Graphical abstract
Keywords: SARS-CoV-2, Voltametric biosensor, Impedimetric biosensor, Immunosensor, DNA-sensor, Aptasensor, MIP-sensor
Abstract
During the COVID-19 pandemic, electrochemical biosensors have shown several advantages including accuracy, low cost, possibility of miniaturization and portability, which make them an interesting testing method for rapid point-of-care (POC) detection of SARS-CoV-2 infection, allowing the detection of both viral RNA and viral antigens. Herein, we reviewed advancements in electrochemical biosensing platforms towards the detection of SARS-CoV-2 based on voltametric and impedimetric transduction modes, highlighting the advantages and drawbacks of the two methods.
1. Introduction
COVID-19 pandemic caused by the SARS-CoV-2 virus is the most challenging health issue in recent years, because of its social and economic impact on several aspects of human life [1], [2], [3], [4]. The development of rapid and reliable tests for COVID-19 diagnosis has a crucial role to prevent further infections in order to reach a pandemic control [5], [6], [7], [8]. Although RT-PCR still remains the gold standard method to detect SARS-CoV-2, antigen rapid detection tests are commonly used to detect the viral proteins and, although they are less sensitive than molecular tests, have the advantages to be relatively inexpensive and to give a fast response at the point of care [9], [10], [11], [12], [13], [14], [15]. Most of them are based on immunochromatographic lateral flow assays, which satisfy the so-called ASSURED (affordable, sensitive, specific, user-friendly, rapid and robust, equipment-free and deliverable to end users) criteria, guidelines provided in 2003 by the World Health Organization (WHO) for ideal test that can be used at all levels of the health care system [16].
Land and coworkers in a recent paper published on Nature [17], proposed the acronym REASSURED, adding two additional criteria of R (real-time connectivity) and E (ease of specimen collection) into the original acronym ASSURED. Future diagnostics should fulfill the need of incorporating new technological elements to provide real-time data and to overcome the difficulties in specimen collection and/or processing, which may limit scaling-up of diagnostics in resource-limited areas. With the rapid development of digital technology and mobile health (i-health), a new generation of devices and tests is emerging which combine the ASSURE criteria with the novel needs expressed by the REASSURED criteria, in terms of non-invasive and easy specimen collection and transmission of test data after proper analysis to provide feedback for immediate patient treatment or for surveillance. Electrochemical biosensors can be used as antigen rapid detection devices which fulfill the more recent REASSURED criteria. For this reason, they are attracting considerable attention in the COVID-19 management [18], [19], [20], provided that they yield detection limits in the pico/nanomolar range [19], [20].
Various types of electrochemical biosensors including potentiometric, voltametric, impedimetric and field-effect transistor (FET)-based have been applied to the detection of SARS-CoV-2 [21], [22], [23], [24], [25], [26], [27]. They measure changes in potential, current, resistance and conductance, respectively, as a consequence of the biological binding events at their electrode’s surfaces. This review focuses on voltametric and impedimetric biosensing detection, summarizing the design and features of the biosensors realized in the current pandemic, highlighting the analytical performances, advantages and drawbacks of each of them. Finally, perspectives of voltametric and impedimetric biosensors as a potential detection tool for COVID-19 managing is discussed.
2. Electrochemical biosensors for COVID-19
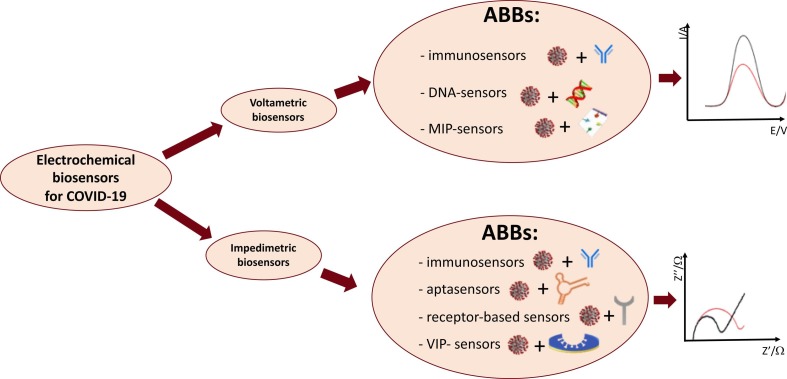
Electrochemical biosensors reported in literature for SARS-CoV-2 detection can be classified by type of transducer or bioreceptor. In particular, they can be divided in voltametric and impedimetric biosensors, depending on the transducer type being used, and in immunosensors, DNA-sensors, and aptasensors depending on the biorecognition element.
In the fabrication of the electrochemical biosensors, conducting nanomaterials have been often used in transducer elements, providing a suitable path for immobilizing the biorecognition elements and a large increase of the catalytic activity of the sensor, in order to overcome sensitivity and selectivity problems [28], [29]. Nanostructures represent important new components in recently developed electrochemical biosensors for COVID-19, such as the use of nanoparticles as electrochemical labels for DNA-sensing, or graphene and carbon nanotubes for electrode materials. Moreover, synergies in nanotechnology and bioelectronics have revealed new possibilities to miniaturize and to optimize existing microscale devices at the nanoscale [30], [31], [32].
The classification in this review is organized by type of transducer. For each transducer a further subdivision is carried out based on type of bioreceptor.
2.1. Voltametric biosensors
Voltametric biosensors are electroanalytical devices where the information about an analyte is obtained by measuring a current as a function of a potential variation. The peak current value obtained over the linear potential range is directly proportional to the analyte bulk concentration. Amperometric biosensors are a particular type of voltametric biosensors, where the current is measured during time at a constant potential. For the development of the voltametric biosensors for SARS-CoV-2 three voltametric techniques have been used, differential pulse voltammetry (DPV), square wave voltammetry (SWV) and crono-amperometry [6].
Classifying by the type of the biorecognition element, the voltametric biosensors reported in literature for COVID-19 con be subdivided in immunosensors for the detection of the viral antigen, molecularly-imprinted (MIP) sensors, DNA-sensors and aptasensors for the detection of the viral RNA, as schematized in Fig. 1 .
Fig. 1.
Schematic classification of electrochemical biosensors for SARS-CoV-2 detection reported in literature. List of abbreviations: ABBs = affinity-based biosensors; MIP = molecular imprinted polymer; VIP = virus imprinted polymer.
2.1.1. Voltametric immunosensors
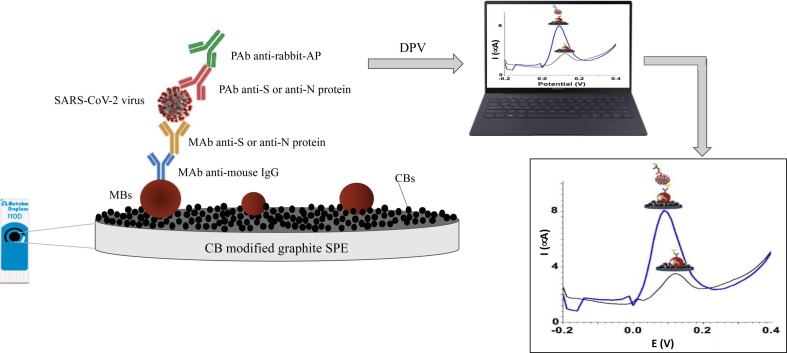
A first immunosensor for SARS-CoV-2 detection was realized by Fabiani et al. [33] using magnetic beads (MBs) as support for the immunological procedure and a carbon black-modified screen-printed electrode for the detection of S-protein and N-protein antigens. A sandwich assay was performed immobilizing antibodies for S and N proteins on MBs and the binding was evaluated by DPV using secondary antibody labelled with alkaline phosphatase enzyme. A scheme of the functioning mechanism of the proposed biosensor is shown in Fig. 2 . The proposed novel sensor configuration demonstrated the capability to detect S and N proteins in untreated saliva with a LOD of 19 ng/mL and 8 ng/mL, respectively, as well as SARS-CoV-2 in saliva clinical samples, showing an agreement in 22/24 samples with the data obtained by RT-PCR using nasopharyngeal swab specimen. Moreover, the sensor showed no cross-reactivity when tested with seasonal H1N1 influenza virus and 2009 pH1N1 influenza pandemic and rapid time of analysis (30 min).
