Abstract
The generation of efficient fluid flow is crucial for organismal development and homeostasis, sexual reproduction, and motility. Multi-ciliated cells possess fields of motile cilia that beat in synchrony to propel fluid. Ciliary arrays are remarkably conserved in their organization and function. Ciliates have polarized multi-ciliary arrays (MCAs) to promote fluid flow for cell motility. The ciliate cortex is decorated with hundreds of basal bodies (BB) forming linear rows along the cell’s anterior-posterior axis. BBs scaffold and position cilia to form the organized ciliary array. Nascent BBs assemble at the base of BBs. As nascent BBs mature, they integrate into the cortical BB and cytoskeletal network and nucleate their own cilium. The organization of MCAs is balanced between cortical stability and cortical dynamism. The cortical cytoskeletal network both establishes and maintains a stable organization of the MCA in the face of mechanical forces exerted by ciliary beating. At the same time, MCA organization is plastic, such that it remodels for optimal ciliary mobility during development and in response to environmental conditions. Such plasticity promotes effective feeding and ecological behavior required for these organisms. Together, these properties allow an organism to effectively sense, adapt to, and move through its environment.
Keywords: Basal body, basal foot, ciliate, cilia, cortex, ciliary rootlet, striated fiber, Tetrahymena
HYDRODYNAMIC flow supports a myriad of biological processes during organismal development, homeostasis, and motility. Most living organisms are equipped with MCAs to promote fluid flow. Despite being evolutionarily distant from multicellular organisms, ciliates possess a remarkably conserved ciliary and cortical architecture and therefore serve as ideal model systems for understanding the universal principles of MCAs. Adopting a ciliate and Tetrahymena thermophila-centric perspective, we highlight cytoskeletal elements that organize and stabilize MCAs. We further discuss the dynamic nature of MCAs in modulating hydrodynamic flow during environmental change.
CILIATE AND VERTEBRATE CORTICAL ARCHITECTURE
Ciliate morphological diversity and its cell cortex
Ciliates are a group of unicellular protists that fall within the Phylum of Ciliophora (Lynn 2008). These free-swimming aquatic organisms possess characteristic arrays of cilia on their cell surface to facilitate cell motility, feeding, and mating (Lynn 2008). Ciliates exhibit remarkably diverse morphology. Examples include the ‘trumpet’ shaped Stentor, the ‘swan tear’ shaped Lacrymaria, the ‘slipper’ shaped Paramecium, and the ‘teardrop’ shaped ciliate Tetrahymena (Allen 1967; Vance 1961; Wan 2019; Woodruff 1937). Ciliates are generally decorated with MCAs in a convex shape that contrasts the concave shape of vertebrate MCAs. Moreover, Tetrahymena and vertebrate MCAs possess conserved structural and molecular elements. Hence, Tetrahymena serve as an excellent model organism to understand vertebrate MCA architecture and function (Figure 1A).
Figure 1.
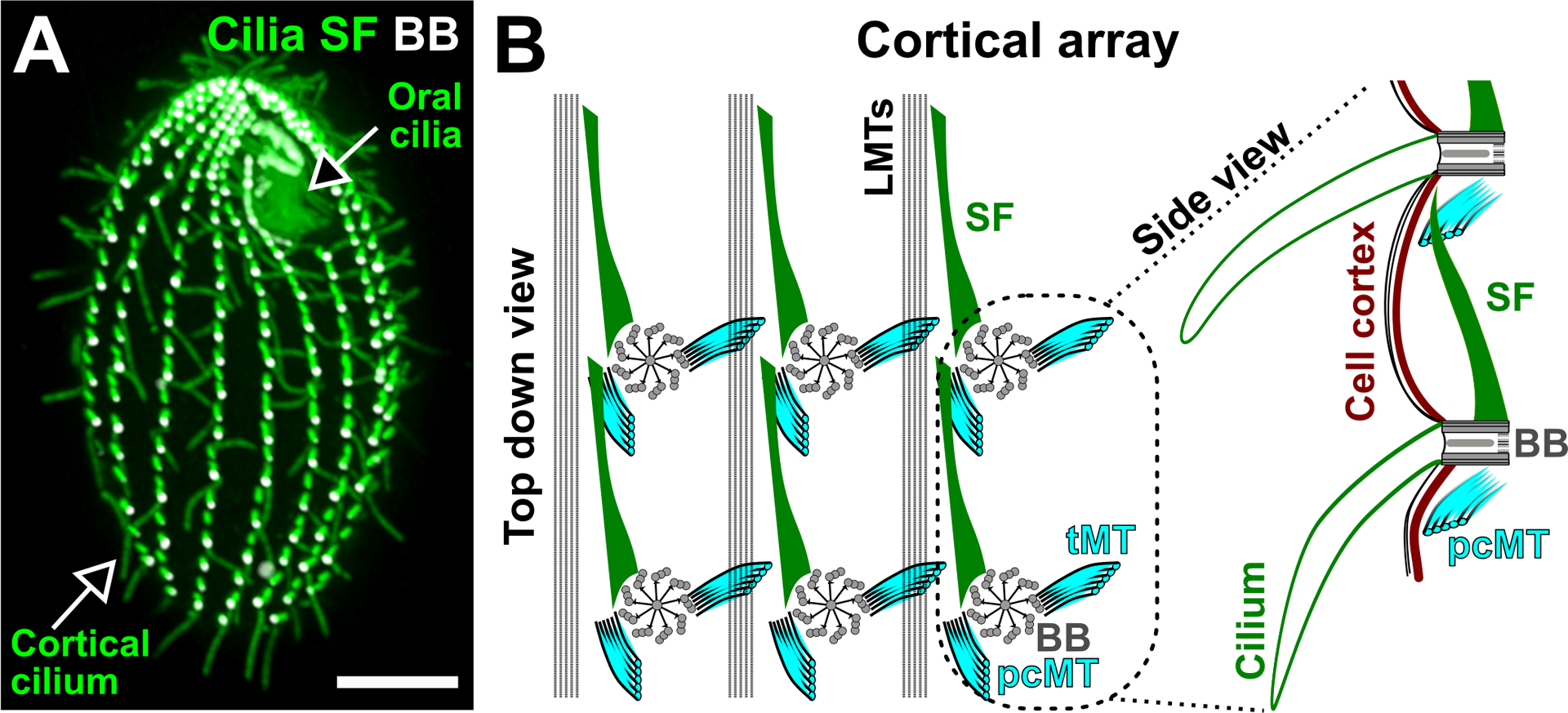
Ciliate cortical architecture. (A) Image illustrates a Tetrahymena thermophila cell. The oral cilia are organized as a cluster at the cell anterior pole to promote feeding. The cortical cilia are arranged into longitudinal rows that are parallel to the cell’s AP axis to promote cell motility. Cilia and SF (green). BB (white). Bar, 10 μm. (B) left: Schematic depicting the top down view of the Tetrahymena cortical array. SFs (green) are oriented anteriorly, pcMTs (cyan) are oriented posteriorly and tMTs (cyan) are projected laterally. LMTs (black) span along the cell’s AP axis on the left of each BB (grey) row. BBs are connected by the BB-associated SF to the pcMT of the BB directly anterior to it. right: Schematic depicting the side view of two connected BBs and their associated cilia (green). The SF distal tip approaches the cell cortex (brown). Models are adapted from Soh et al. (2020).
The organization of ciliary arrays is fundamental to cilia anchorage, coordination, and production of fluid flow (Bayless et al. 2015; Gilpin et al. 2020). The cell cortex of Tetrahymena cells consists of both an oral apparatus or feeding structure and fields of cilia referred to as cortical cilia. The cortical cilia are organized into longitudinal rows that are oriented to beat in the same direction (Figure 1A; Allen 1967). A ciliary row, also known as a kinety, runs along bands of longitudinal microtubules (MT) that span the anterior-posterior (AP) axis of the cell. Within a ciliary row, each BB, or kinetid, is associated with a set of BB-appendages (Figure 1B; Allen 1967; Pitelka 1961). Each BB extends a striated fiber (SF) and a bundle of post-ciliary microtubules (pcMTs) that orient anteriorly and posteriorly, respectively, relative to the cell’s AP axis (Figure 1B; Pitelka 1961). In addition, each BB extends a bundle of transverse microtubules (tMTs) to the right (Figure 1B; when viewed from outside the cell). BBs within a ciliary row are connected via the SF and pcMT of adjacent BBs. This creates a longitudinal connector between BBs within their respective ciliary rows (Allen 1967). BBs are positioned closer to one another on the cell anterior pole than the medial and posterior regions of the cell (Galati et al. 2015). Between adjacent ciliary rows, the BB-associated tMTs extend toward the cell cortex and the BBs in the row to the right (Allen 1967). BB-appendages also link to the cell cortex, which consists of actin filaments and an intermediate filament-like meshwork called the epiplasm (Honts and Williams 2003; Junker et al. 2019; Soh et al. 2020; Williams et al. 2006). Such linkages have also been observed in other ciliates such as Paramecium (Iftode et al. 1996). The molecular nature of the linkages between BBs and the cell cortex remains to be studied. In summary, the Tetrahymena cell cortex comprises BBs and ciliary rows that are longitudinally and laterally connected to form a grid-like and polarized organization (Figure 1; Allen 1967).
The vertebrate cell cortex
Like the arrangement in ciliates, cilia of vertebrate MCAs are also regularly spaced across the apical cell surface (Sandoz et al. 1988). Cilia are nucleated and organized by BBs with BB-appendages that position these structures at the cell cortex. Based on their orientation relative to the cilium power stroke axis, the BB-associated basal foot appears analogous to the pcMTs and an oppositely oriented ciliary rootlet, appears analogous to the ciliate SF (Figures 1 and 5B; Allen 1967; Hard and Rieder 1983; Sandoz et al. 1988). Moreover, both ciliate SF and vertebrate ciliary rootlet display periodic striations along their length. However, the striation periodicity and molecular composition are variable between organisms (Hard and Rieder 1983; Hufnagel 1969; Metz and Westfall 1954; Soh et al. 2020; Vlijm et al. 2018). Unlike ciliate BBs, vertebrate BBs establish connections to each other via MTs nucleated by the basal foot (Figure 3; Gordon 1982; Hard and Rieder 1983; Kunimoto et al. 2012; Sandoz et al. 1988). In addition, BBs are embedded within an actin meshwork, which includes an apical and sub-apical network, that anchors cilia (Hard and Rieder 1983; Mahuzier et al. 2018; Sandoz et al. 1988). The sub-apical actin network interconnects BBs by interacting with ciliary rootlets (Figure 5B (bottom); Antoniades et al. 2014; Werner et al. 2011). Intermediate filaments are also present at the cell cortex of vertebrate MCAs but, like ciliates, little is known about their role in ciliary organization (Lemullois et al. 1987). Inter-BB and BB-cortex connections give rise to an intricate scaffolding network that underlies MCAs. Although the cytoskeletal elements within MCAs of ciliates and vertebrates display unique features, the overall MCA architecture is similar, whereby BBs and their appendages link to each other and to the cell cortex so that cilia are organized, polarized, and anchored at the cell cortex (Figures 1 and 3).
Figure 5.
