Abstract
Background
External cephalic version (ECV) of the breech fetus at term (after 37 weeks) has been shown to be effective in reducing the number of breech presentations and caesarean sections, but the rates of success are relatively low. This review examines studies initiating ECV prior to term (before 37 weeks' gestation).
Objectives
To assess the effectiveness of a policy of beginning ECV before term (before 37 weeks' gestation) for breech presentation on fetal presentation at birth, method of delivery, and the rate of preterm birth, perinatal morbidity, stillbirth or neonatal mortality.
Search methods
We searched the Cochrane Pregnancy and Childbirth Group's Trials Register (31 March 2015) and reference lists of retrieved studies.
Selection criteria
Randomised controlled trials (RCTs) of ECV attempted before term (37 weeks' gestation) or commenced before term, compared with a control group of women (in breech presentation) in which either no ECV attempted or ECV was attempted at term. Cluster‐randomised trials were eligible for inclusion but none were identified. Quasi‐RCTs or studies using a cross‐over design were not eligible for inclusion.
Data collection and analysis
Two review authors independently assessed trials for inclusion and risk of bias, extracted data and checked for accuracy. Studies were assessed for risk of bias and for important outcomes the overall quality of the evidence was assessed using the GRADE approach.
Main results
Five studies are included (2187 women). It was not possible for the intervention to be blinded, and it is not clear what impact lack of blinding would have on the outcomes reported. For other 'Risk of bias' domains studies were either at low or unclear risk of bias.
One study reported on ECV that was undertaken and completed before 37 weeks' gestation compared with no ECV. No difference was found in the rate of non‐cephalic presentation at birth (risk ratio (RR) 1.04, 95% confidence interval (CI) 0.64 to 1.69; participants = 102). One study reported on a policy of ECV that was initiated before term (33 weeks) and up until 40 weeks' gestation and which could be repeated up until delivery compared with no ECV. This study showed a decrease in the rate of non‐cephalic presentation at birth (RR 0.59, 95% CI 0.45 to 0.77; participants = 179).
Three studies reported on ECV started at between 34 to 35 weeks' gestation compared with beginning at 37 to 38 weeks' gestation. Pooled results suggested that early ECV reduced the risk of non‐cephalic presentation at birth (RR 0.81, 95% CI 0.74 to 0.90; participants = 1906; studies = three; I² = 0%, evidence graded high quality), failure to achieve vaginal cephalic birth (RR 0.90, 95% CI 0.83 to 0.97; participants = 1888; studies = three; I² = 0%, evidence graded high quality), and vaginal breech delivery (RR 0.44, 95% CI 0.25 to 0.78; participants = 1888; studies = three; I² = 0%, evidence graded high quality). The difference between groups for risk of caesarean was not statistically significant (RR 0.92, 95% CI 0.85 to 1.00; participants = 1888; studies = three; I² = 0%, evidence graded high quality). There was evidence that risk of preterm labour was increased with early ECV compared with ECV after 37 weeks (6.6% in the ECV group and 4.3% for controls) (RR 1.51, 95% CI 1.03 to 2.21; participants = 1888; studies = three; I² = 0%, evidence graded high quality). There was no clear difference between groups for low infant Apgar score at five minutes or perinatal death (stillbirth plus neonatal mortality up to seven days) (evidence graded as low quality for both outcomes).
Authors' conclusions
Compared with no ECV attempt, ECV commenced before term reduces non‐cephalic presentation at birth. Compared with ECV at term, beginning ECV at between 34 to 35 weeks may have some benefit in terms of decreasing the rate of non‐cephalic presentation, and risk of vaginal breech birth. However, early ECV may increase risk of late preterm birth, and it is important that any future research reports infant morbidity outcomes. Results of the review suggest that there is a need for careful discussion with women about the timing of the ECV procedure so that they can make informed decisions.
Plain language summary
External cephalic version for breech presentation before term
Babies born bottom first (in the breech position) may have more problems during birth than those who are born head first (in the cephalic position) because there may be some delay in birth of the head and pressure on the umbilical cord as the head passes through the birth canal. During an external cephalic version (ECV) a breech baby is turned to the head down position by gently pushing on the mother's abdomen. Research shows that ECV after 37 weeks reduces the number of babies in the breech position at full term, and the number of caesarean sections.
This review included five randomised controlled studies with an overall total of 2187 women, the studies were at low or unclear risk of bias although it was not possible to "blind" women and staff to this intervention. Results showed that if ECV is done near the middle of the third trimester (32 to 34 weeks), it increases the chances that the baby will be lying head down at full term. Three trials including 1888 women found that beginning ECV at between 34 and 36 weeks compared with beginning ECV after 37 weeks (at term) had a 19% decrease in the rate of non‐cephalic presentation at birth, a 10% reduction in the risk of failing to achieve a cephalic vaginal birth, and a considerably reduced chance of a breech vaginal delivery, however, early ECV may significantly increase the chances of late preterm birth. The quality of the evidence for these outcomes was graded as high. The evidence on the possible advantages and disadvantages of early (before 37 weeks) external cephalic version (ECV) will require careful discussion with women about the timing of the ECV procedure so that they can make informed decisions.
Summary of findings
Summary of findings for the main comparison. External cephalic version (ECV) commenced before term versus ECV at term for breech presentation before term.
| External cephalic version (ECV) commenced before term versus ECV at term for breech presentation before term | ||||||
| Population: women with breech presentation before term Settings: 3 trials, 2 multicentre and 1 in Pakistan Intervention: external cephalic version (ECV) commenced before term Comparison: external cephalic version at term | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| External cephalic version at term | External cephalic version (ECV) commenced before term | |||||
| Non‐cephalic presentation at the birth | Study population | RR 0.81 (0.74 to 0.9) | 1906 (3 studies) | ⊕⊕⊕⊕ high | ||
| 523 per 1000 | 424 per 1000 (387 to 471) | |||||
| Moderate | ||||||
| 517 per 1000 | 419 per 1000 (383 to 465) | |||||
| Vaginal cephalic birth not achieved (caesarean section + vaginal breech birth) | Study population | RR 0.9 (0.83 to 0.97) | 1888 (3 studies) | ⊕⊕⊕⊕ high | ||
| 600 per 1000 | 540 per 1000 (498 to 582) | |||||
| Moderate | ||||||
| 633 per 1000 | 570 per 1000 (525 to 614) | |||||
| Caesarean section | Study population | RR 0.92 (0.85 to 1) | 1888 (3 studies) | ⊕⊕⊕⊕ high | ||
| 565 per 1000 | 519 per 1000 (480 to 565) | |||||
| Moderate | ||||||
| 560 per 1000 | 515 per 1000 (476 to 560) | |||||
| Vaginal breech birth | Study population | RR 0.44 (0.25 to 0.78) | 1888 (3 studies) | ⊕⊕⊕⊕ high | ||
| 35 per 1000 | 15 per 1000 (9 to 27) | |||||
| Moderate | ||||||
| 26 per 1000 | 11 per 1000 (6 to 20) | |||||
| Apgar score < 7 at 5 minutes | Study population | RR 1.16 (0.39 to 3.44) | 1759 (2 studies) | ⊕⊕⊝⊝ low1 | ||
| 7 per 1000 | 8 per 1000 (3 to 23) | |||||
| Moderate | ||||||
| 11 per 1000 | 13 per 1000 (4 to 38) | |||||
| Perinatal mortality (Stillbirth or neonatal mortality < 7 days) | Study population | RR 0.23 (0.04 to 1.34) | 1887 (3 studies) | ⊕⊕⊝⊝ low1 | ||
| 5 per 1000 | 1 per 1000 (0 to 7) | |||||
| Moderate | ||||||
| 9 per 1000 | 2 per 1000 (0 to 12) | |||||
| Preterm birth < 37 weeks | Study population | RR 1.51 (1.03 to 2.21) | 1888 (3 studies) | ⊕⊕⊕⊕ high | ||
| 43 per 1000 | 66 per 1000 (45 to 96) | |||||
| Moderate | ||||||
| 44 per 1000 | 66 per 1000 (45 to 97) | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Wide 95% CI crossing the line of no effect and low event rate.
Background
Description of the condition
By late pregnancy most babies in singleton pregnancies are positioned with their heads down ready for the birth; babies presenting in the breech position (bottom first) may be at increased risk during vaginal birth as there may be delay in birth of the head and compression of the umbilical cord as the head passes through the bony pelvis. About 3% to 4% of all pregnant women who reach full term will have a fetus presenting by the breech, and breech birth is associated as having a higher risk for the neonate regardless of mode of birth (Schutte 1985).
Breech presentation may be caused by an underlying fetal or maternal abnormality, or may be an apparently chance occurrence, or related to an otherwise benign variant such as cornual placental position. In the latter instances, breech presentation places a healthy fetus and mother at increased risk of a complicated vaginal delivery or caesarean section. It is not surprising that, over the years, the possibility of manipulating the baby from the breech to the cephalic presentation has intrigued obstetric caregivers.
Description of the intervention
During an external cephalic version (ECV) a breech baby is turned to the head down position by gently pushing on the mother's abdomen. ECV before term came into routine obstetric practice on the basis of the self‐evident immediate effectiveness of the procedure as well as reassuring results from several non‐randomised studies, and in spite of the negative results of the only controlled trial reported prior to 1980 (Brosset 1956). The popularity of ECV before term waned after the mid‐1970s, partly because of reports of a substantial perinatal mortality associated with the procedure (Bradley‐Watson 1975), and the increasing perception of caesarean section as a safer option than ECV or breech delivery.
How the intervention might work
For the singleton fetus in breech presentation, caesarean section has been shown to be safer for the fetus than vaginal birth (Hofmeyr 2003). Even though many women would prefer a vaginal birth (Gamble 2000; Geary 1997; Hildingsson 2002; Turnbull 1999), most would choose caesarean section if there is a medical indication, resulting in the majority of fetuses in breech presentation now being born by caesarean. The risks associated with caesarean section are low, but caesarean section is not without maternal risk and, in developed countries, remains the largest contributing factor to the incidence of maternal mortality and morbidity following childbirth (Liu 2007; Minkoff 2003). Estimates of the incidence of mortality associated with elective caesarean section were nearly tripled compared to vaginal birth (Cooper 2002; Hall 1999), and severe maternal morbidity has been shown to be increased five fold (Liu 2007). Among breech presenting fetuses, a Cochrane review of planned caesarean section versus planned vaginal delivery for breech pregnancy at term, reported that even though 45% of women in the planned vaginal delivery group were delivered by caesarean section, planned caesarean section was associated with an increase in maternal morbidity (risk ratio 1.29, 95% confidence interval 1.03 to 1.61) (Hofmeyr 2003). In addition to the increase in immediate morbidity following caesarean section, intra‐abdominal adhesions may occur after caesarean section resulting in subsequent infertility (LaSala 1987). The presence of the uterine scar puts future pregnancies at increased risk of complications such as ectopic pregnancy, placenta previa, accreta and abruptio, and uterine rupture (Dashe 2002; Gilliam 2002; Lydon‐Rochelle 2001; Minkoff 2003). A further deterrent to caesarean section is that the procedure requires the expertise of an obstetrician or other physician with surgical training, and limits the role for low‐risk obstetrical care providers such as midwives and family practitioners
A review of strategies to reduce caesarean section rates identified external cephalic version (ECV) as the only clinical intervention with demonstrated Level 1 evidence for reducing primary caesarean section rates overall (Walker 2002). ECV undertaken at term has been shown to be effective in moderately decreasing the rate of non‐cephalic presentation at birth and in avoiding caesarean section (Hofmeyr 2015).