Fig. 2.
Schematic representation of the electrochemical immunosensors for SARS-CoV-2 detection proposed by Fabiani et al. [32]. List of abbreviations: CB = carbon black; SPE = screen printed electrode; MBs = magnetic beads; MAb = monoclonal antibodies; PAb = polyclonal antibodies anti-S = antibodies against Spike protein; anti-N = antibodies against Nucleocapsid protein; AP = alkaline phosphatase.
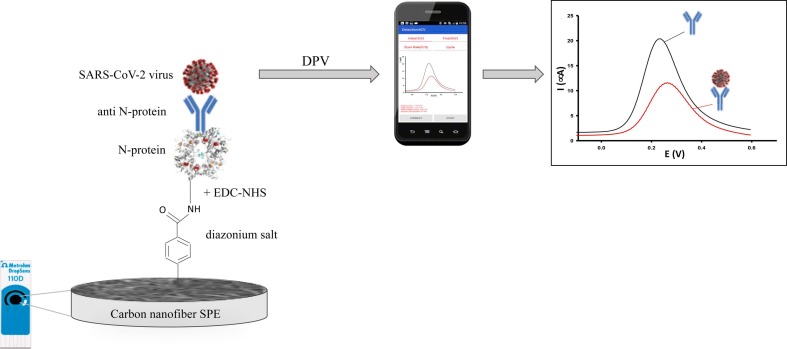
Eissa and Zourob [34] developed a cotton-tipped dual-function immunosensor for the detection of N-protein antigen. The immunosensor was fabricated by immobilizing the virus antigen on a carbon nanofiber-modified screen-printed electrode, functionalized using electrografting of carboxyphenyl groups via the reduction of diazonium salt. The detection of the virus antigen was achieved via swabbing followed by competitive assay using a fixed amount of N-protein antibody in the solution. A ferro/ferricyanide redox probe was used for the detection using SWV technique. The reduction signal of the redox probe at the functionalized electrode was almost disappeared after the formation of the carboxyphenyl layer on the electrode surface. The binding of the N-protein to the modified electrode led to an increase of the reduction current, because of the shielding of the negatively charged carboxylic groups of the surface with the antigen, which is positively charged at pH 7.4. The mechanism of the proposed biosensor is schematized in Fig. 3 . The novelty of the proposed dual-function sensor is the versatility of the platform, which can be used both as sample collector and detection mode. The immunosensor showed a LOD of 0.8 pg/mL, high selectivity, as no cross-reactivity with antigens from other viruses such as influenza A and HCoV.
Fig. 3.
Schematic representation of the electrochemical immunosensors for SARS-CoV-2 detection proposed by Eissa & Zourub [33]. List of abbreviations: SPE = screen printed electrode; EDC-NHS = ethylcarbodiimide (EDC) and N-hydroxysuccinimide (NHS); N-protein = nucleocapsid protein; anti N-protein = antibodies against Nucleocapsid protein.
An ultrasensitive and low-cost telemedicine platform, called SARS-CoV-2 RapidPlex, has been developed by Torrente-Rodrigues et al. [35] for simultaneous rapid and remote detection of four COVID-19 biomarkers, N-protein, anti-spike IgG and IgM proteins, and C-reactive protein (CRP). This multiplex nano-immunosensor provides information on three key aspects of COVID-19 disease: viral infection (N-protein), immune response (IgG and IgM), and disease severity (CRP). The detection of the four selected targets proteins is achieved through sandwich- (N-protein and CRP) and indirect-based (IgG and IgM) immuno-sensing strategies onto laser-engraved graphene electrodes (LEG), modified with 1-pyrenebutyric acid (PBA), used as linker to anchor the required receptors (specific antibodies) to the graphene layer. The nanosensor consists of a four-working-electrode graphene array designed with an Ag/AgCl reference electrode and a graphene counter electrode. Chrono-amperometric readings from the four channels are concurrently taken and data are wirelessly transmitted to a user device via Bluetooth. Moreover, the sensor showed high sensitivity in spiked serum and saliva samples, negligible crosstalk between different working surfaces and no significant cross-reaction for NP, S1-IgG, S1-IgM, and CRP assays against SARS-CoV and MERS-CoV. Finally, the immunosensor was tested in serum and saliva samples from RT-PCR-confirmed COVID-19-positive and negative subjects. All positive samples showed higher signals compared to negative samples, attesting the accurate evaluation of the COVID-19 biomarkers in both biofluids using the proposed sensor. Moreover, the elevated levels of the selected targets found in saliva samples demonstrated the exceptional utility of this biofluid as a valuable source for non-invasively diagnosing and monitoring of SARS-CoV-2 infection.
Another nanosensor based on graphene has been constructed by Mojsoska et at. [36] for the detection of SARS-CoV-2 spike S1 protein. The immunosensor was produced by coating the graphene electrode with a linker suitable to bind the specific antibody anti-spike S1. The sensor, based on the decrease of the SWV signal of a ferri/ferrocyanide solution after the binding of the antigen to the modified electrode, allowed the detection of SARS-CoV- 2 spike S1 protein with a LOD of 260 nM. The sensor described is a proof-of-concept for a fast and simple immunosensor for SARS-CoV-2 detection but it has not been tested on real clinical samples.
In a more recent paper, published by Liv et al. [37], a classical glassy carbon electrode (GCE) and a carbon ink screen printed electrode (CSPE) were modified with graphene oxide (GO) and successively functionalized with the classical ethylcarbodiimide (EDC) and N-hydroxysuccinimide (NHS) to obtain sensitive sensing platforms for SARS-CoV-2 spike protein detection. Unlike the DPV signal of the SARS-CoV-2 spike protein in presence of a redox probe in previous works, the authors noted that anodic peak current at 1430 mV increased with increasing SARS-CoV-2 antigen concentrations, due to the oxidation of the hydroxyl groups of the spike antibody bound on the screen-printed graphene electrode surface. This can be ascribed to the increasing oxidation ability belonging to the antigen/antibody specific interaction. Both biosensors showed a dynamic linear range between 1 ag/ml and 10 fg/ml with a LOD of 1 ag/ml in PBS, saliva and oropharyngeal swab samples. However, the GO/CSPE remarked in terms of cheapness, rapidity and sensor disposability, whereas the GO/GCE in terms of clearness of the voltammograms registered. The nano-immunosensor showed no cross-reactivity towards MERS-CoV, pneumonia and influenza A spike proteins. Although the high overpotential utilized in this study, no other interaction-disrupting interference effects caused by other interfering species present in the complex biological matrices analyzed (saliva and oropharyngeal swab samples) have been registered, attesting that the proposed immunosensor selectively responds to the SARS-CoV-2 spike protein. Moreover, it showed 92.5% specificity and 93.3% sensitivity when compared to RT-PCR, providing great potential for the diagnosis of COVID-19 in real samples.
2.1.2. Voltametric MIP-sensors
Molecularly imprinted polymers (MIP)-based sensors have also been studied in recent decades for detection of various biomarkers [38], [39], [40]. Raziq et al. [41] developed the first portable electrochemical sensor integrated with a molecular imprinted polymer (MIP) as a synthetic recognition element capable of selective detection of SARS-CoV-2 antigen, in particular the 2-nucleoprotein (ncovNP). The sensor was developed by electrodeposition of poly-m-phenylenediamine on gold-based thin-film electrodes, successively modified through the generation of molecular imprints of ncovNP in the polymer film. The rebinding of ncovNP on the prepared ncovNP sensors was measured by DPV. The sensor showed a linear response to ncovNP in the range 2.22–111 fM, with LOD value of 15 fM in PBS spiked with ncovNP. Moreover, it was able to signaling ncovNP presence in nasopharyngeal swab samples of COVID-19 positive patients, differentiating ncovNP from spike protein S1 and hepatitis C virus. The MIP-based sensor relies on a completely different approach compared to currently developed SARS-CoV-2 antigen sensors based on biological receptors, and therefore represents an interesting alternative for the rapid screening of COVID-19.
