Abstract
BACKGROUND:
The investigation of autoantibodies which may play a role in the processes of angiogenesis and tumorogenesis is important in the early diagnostis of cancer.
OBJECTIVE:
This study aimed to investigate the levels of autoantibodies to Glu-plasminogen (Pg) in plasma of patients with tumors.
METHODS:
Plasma samples from healthy volunteers were compared with samples from patients with prostate cancer using 2D electrophoresis and MALDI-TOF mass spectrometry. Plasma samples from 25 patients with prostate cancer, 15 patients with benign prostatic hyperplasia (BPH), 29 patients with breast cancer, and 43 healthy volunteers were tested using ELISA to anti-Pg IgG autoantibodies. Affinity chromatography on Pg-sepharoses was used to assess the quantity of anti-Pg IgG in control plasma and plasma of prostate cancer patients. ATTESTAT program was used for nonparametric analysis.
RESULTS:
Using 2D electrophoresis, marker spots below 50 kD were detected in prostate cancer samples. These spots were identified as fragments of Pg and IgG. Using affinity chromatography on Pg-sepharose, the quantity of IgG bound to Pg versus total IgG was determined to be 9% in control and 27% in prostate cancer samples. The frequency of occurence of elevated levels of anti-Pg IgG was 84% in prostate cancer samples, 69% in breast cancer samples, 40% in BPH samples, and 11% in healthy plasma.
CONCLUSIONS:
Autoantibodies to Pg may be involved in tumorogenesis and elevated levels of anti-Pg IgG antibodies may be a risk factor for tumor development.
Keywords: Plasminogen, autoantibodies to Pg, cancer, ELISA, biomarkers
1. Introduction
Early diagnosis of cancer is one of the most important problems facing modern medicine. The detection of cancers at stages I and II can help to start treatment as soon as possible and, in most cases, can lead to full recovery and rehabilitation of the patient. The main problem of early diagnosis is to find biomarkers reflecting the early stages of cancer. Most biomarkers are molecules connected with secretion and decay of tumor cells and none reflect systemic response to tumor growth. It is known that the components of the fibrinolytic system and angiogenic factors play a role in tumor development and progression [4,5]. There is evidence that cancer at the late stages is accompanied by thrombosis, which may be due to an imbalance of the fibrinolytic system [11]. On the other hand, it has been reported that fibrinolytic and angiogenic factors are actively involved in the promotion of “dormant” tumor growth [2,5]. Fibrinolysis is initiated by tissue plasminogen activator (tPA) or urokinase-like plasminogen activator (uPA), which convert Glu-plasminogen (Pg) to plasmin (Pm) in the presence of fibrin. Pm plays a role in the degradation of the endothelial extracellular matrix, which leads to the invasion and dissemination of tumors cells [2,16]. Tumor growth is accompanied by enhanced neovascularization that helps to supply oxygen and nutrients to the tumor cells and to remove metabolic products [5]. Cleavage products of Pg are known to be involved in the process of regulation of angiogenesis [12]. Angiostatin is one of these proteins and inhibits neovascularization [12]. We hypothesized that autoantibodies to Pg may block angiostatin, and lead to tumor growth. Elevated levels of these autoantibodies have been previously reported in patients with rheumatoid arthritis and systemic lupus [6,15]. Meanwhile, there is no data of the level of autoantibodies to Pg in patients with cancer. This study aimed to investigate the levels of anti-Pg autoantibodies in plasma of patients with tumors.
2. Materials and methods
2.1. Patients and control subjects
The samples were obtained from the Moscow Cancer Center. All participants gave informed consent for the use of plasma samples in the experiments. Citrate plasma samples were obtained from 25 patients with prostate cancer stage II–IV, 15 patients with benign prostatic hyperplasia (BPH), and 29 breast cancer patients stage II-IV. The diagnosis was confirmed by histological examination of material obtained by biopsy. The control group included 44 healthy volunteers: 17 women and 26 men (Table 1). Samples were stored at −70°C.
Table 1.
