Dear Editor:
Initial data after the rollout of COVID-19 vaccines revealed more than 90% effectiveness with infrequent breakthrough infection (1). Furthermore, individuals with breakthrough infection had a lower initial genomic viral load than unvaccinated individuals with severe acute respiratory syndrome-coronavirus-2 (SARS-CoV-2) infection, so transmission potential from those with breakthrough infection was low (2). However, data on the comparison of transmission potential between fully and partially or unvaccinated individuals with SARS-CoV-2 infections in the era of the delta variant are lacking. We thus compared the secondary attack rate and viable viral shedding kinetics with viral culture and subgenomic RNA detection through the dense sampling of respiratory specimens between fully vaccinated individuals and partially or unvaccinated individuals with SARS-CoV-2 delta variant infection.
This prospective study was conducted during the delta variant-dominant era (September 1, 2021 to January 31, 2022) in South Korea in patients infected with SARS-CoV-2 admitted to Asan Medical Center in Seoul, South Korea. Patients infected with the delta variant aged >18 years were included. All enrolled patients were instructed to submit daily saliva samples. SARS-CoV-2 genomic RT-PCR and subgenomic RT-PCR were performed on the collected saliva samples, and culture-based virus isolation was performed on genomic RT-PCR positive samples. The study protocols were approved by the institutional review board of Asan Medical Center (IRB No 2020-0297).
Genomic and subgenomic PCR was performed the same method as described in our previous studies (3). A cut-off value of negative conversion in genomic and subgenomic PCR was a cycle threshold (Ct) of 36, equivalent to a viral copy number of 2.6 log copies/mL, a 95% limit of detection. Culture-based isolation of SARS-CoV-2 from saliva samples was performed through a plaque assay using Vero cells. Culture methods followed those detailed in our previous study (4).
All patients were investigated for the number of close contacts during their infectious period (from ≤2 days before symptom onset in symptomatic patients or ≤2 days before the diagnosis day in cases of asymptomatic patients to isolation)(3), and the number of contact individuals who were diagnosed with SARS-CoV-2 infection within 1 week of contact. Definition of close contacts were found in our previous study (3).
A total of 82 patients were enrolled between September 2021 and December 2022 and were initially interviewed about secondary attack rates (Supplemental Fig. 1). Among them, 22 patients who refused to submit daily saliva specimens were excluded. Of the remaining 60 patients, 5 and 6 were excluded due to undetectable or inconclusive RNA PCR results and confirmed non-delta variant infections, respectively. Finally, a total of 49 patients were analyzed in this study. Of these, 19 patients (40%) were vaccinated, and the remaining 30 (60%) were unvaccinated or partially vaccinated groups. Vaccinated patients were older (median 68 years vs. 44 years, p <0.001) and included more males (61% vs. 35%, p=0.03) than the unvaccinated or partially vaccinated group (Supplemental Table 1).
