in California. Enrichment cultures designed for anaerobic thermophiles, both autotrophic and heterotrophic, were successful at temperatures ranging from 60 to 90°C. Heterotrophic enrichments from all sites yielded sheathed rods (Thermotogales), pleomorphic rods resembling Thermoanaerobacter, and Thermococcus-like isolates. The predominant autotrophic microorganisms recovered from inorganic enrichments using H2, acetate, and CO2 as energy and carbon sources were methanogens, including isolates closely related to Methanobacterium, Methanococcus, and Methanoculleus species. Two 16S rRNA gene (rDNA) libraries were generated from total community DNA collected from production wellheads, using either archaeal or universal oligonucleotide primer sets. Sequence analysis of the universal library indicated that a large percentage of clones were highly similar to known bacterial and archaeal isolates recovered from similar habitats. Represented genera in rDNA clone libraries included Thermoanaerobacter, Thermococcus, Desulfothiovibrio, Aminobacterium, Acidaminococcus, Pseudomonas, Halomonas, Acinetobacter, Sphingomonas, Methylobacterium, and Desulfomicrobium. The archaeal library was dominated by methanogen-like rDNAs, with a lower percentage of clones belonging to the Thermococcales. Our results strongly support the hypothesis that sulfur-utilizing and methane-producing thermophilic microorganisms have a widespread distribution in oil reservoirs and the potential to actively participate in the biogeochemical transformation of carbon, hydrogen, and sulfur in situ.
Over the past decade, microbiological investigations of high temperature, petroleum-rich strata from a number of geographically distant sites have revealed physiologically diverse assemblages of thermophilic and hyperthermophilic anaerobic microorganisms. Physiological types isolated from these biotopes include sulfate reducers (54, 58), sulfidogens (31, 54), fermentative bacteria (12, 22), manganese and iron reducers (23), methanogens (42, 52), and acetogens (13). Despite the description of an increasing number of new thermophilic species, relatively little information is available on the composition of microbial assemblages in these unique subsurface environments. This is primarily due to the reliance on cultured-based methods for the recovery and identification of individual oil field isolates and the focus on specific physiological groups of microorganisms, such as sulfate-reducing and fermentative microorganisms, rather than the entire subsurface microbial community.
Culture-based approaches, while extremely useful for understanding the physiological potential of isolated organisms, do not necessarily provide comprehensive information on the composition of microbial communities (1, 46). Due to this documented disparity between cultivatable and in situ diversity, it is often difficult to assess the significance of cultured members in resident microbial communities. Recent studies, for example, have employed culture-independent molecular methods to show that cultivated microorganisms from both temperate and extreme environments often may represent very minor components of the microbial community (56, 61) as a whole.
In petroleum microbiology, application of molecular methods has been recently incorporated in current analyses of oil-associated microorganisms. Methods such as reverse genome probing (60) and immunological approaches (9, 43) have allowed quantification of specific target organisms within complex communities. These techniques, however, assay only readily cultivated microorganisms. Molecular techniques have proven effective for characterizing complex microbial assemblages in environmental samples. In particular, the analysis of 16S ribosomal DNA (rDNA) recovered from environmental samples has revealed previously unrecognized microbial diversity in a variety of habitats, including pelagic and coastal ocean systems (14, 20), soils and sediments (57), hot springs (25), and terrestrial subsurface environments (7, 47). 16S rDNA sequence analysis has recently been used to characterize microbial assemblages from a low-temperature (25°C), waterflooded petroleum reservoir (59); however, no comparable studies have been reported in high-temperature oil-bearing formations to date.
The discovery of a number of novel archaeal and bacterial rDNA phylotypes during molecular diversity surveys of other high temperature habitats (25, 61) strongly suggests the existence of as-yet-undetected microbial assemblages in geothermally heated, deep-seated petroleum reservoirs. Using both 16S rDNA phylogenetic analysis and enrichment culture techniques, we characterized the microbial diversity and culture characteristics of thermophilic assemblages in the Miocene Monterey formation, a prominent high-temperature, oil-bearing formation in California. We compared 16S rDNA recovered to rDNA from thermophilic enrichments and isolates from the same formation. Results from this study revealed diverse assemblages of thermophilic and mesophilic microorganisms, many of which are closely related to thermophilic strains cultured from both the Monterey and other high-temperature oil-bearing formations.
MATERIALS AND METHODS
Site description and reservoir conditions.
All inoculum sources and total microbial nucleic acid samples originated from production wellhead samples from two offshore oil fields, the South Elwood field (34°23.4′N, 119°54.3′W) and the Santa Clara field (34°10.9′N, 119°25.2′W), and two fields onshore within the San Joaquin Basin, the North Coles Levee field (35°27′N, 119°33′E) and the Yowlumne field (35°04′N, 119°19′E). The South Elwood field produces oil from two geochemically distinct marine formations, the middle Miocene Monterey and the lower Miocene Rincon formation, with average production depths between 1,500 and 1,700 m. The bottom hole temperature of production wells at this site is between 70 and 75°C, and the secondary recovery of oil is by natural gas lift. The Santa Clara field, approximately 50 km southwest of the South Elwood field, consists of four major production horizons: the Miocene Monterey, the Pliocene Upper and Lower Repetto, and the Pliocene-Pleistocene Pico formation. The Monterey, Lower Repetto, and Pico formations have not been waterflooded, whereas the Upper Repetto (well no. S-24) has been injected with seawater since 1984. Production depths at this site range from 1,500 to 2,400 m and bottom hole temperatures are between 50 and 81°C. Within the San Joaquin Basin, the North Coles Levee and Yowlumne fields produce Monterey-sourced oil from the Stevens sand. North Coles Levee, located in the central basin area, produces oil at depths between 2,600 and 2,900 m, with an average temperature of 105°C. The Yowlumne field, found 28 km to the southeast of the North Coles Levee field, has production depths between 3,500 and 4,100 m at temperatures nearing 125°C (18).
Sample collection.
Production fluids used for microbial assemblage analysis were collected directly from production wellheads into prerinsed 20-liter carboys (Nalgene; Nalge Nunc International, Rochester, N.Y.). Samples from the carboys were immediately prepared onsite for chemical analyses, direct cell counts, and culture studies. The remaining fluid was transported at ambient temperature back to the laboratory for separation and concentration of microbial biomass, which began within a few hours of collection. A composite sample of Monterey formation water taken from a representative number of production wellheads from the South Elwood field in January 1996 was used in the construction of 16S rDNA libraries O1 and O2. The criteria for selecting production wells were based on the samples' water content (>50%) and lack of chemical treatment. Production well samples used for enrichment cultures, direct counts, and chemical analysis were also taken from the South Elwood field on October, May, and June 1997 and June 1998. Sample collection from the Santa Clara field occurred on March 1997 and samples from San Joaquin Basin's Yowlumne and North Coles Levee fields were taken on February and March 1998.
Chemical analysis.
Total hydrogen sulfide (H2S, HS−, and S−2) of formation water samples was measured in 0.5-ml aliquots with a model 5890 gas chromatograph (Hewlett Packard, Santa Clarita, Calif.) (8). Sulfate concentrations were determined using single-column ion chromatography (21) with a Shimadzu VP-series high-performance liquid chromatography system and a CDD-6A conductivity detector (Kyoto, Japan). Standard solutions and formation water samples were centrifuged at 10,000 × g for 10 min, and the supernatant was diluted 1:19 with high-performance liquid chromatography-grade water. Twenty-microliter samples and standards were injected through a guard column and onto a Wescan anion column with 4 mM potassium hydrogen phthalic acid as the eluant (5% methanol [pH 4.5]; filtered [0.45-μm pore size] and pumped at a rate of 2.5 ml min−1). Formation water pH measurements were made with a double-junction combination electrode (Broadley-James, Irvine, Calif.) connected to a model PHM93 pH meter (Radiometer, Lyon, France).
Cell counts.
Formation water used for determining cell abundance was processed on site, either fixed directly with formaldehyde (3.7% final concentration) or first separated from the oil phase by heating 60°C for 20 min, followed by a centrifugation step (10 min at 6,000 × g). Fixed samples were stored at 4°C overnight and then prefiltered through a GF/D glass fiber filter (Whatman, Maidstone, United Kingdom) to remove any remaining oil particles. Cells were stained with DAPI (4′,6′-diamidino-2-phenylindole) and counted in an Axioskop 20 epifluorescence microscope (Carl Zeiss, Inc., Thornwood, N.Y.) (48).
Nucleic acid extraction.
Phase separation of the oil-water emulsion was accomplished by heating to 70°C for 10 to 20 min in 2-liter Teflon separatory funnels (Fisher, Houston, Tex.). Microbial biomass from approximately 3.5 liters of the water phase was collected by filtration onto 0.22-μm Sterivex filters (Millipore, Bedford, Mass.) with an in-line 46-mm GF/D filter (Whatman), with a peristaltic pump. Sterivex filters were filled with 1.8 ml of lysis buffer (40 mM EDTA, 750 mM sucrose, and 50 mM Tris-HCl) and stored at −80°C. Natural microbial assemblages were lysed on the filters with proteinase K, lysozyme, and sodium dodecyl sulfate treatment, and nucleic acids were extracted by a standard phenol-chloroform method (39). Nucleic acids were purified on a small-scale CsCl density gradient as previously described (14) and used to construct universal (O1) and archaeal (O2) 16S rDNA libraries.
Construction and screening of 16S rDNA libraries.
Small-subunit (SSU) rRNA genes were amplified by PCR with pooled DNA samples from composite Monterey production fluids. Fifty-microliter reaction mixtures contained 0.2 μM either universal (519fta and 1390rta; see below) or archaea-specific (20f and 958r) primers. Reactions also contained 5 μl of PCR buffer (containing 2 mM MgCl2), 2.5 μM deoxynucleoside triphosphates, 0.025 U of Taq polymerase (Fisher), and acetamide (5% final concentration) in reaction mixtures with universal primers.