2.1.3. Voltametric DNA-sensors
The first DNA-sensor for the detection of the SARS-CoV-2 viral N and S genes realized by Chaibun et al. [42] is based on isothermal rolling circle amplification (RCA) [43], for the simultaneous amplification of two genes, in order to increase the sensitivity and specificity of the SARS-CoV-2 detection. The assay is based on the hybridization of the RCA amplicons with probes functionalized with electrochemically detectable labels. Firstly, circular DNA templates have been designed with the same capture probe binding sequence for both N and S genes in order to allow the binding of the probe-conjugated magnetic beads (CP-MNB) to the RCA amplicons of both genes. Successively, the RCA amplicons containing both N and S genes were electrochemically detected by DPV using the respective redox-labeled silica nanoparticles reported probe (SiNPs-RP). In comparison with PCR-based assays, RCA can be performed under isothermal conditions with minimal reagents and avoids false-positive results. Moreover, RCA assay is less complicated compared with other isothermal amplification methods and can be performed by non-skilled users. The biosensor showed a LOD of 1 copy/μL, which is a lower value than the average viral load in clinical sample after early onset (>1x106 copies/ml) [44] and therefore the proposed DNA-nanosensor can be successfully used to COVID-19 diagnosis at early stages.
The coupling of smartphones with biosensors allows to deliver in real time the health data, collected by a POC biosensor, remotely to the physician. The combination of telemedicine and biosensing technologies collecting and transmitting real-time health information may provide numerous benefits to both health providers and patients, especially during the highly contagious COVID-19 pandemic [45].
The first electrochemical detection of SARS-CoV-2 with a smartphone [46] is reported by Zhao and coworkers [47]. A supersandwich-type DNA-sensor based on p-sulfocalix[8]arene (SCX8) functionalized graphene (SCX8-RGO) for SARS-CoV-2 detection of ORF1ab gene has been developed. The method does not require nucleic acid amplification and reverse transcription, thus avoiding the need to send the samples to external equipped laboratories. The nanosensor represents the first plug-and-play diagnostic device for low cost POC testing of COVID-19. The sensor was initially tested using artificial targets and showed a good linear range between 1x10-17 to 1x10-12 M with a LOD of 3 aM. Finally, the sensor was tested in clinical samples samples from RT-PCR-confirmed COVID-19-positive and negative patients. The detectable positive rate achieved 85.5% in confirmed patients, attesting the superior sensitivity of the proposed assay compared to Rt-PCR method. The LOD of the proposed sensor resulted to be 200 copies/mM, which is the lowest LOD value reported in literature so far.
Alafeef and coworkers [48] developed a biosensor chip for POC use for COVID-19 detection, by using gold nanoparticles (AuNPs) capped with highly specific antisense oligonucleotides (ssDNA) targeting SARS-CoV-2 viral nucleocapsid phosphoprotein (N-gene), as recognition element. The nanosensor is composed of a filter paper coated by graphene nanoplatelets to form a conductive film. A gold electrode was covered by a graphene film and, successively, by gold nanoparticles (AuNPs) capped with ssDNA probes specific to the SARS- CoV-2 RNA. The first advantage of the proposed DNA-sensor is that the authors eliminated the complex RNA amplification step using PCR and introduced the electrical signal amplification from AuNPs. Further, they eliminated the need for the conventional techniques used for impedance recordings, such as electrochemiluminescence, cyclic voltammetry, and EIS, and replaced them by a simple signal conditioning circuit, integrated with a microcontroller and an algorithm for the computer interface. In the presence of SARS-CoV-2 RNA, the specific RNA-DNA hybridization led to the change in charge and electron mobility on the graphene surface, which causes the change in sensor output voltage, which reached stability in less than 5 min, allowing real time detection. The sensor provides a broad linear detection range from 584.4 copies/μl to 5854x107 copies/μl and a limit of detection of 6.9 copies/μL without the need for any further amplification. The enhanced sensitivity of the proposed sensor can be explained by the graphene conductive nanoplatelet film and the signal amplifying gold nanoparticles. The sensor is portable and can be integrated with smartphones [46] for easy and rapid diagnosis of SARS-CoV-2.
Immunosensors and DNA-sensors described in this review are based on biological reagents (antibody, antigen, DNA, etc.) and therefore require time consuming and costly processes for the extraction and/or fabrication of the biological compounds. Hashemi et al. [49] realized a nanosensor which does not require any extraction or biological marker to detect SARS-CoV-2 S-glycoprotein. The proposed device should not, in principle, be included in the present review, but it has been reported because of its peculiar characteristics. The sensor is developed on a carbon screen printed electrode activated upon coating a layer of graphene oxide decorated with 8-hydroxyquinoline (8H), EDC and NHS coupled with gold nanostars (AuNS). The AuNS/EDC-NHS-8H/GO/SPE platform can provide more adsorptive capability via various interactions, such as formation of hydrogen bonds and electrostatic interactions between the nano-based catalyst and the functional groups of the specific viral S-glycoprotein. Moreover, the platform increases the effective surface area of the nanosensor and catalyzed the surface-confined oxidation reaction of the adsorbed glycoprotein. The advantage of this sensor is that it does not require any biological marker, such as antibody, DNA, cells, etc., and the relative processes of extraction and/or fabrication. It allows the detection of different viruses via the differentiable DPV pattern as a fingerprint of each specific viral glycoprotein at different voltage positions. The sensor is governed by an adsorption process and allows the detection of SARS-CoV-2 S-glycoprotein with a LOD of 1.68x10-22 μg/ml in 1 min. Moreover, the nanosensor showed great performance compared to gold-standard RT-PCR, showing a sensitivity of about 95% and a specificity of 60%. The excellent performances of this device achieved without any biological marker can be totally ascribed to the superior conductivity of the nanostructured platform which not only increases the electron transfer rate and electrode surface area of the nanosensor, but also catalyzes the surface-confined redox reaction of the adsorbed glycoproteins to the activated electrode surface.
2.1.4. Voltametric aptasensors
Aptasensors are based on “aptamers”, single-stranded oligonucleotide sequences which are able of binding specific targets with high affinity, specificity and selectivity. Compared to antibodies, aptamers show numerous advantages, such as high stability, sensitivity, low cost of synthesis and easy modification process. Because of these properties, the use of aptamers has increased exponentially in the design of biosensors in the last decade [50], [51], [52], [53].
Tian et al. [54] realized the first aptasensor based on a voltametric mode with high sensitivity and selectivity for detection of SARS-CoV-2 N-protein. A sandwich structure was fabricated on the surface of a glassy carbon electrode, modified with thiolated dual aptamers: the nanoprobe, composed by hemin/G-quadruplex DNAzymes, HRP and Au@Pt NPs, was dropped onto the GC surface to realize the aptamer-protein-nanoprobe sandwich structure and catalyzed the oxidation of hydroquinone with H2O2, giving rise to an amplified electrochemical signal, as a result of a synergistic catalysis of Au@Pt NPs, HRP and GQH DNAzyme. The proposed aptasensor showed a LOD of 8.33 pg/mL, remarkable selectivity and good repeatability. Moreover, the sensor was tested in human serum samples, diluted with PBS, using a commercial ELISA kit as control showing a good recovery in the range 92–110%.
