Abstract
There is an urgent need for better treatment of lung diseases that are a major cause of morbidity and mortality worldwide. This urgency is illustrated by the current COVID-19 health crisis. Moderate-to-extensive lung injury characterizes several lung diseases, and not only therapies that reduce such lung injury are needed but also those that regenerate lung tissue and repair existing lung injury. At present, such therapies are not available, but as a result of a rapid increase in our understanding of lung development and repair, lung regenerative therapies are on the horizon. Here, we discuss existing targets for treatment, as well as novel strategies for development of pharmacological and cell therapy–based regenerative treatment for a variety of lung diseases and clinical studies. We discuss how both patient-relevant in vitro disease models using innovative culture techniques and other advanced new technologies aid in the development of pulmonary regenerative medicine.
Introduction
Loss of functional lung tissue is a characteristic of a range of acute and chronic lung diseases. In patients with severe end-stage chronic lung disease who are refractory to treatment, the only solution is lung transplantation. The type of injury that results in loss of lung function varies between these disease entities and may include destruction or remodeling of the distal lung parenchyma, where gas exchange occurs in the alveoli. Alveolar damage occurs in acute respiratory disease (ARDS), whereas repetitive alveolar damage can result in parenchymal destruction as observed in emphysema in patients with chronic obstructive pulmonary disease (COPD). In contrast, extensive remodeling due to fibrotic processes is observed in idiopathic pulmonary fibrosis (IPF) and other interstitial lung diseases. The current COVID-19 crisis highlights the range of processes leading to loss of functional lung tissue because some patients with COVID-19 (especially those with severe disease) may not only suffer from acute alveolar damage but also suffer from subsequent fibrotic processes [1]. Airway remodeling is also an important cause of lung function loss and is observed in COPD, asthma, and cystic fibrosis or may result from stenosis due to, for example, tumor formation. Finally, vascular remodeling in patients with pulmonary arterial hypertension also results in not only problems in the lungs but also in the heart and may be a cause of the need for lung transplantation.
Lung transplantation can be a life-saving procedure in patients with end-stage lung disease, but shortage of donor lungs and rejection of the transplanted lungs are important limitations. Pulmonary regenerative medicine holds promise for patients with severe loss of lung function requiring transplantation and also for those with progressive loss of lung function. In many of these lung diseases, including COPD, IPF, ARDS, cystic fibrosis, and COVID-19, the epithelium is affected. This epithelium covers the conducting airways, as well as the alveoli located in the distal lung parenchyma.
In the last decade, our understanding of lung development and lung repair has markedly increased, and much of this research has focused on the epithelium and its stem or progenitor cells and the niche (i.e. extracellular matrix and surrounding cells) these cells reside in. Injury models in animals and cell culture models have shown that the adult lung has marked repair capacity. Interestingly, this is supported by observations in patients after pneumonectomy [2]. Use of this increased knowledge of lung development and repair has resulted in the realization that pharmacological approaches, cell therapy, and tissue engineering are realistic options for a better treatment of a range of lung diseases. Therapeutic strategies to repair lung tissue include those aimed at regeneration (cell therapy, extracellular vesicles, and pharmacological interventions) and replacement (cell therapy and tissue engineering) [3]. In this review, we will focus on regenerative pharmacology and cell therapy as a regenerative approach. Readers interested in tissue engineering are referred to other excellent reviews on this topic [4].
Lung stem cells and their role in repair and regeneration
Injury models in animals, mainly mice, have markedly increased our insight into the identity and role of lung stem cell populations in repair. However, such models are increasingly replaced by cell culture models, using primary cells isolated from lung tissue or by using (human) induced pluripotent stem cells that can be differentiated into the various lung cell populations [5]. The need to use more human cell and tissue culture models also arises from the realization that there are limitations in the translation of findings from murine studies to the human situation, resulting from differences in anatomical organization, biology/physiology, and cell-ratio differences along the epithelium lining the lungs [6].
Recent reviews provide a detailed overview of the rapidly evolving field of research into lung stem/progenitor cell populations and associated models, to which we refer the interested reader to obtain further detailed information that is beyond the scope of this review [5,7, 8, 9, 10]. Table 1 provides a summary of the main stem/progenitor populations in the mouse and human lung because these species have been most widely studied. Briefly, in the airway epithelium, the basal cells are the most widely recognized cell type that acts as a progenitor cell. These basal cells have the capacity to self-renew and to differentiate into the various types of luminal cells that constitute the airway epithelium: ciliated cells, secretory cells (mucus-producing goblet cells and club cells) and more rare cell types such as the cystic fibrosis transmembrane conductance regulator (CFTR)-expressing ionocytes, the chemosensory tuft or brush cells, and neuroendocrine cells [5,7,10, 11, 12]. At least two subpopulations of basal cells can be recognized, in which one serves as a self-renewing stem cell, whereas the other population mediates formation of the luminal cells during epithelial differentiation [13]. Single-cell analysis has, however, shown that these basal cells consist of various (and likely more than two) subpopulations that may have different characteristics and may differ in abundance in disease, as shown for instance in COPD [14]. A detailed understanding of the function of these subpopulations in health and disease is still missing. Especially after injury, and mainly demonstrated in mouse injury models, also luminal cells may contribute to repair as shown for club cells [15]. Mouse studies showed that these cells may even give rise to basal cells after extensive injury [16], but it is unclear whether this also contributes to repair in homeostatic conditions and after milder forms of injury. Furthermore, to which extent these basal cells are different from ‘conventional’ basal cells remains to be determined. Furthermore, in the distal airways including at the junction between the bronchioli and alveoli, additional stem cell populations have been identified in the mouse that contribute to airway and alveolar (re)generation (reviewed in [5]).
Table 1.