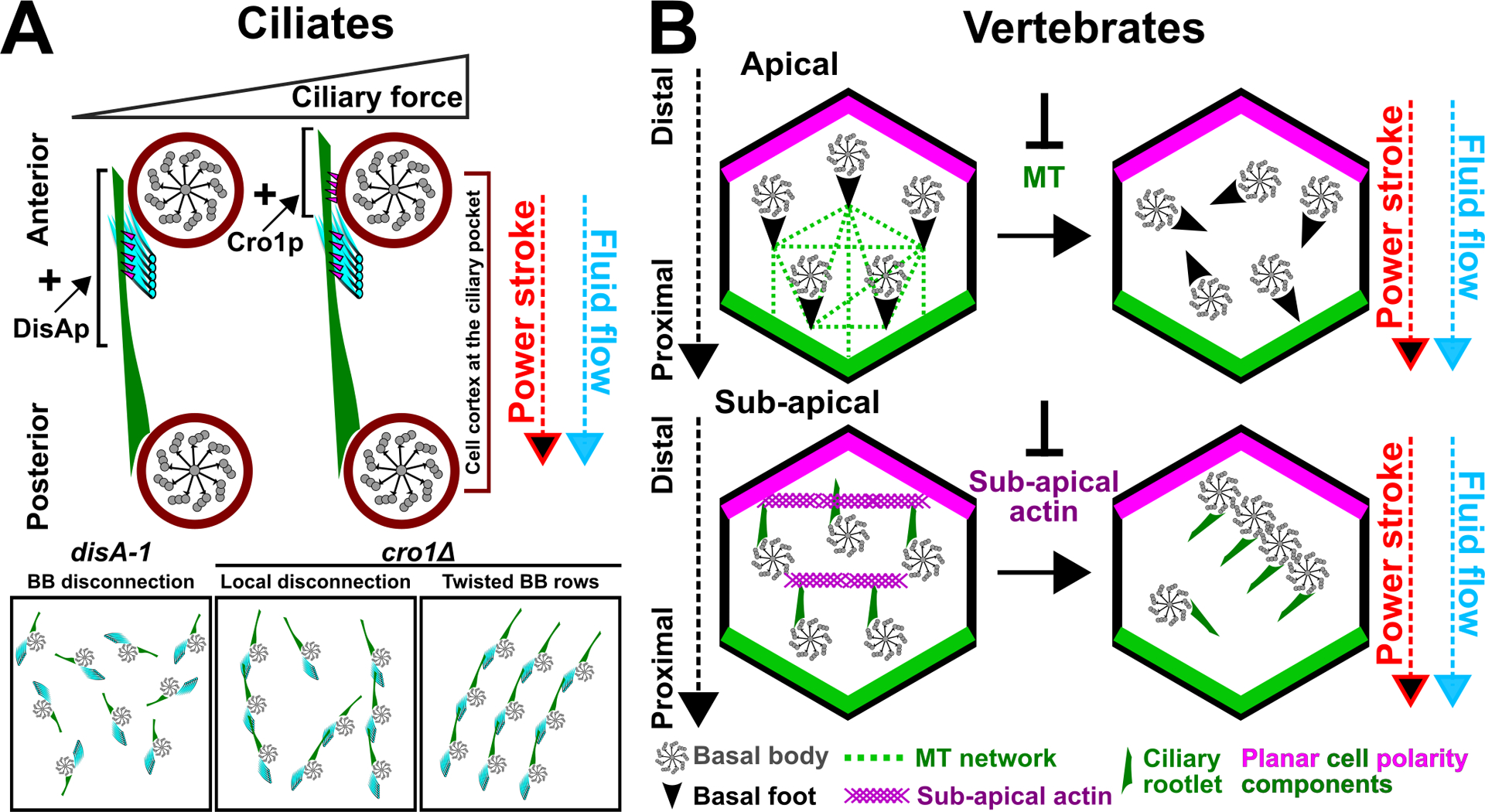
Ciliate and vertebrate BBs are organized and anchored via BB-appendages. (A) Tetrahymena SFs are ciliary force-responsive structures. SFs (green) elongate at elevated ciliary forces to establish connections with both the anterior BB’s pcMTs (cyan) and the cell cortex where the anterior BB’s ciliary pocket resides (brown circle). The SF length mutant, disA-1, possesses short SFs that causes BB (grey) disconnection, disorganization, and disorientation. Inhibition of SF elongation at high ciliary force (cro1) leads to local BB disconnection and twisted BB rows. Arrows (red and blue) depict the direction of the cilium power stroke and fluid flow. Schematic is adapted from Soh et al. (2020). (B) Vertebrate MCAs are organized by cortical MTs (green dotted line) and sub-apical actin network (purple). Each BB (grey) has a basal foot (black arrowhead) and a ciliary rootlet (green) that are oriented in opposite directions. These appendages are oriented along the axis of the cilium power stroke. top: Cortical MTs (green dotted line) interact with the BB-associated basal foot. Disruption to the cortical MT network leads to the loss of global and local BB polarities. Ciliary rootlet is not shown. bottom: The sub-apical actin network (purple) interacts with the ciliary rootlet (green). Disruption to the sub-apical actin network causes the loss of global BB polarity, but BBs remain locally polarized. BBs also form clusters. Basal foot is not shown. Arrows (red and blue) depict the direction of the cilium power stroke and fluid flow.
Figure 3.
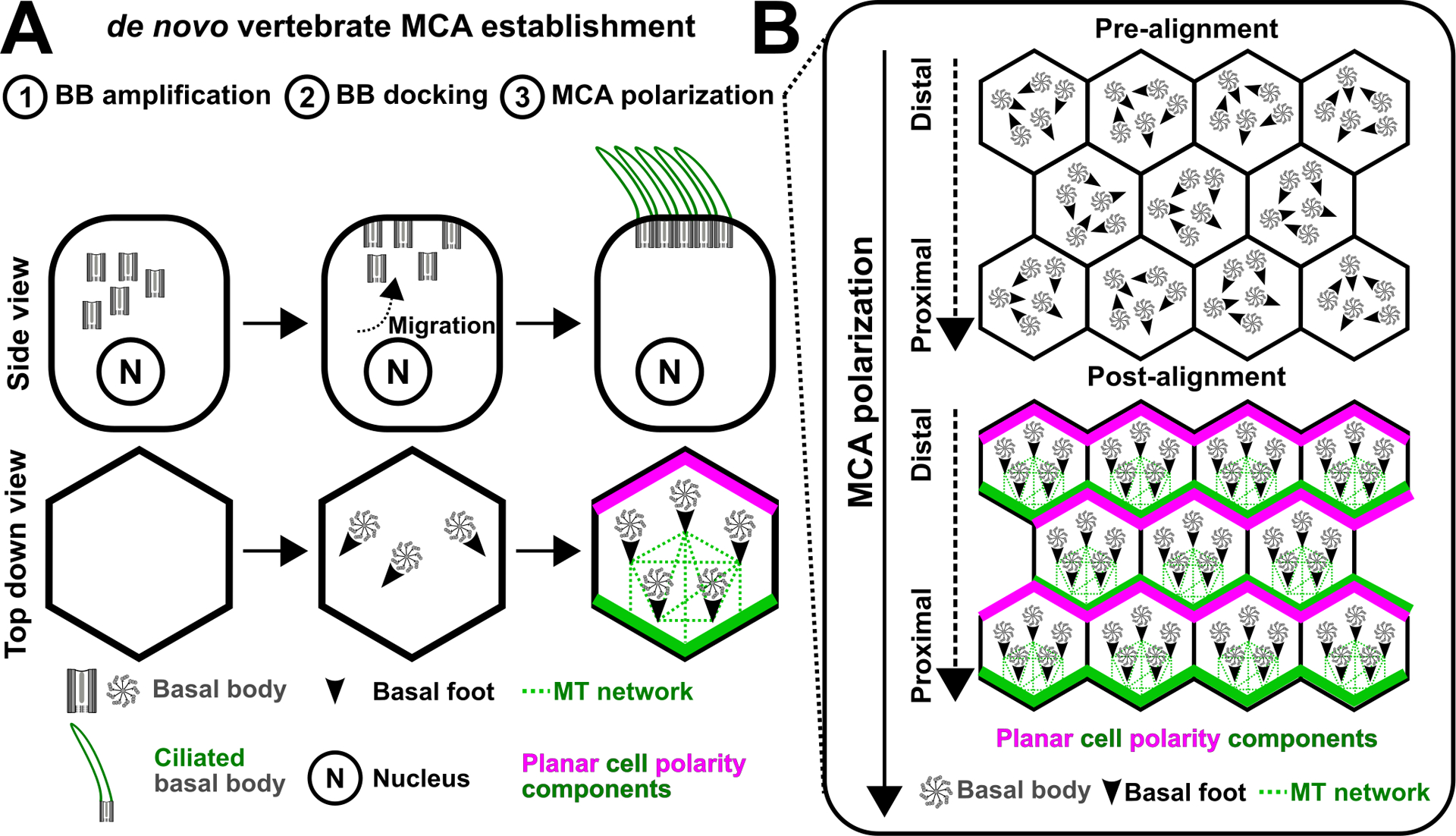
The establishment of the vertebrate MCA cortical organization (A) The organization of the vertebrate MCA is established de novo. This process includes BB (grey) amplification, BB migration, BB docking at the apical cell surface, and ciliogenesis. (B) The polarization of BBs requires an interplay between the planar cell polarity pathway and cortical MTs (green dotted lines), which interact with the BB-associated basal foot.
BUILDING THE MCA ORGANIZATION
The pre-established epigenetic architecture of the ciliate cell cortex
The organization of the ciliate cell cortex persists through the life of a ciliate cell, and it is propagated to daughter cells during cell division (Figure 2A). This concept was termed, ‘structural inheritance’ by ciliatologists, Janine Beisson and Tracy Sonneborn, who described an epigenetic mechanism whereby the pre-existing cortical organization defines the cortical organization of future cells. For discussion on epigenetic regulation of gene expression, we refer readers to the chapter entitled ‘Epigenetic mechanisms in ciliates’ in this special issue. Beisson and Sonneborn made this important discovery by inverting a subpopulation of BBs and tracking the persistence of those inverted BBs in Paramecium cells across multiple cell generations (Beisson & Sonneborn, 1965; Sonneborn, 1964). They discovered that the inverted BB polarity is stably propagated as it persists for multiple cell divisions. ‘Structural inheritance’ was observed in a variety of ciliates, including Tetrahymena and Stentor (Beisson and Sonneborn 1965; Ng and Frankel 1977; Sonneborn 1964; Tartar 1956). Electron microscopy studies that characterized the maturation of nascent BBs suggest a mechanism for ‘structural inheritance’. These studies demonstrated that nascent BBs are assembled at the base of a mother BB, guided along the mother BB’s SF before inserting anteriorly into the cell cortex (Figure 2A; Allen 1967; Dippell 1968; Iftode and Fleury-Aubusson 2003). This ensures that nascent BBs are correctly placed within the pre-existing ciliary architecture. Thus, nascent BBs and cilia acquire their own organization and orientation from the structural information of their local environment. Consistent with this, Tetrahymena mutants with local polarity defects, such as sfi13, disA-1 and cro1, display BB disorientation that is propagated over multiple cell generations (Galati et al. 2014; Soh et al. 2020; Stemm-Wolf et al. 2013). Notably, locally disoriented BBs reorient towards the global AP axis of the cell when the respective wild-type genes were reintroduced in these mutants (Galati et al. 2014; Soh et al. 2020; Stemm-Wolf et al. 2013). This indicates that by reintroducing factors that promote local polarity, BBs can interpret and reorient to global polarity cues to ensure the overall landscape of polarized rows of beating cilia.
Figure 2.