2.2. Impedimetric biosensors
In the impedimetric-mode biosensing the information about the analyte is obtained by measuring the impedance using the electrochemical impedance spectroscopy (EIS) technique [23]. The impedance is the ratio between the sinusoidal potential applied and the current and represents the opposition of an electrical circuit to the flow of electrons in an alternating current (AC) circuit, when the electrode is immersed in a solution containing a redox probe. The results of the impedance measurement can be graphed using the Nyquist plot for all the applied frequencies, with the imaginary part of the impedance Z, out of phase, plotted against the real component, in phase, at each excitation frequency [27]. The interaction between the bioreceptor and the analyte causes changes in the impedance at the working electrode surface, with a general trend of the impedance value to increase at increasing the complexity of the functionalized electrode surface, included analyte binding, as a consequence of the blocking of the electron transfer sites on the electrode surface. The ΔRct (difference between the charge transfer resistance Rct, extrapolated from the semicircle of the Nyquist plot, after and before analyte binding) is directly proportional to the analyte bulk concentration. Unlike voltametric devices, impedance biosensors are label free [25].
On the basis of the biorecognition element used, the impedimetric biosensors reported in literature for COVID-19 belong to the general class of “affinity-based biosensors” (ABBs) [55], typically with antibodies, aptamers or other specific biological receptors, as illustrated in Fig. 1.
2.2.1. Impedimetric immunosensors
Zaccariotto et al. [56] utilized the advantageous immobilization of SARS-CoV-2 antibodies on the reduced graphene oxide electrode to develop an impedimetric immunosensor for SARS-CoV-2 S-protein detection. The EIS technique was utilized using the redox couple [Fe(CN)6]3-/4-]. The sensor showed two linear segments in the calibration plot with different slopes. The first segment resulted to be linear for a S-protein concentration between 0.16 and 1.25 μg/mL, while the second segment resulted linear for a range of 2.5 to 40 μg/mL S-protein concentration, with a LOD of 150 ng/mL. The advantage of the proposed sensor is the easy functionalization of the GC surface, requiring four simple steps which can be easily transported to printed carbon for POC use: i) drop-casting of rGO solution; ii) incubation in EDC-NHS solution; iii) drop-casting of anti-spike glycoprotein antibody solution; iv) blocking step with BSA. The immunosensor was tested in spiked saliva samples showing promising results.
Another impedimetric immunosensor has been developed by Rahmati and coworkers [57], by using a screen-printed carbon electrode modified with proteinA/Cu2O nanocubes for the ordered immobilization of the anti-spike protein antibodies. The nanosensor has the advantage to require no sample pretreatment or labeling. It showed a very good relationship between RCT and S-protein in the range 0.25 fg/mL to 1 μg/mL with a LOD of 0.04 fg/mL without any cross-reactivity. The nanosensor was also tested in biological fluids, such as saliva, artificial nasal samples spiked with S-protein and UTM (universal transport medium for viruses), showing satisfactory results.
2.2.2. Impedimetric aptasensors
The only aptamer-based impedimetric sensor for COVID-19 detection has been developed by Lassere et al. [58] for the sensitive and selective determination of SARS-CoV-2 spike protein S1. The SARS-CoV-2 Optimer sequence has been synthesized at a very low cost (0.01–0.03 UK pence per test), after validation for target affinity and functional binding to the SARS-CoV-2 S1 domain. The system presented several key advantages: i) the use of a simple impedance measurement to determine the S-protein binding; ii) the use of an Optimer (high stability receptor) for S-protein detection rather than an antibody, which is known to be less stable; iii) the use of low cost gold electrodes, which can be easily produced and functionalized at high volume for mass manufacture. Moreover, the sensor can be produced at scale, at an ultra-low cost. The sensor was used for testing in clinical positive and negative samples. The results show that a significant degree of change in Rct occurs between the positive and negative samples, in perfect agreement with the trend obtained with the laboratory-based techniques.
2.2.3. Impedimetric VIP-sensors
Similarly to MIP-based sensors, also virus-imprinted sensors have been realized for detection of several viruses [59], [60], [61]. Hussein et al. [62] constructed the first impedimetric nanosensor based on a screen-printed carbon electrode modified with carbon nanotubes/tungsten oxide (CNTs/WO3) for imprinting the complete SARS-CoV-2 particles within the polymer to create virus complementary binding sites. The CNTs/WO3 platform was used to enhance the surface performance and whole virus imprinting. The virus imprinted matrix has been realized by in situ electrodeposition of polymeric films of poly(meta-aminophenol) in presence of the whole SARS-CoV-2 particles. EIS responses were measured before and after the virus binding, and the charge transfer resistance (ΔRct) was used to draw the calibration curve, by using two redox mediators, potassium ferrocyanide and 2,6-dichlorophenolindophenol. The sensor reached a satisfied low detection limit of 57 pg/mL, resulting 27-fold more sensitive than RT-PCR. The advantages of this sensor include the short time of analysis (10 min) and simplicity of analysis, as the nasopharyngeal swab can be applied directly on the VIP chip with no need of equipped laboratory, and possibility of integration of the sensor chip with a portable electrochemical device, for instantaneous and simple POC detection.
The MIP sensor based on a voltametric mode described in the previous paragraph showed a much higher sensitivity, with a LOD of 15 fM. However, a nucleic acid extraction step was utilized, whereas the designer VIP-sensor does not rely on any sample preparation/extraction.
2.2.4. Impedimetric receptor-based sensors
In some cases, immunosensors based on antigen–antibody interaction may lack high accuracy and effectiveness towards COVID-19 mutations. More accurate interactions are required for a more sensitive and selective detection [63]. Torres and coworkers [64] proposed “RAPID”, an impedimetric biosensor able to detect SARS-CoV-2 S-protein within 4 min, by transforming the biochemical information from the specific molecular binding event between SARS-CoV-2 S-protein and angiotensin-converting enzyme-2 (ACE2) into an electrical signal. The binding between these two molecules causes a change in interfacial electron transfer kinetics of the redox probe and the electrode surface, detectable by measuring the Rct value. The electrodes were prepared by a screen-printing process on a phenolic paper using electrically conductive carbon and successively functionalized by drop-casting method by cross-linking ACE2 using glutaraldeyde, BSA as blocking agent and Nafion solution to protect against electrode surface biofouling. The sensor was firstly tested in spiked PBS and saliva solutions showing a LOD of 2.18 fg/mL and 1.39 pg/mL, respectively. Successively, its diagnostic capability was assessed in titrated solutions of inactivated SARS-CoV-2 and it showed a LOD of 1.16 PFU/mL, which corresponds to a viral load corresponding to day 2 or 3 after symptoms onset. Finally, the performance of RAPID was assessed in positive and negative clinical saliva samples, previously tested by RT-PCR. The sensitivity of RAPID remains high also in real saliva samples (100%), with high specificity (86.5%) and high accuracy (90%), thus highlighting the reliability of the proposed method. Moreover, the authors demonstrated the applicability of the sensor in a portable potentiostat connected to a smart device.
Another impedimetric biosensor based on the ACE2/S-protein interaction has been developed by Büyüksünetçi and coworkers [65] for SARS-CoV-2 S-protein detection. The biosensing platform and the sensor mechanism were slightly different: a gold screen printed working electrode was modified by adding ACE2 using EDC/NHS as linker mixture, as already used by Liv et al. [37], and after the S-protein/receptor binding the trasmembrane protease serine 2 (TMPRSS2) enzyme was put onto the electrode surface to cleave the S2 subunit of the SARS-CoV-2 protein. The biosensor allowed the detection of SARS-CoV-2 spike protein in a large linear range interval with two different linearity slopes, from 700 to 1500 ng/mL and from 1500 to 7000 ng/mL with a LOD of 299.30 ng/mL. The sensor showed no cross-reactivity towards H1N1, H3N2 and influenza A spike proteins and very good correlation with the results obtained with RT-PCR on real samples of positive and negative patients.