It has been hypothesised that compared to waiting until term, beginning the ECV procedure somewhat earlier in pregnancy before the breech is engaged in the pelvis and while there are maximal levels of amniotic fluid present may further decrease the rate of non‐cephalic presentation at birth and promote cephalic vaginal birth (Hutton 2011b).
Why it is important to do this review
Prior to the mid‐1970s, ECV was usually attempted before term because of the belief that the procedure would seldom be successful at term. Subsequent studies showed that with the use of tocolysis, ECV could be achieved in a substantial proportion of women with breech presentation at term (Cluver 2015). ECV at term differs in many fundamental ways from that performed before term. These include the fact that the fetus is mature and may be delivered more readily in the event of complications, and that spontaneous version without ECV attempt, or reversion after successful ECV, may be less common at term. A Cochrane review of ECV at term (beginning at 37 weeks) reported an increased likelihood that the fetus will be cephalic at delivery, and reduced caesarean sections (Hofmeyr 2015). Thus ECV has been recommended for all women with a breech fetus at term, where there is no contraindication. However, the procedure is often unsuccessful, particularly in North American and European settings, (Hofmeyr 2015; Hutton 1999) and in a study comparing outcomes when ECV was begun earlier (34 to 35 weeks' gestation) compared to at term (after 37 weeks' gestation) reported a clinically important decrease in the proportion of women with non‐cephalic presentation at birth (Hutton 2011b).
Readers are referred to other reviews of the topic (Hofmeyr 1989; Hofmeyr 1991; Hofmeyr 1992; Hofmeyr 1993). See also related Cochrane reviews: 'External cephalic version for breech presentation at term' (Hofmeyr 2015); 'Interventions for helping to turn breech babies to head first presentation when using external cephalic version' (Cluver 2015); and, 'Cephalic version by postural management for breech presentation' (Hofmeyr 2012).
Objectives
To assess the effectiveness of a policy of beginning external cephalic version (ECV) before term for breech presentation on fetal presentation at birth, method of delivery, and the rate of preterm birth, perinatal morbidity, stillbirth and neonatal mortality, using the best available evidence.
Methods
Criteria for considering studies for this review
Types of studies
Randomised clinical trials, comparing the effects of external cephalic version (ECV) before term or commenced before term with a control group (no ECV attempt or ECV at term). Cluster‐randomised trials were eligible for inclusion but none were identified. Quasi‐RCTs or studies using a cross‐over design were not eligible for inclusion. We planned to include studies reported in abstract form provided sufficient information was provided to allow us to assess risk of bias.
Types of participants
Women with a live singleton fetus in breech presentation before term.
Types of interventions
External cephalic version attempt before term (37 weeks' gestation) or commenced before term, compared with a no ECV attempt or ECV at term. The comparisons fall into the following three categories.
ECV before term compared with no ECV.
A policy of initiating ECV before term but continuing if necessary up to term compared with no ECV.
A policy of beginning ECV before term compared with a policy of beginning ECV after 37 weeks.
Studies recruiting women both before and at term would be eligible for inclusion in comparison one provided results were reported separately for women in the preterm group.
Types of outcome measures
Outcomes were included if they were determined to be clinically meaningful, data were available for analysis according to original allocation, irrespective of protocol violations and data were available in format suitable for analysis. As part of the assessment of risk of bias we assessed whether reasonable measures were taken to minimise observer bias, and confirmed that missing data were insufficient to materially influence conclusions.
Primary outcomes
Rate of non‐cephalic presentation at birth
Vaginal cephalic birth not achieved (caesarean section plus vaginal breech delivery)
Method of delivery (caesarean section, breech vaginal birth, vaginal cephalic birth)
Secondary outcomes
Preterm birth
Perinatal outcomes including serious morbidity (trialist defined), stillbirth, neonatal mortality and perinatal mortality
Infant Apgar score < seven at five minutes
Search methods for identification of studies
Electronic searches
We searched the Cochrane Pregnancy and Childbirth Group’s Trials Register by contacting the Trials Search Co‐ordinator (31 March 2015).
The Cochrane Pregnancy and Childbirth Group’s Trials Register is maintained by the Trials Search Co‐ordinator and contains trials identified from:
monthly searches of the Cochrane Central Register of Controlled Trials (CENTRAL);
weekly searches of MEDLINE (Ovid);
weekly searches of Embase (Ovid);
monthly searches of CINAHL (EBSCO);
handsearches of 30 journals and the proceedings of major conferences;
weekly current awareness alerts for a further 44 journals plus monthly BioMed Central email alerts.
Details of the search strategies for CENTRAL, MEDLINE, Embase and CINAHL, the list of handsearched journals and conference proceedings, and the list of journals reviewed via the current awareness service can be found in the ‘Specialized Register’ section within the editorial information about the Cochrane Pregnancy and Childbirth Group.
Trials identified through the searching activities described above are each assigned to a review topic (or topics). The Trials Search Co‐ordinator searches the register for each review using the topic list rather than keywords.
[For details of additional searching carried out in the previous version of this review (Hutton 2006), see: Appendix 1.]
Searching other resources
We manually searched the reference lists of all retrieved articles and contacted expert in this research field.
We did not apply any language or date restrictions.
Data collection and analysis
For methods used in the previous version of this review, seeHutton 2006.
For this update, the following methods were used for assessing the reports that were identified as a result of the updated search (this section of the review is based on a standard template used by the Cochrane Pregnancy and Childbirth Group).
Selection of studies
Two review authors independently assessed for inclusion all the potential studies identified as a result of the search strategy. We resolved any disagreement through discussion or, if required, we consulted the third review author.
Data extraction and management
We designed a form to extract data. For eligible studies, two review authors extracted the data using the agreed form. We resolved discrepancies through discussion or, if required, we consulted the third review author. Data were entered into Review Manager software (RevMan 2014) and checked for accuracy.
When information regarding any of the above was unclear, we planned to contact authors of the original reports to provide further details.
Assessment of risk of bias in included studies
Two review authors independently assessed risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). Any disagreement was resolved by discussion or by involving a third assessor.
(1) Random sequence generation (checking for possible selection bias)
We described for each included study the method used to generate the allocation sequence in sufficient detail to allow an assessment of whether it should produce comparable groups.
We assessed the method as:
low risk of bias (any truly random process, e.g. random number table; computer random number generator);
high risk of bias (any non‐random process, e.g. odd or even date of birth; hospital or clinic record number);
unclear risk of bias.
(2) Allocation concealment (checking for possible selection bias)
We described for each included study the method used to conceal allocation to interventions prior to assignment and assessed whether intervention allocation could have been foreseen in advance of, or during recruitment, or changed after assignment.
We assessed the methods as:
low risk of bias (e.g. telephone or central randomisation; consecutively numbered sealed opaque envelopes);
high risk of bias (open random allocation; unsealed or non‐opaque envelopes, alternation; date of birth);
unclear risk of bias.
(3.1) Blinding of participants and personnel (checking for possible performance bias)
We described for each included study the methods used, if any, to blind study participants and personnel from knowledge of which intervention a participant received. We considered that studies were at low risk of bias if they were blinded, or if we judged that the lack of blinding unlikely to affect results. We assessed blinding separately for different outcomes or classes of outcomes.
We assessed the methods as:
low, high or unclear risk of bias for participants;
low, high or unclear risk of bias for personnel.
(3.2) Blinding of outcome assessment (checking for possible detection bias)
We described for each included study the methods used, if any, to blind outcome assessors from knowledge of which intervention a participant received. We assessed blinding separately for different outcomes or classes of outcomes.
We assessed methods used to blind outcome assessment as:
low, high or unclear risk of bias.
(4) Incomplete outcome data (checking for possible attrition bias due to the amount, nature and handling of incomplete outcome data)
We described for each included study the completeness of data including attrition and exclusions from the analysis. We stated whether attrition and exclusions were reported and the numbers included in the analysis at each stage (compared with the total randomised participants), reasons for attrition or exclusion where reported, and whether missing data were balanced across groups or were related to outcomes. Where sufficient information was reported, or could be supplied by the trial authors, we planned to re‐include missing data in the analyses which we undertook.
We assessed methods as:
low risk of bias (e.g. no missing outcome data; missing outcome data balanced across groups);
high risk of bias (e.g. numbers or reasons for missing data imbalanced across groups; ‘as treated’ analysis done with substantial departure of intervention received from that assigned at randomisation);
unclear risk of bias.
(5) Selective reporting (checking for reporting bias)
We described for each included study how we investigated the possibility of selective outcome reporting bias and what we found.
We assessed the methods as:
low risk of bias (where it is clear that all of the study’s pre‐specified outcomes and all expected outcomes of interest to the review have been reported);
high risk of bias (where not all the study’s pre‐specified outcomes have been reported; one or more reported primary outcomes were not pre‐specified; outcomes of interest are reported incompletely and so cannot be used; study fails to include results of a key outcome that would have been expected to have been reported);
unclear risk of bias.
(6) Other bias (checking for bias due to problems not covered by (1) to (5) above)
We described for each included study any important concerns we had about other possible sources of bias.
(7) Overall risk of bias
We made explicit judgements about whether studies were at high risk of bias, according to the criteria given in the Handbook (Higgins 2011). With reference to (1) to (6) above, we planned to assess the likely magnitude and direction of the bias and whether we considered it is likely to impact on the findings. In future updates, we will explore the impact of the level of bias through undertaking sensitivity analyses.
For this update the quality of the evidence has been assessed using the GRADE approach (Schunemann 2009) for the comparison ECV commenced before term versus ECV at term. This comparison was considered to be the most clinically relevant, as external cephalic version at term has been demonstrated to be effective in reducing the chance of non‐cephalic presentation at birth and caesarean section and should be regarded as the standard of care (Hofmeyr 2015). Comparisons of early ECV with no ECV are now mainly of historical interest. The quality of the evidence was assessed for the following outcomes.
Rate of non‐cephalic presentation at birth.
Vaginal cephalic birth not achieved (caesarean section plus vaginal breech delivery).
Caesarean birth.
Breech vaginal birth.
Preterm birth.
Perinatal mortality (stillbirth plus neonatal death up to seven days).
Apgar score less than seven at five minutes.
GRADE profiler (GRADEpro 2014) was used to import data from Review Manager 5.3 (RevMan 2014) in order to create a ’Summary of findings’ table. A summary of the intervention effect and a measure of quality for each of the above outcomes was produced using the GRADE approach. The GRADE approach uses five considerations (study limitations, consistency of effect, imprecision, indirectness and publication bias) to assess the quality of the body of evidence for each outcome. The evidence can be downgraded from 'high quality' by one level for serious (or by two levels for very serious) limitations, depending on assessments for risk of bias, indirectness of evidence, serious inconsistency, imprecision of effect estimates or potential publication bias.
Measures of treatment effect
Dichotomous data
For dichotomous data, we presented results as summary risk ratio with 95% confidence intervals.
Continuous data
We used the mean difference if outcomes were measured in the same way between trials. We planned to use the standardised mean difference to combine trials that measured the same outcome, but used different methods.