Of the 65 individuals with SARS-CoV-2 infection who had a secondary infection, 39 were transmitted from unvaccinated or partially vaccinated patients, and the remaining 26 patients were from fully vaccinated patients. The fully vaccinated patient group showed a statistically significantly lower secondary attack rate than the unvaccinated or partially vaccinated groups (4% vs. 14%, p<0.001).
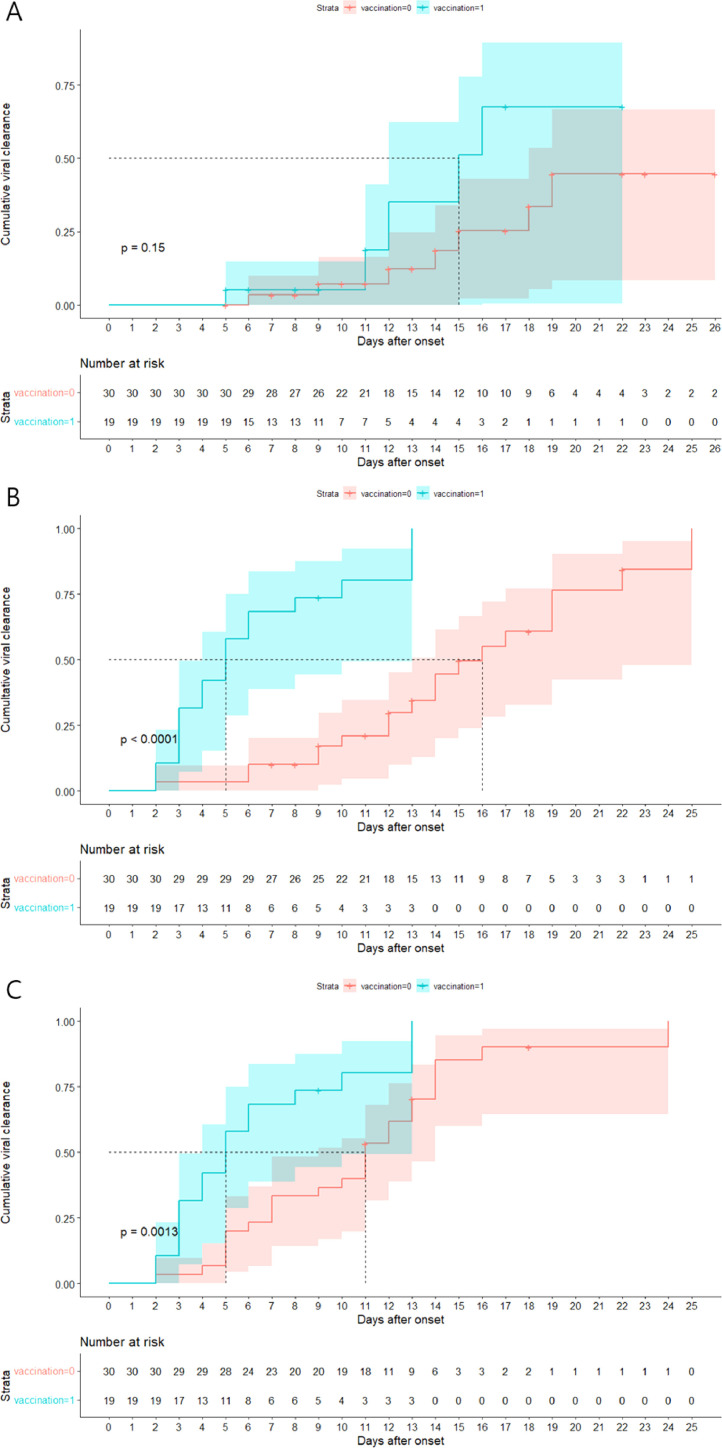
Viral clearance between the vaccinated group and the partially or unvaccinated group was consistently insignificant in genomic RNA PCR (median 15 days, 95% CI [12 – NA] vs. more than 26 days, 95% CI [18 – NA], p=0.15; Fig. 1 A), even after multivariable analysis (HR 2.81, p=0.24; Supplemental Table 2). On the contrary, there were significant differences in the negative conversion of subgenomic RNA detection between the vaccinated and the non-vaccinated groups in the multivariate and univariate analyses (median 5 days, 95% CI [4 – NA] vs. 16 days, 95% CI [13 – NA], p<0.001: Fig. 1B, HR 18.78, p<0.001; Supplemental Table 3 and Fig. 2). Moreover, full vaccination was significantly associated with faster viral clearance (median 5 days, 95% CI [4 - NA] vs. 11 days, 95% CI [9 – 14], p=0.0013: Fig. 1C and Supplemental Fig. 2). This association was consistent in the multivariable analysis (HR 5.18, p=0.001; Supplemental Table 4).
Fig. 1.
Comparison of cumulative viral clearance in vaccinated vs. unvaccinated or partially vaccinated group. Kaplan-Meier curve of daily cumulative negative conversion rate of genomic RNA PCR (A) showed no significant difference between fully vaccinated and not or partially vaccinated group, whereas subgenomic RNA PCR (B) and viable virus isolation by cell culture (C) showed statistically significant differences. Red bar indicate cumulative clearance of viruses in not or partially vaccinated group. Blue bar indicate cumulative viral clearance in fully vaccinated group.
Our previous study reported that fully vaccinated individuals had a lower secondary attack rate than partially vaccinated or unvaccinated individuals (3). However, given the low number of fully vaccinated individuals in this study (n=6), we could not detect a significant difference in the duration of viable viral shedding between the two groups that was a different viral shedding kinetic cohort from the cohort for the calculation of the secondary attack rate (3). Nevertheless, this prospective cohort study builds on our earlier publication (3) with a different independent cohort involving more fully vaccinated or partially vaccinated and unvaccinated hospitalized patients with COVID-19 at varying degrees of severity. We evaluated the secondary attack rate and viral shedding kinetics in the same cohort and found that fully vaccinated individuals with SARS-CoV-2 infection had lower transmissibility than partially vaccinated or unvaccinated individuals. In addition, this study showed that the fully vaccinated individuals with SARS-CoV-2 infection more rapidly achieved viable virus clearance than partially vaccinated or unvaccinated individuals with SARS-CoV-2 infection.