A third biosensor based on ACE2 enzyme immobilized into a layer with amphiphobic character has been realized by Vezza et al. [66] for S1 protein detection. A perfluorocarbon SAM (PFDT-SAM) is deployed on a printed-circuit board (PCB) gold electrode to form a layer which facilitates the immobilization of ACE2 via its hydrophobic tail and at the same time shows anti-fouling properties [67]. The sensor showed a LOD of 1.68 ng/mL with recombinant S-protein and a LOD of 38.6 copies/mL when evaluated with inactivated virus and specificity against IL-6 and streptavidin. As the RNA levels of SARS-CoV-2 in saliva samples have been estimated between 104 and 1013 copies/mL [68], the results of the proposed sensor demonstrated that it can be used for SARS-CoV-2 detection in saliva samples. The only limitation of the proposed sensor is the time of analysis of about 30 min, which is still competitive but further optimization studies are suggested to reduce the overall assay time.
Table 1, Table 2 summarize the voltametric and impedimetric biosensors for SARS-CoV-2 detection scanned in this review, highlighting sensor type, technique, biomarker, sensor platform, and analytical performances.
Table 1.
Comparison of voltametric biosensors reported for COVID-19 diagnosis. List of abbreviations: CNT = carbon nanofiber; MBs = magnetic beads; CB = carbon black;AuNS = gold nanostars; GO = graphene oxide; 8-hydroxyquinoline (8H), 1-ethyl-3-(3-dimethylamino- propyl)carbodiimide (EDC) and N-hydroxysuccinimide (NHS); PBASE = 1-pyrene butyric acid N-hydroxysuccinimide ester; AuTFE = thin film gold electrode; ncovNP = 2 nucleoprotein; MIP = molecular imprinted polymer; LEG = laser engraved graphene; NP = nucleocapsid protein; CRP = C reactive protein; PBA = 1-pyrenebutyric acid; SCX8-RGO = p-sulfocalix[8]arene -graphene oxide; TB = toluidine blue; VIP = virus imprinting polymer; CE = carbon electrode; OPS = oropharyngeal swab; nanoprobes = hemin/GQH DNAzyme, HRP, Au@Pt/MIL-53; CP-MNB = capture probe magnetic beads; SiNPs = silica nanoparticles.
| Sensor | Technique | Biomarker | Biosensor Platform | Nano-sensor | Biosensor format | Sample | Linear range | LOD | References |
|---|---|---|---|---|---|---|---|---|---|
| immunosensor | DPV |
S-protein N-protein |
MBs/CB/SPE/S-protein Ab MBs/CB/SPE/N-protein Ab |
no | label-based sandwich |
saliva | – | 19 ng/mL 8 ng/mL |
[32] |
| immunosensor | SWV |
N-protein |
CNF-SPE/N-protein | no | label-free competitive |
– |
– | 0.8 pg/mL |
[33] |
| immunosensor | amperometry | NP S1-IgG S1-IgM CRP |
LEG/PBA/N-protein Ab LEG/PBA/S1-protein Ab LEG/PBA/S1-protein Ab LEG/PBA/CRP Ab |
yes | double sandwich (label-based) indirect (label-based) indirect (label-based) sandwich (label based) |
serum saliva serum saliva serum saliva serum saliva |
0.1–0.8 μg/mL 0.5–2.0 ng/mL 20–40 μg/mL 0.2–0.5 μg/mL 20–50 μg/mL 0.6–5.0 μg/mL 10–20 μg/mL 0.1–0-5 μg/mL |
– |
[34] |
| immunosensor |
SWV | S1-protein | PBASE-graphene/S1-protein Ab | yes | label free | PBS | – | 260 nM | [35] |
| immunosensor |
DPV | S-protein | BSA/EDC-NHS-GO/SPE/spike Ab | yes | label free | PBS Saliva OPS |
1 ag/mL- 10 fg/mL |
1 ag/mL | [36] |
| MIP-sensor |
DPV | ncovNP | ncovNP-MIP/Au-TFE | no | label free | PBS | 2.22–111 fM | 15fM | [40] |
| DNA-sensor | DPV | N-gene S-gene |
CP/MNB/SiNPs/SPCE/ ssDNA |
yes | sandwich (label-based) |
serum saliva |
1-1x109 copy/ μL |
1 copy/mL |
[41] |
| DNA-sensor | DPV | ORF1ab | SCX8-RGO/TB/ssDNA |
yes | sandwich (label-based) |
PBS clinical samples |
1x10-17-1x10-12 M | 3 aM 200 copies/mL |
[46] |
| DNA-sensor |
voltage output | N-gene |
ssDNA/AuNPs/graphene | yes | label free | saliva | 585.4–5.854x107 copies/{} | 6.9 copies/μL | [47] |
| sensor | DPV |
S-glycoprotein |
AuNS/EDC-NHS-8H/GO/SPE | yes | label free |
PBS |
0.1 pM −1 mM | 1.68x10-22μg/mL |
[48] |
| aptasensor | DPV | N-protein | nanoprobes/N-Protein/MCH/Dual-aptamer/GE | yes | sandwich (label-based) |
PBS |
0.025–50 ng/mL | 8.33 pg/mL |
[53] |
Table 2.
Comparison of impedimetric biosensors reported for COVID-19 diagnosis. List of abbreviations: Cu2O NCs = Cu2O nanocubes; Prot A = protein A; WO3 = tungsten oxide; TFGE = thin film gold electrode; GA = glutaraldehyde; ACE2 = angiotensin-converting enzyme-2; ABB = affinity-based biosensor; PFDT = 1H,1H,2H,2H-perfluorodecanethiol; PCB-AuE = printed circuit board gold electrode; VTM = viral transport medium.
| Sensor | Technique | Biomarker |
Biosen sor Platform |
Nano-sensor | Biosensor format |
Sample |
Linear range | LOD |
References |
|---|---|---|---|---|---|---|---|---|---|
| Immunosensor | EIS |
S-protein |
EDC-NHS/rGO/GC/S-protein Ab | yes | label free |
PBS |
0.16–1.25 μg/mL 2.5–40 μg/mL |
150 ng/mL |
[55] |
| immunosensor | EIS |
S-protein |
BSA/S-protein Ab/ ProtA/Cu2O NCs/SPCE |
yes | label free |
PBS |
0.25 fg/ml −1 μg/mL | 0.04 fg/mL |
[56] |
| aptasensor | EIS | S-protein | TFGE/SARS-CoV-2 Optimer | no | label free | PBS | – | – | [57] |
| VIP-sensor | EIS |
SARS-CoV-2 virus |
CNTs/WO3/SPCE |
yes | label free |
PBS |
– | 57 pg/mL 3.7 copy/mL |
[61] |
| ABB | EIS |
S-protein |
ACE2/GA/SPE/ACE2 | no | label free |
PBS saliva |
10 fg/mL-100 ng/mL 100 fg/mL-100 ng/mL |
2.18 fg/mL 1.39 pg/mL |
[63] |
| ABB | EIS | S-protein | EDC/NHS/AuSPE/ACE2 | no | label free | PBS | 700–1500 ng/mL 1500–7000 ng/mL |
299.3 ng/mL | [64] |
| ABB | EIS | S-protein | PFDT-SAM/PCB-AuE/ ACE2 |
no | label free | PBS VTM |
– | 1.68 ng/mL 38.6 copies/mL |
[65] |
3. Conclusions and future perspectives
This review describes the two main electrochemical sensing modes used in recent electrochemical biosensors for COVID-19 detection, highlighting the significant advances in this field.
Both voltametric and impedimetric sensors are powerful, non-destructive and sensitive techniques which can be used to study the electrical properties of the sensing device interface. Unlike voltametric mode-based biosensors, the application of the impedimetric-mode as a transduction technology has allowed the label-free detection. This is a great advantage as the labeling process may need extra reagents and extra preparation processes, thus enhancing the overall time and costs. However, the impedimetric-mode shows the disadvantages to generally require longer times of analysis (10–30 min, compared to 2–3 min of the voltametric-mode biosensors) and a more expensive instrumentation.