Overview of the various stem/progenitor populations in the mouse and human lung.
| Cell type | Marker expression | Differentiation/cell fate | Location/species | References |
|---|---|---|---|---|
| Basal cell | TP63, KRT5, NGFR | Self; airway luminal cells (including secretory goblet and club cells, and ciliated cells) | Airway Human/mouse |
[11,12,14,49] |
| Club/secretory cell | Scgb1a1 | Self; ciliated cells | Airway Human/mouse |
[15,16] |
| Myoepithelial cell | TP63, Scgb1a1 | Self; basal and luminal cells | Submucosal gland Mouse |
[50] |
| Distal airway cell populations (BASC, DASC, LNEP, MHChigh [H2-K1high]) | Various markers (depending on the subpopulation) | Self; basal, club, ciliated cells; AT1, AT2 | Distal airways Mouse |
Reviewed in [5] |
| AT2 | SFTPC, HT2-280, DC-LAMP, ABCA-3 | Self; AT1 | Alveoli Human/mouse |
[17] |
| AEP | SFTPC, HT2-280, Axin2/TM4SF1 | Self; AT2; AT1 | Alveoli Human/mouse |
[18,51] |
ABCA-3: ATP-binding cassette sub-family A member 3; AEP: alveolar epithelial progenitor cell; BASC: bronchioalveolar stem cell; DASC: distal airway stem cell; DC-LAMP: dendritic cell lysosomal-associated membrane glycoprotein; H2-K1: mouse MHC class I marker; KRT5: keratin 5; LNEP: lineage-negative epithelial progenitor; NGFR: nerve growth factor receptor; Scgb1a1: secretoglobin family 1A member 1; SFTPC: surfactant protein C; TM4SF1: transmembrane 4 L six family member 1; TP63: tumor protein p63.
There has also been a marked progress in our understanding of alveolar development and repair, not only through animal models but also especially through organoid studies including those with human cells [9]. Within the alveolus, the cuboidal alveolar type 2 cell (AT2) serves multiple functions: it produces surfactant to lower surface tension and prevents alveolar collapse, secretes host defense and inflammatory mediators, and serves as a progenitor for the flattened alveolar type 1 cells (AT1) that allow gas exchange. Importantly, AT2 cells also function as stem cells and have the capacity to self-renew [17,18]. WNT/β-catenin activation was reported to be central in self-renewal, whereas inhibition of WNT/β-catenin signaling is important for AT2-to-AT1 differentiation [18,19], although others report that activation is needed for this transition [20]. This illustrates not only that we are only beginning to understand alveolar development and repair but also the challenges and opportunities associated with pharmacological approaches based on this knowledge because the same pathway may regulate self-renewal and differentiation in different ways. Nevertheless, restoring disturbed WNT/β-catenin signaling in mouse emphysema models using blocking of inhibitory LTβR signaling [21] or direct activation of WNT/β-catenin signaling [22] was found to restore alveolar tissue, whereas inhibition of WNT/β-catenin signaling may exert antifibrotic effects in IPF [23,24].
Impact of lung tissue remodeling on repair
Lung epithelial cell function in lung repair and regeneration relies on essential cues from the surrounding niche. In lung diseases, profibrotic and proinflammatory microenvironmental cues, including those resulting from altered mesenchymal cell function, may contribute to dysregulated repair and lung tissue remodeling. Fibroblast activation by transforming growth factor (TGF)-β, a growth factor with increased expression in, for example, lung tissue from patients with COPD or IPF, was found to result in a loss of the ability of these cells to support lung epithelial cell organoid formation [25]. Furthermore, changes in extracellular matrix composition, as well as mechanical cues resulting from increased stiffness in fibrotic areas or alveolar stretch in areas of tissue destruction or mechanical ventilation, may alter repair and differentiation processes, as illustrated by studies on AT2-to-AT1 differentiation [26,27]. Furthermore, although acute proinflammatory cues may promote AT2-to-AT1 differentiation and thus contribute to alveolar repair, prolonged inflammation may impair AT1 maturation and thus regeneration of alveoli [28].
Therapeutic approaches to achieve lung repair and regeneration
A better understanding of mechanisms involved in the respiratory developmental and repair pathways, the stem/progenitor cells and growth factors and other signaling molecules involved, provides clues for intervention strategies. Such interventions have been successfully evaluated in a variety of animal studies [29], but only few of these have reached the stage of clinical evaluation. Table 2 provides an overview of clinical studies using pharmacological or cell therapy approaches that have aimed at regeneration or repair of lung tissue and are discussed in the following.
Table 2.
Clinical studies on regenerative therapies in human lung disease.
| Indication/therapeutic area | Registration nr. and design | Treatment/dosing/timing | Outcome | References |
|---|---|---|---|---|
| LPS-induced human model of lung injury (n = 36) | ISRCTN 98813895 Randomized, double-blind, placebo-controlled trial |
KGF (palifermin) or placebo 60 μg/kg/day (i.v.) 3 days |
Increased Sp-D, increased alveolar IL-1Ra, MMP-9, GM-CSF (pro-repair/pro-resolving effect) | [30] |
| ARDS (n = 29) | ISRCTN95690673 Randomized, double-blind, placebo-controlled trial Phase 2 |
KGF (palifermin) or placebo 60 μg/kg (i.v.) 6 days |
No/worsening effect; fewer ventilator-free first 28 days; higher mortality at day 28 | [31] |
| AATD-emphysema (n = 262) | Randomized, double-blind, placebo-controlled trial Phase 2 |
RARγ-agonist (palovarotene-retinoid) 5 mg/day (oral) 12 months |
No effect | [52] |
| COPD-emphysema (n = 148) | Randomized, double-blind, placebo-controlled trial Phase 2 |
All-trans retinoic acid (ATRA), 13-cis retinoic acid (13-cRA:) or placebo ATRA: 1 mg/kg/d or 2 mg/kg/d (oral) 13-cRA: 1 mg/kg/d (oral) 6 months followed by a 3-month crossover period |
No effect | [53] |
| COPD (n = 6) | Randomized, double-blind, placebo-controlled trial Pilot trial |
FGF-2 2.5 ng by inhalation 3 times a day during two weeks |
Safe and well-tolerated No significant improvements in respiratory symptoms and lung function |
[54] |
| Mild/severe COVID-19 (n = 18) |
NCT04288102 Non-randomized, controlled trial Phase 1 |
UC-MSC 3 × 107 cells/infusion (i.v) Day: 0, 3, and 6 |
Safe and well-tolerated | [55] |
| Advanced COPD-emphysema (n = 4) |
NCT01110252 Single-arm trial Phase 1 |
Mononuclear cells (BMMC) 1x i.v. BMMS (autologous) |
Safe and well-tolerated | [56] |
| Moderate to severe COPD-emphysema (n = 62) |
NCT00683722 Randomized, double-blind, placebo-controlled trial Phase 2 |
BM-MSC 4x 100 × 106 cells/infusion (i.v.; allogeneic) Monthly |