An earlier study on breakthrough infection after the rollout of COVID-19 vaccines revealed a lower initial genomic viral load in vaccinated individuals with SARS-CoV-2 infection than unvaccinated individuals (2). In contrast, several studies conducted in the era of the delta variant revealed that initial or peak genomic viral load was similar between fully vaccinated and partially or unvaccinated individuals with SARS-CoV-2 (5,6). However, data on the role of vaccination in facilitating faster clearance of viable viral shedding are limited (7), although breakthrough infections in fully vaccinated individuals exhibited a faster clearance of genomic viral load than unvaccinated individuals (6,8). Our study demonstrated that vaccination was associated with faster clearance of viable viruses by viral culture and subgenomic RNA detection through the dense sampling of respiratory specimens.
In conclusion, fully vaccinated individuals had a lower transmission potential in secondary attack rate and duration of viable viral shedding than partially or unvaccinated individuals.
Competing Interests
There are no conflicts of interest for any of the authors.
Funding
This work was supported by the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI) funded by the Ministry of Health & Welfare, South Korea [grant number HW20C2062]; and the National Research Foundation of Korea (NRF) funded by the Ministry of Science and ICT, Republic of Korea [grant number NRF-2017M3A9E4061995].
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.jinf.2022.06.002.
Appendix. Supplementary materials
References
- 1.Kuhlmann C, Mayer CK, Claassen M, Maponga T, Burgers WA, Keeton R, et al. Breakthrough infections with SARS-CoV-2 omicron despite mRNA vaccine booster dose. Lancet. 2022 Feb 12;399(10325):625–626. doi: 10.1016/S0140-6736(22)00090-3. PubMed PMID: 35063123. Pubmed Central PMCID: PMC8765759. Epub 2022/01/23. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Levine-Tiefenbrun M, Yelin I, Katz R, Herzel E, Golan Z, Schreiber L, et al. Initial report of decreased SARS-CoV-2 viral load after inoculation with the BNT162b2 vaccine. Nat Med. 2021;27(5):790–792. doi: 10.1038/s41591-021-01316-7. PubMed PMID: 33782619. Epub 2021/03/31. eng. [DOI] [PubMed] [Google Scholar]
- 3.Jung J, Kim JY, Park H, Park S, Lim JS, Lim SY, et al. Transmission and Infectious SARS-CoV-2 Shedding Kinetics in Vaccinated and Unvaccinated Individuals. JAMA Network Open. 2022;5(5) doi: 10.1001/jamanetworkopen.2022.13606. -e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bae S, Kim JY, Lim SY, Park H, Cha HH, Kwon JS, et al. Dynamics of Viral Shedding and Symptoms in Patients with Asymptomatic or Mild COVID-19. Viruses. 2021 Oct 22;13(11) doi: 10.3390/v13112133. PubMed PMID: 34834940. Pubmed Central PMCID: PMC8625453. Epub 2021/11/28. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Singanayagam A, Hakki S, Dunning J, Madon KJ, Crone MA, Koycheva A, et al. Community transmission and viral load kinetics of the SARS-CoV-2 delta (B.1.617.2) variant in vaccinated and unvaccinated individuals in the UK: a prospective, longitudinal, cohort study. Lancet Infect Dis. 2022;22(2):183–195. doi: 10.1016/S1473-3099(21)00648-4. PubMed PMID: 34756186. Pubmed Central PMCID: PMC8554486 National Institute of Health Research, UK Research and Innovation, Community Jameel, Janssen Pharmaceuticals, the Bill & Melinda Gates Foundation, and Gavi, the Vaccine Alliance; consulting fees from the World Bank; payment or honoraria from the Wellcome Trust; travel expenses from WHO; advisory board participation for Takeda; and is a senior editor of the eLife journal. All other authors declare no competing interests. Epub 2021/11/11. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chia PY, Ong SWX, Chiew CJ, Ang LW, Chavatte JM, Mak TM, et al. Virological and serological kinetics of SARS-CoV-2 Delta variant vaccine breakthrough infections: a multicentre cohort study. Clin Microbiol Infect. 2022;28(4):612. doi: 10.1016/j.cmi.2021.11.010. .e1-.e7. PubMed PMID: 34826623. Pubmed Central PMCID: PMC8608661. Epub 2021/11/27. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jung J, Lim SY, Lee J, Bae S, Lim YJ, Hong MJ, et al. Clustering and multiple-spreading events of nosocomial severe acute respiratory syndrome coronavirus 2 infection. J Hosp Infect. 2021;117:28–36. doi: 10.1016/j.jhin.2021.06.012. PubMed PMID: 34453983. Pubmed Central PMCID: PMC8384763. Epub 2021/08/29. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kissler SM, Fauver JR, Mack C, Tai CG, Breban MI, Watkins AE, et al. Viral Dynamics of SARS-CoV-2 Variants in Vaccinated and Unvaccinated Persons. N Engl J Med. 2021 Dec 23;385(26):2489–2491. doi: 10.1056/NEJMc2102507. PubMed PMID: 34941024. Pubmed Central PMCID: PMC8693673. Epub 2021/12/24. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.