Abstract
Adults of the large pine weevil (Hylobius abietis) cause serious damage to coniferous seedlings and are among the most important forest pests in Europe. Seedling protection by chemicals is gradually being restricted or banned for environmental reasons, and non-chemical alternatives are therefore needed. In this 3-year study, we compared the following five treatments for protecting Norway spruce seedlings against H. abietis in the Central European mountains where the weevil is especially abundant: alpha-cypermethrin sprays (the only chemical treatment); coating with sprayed glue (Vermifix); wax coating with C and F types (Norsk Wax); and physical protection with collars. The same block design was set up at a clear-cut site and at a nursery site to compare seedling mortality and wax quality under “wild conditions” with pests and under “ideal conditions” without pests. Repeated application of alpha-cypermethrin was the most effective and least expensive method to protect seedlings against H. abietis. Among the four non-chemical methods, repeated application of glue was the most effective. Because collars were moderately effective but not cost-effective, we do not recommend the use of collars. Wax was inexpensive and environmentally safe but protected seedlings for only 1 year; the newer F type of wax performed better than the C type of wax, and perhaps the F type can be improved. In general we found that seedlings at sites with high numbers of H. abietis require protection for at least 3 years. We conclude by providing an overview of all methods currently available for managing H. abietis in forests.
Subject terms: Agroecology, Forestry
Introduction
In large parts of Europe, successful regeneration of coniferous forests by planting depends on the suppression of damage caused by the large pine weevil, Hylobius abietis (Linnaeus, 1758) (Coleoptera: Curculionidae)1–3. This pest is especially damaging in young forest stands2,4.
Hylobius abietis adults are attracted to the fresh clear-cut by the odours emitted from damaged, dying or newly dead (cut) conifer trees providing the breeding substrate5; they remain at the site through the entire season and also overwinter at the site6,7. The individuals of the parent generation can survive several years and continue to lay eggs in the years following harvest. The new generation of weevils then emerge as adult weevils several years (1–3) later after the initial tree cutting8. Feeding of parental beetles to get reproductively mature when arriving to the clear-cut and losing their flight muscles. Feeding on the mature trees does not cause any economic damage, but beetle’s feeding on planted seedlings causes severe economic damage9, because feeding may cause girdling and often the death of coniferous seedlings7,10,11. A single weevil can damage several seedlings12. Larvae hatch soon after oviposition and develop under the bark of the above-mentioned material13. Development requires 1 to 3 years depending on the conditions10,14, but recent studies indicate that climate change (mainly increasing temperatures) may increase the feeding rate and shorten the weevil’s life cycle15–17. Maturing weevils also feed on the bark of coniferous seedlings18,19. H. abietis adults cause high levels of seedling mortality and economic losses20. In Europe, H. abietis causes annual damage of almost 120 million EUR21.
Depending on population density and weather conditions, H. abietis can kill 50–100% of seedlings at a site11,22,23. Current management relies on silviculture, feeding barriers, and insecticides20.
The use of synthetic insecticides is effective and relatively cheap24. However, because of adverse environmental effects, insecticide use will be increasingly restricted2,19,24–26 and replaced by various physical feeding barriers27. Of the 407 million conifer seedlings delivered in Sweden in 2020, for example, 50% were protected with stem coatings, 47% were unprotected, and only 3% were protected with insecticide28.
Physical barrier systems (glue/sand/wax coatings, paper/plastic guards, shields, collars, clipstops, etc.) are alternatives to synthetic insecticides. Substantial research on physical barriers has been conducted mainly in Scandinavian countries3,27,29–31, but also in the UK11,24,32–34, and Slovakia19,21,37,38.
A relatively new method for the physical protection of coniferous seedlings against the feeding damage caused by H. abietis is the application of Conniflex. In this method, the lower 60% of the seedling stem is protected with a coating containing fine sand embedded in an acrylate dispersion; application of Conniflex is an effective and environmentally friendly alternative to insecticide treatments3,31.
In Europe, wax (names during development: Bugwax, Eco-vax, KVAAE) has been used to protect seedlings against H. abietis for the last 10–15 years, although experiments and development have been ongoing since the 1990s39. This wax is made from natural materials. It does not contain any insecticide or fungicide and is harmless to insects and animals living in the forest. Wax is fully biodegradable, non-reactive, non-toxic, and insoluble in water40. The wax is significantly more elastic than normal wax, provides a physical barrier, reduces emission of volatile attractants, and thereby greatly reduces feeding by H. abietis19,41. In Slovakia, C type of wax (Norsk Wax) has been used to protect seedlings since 2013. The melted wax is manually applied to seedling stem or seedlings are inserted into a “fountain machine” that applies the wax; the wax is applied from the root collar to 15–20 cm above the root collar19. Under ideal conditions, the wax protects the seedlings for about 1.5–2.0 years. That this treatment can be as effective as insecticide application has been demonstrated in Sweden27,30,42 and Slovakia19,21,37,38.
Vermifix is a glue that was developed to protect trees against creeping insects and for use in various glue traps43. Vermifix (Papírna Moudry s.r.o., Czech Republic) has recently been tested for control of H. abietis on conifer seedlings19,21,38. In general, the coating of stems with glue or wax has been found to reduce H. abietis damage and in most cases provided control that was not significantly different from that provided by an insecticide treatment21,38.
Other potential alternatives are application of antifeedant compounds, the plant hormone methyl jasmonate (MJ), or natural product insecticides. The study of Azeem44 suggested that compounds produced by plants that are not hosts of H. abietis might be used to protect seedlings against H. abietis feeding. Unelius45 stated that research is needed to find compatible combinations of coating material and antifeedants that can protect seedlings against H. abietis feeding for two seasons without harming the seedlings. Recent research suggested that a MJ treatment may protect coniferous seedlings against insect herbivory46,47. Natural product insecticides are chemicals derived from plants or microorganisms. Willoughby34 described the effects of azadirachtin extracts from neem trees, pyrethrin extracts from the Dalmatian chrysanthemum, and even sheep fat on H. abietis feeding, but none of them led to protection of seedlings.
Clear-cutting followed by planting is the generally used method for the regeneration of coniferous forests in northern Europe3,48. In contrast, common Slovak forestry management is based on natural regeneration, i.e., a shelter-wood management system49 that normally results in a great number of naturally regenerated trees50. Under such conditions, pests such as H. abietis are not very significant. However, the annual occurrence of wind damage51, particularly in mountainous spruce regions, results in cleared areas that resemble areas subjected to clear-cut forest management. In these areas, the abundance of stumps and harvest residues provide breeding habitat and food for H. abietis18. Wind disturbances are also regularly followed by outbreaks of bark beetles (especially the spruce bark beetle, Ips typographus52), which produce additional material suitable for the development and maintenance of large populations of H. abietis. Damage to seedlings (mainly of spruce) by H. abietis is especially severe in national parks (at elevations up to 1200 m a.s.l., pers. observ.), where the use of insecticides for seedling protection is forbidden21,38. Therefore, researchers have recently investigated non-chemical alternatives for protecting coniferous seedlings against H. abietis feeding18,19,21,37,38,53,54.
In the current study, we conducted a 3-year experiment to compare various chemical (Fig. 1a–c) and non-chemical treatments for protecting seedlings against H. abietis. This experiment was conducted at a site in a mountainous region with high H. abietis abundance and at a nursery site where H. abietis was absent. We evaluated the efficacy, cost, and environmental effects of these treatments. Finally, we provide recommendations for H. abietis management under Central European conditions.
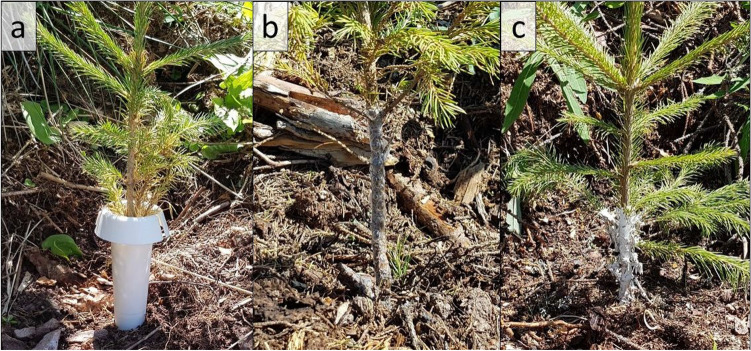
Figure 1.
Spruce seedlings treated with (a) Hylopro, (b) Vermifix, and (c) Eco-wax.
Materials and methods
Study sites
Experiments were performed at two sites. The first site was a forest nursery in Jochy (owner OZ Semenoles, LESY SR, state forest) (hereafter referred to as the nursery site), which is in the northern part of Central Slovakia in the Liptov region (49° 6′ 36.15″ N, 19° 44′ 58.36″ E, 835 m asl).