Characteristics of selected populations
| Attribute╲Cohort | Control | BPH | Prostate cancer | Breast cancer |
|---|---|---|---|---|
| Age, years old | 40–67 | 51–65 | 51–69 | 45–69 |
| N | 43 | 15 | 25 | 29 |
| Male | 26 | 15 | 25 | – |
| Female | 17 | – | – | 29 |
| Smoking status | unknown | unknown | unknown | unknown |
| Race Caucasian | 43 | 15 | 25 | 29 |
2.2. Two-dimensional gel electrophoresis
Two-dimensional gel electrophoresis (2DE) was performed using Protean IEF Cell (Bio-Rad, USA). The separation was performed by isoelectric focusing on IPG strips, pH 3–10 (Bio-Rad) and 9–16% gradient gels were used. Gels were stained using silver and Coomassie blue. They were scanned with a resolution of 300 dots/inch. The images were analyzed using Melanie III software (GeneBio, Switzerland). In order to confirm significant differences between groups of spots, we used the statistical Kruskal-Wallis test. Protein spots (~ 3 mm3) were excised from the gel, washed, and treated with trypsin. Mass spectra results were obtained using MALDI-TOF-mass spectrometer Reflex III (Bruker, USA). Lists of peptide peaks were generated using the Xmass program (Bruker, USA). Identification of proteins after trypsin digestion was performed using Mascot Peptide Fingerprint software (Matrix Science, USA). The error in determining the mass of the ions in peptide fragments was approximately 0.03%.
ELISA. Pg was adsorbed onto 96-well Maxibinding plates from SPL (SPL, Korea) as described previously [15] with some modifications. 100 μl of Pg solution was added to the wells at a concentration of 5 μg/ml in 0.1 M sodium carbonate/bicarbonate buffer (pH 9.6), and incubated overnight at 37°C. Unbound sites were blocked overnight with 1% bovine serum albumin (BSA, MR Medical) in phosphate buffer saline (PBS) at room temperature. The blocking solution was removed and plates were dried at room temperature and stored at 4°C. Plasma samples were diluted 100-fold in PBS containing 0.5% BSA, 100 μl of sample was added to each well in duplicates, and the plates were incubated for 1 h at 37°C. The solution was removed and plates were washed 4 times with 200 μl PBS containing 0.05% Tween-20 (PBST). Monoclonal antibodies to human IgG (100 μl) conjugated with peroxidase (Sigma-Aldrich, USA) were added and incubated for 1 h at 37°C. Plates were washed and 100 μl of tetramethylbenzidine solution (Sigma) was added. The reaction was stopped after 25 min using 100 μl of 2 M H2SO4. Absorbance at 450 nm (A450) was recorded using a spectrophotomer (Bio-Rad).
2.3. Purification of anti-Pg IgG
Glu-plasminogen (Pg) was isolated from plasma of healthy individuals by affinity chromatography using Lys-Sepharose 4B (GE Healthcare) [9]. The purity of the preparation was monitored by SDS-PAGE electrophoresis. Glu-plasminogen (20 mg) were immobilized on 1.8 g rehydrated CNBr-activated Sepharose 4B (GE Healthcare) according to the manufacturer’s instructions. Human plasma in volume 5 ml were added to an equal volume of PBS with 0.01M sodium citrate (PBSC), pH 7.4, at 4°C. Ammonium sulfate precipitation was performed to get a yield at 33% of saturated ammonium sulfate. The solution was centrifuged at 5,000 g, the supernatant was discarded, and the pellet was dissolved in 10 ml of PBSC and dialyzed against PBSC. After dialysis, 2 ml of sample were applied to a column with 1 ml of Protein-G sepharose (GE Healthcare), washed with 10 ml PBSC, and eluted with 3 ml of 0.1 M citrate buffer, pH 2.3. The pH of the eluate was adjusted to pH 7.2 with 0.5 M NaOH. The IgG fraction obtained from Protein-G Sepharose was applied to a 2 ml Pg-sepharose column. The application was performed for 18 hours in a closed loop at 4°C. The column was washed with PBS and specific elution was performed with 0.1 M citrate buffer, pH 2.3. The pH of the eluate was then adjusted to pH 7.2 using 0.5 M NaOH. The specific activity of IgG was assessed by ELISA to Pg. Total protein concentration was measured by the Bradford assay. IgG concentration was assessed using an ELISA kit to IgG (“Hema” Co ltd., Russia). The purity of IgG was monitored by SDS-PAGE electrophoresis.