(i) PCR conditions for archaeal library (O2).
Archaeal 16S rRNA genes from the CsCl-purified nucleic acids were amplified for 30 cycles (1.5 min of denaturation at 94°C, 0.5 min of annealing at 55°C, and 7 min of elongation at 72°C) using archaea-specific primers (20f, 5′ TTC CGG TTG ATC CYG CCR G 3′; 958r, YCC GGC GTT GAM TCC AAT T).
(ii) PCR conditions for universal library (O1).
Modified universal primers (519fta, 5′ GTT TCA GCM GCC GCG GTA ATW C 3′; 1390rta, GTT TGA CGG GCG GTG TGT RCA A) designed to increase ligation efficiency (6) were used in 16S rDNA library construction. Five 50-μl reactions were amended with acetamide and amplified for 20 cycles of annealing at 55°C. In order to minimize PCR bias found to be associated with high-cycle numbers (55), a series of 15-, 20-, 25-, and 30-cycle reactions were run beforehand and used to determine the lowest cycle number possible for construction of the O1 library.
Cloning.
Amplicons were pooled from three reactions for both the O1 and O2 16S rDNA libraries and cloned with a TA cloning vector kit according to the manufacturer's instructions (Invitrogen, Carlsbad, Calif.). A total of 288 and 480 white colonies were selected and screened for the archaeal and universal libraries, respectively. Screening for the libraries was conducted with two separate restriction fragment length polymorphism (RFLP) analyses on M13F- and M13R-amplified products with restriction enzymes HaeIII and RsaI (Promega, Madison, Wis.) according to Massana et al. (36). Unique clones were identified and plasmids were purified either with the Wizard genomic DNA purification kit (Promega) or by electroelution with an automated miniprep protocol (McConnell, La Jolla, Calif.). Cleaned plasmid preparations were quantified and sequenced using the Thermo Sequenase Fluorescent Labeled Primer Cycle Sequencing kit (Amersham, Braunschweig, Germany) and an automated 4000L or 4200 DNA sequencer (LI-COR BioTech, Lincoln, Nebr.). Double-stranded sequencing was completed using a suite of primers targeting 16S rDNA (30).
Phylogenetic analysis.
Sequences from clones and cultured isolates were submitted to GenBank for preliminary analysis using the BLAST program of the Ribosomal Database Project to identify putative close phylogenetic relatives (35). Sequences were aligned to their nearest neighbor with the automated alignment tool of the ARB program package (O. Strunk and W. Ludwig (ed.), submitted for publication). Phylogenetic trees were generated using the SEQBOOT, DNADIST, and NEIGHBOR programs of the PHYLIP version 3.5 software (17). The Kimura two-parameter model (28) was used to estimate evolutionary distance, and 1,000 bootstraps were performed to assign confidence levels to the nodes in the trees.
Medium preparation.
Five basal media amended with various carbon and energy substrates were used in this study. Media included marine-based SSW neutrophile medium (NU) (5), 2216/ASW marine broth (MB) (5), BS medium (BS) (29), Methanosarcina acetovorans medium (MA) (2), and estaurine methanogen medium (MG) (2). Media were prepared using a modified Hungate technique with no initial boiling step (26, 33, 37). Instead, media were flushed with N2 or N2:CO2 (80:20) in 1-liter rubber stopper-sealed glass bottles (Wheaton, Millville, N.J.) for 30 to 40 min, reduced with Na2S, and dispensed into Hungate tubes or serum vials (Bellco Glass, Inc., Vineland, N.J.) in an anaerobic chamber (Anaerobe Systems, San Jose, Calif.). Vials were autoclaved at 121°C for 20 min and stored at room temperature in the dark until use. Medium designed to enrich for H2-utilizing microorganisms (e.g., methanogens) included a 1:4 liquid-to-headspace ratio pressurized to 10 lb/in2. Supplements to media included acetate (0.05%), yeast extract (0.1%), trypticase (0.1%), glucose (0.2%), trimethylamine (0.3%), thiosulfate (0.3%), elemental sulfur (0.5%), and sulfate (0.3 to 0.5%).
Enrichment cultures.
Aliquots of raw production fluids (oil and formation water) were inoculated directly into anaerobic enrichment medium at the platform immediately after collection or stored in 250-ml Wheaton vials containing 0.5 g of dithionite and sealed with butyl rubber stoppers. Production fluid samples were stored at 4°C until use. Functionally distinct groups of thermophilic microorganisms, including sulfur-utilizing heterotrophs, fermentative bacteria, sulfate-reducing bacteria, and methanogens, were cultivated by adding production fluids (2 to 5%) to prereduced media. Incubations between 60 and 70°C were conducted in air incubators, while higher-temperature enrichments (75 to 100°C) were incubated in oil baths. Growth was confirmed by phase-contrast microscopy (Carl Zeiss, Inc.) or, in the case of methanogens, autofluorescent cells were detected by epifluorescence microscopy with a UV excitation filter set.
Identification of enrichment culture microorganisms.
Selected enrichments were subjected to repeated serial dilutions into liquid medium for isolation. Biomass from isolated strains (20 to 100 ml) was collected by centrifugation (10,000 × g at 4°C for 10 to 15 min) and extracted with a proteinase K, lysozyme, sodium dodecyl sulfate, phenol-chloroform procedure (16) or, in some cases (i.e., for Methanobacterium spp.), a modified RNA bead-beating procedure in the presence of phenol (pH 8.0) (24, 53). 16S rDNAs were then amplified by PCR using archaea-specific (20f-958r or 1390r) or bacteria-specific (27f or 8f and 1390r or 1492r) primers. PCR-amplified products were purified with a QIAquick PCR purification kit (Qiagen, Inc., Valencia, Calif.) according to the manufacturer's specifications and sequenced as described previously. Finally, DNAs from selected mixed enrichments were extracted and used to construct three additional clone libraries (M.E1, R.E2, and M.E3). 16S rDNA libraries R.E2 and M.E3 were prepared using the archaea-specific primer set (20f and 958r), and M.E1 was constructed using the universally conserved primer set (519f and 1390r). The amplification of PCR products, cloning, and screening for all three 16S rDNA enrichment libraries were conducted as described earlier for the O2 library.
Nucleotide sequence accession numbers.
The rRNA gene sequences were submitted to GenBank and have been assigned the following accession numbers: AF220303 to AF220350.
RESULTS
Petroleum reservoir characteristics.
Bottom hole temperatures for the sampled petroleum formations ranged from 50 to 125°C. The total dissolved solids of formation water collected from production wellheads varied from approximately 10,000 to 44,000 ppm and were neutral or slightly alkaline in pH. Physicochemical characteristics and microbial counts for the geological formations sampled are listed in Table 1.
TABLE 1.
Geochemical and biological characteristics of formation waters from three high-temperature California oil fieldsa
| Characteristic | Field, geological age, and formation
|
||||||
|---|---|---|---|---|---|---|---|
| South Elwood
|
Santa Clara
|
North Coles Levee
|
Yowlumne
|
||||
| Upper Miocene
|
Lower Miocene
|
Upper Miocene
|
Lower Pliocene
|
Lower Pliocene
|
Middle Miocene
|
Middle Miocene
|
|
| Monterey | Rincon | Monterey | Upper Repetto | Lower Repetto | Stevens-Monterey | Stevens-Monterey | |
| Physicochemical conditions | |||||||
| Depth (m) | 1,500–1,700 | 1,524 | 2,316 | 1,759 | 2,392 | 2,700 | 3,940 |
| Temperature (°C) | 70–75 | 70–75 | 78 | 65 | 81 | 105 | 125 |
| TDS (ppm) | 44,340 | 21,803 | 24,500 | 23,000 | 24,500 | 21,986–30,400 | 9,966–15,974 |
| pH | 7.84 | 7.74 | 8.11 | 7.33 | 7.86 | 7.18 | ND |
| H2S (mM) | 11.2 | 0.94 | 2.4 | 0 | 0 | 0 | ND |
| SO4 (mM) | 0.47–0.9 | 0.71 | 0.29 | 0.6 | BD | 0.45 | 0.75 |
| wt% sulfur oilb | ∼4% | 0.2% | 4.5–5% | ND | ND | ND | ND |
| Direct counts (103 cells/ml) | 54.5 | 1,420 | 2.55 | 18.5 | 529 | 372 | 145 |
| Representative genera enrichment cultures | Thermococcus | Thermococcus | Thermococcus | Thermococcus | Petrotoga | Thermococcusc | Thermococcusc |
| Petrotoga | Thermotogales | Methanococcus | Thermotogac | Anaerobaculum | Thermotogac | Thermotogac | |
| Thermoanaerobacter | Anaerobaculum | Methanobacterium | Methanococcus | Methanobacterium | Methanobacteriumc | Methanobacteriumc | |
| Deferribacter | Methanobacterium | Methanobacterium | |||||
| Thermotogac | Methanoculleus | ||||||
| Methanococcus | |||||||
| Methanoculleus | |||||||
TDS, total dissolved solids; BD, below detection; ND, not determined.
Reference 6a.
Putative identification based on phase microscopy and physiological characteristics.
The direct cell count of the Monterey formation composite wellhead sample used in the construction of the O1 and O2 16S rDNA libraries was 4.13 × 104 cells/ml. Cell numbers determined for individual production wells were highly variable, depending upon the sampling date and origin of the samples. Prokaryotic abundance for the three fields surveyed ranged from 2.5 × 103 to 1.42 × 106 cells ml−1 and are similar to counts reported for other petroleum reservoirs and deep subsurface habitats (19, 40; F. P. Bernard, J. Connan, E. Aquitaine, M. Magot, and S. Elf-Biorecherches, 67th Annu. Tech. Conf. Exhibit. Soc. Petrol. Eng., SPE 24811, 1992).
