Abstract
Background
Some authors secured the membrane during matrix-induced autologous chondrocyte implantation (mACI) with fibrin glue or did not use a formal fixation. The real impact of fibrin glue addition on chondrocytes migration and proliferation has not yet been clarified. This study evaluated the impact of fibrin glue on a chondrocyte loaded collagenic membrane.
Methods
A resorbable collagen I/III porcine derived membrane commonly employed in AMIC was used for all experiments. Chondrocytes from three difference donors were used. At 1-, 2-, 3-, 4-, 6-, and at 8-week the membranes were embedded in Mounting Medium with Dapi (ABCAM, Cambridge, UK). The Dapi contained in the mounting medium ties the DNA of the cell nucleus and emits a blue fluorescence. In this way, the spreading of the cells in the membrane can be easily monitored. The outcomes of interest were to evaluate (1) cell migration and (2) cell proliferation within the porous membrane layer. DAPI/nuclei signals were analysed with fluorescence microscope under a magnification of 100-fold.
Results
The no-fibrin group demonstrated greater migration of the cells within the membrane. Although migration resulted higher in the no-fibrin group at every follow-up, this difference was significant only at week 1 (P < 0.001), 2 (P = 0.004), and 3 (P = 0.03). No difference was found at week 3, 6, and 8. The no-fibrin group demonstrated greater proliferation of the chondrocytes within the membrane. These differences were significant at week 4 (P < 0.0001), 6 (P < 0.0001), 8 (P < 0.0001).
Conclusion
The use of fibrin glue over a resorbable membrane leads to lower in vitro proliferation and migration of chondrocytes.
Keywords: Chondral defects, Autologous chondrocyte implantation, Fibrin
Introduction
Matrix-induced autologous chondrocyte implantation (mACI) has been advocated inpatients with symptomatic chondral defects unresponsive to conservative management [1, 2]. During mACI, autologous chondrocytes are harvested from a non-weight bearing zone of the knee, and expanded over a bioresorbable membrane in an external laboratory [3, 4]. The chondrocyte-loaded membrane is subsequently implanted into the defect in a second surgical session [5, 6]. How such membrane is secured into the defects vary. Initially, the membrane was sutured to the defect to ensure implant stability. However, suture generates partial-thickness lesions of the articular cartilage which may not heal and enlarge with time, leading to persisting symptoms and premature degeneration [7–9]. To avoid membrane suture, fibrin glue has been introduced, although some studies secured the membrane with suture [10, 11], fibrin glue [12–21], or both [22–25]. However, the membrane remains stable in the defects even without formal fixation [26–30]. Such heterogeneity in membrane fixation arises from the limited evidence and lack of consensus. The real impact of fibrin glue addition on chondrocytes migration and proliferation has not yet been clarified. This study evaluated the impact of fibrin glue on a chondrocyte loaded collagenic membrane. An in vitro study was conducted to evaluate chondrocyte migration and proliferation with or without fibrin glue application in a porcine derived collagen I/III membrane commonly employed in mACI.
Methods
Study protocol
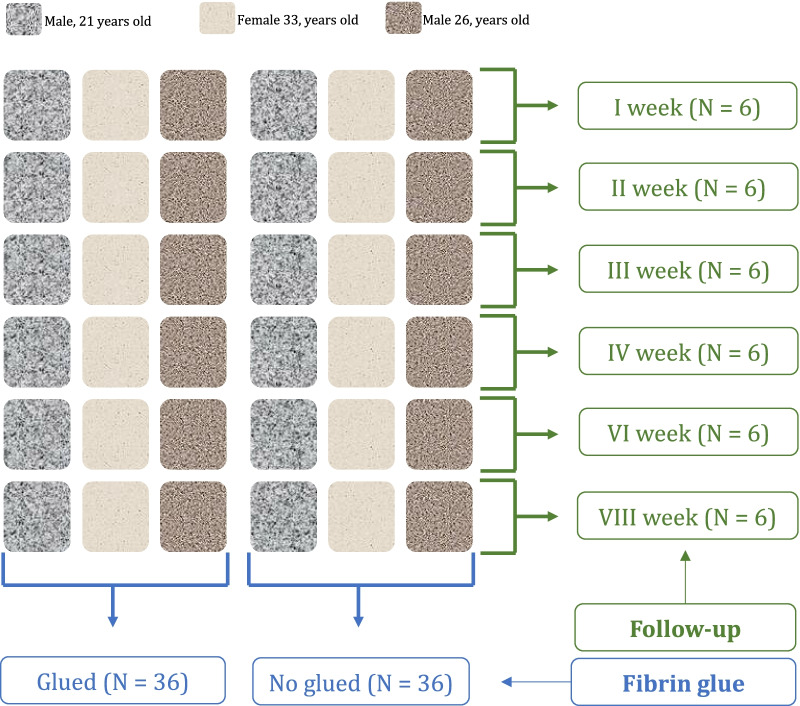
The present study was approved by the ethical committee of the Medical Faculty of the University RWTH of Aachen (ID EK305-13). A resorbable collagen I/III porcine derived membrane (Cartmaix, Matricel GmbH, Herzogenrath, Germany) commonly used in mACI was used for all experiments. Each experiment was repeated three times each containing different donor cells: a 35 years old male, a 34 years old male, and a 21 years old female. The membranes were cut into 0.7 × 0.7 cm (area 0.49 cm2) in a sterile fashion. Overall, 72 membranes were used for the experiments: 36 non-glued and 36 membranes with fibrin glue (Tisseel, Baxter International Inc, Illinois, USA). Cell proliferation and migration were compared at 1-, 2-, 3-, 4-, 6-, and at 8-week follow-up. This process is schematised in Fig. 1.