Statistical analysis was performed using ATTESTAT program. Sensitivity (SN), specificity (SP), and area under curve (AUC) were calculated by receiver operating characteristic (ROC) curves.
3. Results
Plasma samples from five healthy donors and six patients with prostate cancer were used for 2DE. Comparison of 2DE gels of samples of prostate cancer plasma and healthy plasma revealed marker spots below 50 kD in the plasma of cancer patients. MALDI-TOF mass spectrometry identified 90% of marker spots as fragments of Pg and IgG (Fig. 1).
Fig. 1.
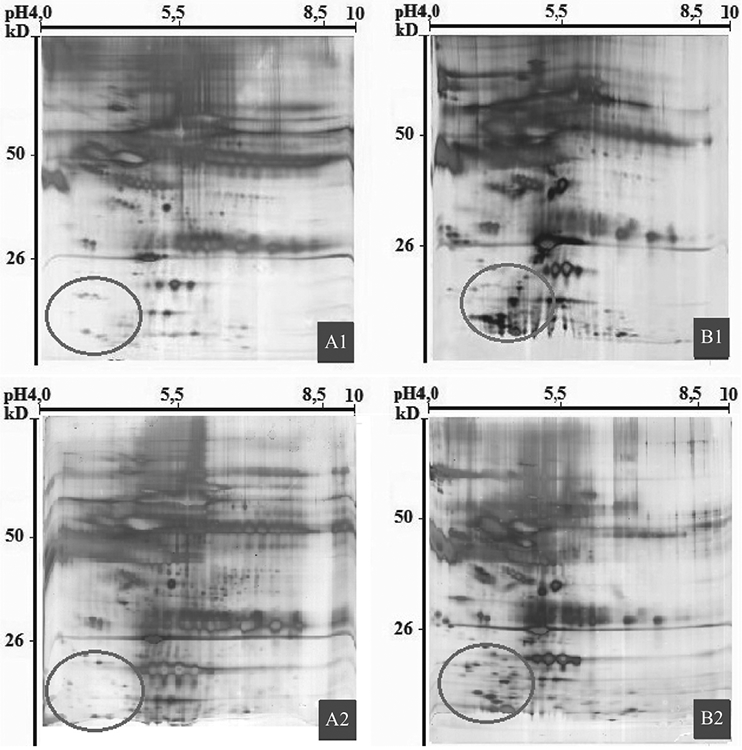
2D electrophoresis of healthy human plasma samples (A1, A2); plasma samples of patients with prostate cancer (B1, B2). Oval area shows marker proteins in cancer patients.
The results of 2DE were a basis to investigate the concentration of anti-Pg IgG in plasma. Five plasma samples of 1 ml each from healthy donors were pooled as well as five plasma samples of patients with prostate cancer. Purification of anti-Pg IgG was performed in 4 independent experiments (Fig. 2, Table 2).
Fig. 2.
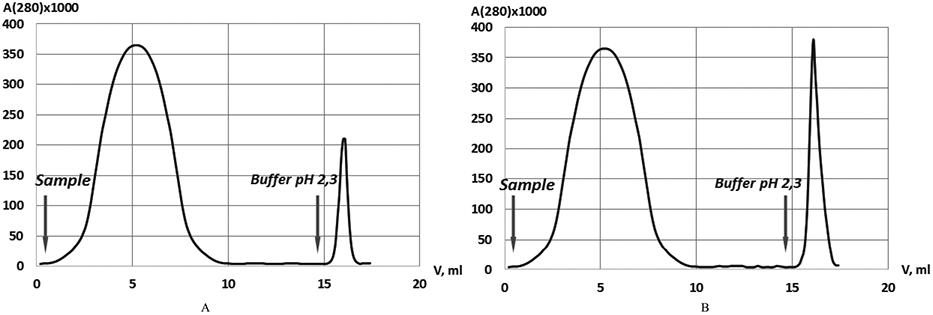
Chromatography using Pg-Sepharose. (A) normal plasma sample; (B) plasma sample of prostate cancer.
Table 2.
