Abstract
Objective
Hypercoagulability and thrombotic complications seen in patients with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), as well as the associated pathophysiology, have been reported extensively. However, there is limited information regarding the factors related to this phenomenon and its association with the Coronavirus disease 2019 (COVID-19) Delta variant.
Methods
A retrospective review including patients admitted to a tertiary center with a COVID-19 positive test and at least one acute thrombotic event confirmed by imaging between June 2020 and August 2021 was performed. We compared the rates of thrombotic events in patients with COVID-19 before and during the Delta peak. We also analyzed the association of the thrombotic complications with demographic characteristics, comorbidities, anticoagulation strategies, and prothrombotic markers while describing other complications secondary to COVID-19 infection.
Results
Of 964 patients admitted with COVID-19 diagnosis, 26.5% (n = 256) had a thrombotic event evidenced by ultrasound or computed tomography scan. Venous thromboembolism was found in 60% (n = 153), arterial thrombosis in 23% (n = 60), and both venous and arterial thromboses in 17% (n = 17) of the study cohort. Of all patients, 94% were not vaccinated. Delta variant wave (DW) patients had thrombotic episodes in 34.7% (n = 50/144) of cases compared with 25% (n = 206/820) of non-Delta wave (NDW) patients, posing an estimated risk 1.36 times higher in patients infected with COVID-19 during the DW than NDW. Overall, DW subjects were significantly younger (P < .001) with lower body mass index (P = .021) compared with NDW patients. Statistical analyses showed African American patients were more likely to have arterial thrombosis compared with the other groups when testing positive for COVID-19 (odds ratio [OR], 1.78; 95% confidence interval [CI], 1.04-3.05; P = .035, whereas immunosuppressed patients had less risk of arterial thrombosis (OR, 0.38; 95% CI, 0.15-0.96; P = .042). Female gender (OR, 2.15; 95% CI, 1.20-3.85; P = .009) and patients with active malignancy (OR, 5.99; 95% CI, 2.14-16.78; P = .001) had an increased risk of having multiple thrombotic events at different locations secondary to COVID-19.
Conclusions
COVID-19 infection is associated with elevated rates of thrombotic complications and an especially higher risk in patients infected during the Delta variant peak. We highlight the importance of vaccination and the development of new anticoagulation strategies for patients with COVID-19 with additional hypercoagulable risk factors to prevent thrombotic complications caused by this disease.
Keywords: Complications, COVID-19 Delta variant, COVID-19 infection, Jypercoagulable state, Thrombotic events, Variants of concern
Article Highlights.
-
•
Type of Research: Single-center retrospective chart review
-
•
Key Findings: Of 964 patients admitted to a tertiary center with Coronavirus disease 2019 (COVID-19) diagnosis, 26.5% (n = 256) had documented thrombotic events; the majority of these patients were unvaccinated. Delta wave patients had thrombotic episodes in 34.7% of cases compared with 25% of non-Delta cases. Delta wave subjects were significantly younger (P < .001) with lower body mass index (P = .021) compared with non-Delta wave patients.
-
•
Take Home Message: COVID-19 infection is associated with elevated rates of thrombotic complications and an estimated higher risk of thrombosis in patients with the Delta variant compared with the non-Delta variants.
Amid the Coronavirus disease 2019 (COVID-19) pandemic, there were multiple concerns regarding the behavior of severe acute respiratory syndrome coronavirus 2 (SARS-Cov-2) infection after a significant spike in the number of COVID-19 cases worldwide during the summer of 2021. Despite the massive vaccination initiative, which was expected to ‘flatten the curve’ throughout the year, the hospital occupancy rate due to COVID-19 continued to increase.1 The Centers for Disease Control and Prevention reported more than 2000 hospital admissions with confirmed COVID-19 from June to September 2021 in Miami-Dade County, Florida.2 , 3 According to the World Health Organization, the best explanation for this trend is the surge of variants of concern (VOC). These are defined as variants with evidence of increased transmissibility, more severe symptoms, decreased effectiveness of treatments and vaccines leading to higher rates of hospitalization and death, or low diagnostic detection.4
From the start of the pandemic, there has been a continued effort to study the unique characteristics of the SARS-Cov-2 virus. As the number of critical patients hospitalized due to COVID-19 increased, a clearer picture of the associated complications began to emerge. One of the most striking patterns seen was that of coagulation abnormalities; specifically, the development of severe hypercoagulable state.5, 6, 7, 8 The mechanism of the hypercoagulable state is thought to be related to an invasion of endothelial cells that causes significant damage and dysfunction, in addition to neutrophil, platelet hyperactivation, and a systemic inflammatory response triggered by the viral infection.9, 10, 11 This phenomenon was seen predominantly in patients who presented with more severe manifestations, and it is currently known to be a significant cause of morbidity and mortality in patients with COVID-19.9 , 12 , 13
Variability in symptoms and severity among patients poses a challenge for health care personnel regarding diagnosis and treatment, facing clinical presentations that range from a single small thrombotic event to disseminated intravascular coagulation, without a pattern that allows predictability of the clinical course.11 , 14 During the recognized COVID-19 Delta variant peak, a higher number of significant thrombotic events due to COVID-19 were seen, along with an increase in consults to the vascular surgery service for multidisciplinary management of those complications. This study reports our experience treating COVID-19 patients with thrombotic episodes before and during the Delta variant wave and reviews the associated factors.