O1 and O2 16S rDNA clone libraries.
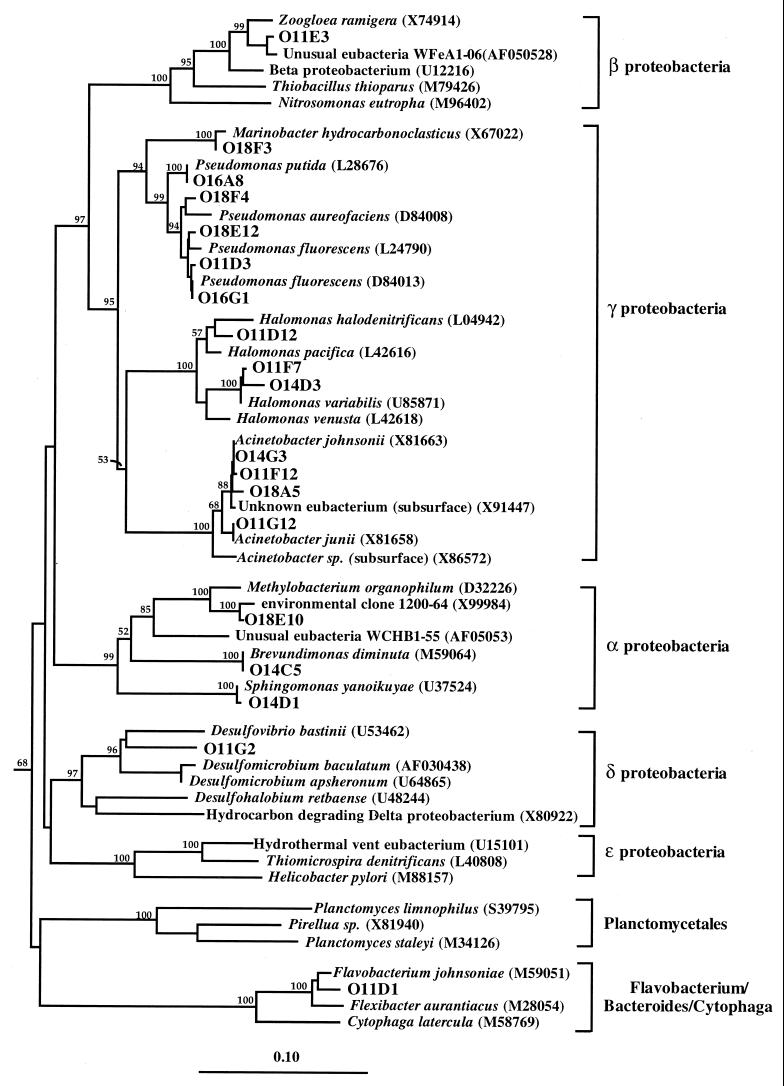
Two 16S rDNA libraries (O1 and O2) were generated from total community DNA collected from production wellheads (mean depth, 1,700 m below sea floor) using universally conserved and archaeal 16S rRNA-targeted PCR primers. RFLP analyses indicated that universal library O1 contained approximately 83 unique clones, while the archaeal O2 library displayed lower diversity with only 9 unique phylotypes. Sequence analysis of 56 unique clones from 159 O1 library clones containing the correctly sized insert revealed that the majority of 16S rDNA clones grouped within the bacterial domain, with 8.8% of the library affiliated with the domain Archaea. Dominant groups with more than one sequence in the universal library were representatives of the low-G+C gram-positive bacteria, gamma proteobacteria, euryarchaeota, alpha proteobacteria, and delta proteobacteria. Represented in these main phylogenetic subdivisions were phylotypes most similar to the bacterial genera Halomonas (9.4%), Pseudomonas (8.2%), Acinetobacter (7.5%), Marinobacter (1.3%), Desulfomicrobium (1.3%), Sphingomonas (0.6%), Brevundimonas (0.6%), Methylobacterium (0.6%), Duganella (0.6%), Acidaminococcus (22%), Thermoanaerobacter (14%), Desulfothiovibrio (1.9%), Aminobacterium (0.6%) and Flexibacter (0.6%) (Table 2). O1 clones related to the Proteobacteria, Flexibacter, Bacteroides, and Cytophaga phyla had high similarities (≥97%) to 16S rDNA phylotypes recovered from similar hydrocarbon-rich environments. These clones were also highly similar to 16S rDNA sequences from microorganisms isolated from petroleum systems, other subsurface habitats, and marine hydrocarbon-contaminated sites (Table 2; Fig. 1) (4, 40).
TABLE 2.
Closest relatives of bacterial phylotypes from O1 16S rRNA gene library
| Division (% representation) | Type sequence | No. of RFLP typesa | No. of total clonesb | Closest cultivated species | Source | % Similarityc |
|---|---|---|---|---|---|---|
| Proteobacteria | ||||||
| γ (35%) | O1 1E2 | 1 | 1 | Marinobacter hydrocarbonoclasticus | Seawater | 99.4 |
| O1 6G1 | 3 | 11 | Pseudomonas fluorescens | Soil | 99.3–99.6 | |
| O1 6A8 | 1 | 2 | Pseudomonas putida | Subsurface | 99.6 | |
| O1 8F4 | 1 | 1 | Pseudomonas aureofaciens | Soil | 99.1 | |
| O1 4B5 | 5 | 20 | Halomonas variabilis | Salt lake | 98.5–99.6 | |
| O1 1D12 | 1 | 1 | Halomonas pacifica | Seawater | 97.2 | |
| O1 8C4 | 3 | 7 | Acinetobacter johnsonii | Sewage | 99.5–99.9 | |
| O1 1G12 | 1 | 2 | Acinetobacter junii | Urine | 100 | |
| O1 8E12 | 1 | 4 | Acinetobacter sp. | Subsurface | 98.9–99.3 | |
| α (3%) | O1 4D1 | 1 | 1 | Sphingomonas yanoikuyae | Soil | 98.9 |
| O1 4C5 | 1 | 2 | Brevundimonas diminuta | Soil | 99.9 | |
| O1 8E10 | 1 | 1 | Methylobacterium radiotolerans | Lake water | 100 | |
| β (0.6%) | O1 1E3 | 1 | 1 | Not cultivated | ||
| δ (1.2%) | O1 1H2 | 1 | 2 | Desulfomicrobium apsheronum | Oil | 91.6 |
| Flavobacterium, Bacteroides, and Cytophaga (0.6%) | O1 1D1 | 1 | 1 | Flexibacter aurantiacus | Soil | 97.6 |
| Low G+C, gram positive (42%) | O1 8G7 | 3 | 40 | Acidaminococcus fermentans | Rumen | 94.7 |
| O1 1B1 | 13 | 18 | Thermoanaerobacter brockii | Oil | 95.8 | |
| O1 1D9 | 1 | 1 | Aminobacterium colombiense | Sewage | 87.1 | |
| O1 8G5 | 1 | 2 | Desulfothiovibrio peptidovorans | Oil | 93.7 |
Number of unique RFLP patterns most closely related to the same cultivated species.
Number of total related clones in SSU O1 library.
16S rDNA E. coli numbering 519 to 1,390.
FIG. 1.
Phylogenetic tree of the proteobacteria and relatives from Monterey-sourced production fluids. 16S rDNA clones from this study are prefaced with O1. A neighbor-joining tree was generated from a mask of 607 nucleotide positions (Escherichia coli numbering 519 to 1,390) with Clostridium formicaceticum (X77836), Clostridium aminobutyricum (X76161), and Desulfotomaculum thermoandovorans (Z26315) serving as outgroups. Bootstrap values (n = 1,000 replicates) of ≥50 are reported as percentages. The scale bar represents the number of changes per nucleotide position.
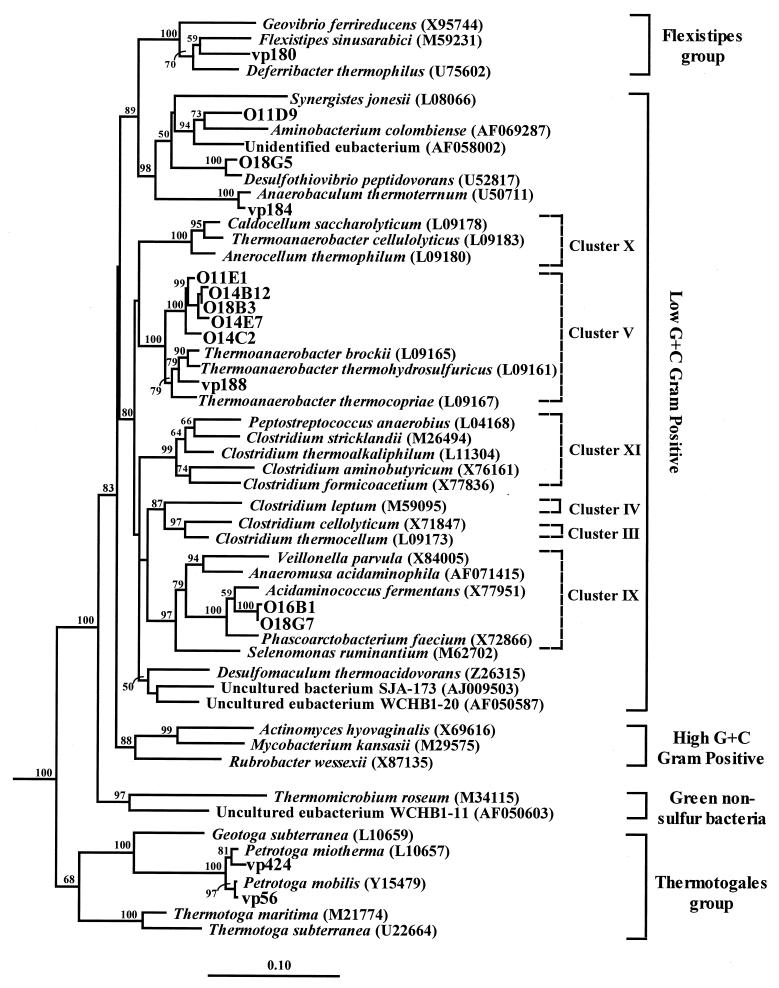
Low-G+C gram-positive and delta proteobacterial rDNA phylotypes were 87 to 96% similar to previously determined rDNA sequences. Although not exhibiting a high degree of similarity to known cultivated species, gram-positive clones were found to cluster within clades containing known hydrogen sulfide-producing fermentative anaerobes (e.g., Acidaminococcus fermentans) (11) and thermophilic thiosulfate-respiring organotrophs (Thermoanaerobacter and Desulfothiovibrio), described previously from high-temperature oil fields (Table 1; Fig. 2). Similarly, the single RFLP type clustering within the Desulfomicrobium group of the delta proteobacterial subdivision was also related (91.7%) to microorganisms recovered from petroleum systems (51).