Results of purification of anti-Pg IgG at each stage. IgG amount is the mean of four independent experiments with the same plasma pool
|
Stage of purification╲Plasma samples, V = 1 ml |
Intact plasma, total IgG, mg |
Ammonium sulfate precipitation. Total IgG, mg |
Protein-G Sepharose, total IgG, mg |
IgG, bound to Pg- sepharose, mg |
IgG, unbound to Pg- sepharose, mg |
|---|---|---|---|---|---|
| Plasma of healthy donors | 9.0 | 8.1 ± 0.4 | 7.5 ± 0.6 | 0.7 ± 0.2 | 6.0 ± 0.5 |
| Plasma of prostate cancer patients | 12.3 | 11.0 ± 0.6 | 10.3 ± 0.9 | 2.8 ± 0.4 | 6.5 ± 0.6 |
The average percentage of IgG bound to Pg-sepharose in pooled samples from healthy donors was 9% of total IgG, while in the pooled samples of plasma from prostate cancer patients it was 27% (Table 2, Fig. 2). The fraction eluted from Pg-sepharose showed only one immunoglobulin band, IgG, in SDS PAGE. The unbound fraction of IgG had no specific activity to Pg as detected by ELISA.
The observed increased concentrations of anti-Pg IgG in the plasma of prostate cancer patients became the basis for a further screening of samples from cancer patients using ELISA. Samples were considered positive (with elevated levels of anti-Pg IgG) at values higher than the cutoff value calculated using ROC curve analysis of control and case of cancer cohorts. Only 2 out of 17 plasma samples from healthy women (or 12%) showed elevated levels of anti-Pg IgG, as well as only 3 out of 27 plasma samples from healthy men (or 11%). Among 25 plasma samples from men with prostate cancer, 21 (or 84%) had elevated levels of anti-Pg IgG. In men with BHP, 6 out of 15 (or 40%) had elevated levels of anti-Pg IgG. And finally, out of 29 plasma samples from patients with breast cancer, elevated level of anti-Pg IgG were detected in 20 cases (69%) (Figs 3,4).
Fig. 3.
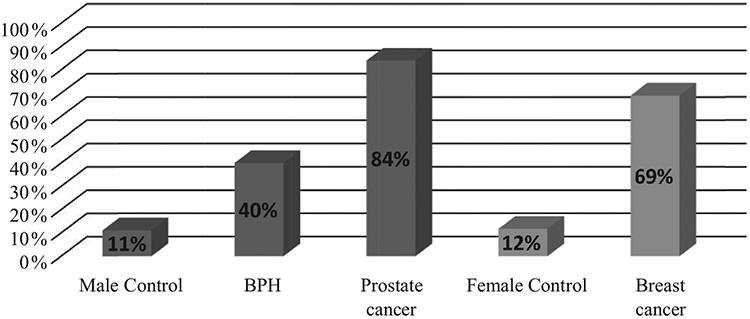
Frequency of occurence of elevated levels of anti-Pg IgG in the cohorts. Cutoff value of A(450) × 1000 for Male Control – 201; BPH – 201; Prostate cancer – 201; Female Control – 187; Breast cancer – 187.
Fig. 4.
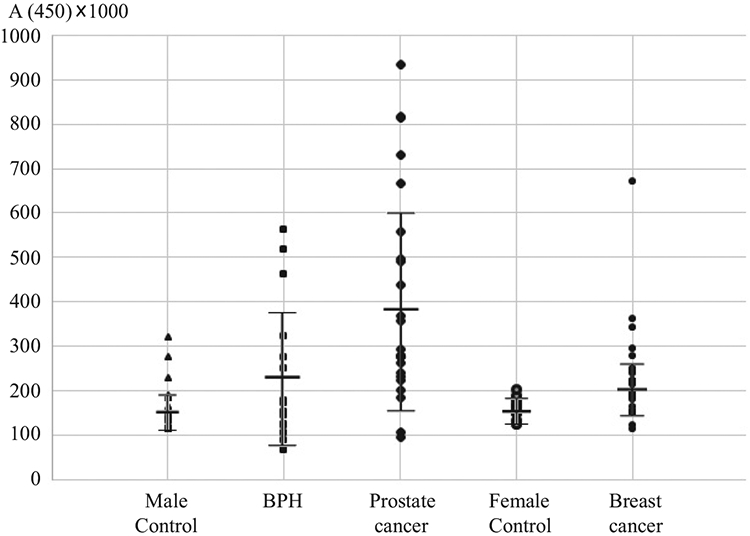
ELISA data of absorbance at 450 nm (A450) in cohorts. Mean ± sd A450 × 1000 of Male Control – 151 ± 51; BHP – 236 ± 161; Prostate cancer – 396 ± 234; Female Control – 187 ± 21; Breast cancer – 200 ± 62.