Methods
A retrospective chart review at the University of Miami Hospital or Jackson Memorial Health System was performed between June 2020 and August 2021. We included in our analysis patients older than 18 years, admitted with a confirmed positive COVID-19 test and at least one acute thrombotic event confirmed by imaging (duplex ultrasonography [US], computed tomography [CT] pulmonary angiography, etc). There was no defined screening protocol in place at any time during the study period, thus the decision to order imaging workup for possible thrombotic complications was left to the provider. Imaging studies were included regardless of indication (ie, screening or symptomatic). We excluded patients with thrombotic events clearly associated with central venous catheters or other indwelling catheters.
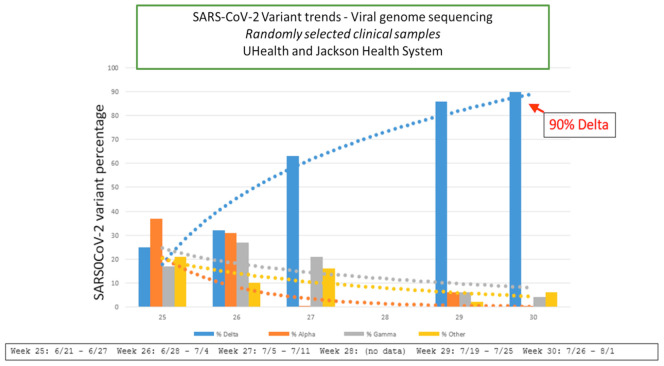
In collaboration with the pathology department, the department of epidemiology performed a detailed analysis of viral genome sequencing in randomly selected clinical samples to detect the SARS-CoV-2 Delta variant progression during the summer peak of 2021. The curve represented in Fig 1 shows the beginning of the Delta variant spike on June 21, 2021, reaching its highest cusp around July 26, 2021. A downtrend of Alpha and Gamma, among other VOCs, is also appreciated in this graph. Based on this analysis, we decided to define the “Delta” period as the time frame between June 1, 2021, which was the time of initiation of the Delta peak, and August 12, 2021, when the data collection ended. A total of 50 patients were identified during this Delta wave (DW), and 206 charts were reviewed throughout the rest of the data collection time and defined as “non-Delta” (NDW) in representation of other VOCs. All COVID-19 diagnoses were confirmed with an United States Food and Drug Administration-approved SARS-CoV2 real-time polymerase chain reaction test.
Fig 1.
Coronavirus disease 2019 (COVID-19) Delta variant peak at University of Miami Health and Jackson Memorial Health System. SARS-CoV-2, Severe acute respiratory syndrome coronavirus 2.
A comparison regarding the thrombotic event rates in patients with suspected Delta variant versus non-Delta variants was performed. Additional logistic and multiple linear regression analyses were executed to test for possible associations between thrombotic complications and variables, including demographic characteristics, comorbidities, anticoagulation strategies, and prothrombotic markers. Data regarding medical history, microbiologic tests, imagining reports, coagulation laboratory on the day of the thrombotic event diagnosis, and events during the hospital stay were also collected.
Statistical analysis was performed with STATA/BE 17.0 (College Station, Tex) and IBM SPSS 28.0.0.0 (Chicago, Ill) statistical software package to assess the possible factors associated with the complications and hypercoagulable state secondary to COVID-19. Endpoints evaluated were the location of thrombi such as venous thromboembolism (VTE) and arterial thromboembolism in upper and lower extremities, pulmonary embolism (PE), myocardial infarction (MI), limb ischemia, and ischemic cerebral infarction. Other outcomes were intensive care unit stay, surgical procedure required, anticoagulation treatment, and other complications associated with COVID-19.
Categorical variables are expressed as the number of subjects (%) and compared using the Fisher exact test with a significance level of 0.05 for all tests. Continuous variables are presented as mean (standard deviation [SD]) or median (interquartile range [IQR]) and were analyzed using the Student t test for normally distributed data or the Mann-Whitney U test for non-normal distribution as appropriate. When adjusting for multiple covariates, logistic regression was used for categorical outcomes and linear regression for continuous outcomes.
The Ethics and Institutional Board Committee approved this study at the University of Miami and Jackson Memorial Hospital, and informed consent exemption was provided.
Results
Baseline demographic and clinical characteristics of the study population
Of 964 patients admitted with COVID-19 diagnosis during the selected dates, 26.5% (n = 256) had a thrombotic event evidenced by US or CT scan and were included in the study. The average age of the population was 63.76 (SD, ±15) years old, with a higher proportion of male (60%) over female (40%) gender and an average body mass index (BMI) of 29.77 (SD, ±7) kg/m2. Hispanic ethnicity accounted for more than one-half of the patients (54%), followed by African American patients (45%) and in a minor percentage of Caucasian patients (1%). The majority of the study cohort were non-smokers, 9% were former smokers, and 5% were current smokers. Only 5% of the patients included had completed their full-dose COVID-19 vaccination records according to instructions from each pharmaceutical company, 1% had only one dose of a two-dose vaccination schedule, and the remaining 94% were not vaccinated at all for COVID-19 (Table I ). COVID-19 boosters were not yet available to the general public during the time of the study.
Table I.