FIG. 2.
Phylogenetic tree of the low-G+C gram-positive division and related bacterial 16S rDNA clones (O1) and isolates (vp) from Monterey, Rincon, and Repetto-sourced production fluids. A neighbor-joining tree was generated from a mask of 609 nucleotide positions (E. coli numbering 519 to 1,390) with T. litoralis (Z70252) and M. thermoautotrophicum (X05482) serving as outgroups. Bootstrap values (n = 1,000 replicates) of ≥50 are reported as percentages. The scale bar represents the number of changes per nucleotide position.
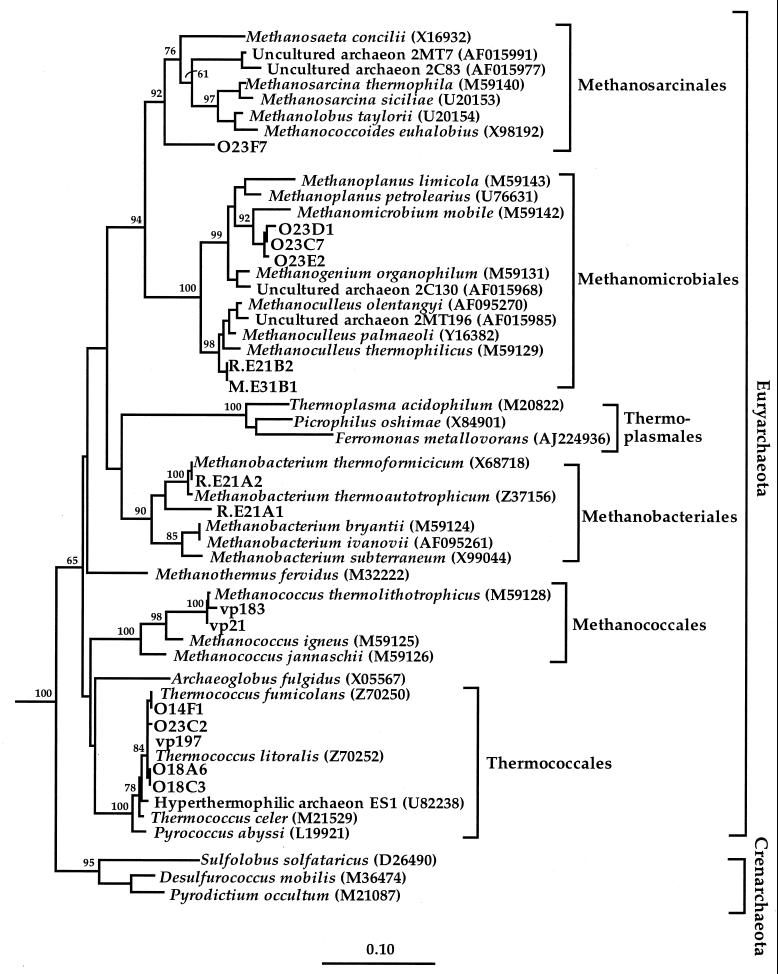
Archaeal phylotypes from the universal (O1) library showed less sequence diversity than the bacterial clones and were represented by five unique RFLP types, all with sequence similarities over 99% to members of the order Thermococcales (Fig. 3). Of the 159 O1 clones screened, two chimeric sequences were detected and excluded from further analyses.
FIG. 3.
Phylogenetic tree of the archaeal domain and archaeal 16S rDNA phylotypes, designated by O1 and O2, from Monterey-sourced production fluids. A neighbor-joining tree was generated from a mask of 331 nucleotide positions (E. coli numbering 20 to 958) with Hydrogenobacter thermophilus (Z30214) and Thermotoga maritima (M21774) serving as outgroups. Bootstrap values (n = 1,000 replicates) of ≥50 are reported as percentages. The scale bar represents the number of changes per nucleotide position.
In contrast to the archaeal component of the universal O1 library, the archaeal O2 library was dominated by methanogen-like rDNAs, with only 1.3% of the clones related to the Thermococcales. In addition to significant differences in the general representation of archaeal diversity in libraries O1 and O2, another marked distinction was the lack of evenness in the O2 library compared to O1. Of the nine unique phylotypes recorded, 89% of the O2 library was represented by a single RFLP type, found to be highly similar (96%) to the oil field methanogen Methanoplanus petrolearius (Table 3) (45). The remaining RFLP type, representing 6% of the archaeal 16S rDNA clones, formed a deep branch within the acetoclastic Methanosarcinales and was only distantly related to known cultured relatives in the database (<88% similarity to Methanosarcina thermophila) (Table 3; Fig. 3).
TABLE 3.
Closest relatives of archaeal phylotypes from O2 and O1 16S rDNA libraries
| Division (% representation) | Type sequence | No. of RFLP types | No. of clones | Closest cultivated species | Source | % Sequence similarity |
|---|---|---|---|---|---|---|
| Methanomicrobiales (96%) | O2 3C7a | 4 | 145 | Methanoplanus petrolearius | Oil | 95.7–96.1 |
| Methanomicrobium mobile | Cow rumen | 93.9–94.4 | ||||
| Methanosarcinales (6%) | O2 3F7a | 1 | 7 | Methanosarcina thermophila | Sewage | 87.6 |
| Thermococcales (1.3%) | O2 3C2a | 1 | 2 | Thermococcus litoralis | Oil | 99.1 |
| Thermococcales (8.8%) | O1 8A6b | 5 | 14 | Thermococcus litoralis | Oil | 99.4–99.9 |
16S rRNA E. coli numbering (20 to 958).
16S rRNA E. coli numbering (519 to 1,390).
Enrichment and cultivation of thermophilic microorganisms.
In addition to the phylogenetic surveys, we used enrichment cultures to directly examine the metabolic diversity of anaerobic isolates cultivated at temperatures between 60 and 100°C and compared these cultured assemblages to the community profiles generated from cloned environmental SSU rDNAs. Using five basal media supplemented with various energy and carbon sources, we detected fermentative, methanogenic, and hydrogen sulfide-producing microorganisms from six different high-temperature petroleum formations in four California oil fields (two offshore and two onshore) (Table 4). The most commonly cultured microorganisms included sulfur-utilizing hyperthermophilic archaea of the genus Thermococcus, fermentative bacteria in the order Thermotogales, and hydrogenotrophic methanogens related to Methanobacterium and Methanococcus. Additional sequenced isolates included a Thermoanaerobacter sp., a Deferribacter sp., Anaerobaculum spp., and Methanoculleus spp. (Table 4). A summary of the results for the most commonly detected groups is reported below.
TABLE 4.