Unit of analysis issues
Cluster‐randomised trials
We planned to include cluster‐randomised trials in the analyses along with individually‐randomised trials if they were otherwise eligible. In this version of the review no such trials were identified. If cluster trials are eligible for future updates we will adjust their sample sizes using the methods described in the Handbook using an estimate of the intracluster correlation co‐efficient (ICC) derived from the trial (if possible), from a similar trial or from a study of a similar population. If we use ICCs from other sources, we will report this and conduct sensitivity analyses to investigate the effect of variation in the ICC. If we identify both cluster‐randomised trials and individually‐randomised trials, we plan to synthesise the relevant information. We will consider it reasonable to combine the results from both if there is little heterogeneity between the study designs and the interaction between the effect of intervention and the choice of randomisation unit is considered to be unlikely.
We will also acknowledge heterogeneity in the randomisation unit and perform a sensitivity analysis to investigate the effects of the randomisation unit.
Cross‐over trials
Cross‐over trials have not been included.
Studies with multiple treatment arms
In this version of the review we have not included any trials with more than two treatment arms; if such trials are included in updates we will use the methods described in the Handbook to analyse findings.
Dealing with missing data
For included studies, we noted levels of attrition. In future updates, if more eligible studies are included, we will explore the impact of including studies with high levels of missing data in the overall assessment of treatment effect by using sensitivity analysis.
For all outcomes, analyses were carried out, as far as possible, on an intention‐to‐treat basis i.e. we attempted to include all participants randomised to each group in the analyses. The denominator for each outcome in each trial was the number randomised minus any participants whose outcomes were known to be missing.
Assessment of heterogeneity
We assessed statistical heterogeneity in each meta‐analysis using the Tau², I² and Chi² statistics. We regarded heterogeneity as substantial if an I² was greater than 30% and either the Tau² was greater than zero, or there was a low P value (less than 0.10) in the Chi² test for heterogeneity. Had we identified substantial heterogeneity (above 30%), we planned to explore it by pre‐specified subgroup analysis.
Assessment of reporting biases
In future updates, if there are 10 or more studies in the meta‐analysis we will investigate reporting biases (such as publication bias) using funnel plots. We will assess funnel plot asymmetry visually. If asymmetry is suggested by a visual assessment, we will perform exploratory analyses to investigate it.
Data synthesis
We carried out statistical analysis using the Review Manager software (RevMan 2014). We used fixed‐effect meta‐analysis for combining data where it was reasonable to assume that studies were estimating the same underlying treatment effect: i.e. where trials were examining the same intervention, and the trials’ populations and methods were judged sufficiently similar.
If there was clinical heterogeneity sufficient to expect that the underlying treatment effects differed between trials, or if substantial statistical heterogeneity was detected, we planned to use random‐effects meta‐analysis to produce an overall summary, if an average treatment effect across trials was considered clinically meaningful. If we use random‐effects analyses in updates, the random‐effects summary will be treated as the average range of possible treatment effects and we will discuss the clinical implications of treatment effects differing between trials. If the average treatment effect is not clinically meaningful, we will not combine trials. If we use random‐effects analyses, the results will be presented as the average treatment effect with 95% confidence intervals, and the estimates of Tau² and I².
Subgroup analysis and investigation of heterogeneity
Had we identified substantial heterogeneity, we planned to investigate it using subgroup analyses. We planned subgroup analysis for:
nulliparous versus multiparous women (as nulliparity is a well established to be associated with decreased likelihood of success of ECV);
type of breech (frank breech, where the fetus has hips flexed and legs extended making the ECV more difficult versus non‐frank);
use of tocolytics versus no tocolytics, (as tocolytics have been shown to increase the likelihood of success in ECV at term, and variation in use may explain heterogeneity between trials);
gestational age at randomisation ( 33 weeks 0 days to 34 weeks 6 days; and 35 weeks 0 days to 36 weeks 6 days).
We planned subgroup analysis for primary outcomes only. In this version of the review the study samples however were insufficient to make this analysis meaningful. We will carry out planned subgroup analysis if more data become available in future updates. We will assess subgroup differences by interaction tests available in RevMan (RevMan 2014). We will report the results of subgroup analyses quoting the Chi² statistic and P value, and the interaction test I² value.
Sensitivity analysis
We planned to carry out sensitivity analyses to explore the effect of trial quality assessed by concealment of allocation, high attrition rates, or both, with poor quality studies being excluded from the analyses in order to assess whether this would make any difference to the overall result. In this version of the review too few studies were included to allow these additional analyses.
Results
Description of studies
Results of the search
In the previous published version of this review, three studies were included (Hutton 2003; Mensink 1980; Van Veelen 1989). In this update, two further studies were identified and were assessed as eligible for inclusion (Akhtar 2013; Hutton 2011). SeeCharacteristics of included studies.
In this version of the review, altogether, five studies were excluded (Brosset 1956; Dafallah 2004; El‐Muzaini 2008; Kasule 1985; Rust 2005) and one is still ongoing (Belizan 1989).Two controlled trials which had been included in an earlier version of this review were subsequently excluded for concerns relating to methodological soundness (Brosset 1956; Kasule 1985). Neither of these trials used random assignment to treatment groups. The Brosset 1956 study states that "cases were divided into two groups" while in the Kasule 1985 trial women were "allocated to a version or non‐version group depending on the day they attended antenatal clinic".
Included studies
Comparison one: ECV before term (from 32 weeks with one repeat attempt) compared with no ECV
Mensink 1980 included women in early third trimester (as early as 32 weeks' gestation) in a randomised controlled trial undertaken in Gronigen, The Netherlands. Allocation was undertaken using randomised sealed envelopes, stratified by parity. Breech was verified by ultrasound. Women with a singleton breech presentation before term (from 32 weeks) were included. Women with any contraindication to external version were excluded. External cephalic version (ECV) was attempted without tocolysis by an assistant in training (n = 50) compared with no ECV attempt (n = 52). If the ECV failed, a further attempt was made by an obstetrician one week later. Outcomes included: non‐cephalic births; caesarean section; one minute Apgar score less than seven; umbilical vein pH less than 7.2; neurological deficit in newborn; and perinatal mortality; neonatal morbidity at the time of delivery was reported, but this was not defined.
Comparison two: ECV commencing before term (33 to 40 weeks with repeated attempts) compared with no ECV
Van Veelen 1989 enrolled 180 healthy white Dutch women with uncomplicated pregnancy at 33 to 40 weeks' gestation and a live singleton breech fetus attending antenatal clinic of Ikazia Hospital, Rotterdam, The Netherlands. Random allocation of women used sealed envelopes, and was stratified by parity. Repeated ECV was performed between 33 and 40 weeks' gestation up to four times with no tocolysis, analgesia or anaesthesia compared with no ECV. The outcomes included: presentation at delivery; mode of delivery; neonatal outcome including perinatal death.
Comparison three: ECV commencing before term compared with ECV commencing after term (37 weeks' gestation)
In this update three studies are now included in this comparison (Akhtar 2013; Hutton 2003; Hutton 2011).
Hutton 2003 is an international multicentre randomised controlled trial (n = 233). All nulliparous women with any breech presentation and multiparous women with a frank breech presentation were eligible for the trial if they had a live singleton fetus and a gestational age of between 34 weeks, 0 days and 36 weeks 0 days. Women were excluded if they had a parity greater than four, if they planned to move to a non‐trial centre, or if there was any contraindication to labour or vaginal birth (such as placenta previa, or previous classical caesarean section), to ECV (such as fetal heart rate abnormalities, abruptio placenta, fetal anomalies, uterine anomalies, oligohydramnios, rupture of membranes, over distended uterus) or to early ECV (such as fetus engaged in the pelvis, an increased risk of preterm labour, increased risk of abruptio placenta). ECV was begun between 34 weeks 0 days and 36 weeks 0 days in the early group (n = 117); and between 37 weeks 0 days and 38 weeks 0 days in the delayed group (n = 116). Tocolysis was recommended to be used either routinely or selectively in both groups; analgesia was permitted. The primary outcome was presentation at delivery; other outcomes included: caesarean section; serious fetal complication; preterm birth less than 37 weeks; women's views about ECV. The study was funded by Canadian Institutes of Health Research.
The Hutton 2011 study included 68 centres in 21 countries with ECV carried out by clinicians who were experienced in the procedure and with birth facilities that were deemed to meet Canadian standards. One‐thousand, five‐hundred and forty‐three women were randomised. The study recruited women with a singleton fetus in a breech presentation, between gestation ages of 33 weeks 0 days and 35 weeks six days. Women with contraindications to ECV (e.g. fetal heart rate abnormalities, placental abruption, major life‐threatening fetal anomalies, uterine anomalies, hyper‐extended fetal head, rupture of fetal membranes, severe oligohydramnios or hydramnios); contraindications to early ECV (e.g. increased risk of preterm labour or placental abruption); or contraindications to labour or vaginal birth (e.g. placenta praevia, previous classical caesarean section); or if they had been prior participants in the trial; were at increased risk of unstable lie (such as grand multiparity); or if they planned to give birth by caesarean section even if the fetus turned to a cephalic position, or if they planned a vaginal birth if the fetus remained breech were excluded. In the early ECV group (n = 767), ECV carried out between 34 weeks 0 days and 35 weeks six days gestation, and within seven days of randomisation. In the delayed ECV group (n = 774) ECV carried out at or after 37 weeks' gestation. In both groups fetal presentation was confirmed by ultrasound, fetal heart rate was monitored before, during and after the procedure. The use of tocolytics and analgesia was left to the discretion of the clinician, and they were directed to use the same approach for women in both arms of the trial. If the procedure was unsuccessful, or if a fetus reverted to non‐cephalic, a repeat ECV procedure could be performed at a later date at the discretion of the care provider in consultation with the woman.
The study by Akhtar 2013 is a single‐centre, parallel‐group randomised controlled trial carried out in a hospital in Pakistan. The study included women with a singleton fetus with breech presentation between 33 and 35 weeks' gestation (n = 123 women). Women with contraindications to ECV, contraindications to early ECV or contraindications to labour or vaginal birth (e.g. fetal heart rate abnormalities, vaginal bleeding, rupture of membranes, placental abruption, fetal growth restriction, previous CS, low amniotic fluid index, fetal weight greater than 4 kg) or women unwilling to undergo ECV were excluded. In the early ECV group, ECV was carried out between 34 (238 days) and 35 weeks of gestation (n = 63). In the delayed ECV group ECV was carried out at or after 37 weeks. No tocolytics were used in either group and women were monitored for three hours before and one hour after the procedure. Up to two‐three attempts were allowed. The procedure was discontinued if there was excessive maternal discomfort or fetal heart rate irregularities (n = 60).
Excluded studies
Five studies were excluded. Two studies were excluded for methodological reasons; it was not clear in the study by Brosset 1956 that allocation to groups was random and in the Kasule 1985 study allocation was by day of the week. Both of these studies are at high risk of selection bias. The remaining studies (Dafallah 2004; El‐Muzaini 2008; Rust 2005) were excluded because the intervention group mainly included women recruited at term and separate results were not available for women with preterm pregnancies. See Characteristics of excluded studies.
Ongoing studies
We have limited information on the study by Belizan 1989; it is not clear whether this study was completed, more information is set out in Characteristics of ongoing studies.
Risk of bias in included studies
See table Characteristics of included studies.
Allocation
Hutton 2003 and Hutton 2011 used a centralised telephone randomisation service and these studies were assessed as low risk of bias for sequence generation and allocation concealment. The remaining studies did not fully describe the methods used for generating the randomisation sequence. Mensink 1980 and Van Veelen 1989 used randomised, sealed envelopes to conceal allocation (it was not clear whether or not envelopes were opaque and sequentially numbered). Akhtar 2013 reported using the same methods as those used in the Hutton 2003 and Hutton 2011 trials but no further information was provided. All studies were stratified for parity at randomisation.