The second site was a clear-cut area located in the forest stand managed by the Forest district of Liptovská Teplička (OZ Liptovský Hrádok, LESY SR, state forest) (hereafter referred to as the clear-cut site) (48° 59′ 59.59″ N, 20° 2′ 14.07″ E, 1070–1100 m asl). The clear-cut site was a 3-ha area that had been recently cleared after sanitation logging of Norway spruce (Picea abies [L.] Karst.) (September 2017) due to I. typographus damage. The surrounding tree species were represented by spruce (95%) and larch (Larix decidua Mill.) (5%). The site contained approximately 230 spruce stumps per ha that had not been removed. Harvest residues has been placed in piles but had not been burned. The site is representative of the vast area of foothills of the Low Tatras Mts., which has a sufficient suitable habitat and food to support high numbers (25,000 weevils per ha) of H. abietis54.
Seedlings, treatments, and experimental design
In spring 2018, 4-year-old Norway spruce seedlings were planted at both sites. The seedlings were supplied by OZ Semenoles Liptovský Hrádok (LESY SR, state forest). The mean height of the planted seedlings was about 36 cm, and the mean root collar diameter at 2 cm above ground level was about 6 mm. All seedlings were bare-root plants and were categorised with regard to size and quality before planting.
Seedlings were planted by a contractor (an external crew) to provide a commercial planting (including its advantages and disadvantages). At both sites, seedlings were planted in holes that were about 40 × 40 cm on the surface and dug 15 cm deep using hoe. Seedlings were planted in rows; the in-row and between-row spacing was 0.5 m at the nursery site (where space was limited) and 1.0 m at the clear-cut site. At each site, 600 seedlings were planted in 10 blocks (i.e., 60 seedlings per block). At the nursery site, blocks were placed next to each other. At the clear-cut site, the blocks were uniformly distributed over an area of 3 ha with 20–30 m between blocks.
Seedlings at the nursery site were irrigated during heatwaves and were fertilized with Konifert Extra produced by Agrofert in an amount of 200 g/m2 and weeded once each year. The weeding was done manually. Seedlings at the clear-cut site were weeded once each year but were not irrigated or fertilised.
Individual rows (10 seedlings per row) in blocks were treated with one of six treatments (Fig. 1a–c, Table 1); the treatments were randomized among the rows in each block.
Table 1.
Description of treatments.
| Treatment | Commercial name (composition) | Usage | Producer/distributor |
|---|---|---|---|
| Control | No treatment applied to the seedlings | ||
| Chemical | Vaztak active (50 g/l alpha-cypermethrin) | Suspension with a 1% concentration was applied in an approximately spray volume of 60 l/ha (1.5–2.0 l of suspension per 100 seedlings). Seedlings were sprayed with a knapsack sprayer (referred to as top-up spraying) 3 times per year (April, June, August) and 9 times during the entire study |
BASF SE Ludwigshafen Germany |
| Collar | Hylopro anti-weevil protective collar (bioplastic collar) |
Biodegradable plastic collar applied in spring 2018. Each collar was opened, placed around the plant base, and then pushed approximately 1 cm into the soil until the lock engaged (Fig. 1a) Locks were checked once each year, at which time open collars were closed |
Grube KG Bispingen Germany |
| Glue | Vermifix (42% polyolefins, 420 g per 1 kg) | One bottle of glue (400 ml) was applied per 70–100 seedlings. Seedlings were treated from the ground to 20 cm height 3 times per year (April, June, August) and 9 times during the entire study (Fig. 1b) |
Papírna Moudrý s.r.o Židlochovice Czech Republic |
|
C wax F wax |
Eco-wax (Paraffin and additives) | Physical protection by wax coating. Before they were planted at the two sites, seedlings were treated with melted wax (approximately 80 °C; about 5–10 g of wax per seedling that coated the stem from soil level to 20 cm above soil level) by the double fountain machine Heco-V-450NW (ZetaEcotech, Italy)35. After wax was applied, the treated stems were immediately cooled with water (Fig. 1c). C wax is standard; the newly developed F wax is more flexible |
Norsk wax AS Larvik Norway |
Assessment of damage and condition of seedlings
Seedlings were assessed every year in October, i.e., at the end of the growing season after pest activity had decreased. Damage to seedlings (feeding scars) caused by H. abietis adults was evaluated only at the clear-cut site (as noted earlier, H. abietis was absent from the nursery site). If adult Hylastes spp. bark beetles were detected on roots or stems (this occurred only at the clear-cut site), damaged areas < 50 mm2 were attributed to Hylastes spp. If the area of stem damage was ≥ 50 mm2, the damage was attributed to H. abietis even if Hylastes spp. beetles were present. The area of damage (mm2) on seedlings caused by H. abietis was determined using transparent millimetre paper. Only new damage that occurred during the growing season was measured.
Mortality of seedlings was evaluated at both sites. At the clear-cut site, seedlings were assessed as alive, dead (because of Hylobius), dead (because of Hylastes), or dead (because of other unknown causes). At the nursery site, the seedlings were assessed as alive, dead (because of human activity), or dead (because of other unknown causes).
Assessment of the condition and quality of wax coating
The state and quality of wax on seedlings treated with C wax and F wax was assessed at both sites at the end of each growing season. For this assessment, we used our own scale (Table 2)38.
Table 2.
Wax condition scale.
| Scale value (rank) | Condition of wax |
|---|---|
| 1 | Excellent (undamaged wax) |
| 2 | Good (cracks in the wax or other damage, but still protecting the seedling) |
| 3 | Average (cracks in the wax or other damage, wax has fallen off ≤ 50% of the seedling circumference) |
| 4 | Poor (wax has fallen off > 50% of the seedling circumference and does not protect the seedling) |
| 5 | Wax missing from the entire circumference of the seedling |
Economic analyses
We also evaluated treatment costs in individual years and total costs (including labour costs). Costs are given in EUR/ha (excluding tax). Individual costs per ha were obtained from three independent sources from the LESY SR state forest and two from private forest sector (Milan Krajči, pers. comm.), and the means of these data were used in the calculations. Costs are based on treating 3000 spruce seedlings/ha.
Statistical analyses
The data were analysed using R 4.0.3 software and RStudio version 1.3.1093. Plots were made using the ggplot2 package55. Data were processed using the dplyr R package version 1.0.2.56. All mixed-effect models were calculated using lmer and glmer functions of the lme4 package, version 1.1-1957.
Damage and mortality of seedlings
Damage and mortality caused by H. abietis adults were evaluated only at the clear-cut site. To study the effect of treated (chemical, collar, glue, C wax, and F wax) and control seedlings on H. abietis feeding damage and mortality, we excluded dead undamaged seedlings and seedlings that died due to Hylastes or “other” factors.
Damages and mortality were measured from multiple blocks: explanatory variable—block (10 levels). In the period 2018–2020: explanatory variable—year (3 levels).
Seedling damages
At the clear-cut site, the effect of treatments on damage by H. abietis feeding was analysed using a linear mixed model (LMM), and the parameters were estimated using the residual maximum likelihood method (REML). The response variable, i.e., feeding damage (mm2), was transformed using the Box–Cox transformation (R, package bestNormalize; version 3.4.1)58 to improve the homogeneity of the variance and the normality of the distribution. Seedlings treated with the chemical suspension were excluded from the analyses because the damage ranged from small to none, with a median value of zero throughout the entire experiment.
We first developed a series of alternative mixed effect models that included different combinations of explanatory variables. Models were compared using the Akaike information criterion (AIC) and ANOVA. Random effects variance components were estimated using REML. Models with various fixed effects were compared using a maximum likelihood estimation (ML).
Random effect: We fitted the maximal complexity of the random effect structure. We tested the hypothesis that the strength of the treatment's effect (slope) on the response variable varies between blocks and years. The least significant terms were dropped. Then the variables block and year were included as crossed random effects (intercept).
Fixed effect: Variable’s treatment and year significantly improved model parameters and were included in the selected model.
Finally, the selected model's variables, treatment, and year were fitted as fixed effects and block as crossed random intercept. The selected model shown in R notation format:
Seedlings mortality
The effects of treatments on seedling mortality were analysed using the generalized linear mixed model (GLMM) fitted using the maximum likelihood Laplace approximation with a binomial distribution and a logit link function. Seedling mortality (alive/dead) was the response variable in the model. Treatment and the study periods were used as a fixed effect, and blocks were used as a random effect.
Treatments were compared using the Tukey method for comparing family estimates. Pairwise differences of least squares means were computed using the emmeans R package (version 1.5.4.)59.
Statistical assumptions of the selected models were simulated and graphically validated using DHARMa package for residual diagnostics of hierarchical regression models. All the assumption tests provided by the package were insignificant60.
Assessment of wax condition and quality
The number of seedlings differed between the years of the experiment. Dead seedlings were excluded from the analyses because they did not grow. Wax quality was compared between wax treatments (C wax and F wax) using the Mann–Whitney U test with the p-value estimated by normal approximation with a continuity correction.
To test how the wax quality deteriorated during the experiment, we used the Wilcoxon–Pratt signed-rank p test with an exact type I error estimate, using the asht R package61. The test assumes that differences between paired samples should be distributed symmetrically around the median. We calculated the differences and plotted them in histograms. All differences were distributed approximately symmetrically.