Fig. 1.
Experimental set-up (N = 72)
Chondrocyte processing
Each membrane was seeded with chondrocytes on the porous side. Following trypsinization (Sigma-Aldrich/Merck KGaA, Darmstadt, Germany) and centrifugation (1500 rmp, 10 min), the chondrocytes were resuspended in a volume of 40 µl per membrane, and spread as homogenous as possible over the membranes of a density per membrane of approximately 100,000 MSCs per cm2. After cultivation of 2 h at 37 °C in the incubator, the wells were filled up with cell culture medium. The cell culture medium was composed as follow: Dulbecco's Modified Eagle’s Medium (DMEM) combined with 4,5 g/l D-Glucose (GlutaMax, high glucose, Gibco/Life Technologies, Paisley, UK), 10% fetal calf serum (FCS, Pan-Biotech, Aidenbach, Germany), 1% penicillin–streptomycin (Pen/Strep, Sigma-Aldrich/Merck KGaA, Darmstadt, Germany). The medium was changed every 3 days.
Experiments
At 1-, 2-, 3-, 4-, 6-, and at 8-week follow-up a membrane was fixed in 4% paraformaldehyde (Merck Schuchardt OHG, Hohenbrunn, Germany) for 12 h. Afterwards, the membranes were dehydrated in an ascending alcohol series (5 min per cuvette) as follow: xylene (3×), 100% ethanol (2×), 96% ethanol, 80% ethanol, 70% ethanol, aqua dest. Subsequently the membranes were embedded in paraffin (Sakura Finetek Europe B.V., Alphen aan den Rijn, Netherlands) and cooled to − 10 °C. 3 µm sized cuts were prepared on a microtome (Schlittenmikrotom PFM Slide 4003E, PFM Medical AG, Cologne, Germany). To allow better adherence on the specimen slides, the cuts were heated at 60 °C for an hour. The paraffin of the slices was removed with xylol (Otto Fischar GmbH&Co KG, Saarbrücken, Germany) and afterwards the slices were carefully rehydrated with a descending alcohol series as follow: xylene (3X), 100% ethanol (2×), 96% ethanol, 80% ethanol, 70% ethanol, aqua dest. The membranes were embedded in Mounting Medium with Dapi (ABCAM, Cambridge, UK) and photographed on the fluorescence microscope (DM/RX, Leica, Wetzlar, Germany). The Dapi contained in the mounting medium ties the DNA of the cell nucleus and emits a blue fluorescence, allowing to detect how the cells in the membrane have spread.
Outcomes of interest
The outcomes of interest were (1) to evaluate cell migration and (2) cell proliferation within the porous membrane layer. DAPI/nuclei signals were analysed with fluorescence microscope at 100-fold magnification and the software Image J version 1.51 (National Institutes of Health, US). Migration was expressed as the percent of ingrowth of such cell within the overall thickness of the membrane. The cells which migrated in the deepest layer of the membrane was used a reference. Proliferation refers to the number of cells per mm3.
Statistics
All statistical analyses were performed using the IBM SPSS Statistics version 28.0 (IBM Corporation, Armonk NY, USA). The Shapiro–Wilk test was performed to investigate data distribution. For normally distributed variables, the t-test (Welch) was used, and the Mann Whitney U test was used for non-parametric data.
Results
Migration
The no-fibrin group demonstrated greater migration of the cells within the membrane. Although migration resulted higher in the no-fibrin group at every follow-up, this difference was significant only at week 1 (P < 0.001), 2 (P = 0.004), and 3 (P = 0.03). No difference was found at week 3, 6, and 8. Figure 2 shows the results of cell migration at each follow-up.
Fig. 2.
