Abstract
Background
: Humoral and cellular immune responses to SARS-CoV-2 vaccination among immunosuppressed patients remain poorly defined, as well as variables associated with poor response.
Methods
: We performed a retrospective observational cohort study at a large Northern California healthcare system of infection-naïve individuals fully vaccinated against SARS-CoV-2 (mRNA-1273, BNT162b2, or Ad26.COV2.S) with clinical SARS-CoV-2 interferon gamma release assay (IGRA) ordered between January through November 2021. Humoral and cellular immune responses were measured by anti-SARS-CoV-2 S1 IgG ELISA (anti-S1 IgG) and IGRA, respectively, following primary and/or booster vaccination.
Results
: 496 immunosuppressed patients (54% female; median age 50 years) were included. 62% (261/419) of patients had positive anti-S1 IgG and 71% (277/389) had positive IGRA after primary vaccination, with 20% of patients having a positive IGRA only. Following booster, 69% (81/118) had positive anti-S1 IgG and 73% (91/124) had positive IGRA. Factors associated with low humoral response rates after primary vaccination included anti-CD20 monoclonal antibodies (P < 0.001), sphingosine 1-phsophate (S1P) receptor modulators (P < 0.001), mycophenolate (P = 0.002), and B cell lymphoma (P = 0.004); those associated with low cellular response rates included S1P receptor modulators (P < 0.001) and mycophenolate (P < 0.001). Of patients who had poor humoral response to primary vaccination, 35% (18/52) developed a significantly higher response after the booster. Only 5% (2/42) of patients developed a significantly higher cellular response to the booster dose compared to primary vaccination.
Conclusions
: Humoral and cellular response rates to primary and booster SARS-CoV-2 vaccination differ among immunosuppressed patient groups. Clinical testing of cellular immunity is important in monitoring vaccine response in vulnerable populations.
Keywords: COVID-19, SARS-CoV-2, Vaccination, IGRA, Serology, Immunosuppression
Abbreviations
- CLL
chronic lymphocytic leukemia
- CVID
common variable immunodeficiency
- HCW
healthcare worker
- HSCT
hematopoietic stem cell transplant
- Ig
immunoglobulins
- IgG assay
anti-S1 IgG enzyme-linked immunosorbent assay (Euroimmun)
- IGRA
interferon-γ (INF-γ) release assay (Stanford)
- ISMT
immune-suppressive/modulatory therapy
- ISP
immunosuppressed patient
- mAbs
monoclonal antibodies
- MBL
monoclonal B cell lymphocytosis
- MDS
myelodysplastic syndrome
- MGUS
monoclonal gammopathy of unknown significance
- MPN
myeloproliferative neoplasm
- MS
multiple sclerosis
- NISP
non-immunosuppressed patient
- NMO
neuromyelitis optica
- S1P
sphingosine 1-phosphate
- SLE
systemic lupus erythematosus
- SLL
small lymphocytic lymphoma
1. Introduction
One of every 25 individuals in the U.S. is immunocompromised[1] and at increased risk for severe COVID-19[2]. Studies have demonstrated poor humoral response to SARS-CoV-2 vaccination in immunosuppressed patients[3,4]. Cellular, or T cell vaccine responses appear to be less impaired in certain immunosuppressed groups, but are less well characterized[5], [6], [7]. The importance of T cell-mediated immunity in protection against emerging SARS-CoV-2 variants is becoming clear, highlighting the need to better characterize this branch of immunity[8,9]. Furthermore, due to the decline in antibody titers over time after vaccination, booster shots have been recommended for all adults[10,11]. Boosters are thought to be especially important for immunosuppressed patients due to their impaired response to primary vaccination[12,13]. Serologic assays and interferon gamma release assays (IGRAs) are robust methods for assessing humoral and cellular response, respectively, to SARS-CoV-2 infection or immunization[14], [15], [16], [17], [18]. Here, we use these assays to compare the humoral and cellular immune responses to SARS-CoV-2 after primary and booster vaccination among immunosuppressed patients. By retrospective analysis, we identify immunosuppressive factors that contribute to impaired response.
2. Methods
2.1. Ethics statement
This study was approved by the Stanford University Institutional Review Board (IRB-60,171 and IRB-57,519). Informed consent was obtained from volunteer healthcare workers before blood collection.
2.2. Study design
Assay design and interpretation for the anti-SARS-CoV-2 S1 IgG (anti-S1 IgG) assay, ACE2 blocking activity assay, and SARS-CoV-2 interferon gamma (IFN-γ) release assay (IGRA) are described in Supplementary Methods. We retrospectively assessed patients with IGRA ordered as part of clinical testing at Stanford Health Care from January 1, 2021 through November 15, 2021, and recorded available anti-S1 IgG antibody results. Those patients with anti-S1 IgG or IGRA results collected at least 14 days following receipt of the second dose of either the Moderna mRNA-1273 or the Pfizer/BioNTech BNT162b2 mRNA vaccines or single dose of the Janssen Ad26.COV2.S vaccine were included in the primary vaccination analysis. One additional dose with any of the three vaccines was considered a booster dose. Patients with anti-S1 IgG or IGRA collected at least seven days following receipt of a booster dose were included in the booster analysis.