Safe and well-tolerated, trend toward lower CRP | [57] |
| Severe COPD-emphysema (n = 8) |
NCT01306513 Single-arm trial Phase 1 |
BM-MSC 2x 1–2 × 106 BM-MSCs/kg (i.v.; autologous) 1 week apart |
Safe and well-tolerated; increase in alveolar septal CD31 | [58] |
| Severe COPD-emphysema (n = 10) |
NCT01872624 Randomized, patient-blinded, placebo-controlled trial Phase 1 |
BM-MSC 1x 108 cells (i.v.; allogeneic) Combined with EBV insertion |
Safe and well-tolerated, increased QoL, decreased CRP, decreased BODE and MMRC. | [59] |
| Mild-to-very severe stable COPD (n = 9) | ANZCTR12614000731695 Single-armed trial Phase I |
BM-MSC 2x 106 cells/kg (i.v.; allogeneic) 1 week apart 111indium-labeled MSC |
Safe and well-tolerated; Reduced MSC uptake in emphysema lungs, decreased systemic inflammation | [60] |
| COPD (n = 5) | Open-label, Single-armed trial Phase (1/2) |
UC-MSC 4x 1‒2x106BM-MSCs/kg (i.v.) |
Safe and well-tolerated; no significant effects | [61] |
| Moderate-to-severe COPD (n = 20) | ISRCTN70443938 Placebo-controlled trial Phase 1/2 |
UC-MSC 1x 106 cells/kg (i.v.) |
Safe and well-tolerated; decreased MMRC and CAT score, lower number of exacerbations | [62] |
| Moderately severe IPF (n = 8) |
NCT01385644 Single-arm Phase 1b |
PL-MSC 2.5 × 106 cells/mL (i.v.) |
Safe and well-tolerated | [63] |
| Moderate and progressive IPF (n = 16) | Single-arm Phase 1 |
Alveolar type II cells 4x 1‒1.2 × 109 cells bronchoscopic instillation 15 days apart |
Safe and well-tolerated | [64] |
| Non-CF bronchiectasis (n = 2) |
NCT02722642 Single-arm Pilot |
SOX9+ basal cells 106/kg body weight bronchoscopic instillation |
Recruitment ongoing (n = 20 estimated) | [65] |
BMMC: bone marrow mononuclear cell; BM-MSC: bone marrow‒derived MSC; PL-MSC: placenta-derived MSC; RARγ: γ-type retinoic acid receptor; UC-MSC: umbilical cord‒derived MSC.
Regenerative pharmacology
Insight into the important role of mesenchymal cell–derived fibroblast growth factors in lung development and repair [8] has resulted in clinical studies. Based on a variety of model studies, including a human lipopolysaccharide challenge model of acute lung injury that showed favorable effects [30], the effect of intravenous FGF7 (formerly named keratinocyte growth factor) was evaluated in patients with ARDS [31]. However, there was no clinical benefit or a change in physiological parameters, and study results even suggested that patients on active treatment did worse than those on placebo. Retinoids and retinoic acid receptor agonists are another class of drugs that have been evaluated in clinical studies aimed at lung repair. These studies were a logical follow-up from the observation that retinoic acid is able to restore alveolar tissue after elastase-induced emphysema in rats [32]. Although these initial observations were confirmed in various—but not all—studies in rodents, it proved difficult to translate these preclinical findings to effective treatment in patients with meaningful outcomes (see Table 2 for details). A third category of drugs that are now close to evaluation for their capacity to stimulate repair and regeneration of lung tissue in clinical trials are those that target senescence. The realization that senescence impairs repair and that lung function declines with aging has led to the discovery that some lung diseases display features of accelerated aging, as demonstrated, for example, in COPD [33] and IPF [34,35]. Accelerated aging is accompanied by increases in senescent cells that have a decreased repair capacity and secrete proinflammatory mediators. These observations have prompted researchers to investigate the effects of senotherapy (using senolytics and senostatics) in animal models of these diseases [33,34], but so far, this has not yet resulted in published clinical studies although some of the drugs used in animal models have been approved for human use for other clinical indications.
Cell therapy
In addition to pharmacotherapy, cell therapy holds promise for pulmonary regenerative medicine. This has been demonstrated in various animal models of lung injury that have been reviewed elsewhere [5,36]. So far, cell therapy related to lung regeneration in patient studies has focused on mesenchymal stromal cells (MSCs) [36], although also other cell types have been evaluated including alveolar epithelial cells (Table 2). MSCs are cells of nonhematopoietic origin that have the capacity to differentiate into multiple mesenchymal cell lineages, that is, chondrocytes, osteoblasts, and adipocytes. Cell culture, animal studies, and clinical studies in graft-versus-host disease and inflammatory bowel disease have shown that MSCs may modify immune and inflammatory responses and possibly increase repair and regeneration. MSCs may help create a more favorable microenvironment for lung repair to occur through their immunomodulatory activity, or they may enhance repair by secreting mediators that enhance repair. MSCs have been used in clinical studies for a variety of indications, including COPD, ARDS, IPF, and COVID-19 (Table 2), but so far, clinical efficacy of MSC treatment for lung diseases remains to be demonstrated.
This lack of a clinical benefit of MSC treatment for lung diseases may be explained by the fact that they enter inflamed lung tissue. Lung inflammation has indeed been found to modify MSC behavior, as demonstrated in a recent study in which human bone marrow–derived MSCs were cultured with bronchoalveolar lavage (BAL) fluid from patients with ARDS or patients with lung diseases other than ARDS (non-ARDS). Incubation of MSCs with BAL from non-ARDS resulted in a different gene expression pattern in MSCs and in a stronger anti-inflammatory phenotype in monocytes exposed to bone marrow–derived MSC conditioned medium than using ARDS BAL [37]. Evidence was provided that especially IL-6 in conditioned medium from ARDS BAL–exposed MSCs contributed to this effect. The authors stressed that also other factors of the (diseased) lung environment may affect MSC behavior, including oxygen levels, tissue stiffness, and mechanical forces such as stretch.
In addition to using, for example, pharmacotherapy to reduce lung inflammation at the time of MSC infusion, there are several other options to improve treatment outcomes of MSC administration. First, the route of administration needs to be considered. Clinical studies have used intravenous administration, whereas local delivery into the lung has been widely used in animal models and only in few human studies (Table 2). Second, conditioning of MSCs by treatment with selected mediators or gene editing may enhance regenerative properties of MSCs [36] and can be considered in clinical studies.