Results
Effectiveness of treatments at the clear-cut site
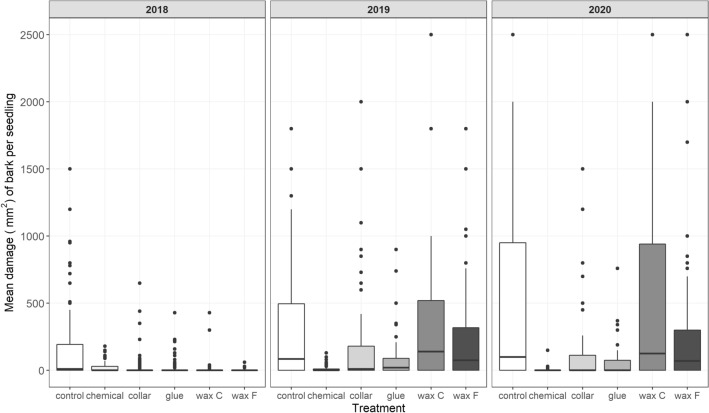
In 2018, the mean damage per seedling across all treatments was relatively low (Fig. 2). Damage was highest for control seedlings and lowest for seedlings treated with F wax and C wax. In 2019, the damage across all treatments except the chemical treatment substantially increased. Seedlings treated with C wax and control seedlings were the most damaged in 2019. In 2020, the mean damage per seedling across all treatments (excluding the chemical treatment) was the highest in the 3 years. Seedlings treated with C wax and control seedlings were again the most damaged. The lowest levels of damage were recorded on seedlings treated with the chemical and the glue.
Figure 2.
Effect of treatments on the area damaged per seedling by H. abietis at the clear-cut site.
Based on the area of feeding scars, the chemical treatment provided the best protection against H. abietis damage (Table 3). For this treatment, we recorded almost no damage, i.e., the median damage was equal to zero during the whole experimental period. Only a few seedlings were attacked. Because we analysed only damaged seedlings, data from the chemical treatment were not included in the LMM.
Table 3.
Areas of feeding scars by Hylobius abietis on seedlings treated with treatments.
| Treatment | 2018 | 2019 | 2020 | |||
|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | |
| Control | 149.1 | 275.1 | 336.2 | 450.4 | 480.7 | 708.1 |
| Chemical | 21.3 | 38.3 | 13.8 | 30.0 | 5.1 | 20.0 |
| Collar | 27.2 | 89.2 | 211.8 | 409.1 | 134.8 | 296.8 |
| Glue | 21.4 | 63.1 | 81.6 | 164.2 | 63.9 | 124.6 |
| Wax C | 8.7 | 52.4 | 338.2 | 497.1 | 501.4 | 669.7 |
| Wax F | 1.1 | 6.9 | 232.7 | 359.8 | 260.9 | 473.2 |
Treatment (p < 0.001, χ2 (5) = 49.28) had a significant effect on the area of feeding scars caused by H. abietis. The average damages (p < 0.001, χ2 (2) = 54.49) were higher each following year of study. Only glue-treated seedlings and those protected with collars had significantly less damage than control seedlings (Table 4). The glue treatment performed significantly better than either of the wax treatments and also significantly better than the collar treatment (Table 5). The difference between the glue and collar treatment was lower than the differences between the glue and the wax treatments. Damage was significantly lower with the collar treatment than with either of the wax treatments.
Table 4.
Results of mixed linear models (LMMs) relating seedling damage to five treatments (excluding the chemical treatment) at the clear-cut site.
| Treatment | Estimate | 95% CI | SE | p |
|---|---|---|---|---|
| Control (Intercept) | − 0.321 | − 0.58 to − 0.06 | 0.133 | 0.023 |
| Collar | − 0.425 | − 0.64 to − 0.17 | 0.135 | 0.006 |
| Glue | − 0.807 | − 1.06 to − 0.55 | 0.132 | < 0.001 |
| F wax | − 0.182 | − 0.44 to 0.12 | 0.133 | 0.282 |
| C wax | − 0.030 | − 0.29 to 0.33 | 0.137 | 0.673 |
| year 2019 | 0.589 | 0.37 to 0.81 | 0.11 | < 0.001 |
| year 2020 | 0.903 | 0.66 to 1.14 | 0.12 | < 0.001 |
Table 5.
Comparison of damage caused by pine weevil between treatments at the clear-cut site; the Tukey method was used to compare family estimates.
| Contrast | Estimate | SE | 95% CI | p |
|---|---|---|---|---|
| Control–Collar | 0.373 | 0.136 | 0.020 to 0.724 | 0.0097 |
| Control–Glue | 0.800 | 0.133 | 0.456 to 1.143 | < 0.0001 |
| Control–C wax | 0.143 | 0.135 | − 0.204 to 0.491 | 0.6420 |
| Control–F wax | − 0.058 | 0.138 | − 0.415 to 0.300 | 0.9994 |
| Collar–Glue | 0.427 | 0.146 | 0.049 to 0.804 | 0.0562 |
| Collar–F wax | − 0.229 | 0.144 | − 0.603 to 0.144 | 0.4158 |
| Collar–C wax | − 0.431 | 0.148 | − 0.81 to 0.048 | 0.0441 |
| Glue–F wax | − 0.656 | 0.142 | − 1.024 to − 0.288 | 0.0001 |
| Glue–C wax | − 0.858 | 0.145 | − 1.233 to 0.481 | < 0.0001 |
| F wax–C wax | − 0.201 | 0.14 | − 0.563 to 0.161 | 0.8062 |
Seedlings protected with C or F wax had lowest damage in the first year of the experiment. Protection by the wax coatings, however, lasted only 1 year. In the second and third years of the experiment, C wax had no protective effect, and C wax-treated seedlings were damaged to the same extent as control seedlings.
Seedling mortality as affected by the treatments
Mortality at the clear-cut site
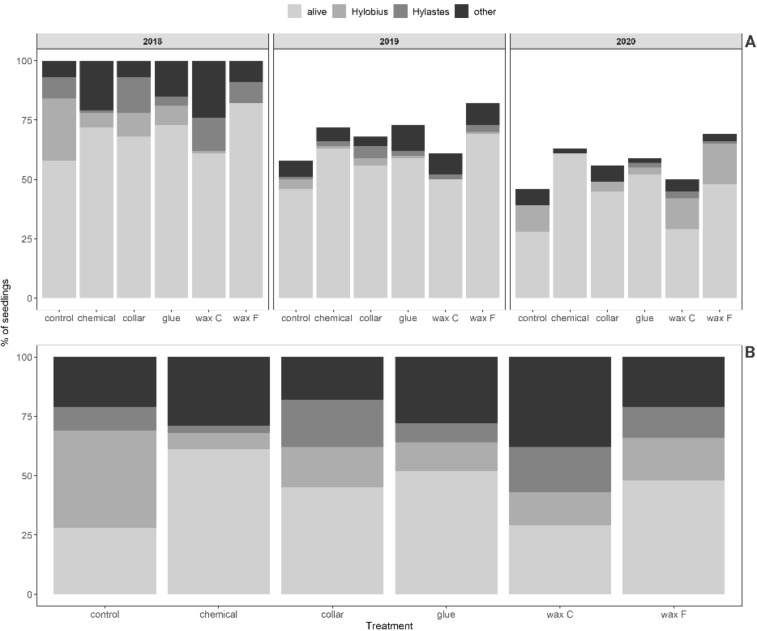
In 2018 at the clear-cut site, the mortality across all treatments including the chemical treatment was 31% (Fig. 3A). Mortality was highest with the control treatment followed by the C wax treatment, and was lowest with the F wax treatment. In 2019, the mortality across all treatments was 17%. Mortality in 2019 was lowest with the chemical treatment (Fig. 3A). In 2020, mortality across all treatments was 23% (Fig. 3A). Mortality in 2020 was highest with the C wax and control treatments and was lowest with the chemical treatment (Fig. 3 A).
Figure 3.
(A) Number of seedlings that remained alive or died (for the indicated reasons) as affected by treatments at the clear-cut site in each of the 3 years of the experiment. (B) Total seedling mortality over the 3 years of the experiment as affected by treatments at the clear-cut site.
In 2018, the highest number of seedlings died because of H. abietis damage to control seedlings. In 2019, H. abietis damage increased (Fig. 2), and resulted in high levels of mortality to control seedlings; H. abietis damage also caused high levels of mortality of control seedlings and seedlings treated with either of the waxes in 2020 (Fig. 3A). Damage caused by Hylastes occurred in all years and with all treatments, and was lowest with the chemical treatment (Fig. 3A,B).
Mortality of seedlings at the end of the 3-year experiment significantly differed among the treatments (p < 0.001, χ2(5) = 31.15) and years (p < 0.001, χ2(2) = 24.21) at the clear-cut site. Mortality was generally lower for seedlings treated with the chemical, waxes, or collar than for the control seedlings (Table 6). The highest odds ratio of survival was found for the seedlings treated with chemicals (Table 6). There was no difference in the mortality of control seedlings and those treated with C wax. Treatments with glue and F wax significantly improved the survival odds. Seedlings with collars had a higher odds ratio than control seedlings. The effect appears to be strong, but because the lower limit of the confidence interval is below 1.0, the overall effect is not that significant (Table 7).