Thermophilic microorganisms cultivated from California onshore and offshore petroleum reservoir production waters
| Field and formation well no.a | Temperature (°C) | Medium type | Sulfur source
|
Carbon source
|
Morphologyc (no. of enrichments/total) | Isolatesd | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| S0 | S2O3 | SO4 | YE-TRPb | Acetate | Glucose | H2-CO2 | N2-CO2 | |||||
| South Elwood | ||||||||||||
| M composite | 80 | NU | x | x | IC, SR | vp198 (T. litoralis) | ||||||
| M 20-9 | 70–85 | MB, NU | x | x | IC (3/5), SR (4/5), R (1/5) | vp204 (T. litoralis) | ||||||
| M 20-9 | 70 | NU | x | x | x | IC | ||||||
| M 20-9 | 70 | NU | x | x | x | IC | ||||||
| M 20-9 | 60 | BS | x | x | SR (3/3), CF SR (1/3), R (2/3) | |||||||
| M 20-9 | 80 | BS | x | x | SR (3/3), CF R (2/3), C (1/3) | vp183 (M. thermolithotrophicus) | ||||||
| M 20-9 and 42-4 | 60–70 | MB, NU, BS | x | x | SR (5/8), R (2/8) | vp424 (P. mobilis) | ||||||
| M 20-9 | 75 | MG | x | x | x | R (2/2) | vp188 (Thermoanaerobacter sp.) | |||||
| M 20-9 | 65 | MA | x | x | SR (3/4), R (1/4) | |||||||
| M 42-16 | 80 | BS | x | VR | vp180 (Deferribacter thermophilus) | |||||||
| M 20-9 | 60–65 | BS | x | AC (1/6), SR (2/6) | M.E3 1B1 (Methanoculleus sp.) | |||||||
| R 20-4 | 70–80 | NU | x | x | x | IC (3/3), SR (3/3) | vp203 (T. litoralis) | |||||
| R 20-4 | 60–65 | NU | x | x | x | SR (1/2), R (1/2) | ||||||
| R 20-4 | 75 | NU | x | x | SR, R | |||||||
| R 20-4 | 75 | NU | x | x | SR, R | |||||||
| R 20-4 | 80 | BS | x | x | CF R | vp184 (A. thermoterrenum) | ||||||
| R 20-4 | 60–75 | NU, BS | x | x | IC (3/3), SR (3/3), R (2/3) | |||||||
| R 20-4 | 70 | MG | x | x | AR (2/2), SR (2/2), C (1/2) | M. thermoautotrophicum | ||||||
| R 20-4 | 65–70 | MA | x | x | SR 2/3 | |||||||
| R 20-4 | 70–80 | BS | x | — | ||||||||
| R 20-4 | 60–70 | BS | x | AR, C | R.E2 1A1, 1A2 (M. thermoautotrophicum) | |||||||
| RE2 2A2 (Methanoculleus sp.) | ||||||||||||
| Santa Clara | ||||||||||||
| M 46 | 60 | NEU | x | x | x | SR, IC, R | ||||||
| M 46 | 60–65 | MG | x | x | x | AR | M. thermoautotrophicum | |||||
| M 46 | 60–75 | BS | x | AC | vp21 (M. thermolithotrophicus) | |||||||
| UR 24 | 78 | NEU | x | x | x | IC, SR | vp197 (T. litoralis) | |||||
| UR 24 | 60 | NEU | x | x | x | IC, SR | ||||||
| UR 24 | 78 | NEU | x | x | SR, IC | |||||||
| UR 24 | 60–65 | MG | x | x | x | AR | M. thermoautotrophicum | |||||
| UR 24 | 60 | BS | x | x | — | |||||||
| UR 24 | 60 | BS | x | AC | M. thermolithotrophicus | |||||||
| LR 57 | 80–85 | NEU | x | x | x | — | ||||||
| LR 57 | 60–70 | NEU | x | x | x | SR (1/2), CF R (2/2), C (1/2) | vp14 (A. thermoterrnum), M.E1 (P. mobilis) | |||||
| LR 57 | 60–70 | MG | x | x | x | SR (2/2), AR (2/2), C (1/2) | M. thermoautotrophicum; vp56 (P. miotherma) | |||||
| LR 57 | 60 | MA | x | x | SR, AC, R | |||||||
| LR 57 | 75 | BS | x | R | ||||||||
| P 75 | 78 | NEU | x | x | x | — | ||||||
| P 75 | 60 | NEU | x | x | x | SR, CF R, IC | ||||||
| P 75 | 60 | BS | x | x | AC | |||||||
| P 75 | 60–75 | BS | x | AC | M. thermolithotrophicus | |||||||
| San Joaquin | ||||||||||||
| M 91, 61, and 29 | 75–85 | 2216 | x | x | x | IC (3/3), R (1/3), SR (1/3) | ||||||
| M 91, 61, 29 | 75–85 | BS | x | x | R (3/5), SR (5/5), AR (1/5), IC (2/5) | |||||||
| M 91 | 73–83 | MG | x | x | x | SR, R | ||||||
| M 91, 61, and 29 | 60–75 | MG | x | x | x | AR (1/4), SR (2/4), R (3/4) | ||||||
| M 61 | 75 | MG | x | x | x | SR, R | ||||||
| M 61 and 29 | 75 | MG | x | R (4/4), C (1/4), AR (1/4) | ||||||||
| M 91 and 61 | 60–75 | BS, MT | x | AR (3/7), SR (2/7), R (3/7), C (1/7) | ||||||||
M, Monterey; R, Rincon; UR, Upper Repetto, LR, Lower Repetto; P, Pico.
YE, 0.1% yeast extract; TRP, 0.1% trypticase.
Morphology in the enrichments: I, irregular; S, sheathed; A, autofluorescent; CF, chain forming; C, cocci; R, rod; —, no growth.
Most closely related sequenced isolate or enrichment 16S rDNA clone.
(i) Thermococcales: sulfidogenic hyperthermophilic archaea.
Successful enrichments of hyperthermophilic sulfur-utilizing irregular cocci were obtained from the Monterey formation in three different California oil fields (two offshore and one onshore), as well as four additional high-temperature, Miocene- and Pliocene-aged, oil-bearing strata (Table 1). These hyperthermophilic sulfidogens were frequently enriched for in marine organic medium containing elemental sulfur at temperatures ranging from 70 to 85°C and appear to be widespread in high-temperature petroleum systems (Table 4). High-temperature, sulfur-based enrichments were dominated by mixed assemblages of sheathed rod-shaped bacteria related to the Thermotogales and Thermococcales-like cocci. Thermococcales-like microorganisms isolated from the Monterey and Rincon formations revealed high similarity to Thermococcus sequences in O1 and O2 clone libraries, with values ranging from 98.2 to 100% (Fig. 3).
(ii) Thermotogales: thermophilic fermentative bacteria.
Sheathed rod-shaped fermentative bacteria related to the order Thermotogales were the most frequent morphotype in production well enrichments (Table 4). Like the hyperthermophilic sulfur-utilizing archeaea, Thermotogales-like bacteria appear to be widely distributed in high-temperature oil-bearing formations both in California and worldwide (12, 22, 27). Cultured isolates and clones recovered from 60°C enrichments from the South Elwood field Monterey formation (vp424), as well as the Santa Clara field's Monterey (M.E1.1F2) and Lower Repetto (vp56) formations, were highly similar to Petrotoga miotherma and Petrotoga mobilis (≥98%), both species isolated from petroleum systems (12, 32). Sheathed rods were most commonly found in coculture with other physiologically diverse microorganisms, including sulfur-utilizing archaea, methanogens, and thermophilic rod-shaped bacteria. These thermophilic bacteria grew in a wide range of culture conditions at temperatures ranging from 60 to 85°C, in salinities between 20 and 46ppt, and in medium designed to enrich for both heterotrophic microorganisms (with and without sulfur) and methanogens (Table 4). As a group, the Thermotogales have a broad range of metabolic capabilities, which may in part explain their widespread distribution and frequent detection (12, 22). Despite their common occurrence in production water enrichments, including the same composite production water sample used for SSU rDNA library construction, no Thermotogales-related 16S rDNA sequences were detected in the universal library (O1). This lack of representation could represent cloning biases, or it may indicate that the Thermotogales are not a dominant component of the subsurface community but rather represent fast-growing opportunists.
(iii) Methanobacteriales and Methanococcales: thermophilic methanogens.
Hydrogenotrophic methanogens related to the genera Methanobacterium, Methanococcus, and Methanoculleus were recovered from 60 to 80°C enrichment cultures from both offshore and continental Californian petroleum reservoirs. Methanogens from the orders Methanobacteriales and Methanococcales were present in multiple high-temperature Miocene- and Pliocene-aged strata from at least two different oil fields, while Methanoculleus spp. were only detected in Rincon and Monterey formations at the South Elwood site. Despite the high percentage of rDNA clones related to methanogens in the O2 library, there was no overlap in the O2 16S rDNA phylotypes and the thermophilic methanogen isolates (Fig. 3). The cultivation and molecular-based approaches each sampled a different subset of the community.
(a) Methanobacterium spp.
Methanogenic rod-shaped microorganisms related to Methanobacterium thermoautotrophicum were isolated from production fluids originating from the South Elwood field Rincon formation as well as the Monterey and the Upper and Lower Repetto formations of the Santa Clara field. Successful enrichments were obtained primarily with low-salinity (9.5-ppt) medium incubated at 60 and 70°C (Table 4). 16S rDNA sequences from isolates originating from the Monterey and Rincon formations demonstrated 95 to 98.7% similarity to M. thermoautotrophicum and 96.3% similarity to each other. All methanobacterium-like isolates from individual oil-bearing formations had identical RFLP patterns (data not shown), suggesting a high degree of SSU rRNA relatedness between the isolates, despite the physicochemical differences between formations. In addition to the offshore production sites, unidentified methane-producing autofluorescent rods were also observed in 73°C enrichments (low-salinity medium) from the San Joaquin Basin (Table 4).
(b) Methanococcus spp.
Methanogenic cocci related to Methanococcus thermolithotrophicus were detected in 60°C marine autotrophic enrichments from all four production horizons in the Santa Clara field, as well as in a 80°C single mixed enrichment culture from the South Elwood field Monterey formation (vp183) (Table 4). Enrichment vp183 was comprised primarily of sheathed rods resembling Thermotogales bacteria, with a smaller percentage of cocci and rods. Amplification of nucleic acid extracts from vp183 with 16S rDNA archaea-specific primers (21f and 958r) revealed the presence of a methanogen highly related (97%) to an M. thermolithotrophicus strain isolated from a North Sea production facility (44). Methanococcus sequence vp183 was 96.7% similar to a sequenced isolate obtained from a Miocene Monterey production well located in the Santa Clara field. Autofluorescent regular and irregular cocci (1 to 2 μm in diameter) were also observed in a number of 60 to 80°C inorganic marine and brackish enrichments from the continental Yowlumne and North Coles Levee oil fields in the San Joaquin Basin. Hydrogen-consuming methanogenic cocci appear to be widespread in marine-based California petroleum systems.
Phylogenetic diversity in enrichment cultures.