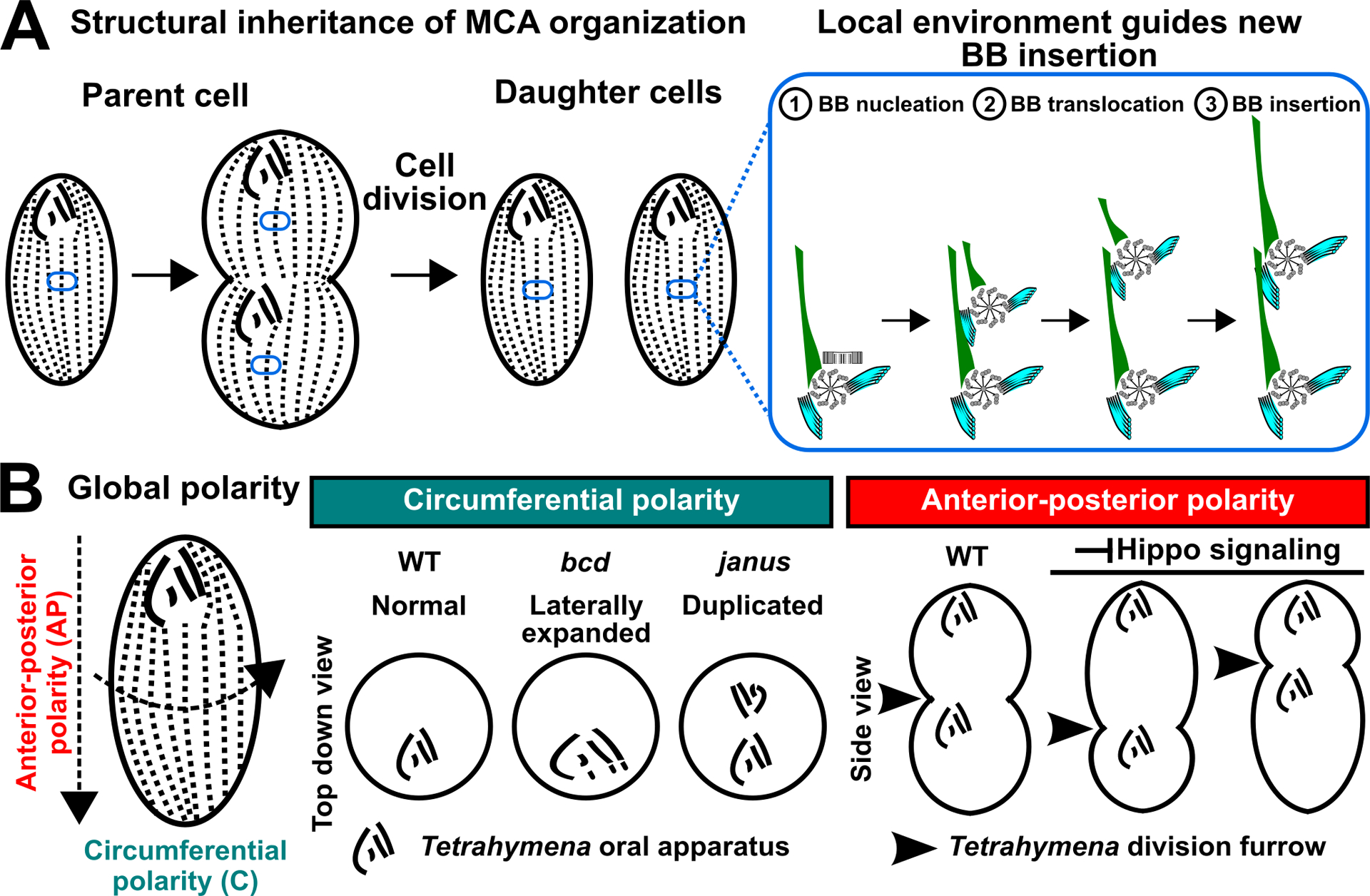
Propagation and establishment of the cortical organization in ciliates. (A) left: During Tetrahymena cell division, BB polarity is propagated from the parent cell to daughter cells via ‘structural inheritance’. right: The mature BB guides the insertion of the nascent BB into the cell cortex (blue oval). (B) Global polarity pathways define the circumferential and AP axes of Tetrahymena cells. left: Defects in circumferential polarity will lead to abnormal patterning of the oral apparatus. right: Defects to Hippo signaling result in improper placement of the oral apparatus and division furrow along the cell’s AP axis.
To uncover the genetic determinants of the global polarity of the cell, mutagenesis screens in Tetrahymena thermophila and Paramecium tetraurelia enabled the discovery of components responsible for circumferential and AP polarities of the cell cortex (Frankel 2008; Jerka-Dziadosz and Beisson 1990; Jones and Berger 1982). Tetrahymena mutants with circumferential polarity defects exhibit abnormal feeding structures (Figure 2B; Cole et al. 1987; Frankel 2008; Frankel, Jenkins, and Bakowska 1984). Also, AP axis polarity mutants improperly position the division furrow and feeding structure during cell division such that they are placed either above or below the medial region of dividing cells, creating cells of different sizes (Figure 2B). The Hippo/MST signaling pathway determines the cell division plane in both vertebrates and ciliates (Bui et al. 2016; Jiang et al. 2019; Tavares et al. 2012). Hippo/MST signaling pathway mutants in Tetrahymena cells disrupt oral apparatus and division furrow positioning, thereby creating both cortical errors. The Hippo signaling molecule, Mob1, is enriched on the cell’s posterior pole by localizing to BBs and BB-associated appendages (Jiang et al. 2019; Jiang et al. 2017; Ruehle et al. 2020; Tavares et al. 2012). Thus, Hippo signaling may transmit AP axis information to a subset of local BBs to regulate the global organization of the cell cortex along the AP axis. Taken together, the organization of the ciliate cell cortex is propagated via ‘structural inheritance’ that is guided by global polarity pathways (Figure 2). Molecules involved in global polarity pathways accumulate at specific BB populations that may function as scaffolding platforms for polarity cues, to facilitate faithful propagation of the ciliate cortical organization.
De novo establishment of the vertebrate MCA organization
In contrast to the epigenetically sustained cellular architecture in ciliates, the organization of the vertebrate MCA is established de novo in the absence of preexisting cortical BBs (Figure 3). During brain, airway, and skin epithelial development, progenitor cells of the MCA lineage undergo a series of developmental events including de novo BB assembly, BB migration and docking at the apical cell surface, BB reorganization and polarization, and ciliogenesis (Figure 3A; Herawati et al. 2016; Spassky and Meunier 2017; Steinman 1968). Prior to ciliogenesis, BBs become polarized along the global AP axis. This is promoted by a combination of mechanical and planar cell polarity pathway cues. During early Xenopus epithelium development, gastrulation induces tissue strain that polarizes cortical MTs along the organism’s AP axis (Chien et al. 2015). Cortical MT polarization promotes the establishment of the planar cell polarity pathway, which transmits global polarity cues onto the cellular level (Figure 3B). This is presumably promoted by polarized MTs that serve as trafficking tracks for the asymmetric deposition of planar cell polarity components at the distal and proximal ends of the cell apical junctions (Shimada et al. 2006). This initiates a positive feedback loop whereby the planar cell polarity pathway further polarizes cortical MTs (Chien et al. 2015). Planar polarized MTs orient BBs locally from the proximal cell junction by interacting with the basal foot-nucleated cortical microtubules to align BBs along the AP axis (Figure 3B; Nakayama et al. 2021; Vladar et al. 2012). Thus, the planar cell polarity pathway and cortical MTs are co-regulated during the establishment of the organism’s AP axis. Together, the de novo established vertebrate MCA is polarized by tissue strain and the planar cell polarity pathway.
The vertebrate MCA is a dynamic system that remodels through the influence of the environment during late stages of development (Guirao et al. 2010; Mitchell et al. 2007). Upon the formation of motile cilia, cilia-driven fluid flow from neighboring cells produces shear forces. As observed in the Xenopus epithelium, fluid shear forces refine the polarity of adjacent cilia so that they beat in a uniform direction for efficient fluid flow (Mitchell et al. 2007). Whether the refinement of cilia orientation involves mechanosensory ion channels and how mechanical cues are then communicated from fluid flow to the cell cortex are current avenues of research (Borovina et al. 2010; Guirao et al. 2010; Mitchell et al. 2007; Praetorius and Spring 2001). Thus, vertebrate MCA is a dynamic system that remodels in response to extrinsic mechanical cues during late developmental stages.
In summary, ciliate and vertebrate MCAs are established via distinct mechanisms. The ciliate cortical organization is sustained and propagated to future progeny (Figure 2A). Conversely, the vertebrate MCA is assembled de novo (Figure 3A). While the establishment of the ciliate cortical architecture appears to be stable and inert, subsequent sections of this review will highlight elements of cortical dynamics that is critical for maintaining homeostatic control over cortical organization.
BB NUMBER AND CORTICAL ORGANIZATION CORRESPONDS WITH CELL SIZE AND MORPHOLOGY
Homeostatic control of BB number in ciliates
Tetrahymena cells maintain control over the number of BBs in each cell. Non-dividing cells possess a consistent total BB number of approximately 500–600 (Nanney 1971). Oral apparatus BBs assemble during cell division with a consistent number of new BBs that form from one ciliary row and organize into four membranelles, hence the name Tetrahymena. Combined with the cortical (non-oral apparatus) BBs, the total number of BBs in Tetrahymena cells nearly doubles during the cell cycle prior to cell division (Galati et al. 2015; Nanney 1971). To ensure that daughter cells inherit the same number of BBs as their parental cells, Tetrahymena cells must be able to count and adjust their BB number via BB assembly and/or loss. In addition, Tetrahymena BBs are typically distributed across 18–21 ciliary rows (Nanney 1966). Tetrahymena cells with ciliary row numbers that fall outside this range reduce or increase their ciliary row numbers, indicating that Tetrahymena cells are also equipped to count and regulate their ciliary row number (Nanney 1966; Nanney and Chow 1974). Notably, the total BB number between cells remains relatively consistent despite differences in the ciliary row number such that cells with more ciliary rows possess fewer BBs per row (Nanney 1971). How cells integrate and process information on the number and organization of BBs remains to be understood. Together, this suggests that the Tetrahymena cell cortex is dynamic and possesses ‘quantitative sensory capacities’ to ensure homeostatic control over BB number and organization (Nanney 1971).
The rules for BB number regulation appear to be broken in Tetrahymena big1–1 mutants. big1–1 cells have more BBs than wildtype cells ((Frankel, Jenkins, Bakowska, et al. 1984); AWJS and CGP unpublished). Moreover, big1–1 cells are larger in size. Whether more BBs directly lead to increased cell surface area, or vice-versa, remains to be tested. In addition, big1–1 mutant cells have more ciliary rows than wild-type cells (Frankel 2008; Nanney and Chow 1974). This suggests that big1–1 cells possess less stringent BB and ciliary row number regulation. Moreover, it also reflects a mechanism whereby the ciliate cortical architecture reorganizes to accommodate more BBs. Ciliary rows become longer with more BBs causing cells to elongate and more ciliary rows are assembled causing cells to become wider. Hence, the dynamic Tetrahymena cell cortex remodels in size and morphology, presumably to accommodate increases and decreases in BB number (Nanney and Chow 1974).
BB number and apical cell surface area are co-regulated in vertebrate MCAs
Like in ciliates, vertebrate MCAs tightly control the number of BBs in each cell. The apical cell surface area develops before BB-genesis and ciliogenesis programs in mouse airway in vitro cultures. By leveraging the temporal difference between the onset of these programs, the apical cell surface area in mouse MCAs correlates with the number of BBs such that larger apical cell surface area leads to more BB assembly (Nanjundappa et al. 2019). In Xenopus skin epithelia, the apical cell surface area also scales with BB number. Notably, BB assembly drives the enlargement of apical cell surface (Kulkarni et al. 2021). Such differences potentially reflect organism specific mechanisms in co-regulating the apical cell surface area and BB number. Nonetheless, it argues that the vertebrate MCA, like ciliates, possesses sensory mechanisms to count and adjust BB number and the apical cell surface area to ensure an optimal cilia density to support fluid flow (Omori et al. 2020).
CILIATE BBS AND ASSOCIATED APPENDAGES DETECT AND RESPOND TO CILIARY FORCES
During ciliary beating, the cell cortex is subjected to constant mechanical stress as motile cilia beat tens of times per second. To maintain BB integrity, BBs are stabilized via triplet MTs, MT post-translational modifications and structural elements interconnecting triplet MTs. In addition, BB-associated appendages maintain BB organization and orientation within the cell cortex. This enables MCAs to resist ciliary forces at the cell cortex, thereby driving fluid flow and cellular motility.
Ciliate and vertebrate MCA BBs are structurally reinforced to transmit ciliary forces to the cell
Mechanical forces from ciliary beating are directly transmitted to BBs (Brokaw 1972; Lindemann 1994; Pearson et al. 2009; Riedel-Kruse et al. 2007; Summers and Gibbons 1971). BBs are MT-based organelles comprised of nine symmetrically arranged triplet MTs. Unlike cytoplasmic MTs, BBs are stable structures (Odde et al. 1999; Schaedel et al. 2015). Lateral interactions between the A-, B-, and C-tubules of triplet MTs stabilize BB MTs against ciliary forces in a manner that is akin to MT bundling (Chapin et al. 1991).
MTs are commonly modified by a series of post-translational modifications that control their physical properties. BB and centriole MTs are stabilized via polyglutamylation (Abal et al. 2005; Bayless et al. 2016; Bobinnec et al. 1998). During cell division, MT polyglutamylation reinforces centrioles and centrosomes against physical forces exerted by the mitotic spindle (Abal et al. 2005; Bobinnec et al. 1998). In Tetrahymena cells, MT polyglutamylation stabilizes BBs against ciliary forces (Bayless et al. 2016). Polyglutamylation can either directly stiffen the MT lattice or indirectly stiffens MTs via the recruitment of MT-associated proteins such as Tau (Felgner et al. 1997; Mickey and Howard 1995; Wall et al. 2020). Thus, MT polyglutamylation potentially stiffens the triplet MTs of Tetrahymena BBs.