Electronic medical record (EMR) data collection was performed by one of five physician authors (NB, PB, CC, MR, LY) on underlying disease and immune-suppressive/modulatory therapy (ISMT), including chemical drugs, biologics, and cellular therapy, such as hematopoietic stem cell transplant (HSCT) and CAR-T. Patients without immunosuppressive diseases or history of ISMT use were included in the NISP (non-immunosuppressed patient) cohort. All other patients were included in the ISP (immunosuppressed patient) cohort. Patients with a documented history of SARS-CoV-2 infection or without vaccination, disease, or therapy documentation in the EMR were excluded from the analysis. Immunocompetent healthcare worker (HCW) volunteers without known history of SARS-CoV-2 infection served as reference. Anti-S1 IgG and IGRA were collected between 14 and 25 days and then at approximately 5- and 9-months following primary vaccination with BNT162b2. Anti-S1 IgG and IGRA were collected between 8- and 34-days following receipt of a booster dose of the BNT162b2 vaccine in a subset of these HCWs. Additional details can be found in Supplementary Methods.
2.3. Statistical analysis
Statistical analyses and graphing were performed in Python version 3.8.5 using the packages pandas, matplotlib, seaborn, numpy, scipy, and statsmodels. Linear regression was performed by method of ordinary least squares. Unless otherwise indicated, Fisher exact test was used for all statistical comparisons, and α = 0.05. See Supplementary Methods for details.
We focused the analysis on anti-S1 IgG and IGRA positivity rates, rather than quantitative values, which decline over time (Supplementary Figure 1)[10,19]. The HCW cohort anti-S1 IgG values were used to establish a reference range for expected (immunocompetent) levels over time after primary vaccination. A cutoff was established based on this reference range for “high” IgG, indicating expected IgG levels over time, and “low” IgG (Supplementary Methods). The same cutoff was not established for IGRA results due to high inter-individual variability.
3. Results
A total of 496 patients were included in this study. Cohort sample size and assay result availability are presented in Supplementary Table 5.
3.1. Vaccine response after primary vaccination
Demographic information and assay results from 18 HCWs, 28 NISPs, and 427 ISPs following primary vaccination are displayed in Table 1 (see also Supplementary Tables 6,7). In 381 ISPs with both anti-S1 IgG and IGRA results available, 51.4% were positive for both (196), 17.6% were negative for both (67), 19.7% were IGRA positive only (75), and 11.3% were IgG positive only (43).
Table 1.
Demographic information and assay results for cohorts after primary vaccination.
| Disease Categories | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| HCW (n = 18) | NISP (n = 28) | ISP (n = 427) | Active Heme Malignancy (n = 29) | Inactive Heme Malignancy (n = 65) | Primary anemia (n = 9) | Solid Malignancy (n = 24) | Solid Organ Transplant (n = 67) | Autoimmune Disease (n = 149) | 1° Immuno-deficiency (n = 42) | Multiple Categories (n = 42) | |
| On ISMT,% (n) | 0 (0) | 0 (0) | 69 (295) | 59 (17) | 45 (29) | 11 (1) | 46 (11) | 100 (67) | 89 (133) | 2 (1) | 86 (36) |
| Male:female ratio | 0.5:1 | 1.5:1 | 0.8:1 | 1.9:1 | 1.3:1 | 0.5:1 | 0.8:1 | 1.5:1 | 0.5:1 | 1.0:1 | 0.6:1 |
| Age, median (IQR), years | 43.5 (38.2–47.8) | 44.0 (35.8–64.2) | 49.0 (24.0–64.0) | 56.0 (29.0–70.0) | 39.0 (19.0–65.0) | 19.0 (16.0–24.0) | 20.5 (15.5–63.2) | 41.0 (17.0–59.5) | 53.0 (38.0–63.0) | 47.0 (32.8–65.5) | 57.5 (35.0–71.0) |
| IgG days since vaccine*, median (IQR) | 20.0 (17.0–21.0) | 158.0 (62.5–194.5) | 80.0 (41.0–127.0) | 48.0 (32.0–97.0) | 48.0 (32.8–95.8) | 78.0 (41.0–102.0) | 99.0 (53.2–129.5) | 66.0 (39.5–105.0) | 108.5 (62.8–152.2) | 81.0 (41.0–118.0) | 81.0 (45.5–122.5) |
| Anti-S1 IgG, median (IQR), OD ratio | 9.6 (9.3–9.9) | 5.7 (3.6–8.2) | 2.8 (0.2–7.1) | 0.7 (0.1–3.9) | 5.1 (0.3–8.4) | 8.5 (6.8–8.9) | 7.2 (4.9–8.7) | 3.3 (0.3–7.7) | 1.2 (0.2–5.2) | 3.5 (1.8–7.0) | 1.6 (0.2–6.1) |
| Anti-S1 IgG,% positive (n) | 100 (18/18) | 100 (27/27) | 62.3 (261/419) | 50 (14/28) | 69 (44/64) | 89 (8/9) | 88 (21/24) | 66 (44/67) | 53 (76/144) | 78 (32/41) | 52 (22/42) |
| ACE2 Blocking%, median (IQR) | 68.0 (50.0–90.8) | 12.0 (0.0–85.0) | 0.0 (0.0–43.8) | 0.0 (0.0–4.0) | 14.5 (0.0–94.8) | 100.0 (33.0–100.0) | 28.0 (0.0–85.5) | 0.5 (0.0–62.2) | 0.0 (0.0–10.2) | 0.0 (0.0–43.0) | 0.0 (0.0–22.0) |
| ACE2 Blocking,% positive (n) | 94 (17/18) | 44 (12/27) | 32.0 (134/419) | 18 (5/28) | 47 (30/64) | 78 (7/9) | 54 (13/24) | 34 (23/67) | 22 (31/144) | 34 (14/41) | 26 (11/42) |
| IGRA days since vaccine†, median (IQR) | 20.0 (17.0–21.0) | 185.0 (148.8–203.5) | 98.0 (56.0–142.0) | 86.5 (47.0–131.5) | 70.0 (41.8–121.2) | 78.0 (41.0–102.0) | 99.0 (60.2–137.2) | 81.0 (58.0–119.5) | 115.0 (78.0–156.0) | 83.5 (49.0–119.2) | 99.5 (64.0–147.5) |
| IFN-γ Response median (IQR), IU/mL | 1.3 (1.1–5.6) | 2.3 (1.4–4.3) | 1.9 (0.2–5.2) | 0.5 (0.1–4.4) | 2.8 (0.7–6.4) | 3.3 (3.0–8.3) | 4.6 (1.1–9.4) | 0.8 (0.1–2.3) | 2.2 (0.5–5.7) | 3.2 (0.4–7.0) | 0.4 (0.1–3.1) |
| IFN-γ Response,% positive (n) | 100 (18/18) | 92 (24/26) | 71.2 (277/389) | 58 (15/26) | 80 (45/56) | 100 (9/9) | 82 (18/22) | 58 (34/59) | 77 (106/137) | 75 (30/40) | 50 (20/40) |
Note: not all patients had both anti-S1 IgG and IGRA results available.