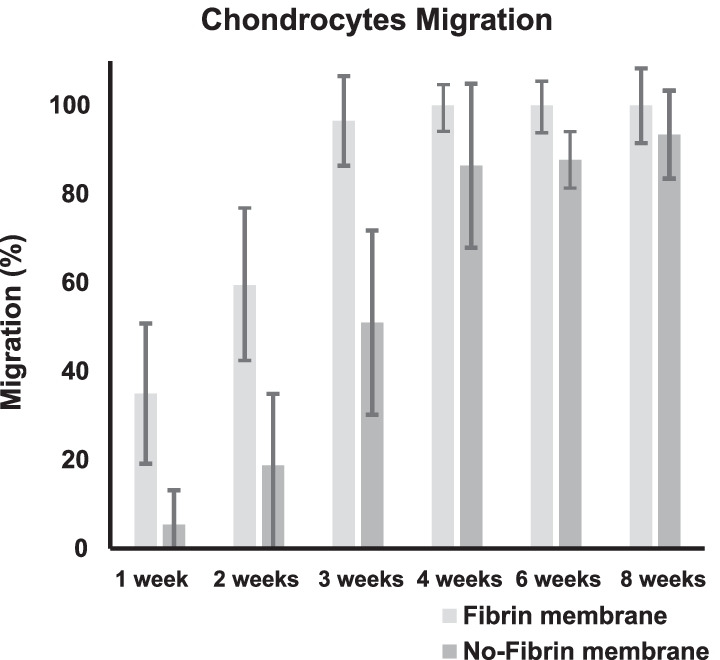
Chondrocytes migration within the membrane
Proliferation
The no-fibrin group demonstrated greater proliferation of the chondrocytes within the membrane. These differences were significant at week 4 (P < 0.0001), 6 (P < 0.0001), 8 (P < 0.0001). Figure 3 shows the results of cell proliferation at each follow-up.
Fig. 3.
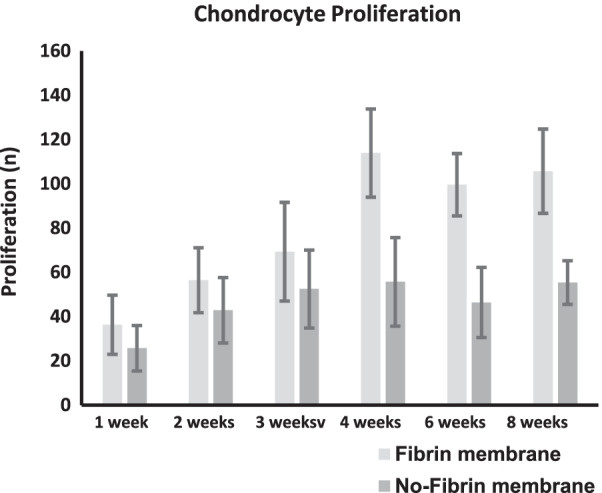
Chondrocytes proliferation within the membrane
Discussion
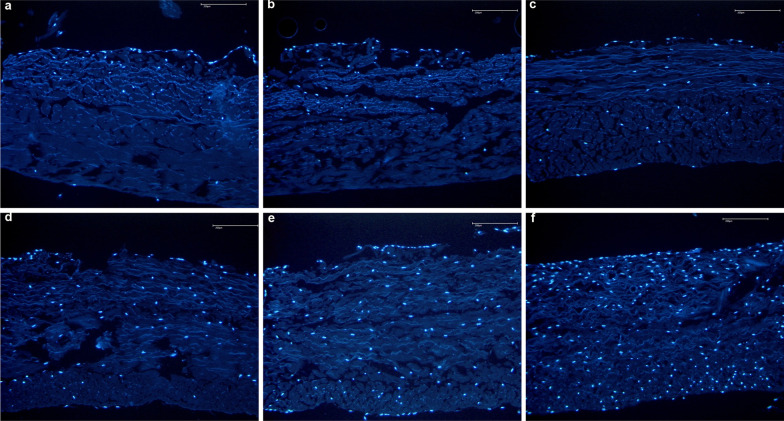
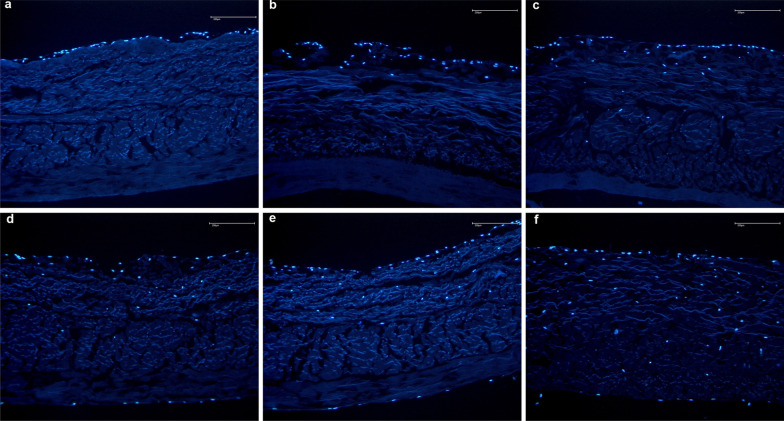
According to the main findings of the present study, the use of fibrin glue over a resorbable membrane leads to lower in vitro proliferation and migration of chondrocytes (Figs. 4 and 5). The membranes without fibrin glue demonstrated greater chondrocytes migration of the cells within the first three weeks, and greater proliferation during the last five weeks.
Fig. 4.
Proliferation and migration of chondrocytes in the membrane without fibrin using the DAPI stained cell nuclei of chondrocytes (blue). Time dependent proliferation and migration of chondrocytes into the non-fibrin treated membrane over the 1, 2, 3, 4, 6, and 8 weeks (picture a–f, respectively)
Fig. 5.