Specific BB triplet MTs are predicted to experience more ciliary forces (Figure 4A and B; Bayless et al. 2016; Meehl et al. 2016; Pearson et al. 2009; Riedel-Kruse et al. 2007). Interestingly, MT polyglutamylation localizes asymmetrically to specific BB triplet MTs (Figure 4A; Bayless et al. 2016). It is plausible that the asymmetric localization of MT polyglutamylation stiffens BB triplet MTs that are exposed to increased mechanical forces, presumably to ensure BB integrity. Alternatively, increased BB triplet MT stiffness may provide a mechanism for control of the ciliary waveform (Narematsu et al. 2015; Tamm 1999). Notably, the asymmetric localization of MT polyglutamylation at BBs is dynamic. Reduction in ciliary force leads to the redistribution of glutamylation at specific triplet MTs (Figure 4A; Bayless et al. 2016). This suggests that BBs are force-responsive structures that can modulate their physical properties, such as stiffness, depending on the level of ciliary force (Bayless et al. 2016). It remains to be understood how BBs sense ciliary force. Other BB MT post-translation modifications such as acetylation, glycylation, and detyrosination have been reported but their functions remain to be uncovered (Akella et al. 2010; Junker et al. 2019; Thazhath et al. 2004). Thus, BBs are ciliary force-responsive structures that undergo dynamic MT polyglutamylation redistribution, presumably to reinforce distinct BB domains against ciliary forces.
Figure 4.
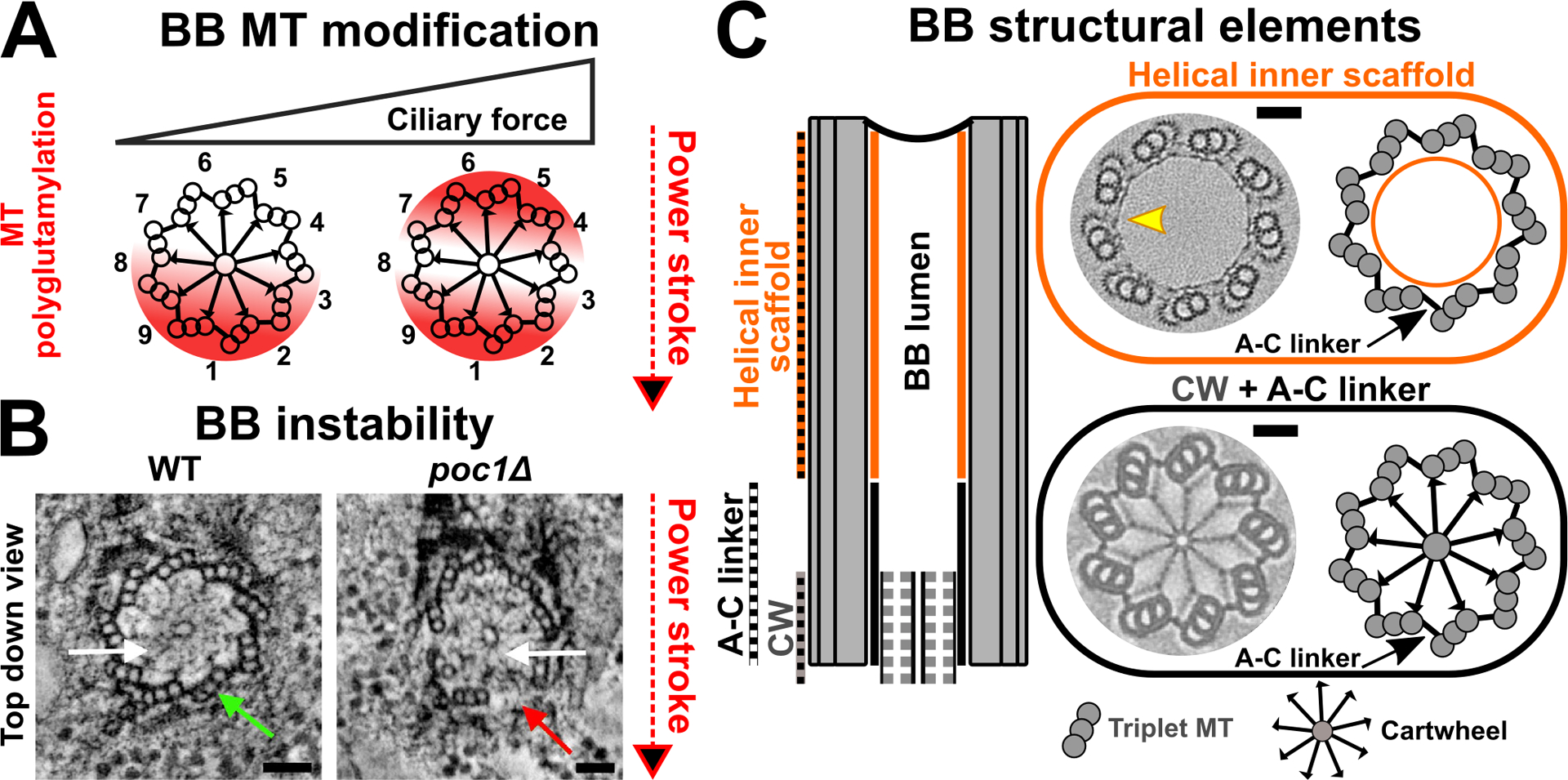
BBs are structurally reinforced via stability elements. (A) BB triplet MTs are asymmetrically polyglutamylated (red) at specific triplet MTs (Bayless et al. 2016). The distribution of triplet MT polyglutamylation is dependent upon the level of ciliary forces. Triplet MTs are numbered. Red arrow marks the axis of the cilium power stroke. (B) BBs become unstable in the absence of Tetrahymena BB stability protein, Poc1. Breakage occurs at specific triplet MTs. White arrows mark the BB cartwheel position. Green arrow marks intact triplet MT at position 1. Red arrow marks triplet MT breakage at specific triplet MT. Images are adapted from Meehl et al. (2016). Bar, 50 nm. (C) BBs are stabilized via structural elements. left: Schematic represents the longitudinal view of a BB. right: The medial-distal BB region is postulated to be stabilized by the helical inner scaffold (orange). EM image is adapted from Le Guennec et al. (2020). Bar, 50 nm. On the BB proximal end, A-C linkers (black) radially organize triplet MTs. The cartwheel (CW; grey) radially extends linkages towards the BB triplet MT wall. Image illustrates the Trichonympha proximal BB region. EM image is adapted from Nazarov et al. (2020). Bar, 50 nm.
BB triplet MTs are radially organized and laterally coupled via A-C linkers (Figure 4C; Gibbons and Grimstone 1960; Klena et al. 2020). Each A-C linker connects the A- and C-tubule of neighboring triplet MTs to form connections that extend approximately one-third up from the BB proximal end (Figure 4C; Klena et al. 2020). Recent work predicts that the BB stability protein, Poc1, is a component of the A-C linkers in Chlamydomonas (Li et al. 2019). Consistent with this, A-C linker structures are disrupted and triplet MT walls break in Tetrahymena poc1Δ cells (Meehl et al. 2016; Pearson et al. 2009). Notably, this typically occurs at specific triplet MTs (Figure 4B; Meehl et al. 2016; Pearson et al. 2009; Riedel-Kruse et al. 2007; Summers and Gibbons 1971). Therefore, BBs are reinforced against ciliary force by A-C linkers at the proximal half of BBs.
While the A-C linkers stabilize the BB proximal end, recent work suggests that a BB inner helical scaffold reinforces the medial to distal region of BBs (Figure 4C; Le Guennec et al. 2020). The inner helical scaffold forms a meshwork that lies along the inner surface of the BB triplet MT wall (Figure 4C). The Poc1 protein is also suggested to localize to the inner helical scaffold in Paramecium and human cells. Whether Poc1 displays distinct roles at the A-C linkers and helical inner scaffold remains to be investigated. Further work is required to understand how the inner helical scaffold interacts with the other BB structural elements to stabilize BBs.
In addition to the above-mentioned structural elements that stabilize BBs via interactions between triplet MTs, the cartwheel structure at the proximal end of BBs reinforces BBs (Figure 4C; Bayless et al. 2012; Culver et al. 2009; Gönczy 2012; Hiraki et al. 2007; Jerka-Dziadosz et al. 2010; Nakazawa et al. 2007). The inner cartwheel is assembled from the Sas6 (Spindle assembly abnormal protein 6) protein that oligomerizes to create a central hub that projects spoke-like structures radially outward to connect to the triplet MTs (Figure 4C; Nakazawa et al. 2007; Nievergelt et al. 2018). A pinhead structure, consisting of at least the CEP135/Bld10 protein (Centrosome-associated protein and Bald, flagella-less mutant), attaches to each spoke of the Sas6 cartwheel and connects to BB triplet MTs (Hiraki et al. 2007; Matsuura et al. 2004). While cartwheels are critical for BB assembly, their role in BB stability is less well understood. Consistent with its role as the integral component of the cartwheel, Sas6 loss results in fewer BBs in Tetrahymena cells, suggesting a defect in BB assembly and/ or BB stability (Culver et al. 2009). Notably, cells that lack Sas6 are devoid of the cartwheel, and they accumulate ribosomes that are normally excluded from the BB lumen. This suggests that the structural integrity of BBs is disrupted in the absence of the cartwheel (Culver et al. 2009; Gönczy 2012; Nakazawa et al. 2007). In addition, the loss of the pinhead protein, CEP135/Bld10, leads to improper cartwheel-triplet MT connections and BB instability (Bayless et al. 2012; Hiraki et al. 2007). Collectively, this supports the model that the cartwheel is not only required for BB assembly but also stabilizes the proximal end of BBs. The size of the cartwheel stack varies between organisms, leading to the speculation that the BB cartwheel stack may modulate physical properties such as BB stiffness (Guichard et al. 2012; Hirono 2014; Klena et al. 2020; O’Toole and Dutcher 2014). We predict that a larger cartwheel stack promotes more radial connections to the BB triplet MTs and potentially stiffens the BB proximal region. It will be interesting to investigate if the BBs of organisms such as Trichonympha, which possess large cartwheel stacks, are indeed stiffer than BB in organisms with a smaller cartwheel stack (Guichard and Gönczy 2016). Therefore, changes to the BB cartwheel stack potentially reflect a mechanism whereby organisms dynamically load or remove cartwheels to tune the stiffness of their BBs in response to ciliary forces.
In summary, BBs comprise structural elements that confer stability to resist mechanical forces from ciliary beating. They are dynamic structures with an ability to adapt to different levels of ciliary force. Additional structural elements such as the MT inner proteins likely impact BB stability and dynamics, and these remain to be understood (Fabritius et al. 2021; Ma et al. 2019).
BB-appendages anchor BBs and transmit ciliary forces to the cell cortex in ciliates and vertebrates
The stabilization of the core BB architecture is complemented by BB-appendages that connect BBs to the cell cortex via physical linkages. This is critical for maintaining cortical organization and effective transmission of ciliary forces to the cell for fluid flow and motility.
Mechanosensation of striated BB-appendages
Ciliate SFs are dynamic structures that modulate BB anchorage in response to ciliary forces. Under normal conditions, SFs anchor BBs at the cell cortex by interacting with the pcMTs of the anterior BB (Figure 1B; Allen 1967; Pitelka 1961). Tetrahymena and Paramecium mutants with reduced SF length exhibit BB disconnection (Galati et al. 2014; Jerka-Dziadosz et al. 1995; Nabi et al. 2019). Disconnected BBs become susceptible to ciliary forces that lead to BB disorganization and disorientation, which refers to BBs that do not remain within ciliary rows or display polarity defects, respectively (Figure 5A; Galati et al. 2014; Jerka-Dziadosz et al. 1995; Soh et al. 2020). Moreover, these cells display swimming defects. Thus, proper SF length facilitates BB connection, thereby ensuring that BBs are anchored against ciliary forces for effective motility. At elevated temperatures, Tetrahymena cells display faster motility, which exerts more ciliary forces on the cell cortex (Galati et al. 2014; Goto et al. 1982; Pearson et al. 2009). Notably, SFs elongate when ciliary force is increased for a sustained duration of at least two hours, suggesting that they are dynamic structures that respond to mechanical forces (Figure 5A; Galati et al. 2014). Elongated SFs interact with both the anterior BB via SF-pcMT interactions and to the cell cortex associated with the anterior BB’s ciliary pocket (Figure 5A). The additional SF-mediated interactions reinforce BB anchorage at the cell cortex. The Tetrahymena mutant, cro1, that fails to elongate its SFs with elevated ciliary forces, displays ciliary rows no longer aligned with the cell AP axis (Figure 5A; Soh et al. 2020). Thus, SF elongation strengthens BB anchorage by further coupling BBs to the cell cortex. In addition, this potentially promotes more effective ciliary force transmission into the cell to support faster cell motility. Together, the length response to ciliary forces suggests that SFs are mechanosensory structures. How SFs integrate mechanical cues to adjust their length remains unclear. We posit that Tetrahymena cells remodel BB anchorage at the cell cortex via dynamic SF length regulation to adapt to changing ciliary force conditions (Figure 5A).