*Number of days elapsed between primary vaccination and anti-S1 IgG testing.
†Number of days elapsed between primary vaccination and IGRA testing.
3.2. Immunosuppressive factors associated with poor vaccine response
Results of a systematic screen of immunosuppressive factors associated with low and high rates of humoral and cellular response are shown in Supplementary Tables 8–11 and Supplementary Figures 3,4. Notable factors associated with a low rate of humoral and cellular response include S1P receptor modulators (IgG: n = 11, P < 0.001; IGRA: n = 11, P < 0.001), mycophenolate (IgG: n = 78, P=.002; IGRA: n = 69, P < 0.001), and systemic steroids (IgG: n = 103, P=.002; IGRA: n = 93, P < 0.001). Anti-CD20 mAbs (n = 48, P < 0.001), B cell lymphoma (n = 55, P = 0.004), and immunoglobulins (n = 50, P = 0.01) were associated with a low rate of humoral response only, while calcineurin inhibitors (n = 89, P < 0.001) and antimitotics (n = 13, P < 0.009) were associated with a low rate of cellular response only.
Results of multivariable linear regression analysis to identify factors associated with humoral and cellular response are presented in Supplementary Table 12,13.
3.3. Subgroup analysis to verify findings of immunosuppressive screen and regression modeling
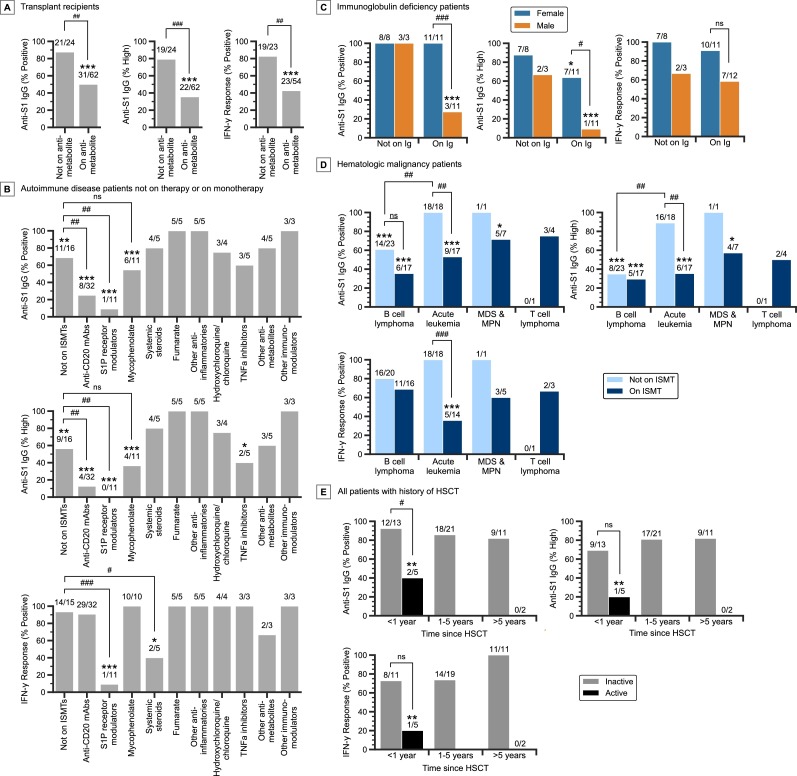
86 solid organ transplant recipients were on ISMT regimens consisting of tacrolimus (n = 75), mycophenolate (61), systemic steroids (52), sirolimus (5), cyclosporine (5), everolimus (3), or azathioprine (1). Of these patients, the addition of antimetabolites to a drug regimen consisting of calcineurin or mTOR inhibitors, with or without systemic steroids, is associated with low humoral and cellular response, as patients not receiving antimetabolites were 88% IgG positive (21/24), 79% IgG high (19/24), and 83% IGRA positive (19/23), while those on antimetabolites (n = 61 mycophenolate, n = 1 azathioprine) were 50% IgG positive (31/62, P = .001), 35% IgG high (22/62, P < 0.001), and 43% IGRA positive (23/54, P = 0.001) (Fig. 1 A). Further stratifying transplant recipients by age, time between transplantation and vaccination, and organ type showed that age and the amount of time between transplant and vaccination may have small effects on immune response (Supplementary Figures 5,6).
Fig. 1.