Due to their simple design and tunable properties, both methods can utilize the classical capture elements as biorecognition elements, such as antibody and DNA, but also different biomolecules such as aptamers, receptors, which are more suitable to cope with the different mutations of the SARS-CoV-2 virus, thus enhancing the accuracy of the sensors. Voltametric aptasensors are among the most sensitive biosensors, allowing the detection of the femtomolar. Similar sensitivity was reached with the impedimetric biosensor based on the specific ACE2 receptor [64].
The incorporation of nanomaterials (graphene, carbon nanotubes) has resulted in their improved sensitivity and selectivity [69], [70]. Biosensors with biomolecules immobilized on different functional nanomaterials display an increased number of binding sites, and therefore enhanced stability, and facile electron transfer. The interaction between two unique materials, nano and biological, represents the key point of the biosensor performances, as demonstrated by Liv et al. [37] with the nano-immunosensor, which yielded attomolar range.
In conclusion, both voltametric and impedimetric-mode based biosensors for COVID-19 are promising alternatives to currently available point-of-care (POC) tests [71]. However, in addition to the limitations highlighted, none of the reviewed sensors met the WHO acceptable minimum requirement for true positive and true negative detection rates, stated as 80% and 97%, respectively [72], [73]. Some of the sensors met the former requirement, but none met the latter. Therefore, the detection accuracies (particularly the true negative/false positive rate) need to be significantly improved before the sensors could be translated into POC devices for commercial use.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The author thanks Sapienza University of Rome for financial support.
References
- 1.Zhu N., Zhang D., Wang W., Li X., Yang B., Song J., Zhao X., Huang B., Shi W., Lu R., Niu P., Zhan F., Ma X., Wang D., Xu W., Wu G., Gao G.F., Tan W. A novel coronavirus from patients with pneumonia in China, 2019. N. Engl. J. Med. 2020;382:727–733. doi: 10.1056/nejmoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang L., Wang Y., Ye D., Liu Q. Review of the 2019 novel Coronavirus (SARS-CoV-2) based on current evidence. Int. J. Ant. Agents. 2020;55 doi: 10.1016/j.ijantimicag.2020.105948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.WHO, COVID-19 Weekly Epidemiological Update 35, World Heal. Organ., 2021, pp. 1–3, https://www.who.int/docs/default-source/coronaviruse/situation reports/weekly_epidemiological_update_22.pdf.
- 4.Wang C., Horby P.W., Hayden F.G., Gao G.F. A novel coronavirus outbreak of global health concern. Lancet. 2020;395:470–473. doi: 10.1016/S0140-6736(20)30185-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Axell-House D.B., Lavingia R., Rafferty M., Clark E., Amirian E.S., Chiao E.Y. The estimation of diagnostic accuracy of tests for COVID-19: a scoping review. J. Infect. 2020;81:681–697. doi: 10.1016/j.jinf.2020.08.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Udugama B., Kadhiresan P., Kozlowski H.N., Malekjahani A., Osborne M., Li V.Y.C., Chen H., Mubareka S., Gubbay J.B., Chan W.C.W. Diagnosing COVID-19: The disease and tools for detection. ACS Nano. 2020;14:3822–3835. doi: 10.1021/acsnano.0c02624. [DOI] [PubMed] [Google Scholar]
- 7.Cui F., Zhou H.S. Diagnostic methods and potential portable biosensors for coronavirus disease 2019. Bios. Bioelectron. 2020;165 doi: 10.1016/j.bios.2020.112349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nguyen N.N.T., McCarthy C., Lantigua D., Camci-Unal G. Development of Diagnostic Tests for Detection of SARS-CoV-2. Diagnostics. 2020;10:905. doi: 10.3390/diagnostics10110905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.R. Antiochia, Paper-based biosensors: Frontiers in Point-of-Care detection of COVID-19 disease biosensors, 11 (2021) 110, https://doi.org/10.3390/bios11040110. [DOI] [PMC free article] [PubMed]
- 10.Antiochia R. Nanobiosensors as new diagnostic tools for SARS, MERS and COVID-19: From past to perspectives. Microchim. Acta. 2020;187:639. doi: 10.1007/s00604-020-04615-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mak G.C., Cheng P.K., Lau S.S., Wong K.K., Lau C., Lam E.T., Chan R.C., Tsang D.N. Evaluation of rapid antigen test for detection of SARS-CoV-2 virus. J. Clin. Virol. 2020;129 doi: 10.1016/j.jcv.2020.104500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Scohy A., Anantharajah A., Bodéus M., Kabamba-Mukadi B., Verroken A., Rodriguez-Villalobos H. Low performance of rapid antigen detection test as frontline testing for COVID-19 diagnosis. J. Clin. Virol. 2020;129 doi: 10.1016/j.jcv.2020.104455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Antigen-Detection in the Diagnosis of SARS-CoV-2 infection using rapid immunoassays, https://www.who.int/publications/i/item/antigen-detection-in-the-diagnosis-of-sars-cov-2 infection-using-rapid-immunoassays.
- 14.Drain P.K., Hyle E.P., Noubary F., Freedberg K.A., Wilson D., Bishai W., Rodriguez W., Bassett I.V. Evaluating diagnostic Point-of-Care tests in resource-limited settings. Lancet Inf. Dis. 2014;14:239–249. doi: 10.1016/S1473-3099(13)70250-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gubala V., Harris L.F., Ricco A.J., Tan M.X., Williams D.E. Point of care diagnostics: Status and future. Anal. Chem. 2012;84:487–515. doi: 10.1021/ac2030199. [DOI] [PubMed] [Google Scholar]
- 16.Naseri M., Zioria Z.M., Simon G.P., Batchelor W. ASSURE-complisnt point-of-care diagnostics for the detection of human viral infections. Rev. Med. Virol. 2021;32 doi: 10.1002/rmv.2263. [DOI] [Google Scholar]
- 17.K.J. Land, D.I. Boeras, X.-S. Chen, A.R. Ramsay, W. R. Peeling, REASSURED diagnostics to inform disease control strategies, strengthen health systems and improve patient outcomes, Nature Microbiology 4 (2019) 46-54, https://doi.org/10.1038/s41564-018-0295-3. 10. 110.1038/s41564-018-0295-3038/s41564-018-0295-3. [DOI] [PMC free article] [PubMed]
- 18.Imran S., Ahmadi S., Kerman K. Electrochemical biosensors for the detection of SARS-CoV-2 and other viruses. Micromachines. 2021;12:174. doi: 10.3390/mi12020174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kumar N., Shetti N.P., Jagannath S., Aminabhavi T.M. Electrochemical sensors for the detection of SARS-CoV-2 virus. Chem. Eng. J. 2022;430 doi: 10.1016/j.cej.2021.132966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lim W.Y., Lan B.L., Ramakrishnan N. Emerging biosensors to detect severea Respiratory syndrome Coronavirus 2 (SARS-CoV-2): A Review. Biosensors. 2021;11:434. doi: 10.3390/bios11110434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sheikhazadeh E. Electrochemical biosensors for COVID-19. Austin J. Infect. Diseases. 2021;8:1051. [Google Scholar]
- 22.Drobysh M., Ramanaviciene A., Viter R., Chen C.-F., Samukaite-Bubniene U., Ratautaite V., Ramanavicius A. Biosensors for the determination of SARS-CoV-2 virus and diagnosis of COVID-19 infection. Int. J. Mol. Sci. 2022;23:666. doi: 10.3390/ijms23020666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Leva-Bueno J., Peyman S., Millner P.A. A review on impedimetric immunosensors for pathogen and biomarker detection. Med. Microbiol. Immunol. 2020;209:343–362. doi: 10.1007/s00430-020-00668-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Seo G., Lee G., Kim M.J., Baek S.-H., Choi M., Ku K.B., Lee C.-S., Jun S., Park D., Kim H.G., Kim S.-J., Lee J.-O., Kim B.T., Park E.G., Kim S.I. Rapid detection of COVID-19 causative virus (SARS-CoV-2) in human nasopharyngeal swab specimens using field-effect transistor-based biosensor. ACS Nano. 2020;14:5135–5142. doi: 10.1021/acsnano.0c02823. [DOI] [PubMed] [Google Scholar]
- 25.Bertok T., Lorencova L., Chocholova E., Jane E., Vikartovska A., Kasak P., Tkac J. Electrochemical impedance spectroscopy based biosensors: mechanistic principles, analytical examples and challenges towards commercialization for assays of protein cancer biomarkers. ChemElectroChem. 2019;6:989–1003. doi: 10.1002/celc.201800848. [DOI] [Google Scholar]
- 26.Antiochia R. Developments in biosensors for CoV detection and future trends. Bios. Bioelectron. 2021;173 doi: 10.1016/j.bios.2020.112777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Z.O. Uygun, H.D.E. Uygun A short footnote: circuit design for Faradaic impedimetric sensors and biosensors, Sensors Actuators B Chem. 202 (2014) 448–453. https://doi.org/10.1016/j.snb.2014.05.029.