The vertebrate structural SF analog, termed the ciliary rootlet, also anchors BBs to the cell cortex. In vertebrates, the ciliary rootlet interacts with the sub-apical actin network (Figure 5B (bottom); Antoniades et al. 2014; Werner et al. 2011). This interaction is mediated by focal adhesion proteins such as vinculin that localize to the ciliary rootlet-actin filament interface (Antoniades et al. 2014). The loss of function of specific focal adhesion proteins abolishes the sub-apical actin network leading to disrupted BB spacing and BB undocking from the cell cortex (Figure 5B (bottom); Antoniades et al. 2014). While it is unclear whether the ciliary rootlet exhibits ciliary force-responsive length regulation like the ciliate SF, the presence of tension sensitive proteins like vinculin within the ciliary rootlet and sub-apical actin network suggests that the MCA cortex experiences and senses tension. This model is supported by the observation that the Xenopus MCA epithelium is subjected to cortical tension (Kulkarni et al. 2021). However, it remains unclear whether ciliary forces contribute to tension at the cell cortex. It is tempting to speculate that cortical tension could serve as a signal that MCAs sense to remodel their ciliary rootlet and sub-apical actin interactions to cater to different levels of ciliary forces. Together, while the nature of the ciliate SF- and vertebrate ciliary rootlet-mediated BB connections is different, these BB-appendages maintain BB organization and orientation in the face of ciliary beating (Figure 5).
BB-associated MT appendages both anchor and modulate BB organization
The Tetrahymena pcMT and tMT are MT-based BB-appendages that anchor BBs at the cell cortex (Figure 1B). Like SFs, the length of pcMTs and tMTs is critical for ensuring proper BB-BB and BB-cell cortex interactions. Both MT-based BB-appendages are subjected to a variety of MT post-translational modifications (Akella et al. 2010; Junker et al. 2019; Wloga et al. 2008; Wloga et al. 2009). Notably, impaired MT glycylation shortens pcMTs and tMTs (Junker et al. 2019). Short pcMTs result in BB disconnection within a ciliary row. EM tomography analysis revealed that the distal ends of pcMTs and tMTs interact with the cell cortex via electron dense linkages (Junker et al. 2019). Reduced pcMT and tMT length also causes BBs to undock from the cell cortex (Junker et al. 2019). Therefore, like SFs, proper pcMT and tMT length is required for establishing BB connections and anchoring BBs to the cell cortex. Whether these MT BB-appendages display mechanosensory ability and length dynamics like the SF at varying ciliary force conditions remains to be investigated.
Like ciliate MT-based BB-appendages, vertebrate BB-associated basal foot promotes BB association with the cell cortex. The basal foot nucleates MTs, which facilitate interactions between BBs and the cell cortex (Gordon 1982; Hard and Rieder 1983; Reed et al. 1984). These interactions organize and anchor BBs at the apical cell surface (Figure 5B (top)). Abolishing the MT nucleation capacity of the basal foot causes BB disorientation and BBs to undock from the cell cortex (Figure 5B (top); Clare et al. 2014; Herawati et al. 2016; Kunimoto et al. 2012; Tateishi et al. 2013; Turk et al. 2015). In the mouse airway MCA, the organization of BBs is dynamic during development. Upon docking at the apical cell surface, BBs are first arranged in a ‘floret pattern’, which represents a cluster of BBs, before they are reorganized to form uniformly spaced BB arrays (Herawati et al. 2016). This transition in BB organization requires MTs (Herawati et al. 2016). The basal foot in the newt airway MCA is associated with stable MTs (Hard and Rieder 1983). Since the transition from a ‘floret pattern’ to uniformly spaced BB arrays undergoes extensive BB reorganization, stable MTs alone are unlikely to promote this reorganization. We speculate that the basal foot interacts with both dynamic and stable MTs to promote BB reorganization and anchorage, respectively. Also, the basal foot interacts with intermediate filaments and actin filaments (Hard and Rieder 1983; Herawati et al. 2016; Sandoz et al. 1988), suggesting that, in addition to MT organization, the basal foot also organizes and cooperates with other cytoskeletal networks to organize and anchor BBs. Thus, the vertebrate basal foot, like the ciliate MT appendages, positions and anchors BBs at the cell cortex (Figure 5).
Apart from its role in maintaining BB organization and orientation, the cortical BB and cytoskeletal networks likely coordinate ciliary beating. Hydrodynamic coupling drives synchronous ciliary beat by transmitting ciliary forces, or cilia coordination cues, between adjacent cilia via the fluid medium (Brumley et al. 2014; Elgeti and Gompper 2013; Herawati et al. 2016; Maestro et al. 2018; Riedel et al. 2005; Tamm 1984). However, hydrodynamic coupling alone appears to be insufficient to coordinate ciliary beating (Narematsu et al. 2015; Wan and Goldstein 2016). It is hypothesized that the cell cortex also serves as a conduit to transmit cilia coordination cues via the underlying interconnected BBs to synchronize ciliary beating (Narematsu et al. 2015; Tamm 1999). Computational modelling of the biflagellate algae, Chlamydomonas, suggests that the coupling strength between adjacent BBs can influence cilia synchrony (Guo et al. 2021). By modulating the extent of coupling between BBs, such as changing the number of interactions that BB-appendages establish between a pair of BBs, this may strengthen or weaken the coupling between BBs and serve as a mechanism to regulate the coordination of ciliary beating (Figure 5A). Since ciliary forces are exerted onto the underlying BB network, such forces could serve as coordination cues to synchronize ciliary beating (Werner et al. 2011). Thus, the cortical BB network not only anchors BBs against ciliary forces but it may also be involved in coordinating ciliary beating.
In summary, the underlying cortical BB network of MCAs is designed to resist and transmit ciliary forces. The core BB architecture is reinforced at distinct domains via stability elements, and BBs are organized and oriented at the cell cortex via BB-appendages. In addition, MCAs are plastic systems that adapt to different levels of ciliary forces. This may impact how ciliary forces are transmitted into the cell to modulate fluid flow and cellular motility.
PLASTICITY OF THE MCA ARCHITECTURE
MCAs are dynamic systems that sense, tune, and adapt to promote fluid flow. Ciliate and vertebrate MCAs exhibit cortical plasticity or remodeling, which refers to the ability of the cell cortex to change its organization in response to intrinsic and/or environmental differences (Figures 3 and 5A). Cortical plasticity enables ciliates to respond dynamically to fluctuating environmental conditions like temperature and nutrient availability, thereby ensuring their survival.
Harsh environments like poor nutrient conditions often drive organisms, including Tetrahymena, to enter a state of dispersal whereby they migrate, colonize new and favorable habitats, and promote gene flow (Figure 6A; Jacob et al. 2017; Van Dyck and Baguette 2005). Ecology studies revealed that a dispersing individual possesses unique morphological traits that distinguish it from a non-dispersing, or resident, individual (Clobert et al. 2009; Jacob et al. 2019; Junker et al. 2021; Stevens et al. 2013). Consistent with this, low nutrient conditions induce Tetrahymena cells to enter a dispersal state whereby they remodel their cell cortex (Nelsen 1978; Nelsen and Debault 1978). Dispersing Tetrahymena cells, known as dispersers, undergo morphological changes that include shorter cell length and more closely positioned cilia within ciliary rows (Figure 6; Junker et al. 2021; Nelsen 1978). Reduced cilia spacing is typically associated with faster fluid flow (Elgeti and Gompper 2013; Gu et al. 2020; Osterman and Vilfan 2011). Consistent with this, dispersers swim at faster rates than resident Tetrahymena cells (Junker et al. 2021). Thus, the Tetrahymena cell cortex reorganizes its BBs and cilia under nutrient poor conditions to promote dispersal (Junker et al. 2021; Nelsen 1978). The plastic nature of the cell cortex enables ciliates to modulate their motility behavior, thereby supporting elevated swimming and mobility for efficient migration to nutrient rich habitats (Figure 6).
Figure 6.
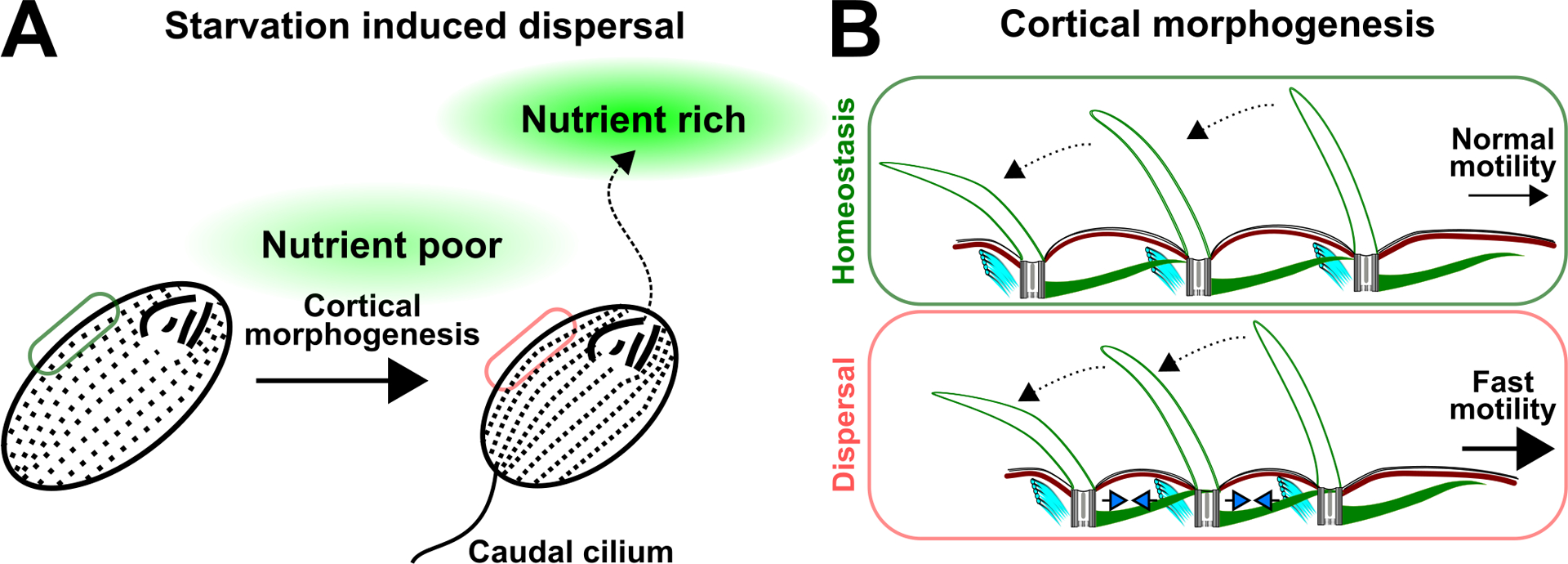
Tetrahymena cells undergo dynamic cortical reorganization during nutrient poor condition. (A) Starvation induces Tetrahymena dispersal from a nutrient-poor environment towards a nutrient-rich environment. This is facilitated by cortical remodeling, which includes the reduction in cell length and the formation of a caudal cilium at the posterior end of the cell. (B) Schematic illustrates the reduction in inter-BB distance (blue arrowheads) upon the starvation of Tetrahymena cells. This is predicted to support faster cell motility. Dotted black arrows represent ciliary beating. Large black arrow indicates faster cell motility.