Conclusions and future directions
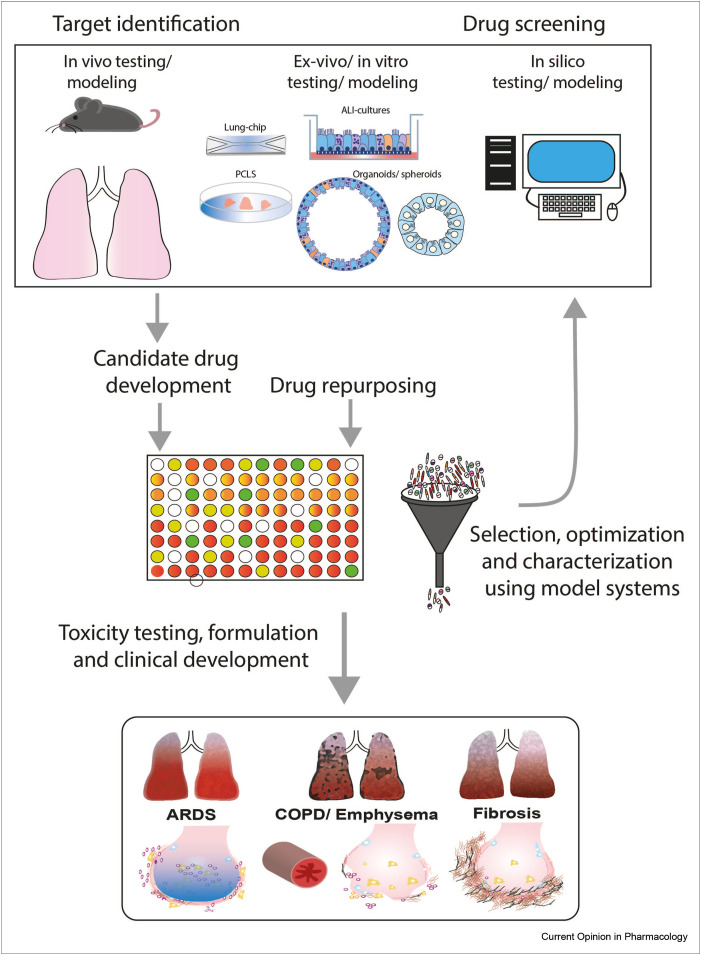
Although a multitude of pharmacological and cell therapy approaches have been evaluated in animal models (reviewed in several recent reviews; [8,29,38]), the number of regenerative approaches evaluated in clinical trials for acute or chronic lung disease has so far been limited. Clearly, there is difficulty in translating outcomes from animal models to the human situation, and there are ethical concerns regarding the use of animals in research. This has important consequences for drug discovery, which previously largely relied on animal models and observations in human lung tissue. Rapid developments in in silico approaches and culture methods, such as organoids, lung-on-chip, and precision-cut lung slices (PCLSs), have markedly contributed to the tool box for regenerative drug development (Figure 1 ). PCLSs provide an ex vivo tool to mimic and study mechanisms operational in pulmonary diseases and provide a platform to screen (novel) regenerative medicine approaches. PCLSs enable studying the various cell types present in the lung in their inherent surrounding, while maintaining tissue structure and integrity, as reviewed in [39]. However, the contribution of, for example, circulating factors and exposure to air cannot be included in PCLS models, but this limitation is shared with conventional cell culture models. Furthermore, so far, PCLSs are mainly used in short-term studies because prolonged culturing of these ex vivo tissue slices proved to be difficult. However, recent studies have shown that improvements such as use of customized hydrogels may support extended culture duration [40]. The advantage of using the PCLS derived from patient tissue for studying lung regeneration is illustrated by a study showing that activation of WNT/β-catenin signaling in the PCLS from patients with COPD resulted in an increase in markers of alveolar repair [41]. In addition, in cell culture models, inclusion of patient-derived cells may increase their relevance. Depending on availability, such cells can be obtained from patients, but (human) induced pluripotent stem cells have also shown potential for the development of patient-specific and disease-relevant in vitro models, as illustrated for Herman-Pudlak syndrome type 2, associated with pulmonary fibrosis [42]. As shown in Figure 1, these complementary approaches contribute to target discovery and development of new drug candidates, and culture methods can be adapted for use in (semi) high-throughput screening approaches using, for example, WNT/β-catenin reporters or, for instance, functional assays for which organoids may be suitable. Such screening approaches are also particularly worthwhile for drug repurposing. Candidate drugs selected using such screens can be further analyzed and validated using the abovementioned in vivo, in vitro, and in silico approaches. More advanced culture models, such as lung-on-chip models in which mechanical forces of breathing (airflow and stretch) [43,44] or culture of, for example, alveolar cells on curved membranes to mimic the morphology of the alveolus [45], have the potential to better mimic the lung environment and may help reducing the need for animal models.
Figure 1.
Approaches to drug discovery in pulmonary regenerative medicine. In vitro, in vivo, exvivo, and in silico modeling of respiratory lung diseases may be used for target identification as well as serve as the model to test drug efficacy. Thus, these complementary approaches contribute to development of new drug candidates, and these can be used in (semi) high-throughput screening approaches (depending on the technique). Such screening approaches are also particularly worthwhile for drug repurposing. Candidate drugs selected using such screens can be further analyzed and validated using the abovementioned in vivo, in vitro, and in silico approaches and lead to regenerative pulmonary medicine for a variety of lung diseases.
These new approaches may help to improve the selection of candidate drugs for human studies. Here, there is also a need for better outcome markers. Especially in COPD, relevant clinical outcomes often require treatment for years to observe, for example, a slowdown of the progressive decline in lung function. This illustrates the need for surrogate (bio)markers of repair or a focus on the exacerbation of disease as a window of accelerated lung function loss and intensified disease burden. Analysis techniques such as proteomics, metabolomics, single-cell/nucleus RNA sequencing, mass cytometry, and advanced imaging techniques also hold great promise for target discovery and biomarker identification.