- 28.Holzinger M., Le Goff A., Cosnier S. Nanomaterials for biosensing applications: A review. Front. Chem. 2014;2:63. doi: 10.3389/fchem.2014.00063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhu C., Yang G., Li H., Du D., Lin Y. Electrochemical sensors and biosensors based on nanomaterials and nanostructures. Anal. Chem. 2015;87:230–249. doi: 10.1021/ac5039863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Balkourani G., Brouzgou A., Archonti M., Papandrianos N., Song S., Tsiakaras P. Emerging materials for the electrochemical detection of COVID-19. J. Electroanal. Chem. 2021;893 doi: 10.1016/j.jelechem.2021.115289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Alhalaili B., Popescu I.N., Kamoun O., Alzubi F., Alawadhia S., Vidu R. Nanobiosensors for the detection of novel Coronavirus 2019-nCoV and other pandemic/epidemic respiratory viruses: A review. Sensors. 2020;20:6591. doi: 10.3390/s20226591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Munoz J., Montes R., Baeza M. Trends in electrochemical impedance spectroscopy involving nanocomposite transducers: characterization, architecture surface and Bio-Sensing. TrAC, Trends Anal. Chem. 2017;97:201–215. [Google Scholar]
- 33.Fabiani L., Saroglia M., Galatà G., De Santis R., Fillo S., Luca V., Faggioni G., D’Amore N., Regalbuto E., Salvatori P., Terova G., Moscone D., Lista F., Arduini F. Magnetic beads combined with carbon black-based screen-printed electrodes for COVID-19: A reliable and miniaturized electrochemical immunosensor for SARS-CoV-2 detection in saliva. Biosens. Bioelectron. 2021;171 doi: 10.1016/j.bios.2020.112686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Eissa S., Zourub M. Development of a Low-Cost Cotton-Tipped Electrochemical immunosensor for the detection of SARS-CoV-2. Anal. Chem. 2021;93:1826–1833. doi: 10.1021/acs.analchem.0c04719. [DOI] [PubMed] [Google Scholar]
- 35.Torrente-Rodrıguez R.M., Lukas H., Tu J., Min J., Yang Y., Xu C., Rossiter H.B., Gao W. SARS-CoV-2 RapidPlex: a graphene-based multiplexed telemedicine platform for rapid and low-cost COVID-19 diagnosis and monitoring. Matter. 2020;3:1981–1998. doi: 10.1016/j.matt.2020.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mojsoska B., Larsen S., Olsen D.A., Madsen J.S., Brandslund I., Alatraktchi F.A. Rapid SARS-CoV-2 detection using electrochemical immunosensor. Sensors. 2021;21:390. doi: 10.3390/s21020390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liv L., Çoban G., Nakiboğlu N., Kocagöz T. A rapid, ultrasensitive voltammetric biosensor for determining SARS-CoV-2 spike protein in real samples. Biosens. Bioelectron. 2021;192:113497. doi: 10.1016/j.bios.2021.113497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Viswanathan S., Rani C., Ribeiro S., Delerue-Matos C. Molecular imprinted nanoelectrodes for ultrasensitive detection of ovarian cancer marker. Biosens. Bioelectron. 2012;33:179–183. doi: 10.1016/j.bios.2011.12.049. [DOI] [PubMed] [Google Scholar]
- 39.Jolly P., Tamboli V., Harniman R.L., Estrela P., Allender C.J., Bowen J.L. Aptamer–MIP hybrid receptor for highly sensitive electrochemical detection of prostate specific antigen. Biosens. Bioelectron. 2016;75:188–195. doi: 10.1016/j.bios.2015.08.043. [DOI] [PubMed] [Google Scholar]
- 40.Kidakova A., Reut J., Boroznjak R., Opik A., Syritski V. Advanced sensing materials based on molecularly imprinted polymers towards developing point-of- care diagnostics devices. Proc. Est. Acad. Sci. 2019;68:158–167. doi: 10.3176/proc.2019.2.07. [DOI] [Google Scholar]
- 41.Raziq A., Kidakova A., Boroznjak R., Reut J., Öpik A., Syritski V. Development of a portable MIP-based electrochemical sensor for detection of SARS-CoV-2 antigen. Biosens. Bioelectron. 2021;178 doi: 10.1016/j.bios.2021.113029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chaibun T., Puenpa J., Ngamdee T., Boonapatcharoen N., Athamanolap P., O’Mullane A.P., Vongpunsawad S., Poovorawan Y., Lee S.Y., Lertanantawong B. Rapid electrochemical detection of coronavirus SARS-CoV-2. Nat. Commun. 2021;12 doi: 10.1038/s.41467-021-2121-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Khan P., Aufdembrink L.M., Engelhart A.E. Isothermal SARS-CoV-2 diagnostics: Tools for enabling distributed pandemic testing as a means of supporting safe reopenings. ACS Synth. Biol. 2020;9:2861–2880. doi: 10.1021/acssynbio.0c00359. [DOI] [PubMed] [Google Scholar]
- 44.Pan Y., Zhang D., Yang P., Poon L.L.M., Wang Q. Viral load of SARS- CoV-2 in clinical samples. Lancet Infect. Dis. 2020;20:411–412. doi: 10.1016/S1473-3099(20)30113-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lukas H., Yu C., Yu Y., Gao W. Emergin telemedicine tolls for remote COVID-19 diagnosis, monitoring and management. ACSNano. 2020;14:16180–16193. doi: 10.1021/acsnano.0c08494. [DOI] [PubMed] [Google Scholar]
- 46.Madrid R.E., Ashur Ramallo F., Barraza D.E., Chaile R.E. Smartphone-based biosensor devices for healthcare: Technologies, trends, and adoption by end-users. Bioengineering. 2022;9(3):101. doi: 10.3390/bioengineering9030101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhao H., Liu F., Xie W., Zhou T.-C., OuYang J., Jin L., Li H., Zhao C.-Y., Zhang L., Wei J. Ultrasensitive supersandwich-type electrochemical sensor for SARS-CoV-2 from the infected COVID-19 patients using a smartphone. Sens. Actuators, B. 2021;327 doi: 10.1016/j.snb.2020.128899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Alafeef M., Dighe K., Moitra P., Pan D. Rapid, ultrasensitive, and quantitative detection of SARS-CoV-2 using antisense oligonucleotides directed electrochemical biosensor chip. ACS Nano. 2020;14:17028–17045. doi: 10.1021/acsnano.0c06392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hashemi S.A., Golab Behbahan N.G., Bahrani S., Mousavi S.M., Gholami A., Ramakrishna S., Firoozsani M., Moghadami M., Lankarani K.B., Omidifar N. Ultra-sensitive viral glycoprotein detection NanoSystem toward accurate tracing SARS-CoV-2 in biological/non-biological media. Biosens. Bioelectron. 2021;171 doi: 10.1016/j.bios.2020.112731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chang C.-C. Recent advancements in aptamer-based surface resonance biosensing. Biosensors. 2021;11:233. doi: 10.3390/bios11070233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Krishnan S., Narasimhan A.K., Gangodkar D., Dhanasekaran S., Jha N.K., Dua K., Thakur V.K., Gupta P.K. Aptameric nanobiosensors for the diagnosis of COVID-19: An update. Mater. Lett. 2022;308 doi: 10.1016/j.matlet.2021.131237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Divya, D.S. Dkhar, R. Kumari, S. Mahapatra, R. Kumar, P. Chandra, Ultrasensitive Aptasensors for the Detection of Viruses Based on Opto-Electrochemical Readout Systems, Biosensors 12 (2022) 81 https:// doi.org/10.3390/bios12020081. [DOI] [PMC free article] [PubMed]