Blinding
Blinding women and care providers is not feasible for the intervention under study. It was not clear whether there was any attempt to achieve observer blinding in the collection of the outcome data in any of the studies. Although lack of blinding would not be likely to effect outcomes such as presentation at delivery, it is not clear whether lack of blinding had an impact on some of the other outcomes reported such as caesarean section.
Incomplete outcome data
All included studies were assessed to be at low risk of bias for this domain. There were no losses to follow‐up in Akhtar 2013, Mensink 1980 or Van Veelen 1989. Hutton 2003 reported one loss to follow‐up in the early ECV group following randomisation but prior to any ECV procedure being done. Hutton 2011 included more than 99% of women randomised in the analysis. All used an intention‐to‐treat approach to analyses.
Selective reporting
In the two multicentre trials (Hutton 2003; Hutton 2011) study protocols were available and there did not appear to have been any outcome reporting bias. In the remaining studies assessment of bias was from published reports and it was not clear whether all outcome data were reported.
Other potential sources of bias
In the Akhtar 2013 trial it was reported that methods and allocation was "in accordance with the two major multicenter trials conducted on the same subject" (Hutton 2003; Hutton 2011). There was no further description of methods used. We contacted the author for more information but have not yet had a response (September 2014).
See Figure 1 and Figure 2 for a summary of findings for risk of bias.
1.
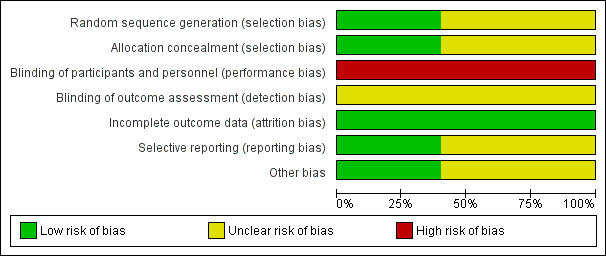
'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
2.
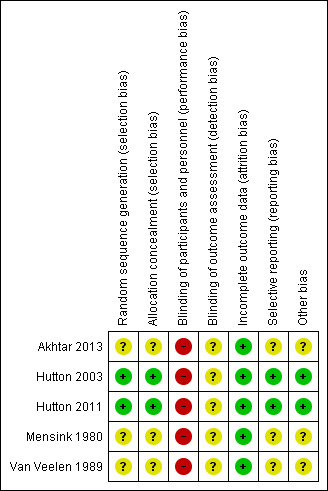
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
Effects of interventions
See: Table 1
Comparison one: ECV attempt before term (with one repeat attempt) compared with no ECV: one trial involving 102 women (Mensink 1980)
Primary outcomes
The rate of non‐cephalic presentation at birth in the ECV group was 40% and in the no ECV group was 39% (risk ratio (RR) 1.04, 95% confidence interval (CI) 0.64 to 1.69) Analysis 1.1. (The trial authors ascribe the low success rate to the gentleness with which ECV was attempted.) There was no clear difference between groups for failure to achieve vaginal cephalic birth (caesarean section plus breech vaginal birth) (RR 1.04, 95% CI 0.67 to 1.62) Analysis 1.2. (The rate of caesarean section was 14% in the ECV group and 8% in the no ECV group (RR 1.82, 95% CI 0.57 to 5.84) Analysis 1.3. The number of women undergoing vaginal breech delivery was comparable in the two groups (RR 0.87, 95% CI 0.49 to 1.52) Analysis 1.4.
1.1. Analysis.
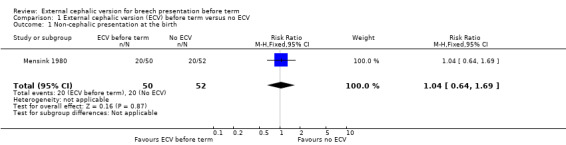
Comparison 1 External cephalic version (ECV) before term versus no ECV, Outcome 1 Non‐cephalic presentation at the birth.
1.2. Analysis.
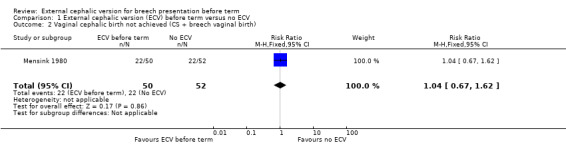
Comparison 1 External cephalic version (ECV) before term versus no ECV, Outcome 2 Vaginal cephalic birth not achieved (CS + breech vaginal birth).
1.3. Analysis.
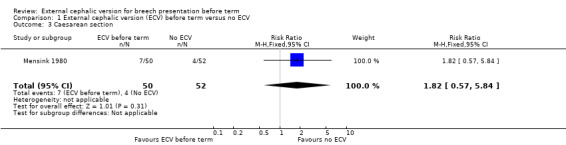
Comparison 1 External cephalic version (ECV) before term versus no ECV, Outcome 3 Caesarean section.
1.4. Analysis.
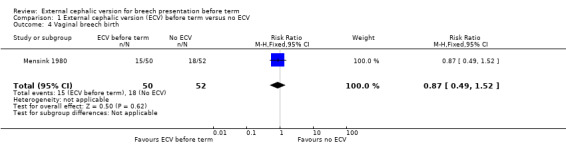
Comparison 1 External cephalic version (ECV) before term versus no ECV, Outcome 4 Vaginal breech birth.
Secondary outcomes
There was no clear evidence of differences between groups for other outcomes.
The rate of one minute Apgar scores less than seven (RR 0.62, 95% CI 0.25 to 1.59) Analysis 1.5.
The rate of stillbirth or neonatal mortality less than seven days (RR 0.35, 95% CI 0.04 to 3.22) Analysis 1.6.
1.5. Analysis.
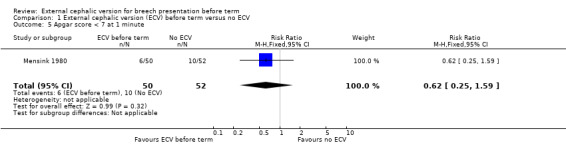
Comparison 1 External cephalic version (ECV) before term versus no ECV, Outcome 5 Apgar score < 7 at 1 minute.
1.6. Analysis.
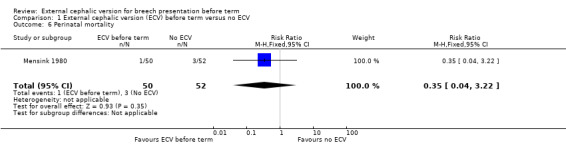
Comparison 1 External cephalic version (ECV) before term versus no ECV, Outcome 6 Perinatal mortality.
Rates of premature delivery were similar in the two groups (two premature deliveries in the intervention group and three in the control group). "Perinatal morbidity" at the time of delivery was also reported although this was not defined (with one event in the intervention group and three in the control group).
Comparison two: ECV commencing before term compared with no ECV (repeated attempts): one trial involving 179 women (Van Veelen 1989)
Primary outcomes
The ECV group had 44% non‐cephalic presentation at birth compared to 74% in the no ECV group (RR 0.59, 95% CI 0.45 to 0.77); this difference between groups was statistically significant Analysis 2.1 . Women in the ECV group were at reduced risk of failing to achieve cephalic vaginal birth (RR 0.62, 95% CI 0.49 to 0.80) Analysis 2.2. The rate of caesarean section delivery was 11% in the ECV group compared to 14% in the no ECV group (RR 0.62, 95% CI 0.27 to 1.43) Analysis 2.3. The frequency of vaginal breech delivery was reduced in the ECV group (RR 0.63, 95% CI 0.46 to 0.85) Analysis 2.4.
2.1. Analysis.
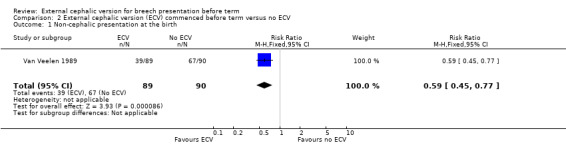
Comparison 2 External cephalic version (ECV) commenced before term versus no ECV, Outcome 1 Non‐cephalic presentation at the birth.
2.2. Analysis.
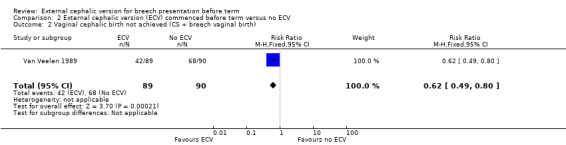
Comparison 2 External cephalic version (ECV) commenced before term versus no ECV, Outcome 2 Vaginal cephalic birth not achieved (CS + breech vaginal birth).
2.3. Analysis.
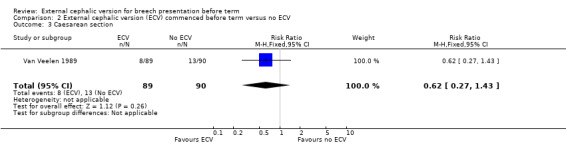
Comparison 2 External cephalic version (ECV) commenced before term versus no ECV, Outcome 3 Caesarean section.
2.4. Analysis.
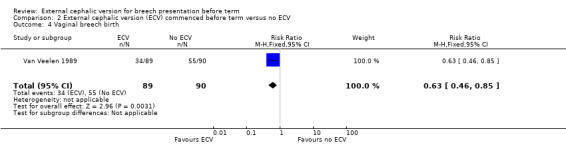
Comparison 2 External cephalic version (ECV) commenced before term versus no ECV, Outcome 4 Vaginal breech birth.
Secondary outcomes
There was insufficient information on other outcomes.
The rate of five minute Apgar scores less than seven (one event in the intervention group) Analysis 2.5.
The rate of stillbirth or neonatal mortality less than seven days (one event in the control group) Analysis 2.6.
2.5. Analysis.
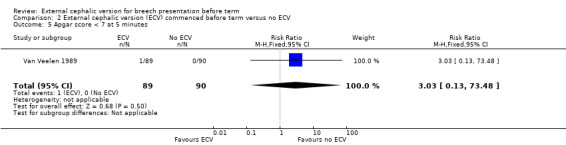
Comparison 2 External cephalic version (ECV) commenced before term versus no ECV, Outcome 5 Apgar score < 7 at 5 minutes.
2.6. Analysis.
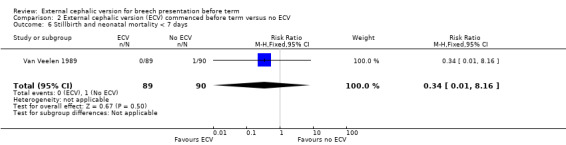
Comparison 2 External cephalic version (ECV) commenced before term versus no ECV, Outcome 6 Stillbirth and neonatal mortality < 7 days.
The authors reported no "major complications" in either group.
Comparison three: ECV commencing before term compared with ECV commencing after term (37 weeks' gestation): three trials involving 1906 women (Akhtar 2013; Hutton 2003; Hutton 2011)
Primary outcomes
The rate of non‐cephalic presentation at birth was lower when ECV was started before term (RR 0.81, 95% CI 0.74 to 0.90; participants = 1906; studies = three; I2 = 0%, evidence graded high quality) Analysis 3.1. Women who had early ECV were at slightly less risk of failing to achieve a cephalic vaginal birth (RR 0.90, 95% CI 0.83 to 0.97; participants = 1888; studies = three; I2 = 0%, evidence graded high quality) Analysis 3.2. The rate of caesarean section was reduced when ECV was started before 37 weeks' gestation although the difference between groups did not reach statistic significance (RR 0.92, 95% CI 0.85 to 1.00; participants = 1888; studies = three; I2 = 0%, evidence graded high quality) Analysis 3.3. Women who were randomised to early ECV were at a considerably reduced risk of having a vaginal breech birth; the difference between groups for this outcome was statistically significant (RR 0.44, 95% CI 0.25 to 0.78; participants = 1888; studies = three; I2 = 0%, evidence graded high quality) Analysis 3.4.