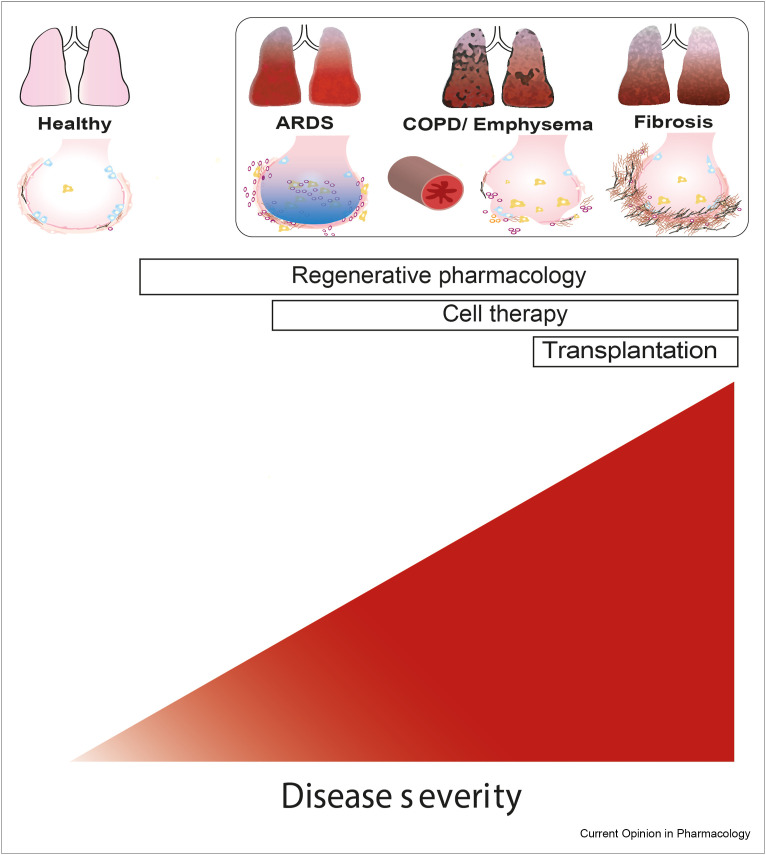
The type of regenerative treatment may depend on the stage of the disease, with pharmacological interventions being feasible at earlier disease stages than cell therapy (Figure 2 ). Timing and duration of treatment may be crucial because some compounds have differential effects on differentiation and proliferation, as shown for retinoic acid [46]. The same holds true for anti-inflammatory treatment that may preferentially be used before starting a repair-inducing treatment. Combined treatments indeed have the potential to enhance the efficacy of therapies. Cell therapy with MSCs or lung progenitors, either derived from induced pluripotent stem cells or from progenitor populations isolated from tissue, may be more effective when combined with pharmacological approaches aimed for instance at improving the microenvironment or niche in which these cells are supposed to act. Furthermore, clearly, the development of additional lung injury should be slowed down or stopped when applying regenerative treatment. In emphysema, alveolar destruction needs to be halted, and repair and regeneration need to be stimulated. Smoking cessation is the most effective intervention to slow down lung function decline in COPD and restrict alveolar destruction, but inflammation does persist for at least some time after cessation [47]. Interestingly, some interventions may have the opportunity to inhibit ongoing destruction while also enhancing repair. This is, for instance, not only likely for anti-inflammatory treatments but also for those targeting mitochondrial dysfunction because this may both trigger inflammation and restrict repair [48]. In lung fibrosis, there is an urgent need to block ongoing fibrosis, and new classes of antifibrotics have shown promise in slowing down progression in, for example, patients with IPF. Combining such an antifibrotic approach with treatment that helps restoring or repairing damaged lung tissue may be most suitable for IPF and other interstitial lung diseases. The need to consider different treatment approaches and combinations for pulmonary regenerative medicine is illustrated in Figure 2.
Figure 2.
Regenerative approaches for lung diseases and the role of disease state. Lung diseases present with heterogenous characteristics, which emphasizes the need to consider different treatment approaches and combinations for pulmonary regenerative medicine. In addition, the choice for a therapy may be determined by the stage of the disease. ARDS, COPD, and fibrosis are presented as examples of diseases that may benefit from a regenerative therapy approach. See text for further details.
CRediT author contribution statement
Padmini Khedoe: Writing – original draft, Writing – review & editing. Xinhui Wu: Writing – review & editing, Reinoud Gosens: Conceptualization, Writing – review & editing. Pieter Hiemstra: Conceptualization, Writing – original draft, Writing – review & editing.
Conflict of interest statement
R.G. received grants from Aquilo BV, Boehringer Ingelheim, Novartis, and Sanofi-Genzyme outside the submitted work; P.S.H. received grants from Boehringer Ingelheim and Galapagos outside the submitted work.
Acknowledgements
The studies in the laboratories of the authors on pulmonary regenerative medicine are supported by grants from the Lung Foundation Netherlands (grant #4.1.19.021) and The Netherlands Research Council—TAS-ZonMW (grant #40-41400-98-16007).
This review comes from a themed issue on Pulmonary (2021)
Edited by Paola Rogliani, Mario Cazzaola and Luigino Calzetta
References
- 1.George P.M., Wells A.U., Jenkins R.G. Pulmonary fibrosis and COVID-19: the potential role for antifibrotic therapy. Lancet Respir Med. 2020;8:807–815. doi: 10.1016/S2213-2600(20)30225-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Butler J.P., Loring S.H., Patz S., Tsuda A., Yablonskiy D.A., Mentzer S.J. Evidence for adult lung growth in humans. N Engl J Med. 2012;367:244–247. doi: 10.1056/NEJMoa1203983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Melo-Narváez M.C., Stegmayr J., Wagner D.E., Lehmann M. Lung regeneration: implications of the diseased niche and ageing. Eur Respir Rev. 2020;29:200222. doi: 10.1183/16000617.0222-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Santis M.M., Bölükbas D.A., Lindstedt S., Wagner Darcy E. How to build a lung: latest advances and emerging themes in lung bioengineering. Eur Respir J. 2018;52:1601355. doi: 10.1183/13993003.01355-2016. [DOI] [PubMed] [Google Scholar]; Comprehensive overview of different approaches to bioenigneering of lung tissue.
- Basil M.C., Katzen J., Engler A.E., Guo M., Herriges M.J., Kathiriya J.J., et al. The cellular and physiological basis for lung repair and regeneration: past, present, and future. Cell Stem Cell. 2020;26:482–502. doi: 10.1016/j.stem.2020.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]; Comprehensive overview and introduction into the basis for lung regenerative medicine by key opinion leaders united in the NIH/NHLBI-supported progenitor cell translational consortium.