Subgroup analysis of immunosuppressed patients following primary vaccination. A)Vaccine response rates in transplant recipients not on antimetabolites and those on antimetabolites (61 on mycophenolate, 1 on azathioprine). Only transplant recipients on a calcineurin inhibitor, mTOR inhibitor, mycophenolate, or other antimetabolites are plotted. B) Vaccine response rates of autoimmune disease patients, without other immunosuppressive conditions, on monotherapy with various ISMTs. Only ISMTs that apply to three or more patients are plotted. C) Vaccine response rates of immunoglobulin deficiency patients, without other immunosuppressive conditions, stratified by immunoglobulin use and sex. D) Vaccine response rates of patients with active and inactive heme malignancy, without other immunosuppressive conditions, stratified by disease subcategory and therapy status. Only disease subcategories that apply to three or more patients are plotted. E) Vaccine response rates of all patients with a history of HSCT, stratified by time between HSCT and vaccination, and activity of the malignancy. Patients with inactive disease include nine patients with primary anemia and one patient with germ cell tumor. ns, not significant; #P < 0.05, ##P < 0.01, ###P < 0.001 for pairwise comparisons as indicated; *P < 0.05, **P < 0.01, ***P < 0.001 compared to NISP, Fisher exact test. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Vaccine responses of 110 autoimmune disease patients, not affected by diseases of any other category and either on monotherapy or not actively on ISMT, are shown in Fig. 1B. Compared to autoimmune patients not on ISMT, patients on S1P receptor modulators had low humoral response rates, at 9% IgG positive (1/11, P = 0.004) and 0% IgG high (0/11, P = 0.003). Likewise, patients on anti-CD20 mAbs were 25% IgG positive (8/32, P = 0.005) and 13% IgG high (4/32, P = 0.004). Importantly, while patients on S1P receptor modulators were 9% IGRA positive (1/11, P < 0.001), those on anti-CD20 mAbs were 91% IGRA positive (29/32). Compared to autoimmune disease patients not on ISMT, those on systemic steroids had low cellular response, at 40% IGRA positive (2/5, P = 0.03), while those on fumarate (5/5) and other anti-inflammatories (5/5) had 100% positive humoral and cellular responses. Autoimmune patients on mycophenolate had lower rates of humoral response compared to NISPs, at 55% IgG positive (6/11, P < 0.001) and 36% IgG high (4/11, P < 0.001), but were 100% IGRA positive (10/10). Notably, the vaccine response rates in patients on mycophenolate are comparable to those of autoimmune disease patients not on ISMTs.
Analysis of 41 patients with immunoglobulin deficiency, without other immunosuppressive diseases and not on any ISMT, showed that female patients on immunoglobulin therapy were 100% IgG positive (11/11), 64% IgG high (7/11), and 91% IGRA positive (10/11). However, male patients had much lower rates especially with humoral response, at 27% IgG positive (3/11, P = 0.001), 9% IgG high (1/11, P = 0.02), and 58% IGRA positive (7/12, P = 0.15) (Fig. 1C, Supplementary Figure 7).
Analysis of 94 patients with active and inactive hematologic malignancy showed that in patients not on ISMT, those with B cell lymphoma were 61% IgG positive (14/23) and 35% IgG high (8/23), while those with acute leukemia (12 B-ALL, 5 AML, and 1 MPAL) had much higher rates, at 100% IgG positive (18/18, P = 0.002) and 89% IgG high (16/18, P < 0.001) (Fig. 1D, Supplementary Figure 8). The cellular response rates in these patients were both high, however, with B cell lymphoma patients at 80% IGRA positive (16/20) and acute leukemia patients at 100% IGRA positive (18/18, P = 0.11).
While acute leukemia patients not on ISMTs had high rates of humoral and cellular response, those on ISMTs (9 B-ALL, 5 AML, and 3 T-ALL) had much lower rates, at 53% IgG positive (9/17, P = 0.001), 35% IgG high (6/17, P = 0.002), and 36% IGRA positive (5/14, P < 0.001) (Fig. 1D).
For 52 patients with a history of HSCT, which included 42 patients with hematologic malignancy, nine with primary anemia, and one with germ cell tumor, analysis showed that those without active hematologic malignancy had high rates of humoral and cellular response, at 87% IgG positive (39/45), 78% IgG high (35/45), and 80% IGRA positive (33/41). By comparison, HSCT patients with active heme malignancy (either newly diagnosed or relapsed/residual disease) had much lower rates of response, at 29% IgG positive (2/7, P = 0.003), 14% IgG high (1/7, P = 0.002), and 14% IGRA positive (1/7, P = 0.001). Patients who received HSCT within one year prior to vaccination, without active hematologic malignancy, were 92% IgG positive (12/13), 69% IgG high (9/13), 73% IGRA positive (8/11) (Fig. 1E). These response rates were comparable to those who received HSCT greater than one year prior to vaccination.
3.4. Analysis of the rate of decline in quantitative vaccine response over time after primary vaccination
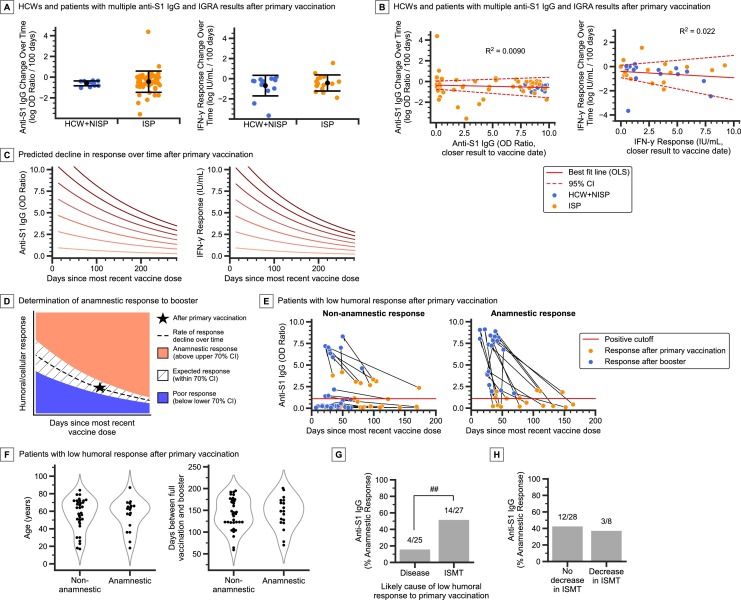
Patients with multiple anti-S1 IgG and IGRA results after primary vaccination showed that the average rates of decline for both humoral and cellular response in ISPs were comparable to those of HCWs and NISPs (Fig. 2 A, Supplementary Figure 9). The average rate of change of log transformed anti-S1 IgG OD ratio is -0.602 per 100 days in HCWs and NISPs (SD = 0.264), and -0.434 per 100 days in ISPs (SD = 1.05, P = 0.56, two-tailed t-test). The average rate of change of log IFN-γ response (IU/mL) is -0.682 per 100 days in HCWs and NISPs (SD = 1.02), and -0.411 per 100 days in ISPs (SD = 0.849, P = 0.43, two-tailed t-test).