Tetrahymena cell cortex can also dynamically adjust the number and composition of its cilia between nutrient rich and poor conditions (Junker et al. 2021; Nelsen 1978). In nutrient poor conditions, dispersing Tetrahymena cells increase their number of cilia by promoting ciliogenesis at unciliated BBs (Nelsen 1978). The increase in cilia number facilitates faster cell motility, which is one of the characteristics of dispersing Tetrahymena cells (Junker et al. 2021; Omori et al. 2020). In addition, a subpopulation of dispersers generates an additional extended cilium, referred to as the caudal cilium, that is positioned on the posterior cell pole (Figure 6A; Nelsen 1978). The caudal cilium is variable in length, and it is approximately 1.2–2.3-fold longer than the average motile cilium that is positioned at the medial region of the cell (Junker et al. 2021). Although its role is unclear, the caudal cilium is speculated to function as a rudder for steering function (Nelsen 1978). Alternative possibilities include a role for the caudal cilium to stabilize rapid cell swimming or in serving as a signaling structure. The emergence of a cilium that is morphologically and functionally distinct from a motile cilium during nutrient poor conditions suggests that cells can dynamically change their cilia composition. This may serve to support motility in dispersers when nutrient availability is low. Collectively, the Tetrahymena cell cortex is plastic. This enables ciliates to sense, respond to, and disperse when environmental conditions, such as nutrient availability, become unfavorable.
Although cortical plasticity offers benefits to the survival of ciliates, this dynamic mode of cortical regulation needs to be delicately balanced with the propagation of cortical organization through ‘structural inheritance’. Excessive cortical plasticity could lead to an unpolarized MCA, which would negatively impact ciliate motility. Conversely, the propagation of MCA organization via ‘structural inheritance’ alone would restrain the ability of a ciliate to respond dynamically to its environment. Both instances will be detrimental to survival. This suggests that ‘structural inheritance’ serves as a foundation for organizing MCAs in ciliates, and it is complemented with plasticity allowing ciliates to adapt when environmental conditions become unfavorable. Like in ciliates, cortical plasticity appears to be a common trend during vertebrate MCA development.
CONCLUSION
Hydrodynamic flow is fundamental to the development and homeostasis of most living organisms. By contrasting the MCAs of ciliates and vertebrates, we highlighted the structural elements that organize and stabilize the cortical architecture. In addition, MCAs are plastic systems that sense, tune, and adapt to the surrounding conditions. This allows organisms to generate optimal fluid flow during development and environmental change.
Acknowledgment
We are grateful to Dr. Brian Mitchell (Northwestern University), Dr. Marisa Ruehle and Alexander Stemm-Wolf (Pearson lab) for critical reading of this review. We thank the Pearson lab for the discussions during the course of writing this manuscript. In addition, we express our gratitude to Mark Winey (University of California Davis), Janet Meehl (University of Colorado Boulder) and the Guichard/ Hamel lab (University of Geneva) for kindly sharing electron micrographs that we presented in this review. We thank our funding sources NIH/ NIGMS RO1GM099820 and R35 GM140813 that supported our interest in this work.
Abbreviations:
- MCA
multi-ciliary array
- BB
basal body
- MT
microtubule
- pcMT
post-ciliary microtubule
- tMT
transverse microtubule
- SF
striated fiber
References
- Abal M, Keryer G, & Bornens M (2005). Centrioles resist forces applied on centrosomes during G2/M transition. Biol. Cell, 97(6), 425–434. doi: 10.1042/bc20040112 [DOI] [PubMed] [Google Scholar]
- Akella JS, Wloga D, Kim J, Starostina NG, Lyons-Abbott S, Morrissette NS, Dougan ST, Kipreos ET, & Gaertig J (2010). MEC-17 is an alpha-tubulin acetyltransferase. Nature, 467(7312), 218–222. doi: 10.1038/nature09324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen RD (1967). Fine structure, reconstruction and possible functions of components of the cortex of Tetrahymena pyriformis. J. Protozool, 14(4), 553–565. [DOI] [PubMed] [Google Scholar]
- Antoniades I, Stylianou P, & Skourides PA (2014). Making the connection: ciliary adhesion complexes anchor basal bodies to the actin cytoskeleton. Dev. Cell, 28(1), 70–80. doi: 10.1016/j.devcel.2013.12.003 [DOI] [PubMed] [Google Scholar]
- Bayless BA, Galati DF, Junker AD, Backer CB, Gaertig J, & Pearson CG (2016). Asymmetrically localized proteins stabilize basal bodies against ciliary beating forces. J. Cell Biol, 215(4), 457–466. doi: 10.1083/jcb.201604135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayless BA, Galati DF, & Pearson CG (2015). Tetrahymena basal bodies. Cilia, 5, 1. doi: 10.1186/s13630-016-0022-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayless BA, Giddings TH Jr., Winey M, & Pearson CG (2012). Bld10/Cep135 stabilizes basal bodies to resist cilia-generated forces. Mol. Biol. Cell, 23(24), 4820–4832. doi: 10.1091/mbc.E12-08-0577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beisson J, & Sonneborn TM (1965). Cytoplasmic inheritance of the organization of the cell cortex in Paramecium aurelia. Proc. Natl. Acad. Sci. USA, 53, 275–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bobinnec Y, Khodjakov A, Mir LM, Rieder CL, Eddé B, & Bornens M (1998). Centriole disassembly in vivo and its effect on centrosome structure and function in vertebrate cells. J. Cell Biol, 143(6), 1575–1589. doi: 10.1083/jcb.143.6.1575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borovina A, Superina S, Voskas D, & Ciruna B (2010). Vangl2 directs the posterior tilting and asymmetric localization of motile primary cilia. Nat. Cell Biol, 12(4), 407–412. doi: 10.1038/ncb2042 [DOI] [PubMed] [Google Scholar]
- Brokaw CJ (1972). Flagellar Movement: A Sliding Filament Model. Science, 178(4060), 455–462. doi:doi: 10.1126/science.178.4060.455 [DOI] [PubMed] [Google Scholar]
- Brumley DR, Wan KY, Polin M, & Goldstein RE (2014). Flagellar synchronization through direct hydrodynamic interactions. Elife, 3, e02750–e02750. doi: 10.7554/eLife.02750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bui DA, Lee W, White AE, Harper JW, Schackmann RCJ, Overholtzer M, Selfors LM, & Brugge JS (2016). Cytokinesis involves a nontranscriptional function of the Hippo pathway effector YAP. Sci. Signaling, 9(417), ra23–ra23. doi:doi: 10.1126/scisignal.aaa9227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapin SJ, Bulinski JC, & Gundersen GG (1991). Microtubule bundling in cells. Nature, 349(6304), 24. doi: 10.1038/349024a0 [DOI] [PubMed] [Google Scholar]
- Chien YH, Keller R, Kintner C, & Shook DR (2015). Mechanical strain determines the axis of planar polarity in ciliated epithelia. Curr. Biol, 25(21), 2774–2784. doi: 10.1016/j.cub.2015.09.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clare DK, Magescas J, Piolot T, Dumoux M, Vesque C, Pichard E, Dang T, Duvauchelle B, Poirier F, & Delacour D (2014). Basal foot MTOC organizes pillar MTs required for coordination of beating cilia. Nat. Commun, 5, 4888. doi: 10.1038/ncomms5888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clobert J, Le Galliard JF, Cote J, Meylan S, & Massot M (2009). Informed dispersal, heterogeneity in animal dispersal syndromes and the dynamics of spatially structured populations. Ecol. Lett, 12(3), 197–209. doi: 10.1111/j.1461-0248.2008.01267.x [DOI] [PubMed] [Google Scholar]
- Cole ES, Frankel J, & Jenkins LM (1987). bcd: A mutation affecting the width of organelle domains in the cortex of Tetrahymena thermophila. Rouxs Arch. Dev. Biol, 196(7), 421–433. doi: 10.1007/bf00399142 [DOI] [PubMed] [Google Scholar]
- Culver BP, Meehl JB, Giddings TH Jr., & Winey M (2009). The two SAS-6 homologs in Tetrahymena thermophila have distinct functions in basal body assembly. Mol. Biol. Cell, 20(6), 1865–1877. doi: 10.1091/mbc.e08-08-0838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dippell RV (1968). The development of basal bodies in paramecium. Proc. Natl. Acad. Sci. USA, 61(2), 461–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elgeti J, & Gompper G (2013). Emergence of metachronal waves in cilia arrays. Proc. Natl. Acad. Sci. USA, 110(12), 4470–4475. doi: 10.1073/pnas.1218869110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabritius AS, Bayless BA, Li S, Stoddard D, Heydeck W, Ebmeier CC, Anderson L, Gunnels T, Nachiappan C, Whittall JB, Old W, Agard DA, Nicastro D, & Winey M (2021). Proteomic analysis of microtubule inner proteins (MIPs) in Rib72 null Tetrahymena cells reveals functional MIPs. Mol. Biol. Cell, 32(21), br8. doi: 10.1091/mbc.E20-12-0786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felgner H, Frank R, Biernat J, Mandelkow E-M, Mandelkow E, Ludin B, Matus A, & Schliwa M (1997). Domains of Neuronal Microtubule-associated Proteins and Flexural Rigidity of Microtubules. J. of Cell Biol, 138(5), 1067–1075. doi: 10.1083/jcb.138.5.1067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frankel J (2008). What do genic mutations tell us about the structural patterning of a complex single-celled organism? Eukaryot. Cell, 7(10), 1617–1639. doi: 10.1128/EC.00161-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frankel J, Jenkins LM, & Bakowska J (1984). Selective mirror-image reversal of ciliary patterns in Tetrahymena thermophila homozygous for a janus mutation. Wilehm Roux Arch. Dev. Biol, 194(2), 107–120. doi: 10.1007/bf00848350 [DOI] [PubMed] [Google Scholar]
- Frankel J, Jenkins LM, Bakowska J, & Nelsen EM (1984). Mutational analysis of patterning of oral structures in Tetrahymena. I. Effects of increased size on organization. J. Embryol. Exp. Morphol, 82, 41–66. [PubMed] [Google Scholar]
- Galati DF, Abuin DS, Tauber GA, Pham AT, & Pearson CG (2015). Automated image analysis reveals the dynamic 3-dimensional organization of multi-ciliary arrays. Biol. Open, 5(1), 20–31. doi: 10.1242/bio.014951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galati DF, Bonney S, Kronenberg Z, Clarissa C, Yandell M, Elde NC, Jerka-Dziadosz M, Giddings TH, Frankel J, & Pearson CG (2014). DisAp-dependent striated fiber elongation is required to organize ciliary arrays. J. Cell Biol, 207(6), 705–715. doi: 10.1083/jcb.201409123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbons IR, & Grimstone AV (1960). On flagellar structure in certain flagellates. J. Biophys. Biochem. Cytol, 7(4), 697–716. doi: 10.1083/jcb.7.4.697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilpin W, Bull MS, & Prakash M (2020). The multiscale physics of cilia and flagella. Nature Reviews Physics, 2(2), 74–88. doi: 10.1038/s42254-019-0129-0 [DOI] [Google Scholar]
- Gönczy P (2012). Towards a molecular architecture of centriole assembly. Nat. Rev. Mol. Cell Biol, 13(7), 425–435. doi: 10.1038/nrm3373 [DOI] [PubMed] [Google Scholar]
- Gordon RE (1982). Three-dimensional organization of microtubules and microfilaments of the basal body apparatus of ciliated respiratory epithelium. Cell Motil, 2(4), 385–391. doi: 10.1002/cm.970020407 [DOI] [PubMed] [Google Scholar]