Table 6.
Estimated parameters for the effect of treatments on the mortality of seedlings at the clear-cut site.
| Treatment | Odds-Ratio | 95% CI | SE | p |
|---|---|---|---|---|
| Control (intercept) | 0.73 | 0.31–0.89 | 0.146 | 0.140 |
| Chemical | 0.345 | 0.22–0.55 | 0.08 | < 0.001 |
| Glue | 0.458 | 0.30–0.71 | 0.10 | < 0.001 |
| Collar | 0.588 | 0.38–0.91 | 0.13 | 0.016 |
| C wax | 0.866 | 0.57–1.32 | 0.10 | 0.512 |
| F wax | 0.454 | 0.29–0.70 | 0.19 | < 0.001 |
| year 2019 | 0.49 | 0.36–0.68 | 0.08 | < 0.001 |
| year 2020 | 0.75 | 0.55–1.14 | 0.12 | 0.068 |
Table 7.
Comparison of mortality between treatments at the clear-cut site based on the Tukey method for comparing family estimates. The log odds were exponentiated to obtain the odds-ratios (OR).
| Contrast | Odds-Ratio | CI | SE | p |
|---|---|---|---|---|
| Control–Chemical | 2.895 | 1.48–5.65 | 0.6793 | 0.0001 |
| Control–Glue | 2.179 | 1.14–4.13 | 0.4895 | 0.0069 |
| Control–Collar | 1.698 | 0.90–3.17 | 0.3731 | 0.1526 |
| Control–F wax | 2.202 | 1.17–4.12 | 0.4855 | 0.0046 |
| Control–C wax | 1.153 | 0.62–2.11 | 0.2461 | 0.9853 |
| Chemical–Glue | 0.753 | 0.37–1.50 | 0.1828 | 0.8511 |
| Chemical–Collar | 0.586 | 0.29–1.15 | 0.1402 | 0.2227 |
| Chemical–F wax | 0.761 | 0.38–1.50 | 0.1815 | 0.8618 |
| Chemical–C wax | 0.398 | 0.20–0.77 | 0.0927 | 0.0011 |
| Glue–Collar | 0.779 | 0.40–1.49 | 0.1787 | 0.8865 |
| Collar–F wax | 1.011 | 0.52–1.94 | 0.2315 | 1 |
| Glue–C wax | 0.529 | 0.28–0.99 | 0.1179 | 0.0491 |
| Collar–F wax | 1.297 | 0.683–2.46 | 0.292 | 0.8582 |
| Collar–C wax | 0.679 | 0.36–1.26 | 0.1482 | 0.4838 |
| F wax–C wax | 0.524 | 0.28–0.97 | 0.1143 | 0.0359 |
Mortality at the nursery site
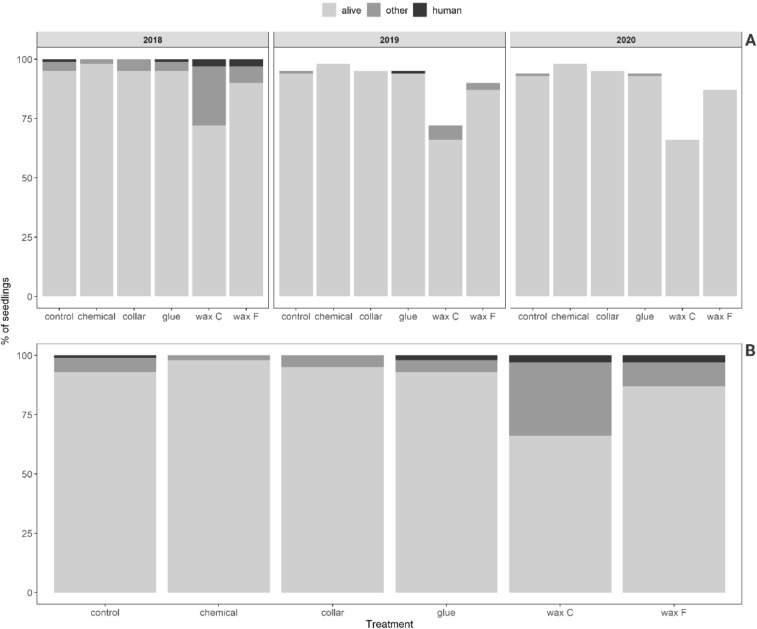
Mortality was lower at the nursery site than at the clear-cut site because of the absence of H. abietis damage or Hylastes damage at the nursery site (Fig. 4A,B). In 2018 at the nursery site, 47 seedlings died due to unknown (other) reasons, and eight died due to accidental damage during handling. One-fourth of the seedlings treated with C wax died in the first year. In 2019, only 10 seedlings (mostly coated with C or F wax) died for unknown reasons, and one seedling died due to handling. In the last year of the experiment (2020), seedlings were well established, and only 2 died (for unknown reasons; Fig. 4A,B).
Figure 4.
(A) Mortality of seedlings at the nursery site in each of 3 years of the experiment as affected by the treatments. (B) Total seedling mortality at the nursery site over the 3 years of the experiment as affected by the treatments.
Wax condition and quality as affected by wax type and year
Effect of wax type on wax quality
At the clear-cut site, there were no significant differences in wax quality between C and F wax in any year of the experiment (2018, W = 2680, p-value = 0.423; 2019, W = 1935, p-value = 0.236; 2020, W = 673.5, p-value = 0.800).
At the nursery site, wax quality significantly differed between C and F wax in 2019 and 2020; quality was higher for F wax than for C wax in both years (2018, W = 3231.5, p-value = 0.8429; 2019, W = 3401, p-value = 0.034; 2020, W = 3505, p-value = 0.01).
Effect of year on wax quality
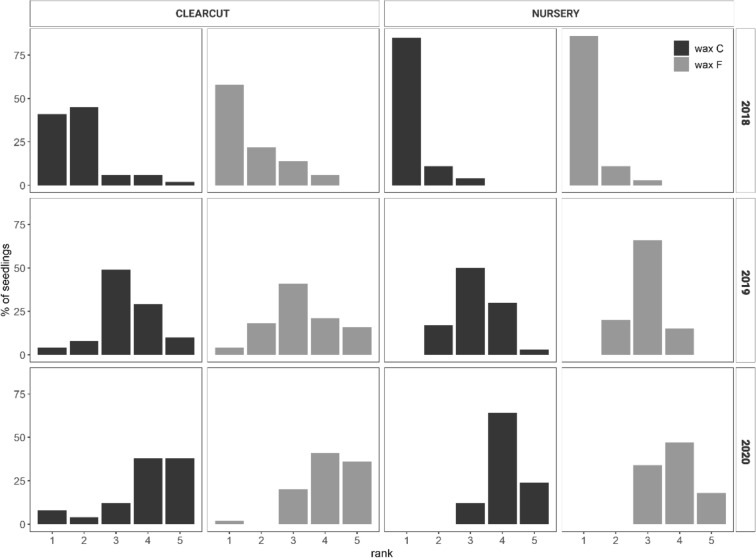
The distribution of wax quality significantly differed among the years of the study for both types of wax and for both sites. At the clear-cut site, the quality of C wax decreased with time (Fig. 5), i.e., wax quality was higher in 2018 than in 2019 (n = 47, Z = − 5.8576, p < 0.001) and was higher in 2019 than in 2020 (n = 24, Z = − 4.2666, p < 0.001). The same reductions were evident for F wax, i.e., wax quality was higher in 2018 than in 2019 (n = 68, Z = − 6.8978, p < 0.001) and was higher in 2019 than in 2020 (n = 44, Z = − 5.2382, p < 0.001).
Figure 5.
Distribution of quality (ranked on a scale from 1 to 5 for the two wax types at two sites and for 3 years of the experiment. Wax quality was ranked on a scale from 1 highest quality to 5 lowest quality).
The results from the nursery site were similar to those from the clear-cut site. The quality of C and F wax significantly decreased with time. For C wax: 2018/2019 (n = 66, Z = − 7.2349, p < 0.001; 2019/2020 (n = 66, Z = − 4.1242, p < 0.001). For F wax: 2018/2019 (n = 87, Z = − 8.3528, p < 0.001); 2019/2020 (n = 87, Z = − 7.4788, p < 0.001). In the first year, quality in the best rank 1 was higher for F wax than for C wax (Fig. 5).
Economic evaluation of the treatments
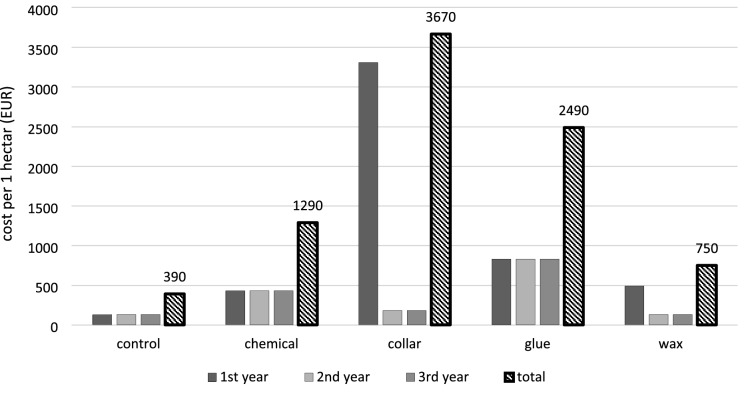
All costs needed for the application of the treatments are indicated in Fig. 6. Weeding once each year costs 130 EUR/ha (in total 390 EUR/ha for 3 years), and these costs were added to all treatment types (including the control).