Demographic characteristics and comorbidities of the cohort population
| N = 256 | |
|---|---|
| Age, years | 63.76 (±15) |
| Sex | |
| Female | 103 (40) |
| Male | 153 (60) |
| Ethnicity | |
| Caucasian | 3 (1) |
| Hispanic | 139 (54) |
| African American | 114 (45) |
| BMI, kg/m2 | 29.77 (±7) |
| Vaccination status | |
| None | 240 (93) |
| One dose | 3 (1) |
| Two doses | 13 (5) |
| Smoking | |
| Never | 192 (75) |
| Former | 20 (9) |
| Current | 10 (5) |
| Comorbidities | |
| Hypertension | 172 (67) |
| Diabetes mellitus | 132 (52) |
| COPD | 21 (8) |
| Asthma | 21 (8) |
| CAD/CHF | 54 (21) |
| Atrial fibrillation | 17 (6) |
| TIA/stroke | 10 (4) |
| Peripheral arterial disease | 7 (3) |
| CKD | 70 (27) |
| Active malignancy | 21 (8) |
| Immunosuppression | 31 (12) |
| Thrombophilia | 4 (1) |
| Previous DVT/PE | 24 (9) |
| Start of COVID-19 symptoms, daysa | 3 (1-7) |
BMI, Body mass index; CAD, coronary arterial disease; CHF, chronic heart failure; CKD, chronic kidney disease; COPD, chronic obstructive pulmonary disease; COVID-19, Coronavirus disease 2019; DVT, deep venous thrombosis; PE, pulmonary embolism; TIA, transient ischemic attack.
Data are presented as number (%), mean (standard deviation), or median (interquartile range).
Start of COVID symptoms in number of days prior to admission.
In terms of comorbidities, hypertension and diabetes mellitus were the most common, with 67% and 52%, respectively. Other conditions present in the medical history were chronic kidney disease in 27%, coronary artery disease or heart failure in 21%, chronic pulmonary obstructive disease, and asthma in the same proportion (8%), and transient ischemic attack or ischemic stroke (TIA/stroke) in 4% of the patients (Table I). Of all COVID-positive imaging confirmed thromboses, 14.8% (n = 38) were asymptomatic or minimally symptomatic for COVID-19.
Baseline demographic and clinical characteristics before and during Delta variants
From a total of 144 admissions at both institutions with COVID-19 positive tests during the time defined as DW, 50 patients (34.7%) developed thrombosis confirmed by imaging, compared with 206 of the 820 COVID-19-positive patients (25.1%) admitted during the NDW. This suggests an estimated 1.36 times higher risk of thrombosis in patients infected with COVID-19 during the DW than the NDW.
The patients admitted during the DW had a median age of 59 years (SD, ±10) and a BMI of 26 kg/m2 (SD, ±4). There were more patients identified with male gender than female gender (42%) and more African American patients (54%) than Hispanic patients (46%). The majority were nonsmokers, with lower rates of former (7%) and current (4%) smokers. Only 10% received either one dose or the full vaccination dose. Overall, the DW subjects were significantly younger (P < .001) and had decreased BMIs (P = .021) than the NDW patients. Also, they were more likely to have at least one dose of any vaccine against COVID-19. Comorbidities such as hypertension, diabetes mellitus, chronic pulmonary obstructive disease, asthma, coronary artery disease or heart failure, atrial fibrillation, TIA or stroke, chronic kidney disease, and active malignancies were less likely in the DW sample than in the NDW population. Only medical history of immunosuppression or DVT/PE was higher in the DW group, without a statistical significance (Table II ).
Table II.
Demographic characteristics compared between patients who were Coronavirus disease 2019 (COVID-19)-positive during Delta variant wave (DW) time and the rest of the cohort (non-Delta wave [NDW])
| NDW (n = 206) | DW (n = 50) | P-value | |
|---|---|---|---|
| Age, years | 66 (±10) | 59 (±10) | <.001 |
| Sex | .78 | ||
| Female | 82 (40) | 21 (42) | |
| Male | 124 (60) | 29 (58) | |
| Ethnicity | .25 | ||
| Caucasian | 3 (1) | 0 (0) | |
| Hispanic | 116 (56) | 23 (46) | |
| African American | 87 (42) | 27 (54) | |
| BMI, kg/m2 | 30 (±5) | 26 (±4) | .021 |
| Vaccination status | .002 | ||
| None | 195 (95) | 45 (90) | |
| One dose | 0 (0) | 3 (6) | |
| Two doses | 11 (5) | 2 (4) | |
| Smoking | .83 | ||
| Never | 152 (73) | 40 (80) | |
| Former | 17 (10) | 3 (7) | |
| Current | 8 (5) | 2 (4) | |
| Comorbidities | |||
| Hypertension | 143 (69) | 29 (58) | .12 |
| Diabetes mellitus | 111 (54) | 21 (42) | .13 |
| COPD | 18 (9) | 3 (6) | .53 |
| Asthma | 19 (9) | 2 (4) | .23 |
| CAD/CHF | 45 (22) | 9 (18) | .55 |
| Atrial fibrillation | 17 (8) | 0 (0) | .036 |
| TIA/stroke | 6 (3) | 4 (8) | .096 |
| Peripheral arterial disease | 5 (2) | 2 (4) | .54 |
| CKD | 58 (28) | 12 (24) | .55 |
| Active malignancy | 19 (9) | 2 (4) | .23 |
| Immunosuppression | 24 (12) | 7 (14) | .65 |
| Thrombophilia | 3 (1) | 1 (2) | .78 |
| Previous DVT/PE | 16 (8) | 8 (16) | .073 |
| Start of COVID symptoms, daysa | 3 (1-7) | 4 (1-7) | .39 |
BMI, Body mass index; CAD, coronary arterial disease; CHF, chronic heart failure; CKD, chronic kidney disease; COPD, chronic obstructive pulmonary disease; DVT, deep venous thrombosis; PE, pulmonary embolism; TIA, transient ischemic attack.