- Goto M, Ohki K, & Nozawa Y (1982). Evidence for a Correlation between Swimming Velocity and Membrane Fluidity of Tetrahymena Cells. Biochimica Et Biophysica Acta, 693(2), 335–340. doi:Doi 10.1016/0005-2736(82)90440-0 [DOI] [PubMed] [Google Scholar]
- Gu H, Boehler Q, Cui H, Secchi E, Savorana G, De Marco C, Gervasoni S, Peyron Q, Huang TY, Pane S, Hirt AM, Ahmed D, & Nelson BJ (2020). Magnetic cilia carpets with programmable metachronal waves. Nat. Commun, 11(1), 2637. doi: 10.1038/s41467-020-16458-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guichard P, Desfosses A, Maheshwari A, Hachet V, Dietrich C, Brune A, Ishikawa T, Sachse C, & Gönczy P (2012). Cartwheel architecture of Trichonympha basal body. Science, 337(6094), 553. doi: 10.1126/science.1222789 [DOI] [PubMed] [Google Scholar]
- Guichard P, & Gönczy P (2016). Basal body structure in Trichonympha. Cilia, 5, 9. doi: 10.1186/s13630-016-0031-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guirao B, Meunier A, Mortaud S, Aguilar A, Corsi J, Strehl L, Hirota Y, Desoeuvre A, Boutin C, Han Y-G, Mirzadeh Z, Cremer H, Montcouquiol M, Sawamoto K, & Spassky N (2010). Coupling between hydrodynamic forces and planar cell polarity orients mammalian motile cilia. Nat. Cell Biol, 12, 341–350. [DOI] [PubMed] [Google Scholar]
- Guo H, Man Y, Wan KY, & Kanso E (2021). Intracellular coupling modulates biflagellar synchrony. J. R. Soc. Interface, 18(174), 20200660. doi: 10.1098/rsif.2020.0660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hard R, & Rieder CL (1983). Muciliary transport in newt lungs: the ultrastructure of the ciliary apparatus in isolated epithelial sheets and in functional triton-extracted models. Tissue Cell, 15(2), 227–243. [DOI] [PubMed] [Google Scholar]
- Herawati E, Taniguchi D, Kanoh H, Tateishi K, Ishihara S, & Tsukita S (2016). Multiciliated cell basal bodies align in stereotypical patterns coordinated by the apical cytoskeleton. J. Cell. Biol, 214(5), 571–586. doi: 10.1083/jcb.201601023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiraki M, Nakazawa Y, Kamiya R, & Hirono M (2007). Bld10p constitutes the cartwheel-spoke tip and stabilizes the 9-fold symmetry of the centriole. Curr. Biol, 17(20), 1778–1783. doi: 10.1016/j.cub.2007.09.021 [DOI] [PubMed] [Google Scholar]
- Hirono M (2014). Cartwheel assembly. Philos Trans R Soc Lond B Biol Sci, 369(1650). doi: 10.1098/rstb.2013.0458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honts JE, & Williams NE (2003). Novel cytoskeletal proteins in the cortex of Tetrahymena. J. Eukaryot. Microbiol, 50(1), 9–14. doi: 10.1111/j.1550-7408.2003.tb00100.x [DOI] [PubMed] [Google Scholar]
- Hufnagel LA (1969). Cortical ultrastructure of Paramecium aurelia. Studies on isolated pellicles. J. Cell Biol, 40(3), 779–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iftode F, Adoutte A, & Fleury-Aubusson A (1996). The surface pattern of Paramecium tetraurelia in interphase: an electron microscopic study of basal body variability, connections with associated ribbons and their epiplasmic environment. European J. of Protist, 32, 46–57. doi: 10.1016/s0932-4739(96)80076-9 [DOI] [Google Scholar]
- Iftode F, & Fleury-Aubusson A (2003). Structural inheritance in Paramecium: ultrastructural evidence for basal body and associated rootlets polarity transmission through binary fission. Biol. Cell, 95(1), 39–51. doi: 10.1016/s0248-4900(03)00005-4 [DOI] [PubMed] [Google Scholar]
- Jacob S, Chaine AS, Huet M, Clobert J, & Legrand D (2019). Variability in Dispersal Syndromes Is a Key Driver of Metapopulation Dynamics in Experimental Microcosms. Am. Nat, 194(5), 613–626. doi: 10.1086/705410 [DOI] [PubMed] [Google Scholar]
- Jacob S, Legrand D, Chaine AS, Bonte D, Schtickzelle N, Huet M, & Clobert J (2017). Gene flow favours local adaptation under habitat choice in ciliate microcosms. Nat. Ecol. Evol, 1(9), 1407–1410. doi: 10.1038/s41559-017-0269-5 [DOI] [PubMed] [Google Scholar]
- Jerka-Dziadosz M, & Beisson J (1990). Genetic approaches to ciliate pattern formation: from self-assembly to morphogenesis. Trends Genet, 6(2), 41–45. doi: 10.1016/0168-9525(90)90072-e [DOI] [PubMed] [Google Scholar]
- Jerka-Dziadosz M, Gogendeau D, Klotz C, Cohen J, Beisson J, & Koll F (2010). Basal body duplication in Paramecium: the key role of Bld10 in assembly and stability of the cartwheel. Cytoskeleton (Hoboken), 67(3), 161–171. doi: 10.1002/cm.20433 [DOI] [PubMed] [Google Scholar]
- Jerka-Dziadosz M, Jenkins LM, Nelsen EM, Williams NE, Jaeckel-Williams R, & Frankel J (1995). Cellular polarity in ciliates: persistence of global polarity in a disorganized mutant of Tetrahymena thermophila that disrupts cytoskeletal organization. Dev. Biol, 169(2), 644–661. doi: 10.1006/dbio.1995.1176 [DOI] [PubMed] [Google Scholar]
- Jiang YY, Maier W, Baumeister R, Joachimiak E, Ruan Z, Kannan N, Clarke D, Louka P, Guha M, Frankel J, & Gaertig J (2019). Two antagonistic hippo signaling circuits set the division plane at the medial position in the ciliate Tetrahymena. Genetics, 211(2), 651–663. doi: 10.1534/genetics.118.301889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang YY, Maier W, Baumeister R, Minevich G, Joachimiak E, Ruan Z, Kannan N, Clarke D, Frankel J, & Gaertig J (2017). The Hippo Pathway Maintains the Equatorial Division Plane in the Ciliate Tetrahymena. Genetics, 206(2), 873–888. doi: 10.1534/genetics.117.200766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones D, & Berger JD (1982). Temperature-sensitive mutations affecting cortical morphogenesis and cell division in Paramecium tetraurelia. Canadian Journal of Zoology, 60(10), 2296–2308. doi: 10.1139/z82-296 [DOI] [Google Scholar]
- Junker AD, Jacob S, Philippe H, Legrand D, & Pearson CG (2021). Plastic cell morphology changes during dispersal. iScience, 24(8), 102915. doi: 10.1016/j.isci.2021.102915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Junker AD, Soh AWJ, O’Toole ET, Meehl JB, Guha M, Winey M, Honts JE, Gaertig J, & Pearson CG (2019). Microtubule glycylation promotes attachment of basal bodies to the cell cortex. J. Cell Sci, 132(15). doi: 10.1242/jcs.233726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klena N, Le Guennec M, Tassin AM, van den Hoek H, Erdmann PS, Schaffer M, Geimer S, Aeschlimann G, Kovacik L, Sadian Y, Goldie KN, Stahlberg H, Engel BD, Hamel V, & Guichard P (2020). Architecture of the centriole cartwheel-containing region revealed by cryo-electron tomography. Embo J, 39(22), e106246. doi: 10.15252/embj.2020106246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulkarni S, Marquez J, Date P, Ventrella R, Mitchell BJ, & Khokha MK (2021). Mechanical stretch scales centriole number to apical area via Piezo1 in multiciliated cells. Elife, 10. doi: 10.7554/eLife.66076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunimoto K, Yamazaki Y, Nishida T, Shinohara K, Ishikawa H, Hasegawa T, Okanoue T, Hamada H, Noda T, Tamura A, Tsukita S, & Tsukita S (2012). Coordinated ciliary beating requires Odf2-mediated polarization of basal bodies via basal feet. Cell, 148(1–2), 189–200. doi: 10.1016/j.cell.2011.10.052 [DOI] [PubMed] [Google Scholar]
- Le Guennec M, Klena N, Gambarotto D, Laporte MH, Tassin AM, van den Hoek H, Erdmann PS, Schaffer M, Kovacik L, Borgers S, Goldie KN, Stahlberg H, Bornens M, Azimzadeh J, Engel BD, Hamel V, & Guichard P (2020). A helical inner scaffold provides a structural basis for centriole cohesion. Sci. Adv, 6(7), eaaz4137. doi: 10.1126/sciadv.aaz4137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemullois M, Gounon P, & Sandoz D (1987). Relationships between cytokeratin filaments and centriolar derivatives during ciliogenesis in the quail oviduct. Biol Cell, 61(1–2), 39–49. [DOI] [PubMed] [Google Scholar]
- Li S, Fernandez JJ, Marshall WF, & Agard DA (2019). Electron cryo-tomography provides insight into procentriole architecture and assembly mechanism. Elife, 8. doi: 10.7554/eLife.43434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindemann CB (1994). A “Geometric Clutch” Hypothesis to Explain Oscillations of the Axoneme of Cilia and Flagella. Journal of Theoretical Biology, 168(2), 175–189. doi: 10.1006/jtbi.1994.1097 [DOI] [Google Scholar]
- Lynn D (2008). The ciliated protozoa: characterization, classification, and guide to the literature: Springer Science & Business Media. [Google Scholar]
- Ma M, Stoyanova M, Rademacher G, Dutcher SK, Brown A, & Zhang R (2019). Structure of the Decorated Ciliary Doublet Microtubule. Cell, 179(4), 909–922.e912. doi: 10.1016/j.cell.2019.09.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maestro A, Bruot N, Kotar J, Uchida N, Golestanian R, & Cicuta P (2018). Control of synchronization in models of hydrodynamically coupled motile cilia. Communications Physics, 1(1), 28. doi: 10.1038/s42005-018-0031-6 [DOI] [Google Scholar]
- Mahuzier A, Shihavuddin A, Fournier C, Lansade P, Faucourt M, Menezes N, Meunier A, Garfa-Traore M, Carlier MF, Voituriez R, Genovesio A, Spassky N, & Delgehyr N (2018). Ependymal cilia beating induces an actin network to protect centrioles against shear stress. Nat. Commun, 9(1), 2279. doi: 10.1038/s41467-018-04676-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuura K, Lefebvre PA, Kamiya R, & Hirono M (2004). Bld10p, a novel protein essential for basal body assembly in Chlamydomonas : localization to the cartwheel, the first ninefold symmetrical structure appearing during assembly. J. Cell Biol, 165(5), 663–671. doi: 10.1083/jcb.200402022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meehl JB, Bayless BA, Giddings TH Jr., Pearson CG, & Winey M (2016). Tetrahymena Poc1 ensures proper intertriplet microtubule linkages to maintain basal body integrity. Mol. Biol. Cell, 27(15), 2394–2403. doi: 10.1091/mbc.E16-03-0165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metz CB, & Westfall JA (1954). The Fibrillar Systems of Ciliates as Revealed by the Electron Microscope. II. Tetrahymena. Biological Bulletin, 107(1), 106–122. doi: 10.2307/1538634 [DOI] [Google Scholar]
- Mickey B, & Howard J (1995). Rigidity of microtubules is increased by stabilizing agents. J. Cell Biol, 130(4), 909–917. doi: 10.1083/jcb.130.4.909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell B, Jacobs R, Li J, Chien S, & Kintner C (2007). A positive feedback mechanism governs the polarity and motion of motile cilia. Nature, 447(7140), 97–101. doi: 10.1038/nature05771 [DOI] [PubMed] [Google Scholar]
- Nabi A, Yano J, Valentine MS, Picariello T, & Van Houten JL (2019). SF-Assemblin genes in Paramecium: phylogeny and phenotypes of RNAi silencing on the ciliary-striated rootlets and surface organization. Cilia, 8, 2. doi: 10.1186/s13630-019-0062-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakayama S, Yano T, Namba T, Konishi S, Takagishi M, Herawati E, Nishida T, Imoto Y, Ishihara S, Takahashi M, Furuta K. y., Oiwa K, Tamura A, & Tsukita S (2021). Planar cell polarity induces local microtubule bundling for coordinated ciliary beating. J. Cell Biol, 220(7). doi: 10.1083/jcb.202010034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakazawa Y, Hiraki M, Kamiya R, & Hirono M (2007). SAS-6 is a cartwheel protein that establishes the 9-fold symmetry of the centriole. Curr. Biol, 17(24), 2169–2174. doi: 10.1016/j.cub.2007.11.046 [DOI] [PubMed] [Google Scholar]