3.1. Analysis.
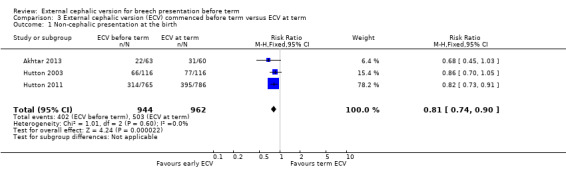
Comparison 3 External cephalic version (ECV) commenced before term versus ECV at term, Outcome 1 Non‐cephalic presentation at the birth.
3.2. Analysis.
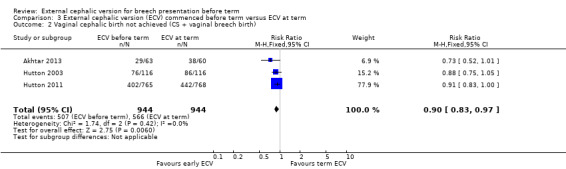
Comparison 3 External cephalic version (ECV) commenced before term versus ECV at term, Outcome 2 Vaginal cephalic birth not achieved (CS + vaginal breech birth).
3.3. Analysis.
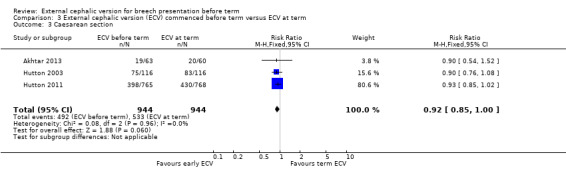
Comparison 3 External cephalic version (ECV) commenced before term versus ECV at term, Outcome 3 Caesarean section.
3.4. Analysis.
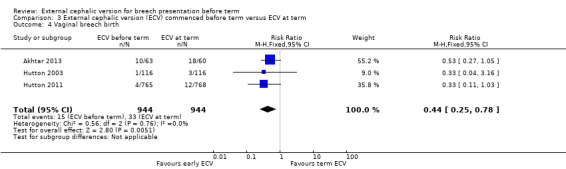
Comparison 3 External cephalic version (ECV) commenced before term versus ECV at term, Outcome 4 Vaginal breech birth.
Secondary outcomes
The rate of preterm birth less than 37 weeks was increased in the early ECV group (RR 1.51, 95% CI 1.03 to 2.21; participants = 1888; studies = three; I2 = 0%, evidence graded high quality) Analysis 3.7.
3.7. Analysis.
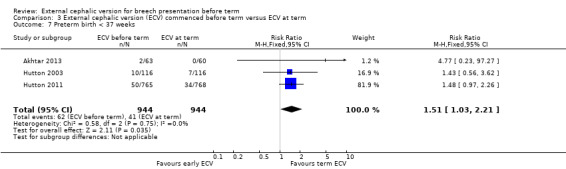
Comparison 3 External cephalic version (ECV) commenced before term versus ECV at term, Outcome 7 Preterm birth < 37 weeks.
There was no strong evidence of differences between groups identified for:
the rate of five minute Apgar scores less than seven (RR 1.16, 95% CI 0.39 to 3.44; participants = 1759; studies = two; I2 = 0%, evidence graded low quality due to imprecision) Analysis 3.5;
the rate of stillbirth or neonatal mortality less than seven days (RR 0.23, 95% CI 0.04 to 1.34; participants = 1887; studies = three; I2 = 0%, evidence graded low quality due to imprecision) Analysis 3.6;
The studies by Hutton 2003 and Hutton 2011 reported several outcomes relating to neonatal outcome but these were not mutually exclusive and so a single composite outcome was reported: one or more serious fetal complication (RR 0.87, 95% CI 0.42 to 1.79; participants = 1761; studies = two; I2 = 0%) Analysis 3.8.
There no clear difference between groups for NICU stay for four days or longer (RR 2.50, 95% CI 0.49 to 12.63; participants = 232; studies = 1) Analysis 3.9.
3.5. Analysis.
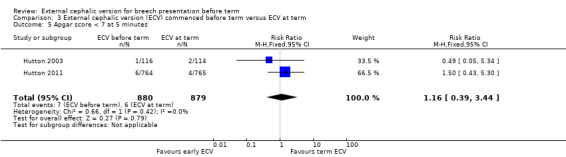
Comparison 3 External cephalic version (ECV) commenced before term versus ECV at term, Outcome 5 Apgar score < 7 at 5 minutes.
3.6. Analysis.
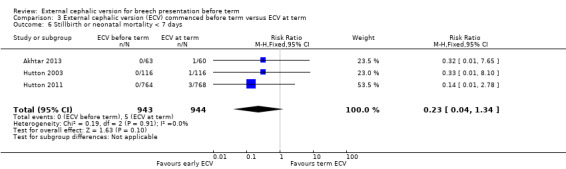
Comparison 3 External cephalic version (ECV) commenced before term versus ECV at term, Outcome 6 Stillbirth or neonatal mortality < 7 days.
3.8. Analysis.
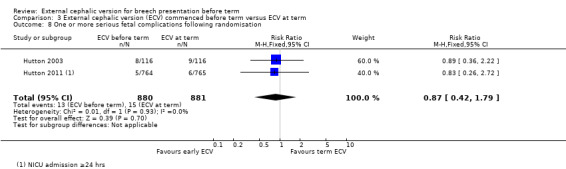
Comparison 3 External cephalic version (ECV) commenced before term versus ECV at term, Outcome 8 One or more serious fetal complications following randomisation.
3.9. Analysis.
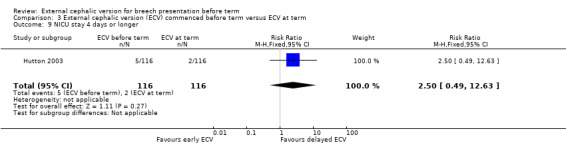
Comparison 3 External cephalic version (ECV) commenced before term versus ECV at term, Outcome 9 NICU stay 4 days or longer.
Non‐prespecified outcome
One study reported maternal pain scores following the ECV attempt; pain scores were lower when ECV was commenced before term (mean difference (MD) ‐4.60, 95% CI ‐7.74 to ‐1.46; participants = 1533).
SeeTable 1.
Discussion
Summary of main results
We have good evidence to support external cephalic version (ECV) beginning at term, that is after 37 weeks' gestation. A Cochrane review of ECV concluded that ECV is a useful manoeuvre to decrease both the rate of non‐cephalic presentation and caesarean section when it is begun after 37 weeks' gestation (Hofmeyr 2015), and the major obstetrical societies recommend that ECV be offered to low‐risk women with singleton breech pregnancies. Of the studies of ECV at term, those undertaken in European or American centres report a relatively low rate of success with ECV and a remarkably higher rate of non‐cephalic presentation at birth compared to the African trials. It is possible that there is a difference in the population characteristics. In a cohort study Hofmeyr 1986 reported higher rates of success with the ECV procedure in a group of African women compared to Caucasian women.
The studies of ECV before term are less straightforward.The Mensink 1980 trial which compared ECV prior to term with no ECV, undertook the procedure at an early stage in pregnancy (32 weeks' gestation), when the rates of spontaneous version remain high. Despite the findings from this early study of ECV before term which clearly showed no difference between the ECV and no ECV group, the more recent trials suggest that there may be benefit to beginning ECV prior to, but near term, particularly amongst those populations where success rates at term are low. The Van Veelen 1989 study beginning ECV as early as 33 weeks (but up to 40 weeks, with a mean of gestational age at ECV of 35 weeks) compared with no ECV showed a 30% decrease in the rate on non‐cephalic presentation. This trial showed that women in the ECV group were at reduced risk of failing to achieve cephalic vaginal birth, even though no difference was found in the rate of caesarean section. This is likely due to the higher proportion of women planning a vaginal breech birth when the fetus remained breech at term, as the study was undertaken prior to publication of findings from the Term Breech Trial (Hannah 2000), and a policy of vaginal breech delivery is evident. The study was too small to meaningfully rule out differences in Apgar scores less than seven at five minutes or in stillbirth or neonatal mortality less than seven days. In the Van Veelen 1989 study, the mean time of beginning ECV was 35 weeks' gestation, and it is unclear if the benefit that was found could be attributed to beginning the procedure earlier in pregnancy, or because some of the procedures were not initiated until after term.
Three trials compared beginning ECV early at between 34 and 36 weeks' gestation with ECV beginning at between 37 and 38 weeks' gestation (Akhtar 2013; Hutton 2003; Hutton 2011). Compared with women undergoing ECV at term, women who were randomised to ECV before term had a 19% decrease in the rate of non‐cephalic presentation at birth, a 10% reduction in the risk of failing to achieve a cephalic vaginal birth, a 8% decrease in the caesarean section rate, and a considerably reduced risk of undergoing a vaginal breech birth. The quality of the evidence for all of these outcomes was graded high quality. These findings are clinically important, and except for the finding relating to caesarean section these differences (favouring early ECV) were statistically significant. However, women randomised to early ECV appeared to be at increased risk of late preterm birth (risk increased by 51%), and although, overall, the number of women delivering their babies before term was relatively small (6.6% in the ECV group and 4.3% for controls) the possible increase in late preterm birth needs to be set against the positive outcomes associated with ECV.
Quality of the evidence
The trials included in the review were of mixed methodological quality. Three of the studies did not provide good descriptions of the methods used (Akhtar 2013; Mensink 1980; Van Veelen 1989). Blinding was not possible in these studies and it is difficult to know what impact lack of blinding had on outcomes. While the outcomes measured were objective and may not have been subject to detection bias it is possible that lack of blinding may have affected women's and care providers' behaviour and this may have had an effect on clinical decision‐making which could have influenced outcomes such as the decision whether or not to carry out a caesarean section. In two studies trial protocols were available; without this information it is difficult to assess possible outcome reporting bias.
Potential biases in the review process
The review process is subject to bias. We attempted to minimise bias by having two review authors independently involved in assessing risk of bias and carrying out data extraction. One of the review authors (E Hutton) was involved in two of the included trials (Hutton 2003; Hutton 2011); this author was not involved in data extraction or in assessing bias for these trials.
Authors' conclusions
Implications for practice.
The evidence on the benefits of early (before 37 weeks) external cephalic version (ECV) will require careful discussion with women to adequately inform decisions around timing of the ECV procedure. While a woman undergoing early ECV is more likely to have cephalic presentation at delivery, have reduced risk of failing to achieve a cephalic vaginal birth and of undergoing a breech vaginal birth, her risk of preterm birth will be increased.
Implications for research.
Because of the known benefits of ECV, it would now be unethical to recruit women to studies comparing ECV before term with no ECV. Studies comparing early ECV (begun 34 to 35 completed weeks) with ECV begun after 37 weeks have shown that beginning ECV earlier decreases the risk of a non‐cephalic presentation at birth, and of failing to achieve a cephalic vaginal birth but increases the risk of having a preterm birth. It is unlikely that additional research will alter these findings substantially and although additional studies may provide more refined estimates of neonatal outcomes, the value of such information may not be needed in light of the findings regarding preterm birth.