- 53.Mandal M., Dutta N., Dutta G. Aptamer-based biosensors and their implications in COVID-19 diagnosis. Anal. Methods. 2021;13:5400–5417. doi: 10.1039/D1AY01519B. [DOI] [PubMed] [Google Scholar]
- 54.Tian J., Liang Z., Hu O., He Q., Sun D., Chen Z. An electrochemical dual-aptamer biosensor based on metal-organic frameworks MIL-53 decorated with Au@ Pt nanoparticles and enzymes for detection of COVID-19 nucleocapsid protein. Electrochim. Acta. 2021;387 doi: 10.1016/j.electacta.2021.138553. [DOI] [Google Scholar]
- 55.Antiochia R., Favero G., Conti M.E., Mazzei F., Tortolini C. Affinity-based biosensors for pathogenic bacteria detection. Int. J. Environmental. Technol. Manag. 2015;18:185–206. [Google Scholar]
- 56.Zaccariotto G., Silva M.K.L., Rocha G.S., Cesarino I. A novel method for the detection of SARS-CoV-2 based on graphene-impedimetric immunosensor. Materials. 2021;14:4230. doi: 10.1016/j.jviromet.2021.114163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rahmati Z., Roushani M., Hosseini H., Choobin H. Electrochemical immunosensor with Cu2O nanocube coating for detection of SARS-CoV-2 spike protein. Microchim. Acta. 2021;188:105. doi: 10.1007/s00604-021-04762-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lasserre P., Balansethupathy B., Vezza V.J., Butterworth A., Macdonald A., Blair E.O., McAteer L., Hannah S., Ward A.C., Hoskisson P.A., Longmuir A., Setford S., Farmer E.C.W., Murphy M.E., Flynn H., Corrigan D.K. SARS-CoV–2 aptasensors based on electrochemical impedance spectroscopy and low-cost gold electrode substrates. Anal. Chem. 2022;94:2126–2133. doi: 10.1021/acs.analchem.1c04456è. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Crapnell R.D., Dempsey-Hibbert N.C., Peeters M., Tridente A., Banks C.E. Molecularly imprinted polymer based electrochemical biosensors: Overcoming the challenges of detecting vital biomarkers and speeding up diagnosis. Talanta. 2020;2 doi: 10.1016/j.talo.2020.100018. [DOI] [Google Scholar]
- 60.Nawaz N., Bakar N.K.A., Mahmud H.N.M.E., Jamaludin N.S. Molecularly imprinted polymers-based DNA biosensors. Anal. Biochem. 2021;630 doi: 10.1016/j.ab.2021.114328. [DOI] [PubMed] [Google Scholar]
- 61.Ayankojo K.G., Boroznjak R., Reut J., Opik A., Syritsk V. Molecularly imprinted polymer based electrochemical sensor for quantitative detection of SARS-CoV-2 spike protein. Sens. Act. B. 2022;353 doi: 10.1016/j.snb.2021.131160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hussein H.A., Kandeil A., Gomaa M., El Nashar R.M., El-Sherbiny I.M., Hassan R.Y.A. SARS-CoV-2-impedimetric biosensor: virus-imprinted chips for early and rapid diagnosis. ACS Sensors. 2021;6:4098–4107. doi: 10.1021/acssensors.1c01614. [DOI] [PubMed] [Google Scholar]
- 63.Limsakul P., Charupanit K., Moonla C., Jeerapan I. Advances in emergent biological recognition elements and bioelectronics for diagnosing COVID-19. Emergent Mater. 2021;4:231–247. doi: 10.1007/s42247-021-00175-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Torres M.D.T., de Araujo W.R., de Lima L.F., Ferreira A.L., de la Fuente-Nunez C. Low-cost biosensor for rapid detection of SARS-CoV-2 at the point of care. Matter. 2021;4:2403–2416. doi: 10.1016/j.matt.2021.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tepeli Büyüksünetçi Y., Çitil B.E., Anık Ü. An impedimetric approach for COVID-19 detection. Analyst. 2021;147(1):130–138. doi: 10.1039/d1an01718g. [DOI] [PubMed] [Google Scholar]
- 66.Vezza V.J., Butterworth A., Lasserre P., Blair E.O., MacDonald A., Hannah S., Rinaldi C., Hoskisson P.A., Ward A.C., Longmuir A., Setford S., Farmer E.C.W., Murphy M.E., Corrigan D.K. An electrochemical SARS-CoV-2 biosensor inspired by glucose test strip manufacturing processes. ChemComm. 2021;57(30):3704–3707. doi: 10.1039/d1cc00936b. [DOI] [PubMed] [Google Scholar]
- 67.Miodek A., Regan E.M., Bhalla N., Hopkins N.A.E., Goodchild S.A., Estrela P. Optimization and characterization of anti-fouling ternary SAM layers for impedance-based aptasensors. Sensors. 2015;15:25015–25032. doi: 10.3390/s151025015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hill C., Thuret J.-Y. The sensitivity and costs of testing for SARS-CoV-2 infection with saliva versus nasopharyngeal swabs. Ann. Intern. Med. 2021;174:582. doi: 10.7326/L21-0092. [DOI] [PubMed] [Google Scholar]
- 69.del Caño R., García-Mendiola T., García-Nieto D., Álvaro R., Luna M., Iniesta H.A., Coloma R., Diaz C.R., Milán-Rois P., Castellanos M., Abreu M., Cantón R., Galán J.C., Pineda T., Pariente F., Miranda R., Somoza Á., Lorenzo E. Amplification-free detection of SARS-CoV-2 using gold nanotriangles functionalized with oligonucleotides. Microchim. Acta. 2022;189(4) doi: 10.1007/s00604-022-05272-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Martínez-Periñán E., García-Mendiola T., Enebral-Romero E., del Caño R., Vera-Hidalgo M., Vázquez Sulleiro M., Navío C., Pariente F., Pérez E.M., Lorenzo E. A MoS2 platform and thionine-carbon nanodots for sensitive and selective detection of pathogens. Biosens. Bioelectron. 2021;189:113375. doi: 10.1016/j.bios.2021.113375. [DOI] [PubMed] [Google Scholar]
- 71.Kaci K., del Caño R., Luna M., Milán-Rois P., Castellanos M., Abreu M., Cantón R., Galán J.C., Somoza Á., Miranda R., González de Rivera G., García-Mendiola T., Lorenzo E. Paving the way to point of care (POC) devices for SARS-CoV-2 detection. Talanta. 2022;247:123542. doi: 10.1016/j.talanta.2022.123542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Antigen-Detection in the Diagnosis of SARS-CoV-2 Infection Using Rapid Immunoassays, https://www.who. int/publications/i/item/antigen-detection-in-the-diagnosis-of-sars-cov-2infection-using-rapid-immunoassays (accessed on 19 May 2021).
- 73.WHO, Recommendations for national SARS-CoV-2 testing strategies and diagnostic capacities, 2021, https://apps.who.int/iris/bitstream/handle/10665/342002/WHO-2019-nCoV-lab-testing-2021.1-eng.pdf?sequence=1&isAllowed=y.