Thermophilic (60 to 65°C) methane-producing autotrophic marine enrichments from both the Monterey (M.E3) and Rincon (R.E2) formations at the South Elwood field site contained mixed assemblages of autofluorescent irregular cocci and sheathed rods (Table 4). Enrichment R.E2 also contained autofluorescent rods resembling Methanobacterium spp. Repeated attempts to isolate the irregular cocci from both enrichments were unsuccessful. To identify these thermophilic methanogenic assemblages, nucleic acids were extracted from primary enrichments and used to construct two clone libraries (R.E2 and M.E3) consisting of 96 and 42 clones, respectively. RFLP analysis of 24 16S rDNA clones from library M.E3 revealed a single RFLP type. Library R.E2 contained an RFLP type identical to that of M.E3, as well as two additional RFLP patterns. Sequencing of unique clones from libraries R.E2 and M.E3 revealed the enrichments from both South Elwood field-producing horizons contained methanogens related to Methanoculleus thermophilicus (>96% similarity), a moderately thermophilic hydrogenotrophic methanogen (Fig. 3). The two remaining phylotypes from rDNA clone library R.E2 were found to be highly similar to M. thermoautotrophicum (99.9 and 95.9%).
Comparison between 16S rDNAs from isolates to those directly recovered in libraries.
Cultured thermophilic isolates showed genus-level similarity in 2 out of 10 genera detected by cultivation-independent techniques in the South Elwood production field. These included isolates in the archaeal genus Thermococcus and the bacterial genus Thermoanaerobacter (Fig. 2 and 3). Thermococcales-related 16S rDNA clones from both the universal and archaeal libraries as well as cultured isolates from the same sample (vp198) and from other offshore oil fields in California (vp197) all demonstrated a high degree of sequence similarity. These sequences were most similar to the sulfidogenic hyperthermophile Thermococcus litoralis (99.5 to 99.9%), originally described from a hot sulfur spring and more recently recovered from high-temperature onshore petroleum fields in France (31, 41).
In contrast to the high sequence similarity between Thermococcus 16S rDNA clones and cultured isolates, Thermoanaerobacter representatives (13 16S rDNA clones and one cultured isolate) did not form a coherent clade but rather were loosely affiliated with other Thermoanaerobacter species (92.4% similarity within the group). Thermoanaerobacter 16S rDNA phylotypes formed four distinct groups of highly related (≥99%) clones. These groups clustered tightly together, displaying greater similarity to each other (95.7 to 99.9%) than to known cultured strains in the database, including the single isolate (vp188) obtained from the South Elwood Monterey formation (similarity of 93.4 to 94.9%) (Fig. 2). This cluster of 13 highly related phylotypes most likely represents a new group of uncultivated species and/or strains within Thermoanaerobacter cluster V.
DISCUSSION
The Miocene Monterey formation consists of sulfur-rich, diatomaceous shale and is the principal source of fossil fuels in California. This high-temperature (70°C) petroleum system is widespread in California and is characterized by elevated levels of organic acids (up to 5,800 mg/liter) and hydrogen sulfide (up to 83 mM) in the associated formation water (J. R. Boles, personal communication). Because of its unusual composition and significance in California oil production, the Miocene Monterey formation has been of interest to geologists for decades. However, the associated microbiota, which can have dramatic impacts on oil quality and reservoir permeability, are not well characterized.
The majority of clones obtained in oil reservoir universal 16S rDNA clone libraries were dominated by phylotypes from three major bacterial divisions, with only 8.8% of the library affiliated with the Archaea. We could not amplify eucaryal rDNA with universal primers for this group. These findings suggest that the majority of the microbial community associated with formation waters in this high-temperature petroleum system are members of the domain Bacteria. Low percentages of archaeal clones in universal 16S rDNA libraries have also been observed in other high-temperature or hydrocarbon-rich environments, such as hot springs (25) and hydrocarbon-contaminated aquifers (15).
Archaeal phylotypes represented in the O1 and O2 clone libraries were related to functionally diverse groups, including the hyperthermophilic sulfide-producing Thermococcales, the hydrogenotrophic Methanomicrobiales, and the methylotrophic and acetoclastic Methanosarcinales. Archaeal diversity in the O1 and O2 16S rDNA libraries revealed a marked disparity in representation, with 100% of the universal O1 archaeal clones clustering within the Thermococcales, compared to only 1.3% of phylotypes related to the Thermococcales in the methanogen-dominated O2 library. Reasons for this disparity may include differences in universal and archaea-specific primer annealing, differences in PCR conditions (i.e., low cycle number and acetamide addition) (50), or an increase in diversity in the archaeal library, possibly caused by template reannealing bias (55).
Representative phylotypes recovered from the Miocene Monterey formation were related to known mesophilic (e.g., Pseudomonas and Acinetobacter) and thermophilic (e.g., Thermoanaerobacter and Thermococcus) genera. Out of 17 genera represented in the O1 and O2 16S rDNA libraries, 12 sequence clusters displayed a high similarity (≥96%) to cultured isolates recovered from similar environments (Table 2 and 3). Phylotypes from the O1 library related to the Proteobacterial subdivision were highly similar (≥97%) to isolates obtained from oil fields or hydrocarbon-rich environments (Table 2; Fig. 1). These Proteobacterial phylotypes are not likely derived from thermophilic microorganisms and may be representative of mesophilic microorganisms residing in cooler portions of the reservoir or along the walls or openings of the production well tubing. Sequence types clustering within known thermophilic groups include the low-G+C gram-positive genera Desulfothiovibrio and Thermoanaerobacter and the hyperthermophilic archaeal genus Thermococcus, all of which have been previously isolated from a number of high-temperature petroleum systems worldwide (16, 31, 34). Additional sequence types with potential thermophilic phenotypes include O1 library clone O11D9, whose closest relatives include the amino acid-degrading Aminobacterium colombiense (87.1% similarity) and the thermophilic petroleum reservoir isolate Anaerobaculum thermoterrenum (3, 49) as well as the O2 library clone O23F7, most similar (87.6%) to the thermophilic acetoclastic methanogen M. thermophila.
Thermoanaerobacter-related clones were second in abundance in the O1 library and displayed a large amount of 16S rDNA sequence variability (95.6 to 99.5% similarity). These phylotypes formed a coherent cluster adjacent to Thermoanaerobacter brockii and Thermoanaerobacter ethanolicus within cluster V of the low-G+C gram-positive division (10) (Fig. 2). The high-sequence diversity observed within this novel cluster of as-yet-uncultivated Thermoanaerobacter relatives might be the result of niche diversification (38) or multiple rRNA operons within a few representative species. Considering the physicochemical heterogeneity of the Miocene Monterey shale and the fact that the environmental 16S rDNA libraries were constructed from a composite sample taken from multiple production wells, it is plausible that individual populations of this new group of Thermoanaerobacter-like microorganisms reside in different microhabitats within the reservoir.
Anaerobic thermophilic enrichments from six geochemically distinct, high-temperature, oil-bearing formations revealed eight distinct thermophilic and hyperthermophilic bacterial and archaeal genera phenotypically described as sulfidogens, methanogens, and fermentative microorganisms. Of the cultured isolates and enrichment library clones sequenced, seven of the eight unique genera were related to thermophilic microorganisms previously isolated from oil reservoirs, suggesting that these cultivatable thermophiles may be a common component of these geothermally heated specialized subsurface environments. In particular, thermophiles related to the sulfidogenic and fermentative genera Thermotoga, Petrotoga, Thermococcus, and the H2-CO2-utilizing Methanobacterium and Methanococcus were shown to be widespread within both Californian petroleum systems and worldwide (22, 31, 42, 44).
The major types of microorganisms detected both in enrichments and in 16S rDNA clone libraries are consistent with the physicochemical conditions present in the petroleum system under investigation. The anaerobic sulfur and organic acid-rich formation fluids associated with the high-temperature Miocene Monterey shales (South Elwood field) contained a diverse number of phylotypes, a significant percentage of which were highly similar to known thermophilic fermentative and organotrophic sulfur-respiring bacteria and archaea (e.g., Thermoanaerobacter and Thermococcus). Representatives of these genera were also isolated from enrichment cultures inoculated with Monterey-sourced production fluids, further suggesting that these thermophilic sulfur-utilizing groups are significant components of the resident subsurface microbial assemblage and may be contributing to active production of hydrogen sulfide in the reservoir. Also shown to produce H2S from sulfur oxyanions, members of the Thermotogales order were detected in enrichments from all wells tested with a wide range of culture media. Although these seemingly abundant sheathed rods were present in the majority of enrichments, Thermotogales-related phylotypes were not detected in the O1 library. This data may indicate that these fast-growing thermophiles are not a major component of the in situ reservoir assemblage. Alternatively, they may have been missed due to PCR biases in the mixed assemblage DNA amplification, although the universal primers used were found to be compatible with the Thermotogales 16S rDNA sequences in the database.
Due to the numerous problems, both health and economic, associated with oil reservoir souring, a significant portion of petroleum microbiology research has focused on the activities and identification of sulfate-reducing bacteria, long considered the major culprit in hydrogen sulfide production within waterflooded strata. We found little evidence for sulfate-reducing bacteria or archaea in 16S rDNA clone libraries and enrichments. However, recent isolation of sulfidogens, such as sulfur-utilizing Thermococcales and Thermotogales and thiosulfate-respiring Desulfothiovibrio and Thermoanaerobacter spp., from high-temperature petroleum systems has introduced the potential for biological souring from alternative sulfur sources. In addition to potential H2S production from elemental sulfur and thiosulfate, recent work by Stetter and colleagues (53a) has shown evidence for active desulfurization of crude oils by strains of Thermococcales and Thermotogales. With the high levels of sulfur and organic acids associated with the Miocene Monterey production fluids, it is likely that heterotrophic sulfidogenic thermophiles exploiting organosulfur compounds in crude oil may be the predominant source of biological H2S production, rather than sulfate-reducing microorganisms.