Using ROC curve analysis, we demonstrate a significant difference in anti-Pg IgG levels between BPH and prostate cancer, with SN/SP/AUC – 92/60/0.73. For breast cancer versus control, the values were SN/SP/AUC – 72/88/0.80 (Fig. 5, Table 3).
Fig. 5.
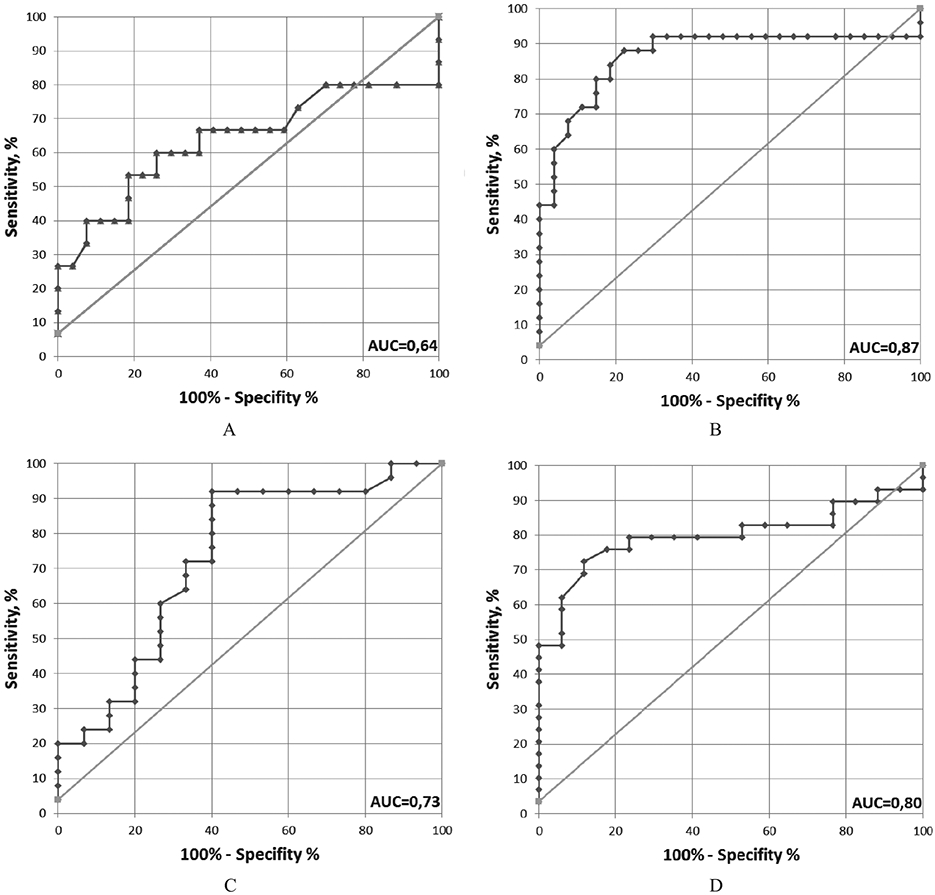
ROC curves. ROC curves reflect differences in level of anti-Pg IgG in Case/Control cohorts. A. BPH/Control Male, B. Prostate cancer/Control Male, C. Prostate cancer/BPH, D. Breast cancer/Control Female.
Table 3.