Data are presented as number (%), mean (standard deviation), or median (interquartile range).
Start of COVID symptoms in number of days prior to admission.
Thrombotic complications and outcomes in the study cohort
The most frequent acute thrombotic complication for the whole study cohort was VTE, found in 60% (n = 153), followed by arterial thrombosis in 23% (n = 60) and both venous and arterial thrombi combined in 17% (n = 43) of the patients. Our results show that 66% of the venous and 78% of the arterial thromboses were located in the lower extremities, whereas 20% and 13% were located in the upper extremities. The remaining events had thromboses in both upper and lower extremities simultaneously. The majority of the lower extremity VTE were located above the knee (79%) and the rest below the knee (14%), including soleus and gastrocnemius VTE, or both above and below the knee combined (7%). Other thrombotic complications were PE in 28% (n = 72), MI in 5% (n = 14), aortic thrombus in 2% (n = 4), ischemic stroke in 1% (n = 3), and visceral thrombus in 1% (n = 2). Only three patients of the 14 who presented with MI had previously documented coronary artery disease, and one of the three patients who had an ischemic stroke had medical history of TIA. A total of 90 patients (35%) had multiple thrombotic events simultaneously, including venous and arterial in different locations. Only 9% of all the patients (n = 24) had previous DVT/PE events (Table III ).
Table III.
Thrombotic events and procoagulant markers
| N = 256 | |
|---|---|
| Venous thrombosis | 153 (60%) |
| Upper extremity | 30 (20%) |
| Lower extremity | 101 (66%) |
| Upper and lower extremities | 13 (8%) |
| Neck and thoracic veins | 9 (6%) |
| Arterial thrombosis | 60 (23%) |
| Upper extremity | 8 (14%) |
| Lower extremity | 47 (78%) |
| Upper and lower extremity | 3 (5%) |
| Visceral thrombus | 2 (3%) |
| Arterial and venous thrombosis | 43 (17%) |
| Pulmonary embolism | 72 (28%) |
| Myocardial infarction | 14 (5%) |
| Aortic thrombus | 4 (2%) |
| Stroke | 3 (1%) |
| Multiple thrombotic locationsa | 91 (35%) |
| Time of thrombosis from admission, daysa | 4 [1-12] |
| Anticoagulation treatment | 228 (89%) |
| Start of anticoagulation, daysb | 2 [1-9] |
| DVT prophylaxis | 157 (61%) |
| Surgery | 30 (12%) |
| ICU | 195 (76%) |
| Deceased | 99 (39%) |
| D-dimer, μg/mL | 8 (3-20) |
| Fibrinogen, μg/mL | 383 (246-535) |
| PT, seconds | 15 (15-18) |
| aPTT, seconds | 38 (31-65) |
| Ferritin, μg/mL | 808 (398-1409) |
| Antiphospholipid antibody, GLP | 32 (1-35) |
DVT, Deep venous thrombosis; GLP, immunoglobulin G phospholipid units; ICU, intensive care unit; PT, prothrombin time; aPTT, partial thromboplastin time.
Data are presented as number (%), mean (standard deviation), or median (interquartile range).
Time of thrombosis detection in days since hospital admission.
Start of anticoagulation treatment in days since hospital admission.
The statistical analysis showed African American patients were more likely to have arterial thromboembolic events than other groups when testing positive for COVID-19 (odds ratio [OR], 1.78; 95% CI, 1.04-3.05; P = .035). Conversely, immunosuppressed patients with COVID-19 were less likely to have arterial thromboembolic complications (OR, 0.38; 95% CI, 0.15-0.96; P = .042). Also, female gender (OR, 2.15; 95% CI, 1.20-3.85; P = .009) and patients with active malignancy (OR, 5.99; 95% CI, 2.14-16.78; P = .001) were more likely to present multiple thrombi at different locations simultaneously due to COVID-19 infection. No other demographic variables or comorbidities were significantly associated with the thrombotic complications due to COVID-19.
The thrombotic events documented in these cases occurred in a median time of 4 days (IQR, 1-12 days) since hospital admission with a COVID-positive test (Table III). Almost one-half of the study cohort had the thrombotic event diagnosed at admission. A total of 157 patients (61%) received DVT prophylaxis before any thrombotic complication was detected. The medications most frequently used for this purpose were subcutaneous heparin (36.5%), followed by enoxaparin (28%), and occasionally fondaparinux (0.5%) (Supplementary Table, online only). The remaining patients without DVT prophylaxis were already on anticoagulation, started anticoagulation therapy at admission (n = 88), or had a contraindication for DVT prophylaxis due to high risk of bleeding (n = 11).
For anticoagulation strategies, 228 patients were started on anticoagulant medication in a median time of 2 days (IQR, 1-9 days) after the emergency room admission (Table III), most commonly with heparin drip (51.4%) or therapeutic enoxaparin (23.8%), as well as apixaban (10.1%). Other agents used were argatroban (2%), rivaroxaban (0.9%), and warfarin (0.9%). Most of the patients were either prescribed apixaban on discharge (35%) or continued care with enoxaparin (8%), heparin (5%), warfarin (3.8%), rivaroxaban (2%), or argatroban (0.9%). Additional treatment with aspirin was given in 70 patients (28.2%) (Supplementary Table, online only). None of the antithrombotic treatments mentioned above were statistically associated with the severity of the thrombotic complications (P = .32).