Figure 6.
Costs per ha to protect spruce seedlings with the treatments. Treatment costs per ha are for the standard number of 3000 seedlings/ha. All columns include standard costs for weeding once each year (130 EUR/ha). Price for 1 l of Vaztak Active is approximately 32 EUR (required amount = 0.6 l/ha). Discount price for 1 bottle of Vermifix spray is approximately 2.3 EUR (required amount = 45 bootles/ha). Price for 1 collar is approximately 1 EUR (required amount = 3000 collars/ha). Costs per 1 waxed seedling are 0.12 EUR (material and service). The presented prices are valid for Slovakia (year 2021).
The costs were lowest for the wax treatments (750 EUR/ha for 3 years). Wax coating was applied in the first year (360 EUR/ha), and was not applied again because this treatment cannot be repeated in the field.
Chemical treatment of seedlings was relatively inexpensive (1290 EUR/ha for 3 years), even though it was applied three times each year. A single chemical treatment (material and labour) costs 100 EUR/ha.
Treatment of seedlings with glue was relatively expensive (2490 EUR/ha for 3 years). According to our calculations, a single treatment costs approximately 233 EUR/ha (material and labour).
Mechanical protection of seedlings with collars was the most expensive (3670 EUR/ha for 3 years). These biodegradable collars are very expensive (ca. 1 EUR each), and they must be checked at least once each year (approximately 50 EUR).
Discussion
Most recent experiments that compared chemical and non-chemical treatments for protection of seedlings against H. abietis have been conducted in the UK11,24,26,32,33 and Slovakia19,21,38. A comprehensive comparison of efficacy based on our results and our experience with all available and possible protection methods in Central Europe is provided in the following sections.
Chemical treatment
The application of pesticides in forestry will be gradually restricted or forbidden (different certification schemes such as Forest Stewardship Council (FSC) and Programme for the Endorsement of Forest Certification (PEFC); European Commission policies described in Refs.11,19,24,26 even though much less pesticide is applied to forests than to agricultural crops26,62. Some effective insecticides (cypermethrin, alpha-cypermethrin) have been replaced by less toxic insecticides11,26,33 and by ULV (ultra-low volume) application techniques24. Using of insecticides is restricted in national parks19 and is restricted to treat trees only in nurseries before they are planted in the forest26. In our study, we used a non-systemic pyrethroid insecticide, alpha-cypermethrin. Despite its disadvantages (described above and in Table 8), alpha-cypermethrin provided the best protection against H. abietis (Figs. 2, 3A,B). In the third year, alpha-cypermethrin application resulted in almost zero damage by H. abietis to seedlings (Fig. 2), and the lowest mortality of seedlings at both sites (Fig. 4). The high effectiveness of insecticides has been described by the authors cited above, which indicates that a similarly effective alternative may be difficult to find. Moreover, chemically treated seedlings were the least damaged by Hylastes spp., which attack the roots (Figs. 3, 4). We suspect that the alpha-cypermethrin provided protection against Hylastes spp. by running down the stem to the root neck.
Table 8.
Strengths and weaknesses of the treatments used to reduce H. abietis damage to seedlings in this study.
| Treatment mark | Strengths | Weaknesses |
|---|---|---|
| Chemical |
Cheap and effective (partly effective also against Hylastes spp.) Simple and fast application Different forms of application (pre-planting, post-planting) Treatment can be repeated in the field |
Environmental impact Staff safety Chemicals are restricted and will be probably banned in forestry in some countries (see cited works in Introduction) Need to repeat treatments (2–3 times per season) |
| Glue |
Good effectiveness Simple application New method |
Costs per ha Potential phytotoxicity Need for more tests |
| Collar |
Low environmental impact Easy to set up |
Costs per ha Some collars do not remain locked, checking once a year is necessary If the pine weevil gets into the collar, plant damage can be substantial Biodegradability is debatable |
| Wax type C and F |
Low environmental impact Costs per ha (only one treatment) Excellent effectiveness in the first year F composition seems better More layers can be put on |
Not suitable at sites with high population densities Quality of treatment—staff needs to be careful during the whole process from waxing up to planting Phytotoxicity in the case of insufficient cooling Wax cracking and falling off in the second season Treatment can not be repeated in the field |
Wax treatment
In the first year of the study, wax provided the best protection among all of the treatments; in that year, seedlings treated with either wax type had the lowest mortality caused by H. abietis and the smallest area of feeding scars (Fig. 2). In the second year, the wax began to crack and fall off (Fig. 5). In the third year, the wax provided no protection, and damage for wax-treated seedlings was similar to damage for untreated (control) seedlings (Fig. 2). Although no significant difference was found between the damage of seedlings treated with different wax types against H. abietis (Table 5), seedling mortality was lower for F wax than for C wax (Table 7), probably because of the better elasticity of F wax (J.M. Petersson, pers. comm.). This difference in the efficacy of the two wax types was confirmed at the nursery site in 2019 and 2020, where significant differences in quality were found between F and C wax types. At the clear-cut site, however, the two types of waxes did not differ in quality. Moreover, the mortality of seedlings was lower for F wax than C wax at both sites (Figs. 3, 4), probably because of higher phytotoxicity (as indicated by mortality caused by “other, unknown factors”) with C wax. In contrast, Eriksson27 found that seedlings treated with the F type had a significantly higher rate of damage caused by “other, unknown factors” than seedlings treated with the C type. Apart from our work, Eriksson27 has been the only study that tested the F type of wax. Many studies have compared wax with other treatments and have found that wax had promising effectiveness against H. abietis19,27,30,38,42,62–65, including our studies. This was confirmed in the first year of our study, but the quality and hence the protective characteristics of wax rapidly decreased in subsequent years. Most of the cited works monitored wax effectiveness only for 1 or 2 years. H. abietis imagoes, however, may remain at the site for up to 4 years8, which can be a problem for seedlings treated with wax, especially in the third year (as in our case); when the wax falls off (Fig. 5), the seedlings are no longer protected (Fig. 2). This is the greatest weakness of wax treatments (Table 8). Problems with cracks and falling off have been reported by forest managers from Slovakia, the Czech Republic, and Austria (pers. obs.). Similar results have been presented26,32 who found that wax quality declined in the cold weather of the UK. This is most probably the reason for wax cracking in our study (Fig. 5), because the chosen sites (nursery and clear-cut) are located in areas with harsh mountainous conditions (above 800 and 1000 masl, respectively).
The producer suggests that the ideal thickness of the wax layer is 1.5 mm (J. M. Peterssen, pers. comm.), because this thickness resulted in less cracking than thinner layers66. The thickness of the wax layer in this study was consistent with the producer’s recommendations.
Wax temperature during the application is approximately 80 °C, and treated seedlings must be quickly cooled with water to avoid damage to the seedlings36. During this procedure, mistakes can occur, which could have caused increased phytotoxicity and mortality at both sites in the first year of our study (Figs. 3A, 4A). We observed that the staff handling the seedlings damaged them during waxing, transport, storage and planting (Table 7). It is necessary to increase staff awareness of the need for careful handling of seedlings during and after waxing. In other experiments, we frequently observed that the wax was damaged immediately after planting. Another frequent mistake is that the seedlings are planted too deep, and only few centimetres of the wax layer remain above the ground. These mistakes may be reduced by staff training, which should include the reading and reviewing of a manual/leaflet that explains potential problems. Wax cracking can be partially reduced by applying additional layers (two layers crack less than one layer) (G. Nordlander, pers. comm.). A further detailed comparison of wax and insecticides based on reports27,30,42,62–64 is discussed in our previous study19.
Willoughby26 found that wax provided variable protection against H. abietis and that protection was poor at sites with abundant H. abietis populations. At sites with low to intermediate populations of H. abietis, however, protection was sufficiently promising to warrant further experiments with wax26,32. The cracking of the wax layer and the high population of H. abietis at the clear-cut site54 could explain the weak protection provided by wax in the current study.
Glue treatment
Vermifix glue has been studied only by the authors of the current study19,38. During the first experiments in 2015 and 2016 (unpubl. data), the glue caused relatively high phytotoxicity on spruce seedlings. The producer subsequently managed to reduce this problem because, in the current study, seedling mortality was not higher with glue than with other treatments at either site even though multiple coatings of glue were applied (3 times every year, 9 layers in total) (Figs. 3, 4). Moreover, glue significantly reduced H. abietis feeding damage, and provided the second-best protection (Fig. 2). To date, only a few other studies have evaluated the effects of glue treatment of seedlings on H. abietis feeding damage. Eriksson27,62 found that glue (Bayer) prevented adults from feeding on seedlings. Similar feeding barriers, which are applied by coating, and which were tested against H. abietis, include Polymer24, Flexcoat26,34, Södra27, and Conniflex; the latter material is widely used in Sweden3,31.