Proliferation and migration of chondrocytes in the fibrin glued membrane using the DAPI stained cell nuclei of chondrocytes (blue). Time dependent proliferation and migration of chondrocytes into the non-fibrin treated membrane over the 1, 2, 3, 4, 6, and 8 weeks (picture a–f, respectively)
Initially, it was believed that such membranes should be fixed using sutures in autologous chondrocyte implantation. However, studies demonstrated that suture can irreversibly damage the cartilage. Suturing the membrane generates partial-thickness lesions of the articular cartilage which may not heal and enlarge with time [7, 8]. Hunzinker et al. [9], to establish the potential damage of sutures in cartilage, sutured the surrounding articular cartilage of large, partial-thickness trochlear defects in 18 adult goats. The perisutural area underwent histological, histochemical and histomorphometrical analysis: suturing induced severe local cartilage impairment which may lead to pain, reduced healing and premature osteoarthritis [9]. To avoid this, membrane suture fibrin glue has been introduced. Fibrin glue has been widely employed given its biological sealing, haemostatic and adhesive proprieties [31, 32]. Its primary use is as a biological sealant, and it also promotes chondrocytes migration and proliferation [33–35]. Mainly through the action of thrombin, fibrin glue is believed to promote a variety of cellular responses, increasing cell migration, proliferation and survival [36–40].
The success of chondrocyte cultivation in a matrix depends on the ability of the implanted chondrocytes to proliferate and synthesize cartilage matrix [41]. For cartilage, platelet derivates such as fibrin directly induce regeneration of the injured tissue [42], but also provide a scaffold carrying biochemical stimuli [43]. The ideal injectable hydrogel for neocartilage formation would be non-reactive, biocompatible, biodegradable at an appropriate rate, and able to support growth factor delivery [44, 45]. To date, numerous natural and synthetic biomaterials have been used for neocartilage tissue engineering, including chitosan [46], collagen/gelatin [47], alginate [48], and fibrin [49].
Fibrin glue is a biological tissue adhesive imitating the final stages of the blood coagulation cascade [50]. Fibrin glue contains a fibrinogen component and a thrombin component which are prepared by processing blood plasma [51]. Previous studies demonstrated fibrin glue to provide mitogenic and chemotactic stimuli for mesenchymal stem cells by releasing platelet-derived growth factor, which promotes cell proliferation, migration, and matrix synthesis [52]. Therefore, fibrin glue has been suggested to sustain isolated chondrocytes, promoting cell proliferation and matrix synthesis [53]. Fibrin glue has been shown to affect extracellular matrix formation, accumulation of collagen, and increase of spherical-shaped chondrocytes in vitro [41]. Commercially available fibrin glue has been applied in clinical practice for some decades [54]. However, fibrinolysis in vivo leads to rapid resorption of the fibrin glue [54]. In 2007, Eyrich et al. [55] developed a long-stable fibrin glue by a modification of buffer substances and supplementation of antifibrinolytic agents. Their fibrin glue hydrogel has been used for tissue engineering of cartilaginous tissues with cultured chondrocytes [56]. Homminga et al. [57] reported that chondrocytes encapsulated in a fibrin sealant retained their morphology and synthetized matrix in vitro. However, Brittberg et al. suggested that fibrin sealants were not stable for osteochondral healing in vivo [58]. In a second in vitro experiment, chondrocyte migration into the fibrin sealant was evaluated in comparison to the chondrocyte migration into rabbit and human blood clots. Whereas, a migration of chondrocytes into blood clots was observed, no chondrocyte migration into the fibrin sealant occurred [58]. Fbrin sealant contains a high concentration of clotting factors but an insufficient amount of stimulating factors [58]. Furthermore, Cheung et al. suggested that fibrin sealants might impair the migration of chondrocytes via a barrier effect [59]. Similarly, no effect on chondrocyte growth and proliferation by fibrin glue has been observed in other studies [60, 61].
Growth factors have been widely used in neocartilage production in vitro [60]. IGF-1 and 2 promote differentiation of immature chondrocytes [60]. b-FGF exerts mitogenic effects and promotes cell survival [60]. Fibrin glue stabilizes growth factors and other proteins, which prevents natural enzymatic degradation [62]. However, a previous study demonstrated a negative effect of growth factors on the production of neocartilage in vitro [60]. They assumed dose-dependent effects as the in vitro concentrations of growth factors have not been examined [60]. For example, a high concentration of platelets was reported to inhibit proliferation, migration, and the production of collagen type I in human tenocytes [63]. Therefore, the inhibitory effects observed in vitro using high concentrations of fibrin or growth factors strongly suggest impairment of wound healing in vivo.
This study has certainly several limitations. The intraarticular environment is rich of proteins, cells, and cytokines which may influence chondrocytes migration and proliferation. Moreover, the repetitive cycles of weight bearing and motion occurring in the postoperative period have not been considered in the present investigation. Further, the chondrocytes were obtained by only three different donors, which may also not reflect the biological variability of human. Finally, the follow-up was eight weeks, which may limit the capability to identify long-term migration and proliferation of chondrocytes. These limitations should be overcome by future studies, which should also compare the histological changes in vivo of fibrin adduction.