ROC curve analysis of ELISA data for anti-Pg IgG
| Cancer/Healthy | BPH/Control male |
Prostate cancer/Control male |
Prostate cancer/BPH | Breast cancer/Control female |
|---|---|---|---|---|
| SN/SP/AUC | 53/81/0.64 | 88/78/0.87 | 92/60/0.73 | 72/88/0.80 |
| Cutoff value of A(450) × 1000 | 201 | 201 | 201 | 187 |
4. Discussion
Using chromatography methods, we have shown that the proportion of anti-Pg IgG versus total IgG in the plasma of prostate cancer patients is 27%, compared to 9% in the plasma of healthy subjects. According to the literature, the proportion of anti-Pg IgG ranges from 0.2% to 1% of total IgG in control subjects and up to 1.4% in subjects with autoimmune disease [6,8]. The difference of anti-Pg to total IgG ratio in healthy subjects between our study and previously published data could be explained by a difference in applying conditions and elution of the samples. We have applied a sample for overnight by closed loop procedure whereas other investigators performed this for 2 hours. The elution was performed by 0.1M citrate buffer, pH 2.3, but according to previously published data 0.1 M glycin-HCl buffer pH 2,7 was used. Moreover we have tried to use 0.1 M glycin-HCl buffer pH 2.7, but the yeld of IgG was low (unpublished data).
The presence of such a high proportion of anti-Pg IgG in the plasma of healthy individuals (9% of total IgG), and the significant increase in autoantibody levels in plasma of cancer patients (27% of total IgG) suggests a new mechanism of regulation of homeostasis, especially in the processes of coagulation and angiogenesis. It is possible that anti-Pg IgG normally acts as a buffer system that maintains balance during sudden changes in activity or concentration of components of the fibrinolytic system and angiogenic factors. Anti-Pg IgG could also regulate other components of these systems, as it has been suggested to cross-react with other fibrinolytic and angiogenic factors, such as prothrombin [13], tissue plasminogen activator (tPA), apolipoprotein A (ApoA) and hepatocyte growth factor (HGF) (7, 10, 14). All of these proteins demonstrate a structural homology (1, 3, 10).
Using ELISA, we showed that 84% of prostate cancer samples and 69% of breast cancer samples had high levels of anti-Pg IgG, compared to 40% in non-cancer samples such as BHP. The frequency of occurrence of elevated levels of anti-Pg IgG in healthy controls (11% of healthy males and 12% of healthy females) is similar to data reported in the literature [2]. According to previous reports, elevated levels of anti-Pg IgG were also found in plasma of patients with systemic autoimmune diseases such as rheumatoid arthritis and diseases associated with antiphospholipid syndrome. The frequency of occurrence of positive samples in these cases was up to 30% [6,8]. Roc curve analysis shows significant difference between BPH and prostate cancer groups, with SP/SN/AUC values of 60%/90%/0.73. This data supports the idea that elevated levels of anti-Pg IgG may be a marker of prostate oncogenesis. In samples from breast cancer patients, SP/SN/AUC values were 88%/72/0.80, showing good correlation between elevated levels of anti-Pg IgG and the process of carcinogenesis.
These results are also consistent with a model where anti-Pg autoantibodies play a role in angiogenesis in healthy individuals and in cancer patients. The elevated levels of autoantibodies to Pg can block the action of angiostatin, which in turn can lead to tumor capillarization and growth.
The presence of high levels of anti-Pg IgG may be a risk factor for tumor development. It may help to both identify individuals with this risk as well as to diagnose the type of cancer when used in combination with other type-associated tumor markers. We suggest using different parts of the Pg molecule (i.e. angiostatin) as an antigen, as well as detection of other subclasses and classes of immunoglobulins (IgG1; IgG2; IgG3; IgA; IgM) in ELISA to determine a specific association with specific types of cancer or autoimmune disease. These studies may provide the basis for the differential diagnosis of these diseases.