VTE was the most common type of thrombotic complication (82%) during the DW compared with the NDW (75%; P = .31). On the other hand, slightly higher rates of arterial thrombosis were seen during the NDW (36% vs 41%; P = .50). The thrombus location, surgical management, ICU stay, and mortality rates were similar across both groups. In addition, the DW group showed earlier anticoagulation treatment initiated with a median of 1 day (IQR, 1-5 days) since the admission (P = .22), with consequent smaller DVT prophylaxis rates (P = .057), compared with the NDW (Table IV ). However, none of the associations were significant.
Table IV.
Thrombotic events and procoagulant markers compared between patients who were Coronavirus disease 2019 (COVID-19) positive during Delta variant wave time (DW) and the rest of the cohort (non-Delta [NDW])
| NDW (n = 206) | DW (n = 50) | P-value | |
|---|---|---|---|
| Venous thrombosis | 155 (75) | 41 (82) | .31 |
| Arterial thrombosis | 85 (41) | 18 (36) | .50 |
| Thrombi location | |||
| Upper extremity | 43 (21) | 11 (22) | .86 |
| Lower extremity | 131 (63) | 33 (66) | .75 |
| Pulmonary embolism | 58 (28) | 14 (28) | .98 |
| Myocardial infarction | 12 (6) | 2 (4) | .61 |
| Aortic thrombi | 3 (1) | 1 (2) | .78 |
| Stroke | 3 (1) | 0 (0) | .39 |
| Multiple thrombotic locationsa | 74 (36) | 16 (32) | .60 |
| Time of thrombosis, daysa | 5 (1-11) | 2 (1-13) | .92 |
| Anticoagulation treatment | 185 (90) | 44 (88) | .71 |
| Start of anticoagulation, daysb | 2 (1-10) | 1 (1-5) | .22 |
| DVT prophylaxis | 134 (65) | 23 (46) | .057 |
| Surgery | 25 (12) | 5 (10) | .67 |
| ICU | 157 (76) | 38 (76) | .97 |
| Deceased | 78 (38) | 21 (42) | .61 |
| D-dimer, μg/mL | 9 (3-2) | 6 (3-13) | .16 |
| Fibrinogen, μg/mL | 384 (280-546) | 355 (150-518) | .29 |
| PT, seconds | 15 (15-17) | 16 (15-18) | .33 |
| aPTT, seconds | 37 (30 – 59) | 50 (35-87) | .008 |
| Ferritin, μg/mL | 794 (412-1365) | 861 (327-1892) | .72 |
DVT, Deep venous thrombosis; ICU, intensive care unit; PT, prothrombin time; aPTT, partial thromboplastin time.
Data are presented as number (%), mean (standard deviation), or median (interquartile range).
Time of thrombosis detection in days since hospital admission.
Start of anticoagulation treatment in days since hospital admission.
Data on prothrombotic markers from blood samples drawn nearest to the time thrombosis was suspected showed a median elevated d-dimer of 8 μg/mL (IQR, 3-20 μg/mL), fibrinogen of 383 μg/mL (IQR, 246-535 μg/mL), and ferritin of 808 μg/mL (IQR, 398-1409 μg/mL). The median coagulation parameters detected were prothrombin time of 15 seconds (IQR, 15-18 seconds) and activated partial thromboplastin time (aPTT) of 32 seconds (IQR, 1-35 seconds) (Table III). A linear regression showed significant correlation between the d-dimer value, measured in a total of 238 observations, and thrombosis in lower extremity (risk ratio [RR], 4.74; 95% CI, 1.96-7.53; P = .001), PE (RR, 5.63; 95% CI, 2.13-9.14; P = .002), as well as ICU stay (RR, 4.79; 95% CI, 2.47-7.13; P < .001).
Although there was no statistically significant difference in the levels of the prothrombotic markers between the DW and NDW groups, the coagulation time measurements showed a higher aPTT in the DW group compared with the NDW group (P = .008), with a median of 50 seconds (IQR, 35-87 seconds) and 37 seconds (IQR, 30-59 seconds), respectively (Table IV).
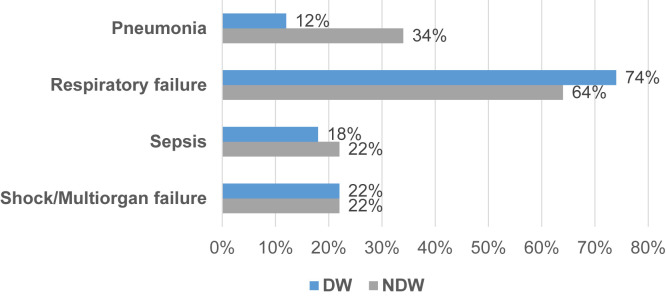
Regarding the treatment of the ischemic complications, 30 cases (12%) required management with one or more surgical interventions, such as 12 inferior vena cava filters placed, two percutaneous coronary interventions, six thrombolysis, eight thrombectomies, five embolectomies, one extremity bypass creation, and in the most severe cases, extremity amputation, which was done in two patients. Overall, 195 subjects (76%) required intensive care management, and 99 (39%) expired. Complications such as respiratory failure were more prevalent in DW than NDW (74% vs 64%). However, NDW had a higher proportion of patients with pneumonia (34% vs 12%). Similar rates of sepsis, shock, and multiorgan failure were seen in both groups (Fig 2 ). Other events such as encephalopathy, acute kidney injury, and transaminitis were seen in 30% (n = 76) of the study cohort.