What's new
| Date | Event | Description |
|---|---|---|
| 31 August 2014 | New search has been performed | Search updated and five new studies were identified; two new studies have been included (Akhtar 2013; Hutton 2011) and three excluded (Dafallah 2004; El‐Muzaini 2008; Rust 2005). The review now includes five trials. The methods have been updated and now include the use of GRADE. |
| 31 August 2014 | New citation required but conclusions have not changed | This review has been updated to include a further two new studies. The results and conclusions have not changed. |
History
Protocol first published: Issue 1, 1996 Review first published: Issue 1, 1996
| Date | Event | Description |
|---|---|---|
| 22 October 2012 | Amended | Search updated. One new report of Hutton 2003 added to Studies awaiting classification (Hutton 2008). Three new reports of Hutton 2004 added to Ongoing studies and five new reports added to Studies awaiting classification (Dafallah 2004a; El‐Muzaini 2008a; Hutton 2011a; Murray‐Davis 2012; Rust 2005a) |
| 2 September 2008 | Amended | Converted to new review format. |
| 18 October 2005 | New citation required and conclusions have changed | Substantive amendment |
| 1 April 2005 | New search has been performed | The protocol for the review 'External cephalic version for breech presentation before term' has been updated in order to distinguish between those studies that attempt external cephalic version (ECV) only before term and those that include ECV before term and at term. This distinction has not been made previously. The following comparisons are now included in the review: (1) ECV before term compared to no ECV; (2) ECV commenced before term and continued up until delivery compared to no ECV; (3) ECV commenced before term and continued up until delivery compared with beginning ECV after 37 weeks' gestation. We conducted a new search in April 2005, as a result of which we identified one new trial (Hutton 2003) and one new ongoing study (Hutton 2004). As a result of the changes to the protocol, the Brosset 1956 and Kasule 1985 trials have now been exluded and Van Veelen 1989 has been included. |
Acknowledgements
T Dowswell is supported by the NIHR Cochrane Programme Grant Project: 13/89/05 – Pregnancy and childbirth systematic reviews to support clinical guidelines.
As part of the pre‐publication editorial process, this review has been commented on by three peers (an editor and two referees who are external to the editorial team) and the Group's Statistical Adviser.
This project was supported by the National Institute for Health Research, via Cochrane Infrastructure and Cochrane programme Grant funding to Cochrane Pregnancy and Childbirth. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIIHR, NHS or the Department of Health.
Appendices
Appendix 1. Additional searching carried out in previous version of the review
For Hutton 2006, authors conducted a additional literature search which included electronic databases: The Cochrane Central Register of Controlled Trials (The Cochrane Library, 2005, Issue 1, MEDLINE (1965 to April 2005), EMBASE (1988 to April 2005) and Controlled Clinical Trials randomised controlled trials registry (April 2005), using the search terms: 'external cephalic version or ECV'.
Data and analyses
Comparison 1. External cephalic version (ECV) before term versus no ECV.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Non‐cephalic presentation at the birth | 1 | 102 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.64, 1.69] |
| 2 Vaginal cephalic birth not achieved (CS + breech vaginal birth) | 1 | 102 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.67, 1.62] |
| 3 Caesarean section | 1 | 102 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.82 [0.57, 5.84] |
| 4 Vaginal breech birth | 1 | 102 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.49, 1.52] |
| 5 Apgar score < 7 at 1 minute | 1 | 102 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.62 [0.25, 1.59] |
| 6 Perinatal mortality | 1 | 102 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.35 [0.04, 3.22] |
Comparison 2. External cephalic version (ECV) commenced before term versus no ECV.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Non‐cephalic presentation at the birth | 1 | 179 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.59 [0.45, 0.77] |
| 2 Vaginal cephalic birth not achieved (CS + breech vaginal birth) | 1 | 179 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.62 [0.49, 0.80] |
| 3 Caesarean section | 1 | 179 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.62 [0.27, 1.43] |
| 4 Vaginal breech birth | 1 | 179 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.63 [0.46, 0.85] |
| 5 Apgar score < 7 at 5 minutes | 1 | 179 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.03 [0.13, 73.48] |
| 6 Stillbirth and neonatal mortality < 7 days | 1 | 179 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.34 [0.01, 8.16] |
Comparison 3. External cephalic version (ECV) commenced before term versus ECV at term.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Non‐cephalic presentation at the birth | 3 | 1906 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.74, 0.90] |
| 2 Vaginal cephalic birth not achieved (CS + vaginal breech birth) | 3 | 1888 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.83, 0.97] |
| 3 Caesarean section | 3 | 1888 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.85, 1.00] |
| 4 Vaginal breech birth | 3 | 1888 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.44 [0.25, 0.78] |
| 5 Apgar score < 7 at 5 minutes | 2 | 1759 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.16 [0.39, 3.44] |
| 6 Stillbirth or neonatal mortality < 7 days | 3 | 1887 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.23 [0.04, 1.34] |
| 7 Preterm birth < 37 weeks | 3 | 1888 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.51 [1.03, 2.21] |
| 8 One or more serious fetal complications following randomisation | 2 | 1761 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.42, 1.79] |
| 9 NICU stay 4 days or longer | 1 | 232 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.5 [0.49, 12.63] |
| 10 (Non‐prespecified outcome) Maternal pain score (0‐100; 0 = no pain) | 1 | 1533 | Mean Difference (IV, Fixed, 95% CI) | ‐4.60 [‐7.74, ‐1.46] |
3.10. Analysis.
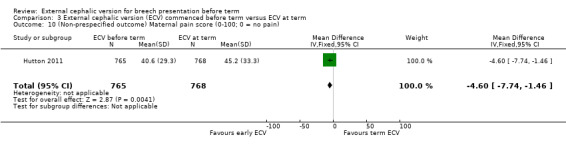
Comparison 3 External cephalic version (ECV) commenced before term versus ECV at term, Outcome 10 (Non‐prespecified outcome) Maternal pain score (0‐100; 0 = no pain).
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Akhtar 2013.
| Methods | Single centre, parallel‐group randomised controlled trial. 2‐arm trial with individual randomisation. | |
| Participants | Setting: Mardan Medical Complex, Pakistan. July 2010 to 31st Dec 2011. Inclusion criteria: singleton fetus in a breech presentation confirmed by ultrasound, between 33 weeks and 35 weeks' gestation. N = 123 women. Exclusion criteria: women with contraindication to ECV; contraindications to early ECV or contraindications to labour or vaginal birth (e.g. fetal heart rate abnormalities, vaginal bleeding, rupture of membranes, placental abruption, fetal growth restriction, previous caesarean section, low amniotic fluid index, fetal weight > 4 kg) or woman unwilling to undergo ECV. |
|
| Interventions | Early ECV: ECV carried out between 34 (238 days) and 35 weeks of gestation. No tocolytics were used. Women were monitored for 3 hours before and 1 hour after the procedure. Up to 2‐3 attempts were allowed. The procedure was discontinued if there was excessive maternal discomfort or fetal heart rate irregularities. N = 63. Control: ECV carried out at or after 37 weeks. No tocolytics were used. Women were monitored for 3 hours before and 1 hour after the procedure. Up to 2‐3 attempts were allowed. The procedure was discontinued if there was excessive maternal discomfort or fetal heart rate irregularities. N = 60. |
|
| Outcomes | Reported number of attempts at ECV, reasons for discontinuing ECV, maternal and fetal complications, presentation at delivery, mode of delivery. | |
| Notes | It was reported that methods and allocation was "in accordance with the two major multicenter trials conducted on the same subject". There was no further description of methods used. We contacted the author for more information but have not yet had a response. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Reported that this was the same as methods used in published multicentre randomised controlled trials. |
| Allocation concealment (selection bias) | Unclear risk | Not described. "Patients were randomly divided into two groups". |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding mentioned. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No blinding mentioned. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All women appeared to be accounted for in the analysis. |
| Selective reporting (reporting bias) | Unclear risk | Assessment from published report. We requested a copy of the protocol but this was not made available to us. |
| Other bias | Unclear risk | It was reported that methods and allocation was "in accordance with the two major multicenter trials conducted on the same subject". There was no further description of methods used. We contacted the author for more information but have not yet had a response. |
Hutton 2003.
| Methods | An international multicentre randomised controlled trial with randomisation stratified by parity using a centralised telephone randomisation system. Breech verified within 4 days of randomisation, and confirmed prior to ECV attempt. | |
| Participants | All nulliparous women with any breech presentation and multiparous women with a frank breech presentation were eligible for the trial if they had a live singleton fetus and a gestational age of between 34 weeks, 0 days and 36 weeks 0 days. Women were excluded if they had a parity > 4, if they planned to move to a non‐trial centre, or if there was any contraindication to labour or vaginal birth (such as placenta previa, or previous classical caesarean section), to ECV (such as fetal heart rate abnormalities, abruptio placenta, fetal anomalies, uterine anomalies, oligohydramnios, rupture of membranes, over distended uterus) or to early ECV (such as fetus engaged in the pelvis, an increased risk of preterm labour, increased risk of abruptio placenta). | |
| Interventions | ECV was begun between 34 weeks 0 days and 36 weeks 0 days in the early group (n = 117); and between 37 weeks 0 days and 38 weeks 0 days in the delayed group (n = 116). Tocolysis recommended either routinely or selectively in both groups; analgesia permitted. | |
| Outcomes | Primary: presentation at delivery. Other: caesarean section rate; serious fetal complication; preterm birth < 37 weeks; women's view's about ECV. | |
| Notes | n = 233. Funded by Canadian Institutes of Health Research; coordinated through the Maternal Infant and Reproductive Health Research Unit (MIRU) at the University of Toronto, Canada. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Stratification by parity, random block sizes. External randomisation service. |
| Allocation concealment (selection bias) | Low risk | External telephone randomisation service. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Unblinded intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Interim analysis was carried out by blinded assessors. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Analysis by intention‐to‐treat. 233 women randomised, outcome data available for 132 women and babies. |
| Selective reporting (reporting bias) | Low risk | Protocol provided by the authors. Outcome reporting bias not apparent. |
| Other bias | Low risk | Other bias not apparent. |
Hutton 2011.