Conclusion
The use of fibrin glue over a resorbable membrane leads to lower in vitro proliferation and migration of chondrocytes.
Acknowledgements
None
Author contributions
FM conception and design, drafting, final approval; NM: supervision, revision, final approval; JE: supervision, final approval; CW: supervision, final approval; SL: experiments; JP: drafting, final approval; HS: drafting, final approval; FH: supervision, final approval; JG: conception, design, analysis, and interpretation of the data, drafting, final approval. All authors read and approved the final manuscript.
Funding
Open Access funding enabled and organized by Projekt DEAL. The authors received no financial support for the research, authorship, and/or publication of this article.
Availability of data and materials
The datasets generated during and/or analysed during the current study are available throughout the manuscript.
Declarations
Ethics approval and consent to participate
The present study was approved by the ethical committee of the Medical Faculty of the University RWTH of Aachen (ID EK305-13).
Consent for publication
Not applicable.
Competing interests
Professor Nicola Maffulli is the Editor in Chief of the Journal of Orthopaedic Surgery and Research.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Filippo Migliorini and Julia Prinz contributed equally
Contributor Information
Filippo Migliorini, Email: migliorini.md@gmail.com.
Nicola Maffulli, Email: n.maffulli@qmul.ac.uk.
References
- 1.Migliorini F, Maffulli N, Baroncini A, et al. Matrix-induced autologous chondrocyte implantation versus autologous matrix-induced chondrogenesis for chondral defects of the talus: a systematic review. Br Med Bull. 2021;138:144–154. doi: 10.1093/bmb/ldab008. [DOI] [PubMed] [Google Scholar]
- 2.Migliorini F, Eschweiler J, Gotze C, et al. Matrix-induced autologous chondrocyte implantation (mACI) versus autologous matrix-induced chondrogenesis (AMIC) for chondral defects of the knee: a systematic review. Br Med Bull. 2021;138(1):144–154. doi: 10.1093/bmb/ldab008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Migliorini F, Eschweiler J, Spiezia F, et al. Arthroscopy versus mini-arthrotomy approach for matrix-induced autologous chondrocyte implantation in the knee: a systematic review. J Orthop Traumatol. 2021;22:23. doi: 10.1186/s10195-021-00588-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Migliorini F, Eschweiler J, Goetze C, et al. Membrane scaffolds for matrix-induced autologous chondrocyte implantation in the knee: a systematic review. Br Med Bull. 2021;140:50–61. doi: 10.1093/bmb/ldab024. [DOI] [PubMed] [Google Scholar]
- 5.Migliorini F, Eschweiler J, Schenker H, et al. Surgical management of focal chondral defects of the knee: a Bayesian network meta-analysis. J Orthop Surg Res. 2021;16:543. doi: 10.1186/s13018-021-02684-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Migliorini F, Berton A, Salvatore G, et al. Autologous chondrocyte implantation and mesenchymal stem cells for the treatments of chondral defects of the knee-a systematic review. Curr Stem Cell Res Ther. 2020;15:547–556. doi: 10.2174/1574888X15666200221122834. [DOI] [PubMed] [Google Scholar]
- 7.Walker EA, Verner A, Flannery CR, et al. Cellular responses of embryonic hyaline cartilage to experimental wounding in vitro. J Orthop Res. 2000;18:25–34. doi: 10.1002/jor.1100180105. [DOI] [PubMed] [Google Scholar]
- 8.Hunziker EB, Quinn TM. Surgical removal of articular cartilage leads to loss of chondrocytes from cartilage bordering the wound edge. J Bone Joint Surg Am. 2003;85(2):85–92. doi: 10.2106/00004623-200300002-00011. [DOI] [PubMed] [Google Scholar]
- 9.Hunziker EB, Stahli A. Surgical suturing of articular cartilage induces osteoarthritis-like changes. Osteoarthritis Cartilage. 2008;16:1067–1073. doi: 10.1016/j.joca.2008.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zeifang F, Oberle D, Nierhoff C, et al. Autologous chondrocyte implantation using the original periosteum-cover technique versus matrix-associated autologous chondrocyte implantation: a randomized clinical trial. Am J Sports Med. 2010;38:924–933. doi: 10.1177/0363546509351499. [DOI] [PubMed] [Google Scholar]
- 11.Meyerkort D, Ebert JR, Ackland TR, et al. Matrix-induced autologous chondrocyte implantation (MACI) for chondral defects in the patellofemoral joint. Knee Surg Sports Traumatol Arthrosc. 2014;22:2522–2530. doi: 10.1007/s00167-014-3046-x. [DOI] [PubMed] [Google Scholar]