Fig. 2.
Change in vaccine response rates over time and after booster vaccination. A) Comparison of the change in natural log transformed anti-S1 IgG and IGRA values over time in HCW, NISP, and ISP cohorts. Error bars, mean ± standard deviation. B) Linear model fitted to the change in natural log transformed vaccine response over time versus the initial vaccine response (using the value of the assay performed closest to the vaccination date). Dotted lines denote the 95% confidence interval (CI) of the linear model mean and intercept. Note the 95% CI for slope includes 0 in both cases. C) Predicted decline in vaccine response over time (Supplementary Methods), at various starting (i.e. theoretical peak) response values after primary vaccination. D) Determination of anamnestic booster response given primary vaccination (primary dose) response (Star), based on the expected rate of change in response over time after primary vaccination (dashed line), with confidence intervals (CI) (Supplementary Methods). Booster responses that fall above the upper bound of the 70% CI (pink region) are determined to be anamnestic, while those that fall below the lower bound (blue region) are determined to be poor. E) ISP (n = 52) cohort with low anti-S1 IgG after primary vaccination separated into non-anamnestic (poor and expected) booster response (n = 34) and anamnestic booster response (n = 18), showing both the response after primary vaccination (orange dots) and after booster (blue dots). F) Distribution of age and days between primary vaccination and booster in non-anamnestic and anamnestic booster response patients. G) Anamnestic booster response rates in patients stratified by the likely primary cause of low humoral (anti-S1 IgG) response after primary vaccination (disease: heme malignancy and primary immunodeficiency patients, ISMT: solid malignancy, solid organ transplant, and autoimmune disease patients) H) Anamnestic booster response rates in patients stratified by whether there was a decrease in ISMT dosage around the time of booster vaccination. Only patients on ISMT during the primary doses are included. Panels A and B: n = 12 HCWs, 2 NISPs, 52 ISPs (IgG) and n = 15 HCWs, 1 NISP, 15 ISPs (IGRA). Panels F-H: only patients with low anti-S1 IgG after primary vaccination are included. ns, not significant; #P < 0.05, ##P < 0.01, ###P < 0.001 for pairwise comparisons as indicated, Fisher exact test. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
3.5. Vaccine response after booster vaccination
Demographic information and assay results from 6 immunocompetent HCWs, 6 NISPs, and 125 ISPs after booster vaccination are displayed in Table 2 (see also Supplementary Tables 14,15). In 117 ISPs that had both anti-S1 IgG and IGRA results available, 55% were positive for both (64), 14% were negative for both (16), 18% were IGRA positive only (21), and 14% were IgG positive only (16).
Table 2.
Demographic information and assay results for cohorts after booster vaccination.
| Disease Categories | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| HCW (n = 6) | NISP (n = 6) | ISP (n = 125) | Active Heme Malignancy (n = 10) | Inactive Heme Malignancy (n = 21) | Solid Malignancy (n = 3) | Solid Organ Transplant (n = 24) | Autoimmune Disease (n = 38) | 1° Immuno-deficiency (n = 17) | Multiple categories (n = 12) | |
| On ISMT,% (n) | 0 (0) | 0 (0) | 71 (89) | 70 (7) | 43 (9) | 33 (1) | 100 (24) | 97 (37) | 6 (1) | 83 (10) |
| Male:female ratio | 0.2:1 | 0.2:1 | 0.8:1 | 1.5:1 | 1.3:1 | 2.0:1 | 2.0:1 | 0.5:1 | 0.4:1 | 0.5:1 |
| Age, median (IQR), years | 54.0 (47.8–57.2) | 51.5 (42.5–58.2) | 58.0 (44.0–68.0) | 57.0 (38.5–69.5) | 65.0 (46.0–70.0) | 64.0 (63.5–73.5) | 50.0 (28.2–58.5) | 55.5 (45.8–64.0) | 63.0 (51.0–71.0) | 63.5 (54.2–71.0) |
| IgG days since vaccine*, median (IQR) | 22.0 (16.2–29.2) | 34.0 (22.0–52.0) | 36.0 (28.2–49.8) | 27.0 (22.0–43.5) | 34.5 (30.0–49.0) | 27.0 (21.5–32.5) | 37.0 (27.0–46.5) | 37.0 (29.5–55.5) | 35.0 (31.0–42.0) | 44.0 (34.5–60.5) |
| Anti-S1 IgG, median (IQR), OD ratio | 8.7 (7.7–9.7) | 9.8 (9.3–10.0) | 5.2 (0.3–8.6) | 1.5 (0.2–8.9) | 4.9 (0.1–8.7) | 8.1 (7.9–8.2) | 8.3 (0.4–9.1) | 3.3 (1.0–7.9) | 7.0 (4.3–7.8) | 1.7 (0.2–7.5) |
| Anti-S1 IgG,% positive (n) | 100 (6/6) | 100 (5/5) | 69 (81/118) | 50 (5/10) | 55 (11/20) | 100 (2/2) | 70 (16/23) | 71 (25/35) | 88 (15/17) | 64 (7/11) |
| ACE2 Blocking%, median (IQR) | 95.5 (92.0–99.8) | 100.0 (100.0–100.0) | 31.5 (0.0–94.8) | 0.0 (0.0–87.0) | 26.0 (0.0–97.8) | 87.0 (82.5–91.5) | 90.0 (0.0–100.0) | 9.0 (0.0–42.5) | 36.0 (7.0–94.0) | 1.0 (0.0–66.0) |
| ACE2 Blocking,% positive (n) | 100 (6/6) | 100 (5/5) | 53 (62/118) | 40 (4/10) | 50 (10/20) | 100 (2/2) | 65 (15/23) | 49 (17/35) | 59 (10/17) | 36 (4/11) |