- 6.Bonniaud P., Fabre A., Frossard N., Guignabert C., Inman M., Kuebler W.M., et al. Optimising experimental research in respiratory diseases: an ERS statement. Eur Respir J. 2018;51 doi: 10.1183/13993003.02133-2017. [DOI] [PubMed] [Google Scholar]
- 7.Alysandratos K.D., Herriges M.J., Kotton D.N. Epithelial stem and progenitor cells in lung repair and regeneration. Annu Rev Physiol. 2020;83:529–550. doi: 10.1146/annurev-physiol-041520-092904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chu X., Chen C., Chen C., Zhang J.S., Bellusci S., Li X. Evidence for lung repair and regeneration in humans: key stem cells and therapeutic functions of fibroblast growth factors. Front Med. 2020;14:262–272. doi: 10.1007/s11684-019-0717-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Evans K.V., Lee J.H. Alveolar wars: the rise of in vitro models to understand human lung alveolar maintenance, regeneration, and disease. Stem Cells Transl Med. 2020;9:867–881. doi: 10.1002/sctm.19-0433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Whitsett J.A., Kalin T.V., Xu Y., Kalinichenko V.V. Building and regenerating the lung cell by cell. Physiol Rev. 2019;99:513–554. doi: 10.1152/physrev.00001.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Montoro D.T., Haber A.L., Biton M., Vinarsky V., Lin B., Birket S.E., et al. A revised airway epithelial hierarchy includes CFTR-expressing ionocytes. Nature. 2018;560:319–324. doi: 10.1038/s41586-018-0393-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Plasschaert L.W., Zilionis R., Choo-Wing R., Savova V., Knehr J., Roma G., et al. A single-cell atlas of the airway epithelium reveals the CFTR-rich pulmonary ionocyte. Nature. 2018;560:377–381. doi: 10.1038/s41586-018-0394-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Watson J.K., Rulands S., Wilkinson A.C., Wuidart A., Ousset M., Van Keymeulen A., et al. Clonal dynamics reveal two distinct populations of basal cells in slow-turnover airway epithelium. Cell Rep. 2015;12:90–101. doi: 10.1016/j.celrep.2015.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao W., Wang S., Duleba M., Niroula S., Goller K., Xie J., et al. Regenerative metaplastic clones in COPD lung drive inflammation and fibrosis. Cell. 2020;181:848–864 e18. doi: 10.1016/j.cell.2020.03.047. [DOI] [PMC free article] [PubMed] [Google Scholar]; Excellent study using clonal analysis of airway basal progenitors from lungs of COPD patients to demonstrate heterogeneity in the basal cell population and how this may underlie pathological features.
- 15.Rawlins E.L., Okubo T., Xue Y., Brass D.M., Auten R.L., Hasegawa H., et al. The role of Scgb1a1+ Clara cells in the long-term maintenance and repair of lung airway, but not alveolar, epithelium. Cell Stem Cell. 2009;4:525–534. doi: 10.1016/j.stem.2009.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tata P.R., Mou H., Pardo-Saganta A., Zhao R., Prabhu M., Law B.M., et al. Dedifferentiation of committed epithelial cells into stem cells in vivo. Nature. 2013;503:218–223. doi: 10.1038/nature12777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barkauskas C.E., Cronce M.J., Rackley C.R., Bowie E.J., Keene D.R., Stripp B.R., et al. Type 2 alveolar cells are stem cells in adult lung. J Clin Invest. 2013;123:3025–3036. doi: 10.1172/JCI68782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zacharias W.J., Frank D.B., Zepp J.A., Morley M.P., Alkhaleel F.A., Kong J., et al. Regeneration of the lung alveolus by an evolutionarily conserved epithelial progenitor. Nature. 2018;555:251–255. doi: 10.1038/nature25786. [DOI] [PMC free article] [PubMed] [Google Scholar]; Landmark study demonstrating the presence of a Wnt-responsive progenitor lineage within the alveolar type 2 population in mouse lung that is able to rapdily expand after injury but is quiscent in homeostasis. Demonstration of similar population in human alveolus marked by surface expression of TM4SF1.
- 19.Frank D.B., Peng T., Zepp J.A., Snitow M., Vincent T.L., Penkala I.J., et al. Emergence of a wave of Wnt signaling that regulates lung alveologenesis by controlling epithelial self-renewal and differentiation. Cell Rep. 2016;17:2312–2325. doi: 10.1016/j.celrep.2016.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Abdelwahab E.M.M., Rapp J., Feller D., Csongei V., Pal S., Bartis D., et al. Wnt signaling regulates trans-differentiation of stem cell like type 2 alveolar epithelial cells to type 1 epithelial cells. Respir Res. 2019;20:204. doi: 10.1186/s12931-019-1176-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conlon T.M., John-Schuster G., Heide D., Pfister D., Lehmann M., Hu Y., et al. Inhibition of LTβR signalling activates WNT-induced regeneration in lung. Nature. 2020;588:151–156. doi: 10.1038/s41586-020-2882-8. [DOI] [PMC free article] [PubMed] [Google Scholar]; Important study showing how the same triggers that mediate smoke-induced formation of tertiary lymphoid structures in mouse lung impair alveolar repair, which can be reversed by blocking LTbR signaling in mouse and human model systems.