Using both 16S rDNA phylogenetic surveys and culture-based methods, we were able to characterize a number of diverse thermophilic and mesophilic microorganisms associated with high-temperature petroleum systems in California. Phylotypes and cultured thermophilic isolates highly related to the organotrophic and sulfidogenic genera Thermococcus, Thermoanaerobacter, Desulfothiovibrio, Anaerobaculum, Petrotoga, and Deferribacter, as well as the methanogenic Methanobacterium and Methanococcus, have all been previously reported from high-temperature oil field environments (12, 23, 31, 34, 42, 44). This suggests that these groups of thermally adapted microorganisms are widespread within high-temperature petroleum systems and may have significant impacts on reservoir geochemistry. Microorganisms detected in this study, but not yet described from petroleum systems, included sequence types related to the fermentative anaerobe Acidaminococcus, the acetoclastic Methanosarcinales, and thermophilic methanogens clustering within the genus Methanoculleus. Thermophilic microbial assemblages within high-temperature petroleum systems are phylogenetically diverse and tend to be phenotypically associated with fermentative, sulfidogenic, or methanogenic microorganisms. The widespread occurrence of these diverse thermophilic microorganisms from complementary functional groups in deep-seated high-temperature petroleum reservoirs suggests the potential for closely coupled, active biogeochemical cycling of carbon and sulfur in hot subsurface petroleum habitats.
ACKNOWLEDGMENTS
Funding for this project was provided by the University of California Energy Institute, NSF grants OCE95-29804 and OPP94-18442, and the David and Lucile Packard Foundation.
This work could not have been done without the cooperation of Mobil, Torch, and Chevron Oil companies, in particular Mobil's Scott Hornafius, Prentice Patterson, and Geoffrey MacDonald, and Torch's David White and Sabrina Miller. We also thank Anaerobe System's Mike Cox for providing the anaerobic chamber, as well as Jim Boles, Shana Goffredi, Jim Childress, Chad Mireau, Martin Keller, and Marcelino Suzuki for their assistance during the course of this study.
REFERENCES
- 1.Amann R I, Ludwig W, Schleifer K H. Phylogenetic identification and in situ detection of individual microbial cells without cultivation. Microbiol Rev. 1995;59:143–169. doi: 10.1128/mr.59.1.143-169.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.American Type Culture Collection. Catalogue of bacteria and phages. 18th ed. Rockville, Md: American Type Culture Collection; 1992. [Google Scholar]
- 3.Baena S, Fardeau M-L, Labat M, Ollivier B, Thomas P, Garcia J-L, Patel B K C. Aminobacterium colombiense gen. nov., sp. nov., an amino acid-degrading anaerobe isolated from anaerobic sludge. Anaerobe. 1998;4:241–250. doi: 10.1006/anae.1998.0170. [DOI] [PubMed] [Google Scholar]
- 4.Balkwill D L, Reeves R H, Drake G R, Reeves J Y, Crocker F H, King M B, Boone D R. Phylogenetic characterization of bacteria in the subsurface microbial culture collection. FEMS Microbiol Rev. 1997;20:201–216. doi: 10.1111/j.1574-6976.1997.tb00309.x. [DOI] [PubMed] [Google Scholar]
- 5.Baross J A. Isolation, growth, and maintenance of hyperthermophiles. In: Robb F T, Place A R, editors. Archaea: a laboratory manual. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1995. pp. 15–23. [Google Scholar]
- 6.Brownstein M J, Carpten J D, Smith J R. Modulation of non-templated nucleotide addition by Taq DNA polymerase: primer modifications that facilitate genotyping. BioTechniques. 1996;20:1004–1006. doi: 10.2144/96206st01. , 1008–1010. [DOI] [PubMed] [Google Scholar]
- 6a.California Division of Oil and Gas. Division of Oil and Gas. 3rd ed. II. Sacramento: California Division of Oil and Gas; 1991. California oil and gas fields: Southern, Central, Coastal, and Offshore California; p. 689. [Google Scholar]
- 7.Chandler D P, Brockman F J, Bailey T J, Fredrickson J K. Phylogenetic diversity of archaea and bacteria in a deep subsurface paleosol. Microb Ecol. 1998;36:37–50. doi: 10.1007/s002489900091. [DOI] [PubMed] [Google Scholar]
- 8.Childress J J, Arp A J, Fisher C R. Metabolic and blood characteristics of the hydrothermal vent tube worm Riftia pachyptila. Mar Biol. 1984;83:109–124. [Google Scholar]
- 9.Christensen B, Torsvik T, Lien T. Immunomagnetically captured thermophilic sulfate-reducing bacteria from North Sea oil field waters. Appl Environ Microbiol. 1992;58:1244–1248. doi: 10.1128/aem.58.4.1244-1248.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Collins M D, Lawson P A, Willems A, Cordoba J J, Fernandez-Garayzabal J, Garcia P, Cai J, Hippe H, Farrow J A E. The phylogeny of the genus Clostridium: proposal of five new genera and eleven new species combinations. Int J Syst Bacteriol. 1994;44:812–826. doi: 10.1099/00207713-44-4-812. [DOI] [PubMed] [Google Scholar]
- 11.Cook G M, Rainey F A, Chen G, Stackebrandt E, Russell J B. Emendation of the description of Acidaminococcus fermentans, a trans-aconitate- and citrate-oxidizing bacterium. Int J Syst Bacteriol. 1994;44:576–578. doi: 10.1099/00207713-44-3-576. [DOI] [PubMed] [Google Scholar]
- 12.Davey M E, Wood W A, Key R, Nakamura K, Stahl D A. Isolation of three species of Geotoga and Petrotoga: two new genera, representing a new lineage in the bacterial line of descent distantly related to the ‘Thermotogales.’. Syst Appl Microbiol. 1993;16:191–200. [Google Scholar]
- 13.Davydova-Charakhch'yan I A, Mileeva A N, Mityushina L L, Belyaev S S. Acetogenic bacteria from oil fields of Tataria and Western Siberia. Mikrobiologiya. 1993;61:306–315. [Google Scholar]
- 14.DeLong E F. Archaea in coastal marine environments. Proc Natl Acad Sci USA. 1992;89:5685–5689. doi: 10.1073/pnas.89.12.5685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dojka M A, Hugenholtz P, Haack S K, Pace N R. Microbial diversity in a hydrocarbon- and chlorinated-solvent-contaminated aquifer undergoing intrinsic bioremediation. Appl Environ Microbiol. 1998;64:3869–3877. doi: 10.1128/aem.64.10.3869-3877.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fardeau M L, Cayol J L, Magot M, Ollivier B. H-2 oxidation in the presence of thiosulfate, by a Thermoanaerobacter strain isolated from an oil-producing well. FEMS Microbiol Lett. 1993;113:327–332. [Google Scholar]
- 17.Felsenstein J. PHYLIP—phylogeny interference package (version 3.5) Cladistics. 1993;5:164–166. [Google Scholar]
- 18.Fisher J B, Boles J R. Water rock interaction in tertiary sandstones, San Joaquin Basin, California, USA—diagenetic controls on water composition. Chem Geol. 1990;82:83–101. [Google Scholar]
- 19.Ghiorse W C, Wilson J T. Microbial ecology of the terrestrial subsurface. Adv Appl Microbiol. 1988;33:107–172. doi: 10.1016/s0065-2164(08)70206-5. [DOI] [PubMed] [Google Scholar]
- 20.Giovannoni S J, Britschgi T B, Moyer C L, Field K G. Genetic diversity in Sargasso Sea bacterioplankton. Nature. 1990;345:60–63. doi: 10.1038/345060a0. [DOI] [PubMed] [Google Scholar]
- 21.Girard J E, Glatz J A. Ion chromatography with conventional HPLC instrumentation. Am Lab. 1981;13:26–35. [Google Scholar]
- 22.Grassia G S, McLean K M, Glenat P, Bauld J, Sheehy A J. A systematic survey for thermophilic fermentative bacteria and archaea in high temperature petroleum reservoirs. FEMS Microbiol Ecol. 1996;21:47–58. [Google Scholar]
- 23.Greene A D, Patel B K C, Sheehy A J. Deferribacter thermophilus gen. nov., sp. nov., a novel thermophilic manganese- and iron-reducing bacterium isolated from a petroleum reservoir. Int J Syst Bacteriol. 1997;47:505–509. doi: 10.1099/00207713-47-2-505. [DOI] [PubMed] [Google Scholar]
- 24.Hovanec T A, Delong E F. Comparative analysis of nitrifying bacteria associated with freshwater and marine aquaria. Appl Environ Microbiol. 1996;62:2888–2896. doi: 10.1128/aem.62.8.2888-2896.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hugenholtz P, Pitulle C, Hershberger K L, Pace N R. Novel division level bacterial diversity in a Yellowstone hot spring. J Bacteriol. 1998;180:366–376. doi: 10.1128/jb.180.2.366-376.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hungate R E. A roll tube method for cultivation of strict anaerobes. In: Norris J R, Ribbons D W, editors. Methods in Microbiology. 3B. New York, N.Y: Academic Press Inc.; 1969. pp. 117–132. [Google Scholar]
- 27.Jeanthon C, Reysenbach A L, L'Haridon S, Gambacorta A, Pace N R, Glenat P, Prieur D. Thermotoga subterranea sp. nov., a new thermophilic bacterium isolated from a continental oil reservoir. Arch Microbiol. 1995;164:91–97. [PubMed] [Google Scholar]