Fig 2.
Complications secondary to Coronavirus disease 2019 (COVID-19) infection comparing Delta wave (DW) to non-Delta wave (NDW).
Discussion
The mechanisms responsible for the hypercoagulable state secondary to COVID-19 disease vary depending on the disease’s risk factors, presentation, severity, and progression.9 In our experience, we found that 26.5% of the patients admitted with a COVID-19-positive test presented with thrombotic events confirmed by US or CT angiography in a median of 4 days since the admission. This rate includes VTE, arterial thromboembolism in extremities, visceral thrombi, PE, MI, and ischemic stroke. Current literature reports on a VTE prevalence of 21% tp 63.3% and an initial cumulative incidence of 31%, specifically in critically ill patients7 , 15, 16, 17; however, the rate of all specific thrombotic complications related to COVID-19 has not been clearly established.
Our study population showed a rate of 20.3% for VTE, 7.5% for PE, and 10.6% for arterial thrombosis from the total COVID-19-positive admissions during the data collection time. A metanalysis by Malas et al, with 42 studies and 8271 patients enrolled, reported similar VTE rates (21%), higher PE rates (13%), and lower arterial thrombosis rates (2%) compared with our study.15 Other authors who described thromboembolic incidence in specific locations also showed higher PE rates (16.7%) in their population18 and lowered arterial thrombosis rates (4.4%), mainly in critical patients19 compared with our results.
The sample population of this study follows a similar trend in the distribution of demographic traits compared with the target community served by the participating health institutions. According to the 2019 United States Census, the population in Miami-Dade County is 51.4% female gender, with the following race/ethnicity identification: 69.4% Hispanic or Latino, 17.7% African American, and 12.9% Caucasian.20 In general, older Hispanic or African American male patients with slightly high BMI and multiple comorbidities, who are not vaccinated, were more prone to thrombotic complications secondary to COVID-19 infection.
The emergent concept of the VOC, the recent surge of COVID-19 cases in summer 2021, and the need for further information on the behavior of these new genotypes motivated a subgroup analysis in the patients admitted during the estimated time of the COVID-19 Delta variant spike. This variant was reported as more transmissible and prevalent in the Southeastern United States region due to the low vaccination levels,21 reinforcing the relevance of describing the outcomes secondary to this mutation.
Our patients infected during the DW were younger and had lower BMI than the NDW group. In terms of the thrombotic complications, there is a risk of thrombosis 1.36 times higher in patients infected with COVID-19 during the DW than the NDW. Crude rates showed higher overall thrombosis rates and VTE rates than NDW; however, there was no statistical significance when testing across both groups.
In general, older male patients with multiple comorbidities were more at risk of complications secondary to COVID-19 and consequent higher mortality risk.22 These risk factors are supported by a study that evaluated the influence of gender and demographic traits in the COVID-19 positive population in China.23 The subjects described in the present study had similar demographic characteristics; however, our statistical analysis showed a higher risk of multiple simultaneous thrombi in the female gender subgroup, implying a more severe and even fatal thrombotic pattern.
Other demographic variables such as race were equally associated with life-threatening outcomes. In this case, African American patients had higher arterial thrombotic complications with a significant statistical association. It should be noted that this association does not prove any direct causal link between African American race and specific thrombotic complications. The elevated risk is very likely to be explained by other social determinants of health, which can have profound effect on health outcomes and disproportionally affect patients of different race groups.24 , 25 Additional comorbidities such as active malignancy also contributed to the prothrombotic state in COVID-19, unlike smoking and previous DVT/PE, which were not statistically significant when adjusting for these covariates.
Elevated prothrombotic makers and coagulopathy have demonstrated the hypercoagulable state in patients with COVID-19.5 Some studies report a correlation between the D-dimer levels and the severity of the COVID-19 evolution.6 , 15 , 26 Our results show elevated median levels of D-dimer, among other markers such as fibrinogen and ferritin taken at the moment of thrombotic event detection. The D-dimer alone was found to correlate with the thrombotic outcomes, representing a higher risk of lower extremity thromboembolism and PE, disease severity, and patients requiring ICU care.
This study collected data from different time points throughout the pandemic, reflected in the various prophylactic and anticoagulant regimens given to these patients. Consequently, our results did not define a reduction of thrombotic events with different anticoagulation treatments recorded; however, the most common agents used were heparin and apixaban, which in most cases prevented a recurrent thrombotic event. We also found that the DW group was started on anticoagulation earlier, which likely explains the higher aPTT results. Moreover, 69% of the patients on early DVT prophylaxis still showed thrombotic complications despite adequate preventive measures. Previous literature also described this phenomenon27; it highlights the need to develop a standardized protocol for early antithrombotic regimens in patients with COVID-19.
The retrospective nature and single-centered design, which may reduce the generalizability, as well as the introduction of selection and reporting bias, are some of the limitations of this study. Although there were no defined protocols to guide screening for thromboses in patients with COVID-19, there may have been shifts in practice patterns during the course of the pandemic that our study cannot account for. It is also important to mention the variability in the approach of this viral infection due to the lack of evidence-based information available, which acts as an obstacle when defining risk factors and actions that have repercussions on the thrombotic outcomes. Despite these limitations, we performed a detailed description of our experience as a referral center for COVID-19 in the South Florida region,28 finding essential factors associated with the thrombotic complications and providing an estimate regarding the delta variant impact. However, we acknowledge the need for a larger prospective trial to evaluate the long-term anticoagulation effect on the well-known hypercoagulable disorder secondary to COVID-19 infection and validated management strategies to prevent thrombotic complications.