- Nanjundappa R, Kong D, Shim K, Stearns T, Brody SL, Loncarek J, & Mahjoub MR (2019). Regulation of cilia abundance in multiciliated cells. Elife, 8. doi: 10.7554/eLife.44039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nanney DL (1966). Corticotype transmission in Tetrahymena. Genetics, 54(4), 955–968. doi: 10.1093/genetics/54.4.955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nanney DL (1971). The constancy of cortical units in tetrahymena with varying numbers of ciliary rows. Journal of Experimental Zoology, 178, 177–181. [Google Scholar]
- Nanney DL, & Chow M (1974). Basal Body Homeostasis in Tetrahymena. The American Naturalist, 108, 125 – 139. [Google Scholar]
- Narematsu N, Quek R, Chiam KH, & Iwadate Y (2015). Ciliary metachronal wave propagation on the compliant surface of Paramecium cells. Cytoskeleton (Hoboken), 72(12), 633–646. doi: 10.1002/cm.21266 [DOI] [PubMed] [Google Scholar]
- Nelsen EM (1978). Transformation in Tetrahymena thermophila. Development of an inducible phenotype. Dev. Biol, 66(1), 17–31. doi: 10.1016/0012-1606(78)90270-1 [DOI] [PubMed] [Google Scholar]
- Nelsen EM, & Debault LE (1978). Transformation in Tetrahymena pyriformis: description of an inducible phenotype. J. Protozool, 25(1), 113–119. doi: 10.1111/j.1550-7408.1978.tb03880.x [DOI] [PubMed] [Google Scholar]
- Ng SF, & Frankel J (1977). 180 degrees rotation of ciliary rows and its morphogenetic implications in Tetrahymena pyriformis. Proc. Natl. Acad. Sci. USA, 74(3), 1115–1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nievergelt AP, Banterle N, Andany SH, Gönczy P, & Fantner GE (2018). High-speed photothermal off-resonance atomic force microscopy reveals assembly routes of centriolar scaffold protein SAS-6. Nature Nanotechnology, 13(8), 696–701. doi: 10.1038/s41565-018-0149-4 [DOI] [PubMed] [Google Scholar]
- O’Toole ET, & Dutcher SK (2014). Site-specific basal body duplication in Chlamydomonas. Cytoskeleton (Hoboken), 71(2), 108–118. doi: 10.1002/cm.21155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odde DJ, Ma L, Briggs AH, DeMarco A, & Kirschner MW (1999). Microtubule bending and breaking in living fibroblast cells. J. Cell Sci, 112 (Pt 19), 3283–3288. [DOI] [PubMed] [Google Scholar]
- Omori T, Ito H, & Ishikawa T (2020). Swimming microorganisms acquire optimal efficiency with multiple cilia. Proc. Natl. Acad. Sci, 117(48), 30201. doi: 10.1073/pnas.2011146117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osterman N, & Vilfan A (2011). Finding the ciliary beating pattern with optimal efficiency. Proc. Natl. Acad. Sci, 108(38), 15727. doi: 10.1073/pnas.1107889108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson CG, Osborn DP, Giddings TH Jr., Beales PL, & Winey M (2009). Basal body stability and ciliogenesis requires the conserved component Poc1. J. Cell Biol, 187(6), 905–920. doi: 10.1083/jcb.200908019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitelka D (1961). Fine structure of the silverline and fibrillar systems of three Tetrahymenid ciliates. J. Protozool, 8(1), 75–89. doi: 10.1111/j.1550-7408.1961.tb01186.x [DOI] [Google Scholar]
- Praetorius HA, & Spring KR (2001). Bending the MDCK cell primary cilium increases intracellular calcium. J. Membr. Biol, 184(1), 71–79. doi: 10.1007/s00232-001-0075-4 [DOI] [PubMed] [Google Scholar]
- Reed W, Avolio J, & Satir P (1984). The cytoskeleton of the apical border of the lateral cells of freshwater mussel gill: structural integration of microtubule and actin filament-based organelles. J. Cell Sci, 68, 1–33. [DOI] [PubMed] [Google Scholar]
- Riedel-Kruse IH, Hilfinger A, Howard J, & Julicher F (2007). How molecular motors shape the flagellar beat. HFSP J, 1(3), 192–208. doi: 10.2976/1.2773861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riedel IH, Kruse K, & Howard J (2005). A self-organized vortex array of hydrodynamically entrained sperm cells. Science, 309(5732), 300–303. doi: 10.1126/science.1110329 [DOI] [PubMed] [Google Scholar]
- Ruehle MD, Stemm-Wolf AJ, & Pearson CG (2020). Sas4 links basal bodies to cell division via Hippo signaling. J. Cell Biol, 219(8). doi: 10.1083/jcb.201906183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandoz D, Chailley B, Boisvieux-Ulrich E, Lemullois M, Laine MC, & Bautista-Harris G (1988). Organization and functions of cytoskeleton in metazoan ciliated cells. Biol. Cell, 63(2), 183–193. doi: 10.1016/0248-4900(88)90057-3 [DOI] [PubMed] [Google Scholar]
- Schaedel L, John K, Gaillard J, Nachury MV, Blanchoin L, & Théry M (2015). Microtubules self-repair in response to mechanical stress. Nat. Mater, 14(11), 1156–1163. doi: 10.1038/nmat4396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimada Y, Yonemura S, Ohkura H, Strutt D, & Uemura T (2006). Polarized Transport of Frizzled along the Planar Microtubule Arrays in Drosophila Wing Epithelium. Dev. Cell, 10(2), 209–222. doi: 10.1016/j.devcel.2005.11.016 [DOI] [PubMed] [Google Scholar]
- Soh AWJ, van Dam TJP, Stemm-Wolf AJ, Pham AT, Morgan GP, O’Toole ET, & Pearson CG (2020). Ciliary force-responsive striated fibers promote basal body connections and cortical interactions. J. Cell Biol, 219(1). doi: 10.1083/jcb.201904091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonneborn TM (1964). The determinants and evolution of life. The differentiation of cells. Proc. Natl. Acad. Sci. USA, 51, 915–929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spassky N, & Meunier A (2017). The development and functions of multiciliated epithelia. Nat. Rev. Mol. Cell Biol, 18(7), 423–436. doi: 10.1038/nrm.2017.21 [DOI] [PubMed] [Google Scholar]
- Steinman RM (1968). An electron microscopic study of ciliogenesis in developing epidermis and trachea in the embryo of Xenopus laevis. Am. J. Anat, 122(1), 19–55. doi: 10.1002/aja.1001220103 [DOI] [PubMed] [Google Scholar]
- Stemm-Wolf AJ, Meehl JB, & Winey M (2013). Sfr13, a member of a large family of asymmetrically localized Sfi1-repeat proteins, is important for basal body separation and stability in Tetrahymena thermophila. J. Cell Sci, 126(Pt 7), 1659–1671. doi: 10.1242/jcs.120238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens VM, Trochet A, Blanchet S, Moulherat S, Clobert J, & Baguette M (2013). Dispersal syndromes and the use of life-histories to predict dispersal. Evol. Appl, 6(4), 630–642. doi: 10.1111/eva.12049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summers KE, & Gibbons IR (1971). Adenosine Triphosphate-Induced Sliding of Tubules in Trypsin-Treated Flagella of Sea-Urchin Sperm. Proc. Natl. Acad. Sci, 68(12), 3092. doi: 10.1073/pnas.68.12.3092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamm SL (1984). Mechanical synchronization of ciliary beating within comb plates of ctenophores. J. Exp. Biol, 113, 401–408. [DOI] [PubMed] [Google Scholar]
- Tamm SL (1999). Locomotory waves of Koruga and Deltotrichonympha: flagella wag the cell. Cell. Motil. Cytoskeleton, 43(2), 145–158. doi: [DOI] [PubMed] [Google Scholar]
- Tartar V (1956). Pattern and substance in Stentor. Symp. Soc. Dev. Biol, 14, 73–100. [Google Scholar]
- Tateishi K, Yamazaki Y, Nishida T, Watanabe S, Kunimoto K, Ishikawa H, & Tsukita S (2013). Two appendages homologous between basal bodies and centrioles are formed using distinct Odf2 domains. J. Cell Biol, 203(3), 417–425. doi: 10.1083/jcb.201303071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavares A, Gonçalves J, Florindo C, Tavares AA, & Soares H (2012). Mob1: defining cell polarity for proper cell division. J. Cell Sci, 125(Pt 2), 516–527. doi: 10.1242/jcs.096610 [DOI] [PubMed] [Google Scholar]
- Thazhath R, Jerka-Dziadosz M, Duan J, Wloga D, Gorovsky MA, Frankel J, & Gaertig J (2004). Cell Context-specific Effects of the β-Tubulin Glycylation Domain on Assembly and Size of Microtubular Organelles. Mol. Biol. Cell, 15(9), 4136–4147. doi: 10.1091/mbc.e04-03-0247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turk E, Wills AA, Kwon T, Sedzinski J, Wallingford JB, & Stearns T (2015). Zeta-Tubulin Is a Member of a Conserved Tubulin Module and Is a Component of the Centriolar Basal Foot in Multiciliated Cells. Curr. Biol, 25(16), 2177–2183. doi: 10.1016/j.cub.2015.06.063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Dyck H, & Baguette M (2005). Dispersal behaviour in fragmented landscapes: Routine or special movements? Basic and Applied Ecology, 6(6), 535–545. doi: 10.1016/j.baae.2005.03.005 [DOI] [Google Scholar]
- Vance T (1961). The biology of Stentor. Oxford, New York,: Pergammon Press. [Google Scholar]
- Vladar EK, Bayly RD, Sangoram AM, Scott MP, & Axelrod JD (2012). Microtubules enable the planar cell polarity of airway cilia. Curr. Biol, 22(23), 2203–2212. doi: 10.1016/j.cub.2012.09.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlijm R, Li X, Panic M, Ruthnick D, Hata S, Herrmannsdorfer F, Kuner T, Heilemann M, Engelhardt J, Hell SW, & Schiebel E (2018). STED nanoscopy of the centrosome linker reveals a CEP68-organized, periodic rootletin network anchored to a C-Nap1 ring at centrioles. Proc. Natl. Acad. Sci. USA, 115(10), E2246–E2253. doi: 10.1073/pnas.1716840115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wall KP, Hart H, Lee T, Page C, Hawkins TL, & Hough LE (2020). C-Terminal Tail Polyglycylation and Polyglutamylation Alter Microtubule Mechanical Properties. Biophys. J, 119(11), 2219–2230. doi: 10.1016/j.bpj.2020.09.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan KY (2019). Ciliate Biology: The Graceful Hunt of a Shape-Shifting Predator. Curr. Biol, 29(22), R1174–R1176. doi: 10.1016/j.cub.2019.10.013 [DOI] [PubMed] [Google Scholar]
- Wan KY, & Goldstein RE (2016). Coordinated beating of algal flagella is mediated by basal coupling. Proc. Natl. Acad. Sci. USA, 113(20), E2784–2793. doi: 10.1073/pnas.1518527113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner ME, Hwang P, Huisman F, Taborek P, Yu CC, & Mitchell BJ (2011). Actin and microtubules drive differential aspects of planar cell polarity in multiciliated cells. J. Cell Biol, 195(1), 19–26. doi: 10.1083/jcb.201106110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams NE, Tsao CC, Bowen J, Hehman GL, Williams RJ, & Frankel J (2006). The actin gene ACT1 is required for phagocytosis, motility, and cell separation of Tetrahymena thermophila. Eukaryot. Cell, 5(3), 555–567. doi: 10.1128/EC.5.3.555-567.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wloga D, Rogowski K, Sharma N, Van Dijk J, Janke C, Eddé B, Bré MH, Levilliers N, Redeker V, Duan J, Gorovsky MA, Jerka-Dziadosz M, & Gaertig J (2008). Glutamylation on alpha-tubulin is not essential but affects the assembly and functions of a subset of microtubules in Tetrahymena thermophila. Eukaryot. Cell, 7(8), 1362–1372. doi: 10.1128/ec.00084-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wloga D, Webster DM, Rogowski K, Bré MH, Levilliers N, Jerka-Dziadosz M, Janke C, Dougan ST, & Gaertig J (2009). TTLL3 Is a tubulin glycine ligase that regulates the assembly of cilia. Dev. Cell, 16(6), 867–876. doi: 10.1016/j.devcel.2009.04.008 [DOI] [PubMed] [Google Scholar]
- Woodruff LL (1937). Louis Joblot and the Protozoa. The Scientific Monthly, 44(1), 41–47. [Google Scholar]