| IGRA days since vaccine†, median (IQR) | 22.0 (16.2–29.2) | 33.5 (24.8–47.5) | 36.5 (28.0–50.5) | 31.0 (22.0–46.2) | 39.0 (31.0–50.0) | 20.0 (18.0–29.5) | 36.5 (26.2–44.8) | 37.0 (28.0–57.0) | 35.0 (31.0–42.0) | 48.0 (34.8–73.0) |
| IFN-γ Response median (IQR), IU/mL | 1.7 (1.0–4.0) | 4.7 (3.3–7.3) | 2.8 (0.3–8.8) | 9.9 (3.6–10.0) | 2.6 (1.2–6.0) | 9.3 (5.0–9.6) | 1.0 (0.1–3.2) | 3.9 (0.7–9.2) | 4.0 (1.4–8.9) | 0.7 (0.2–2.8) |
| IFN-γ Response,% positive (n) | 100 (6/6) | 83 (5/6) | 73 (91/124) | 80 (8/10) | 76 (16/21) | 100 (3/3) | 54 (13/24) | 84 (31/37) | 82 (14/17) | 50 (6/12) |
Note: not all patients had both anti-S1 IgG and IGRA results available.
*Number of days elapsed between primary vaccination and anti-S1 IgG testing.
†Number of days elapsed between primary vaccination and IGRA testing.
In the booster cohort of 6 HCWs, 1 NISP, and 84 ISPs with anti-S1 IgG or IGRA results collected before and after the booster dose, testing occurred a median of 89 days after primary vaccination, and 36 days after booster. To correct for this collection time difference, we applied the average rate of vaccine response decline over time after the primary dose (Fig. 2C) to predict the expected non-anamnestic booster responses. This allowed us to determine, with statistical confidence, patients who had poor, expected, and anamnestic booster responses (Fig. 2D, Supplementary Figure 10, Supplementary Methods). All HCWs and NISPs included in the analysis had expected booster anti-S1 IgG and IGRA responses. In ISPs, 71% (56/79) had at least the expected humoral response, and 64% (27/42) had at least the expected cellular response (Supplementary Figure 10).
Overall, 23% (18/79) of ISPs had an anamnestic humoral response, and 5% (2/42) had an “anamnestic” cellular response. Of 40 ISPs with both paired anti-S1 IgG and paired IGRA results available, 10 had an anamnestic humoral booster response and two had an “anamnestic” cellular booster response, a statistically significant difference (P = 0.03). Notably, 35% (18/52) ISPs with low humoral response after primary vaccination had an anamnestic booster response (Fig. 2E). We focused the rest of the analysis on humoral booster response, and specifically on patients with low humoral response after the primary doses.
Patient age and duration between primary vaccination and booster doses were not associated with booster response (Fig. 2F). Patients with immune defects due to disease and not necessarily ISMT (hematologic malignancy and primary immunodeficiency patients) had low rates of anamnestic response of 16% (4/25), while patients with ISMT-induced immunodeficiency (solid malignancy, solid organ transplant, and autoimmune disease patients) had higher rates of anamnestic response of 52% (14/27, P = 0.009) (Fig. 2G).
Following boosters, 50% of patients on anti-CD20 mAbs had an anamnestic response (4/8), 0% for S1P receptor modulators (0/2), 56% for mycophenolate (5/9), and 50% for systemic steroids (5/10). 20% of patients with acute leukemia had an anamnestic response (1/5), 18% for B cell lymphoma (2/11), 33% for plasma cell disease (1/3), and 0% for common variable immunodeficiency (0/4) (Supplementary Figure 11).
Eight patients had their ISMT dosage temporarily decreased by their provider to try to elicit an anamnestic booster response. Three of these patients had an anamnestic response (38%), a rate comparable to patients who did not have their ISMT altered (43%, 12/28) (Fig. 2H).
4. Discussion
We identified patient factors affecting immune response to primary and booster SARS-CoV-2 vaccines. In concordance with other studies[20], [21], [22], [23], [24], [25], [26], [27], [28], we found that anti-CD20 mAb and S1P receptor modulator use were associated with decreased humoral response. Unlike anti-CD20 mAb use, S1P receptor modulator use was also associated with low rates of cellular response. We showed that antimetabolite/mycophenolate use in combination with a calcineurin/mTOR inhibitor was the strongest predictor of decreased humoral and cellular response in transplant recipients, in concordance with other studies[3]. HSCT patients without active hematologic malignancy had relatively high rates of humoral and cellular response, even if vaccinated less than one year from transplantation. This is concordant with findings from one study[29], but not another[30]. Hematological malignancies were associated with lower vaccine response than solid malignancies, consistent with other reports[20,21,25,27,31]. Primary hypogammaglobulinemia disorders were associated with decreased humoral response, particularly in male patients. Men typically produce lower antibody responses to vaccination[32], an effect likely amplified in hypogammaglobulinemia.