Conclusions
Concerning our experience as a referral center in the south Florida region, we can conclude that our patient population presents high rates of thrombotic complications secondary to COVID-19. We hope to highlight the importance of vaccination, given that the vast majority of the patients presenting with complications were not vaccinated. Patients admitted during the DW showed an especially elevated risk for thrombotic complications. A high level of suspicion as well as further investigation into an optimal anticoagulation strategy is needed to prevent thrombotic complications caused by this disease.
Author Contributions
Conception and design: KMP, CO, AB, JR
Analysis and interpretation: KMP, CO, AB, TS, NK, SKP, MT, OV, JR
Data collection: KMP, CO, ML
Writing the article: KMP, CO, AB, ML
Critical revision of the article: AB, TS, NK, SKP, MT, OV, JR
Final approval of the article: KMP, CO, AB, ML, TS, NK, SKP, MT, OV, JR
Statistical analysis: KMP, CO
Obtained funding: AB, OV, JR
Overall responsibility: JR
Acknowledgments
The authors thank David Andrews, MD, Associate Professor of Clinical, Department of Pathology, Division of Clinical and Translational Research, Leonard M. Miller School of Medicine, University of Miami, who provided the SARS-CoV-2 Delta variant progression chart.
Footnotes
Author conflict of interest: none.
The editors and reviewers of this article have no relevant financial relationships to disclose per the JVS policy that requires reviewers to decline review of any manuscript for which they may have a conflict of interest.
Additional material for this article may be found online at www.jvascsurg.org.
Additional material for this article may be found online at www.jvascsurg.org.
Appendix (online only).
Supplementary Table (online only).
Anticoagulation and deep vein thrombosis (DVT) prophylaxis management compared between variants
| NDW (n = 206) | DW (n = 50) | P-value | |
|---|---|---|---|
| Anticoagulation agent | |||
| Heparin drip | 106 (51.4) | 20 (40) | .32 |
| Enoxaparin | 49 (23.8) | 16 (32) | |
| Warfarin | 2 (0.9) | 1 (2) | |
| Rivaroxaban | 2 (0.9) | 1 (2) | |
| Apixaban | 21 (10.1) | 8 (16) | |
| DVT prophylaxis | |||
| Heparin SQ | 75 (36.5) | 12 (24) | .98 |
| Enoxaparin SQ | 58 (28) | 10 (20) | |
| Fondaparinux | 1 (0.4) | 1 (2) | |
| Aspirin | 54 (26) | 16 (32) | .38 |
| Discharge anticoagulation agenta | |||
| Heparin drip | 11 (5) | 1 (2) | .14 |
| Enoxaparin | 17 (8) | 8 (16) | |
| Warfarin | 8 (3) | 3 (6) | |
| Rivaroxaban | 5 (2) | 1 (2) | |
| Apixaban | 73 (35) | 14 (28) | |
| Argatroban | 2 (0.9) | 1 (2) |
DW, Delta wave; NDW, non-Delta wave; SQ, subcutaneous.
Data are presented as number (%).
Patients not reported on discharge anticoagulation expired, had the anticoagulant stopped due to drop in hemoglobin or documented bleeding, or were never placed on anticoagulant due to prior contraindications.
References
- 1.WHO Therapeutics Steering Committee . 1st ed. 31 March 2021. Therapeutics and COVID-19. Living Guideline. WHO/2019-nCoV/therapeutics/2021. World Health Organization. [Google Scholar]
- 2.Center of Disease Control and Prevention. COVID-19 Weekly Cases and Death per 100,000 Population by Age, Race/Ethnicity, and Sex COVID Data Tracker 2021. https://covid.cdc.gov/covid-data-tracker/#demographicsovertime Available from:
- 3.Centers for Disease Control and Prevention . COVID Data Tracker; 2021. Trends in number of COVID-19 cases and death in the US reported to CDC, by state/territory.https://covid.cdc.gov/covid-data-tracker/#datatracker-home [Google Scholar]
- 4.Liu Y., Rocklöv J. The reproductive number of the Delta variant of SARS-CoV-2 is far higher compared to the ancestral SARS-CoV-2 virus. J Trav Med. 2021;28:taab124. doi: 10.1093/jtm/taab124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Al-Samakri H., Karp Leaf R., Dzik W.H., Carlson J.C.T., Fogerty A., Waheed A., et al. COVID-19 and coagulation: bleeding and thrombotic manifestations of SARS-CoV-2 infection. Blood. 2020;136:489–500. doi: 10.1182/blood.2020006520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Miltiades A., Houck P., Monteleone M., Harrison N., Cabrera-Garcia D., Roh D., et al. Insight into fibrinogen-mediated COVID-19 hypercoagulability in critically ill patients. J Neurosurg Anesthesiol. 2022;34:136–140. doi: 10.1097/ANA.0000000000000812. [DOI] [PubMed] [Google Scholar]