- 12.Anders S, Goetz J, Schubert T, et al. Treatment of deep articular talus lesions by matrix associated autologous chondrocyte implantation–results at five years. Int Orthop. 2012;36:2279–2285. doi: 10.1007/s00264-012-1635-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aurich M, Bedi HS, Smith PJ, et al. Arthroscopic treatment of osteochondral lesions of the ankle with matrix-associated chondrocyte implantation: early clinical and magnetic resonance imaging results. Am J Sports Med. 2011;39:311–319. doi: 10.1177/0363546510381575. [DOI] [PubMed] [Google Scholar]
- 14.Dixon S, Harvey L, Baddour E, et al. Functional outcome of matrix-associated autologous chondrocyte implantation in the ankle. Foot Ankle Int. 2011;32:368–374. doi: 10.3113/FAI.2011.0368. [DOI] [PubMed] [Google Scholar]
- 15.Giza E, Sullivan M, Ocel D, et al. Matrix-induced autologous chondrocyte implantation of talus articular defects. Foot Ankle Int. 2010;31:747–753. doi: 10.3113/FAI.2010.0747. [DOI] [PubMed] [Google Scholar]
- 16.Kreulen C, Giza E, Walton J, et al. Seven-year follow-up of matrix-induced autologous implantation in talus articular defects. Foot Ankle Spec. 2018;11:133–137. doi: 10.1177/1938640017713614. [DOI] [PubMed] [Google Scholar]
- 17.Magnan B, Samaila E, Bondi M, et al. Three-dimensional matrix-induced autologous chondrocytes implantation for osteochondral lesions of the talus: midterm results. Adv Orthop. 2012;2012:942174. doi: 10.1155/2012/942174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schneider TE, Karaikudi S. Matrix-induced autologous chondrocyte implantation (MACI) grafting for osteochondral lesions of the talus. Foot Ankle Int. 2009;30:810–814. doi: 10.3113/FAI.2009.0810. [DOI] [PubMed] [Google Scholar]
- 19.Migliorini F, Eschweiler J, Maffulli N, et al. Management of patellar chondral defects with autologous matrix induced chondrogenesis (AMIC) compared to microfractures: a four years follow-up clinical trial. Life (Basel) 2021;11(2):141. doi: 10.3390/life11020141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Migliorini F, Eschweiler J, Maffulli N, et al. Autologous matrix-induced chondrogenesis (AMIC) and microfractures for focal chondral defects of the knee: a medium-term comparative study. Life (Basel) 2021;11(3):183. doi: 10.3390/life11030183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Migliorini F, Eschweiler J, Maffulli N, et al. Autologous matrix induced chondrogenesis (AMIC) compared to microfractures for chondral defects of the talar shoulder: a five-year follow-up prospective cohort study. Life (Basel) 2021;11(3):244. doi: 10.3390/life11030244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Akgun I, Unlu MC, Erdal OA, et al. Matrix-induced autologous mesenchymal stem cell implantation versus matrix-induced autologous chondrocyte implantation in the treatment of chondral defects of the knee: a 2-year randomized study. Arch Orthop Trauma Surg. 2015;135:251–263. doi: 10.1007/s00402-014-2136-z. [DOI] [PubMed] [Google Scholar]
- 23.Cvetanovich GL, Riboh JC, Tilton AK, et al. Autologous chondrocyte implantation improves knee-specific functional outcomes and health-related quality of life in adolescent patients. Am J Sports Med. 2017;45:70–76. doi: 10.1177/0363546516663711. [DOI] [PubMed] [Google Scholar]
- 24.Lopez-Alcorocho JM, Aboli L, Guillen-Vicente I, et al. Cartilage defect treatment using high-density autologous chondrocyte implantation: two-year follow-up. Cartilage. 2018;9:363–369. doi: 10.1177/1947603517693045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nejadnik H, Hui JH, Feng Choong EP, et al. Autologous bone marrow-derived mesenchymal stem cells versus autologous chondrocyte implantation: an observational cohort study. Am J Sports Med. 2010;38:1110–1116. doi: 10.1177/0363546509359067. [DOI] [PubMed] [Google Scholar]
- 26.Buda R, Vannini F, Castagnini F, et al. Regenerative treatment in osteochondral lesions of the talus: autologous chondrocyte implantation versus one-step bone marrow derived cells transplantation. Int Orthop. 2015;39:893–900. doi: 10.1007/s00264-015-2685-y. [DOI] [PubMed] [Google Scholar]
- 27.Giannini S, Buda R, Ruffilli A, et al. Arthroscopic autologous chondrocyte implantation in the ankle joint. Knee Surg Sports Traumatol Arthrosc. 2014;22:1311–1319. doi: 10.1007/s00167-013-2640-7. [DOI] [PubMed] [Google Scholar]