| Methods | 2‐arm, unblinded, multicentre, parallel‐group randomised controlled trial. Stratification for parity and centre. Individual randomisation. | |
| Participants | Setting: 68 centres in 21 countries. Hospital setting, with clinicians who were experienced in ECV and birth facilities that were deemed to meet Canadian standards. 1543 women randomised. Inclusion criteria: women with singleton fetus in a breech presentation who had a recent screening ultrasound, between 33+0/7 weeks' and 35+6/7 weeks' gestation. Exclusion criteria: women with contraindications to ECV (e.g. fetal heart rate abnormalities, placental abruption, major life‐threatening fetal anomalies, uterine anomalies, hyper‐extended fetal head, rupture of fetal membranes, severe oligohydramnios or hydramnios); contraindications to early ECV (e.g. increased risk of preterm labour or placental abruption); or contraindications to labour or vaginal birth (e.g. placenta praevia, previous classical caesarean section); or if they had been prior participants in the trial; were at increased risk of unstable lie (such as grand multiparity); or if they planned to give birth by caesarean section even if the fetus turned to a cephalic position, or if they planned a vaginal birth if the fetus remained breech. |
|
| Interventions | Early ECV: (n = 767) ECV carried out between 34+0/7 and 35+6/7 weeks of gestation, and within 7 days of randomisation. Fetal presentation was confirmed by ultrasound immediately before the ECV procedure. Fetal heart rate was monitored before, during and after the procedure. The use of tocolytics and analgesia was left to the discretion of the clinician, and they were directed to use the same approach for women in both arms of the trial. If the procedure was unsuccessful, or if a fetus later reverted to non‐cephalic, a repeat ECV procedure could be performed at a later date at the discretion of the care provider in consultation with the woman. Delayed ECV: (n = 774) ECV carried out at or after 37+0/7. Fetal presentation was confirmed by ultrasound immediately before the ECV procedure. Fetal heart rate was monitored before, during and after the procedure. The use of tocolytics and analgesia was left to the discretion of the clinician, and they were directed to use the same approach for women in both arms of the trial. If the procedure was unsuccessful, or if a fetus later reverted to non‐cephalic, a repeat ECV procedure could be performed at a later date at the discretion of the care provider in consultation with the woman. (Overall, tocolytics were used during all ECV attempts in 68% of cases.) |
|
| Outcomes | Primary: rate of caesarean section. Secondary: rate of preterm birth (< 37 weeks), non‐cephalic presentation at birth, admission to NICU for more than 24 hours, serious neonatal morbidity or death, maternal morbidity or death, pain and maternal satisfaction. |
|
| Notes | 1 of the review authors was an investigator on this trial. Data extraction and assessment of risk of bias were carried out by 2 independent review authors. This study was funded by Canadian institutes of Health Research. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation with a computerised randomisation program, using computer‐generated random block sizes and 1:1 allocation. |
| Allocation concealment (selection bias) | Low risk | Central randomisation by telephone. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | “The nature of the intervention did not lend itself to blinding of either participants or clinicians.” |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Some efforts were made to avoid detection bias. Blinding of initial assessment and recording of outcomes is not described. An independent Data Safety and Monitoring Board “reviewed all stillbirths and neonatal deaths, blinded to allocation group, for the existence of any anomaly considered incompatible with life and to make a determination regarding exclusion of any women from the analysis of perinatal/neonatal outcomes”. An interim analysis of results was also carried out by the independent Data Safety and Monitoring Board, blinded to group assignment. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 2 women, 1 in each group, asked to be removed from the study. 8 women were lost to follow‐up (2 assigned to early ECV, 6 to delayed ECV). This left 1533 women (99.4%), so although the losses to follow‐up were unequal the numbers were small in the context of the whole study. A small amount of missing data (2 early ECV, 1 delayed ECV) accounted for in table 5. An intention‐to‐treat analysis was conducted. Perinatal and neonatal deaths were excluded from the analyses of measures of neonatal morbidity. |
| Selective reporting (reporting bias) | Low risk | All relevant outcomes appear to have been reported, including those showing no differences between groups. Multiple reports available for this study including trial registration. |
| Other bias | Low risk | Baseline characteristics were similar in the 2 groups. No other bias apparent. |
Mensink 1980.
| Methods | Allocation at 32 weeks' gestation by randomised sealed envelopes, stratified by parity. Breech verified by ultrasound. | |
| Participants | Singleton breech presentation before term (from 32 weeks). Exclusion criteria: contraindication to external version. |
|
| Interventions | External cephalic version attempt without tocolysis (n = 50) compared with no ECV attempt (n = 52). ECV was attempted by an assistant in training. If failed, a further attempt was made by an obstetrician 1 week later. |
|
| Outcomes | Non‐cephalic births; caesarean section; 1 minute Apgar score < 7; Umbilical vein pH < 7.2; neurological deficit in newborn; perinatal mortality. The perinatal death was due to placental abruption. | |
| Notes | Groningen, The Netherlands. The authors ascribe the low success rate to the gentleness with which external cephalic version was attempted. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described. |
| Allocation concealment (selection bias) | Unclear risk | Allocations were concealed in sealed envelopes. It was not clear whether all envelopes were used in sequential order and that all were accounted for. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Unblinded trial. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | It was not possible to blind the intervention. It was not clear whether outcomes were recorded by blinded assessors. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 102 women were randomised. All appear to be accounted for in the analysis. |
| Selective reporting (reporting bias) | Unclear risk | Assessment from original paper, translated notes and correspondence. It was not clear whether all outcomes were fully reported. |
| Other bias | Unclear risk | The description of study methods was very brief. Other bias was not apparent. |
Van Veelen 1989.
| Methods | Random allocation of women using sealed envelopes, stratified by parity. | |
| Participants | Healthy white Dutch women with uncomplicated pregnancy of 33‐40 weeks' gestation and a live singleton breech fetus attending antenatal clinic of Ikazia Hospital, Rotterdam, The Netherlands. | |
| Interventions | Repeated ECV performed between 33 and 40 weeks' gestation with no tocolysis, analgesia or anaesthesia compared to no ECV. | |
| Outcomes | Presentation at delivery; mode of delivery; neonatal outcome. | |
| Notes | n = 180. Rotterdam, The Netherlands. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described. Randomisation stratified by parity. |
| Allocation concealment (selection bias) | Unclear risk | "by means of drawing a sealed envelope." Othe information not provided. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | There was no mention of whether or not outcome assessors were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 180 women were randomised. 1 was lost to follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | Assessment from published report. |
| Other bias | Unclear risk | Little information on study methods was provided. Other bias was not apparent. |
ECV: external cephalic version NICU: neonatal intensive care unit
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Brosset 1956 | Method to group allocation is described as "dividing the cases up into two groups". It is highly unlikely that randomisation was used and there is risk of enrolment bias. This study is of historical interest in that it is an early example of a controlled trial. |
| Dafallah 2004 | The methods of randomisation were not described for this study. Participants recruited included women between 36 to 38 weeks' gestation, some of the women were ≥ 37 weeks' gestation; separate data were not reported for women before term. |
| El‐Muzaini 2008 | It was not clear that this was a randomised controlled trial and participants recruited to the study were women with singleton pregnancies with breech presentation at term (≥ 37 weeks). |
| Kasule 1985 | Method to group allocation is described as being dependant on the day that women attended at antenatal clinic (on Monday and Wednesday ECV was performed, whereas on Tuesday and Thursday it was not), and there is significant risk of enrolment bias. It is also unclear in this study how the breech pregnancies were confirmed. In addition, 25% of the study population were grand‐multiparous women who are at increased risk of unstable lie, and are at an increased risk of encountering complications. |
| Rust 2005 | In this trial women were recruited from 36 up to 386/7 weeks. Most included women were at term and separate data were not reported for women less than 37 weeks' gestation. |
ECV: external cephalic version
Characteristics of ongoing studies [ordered by study ID]
Belizan 1989.
| Trial name or title | Early external cephalic version in antenatal care. A randomized trial. |
| Methods | |
| Participants | Women with breech presentation at 31 weeks' pregnancy. |
| Interventions | ECV for breech presentation versus no ECV. |
| Outcomes | Caesarean section; length of postpartum stay. |
| Starting date | June 1989. |
| Contact information | Belizan JM. Centro Rosarino de Estudios Perinatales, Bv OroNo 500, 2000 Rosario, Argentina. Tel +54 41 63745 |
| Notes | It is not clear whether or not this trial was completed. |
ECV: external cephalic version
Differences between protocol and review
The protocol for this review was modified in April 2005 to include comparisons of external cephalic version (ECV) commenced before term but continued if necessary up to term.
In this updated version of the review two additional primary outcomes have been added. The purpose of ECV is to avoid a caesarean birth or a breech vaginal birth; we have therefore added a new composite outcome: cephalic vaginal birth not achieved (caesarean section + breech vaginal birth). The individual outcomes are presented separately; vaginal breech birth is now reported, caesarean section was one of the original outcomes. In this version we have also added an additional secondary outcome: Infant Apgar score < seven at five minutes. Infant morbidity was not consistently reported and low infant Apgar score provides some indication of infant wellbeing at birth. We have now reported more information re neonatal outcome. We also report a non‐prespecified outcome: maternal pain scores.
Contributions of authors
GJ Hofmeyr prepared the initial review of this topic. E Hutton has revised and will maintain the review. T Dowswell was involved in updating the review.
Sources of support
Internal sources
(GJH) Effective Care Research Unit, University of the Witwatersrand/Fort Hare, Eastern Cape Department of Health, South Africa.
(TD) Cochrane Pregnancy and Childbirth Group, Department of Women's and Children's Health, The University of Liverpool, Liverpool, UK.
External sources
-
National Institute for Health Research (NIHR), UK.
NIHR Cochrane Programme Grant Project: 13/89/05 – Pregnancy and childbirth systematic reviews to support clinical guidelines
Declarations of interest
E Hutton is an author of two of the included trials (Hutton 2003; Hutton 2011) but was not involved in data extraction or assessment of risk of bias for these trials.
Justus Hofmeyr: I have received royalties for three chapters that I have authored in Up‐to‐date (http://www.uptodate.com/contents/search).
http://www.uptodate.com/contents/external‐cephalic‐version
http://www.uptodate.com/contents/overview‐of‐breech‐presentation
http://www.uptodate.com/contents/delivery‐of‐the‐fetus‐in‐breech‐presentation
Therese Dowswell: I am employed by the University of Liverpool on an NIHR Cochrane Programme grant to work on a range of Cochrane Reviews. The Funders have no influence on the content or conclusions of the reviews that I work on.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Akhtar 2013 {published data only}
- Akhtar N. Early versus late external cephalic version. Journal of Postgraduate Medical Institute 2013;27(2):164‐9. [Google Scholar]
Hutton 2003 {published data only}
- Hutton EK, Kaufman K, Hodnett E, Amankwah K, Hewson SA, McKay D, et al. External cephalic version beginning at 34 weeks' gestation versus 37 weeks' gestation: a randomized multicenter trial. American Journal of Obstetrics and Gynecology 2003;189(1):245‐54. [DOI] [PubMed] [Google Scholar]
- Hutton EK, Saunders CA, Tu M, Stoll K, Berkowitz J, for the Early External Cephalic Version Trial Collaborators Group. Factors associated with a successful external cephalic version in the early ECV trial. JOGC: Journal of Obstetrics and Gynaecology Canada 2008;30(1):23‐8. [DOI] [PubMed] [Google Scholar]
Hutton 2011 {published data only}
- Hutton E. The Early External Cephalic Version 2 Trial. www.utoronto.ca/MIRU/eecv2 (accessed 19 July 2005).
- Hutton E. The early ECV trial. JOGC: Journal of Obstetrics and Gynaecology Canada 2007;29(6 Suppl 1):S47. [DOI] [PubMed] [Google Scholar]
- Hutton EK. The early ECV‐2 Trial Group. The early external cephalic version ‐ 2 trial. Perinatal Society of Australia and New Zealand 7th Annual Congress; 2003 March 9‐12; Tasmania, Australia 2003:A33.
- Hutton EK. The early ECV‐2 Trial Group. The early external cephalic version ‐ 2 trial. Perinatal Society of Australia and New Zealand 7th Annual Congress; 2003 March 9‐12; Tasmania, Australia 2003:P97.
- Hutton EK, Hannah ME, Ross SJ, Delisle MF, Carson GD, Windrim R, et al. The Early External Cephalic Version (ECV) 2 Trial: an international multicentre randomised controlled trial of timing of ECV for breech pregnancies. BJOG: an international journal of obstetrics and gynaecology 2011;118(5):564‐77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutton EK, Phipps H. Early ECV 2 trial. Perinatal Society of Australia and New Zealand 10th Annual Congress; 2006 April 3‐6; Perth, Australia. 2006:369.