- 7.Wichmann D., Sperhake J.-P., Lutgehetmann M., Steurer S., Elder C., Heinemann A., et al. Autopsy findings and venous thromboembolism in patients with COVID-19: a prospective cohort study. Ann Int Med. 2020;173:268–277. doi: 10.7326/M20-2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Iba T., Levy J.H., Connors J.M., Warkentin T., Thachil J., Levi M. The unique characteristics of COVID-189 coagulopathy. Crit Care. 2020;24:360. doi: 10.1186/s13054-020-03077-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Teuwen L.A., Geldhof V., Pasut A., Carmeliet P. COVID-19: the vasculature unleashed. Nat Rev Immunol. 2020;20:389–391. doi: 10.1038/s41577-020-0343-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Francischetti I., Toomer K., Zhang Y., Jani J., Siddiqui Z., Brotman D.J., et al. Upregulation of pulmonary tissue factor, loss of thrombomodulin and immunothrombosis in SARS-CoV-2 infection. EClinicalMedicine. 2021;39:101069. doi: 10.1016/j.eclinm.2021.101069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McIntosh K. COVID-19: clinical features. https://www.uptodate.com/contents/covid-19-clinical-features Available at:
- 12.Cuker A., Peyvandi F. COVID-19: hypercoagulability. https://www.uptodate.com/contents/covid-19-hypercoagulability Available at:
- 13.Higashikuni Y., Lui W., Obana T., Sata M. Pathogenic basis of thromboinflammation and endothelial injury in COVID-19: current findings and therapeutic implications. Int J Mol Sci. 2021;22:12081. doi: 10.3390/ijms222112081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shao T., In-Bok Lee C., Jabori S., Rey J., Duran E., Kang N. Acute upper limb ischemia as the first manifestation in a patient with COVID-19. J Vasc Surg Cases Innov Tech. 2020;6:674–677. doi: 10.1016/j.jvscit.2020.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Malas M.B., Naazie I.N., Elsayed N., Mathlouthi R.M., Clary B. Thromboembolism risk of COVID-19 is high and associated with a higher risk of mortality: a systematic review and meta-analysis. EClinicalMedicine. 2020;29:100639. doi: 10.1016/j.eclinm.2020.100639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marini C.P., Lewis E., Petrone P., Zenilman A., Lu Z., Rivera A., et al. Incidence and effects of deep vein thrombosis on the outcome of patients with Coronavirus disease 2019 infection. J Vasc Surg Venous Lymphat Disord. 2022;10:803–810. doi: 10.1016/j.jvsv.2021.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Klok F., Kruip M., Van der Meer N., Arbous M., Gommers D., Kant K., et al. Confirmation of the high cumulative incidence of thrombotic complications in critically ill ICU patients with COVID-19: an updated analysis. Thromb Res. 2020;191:148–150. doi: 10.1016/j.thromres.2020.04.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Helms J., Tacquard C., Severac F., Leonard-Lorant I., Ohana M., Delabranche X., et al. High risk of thrombosis in patients with severe SRAS-CoV-2 infection: a multicenter prospective cohort study. Intensive Care Med. 2020;46:1089–1098. doi: 10.1007/s00134-020-06062-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cheruiyot I., Kipkorir V., Ngure B., Misiani M., Munguti J., Ogeng'o J. Arterial thrombosis in Coronavirus disease 2019 patients: a systematic review. Ann Vasc Surg. 2021;70:273–281. doi: 10.1016/j.avsg.2020.08.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.United States Census Bureau QuickFacts. https://www.census.gov/quickfacts/fact/table/miamidadecountyflorida,browardcountyflorida,palmbeachcountyflorida,FL/PST045219 Miami-Dade County, Florida; 2019. Available from:
- 21.Chohan F., Ishak A., Alderette T., Rad P., Michel G. Clinical presentation of a COVID-19 Delta variant patient: case report. Cureus. 2021;13:e18603. doi: 10.7759/cureus.18603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lusczek E., Ingraham N., Karam B., Porper J., Siegel L., Helgenson E.S., et al. Characterizing COVID-19 clinical phenotypes and associated comorbidities and complications profiles. PLoS One. 2021;16:e0248956. doi: 10.1371/journal.pone.0248956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jin J.-M., Bai P., He W., Wu F., Liu X.-F., Han D.-M., et al. Gender differences in patients with COVID-19: focus on severity and mortality. Front Public Health. 2020;8:152. doi: 10.3389/fpubh.2020.00152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yancy C.W. COVID-19 and African Americans. JAMA. 2020;323:1891–1892. doi: 10.1001/jama.2020.6548. [DOI] [PubMed] [Google Scholar]
- 25.Ioannidis J.P.A., Powe N.R., Yancy C. Recalibrating the use of race in medical research. JAMA. 2021;325:623–624. doi: 10.1001/jama.2021.0003. [DOI] [PubMed] [Google Scholar]
- 26.Yu H.-H., Qin C., Chen M., Wang W., Tian D.-S. D-dimer level is associated with the severity of COVID-19. Thromb Res. 2020;195:219–225. doi: 10.1016/j.thromres.2020.07.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Oba S., Hosoya T., Amamiya M., Mitsumure T., Kawata D., Sasaki H., et al. Arterial and venous thrombosis complicated in COVID-19: a retrospective single center analysis in Japan. Front Cardiovasc Med. 2021;8:767074. doi: 10.3389/fcvm.2021.767074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Centers for Disease Control and Prevention . COVID Data Tracker; Miami-Dade County, Florida: 2021. COVID-19 Integrated County View. [Google Scholar]