Collars
Other feeding barriers include collars. The type of the collar used in our study (Table 1, Fig. 1b) was tested under Central European conditions for the first time. We found that the collars protected seedlings better than wax (Fig. 2). The producer states that the type of plastic used to construct the collars is biodegradable and will last in the field for 4 years (Table 1), but our previous experiments (unpubl. data) indicate that these collars do not degrade even after 4–5 years (pers. obs.). The deploying of these collars was also problematic because the locks did not remain closed (the collars were checked once each year). In some cases, H. abietis adults climb over the collar and cause substantial damage before the damage is detected. Similar findings were reported from the UK24. The major disadvantage of collars, however, is their price (Table 8, Fig. 6), and we therefore do not recommend their use.
A detailed comparison of the effectiveness of different types of feeding barriers, which have been developed mainly in Sweden29, has been recently presented21 and is therefore not presented here because of space limitations. In general, these barriers are relatively expensive, must be checked frequently, and are not suitable in the areas with high population densities of H. abietis. The barriers warrant further research, however, as non-chemical alternatives for control of H. abietis.
Practical and economic comparison of the treatments
Based on our previous experiments (cited in this work and unpubl. data) and the current results, we have summarized the weaknesses and strengths of the applied treatments in Table 8. Multiple comparisons with other treatments are presented in new studies24,26,33.
We found that application is substantially cheaper for wax and insecticides than for glue and collars (Fig. 6). In our case, the costs per one waxed seedling was 0.12 EUR using the fountain machine, in addition the producer states that the cost is 0.05 EUR (or 0.05–0.09 EUR per tree39 using the new wax machine (semi-automatised link)66 with costs per ha equal to 110 EUR.
In our study, we applied the insecticide three times per year, and the costs were still low. Our findings also support Hardy24, who found that the tested insecticides were cheap and effective. The collar and glue used in our study are expensive (Fig. 6) (3670 and 2490 EUR per ha, respectively). The total average costs of planting are 1744 EUR per ha in Slovakia (State Forests, pers. comm.). Considering this information and the costs for collar or glue treatments, it may be cheaper to replant an area than to use collars or glue.
The initial costs of seedling protection can be reduced by delaying restocking until H. abietis population levels have fallen32, but this approach generates costs for weeding, increases costs for planting due to worsened conditions, and delays wood production for future generations. Costs could also be reduced using a management support system, which helps foresters predict and reduce damage; such a system has been developed by Moore67 for UK conditions. Another detailed comparison of different treatments can be found in Willoughby et al.11.
Recommendations for forest managers
Based on our experience and considering the pressure to reduce insecticide applications in forests, we recommend the following for forest management in Central Europe:
In spite of their ecological disadvantages, insecticide application will for the near future be the least expensive and most effective way to protect seedlings against H. abietis in areas with high population densities.
The negative effects of insecticides on the forest environment can be reduced by treating seedlings with the insecticides before they are planted in the field (e.g., by dipping them in insecticides in the nursery). Depending on the insecticide, such a treatment remains effective for 5 months68, or for 3–7 months11,34. Viiri69 also found that dipping in an insecticide was superior to spraying an insecticide for controlling H. abietis. According to our experience and those of researchers11,26, spraying is effective for 1–3 months.
Environmental impacts of insecticides could be reduced by applying less toxic insecticides11,26 or by using ULV application methods24.
In areas with low to moderate H. abietis damage, different types of feeding barriers can be used including new types of feeding barriers that are comparable in cost to wax and insecticide treatments.
Wax is an inexpensive and a good alternative to insecticides, but its negatives need to be eliminated (wax cracks, falls off, and can be phytotoxic). Wax is not suitable for sites with extremely high H. abietis population densities. Wax may also be unsuitable for areas with harsh conditions26.
Vermifix glue is the most effective alternative to chemical treatments. Its potential to protect spruce seedlings has been confirmed19,38, but although its spray application is effective, it is relatively expensive. Theoretically, the glue can be applied in a less expensive manner, or eventually can be mixed with sand to provide an abrasive repellent against H. abietis feeding. Further research on glue is warranted.
A combined use of wax in the first year of planting (according to most authors, wax is effective as long as it remains in place) and of insecticides (if allowed) or glue in the second (third) year should be considered. This combination would be cheaper and less harmful to the environment than the repeated application of insecticide. Another possibility is delayed restocking with waxed seedlings.
Research on bio-pesticides and new methods of their application (e.g., using the bio-insecticide carrier described in Lalík70 should be supported).
Maximum use of silvicultural practices that reduce feeding damage by H. abietis on planted seedlings. These practices include natural regeneration, weed removal, delayed restocking, planting mixed forests, scarification, and others that are described in the works cited in the Introduction or in the review21.
Conclusions
In the current study, we found that insecticide application was the most effective and least expensive way to protect seedlings against H. abietis, but the use of insecticides in European forestry will be severely restricted or even banned in the future. The glue spray used in our study showed promising results for seedling protection and can be easily applied.
Collars provided moderate protection, but they are expensive, their locks must be checked annually, and the biodegradability of the collars used is questionable. We therefore do not recommend using this type of collar in its present form.
The major weakness of wax is its potential to crack and fall off the seedling in the second half of the second season after waxing. Despite the promising results, wax effectiveness in this study was not sufficient in the second and the third year. The newer F type achieved better results than the standard C type, and we therefore suspect that further research could improve wax performance; this research could include the application of two wax layers, the development of a more flexible wax, the renewal of the wax layer in the field, and the reduction of wax phytotoxicity.
We also see some promise for combining the tested methods, e.g., wax could be used to protect seedlings in the first year, and the spraying of glue or insecticides (if allowed) could be used to protect seedlings in the second year. Although the current study and the papers cited herein have provided many answers, many questions about how to protect coniferous seedlings against H. abietis remain unanswered. Additional research is clearly needed to improve some of the existing protection methods and to develop new protection methods.
Acknowledgements
We thank all local foresters for their help with the field experiments. The authors also thank Dr. Bruce Jaffee (USA) for linguistic and editorial improvements.
Author contributions
J.G.: Conceptualization, supervision, investigation, methodology, formal analysis, writing—review & editing. M.L.: Conceptualization, formal analysis, investigation, methodology, writing—original draft, writing—review & editing. S.R.: Investigation, methodology, writing—original draft. C.N.: Data curation, formal analysis, validation; visualization. M.B.: Investigation, writing—review & editing. J.P.: Investigation. S.H.: Investigation. M.Z.: Supervision. A.K.: Supervision. J.V.: Investigation. A.G.: Investigation. J.H.: Formal analysis, writing—review & editing.
Funding
This work was supported by the Slovak Research and Development Agency under the contract No. APVV-16-0031, APVV-18-0086, APVV-20-0365, by the Ministry of Agriculture and Rural Development of the Slovak Republic (Promoles project), and by the Ministry of Defence of the Slovak Republic No. VLMSR 0120. This work represents the output of the project “Research and development of contactless methods for the acquisition of geospatial data for the purpose of forest monitoring to streamline forest management and increase forest protection” ITMS 313011V465 supported by the European structural and investment funds for “Operational Programme Integrated Infrastructure”. This research was also partly supported by the grant “Advanced research supporting the forestry and wood‐processing sector's adaptation to global change and the 4th industrial revolution,” No. CZ.02.1.01/0.0/0.0/16_019/0000803, financed by OP RDE.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Day, K. R. & Leather, S. Threats to forestry by insect pests in Europe. In Forests and Insects. (eds. Watt, A. D., Stork, N. E. & Hunter, M. D.) 177–205 (Chapman and Hall, 1997).
- 2.Långström, B. & Day, K.R. Damage, control and management of weevil pests, especially Hylobius abietis. In Bark and Wood Boring Insects in Living Trees in Europe, a Synthesis (eds. Lieutier, F. et al.) 415–444 (Springer, 2007).
- 3.Nordlander G, Hellqvist C, Johansson K, Nordenhem H. Regeneration of European boreal forests: Effectiveness of measures against seedling mortality caused by the pine weevil Hylobius abietis. For. Ecol. Manag. 2011;262:2354–2363. doi: 10.1016/j.foreco.2011.08.033. [DOI] [Google Scholar]
- 4.Leather SR, Day KR, Salisbury A. The biology and ecology of the large pine weevil, Hylobius abietis (Coleoptera: Curculionidae): A problem of dispersal? Bull. Entomol. 1999;89:3–16. doi: 10.1017/S0007485399000024. [DOI] [Google Scholar]
- 5.Moore, R. Managing the threat to restocking posed by the large pine weevil, Hylobius abietis: the importance of time of felling of spruce stands. Forestry Commission Information https://www.forestresearch.gov.uk/documents/323/fcin061_OIvSOQX.pdf (2004). Accessed 29 May 2022.