- 28.Giannini S, Buda R, Vannini F, et al. Arthroscopic autologous chondrocyte implantation in osteochondral lesions of the talus: surgical technique and results. Am J Sports Med. 2008;36:873–880. doi: 10.1177/0363546507312644. [DOI] [PubMed] [Google Scholar]
- 29.Niemeyer P, Laute V, John T, et al. The effect of cell dose on the early magnetic resonance morphological outcomes of autologous cell implantation for articular cartilage defects in the knee: a randomized clinical trial. Am J Sports Med. 2016;44:2005–2014. doi: 10.1177/0363546516646092. [DOI] [PubMed] [Google Scholar]
- 30.Niemeyer P, Laute V, Zinser W, et al. A prospective, randomized, open-label, multicenter, phase III noninferiority trial to compare the clinical efficacy of matrix-associated autologous chondrocyte implantation with spheroid technology versus arthroscopic microfracture for cartilage defects of the knee. Orthop J Sports Med. 2019;7:2325967119854442. doi: 10.1177/2325967119854442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Morales-Conde S, Balla A, Alarcon I, et al. Minimally invasive repair of ventral hernia with one third of tackers and fibrin glue: less pain and same recurrence rate. Minerva Chir. 2020;75:292–297. doi: 10.23736/S0026-4733.20.08468-0. [DOI] [PubMed] [Google Scholar]
- 32.Wong AI, McDonald A, Jones B, et al. Patch-and-glue: novel technique in bronchoesophageal fistula repair and broncholith removal with stent and fibrin glue. J Bronchology Interv Pulmonol. 2021;28(3):e45–e49. doi: 10.1097/LBR.0000000000000732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nair MA, Shaik KV, Kokkiligadda A, et al. Tissue-engineered maxillofacial skeletal defect reconstruction by 3D printed beta-tricalcium phosphate scaffold tethered with growth factors and fibrin glue implanted autologous bone marrow-derived mesenchymal stem cells. J Med Life. 2020;13:418–425. doi: 10.25122/jml-2020-0044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sanghani-Kerai A, Coathup M, Brown R, et al. The development of a novel autologous blood glue aiming to improve osseointegration in the bone-implant interface. Bone Joint Res. 2020;9:402–411. doi: 10.1302/2046-3758.97.BJR-2019-0073.R3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cassaro CV, Justulin LA, Jr, de Lima PR, et al. Fibrin biopolymer as scaffold candidate to treat bone defects in rats. J Venom Anim Toxins Incl Trop Dis. 2019;25:e20190027. doi: 10.1590/1678-9199-jvatitd-2019-0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Karp JM, Tanaka TS, Zohar R, et al. Thrombin mediated migration of osteogenic cells. Bone. 2005;37:337–348. doi: 10.1016/j.bone.2005.04.022. [DOI] [PubMed] [Google Scholar]
- 37.Brown LF, Lanir N, McDonagh J, et al. Fibroblast migration in fibrin gel matrices. Am J Pathol. 1993;142:273–283. [PMC free article] [PubMed] [Google Scholar]
- 38.Tani K, Yasuoka S, Ogushi F, et al. Thrombin enhances lung fibroblast proliferation in bleomycin-induced pulmonary fibrosis. Am J Respir Cell Mol Biol. 1991;5:34–40. doi: 10.1165/ajrcmb/5.1.34. [DOI] [PubMed] [Google Scholar]
- 39.Chinni C, de Niese MR, Tew DJ, et al. Thrombin, a survival factor for cultured myoblasts. J Biol Chem. 1999;274:9169–9174. doi: 10.1074/jbc.274.14.9169. [DOI] [PubMed] [Google Scholar]
- 40.Pagel CN, de Niese MR, Abraham LA, et al. Inhibition of osteoblast apoptosis by thrombin. Bone. 2003;33:733–743. doi: 10.1016/S8756-3282(03)00209-6. [DOI] [PubMed] [Google Scholar]
- 41.Wysocka A, Mann K, Bursig H, et al. Chondrocyte suspension in fibrin glue. Cell Tissue Bank. 2010;11:209–215. doi: 10.1007/s10561-009-9163-y. [DOI] [PubMed] [Google Scholar]
- 42.Zhu Y, Yuan M, Meng HY, et al. Basic science and clinical application of platelet-rich plasma for cartilage defects and osteoarthritis: a review. Osteoarthr Cartil. 2013;21:1627–1637. doi: 10.1016/j.joca.2013.07.017. [DOI] [PubMed] [Google Scholar]
- 43.Giusti I, D'Ascenzo S, Macchiarelli G, et al. In vitro evidence supporting applications of platelet derivatives in regenerative medicine. Blood Transfus. 2020;18:117–129. doi: 10.2450/2019.0164-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Silverman RP, Passaretti D, Huang W, et al. Injectable tissue-engineered cartilage using a fibrin glue polymer. Plast Reconstr Surg. 1999;103:1809–1818. doi: 10.1097/00006534-199906000-00001. [DOI] [PubMed] [Google Scholar]