Importantly, we did not find evidence that the rate of decline in humoral and cellular response over time differ between non-immunosuppressed individuals and ISPs. We found that boosters compensate for the waning of primary vaccine response, as 71% of ISPs had at least the expected rise in humoral response after booster, based on timing since vaccination, and 64% had at least the expected rise in cellular response. However, we found no strong evidence that boosters produce a significantly stronger cellular response in ISPs compared to primary vaccination, in concordance with a previous report in cancer patients[33], but contradicting another study in patients taking rituximab[28]. In terms of humoral response, the rate of anamnestic response after booster in patients with poor humoral response after primary vaccination was 35%. We found that the immunosuppressive effects of ISMTs, such as anti-CD20 mAbs, can be partially overcome with booster immunization.
As the COVID-19 pandemic ensues and new SARS-CoV-2 variants arise, our findings provide an evidence-based framework for determining optimal vaccination strategy in immunosuppressed patients. Immunosuppressive conditions differentially impact humoral and cellular responses to SARS-CoV-2 vaccines. The importance of cellular immunity against SARS-CoV-2 and emerging variants is now well established [[7], [8], [9],34,35]. This emphasizes the importance of monitoring cellular responses to vaccination and the utility of performing cellular immunity testing in the clinical laboratory.
Data availability
De-identified raw data used in the study are available upon request.
Declaration of Competing Interest
The authors declare no competing interests.
Acknowledgements
We thank all study participants.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.jcv.2022.105217.
Appendix. Supplementary materials
References
- 1.Harpaz R., Dahl R.M., Dooling K.L. Prevalence of immunosuppression among US adults, 2013. JAMA. 2016;316:2547–2548. doi: 10.1001/jama.2016.16477. [DOI] [PubMed] [Google Scholar]
- 2.Fung M., Babik J.M. COVID-19 in immunocompromised hosts: what we know so far. Clin. Infect. Dis. 2021;72:340–350. doi: 10.1093/cid/ciaa863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boyarsky B.J., Werbel W.A., Avery R.K., et al. Antibody response to 2-dose SARS-CoV-2 mRNA vaccine series in solid organ transplant recipients. JAMA. 2021;325:2204–2206. doi: 10.1001/jama.2021.7489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Agha M.E., Blake M., Chilleo C., Wells A., Haidar G. Suboptimal response to coronavirus disease 2019 messenger RNA vaccines in patients with hematologic malignancies: a need for vigilance in the postmasking era. Open Forum Infect. Dis. 2021;8:ofab353. doi: 10.1093/ofid/ofab353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Prendecki M., Clarke C., Edwards H., et al. Humoral and T-cell responses to SARS-CoV-2 vaccination in patients receiving immunosuppression. Ann. Rheum. Di.s. 2021;80:1322–1329. doi: 10.1136/annrheumdis-2021-220626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schmidt T., Klemis V., Schub D., et al. Cellular immunity predominates over humoral immunity after homologous and heterologous mRNA and vector-based COVID-19 vaccine regimens in solid organ transplant recipients. Am. J. Transplant. 2021;21:3990–4002. doi: 10.1111/ajt.16818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bertoletti A., Le Bert N., Qui M., Tan A.T. SARS-CoV-2-specific T cells in infection and vaccination. Cell Mol. Immunol. 2021;18:2307–2312. doi: 10.1038/s41423-021-00743-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Geers D., Shamier M.C., Bogers S., et al. SARS-CoV-2 variants of concern partially escape humoral but not T-cell responses in COVID-19 convalescent donors and vaccinees. Sci. Immunol. 2021;6:eabj1750. doi: 10.1126/sciimmunol.abj1750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gao Y., Cai C., Grifoni A., et al. Ancestral SARS-CoV-2-specific T cells cross-recognize omicron. Nat. Med.2022; published online Jan 14. DOI:10.1038/d41591-022-00017-z. [DOI] [PMC free article] [PubMed]
- 10.Levin E.G., Lustig Y., Cohen C., et al. Waning immune humoral response to BNT162b2 Covid-19 vaccine over 6 months. N. Engl. J. Med. 2021;385:e84. doi: 10.1056/NEJMoa2114583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Naaber P., Tserel L., Kangro K., et al. Dynamics of antibody response to BNT162b2 vaccine after six months: a longitudinal prospective study. Lancet Reg. Health Eur. 2021;10 doi: 10.1016/j.lanepe.2021.100208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kamar N., Abravanel F., Marion O., Couat C., Izopet J., Del Bello A. Three doses of an mRNA covid-19 vaccine in solid-organ transplant recipients. N. Engl. J. Med. 2021;385:661–662. doi: 10.1056/NEJMc2108861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hall V.G., Ferreira V.H., Ku T., et al. Randomized trial of a third dose of mRNA-1273 vaccine in transplant recipients. N. Engl. J. Med. 2021;385:1244–1246. doi: 10.1056/NEJMc2111462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Röltgen K., Powell A.E., Wirz O.F., et al. Defining the features and duration of antibody responses to SARS-CoV-2 infection associated with disease severity and outcome. Sci. Immunol. 2020;5:eabe0240. doi: 10.1126/sciimmunol.abe0240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Montesinos I., Dahma H., Wolff F., et al. Neutralizing antibody responses following natural SARS-CoV-2 infection: dynamics and correlation with commercial serologic tests. J. Clin. Virol. 