- 22.Kneidinger N., Yildirim A.O., Callegari J., Takenaka S., Stein M.M., Dumitrascu R., et al. Activation of the WNT/beta-catenin pathway attenuates experimental emphysema. Am J Respir Crit Care Med. 2011;183:723–733. doi: 10.1164/rccm.200910-1560OC. [DOI] [PubMed] [Google Scholar]
- 23.Henderson W.R., Jr., Chi E.Y., Ye X., Nguyen C., Tien Y.T., Zhou B., et al. Inhibition of Wnt/beta-catenin/CREB binding protein (CBP) signaling reverses pulmonary fibrosis. Proc Natl Acad Sci U S A. 2010;107:14309–14314. doi: 10.1073/pnas.1001520107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lehmann M., Hu Q., Hu Y., Hafner K., Costa R., van den Berg A., et al. Chronic WNT/β-catenin signaling induces cellular senescence in lung epithelial cells. Cell Signal. 2020;70:109588. doi: 10.1016/j.cellsig.2020.109588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ng-Blichfeldt J.P., de Jong T., Kortekaas R.K., Wu X., Lindner M., Guryev V., et al. TGF-beta activation impairs fibroblast ability to support adult lung epithelial progenitor cell organoid formation. Am J Physiol Lung Cell Mol Physiol. 2019;317:L14–L28. doi: 10.1152/ajplung.00400.2018. [DOI] [PubMed] [Google Scholar]
- 26.Wu H., Yu Y., Huang H., Hu Y., Fu S., Wang Z., et al. Progressive pulmonary fibrosis is caused by elevated mechanical tension on alveolar stem cells. Cell. 2020;180:107–121. doi: 10.1016/j.cell.2019.11.027. e17. [DOI] [PubMed] [Google Scholar]
- 27.Li J., Wang Z., Chu Q., Jiang K., Li J., Tang N. The strength of mechanical forces determines the differentiation of alveolar epithelial cells. Dev Cell. 2018;44:297–312 e5. doi: 10.1016/j.devcel.2018.01.008. [DOI] [PubMed] [Google Scholar]
- 28.Choi J., Park J.E., Tsagkogeorga G., Yanagita M., Koo B.K., Han N., et al. Inflammatory signals induce AT2 cell-derived damage-associated transient progenitors that mediate alveolar regeneration. Cell Stem Cell. 2020;27:366–382. doi: 10.1016/j.stem.2020.06.020. e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ng-Blichfeldt J.P., Gosens R., Dean C., Griffiths M., Hind M. Regenerative pharmacology for COPD: breathing new life into old lungs. Thorax. 2019;74:890–897. doi: 10.1136/thoraxjnl-2018-212630. [DOI] [PubMed] [Google Scholar]
- 30.Shyamsundar M., McAuley D.F., Ingram R.J., Gibson D.S., O'Kane D., McKeown S.T., et al. Keratinocyte growth factor promotes epithelial survival and resolution in a human model of lung injury. Am J Respir Crit Care Med. 2014;189:1520–1529. doi: 10.1164/rccm.201310-1892OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McAuley D.F., Cross L.M., Hamid U., Gardner E., Elborn J.S., Cullen K.M., et al. Keratinocyte growth factor for the treatment of the acute respiratory distress syndrome (KARE): a randomised, double-blind, placebo-controlled phase 2 trial. Lancet Respir Med. 2017;5:484–491. doi: 10.1016/S2213-2600(17)30171-6. [DOI] [PubMed] [Google Scholar]
- 32.Massaro G.D., Massaro D. Retinoic acid treatment abrogates elastase-induced pulmonary emphysema in rats [see comments] [published erratum appears in Nat Med 1997 Jul;3(7):805] Nat Med. 1997;3:675–677. doi: 10.1038/nm0697-675. [DOI] [PubMed] [Google Scholar]
- 33.Baker J.R., Donnelly L.E., Barnes P.J. Senotherapy: a new horizon for COPD therapy. Chest. 2020;158:562–570. doi: 10.1016/j.chest.2020.01.027. [DOI] [PubMed] [Google Scholar]
- 34.Merkt W., Bueno M., Mora A.L., Lagares D. Senotherapeutics: targeting senescence in idiopathic pulmonary fibrosis. Semin Cell Dev Biol. 2020;101:104–110. doi: 10.1016/j.semcdb.2019.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yao C., Guan X., Carraro G., Parimon T., Liu X., Huang G., et al. Senescence of alveolar type 2 cells drives progressive pulmonary fibrosis. Am J Respir Crit Care Med. 2021;12:65. doi: 10.1164/rccm.202004-1274OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Behnke J., Kremer S., Shahzad T., Chao C.M., Böttcher-Friebertshäuser E., Morty R.E., et al. MSC based therapies-new perspectives for the injured lung. J Clin Med. 2020;9:682. doi: 10.3390/jcm9030682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Abreu S.C., Rolandsson Enes S., Dearborn J., Goodwin M., Coffey A., Borg Z.D., et al. Lung inflammatory environments differentially alter mesenchymal stromal cell behavior. Am J Physiol Lung Cell Mol Physiol. 2019;317 doi: 10.1152/ajplung.00263.2019. L823-l31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Broekman W., Khedoe P., Schepers K., Roelofs H., Stolk J., Hiemstra P.S. Mesenchymal stromal cells: a novel therapy for the treatment of chronic obstructive pulmonary disease? Thorax. 2018;73:565–574. doi: 10.1136/thoraxjnl-2017-210672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Alsafadi H.N., Uhl F.E., Pineda R.H., Bailey K.E., Rojas M., Wagner D.E., et al. Applications and approaches for three-dimensional precision-cut lung slices. Disease modeling and drug discovery. Am J Respir Cell Mol Biol. 2020;62:681–691. doi: 10.1165/rcmb.2019-0276TR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bailey K.E., Pino C., Lennon M.L., Lyons A., Jacot J.G., Lammers S.R., et al. Embedding of precision-cut lung slices in engineered hydrogel biomaterials supports extended ex vivo culture. Am J Respir Cell Mol Biol. 2020;62:14–22. doi: 10.1165/rcmb.2019-0232MA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Uhl F.E., Vierkotten S., Wagner D.E., Burgstaller G., Costa R., Koch I., et al. Preclinical validation and imaging of Wnt-induced repair in human 3D lung tissue cultures. Eur Respir J. 2015;46:1150–1166. doi: 10.1183/09031936.00183214. [DOI] [PubMed] [Google Scholar]
- 42.Korogi Y., Gotoh S., Ikeo S., Yamamoto Y., Sone N., Tamai K., et al. In vitro disease modeling of hermansky-pudlak syndrome type 2 using human induced pluripotent stem cell-derived alveolar organoids. Stem Cell Rep. 2019;12:431–440. doi: 10.1016/j.stemcr.2019.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stucki J.D., Hobi N., Galimov A., Stucki A.O., Schneider-Daum N., Lehr C.M., et al. Medium throughput breathing human primary cell alveolus-on-chip model. Sci Rep. 2018;8:14359. doi: 10.1038/s41598-018-32523-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nawroth J.C., Barrile R., Conegliano D., van Riet S., Hiemstra P.S., Villenave R. Stem cell-based Lung-on-Chips: the best of both worlds? Adv Drug Deliv Rev. 2019;140:12–32. doi: 10.1016/j.addr.2018.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Baptista D., Teixeira L.M., Birgani Z.T., van Riet S., Pasman T., Poot A., et al. 3D alveolar in vitro model based on epithelialized biomimetically curved culture membranes. Biomaterials. 2021;266:120436. doi: 10.1016/j.biomaterials.2020.120436. [DOI] [PubMed] [Google Scholar]
- 46.Ng-Blichfeldt J.P., Schrik A., Kortekaas R.K., Noordhoek J.A., Heijink I.H., Hiemstra P.S., et al. Retinoic acid signaling balances adult distal lung epithelial progenitor cell growth and differentiation. EBioMedicine. 2018;36:461–474. doi: 10.1016/j.ebiom.2018.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lapperre T.S., Postma D.S., Gosman M.M., Snoeck-Stroband J.B., ten Hacken N.H., Hiemstra P.S., et al. Relation between duration of smoking cessation and bronchial inflammation in COPD. Thorax. 2006;61:115–121. doi: 10.1136/thx.2005.040519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hoffmann R.F., Jonker M.R., Brandenburg S.M., de Bruin H.G., Ten Hacken N.H.T., van Oosterhout A.J.M., et al. Mitochondrial dysfunction increases pro-inflammatory cytokine production and impairs repair and corticosteroid responsiveness in lung epithelium. Sci Rep. 2019;9:15047. doi: 10.1038/s41598-019-51517-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rock J.R., Onaitis M.W., Rawlins E.L., Lu Y., Clark C.P., Xue Y., et al. Basal cells as stem cells of the mouse trachea and human airway epithelium. Proc Natl Acad Sci U S A. 2009;106:12771–12775. doi: 10.1073/pnas.0906850106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tata A., Kobayashi Y., Chow R.D., Tran J., Desai A., Massri A.J., et al. Myoepithelial cells of submucosal glands can function as reserve stem cells to regenerate airways after injury. Cell Stem Cell. 2018;22:668–683. doi: 10.1016/j.stem.2018.03.018. e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nabhan A.N., Brownfield D.G., Harbury P.B., Krasnow M.A., Desai T.J. Single-cell Wnt signaling niches maintain stemness of alveolar type 2 cells. Science. 2018;359:1118–1123. doi: 10.1126/science.aam6603. [DOI] [PMC free article] [PubMed] [Google Scholar]; Mouse study showing that alveolar type 2 cells reside in a niche harbored by fibroblasts that provide Wnt ligands for AT2 maintenance, whereas AT2-derived Wnt mediates expansion of the AT2 progenitor pool upon injury. Absence of Wnt signaling leads to differentiation to AT1 cells.