- 28.Kimura M. A simple method for estimating evolutionary rate of base substitutions through comparative studies of nucleotide sequences. J Mol Evol. 1980;16:111–120. doi: 10.1007/BF01731581. [DOI] [PubMed] [Google Scholar]
- 29.Kurr M, Huber R, Konig H, Jannasch H W, Frike H, Trincone A, Kristjansson J K, Stetter K O. Methanopyrus kandleri, gen. and sp. nov., represents a novel group of hyperthermophilic methanogens, growing at 110°C. Arch Microbiol. 1991;156:239–247. [Google Scholar]
- 30.Lane D J, Pace B, Olsen G J, Stahl D A, Sogin M L, Pace N R. Rapid determination of 16S ribosomal RNA sequences for phylogenetic analysis. Proc Natl Acad Sci USA. 1985;82:6955–6959. doi: 10.1073/pnas.82.20.6955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.L'Haridon S, Reysenbach A L, Glenat P, Prieur D, Jeanthon C. Hot subterranean biosphere in a continental oil reservoir. Nature. 1995;377:223–224. [PubMed] [Google Scholar]
- 32.Lien T, Madsen M, Rainey F A, Birkeland N K. Petrotoga mobilis sp. nov., from a North Sea oil-production well. Int J Syst Bacteriol. 1998;48:1007–1013. doi: 10.1099/00207713-48-3-1007. [DOI] [PubMed] [Google Scholar]
- 33.Macy J M, Snellen J E, Hungate R E. Use of syringe methods for anaerobiosis. Am J Clin Nutr. 1972;25:1318–1323. doi: 10.1093/ajcn/25.12.1318. [DOI] [PubMed] [Google Scholar]
- 34.Magot M, Ravot G, Campaignolle X, Ollivier B, Patel B K C, Fardeau M L, Thomas P, Crolet J L, Garcia J L. Dethiosulfovibrio peptidovorans gen. nov., sp. nov., a new anaerobic, slightly halophilic, thiosulfate-reducing bacterium from corroding offshore oil wells. Int J Syst Bacteriol. 1997;47:818–824. doi: 10.1099/00207713-47-3-818. [DOI] [PubMed] [Google Scholar]
- 35.Maidak B L, Larsen N, McCaughey M J, Overbeek R, Olsen G J, Fogel K, Blandy J, Woese C R. The Ribosomal Database Project. Nucleic Acids Res. 1994;22:3485–3487. doi: 10.1093/nar/22.17.3485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Massana R, Murray A E, Preston C M, Delong E F. Vertical distribution and phylogenetic characterization of marine planktonic Archaea in the Santa Barbara channel. Appl Environ Microbiol. 1997;63:50–56. doi: 10.1128/aem.63.1.50-56.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Miller T L, Wolin M J. A serum bottle modification of the Hungate technique for cultivating obligate anaerobes. Appl Microbiol. 1974;27:985–987. doi: 10.1128/am.27.5.985-987.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Moore L R, Rocap G, Chisholm S W. Physiology and molecular phylogeny of coexisting Prochlorococcus ecotypes. Nature. 1998;393:464–467. doi: 10.1038/30965. [DOI] [PubMed] [Google Scholar]
- 39.Murray A E, Preston C M, Massana R, Taylor L T, Blakis A, Wu K, DeLong E F. Seasonal and spatial variability of bacterial and archaeal assemblages in the coastal waters near Anvers Island, Antarctica. Appl Environ Microbiol. 1998;64:2585–2595. doi: 10.1128/aem.64.7.2585-2595.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nazina T N, Rozanova E P, Kuznetsov S I. Microbial oil transformation processes accompanied by methane and hydrogen-sulfide formation. Geomicrobiol J. 1985;4:103–130. [Google Scholar]
- 41.Neuner A, Jannasch H W, Belkin S, Stetter K O. Thermococcus litoralis sp. nov.—a new species of extremely thermophilic marine archaebacteria. Arch Microbiol. 1990;153:205–207. [Google Scholar]
- 42.Ng T K, Weimer P J, Gawel L J. Possible nonanthropogenic origin of two methanogenic isolates from oil-producing wells in the San Miguelito field, Ventura County, California. Geomicrobiol J. 1989;7:185–192. [Google Scholar]
- 43.Nilsen R K, Beeder J, Thorstenson T, Torsvik T. Distribution of thermophilic marine sulfate reducers in North Sea oil field waters and oil reservoirs. Appl Environ Microbiol. 1996;62:1793–1798. doi: 10.1128/aem.62.5.1793-1798.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nilsen R K, Torsvik T. Methanococcus thermolithotrophicus isolated from North Sea oil field reservoir water. Appl Environ Microbiol. 1996;62:728–731. doi: 10.1128/aem.62.2.728-731.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ollivier B, Cayol J L, Patel B K, Magot M, Fardeau M L, Garcia J L. Methanoplanus petrolearius sp. nov., a novel methanogenic bacterium from an oil-producing well. FEMS Microbiol Lett. 1997;147:51–56. doi: 10.1111/j.1574-6968.1997.tb10219.x. [DOI] [PubMed] [Google Scholar]
- 46.Onstott T C, Phelps T J, Colwell F S, Ringelberg D, White D C, Boone D R. Observations pertaining to the origin and ecology of microorganisms recovered from the deep subsurface of Taylorsville Basin, Virginia. Geomicrobiol J. 1998;15:353–385. [Google Scholar]
- 47.Pedersen K, Arlinger J, Ekendahl S, Hallbeck L. 16S rRNA gene diversity of attached and unattached bacteria in boreholes along the access tunnel to the Aspo hard rock laboratory, Sweden. FEMS Microbiol Ecol. 1996;19:249–262. [Google Scholar]
- 48.Porter K G, Feig Y S. The use of DAPI for identifying and counting aquatic microflora. Limnol Oceanogr. 1980;25:943–948. [Google Scholar]
- 49.Rees G N, Patel B K C, Grassia G S, Sheehy A J. Anaerobaculum thermoterrenum gen. nov., sp. nov., a novel, thermophilic bacterium with ferments citrate. Int J Syst Bacteriol. 1997;47:150–154. doi: 10.1099/00207713-47-1-150. [DOI] [PubMed] [Google Scholar]
- 50.Reysenbach A L, Giver L J, Wickham G S, Pace N R. Differential amplification of rRNA genes by polymerase chain reaction. Appl Environ Microbiol. 1992;58:3417–3418. doi: 10.1128/aem.58.10.3417-3418.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rozanova E P, Nazina T N, Galushko A S. Isolation of a new genus of sulfate-reducing bacteria and description of a new species of this genus, Desulfomicrobium apsheronum gen. nov., sp. nov. Microbiology. 1988;57:514–520. [Google Scholar]
- 52.Rozanova E P, Savvichev A S, Miller Yu M, Ivanov M V. Microbial processes in a flooded West Siberian oil field with a complex of organic compounds. Mikrobiologiya. 1997;66:852–859. [Google Scholar]
- 53.Stahl D A, Flesher B, Mansfield H R, Montgomery L. Use of phylogenetically based hybridization probes for studies of ruminal microbial ecology. Appl Environ Microbiol. 1988;54:1079–1084. doi: 10.1128/aem.54.5.1079-1084.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53a.Stetter K O, Huber R. The role of hyperthermophilic prokaryotes in oil fields. In: Bell C R, Brylinsky M, Johnson-Green P, editors. Microbial biosystems: new frontiers. Proceedings of the 8th International Symposium on Microbial Ecology. Halifax, Canada: Atlantic Canada Society for Microbial Ecology; 1999. , in press. [Google Scholar]
- 54.Stetter K O, Huber R, Bloechl E, Kurr M, Eden R D, Fiedler M, Cash H, Vance I. Hyperthermophilic archaea are thriving in deep North Sea and Alaskan oil reservoirs. Nature. 1993;365:743–745. [Google Scholar]
- 55.Suzuki M T, Giovannoni S J. Bias caused by template annealing in the amplification of mixtures of 16S rRNA genes by PCR. Appl Environ Microbiol. 1996;62:625–630. doi: 10.1128/aem.62.2.625-630.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Suzuki M T, Rappé M S, Haimberger Z W, Winfield H, Adair N, Ströbel J, Giovannoni S J. Bacterial diversity among small-subunit rRNA gene clones and cellular isolates from the same seawater sample. Appl Environ Microbiol. 1997;63:983–989. doi: 10.1128/aem.63.3.983-989.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Takami H, Inoue A, Fuji F, Horikoshi K. Microbial flora in the deepest sea mud of the Mariana Trench. FEMS Microbiol Lett. 1997;152:279–285. doi: 10.1111/j.1574-6968.1997.tb10440.x. [DOI] [PubMed] [Google Scholar]
- 58.Tardy-Jacquenod C, Caumette P, Matheron R, Lanau C, Arnauld O, Magot M. Characterization of sulfate-reducing bacteria isolated from oil-field waters. Can J Microbiol. 1996;42:259–266. doi: 10.1139/m96-038. [DOI] [PubMed] [Google Scholar]
- 59.Voordouw G, Armstrong S M, Reimer M F, Fouts B, Telang A J, Shen Y, Gevertz D. Characterization of 16S rRNA genes from oil field microbial communities indicates the presence of a variety of sulfate-reducing, fermentative, and sulfide-oxidizing bacteria. Appl Environ Microbiol. 1996;62:1623–1629. doi: 10.1128/aem.62.5.1623-1629.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Voordouw G, Shen Y, Harrington C S, Telang A J, Jack T R, Westlake D W S. Quantitative reverse sample genome probing of microbial communities and its application to oil field production waters. Appl Environ Microbiol. 1993;59:4101–4114. doi: 10.1128/aem.59.12.4101-4114.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ward D M, Weller R, Bateson M M. 16S rRNA sequences reveal numerous uncultured microorganisms in a natural community. Nature. 1990;345:63–65. doi: 10.1038/345063a0. [DOI] [PubMed] [Google Scholar]