References
- [1].Bussolino F, Di Renzo MF, Ziche M, Bocchietto E, Olivero M, Naldini L, Gaudino G, Tamagnone L, Coffer A and Comoglio PM, Hepatocyte growth factor is a potent angiogenic factor which stimulates endothelial cell motility and growth, J Cell Biol 119 (1992), 629–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Castellino FJ and Ploplis VA, Structure and function of the plasminogen/plasmin system, Thromb Haemost 93 (2005), 647–654. [DOI] [PubMed] [Google Scholar]
- [3].Donate LE, Gherardi E, Srinivasan N, Sowdhamini R, Aparicio S and Blundell TL, Molecular evolution and domain structure of plasminogen-related growth factors (HGF/SF and HGF1/MSP), Protein Sci 3 (1994), 2378–2394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Falanga A, Marchetti M and Vignoli A, Coagulation and cancer: Biological and clinical aspects, J Thromb Haemost 11 (2012), 223–233. [DOI] [PubMed] [Google Scholar]
- [5].Folkman J, Tumor angiogenesis: therapeutic implications, N Engl J Med 285 (1971), 1182–1186. [DOI] [PubMed] [Google Scholar]
- [6].Gonzalez-Gronow M, Cuchacovich M, Grigg DM and Pizzo SV, Analysis of autoantibodies to plasminogen in the serum of patients with rheumatoid arthritis, J Mol Med (Berl) 74 (1996), 463–469. [DOI] [PubMed] [Google Scholar]
- [7].Ikeo K, Takahashi K and Gojobori TJ, Different evolutionary histories of kringle and protease domains in serine proteases: A typical example of domain evolution, Mol Evol 40 (1995), 331–336. [DOI] [PubMed] [Google Scholar]
- [8].Kozmin LD, Shirokova IE, Lisitsina TA, Popkova TV, Reschetnyak TM, Belenkiy AG, Martynov AI and Bliznukov OP, Anti-plasminogen autoantibodies from plasma of patients with systemic lupus erythematosus having anti-phospholipid antibody syndrome: Isolation and some immunochemical properties, Biochemistry (Mosc) 68 (2003), 339–345. [DOI] [PubMed] [Google Scholar]
- [9].Levashov MY, Aisina RB, Gershkovich KB and Varfolomeyev SD, Mechanism of action of omega-amino acids on plasminogen activation and fibrinolysis induced by staphylokinase, Biochemistry (Mosc) 72 (2007), 707–715. [DOI] [PubMed] [Google Scholar]
- [10].Nakamura T and Mizuno S, The discovery of hepatocyte growth factor (HGF) and its significance for cell biology, life sciences and clinical medicine, Proc Jpn Acad Ser B Phys Biol Sci 86 (2010), 588–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Nash GF, Walsh DC and Kakkar AK, The role of the coagulation system in tumour angiogenesis, Lancet Oncol 2 (2001), 608–13. [DOI] [PubMed] [Google Scholar]
- [12].O’Reilly MS, Holmgren L, Shing Y, Chen C, Rosenthal RA, Moses M, Lane WS, Cao Y, Sage EH and Folkman J, Angiostatin: A novel angiogenesis inhibitor that mediates the suppression of metastases by a Lewis lung carcinoma, J Cell 79 (1994), 315–328. [DOI] [PubMed] [Google Scholar]
- [13].Puurunen M, Mänttäri M, Manninen V, Palosuo T and Vaarala O, Antibodies to prothrombin crossreact with plasminogen in patients developing myocardial infarction, Br J Haematol 100 (1998), 374–379. [DOI] [PubMed] [Google Scholar]
- [14].Rouy D, Koschinsky ML, Fleury V, Chapman J and Anglés-Cano E, Apolipoprotein(a) and plasminogen interactions with fibrin: A study with recombinant apolipoprotein(a) and isolated plasminogen fragments, Biochemistry 31 (1992), 6333–6339. [DOI] [PubMed] [Google Scholar]
- [15].Stefãnescu M, Szegli G, Cremer L, Zarma L, Mazilu E, Naghiu M, Niculescu D, Gache A and Onu A, The presence and significance of some anti-enzyme antibodies (anti-plasminogen, anti-trypsin, anti-phospholipase C) in rheumatoid arthritis (RA) and reactive arthritis (rA), Arch Roum Pathol Exp Microbiol 48 (1989), 47–53. [PubMed] [Google Scholar]
- [16].Tarui T, Majumdar M, Miles LA and Ruf W, Plasmininduced migration of endothelial cells. A potential target for the anti-angiogenic action of angiostatin, J Biol Chem 277 (2002), 33564–33570. [DOI] [PubMed] [Google Scholar]