- Hutton EK, for the EECV2 Trial Collaborative Group. Early ECV 2 trial [abstract]. Journal of Paediatrics and Child Health 2007;43(Suppl 1):A117‐8. [Google Scholar]
- Murray‐Davis B, Marion A, Malott A, Reitsma A, Hutton EK, Early ECV2G. Women's experiences of participating in the early external cephalic version 2 trial. Birth 2012;39(1):30‐8. [DOI] [PubMed] [Google Scholar]
Mensink 1980 {published data only}
- Mensink WFA, Huisjes HJ. Is external version useful in breech presentation?. Nederlands Tijdschrift voor Geneeskunde 1980;124:1828‐31. [PubMed] [Google Scholar]
Van Veelen 1989 {published data only}
- Veelen AJ, Cappellen AW, Flu PK, Straub MJPF, Wallenburg HCS. Effect of external cephalic version in late pregnancy on presentation at delivery: a randomized controlled trial. British Journal of Obstetrics and Gynaecology 1989;96(8):916‐21. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Brosset 1956 {published data only}
- Brosset A. The value of prophylactic external version in cases of breech presentation. Acta Obstetricia et Gynecologica Scandinavica 1956;35(4):555‐62. [DOI] [PubMed] [Google Scholar]
Dafallah 2004 {published data only}
- Dafallah SE, Elhag SM. The role of external cephalic version on the presentation at delivery. Saudi Medical Journal 2004;25(3):386‐8. [PubMed] [Google Scholar]
El‐Muzaini 2008 {published data only}
- El‐Muzaini MF, Felimban HM, Al‐Hazmi NM, Shabaan LA, Namankani FY, Fathudein MA. Breech with previous scar, is ECV a good option?. BJOG: an international journal of obstetrics and gynaecology 2008;115(s1):77‐8. [Google Scholar]
Kasule 1985 {published data only}
- Kasule J, Chimbira TH, Brown I. Controlled trial of external cephalic version. British Journal of Obstetrics and Gynaecology 1985;92(1):14‐8. [DOI] [PubMed] [Google Scholar]
Rust 2005 {published data only}
- Rust O, Atlas R, Gersbach E, Roberts W, Larkin R, Hess LW. A randomized trial of late versus early external cephalic version for the treatment of abnormal presentation at term [abstract]. American Journal of Obstetrics and Gynecology 2005;193(6 Suppl):S129. [Google Scholar]
References to ongoing studies
Belizan 1989 {published data only}
- Belizan JM. Early external cephalic version in antenatal care. A randomized trial. Personal communication 1989.
Additional references
Bradley‐Watson 1975
- Bradley‐Watson PJ. The decreasing value of external cephalic version in modern obstetric practice. American Journal of Obstetrics and Gynecology 1975;123(3):237‐40. [DOI] [PubMed] [Google Scholar]
Cluver 2015
- Cluver C, Gyte GML, Sinclair M, Dowswell T, Hofmeyr GJ. Interventions for helping to turn term breech babies to head first presentation when using external cephalic version. Cochrane Database of Systematic Reviews 2015, Issue 2. [DOI: 10.1002/14651858.CD000184.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cooper 2002
- Cooper GM, Lewis G, Neilson J. Editorial: confidential enquiries into maternal deaths. British Journal of Anaesthesia 2002; Vol. 89, issue 3:369‐72. [PubMed]
Dashe 2002
- Dashe JS, McIntire DD, Ramus RM, Santos‐Ramos R, Twickler DM. Persistence of placenta previa according to gestational age at ultrasound. Obstetrics & Gynecology 2002;99(5 Pt 1):692‐7. [DOI] [PubMed] [Google Scholar]
Gamble 2000
- Gamble JA, Creedy DK. Women's request for a cesarean section: a critique of the literature. Birth 2000;27(4):256‐63. [DOI] [PubMed] [Google Scholar]
Geary 1997
- Geary M, Fanagan M, Boylan P. Maternal satisfaction with management of labour and preference for mode of delivery. Journal of Perinatal Medicine 1997;25(5):433‐9. [DOI] [PubMed] [Google Scholar]
Gilliam 2002
- Gilliam M, Rosenberg D, Davis F. The likelihood of placenta previa with greater number of cesarean deliveries and higher parity. Obstetrics & Gynecology 2002;99(6):976‐80. [DOI] [PubMed] [Google Scholar]
GRADEpro 2014 [Computer program]
- GRADEpro. [Computer program on www.gradepro.org]. GRADEpro. [Computer program on www.gradepro.org]. Version 2015. GRADEpro. [Computer program on www.gradepro.org], 2014.
Hall 1999
- Hall MH, Bewley S. Maternal mortality and mode of delivery. Lancet 1999;354(1180):776. [DOI] [PubMed] [Google Scholar]
Hannah 2000
- Hannah ME, Hannah WJ, Hewson SA, Hodnett ED, Saigal S, Willan AR, for the term Breech Trial Collaborative Group. Planned caesarean section versus planned vaginal birth for breech presentation at term: a randomised multicentre trial. Lancet 2000;356(9239):1375‐83. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Hildingsson 2002
- Hildingsson I, Radestad I, Rubertsson C. Waldenstrom U. Few women wish to be delivered by caesarean section. BJOG: an international journal of obstetrics and gynaecology 2002;109(6):618‐23. [PubMed] [Google Scholar]
Hofmeyr 1986
- Hofmeyr GJ, Sadan O, Myer IG, Galal KC, Simko G. External cephalic version and spontaneous version rates: ethnic and other determinants. British Journal Obstetrics Gynaecology 1986;93(1):13‐6. [DOI] [PubMed] [Google Scholar]
Hofmeyr 1989
- Hofmeyr GJ. Breech presentation and abnormal lie in late pregnancy. In: Chalmers I, Enkin MW, Keirse MJNC editor(s). Effective Care in Pregnancy and Childbirth. Oxford: Oxford University Press, 1989:653‐65. [Google Scholar]
Hofmeyr 1991
- Hofmeyr GJ. External cephalic version at term: how high are the stakes?. BJOG: an international journal of obstetrics and gynaecology 1991;98(1):1‐3. [DOI] [PubMed] [Google Scholar]
Hofmeyr 1992
- Hofmeyr GJ. Breech presentation and shoulder dystocia in childbirth. Current Opinion in Obstetrics and Gynecology 1992;4(6):807‐12. [PubMed] [Google Scholar]
Hofmeyr 1993
- Hofmeyr GJ. External cephalic version at term. Fetal Maternal Medicine Review 1993;5:213‐22. [Google Scholar]
Hofmeyr 2003
- Hofmeyr GJ, Hannah M, Lawrie TA. Planned caesarean section for term breech delivery. Cochrane Database of Systematic Reviews 2003, Issue 2. [DOI: 10.1002/14651858.CD000166] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hofmeyr 2012
- Hofmeyr GJ, Kulier R. Cephalic version by postural management for breech presentation. Cochrane Database of Systematic Reviews 2012, Issue 10. [DOI: 10.1002/14651858.CD000051.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hofmeyr 2015
- Hofmeyr GJ, Kulier R, West HM. External cephalic version for breech presentation at term. Cochrane Database of Systematic Reviews 2015, Issue 4. [DOI: 10.1002/14651858.CD000083.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hutton 1999
- Hutton EK, Hannah ME, Amankwah K, Kaufman K, Hodnett ED. External cephalic version (ECV) and the Early ECV Trial. Journal of the Society of Obstetricians and Gynaecologists of Canada 1999;21(14):1316‐26. [Google Scholar]
Hutton 2011b
- Hutton EK, Hannah ME, Ross SJ, Delisle MF, Carson GD, Windrim R, et al. The Early External Cephalic Version (ECV) 2 Trial: an international multicentre randomised controlled trial of timing of ECV for breech pregnancies. BJOG: an international journal of obstetrics and gynaecology 2011;118(5):564‐77. [DOI] [PMC free article] [PubMed] [Google Scholar]
LaSala 1987
- LaSala AP, Berkeley AS. Primary cesarean section and subsequent fertility. American Journal of Obstetrics and Gynecology 1987;157(2):379‐83. [DOI] [PubMed] [Google Scholar]
Liu 2007
- Liu S, Liston RM, Joseph KS, Heaman M, Sauve R, Kramer MS, for the Maternal Health Study Group of the Canadian Perinatal Surveillance System. Maternal mortality and severe morbidity associated with low‐risk planned cesarean delivery versus planned vaginal delivery at term. CMAJ: Canadian Medical Association Journal 2007;176(4):455‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lydon‐Rochelle 2001
- Lydon‐Rochelle M, Holt VL, Easterling TR, Martin DP. First‐birth cesarean and placental abruption or previa at second birth. Obstetrics & Gynecology 2001;97(5 Pt 1):765‐9. [PubMed] [Google Scholar]
Minkoff 2003
- Minkoff H, Chervenak FA. Elective primary cesarean delivery. New England Journal of Medicine 2003;384(10):946‐50. [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Schunemann 2009
- Schunemann HJ. GRADE: from grading the evidence to developing recommendations. A description of the system and a proposal regarding the transferability of the results of clinical research to clinical practice [GRADE: Von der Evidenz zur Empfehlung. Beschreibung des Systems und Losungsbeitrag zur Ubertragbarkeit von Studienergebnissen]. Zeitschrift fur Evidenz, Fortbildung und Qualitat im Gesundheitswesen 2009;103(6):391‐400. [PUBMED: 19839216] [DOI] [PubMed] [Google Scholar]
Schutte 1985
- Schutte MF, Hemel OJS, Berg C, Pol A. Perinatal mortality in breech presentation as compared with vertex presentation in singleton pregnancies: an analysis based on 57819 computer‐registered pregnancies in The Netherlands. European Journal of Obstetrics, Gynecology, and Reproductive Biology 1985;19:391‐400. [DOI] [PubMed] [Google Scholar]
Turnbull 1999
- Turnbull DA, Wilkinson C, Yaser A, Carty V, Svigos JM, Robinson JS. Women's role and satisfaction in the decision to have a caesarean section. Medical Journal of Australia 1999;170(12):580‐3. [DOI] [PubMed] [Google Scholar]
Walker 2002
- Walker R, Turnbull D, Wilkinson C. Strategies to address global cesarean section rates: a review of the evidence. Birth 2002;29(1):28‐39. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Hofmeyr 1995
- Hofmeyr GJ. External cephalic version before term. [revised 04 October 1993]. In: Enkin MW, Keirse MJNC, Renfrew MJ, Neilson JP, Crowther C (eds.) Pregnancy and Childbirth Module. In: The Cochrane Pregnancy and Childbirth Database [database on disk and CDROM]. The Cochrane Collaboration; Issue 2, Oxford: Update Software; 1995.
Hofmeyr 1996b
- Hofmeyr GJ. External cephalic version for breech presentation before term. Cochrane Database of Systematic Reviews 1996, Issue 1. [DOI: 10.1002/14651858.CD000084] [DOI] [PubMed] [Google Scholar]
Hutton 2006
- Hutton EK, Hofmeyr GJ. External cephalic version for breech presentation before term. Cochrane Database of Systematic Reviews 2006, Issue 1. [DOI: 10.1002/14651858.CD000084.pub2] [DOI] [PubMed] [Google Scholar]