- 6.Nordlander G, Nordenhem H, Bylund H. Oviposition patterns of the pine weevil Hylobius abietis. Entomol. Exp. Appl. 1997;85:1–9. doi: 10.1046/j.1570-7458.1997.00229.x. [DOI] [PubMed] [Google Scholar]
- 7.Örlander G, Nilsson U, Nordlander G. Pine weevil abundance on clearcuts of different ages: A 6-year study using pitfall traps. Scand. J. For. Res. 1997;12:225–240. doi: 10.1080/02827589709355405. [DOI] [Google Scholar]
- 8.Nordlander G, Hellqvist C, Hjelm K. Replanting conifer seedlings after pine weevil emigration in spring decreases feeding damage and seedling mortality. Scand. J. For. Res. 2017;32:60–67. doi: 10.1080/02827581.2016.1186220. [DOI] [Google Scholar]
- 9.Fedderwitz F, Björklund N, Ninkovic V, Nordlander G. Does the pine weevil (Hylobius abietis) prefer conifer seedlings over other main food sources? Silva Fenn. 2018;52:9946. doi: 10.14214/sf.9946. [DOI] [Google Scholar]
- 10.Day, K. R., Nordlander, G., Kenis, M., Halldórsson, G. General biology and life cycles of bark weevils. In Bark and Wood Boring Insects in Living Trees in Europe, a Synthesis (eds. Lieutier, F., et al.) 331–349 (Springer, 2007).
- 11.Willoughby, I., Moore, R. & Nisbet, T. Interim guidance on the integrated management of Hylobius abietis in UK forestry. Forest Research Research Note, https://www.forestresearch.gov.uk/documents/607/FR_InterimguidanceonmanagementHylobiusabietis_2017.pdf (2017). Accessed 29 May 2022.
- 12.Eidmann HH, Lindelöw A. Estimates and measurements of pine weevil feeding on conifer seedlings: Their relationships and application. Can. J. For. Res. 1997;27:1068–1073. doi: 10.1139/x97-047. [DOI] [Google Scholar]
- 13.Dillon AB, Moore CP, Downes MJ, Griffin CT. Evict of infect? Managing populations of the large pine weevil, Hylobius abietis, using bottom-up and topdown approach. For. Ecol. Manag. 2008;255:2634–2642. doi: 10.1016/j.foreco.2008.01.021. [DOI] [Google Scholar]
- 14.Moore R, Brixey JM, Milner AD. Effect of time of year on the development of immature stages of the large pine weevil (Hylobius abietis L.) in stumps of Sitka spruce (Picea sitchensis Carr.) and influence of felling date on their growth, density and distribution. J. Appl. Entomol. 2004;128:167–176. doi: 10.1111/j.1439-0418.2004.00828.x. [DOI] [Google Scholar]
- 15.Inward DJG, Wainhouse D, Peace A. The effect on temperature on the development and life cycle regulation of the pine weevil Hylobius abietis and potential impacts of climate change. Agric. For. Entomol. 2012;14:348–357. doi: 10.1111/j.1461-9563.2012.00575.x. [DOI] [Google Scholar]
- 16.Tudoran A, Oltean I, Varga M. Control and management of the pine weevil Hylobius abietis L. Bull. UASVM Hortic. 2019;76:1. [Google Scholar]
- 17.Wainhouse D, Inward G, Morgan G. Modelling geographical variation in voltinism of Hylobius abietis under climate change and implications for management. Agric. For. Entomol. 2014;16:136–146. doi: 10.1111/afe.12043. [DOI] [Google Scholar]
- 18.Galko, J., Vakula, J., Kunca, A., Rell, S. & Gubka, A. Slovak technical standard no. 48 2712, Ochrana lesa proti tvrdoňom a lykokazom na sadeniciach (Forest protection against large pine weevil and bark beetles from genus Hylastes on seedlings), Slovak Office of Standards, Metrology and Testing, Bratislava (2016).
- 19.Lalík M, et al. Non-pesticide alternatives for reducing feeding damage caused by the large pine weevil (Hylobius abietis L.) Ann. Appl. Biol. 2020;177:132–142. doi: 10.1111/aab.12594. [DOI] [Google Scholar]
- 20.Tudoran A, Nordlander G, Karlberg A, Puentes A. A major forest insect pest, the pine weevil Hylobius abietis, is more susceptible to Diptera-than Coleoptera-targeted Bacillus thuringiensis strains. Pest Manag. Sci. 2020;77:1303–1315. doi: 10.1002/ps.6144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lalík M, et al. Ecology, management and damage by the large pine weevil (Hylobius abietis) (Coleoptera: Curculionidae) in coniferous forests within Europe. Centr. Eur. For. J. 2021;67:91–107. [Google Scholar]
- 22.Örlander G, Nilsson U. Effect of reforestation methods on pine weevil (Hylobius abietis) damage and seedling survival. Scand. J. For. Res. 1999;14:341–354. doi: 10.1080/02827589950152665. [DOI] [Google Scholar]
- 23.Petersson M, Örlander G. Effectiveness of combinations of shelterwood, scarification, and feeding barriers to reduce pine weevil damage. Can. J. For. Res. 2003;33:64–73. doi: 10.1139/x02-156. [DOI] [Google Scholar]
- 24.Hardy C, Sayyed I, Leslie AD, Dittrich AD. Effectiveness of insecticides, physical barriers and size of planting stock against damage by the pine weevil (Hylobius abietis) Crop Prot. 2020;137:105307. doi: 10.1016/j.cropro.2020.105307. [DOI] [Google Scholar]
- 25.Harvey CD, Williams CD, Dillon AB, Griffin CT. Inundative pest control: How risky is it? A case study using entomopathogenic nematodes in a forest ecosystem. For. Ecol. Manag. 2016;380:242–251. doi: 10.1016/j.foreco.2016.08.018. [DOI] [Google Scholar]
- 26.Willoughby IH, Moore R, Moffat AJ, Forster J, Sayyed I, Leslie K. Are there viable chemical and non-chemical alternatives to the use of conventional insecticides for the protection of young trees from damage by the large pine weevil Hylobius abietis L. in UK forestry? Forestry. 2020;93:694–712. doi: 10.1093/forestry/cpaa013. [DOI] [Google Scholar]
- 27.Eriksson, S., Wallertz, K. & Karlsson, A.-B. Test av mekaniska plantskyddmot snytbaggar i omarkberedd ochmarkberedd mark, anlagt våren 2015. Sveriges Lantbruksuniversitet Rapport 16. (2018) https://pub.epsilon.slu.se/15698/1/eriksson_s_et_al_181010.pdf (accessed 8 Dec 2021).
- 28.Swedish Forest Agency [Skogsstyrelsen] Forest seedlings delivered for planting 2020. Sveriges Officiella Statistik, Statistiska Meddelanden JO 0313 SM 2001. (2021) https://www.skogsstyrelsen.se/globalassets/statistik/statistiska-meddelanden/sm-levererade-skogsplantor-2020.pdf (accessed 8 Dec 2021).
- 29.Petersson M, Örlander G, Nilsson U. Feeding barriers to reduce damage by pine weevil (Hylobius abietis) Scand. J. For. Res. 2004;19:48–59. doi: 10.1080/02827580310019554. [DOI] [Google Scholar]
- 30.Petersson, M., Eriksson, S. & Zetterqvist, F. Mekaniska plantskydd mot snytbaggeskador, anlagt 2003. Slutrapport SLU, Asa försökspark, Rapport, 3. (2006) https://docplayer.se/137316277-Mekaniska-plantskydd-mot-snytbaggeskador-anlagt-2006.html (accessed 10 Dec 2021).
- 31.Nordlander G, Nordenhem H, Hellqvist C. A flexible sand coating (Conniflex) for the protection of conifer seedlings against damage by the pine weevil Hylobius abietis. Agric. For. Entomol. 2009;11:91–100. doi: 10.1111/j.1461-9563.2008.00413.x. [DOI] [Google Scholar]
- 32.Leslie, K. & Liddon, T. An integrated pest management strategy for Hylobius—the holy grail of forestry? Forest and Timber News, April 2017, 44–45. https://www.confor.org.uk/media/246596/integrated-pest-managment-strategy-for-hylobius-april-2017.pdf (2017). Accessed 29 May 2022.
- 33.Moore R, Willoughby IH, Moffat AJ, Forster J. Acetamiprid, chlorantraniliprole, and in some situations the physical barriers MultiPro® or Kvaae® wax, can be alternatives to traditional synthetic pyrethroid insecticides for the protection of young conifers from damage by the large pine weevil Hylobius abietis L. Scand. J. For. Res. 2021;36:230–248. doi: 10.1080/02827581.2021.1906313. [DOI] [Google Scholar]
- 34.Willoughby IH, et al. Are there viable chemical and non-chemical alternatives to the use of conventional insecticides for the protection of young trees from damage by the large pine weevil Hylobius abietis L. in UK forestry? Forestry. 2020;93:694–712. doi: 10.1093/forestry/cpaa013. [DOI] [Google Scholar]
- 35.Galko, J., Kunca, A., Gubka, A. & Vakula, J. Predstavenie nového spôsobu ošetrenia sadeníc voskom ako účinnej ochrany pred tvrdoňom smrekovým (Introduction of a new method of wax seedling treatment as an effective protection against large pine weevil). In Actual Problems in Forest Protections 2013 (Conference Proceedings), National Forest Centre, Zvolen, vol. 22 (ed. Kunca, A.) 86–89. (2013) http://www.los.sk/data/DownloadHandler.ashx?id=38&filename=15.pdf (accessed 1 Dec 2021).