2021;144 doi: 10.1016/j.jcv.2021.104988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Murugesan K., Jagannathan P., Pham T.D., et al. Interferon-γ release assay for accurate detection of severe acute respiratory syndrome coronavirus 2 T-cell response. Clin. Infect. Dis. 2021;73:e3130–e3132. doi: 10.1093/cid/ciaa1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Murugesan K., Jagannathan P., Altamirano J., et al. Long term accuracy of SARS-CoV-2 interferon-γ release assay and its application in household investigation. Clin. Infect. Dis. 2022:ciac045. doi: 10.1093/cid/ciac045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fernández-González M., Agulló V., Padilla S., et al. Clinical performance of a standardized SARS-CoV-2 interferon-γ release assay for simple detection of T-cell responses after infection or vaccination. Clin. Infect. Dis. 2021:ciab1021. doi: 10.1093/cid/ciab1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chia W.N., Zhu F., Ong S.W.X., et al. Dynamics of SARS-CoV-2 neutralising antibody responses and duration of immunity: a longitudinal study. Lancet Microbe. 2021;2:e240–e249. doi: 10.1016/S2666-5247(21)00025-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Addeo A., Shah P.K., Bordry N., et al. Immunogenicity of SARS-CoV-2 messenger RNA vaccines in patients with cancer. Cancer Cell. 2021;39:1091–1098. doi: 10.1016/j.ccell.2021.06.009. e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Greenberger L.M., Saltzman L.A., Senefeld J.W., Johnson P.W., DeGennaro L.J., Nichols G.L. Antibody response to SARS-CoV-2 vaccines in patients with hematologic malignancies. Cancer Cell. 2021;39:1031–1033. doi: 10.1016/j.ccell.2021.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Achiron A., Mandel M., Dreyer-Alster S., et al. Humoral immune response to COVID-19 mRNA vaccine in patients with multiple sclerosis treated with high-efficacy disease-modifying therapies. Ther. Adv. Neurol. Disord. 2021;14 doi: 10.1177/17562864211012835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Apostolidis S.A., Kakara M., Painter M.M., et al. Cellular and humoral immune responses following SARS-CoV-2 mRNA vaccination in patients with multiple sclerosis on anti-CD20 therapy. Nat. Med. 2021;27:1990–2001. doi: 10.1038/s41591-021-01507-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Deepak P., Kim W., Paley M.A., et al. Effect of Immunosuppression on the Immunogenicity of mRNA Vaccines to SARS-CoV-2 : a prospective cohort study. Ann. Intern. Med. 2021;174:1572–1585. doi: 10.7326/M21-1757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ehmsen S., Asmussen A., Jeppesen S.S., et al. Antibody and T cell immune responses following mRNA COVID-19 vaccination in patients with cancer. Cancer Cell. 2021;39:1034–1036. doi: 10.1016/j.ccell.2021.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sormani M.P., Inglese M., Schiavetti I., et al. Effect of SARS-CoV-2 mRNA vaccination in MS patients treated with disease modifying therapies. EBioMedicine. 2021;72 doi: 10.1016/j.ebiom.2021.103581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thakkar A., Gonzalez-Lugo J.D., Goradia N., et al. Seroconversion rates following COVID-19 vaccination among patients with cancer. Cancer Cell. 2021;39:1081–1090. doi: 10.1016/j.ccell.2021.06.002. e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jyssum I., Kared H., Tran T.T., et al. Humoral and cellular immune responses to two and three doses of SARS-CoV-2 vaccines in rituximab-treated patients with rheumatoid arthritis: a prospective, cohort study. The Lancet Rheumatol. 2021;0 doi: 10.1016/S2665-9913(21)00394-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Harrington P., Doores K.J., Saha C., et al. Repeated vaccination against SARS-CoV-2 elicits robust polyfunctional T cell response in allogeneic stem cell transplantation recipients. Cancer Cell. 2021;39:1448–1449. doi: 10.1016/j.ccell.2021.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tamari R., Politikos I., Knorr D.A., et al. Predictors of humoral response to SARS-CoV-2 vaccination after hematopoietic cell transplantation and CAR T-cell therapy. Blood Cancer Discov. 2021;2:577–585. doi: 10.1158/2643-3230.BCD-21-0142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Greenberger L.M., Saltzman L.A., Senefeld J.W., Johnson P.W., DeGennaro L.J., Nichols G.L. Anti-spike antibody response to SARS-CoV-2 booster vaccination in patients with B cell-derived hematologic malignancies. Cancer Cell. 2021;39:1297–1299. doi: 10.1016/j.ccell.2021.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fischinger S., Boudreau C.M., Butler A.L., Streeck H., Alter G. Sex differences in vaccine-induced humoral immunity. Semin Immunopathol. 2019;41:239–249. doi: 10.1007/s00281-018-0726-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shroff R.T., Chalasani P., Wei R., et al. Immune responses to two and three doses of the BNT162b2 mRNA vaccine in adults with solid tumors. Nat. Med. 2021;27:2002–2011. doi: 10.1038/s41591-021-01542-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bange E.M., Han N.A., Wileyto P., et al. CD8+ T cells contribute to survival in patients with COVID-19 and hematologic cancer. Nat. Med. 2021;27:1280–1289. doi: 10.1038/s41591-021-01386-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Le Bert N., Tan A.T., Kunasegaran K., et al. SARS-CoV-2-specific T cell immunity in cases of COVID-19 and SARS, and uninfected controls. Nature. 2020;584:457–462. doi: 10.1038/s41586-020-2550-z. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
De-identified raw data used in the study are available upon request.