- 52.Stolk J., Stockley R.A., Stoel B.C., Cooper B.G., Piitulainen E., Seersholm N., et al. Randomised controlled trial for emphysema with a selective agonist of the gamma-type retinoic acid receptor. Eur Respir J. 2012;40:306–312. doi: 10.1183/09031936.00161911. [DOI] [PubMed] [Google Scholar]
- 53.Roth M.D., Connett J.E., D'Armiento J.M., Foronjy R.F., Friedman P.J., Goldin J.G., et al. Feasibility of retinoids for the treatment of emphysema study. Chest. 2006;130:1334–1345. doi: 10.1378/chest.130.5.1334. [DOI] [PubMed] [Google Scholar]
- 54.Kim Y.S., Hong G., Kim D.H., Kim Y.M., Kim Y.K., Oh Y.M., et al. The role of FGF-2 in smoke-induced emphysema and the therapeutic potential of recombinant FGF-2 in patients with COPD. Exp Mol Med. 2018;50:1–10. doi: 10.1038/s12276-018-0178-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Meng F., Xu R., Wang S., Xu Z., Zhang C., Li Y., et al. Human umbilical cord-derived mesenchymal stem cell therapy in patients with COVID-19: a phase 1 clinical trial. Signal Transduct Target Ther. 2020;5:172. doi: 10.1038/s41392-020-00286-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ribeiro-Paes J.T., Bilaqui A., Greco O.T., Ruiz M.A., Marcelino M.Y., Stessuk T., et al. Unicentric study of cell therapy in chronic obstructive pulmonary disease/pulmonary emphysema. Int J Chron Obstruct Pulmon Dis. 2011;6:63–71. doi: 10.2147/COPD.S15292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Weiss D.J., Casaburi R., Flannery R., LeRoux-Williams M., Tashkin D.P. A placebo-controlled, randomized trial of mesenchymal stem cells in COPD. Chest. 2013;143:1590–1598. doi: 10.1378/chest.12-2094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Stolk J., Broekman W., Mauad T., Zwaginga J.J., Roelofs H., Fibbe W.E., et al. A phase I study for intravenous autologous mesenchymal stromal cell administration to patients with severe emphysema. QJM. 2016;109:331–336. doi: 10.1093/qjmed/hcw001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.de Oliveira H.G., Cruz F.F., Antunes M.A., de Macedo Neto A.V., Oliveira G.A., Svartman F.M., et al. Combined bone marrow-derived mesenchymal stromal cell therapy and one-way endobronchial valve placement in patients with pulmonary emphysema: a phase I clinical trial. Stem Cells Transl Med. 2017;6:962–969. doi: 10.1002/sctm.16-0315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Armitage J., Tan D.B.A., Troedson R., Young P., Lam K.V., Shaw K., et al. Mesenchymal stromal cell infusion modulates systemic immunological responses in stable COPD patients: a phase I pilot study. Eur Respir J. 2018;51 doi: 10.1183/13993003.02369-2017. [DOI] [PubMed] [Google Scholar]
- 61.Karaoz E., Kalemci S., Ece F. Improving effects of mesenchymal stem cells on symptoms of chronic obstructive pulmonary disease. Bratisl Lek Listy. 2020;121:188–191. doi: 10.4149/BLL_2020_028. [DOI] [PubMed] [Google Scholar]
- 62.Le Thi Bich P., Nguyen Thi H., Dang Ngo Chau H., Phan Van T., Do Q., Dong Khac H., et al. Allogeneic umbilical cord-derived mesenchymal stem cell transplantation for treating chronic obstructive pulmonary disease: a pilot clinical study. Stem Cell Res Ther. 2020;11:60. doi: 10.1186/s13287-020-1583-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chambers D.C., Enever D., Ilic N., Sparks L., Whitelaw K., Ayres J., et al. A phase 1b study of placenta-derived mesenchymal stromal cells in patients with idiopathic pulmonary fibrosis. Respirology. 2014;19:1013–1018. doi: 10.1111/resp.12343. [DOI] [PubMed] [Google Scholar]
- 64.Serrano-Mollar A., Gay-Jordi G., Guillamat-Prats R., Closa D., Hernandez-Gonzalez F., Marin P., et al. Safety and tolerability of alveolar type II cell transplantation in idiopathic pulmonary fibrosis. Chest. 2016;150:533–543. doi: 10.1016/j.chest.2016.03.021. [DOI] [PubMed] [Google Scholar]
- 65.Ma Q., Ma Y., Dai X., Ren T., Fu Y., Liu W., et al. Regeneration of functional alveoli by adult human SOX9(+) airway basal cell transplantation. Protein Cell. 2018;9:267–282. doi: 10.1007/s13238-018-0506-y. [DOI] [PMC free article] [PubMed] [Google Scholar]