- 45.Liu M, Zeng X, Ma C, et al. Injectable hydrogels for cartilage and bone tissue engineering. Bone Res. 2017;5:17014. doi: 10.1038/boneres.2017.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Naderi-Meshkin H, Andreas K, Matin MM, et al. Chitosan-based injectable hydrogel as a promising in situ forming scaffold for cartilage tissue engineering. Cell Biol Int. 2014;38:72–84. doi: 10.1002/cbin.10181. [DOI] [PubMed] [Google Scholar]
- 47.Yuan L, Li B, Yang J, et al. Effects of composition and mechanical property of injectable collagen I/II composite hydrogels on chondrocyte behaviors. Tissue Eng Part A. 2016;22:899–906. doi: 10.1089/ten.tea.2015.0513. [DOI] [PubMed] [Google Scholar]
- 48.Balakrishnan B, Joshi N, Jayakrishnan A, et al. Self-crosslinked oxidized alginate/gelatin hydrogel as injectable, adhesive biomimetic scaffolds for cartilage regeneration. Acta Biomater. 2014;10:3650–3663. doi: 10.1016/j.actbio.2014.04.031. [DOI] [PubMed] [Google Scholar]
- 49.Benavides OM, Brooks AR, Cho SK, et al. In situ vascularization of injectable fibrin/poly(ethylene glycol) hydrogels by human amniotic fluid-derived stem cells. J Biomed Mater Res A. 2015;103:2645–2653. doi: 10.1002/jbm.a.35402. [DOI] [PubMed] [Google Scholar]
- 50.Le Guéhennec L, Layrolle P, Daculsi G. A review of bioceramics and fibrin sealant. Eur Cell Mater. 2004;8:1–10. doi: 10.22203/eCM.v008a01. [DOI] [PubMed] [Google Scholar]
- 51.Panda A, Kumar S, Kumar A, et al. Fibrin glue in ophthalmology. Indian J Ophthalmol. 2009;57:371–379. doi: 10.4103/0301-4738.55079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.McGrath MH. Peptide growth factors and wound healing. Clin Plast Surg. 1990;17:421–432. doi: 10.1016/S0094-1298(20)30617-9. [DOI] [PubMed] [Google Scholar]
- 53.Sims CD, Butler PE, Cao YL, et al. Tissue engineered neocartilage using plasma derived polymer substrates and chondrocytes. Plast Reconstr Surg. 1998;101:1580–1585. doi: 10.1097/00006534-199805000-00022. [DOI] [PubMed] [Google Scholar]
- 54.Skodacek D, Arnold U, Storck K, et al. Chondrocytes suspended in modified fibrin glue for vocal fold augmentation: an in vitro study in a porcine larynx model. Head Neck. 2012;34:667–673. doi: 10.1002/hed.21789. [DOI] [PubMed] [Google Scholar]
- 55.Eyrich D, Brandl F, Appel B, et al. Long-term stable fibrin gels for cartilage engineering. Biomaterials. 2007;28:55–65. doi: 10.1016/j.biomaterials.2006.08.027. [DOI] [PubMed] [Google Scholar]
- 56.Kwon TK, Buckmire R. Injection laryngoplasty for management of unilateral vocal fold paralysis. Curr Opin Otolaryngol Head Neck Surg. 2004;12:538–542. doi: 10.1097/01.moo.0000144393.40874.98. [DOI] [PubMed] [Google Scholar]
- 57.Homminga GN, Buma P, Koot HW, et al. Chondrocyte behavior in fibrin glue in vitro. Acta Orthop Scand. 1993;64:441–445. doi: 10.3109/17453679308993663. [DOI] [PubMed] [Google Scholar]
- 58.Brittberg M, Sjögren-Jansson E, Lindahl A, et al. Influence of fibrin sealant (Tisseel) on osteochondral defect repair in the rabbit knee. Biomaterials. 1997;18:235–242. doi: 10.1016/S0142-9612(96)00117-2. [DOI] [PubMed] [Google Scholar]
- 59.Cheung HS, Lynch KL, Johnson RP, et al. In vitro synthesis of tissue-specific type II collagen by healing cartilage. I. Short-term repair of cartilage by mature rabbits. Arthritis Rheum. 1980;23:211–219. doi: 10.1002/art.1780230212. [DOI] [PubMed] [Google Scholar]
- 60.Westreich R, Kaufman M, Gannon P, et al. Validating the subcutaneous model of injectable autologous cartilage using a fibrin glue scaffold. Laryngoscope. 2004;114:2154–2160. doi: 10.1097/10.mlg.0000149449.37640.0d. [DOI] [PubMed] [Google Scholar]
- 61.Visna P, Pasa L, Cizmár I, et al. Treatment of deep cartilage defects of the knee using autologous chondrograft transplantation and by abrasive techniques–a randomized controlled study. Acta Chir Belg. 2004;104:709–714. doi: 10.1080/00015458.2004.11679648. [DOI] [PubMed] [Google Scholar]
- 62.Kaufman MR, Westreich R, Ammar SM, et al. Autologous cartilage grafts enhanced by a novel transplant medium using fibrin sealant and fibroblast growth factor. Arch Facial Plast Surg. 2004;6:94–100. doi: 10.1001/archfaci.6.2.94. [DOI] [PubMed] [Google Scholar]
- 63.Giusti I, D'Ascenzo S, Mancò A, et al. Platelet concentration in platelet-rich plasma affects tenocyte behavior in vitro. Biomed Res Int. 2014;2014:630870. doi: 10.1155/2014/630870. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and/or analysed during the current study are available throughout the manuscript.