- 36.Galko, J. et al. Vyhodnotenie experimentov voskom ošetrených sadeníc, ako mechanickej ochrany proti tvrdoňovi smrekovému a návrh technologického postupu voskovania (Evaluation of experiments of wax-treated seedlings as mechanical protection against large pine weevil and design of technological process of waxing). In Actual Problems in Forest Protections 2015 (Conference Proceedings), National Forest Centre, Zvolen, vol. 24 (ed. Kunca, A.) 21–30. (2015) http://www.los.sk/data/DownloadHandler.ashx?id=512&filename=Galko.4-2015.pdf (accessed 8 June, 2021).
- 37.Lalík, M. Modern biotechnological control of pine weevil (Hylobius abietis). Ph.D. thesis. Czech University of Life Sciences Prague (2021).
- 38.Rell, S. Alternative methods of seedling protection against the large pine weevil (Hylobius abietis). PhD. thesis Technical University Zvolen. (2018).
- 39.Norsk Wax, Substitution of insecticides with wax. (2016) http://www.metla.fi/tapahtumat/2016/taimitarhapaivat/Pettersen.pdf (accessed 29 Nov 2021).
- 40.Wax information. https://www.norsk-wax.no/forestry (accessed 28 Nov 2021).
- 41.Watson PG. Influence of insecticide, wax and biofungicide treatments, applied to Pinus sylvestris and Picea abies, on the olfactory orientation of the large pine weevil, Hylobius abietis L. (Coleoptera: Curculionidae) Agric. For. Entomol. 1999;1:171–177. doi: 10.1046/j.1461-9563.1999.00023.x. [DOI] [Google Scholar]
- 42.Härlin, C. & Eriksson, S. Test av mekaniska plantskydd mot snytbaggar i omarkberedd och markberedd mark, anlagt våren 2012. Slutrapport. SLU, Enheten för skoglig fältforskning, Rapport 12. (2016) https://pub.epsilon.slu.se/13059/7/harlin_c_eriksson_s_160223.pdf (accessed 1 Dec 2021).
- 43.Glue information. https://www.moudry-cz.com/cs/vermifix-lepidlo-ve-spreji-na-ochranu-stromu-a-rostlin/ (accessed 27 Nov 2021).
- 44.Azeem M, et al. Chemical composition and antifeedant activity of some aromatic plants against pine weevil (Hylobius abietis) Ann. Appl. Biol. 2020;177:121–131. doi: 10.1111/aab.12586. [DOI] [Google Scholar]
- 45.Unelius C, Bohman B, Nordlander G. Comparison of phenylacetates with benzoates and phenylpropanoates as antifeedants for the pine weevil, Hylobius abietis. J. Agric. Food Chem. 2018;66:11797–11805. doi: 10.1021/acs.jafc.8b03830. [DOI] [PubMed] [Google Scholar]
- 46.Fedderwitz F, Björklund N, Anngren R, Lindström A, Nordlander G. Can methyl jasmonate treatment of conifer seedlings be used as a tool to stop height growth in nursery forest trees? New For. 2020;51:379–394. doi: 10.1007/s11056-019-09737-6. [DOI] [Google Scholar]
- 47.Chen Y, Bylund H, Björkman C, Fedderwitz F, Puentes A. Seasonal timing and recurrence of methyl jasmonate treatment influence pine weevil damage to Norway spruce seedlings. New For. 2021;52:431–448. doi: 10.1007/s11056-020-09803-4. [DOI] [Google Scholar]
- 48.Tahvonen O, Pukkala T, Laiho O, Lähde E, Niinimäki S. Optimal forest management of uneven-aged Norway spruce stands. For. Ecol. Manag. 2010;260:106–115. doi: 10.1016/j.foreco.2010.04.006. [DOI] [Google Scholar]
- 49.Saniga, M. & Kucbel, S. Prírode blízke pestovanie lesa. Technická univerzita vo Zvolene, Zvolen. ISBN 978-80-228-2411-8 (2012).
- 50.Vencúrik, J., Jaloviar, P., Saniga, M. & Kucbel, S. Rast prirodzenej a umelej obnovy vo vybraných rekonštruovaných smrekových porastoch Oravských Beskýd: vedecká monografia. Technická univerzita vo Zvolene, Zvolen. ISBN 978-80-228-3017-1 (2017).
- 51.Kunca A, et al. Salvage felling in the Slovak Republic’s forests during the last twenty years (1998–2017) Cent. Eur. For. J. 2019;65:3–11. [Google Scholar]
- 52.Vakula J, et al. Influence of selected factors on bark beetle outbreak dynamics in the Western Carpathians. Cent. Eur. For. J. 2015;61:149–156. [Google Scholar]
- 53.Barta M, et al. Hypocrealean fungi associated with Hylobius abietis in Slovakia, their virulence against weevil adults and effect on feeding damage in laboratory. Forests. 2019;10:634. doi: 10.3390/f10080634. [DOI] [Google Scholar]
- 54.Lalík M, et al. Simple is best: Pine twigs are better than artificial lures for trapping of pine weevils in pitfall traps. Forests. 2019;10:642. doi: 10.3390/f10080642. [DOI] [Google Scholar]
- 55.Wickham H. ggplot2: Elegant Graphics for Data Analysis. Springer; 2016. [Google Scholar]
- 56.Wickham, H., François, R., Henry, L. & Müller, K. dplyr: A Grammar of Data Manipulation. R package version 1.0.2. (2020) https://CRAN.R-project.org/package=dplyr (accessed 23 Nov 2021).
- 57.Bates D, Mächler M, Bolker B, Walker S. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 2015;67:1–48. doi: 10.18637/jss.v067.i01. [DOI] [Google Scholar]
- 58.Petersson RA, Cavanaugh JE. Ordered quantile normalization: A semiparametric transformation built for the cross-validation era. J. Appl. Stat. 2019;47:2312–2327. doi: 10.1080/02664763.2019.1630372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lenth, R. V. et al. emmeans: Estimated Marginal Means, aka Least-Squares Means. (2021) https://CRAN.R-project.org/package=emmeans (accessed 23 Nov 2021).
- 60.Hartig, F. DHARMa: Residual Diagnostics for Hierarchical (Multi-Level/Mixed) Regression Models. R package version 0.4.5. (2022) https://CRAN.R-project.org/package=DHARMa.
- 61.Fay, M. P. Applied Statistical Hypothesis Tests. R package version 0.9.6. (2020) https://CRAN.R-project.org/package=asht (accessed 15 Nov 2021).
- 62.Eriksson, S., Karlsson, A. & Härlin, C. Test av mekaniska plantskydd mot snytbaggar i omarkberedd och markberedd mark, anlagt våren 2013. Slutrapport Sveriges lantbruksuniversitet, Report, 15. (2017)https://pub.epsilon.slu.se/14040/7/eriksson_s_et_al_170214.pdf (accessed 17 Nov 2021).
- 63.Härlin, C. & Eriksson, S. Test av mekaniska plantskydd och insekticider mot snytbaggar, anlagt våren 2010. Slutrapport. SLU, Enheten för skoglig fältforskning, Rapport 7. (2013) https://pub.epsilon.slu.se/10901/7/harlin_c_eriksson_s_131121.pdf (accessed 22 Nov 2021).
- 64.Härlin, C. & Eriksson, S. Test av mekaniska plantskydd och insekticider mot snytbaggar i omarkberedd och markberedd mark, anlagt våren 2011. Slutrapport. SLU, Enheten för skoglig fältforskning, Rapport 10. (2014) https://pub.epsilon.slu.se/11603/7/harlin_c_etal_141023.pdf (accessed 19 Nov 2021).
- 65.Öhrn, P. & Nordlander, G. WeevilSTOP—Field activities in Sweden 2015. (2015) https://www.weevilstop.com/project-results (accessed 30 Oct 2021).
- 66.Final Report Summary—WEEVIL STOP https://cordis.europa.eu/project/id/315404/reporting/fr (accessed 3 Dec 2021).
- 67.Moore, R., 2018. Hylobius Management Support System (MSS). https://www.forestresearch.gov.uk/tools-and-resources/tree-healthand-protection-services/hylobius-management-support-system/.
- 68.Olenici N, Bouriaud O, Manea IA. Efficient conifer seedling protection against pine weevil damage using neonicotinoids. Baltic For. 2018;24:201–209. [Google Scholar]
- 69.Viiri H, Tuomainen A, Tervo L. Persistence of deltamethrin against Hylobius abietis on Norway spruce seedlings. Scand. J. For. Res. 2007;22:128–135. doi: 10.1080/02827580701224113. [DOI] [Google Scholar]
- 70.Lalík M, et al. Potential of Beauveria bassiana application via a carrier to control the large pine weevil. Crop Prot. 2021;143:105563. doi: 10.1016/j.cropro.2021.105563. [DOI] [Google Scholar]