Abstract
Purpose
To assess the change in corneal pachymetry after a novel epithelium-on (EpiSmart®) corneal crosslinking procedure (CXL).
Methods
Eyes treated as part of the open-label, non-controlled arm of the study “Collagen Crosslinking with Ultraviolet-A in Asymmetric Corneas” (NCT01097447) were examined at baseline, 3-, 6- and 12-months post-CXL. Thinnest pachymetry readings based on Pentacam (OCULUS GmbH, Wetzlar, Germany) were recorded.
Results
A total of 101 eyes met the study inclusion criteria. Thinnest pachymetric readings at baseline averaged 451 ± 50 microns. The mean (± SD) minimum thickness was 450 ± 46 microns at 3 months, 452 ± 47 microns at 6 months, and 451 ± 48 microns at 12 months post-CXL. The changes from baseline (mean ± SE) at 3, 6, and 12 months post-CXL were −1.2 ± 1.5 microns, 0.5 ± 1.6 microns, and 0.4 ± 1.6 microns, respectively. Student’s t-tests showed no statistically significant change in pachymetry from baseline for any exam period.
Conclusion
This study demonstrated that, after EpiSmart® epithelium-on CXL, there was no substantial corneal thinning observable on Scheimpflug tomography out to 12 months.
Keywords: corneal thinning, cross-linking, epithelium-on, keratoconus, pachymetry, post-surgical ectasia
Introduction
Corneal thinning is a hallmark of keratoconus and other ectatic diseases (pellucid marginal degeneration, keratoglobus, and post-surgical ectasia). Corneal thinning may also be seen in any condition associated with keratocyte apoptosis and/or epithelial injury.1
Corneal thickness is one of the determinants of keratoconus disease severity2,3 and is used in the historical Amsler-Krumeich classification and the newer Belin ABCD keratoconus grading system.4 Progressive thinning, in addition to steepening of the anterior and/or posterior corneal surfaces is recognized as a sign of advancing disease severity.4,5
Corneal thinning is commonly seen in keratoconic eyes after epithelium-off crosslinking and is hypothesized to be due to either a direct effect of corneal crosslinking (CXL), secondary to keratocyte death, epithelial removal, and/or stromal loss secondary to inflammation.1,6,7 Post epithelium-off CXL thinning,8–10 however, can confound the evaluation of the procedure’s safety and efficacy during the healing period. This study assesses changes in corneal pachymetry one year after a novel epithelium-on CXL treatment.
A number of transepithelial crosslinking methods have been tested in an attempt to find a non-invasive alternative to the Dresden (“epi-off”) protocol.11 In general, efficacy has been variable, with benefits in visual acuity but smaller changes in Kmax than observed with epi-off CXL.12,13 More recent efforts have explored different UVA fluence protocols with transepithelial CXL,14 and combined CXL with refractive procedures.15 The EpiSmart® crosslinking treatment (CXL Ophthalmics, Encinitas, CA, USA) has emerged as a promising epi-on protocol providing benefits in visual acuity comparable to epi-off with reduced rates of adverse events.16,17 It was recently evaluated in a Phase 2 study of over 2000 patients, with efficacy similar to epi-off CXL and epithelial defect rate <2%.17
Materials and Methods
This is a retrospective, non-interventional case series in which consecutive eyes of patients who underwent EpiSmart crosslinking for keratoconus or post-surgical ectasia performed at a single location were analyzed. All eyes were treated as part of the open-label, non-controlled arm study under “Collagen Crosslinking with Ultraviolet-A in Asymmetric Corneas” (NCT01097447) sponsored by CXLUSA USA, LLC (Bethesda, MD) under ethics committee approval (IRB Company, Inc., Buena Park, CA) between January 1, 2015 and December 31, 2016. The study was conducted in accordance with the Health Insurance Portability and Accountability Act of 1996 (HIPPA). While the protocol allowed for bilateral simultaneous treatments if both eyes met entrance criteria, the more severely diseased eye (as evaluated by higher maximal corneal curvature (Kmax) and/or worse best spectacle corrected visual acuity (BSCVA)) was chosen for analysis. Baseline clinical data collected included age, self-reported patient sex, BSCVA, Kmax, thinnest pachymetry (TP), and the final “D” value from the Belin/Ambrosio Enhanced Ectasia Display (OCULUS Pentacam, Wetzlar, Germany).18,19 The “D” index has been shown to be a sensitive indicator of keratoconus.18,19 TP is the corneal thickness at its thinnest point. Pentacam-derived TP values were measured at baseline and at post-operative follow-up visits.
Surgical Technique
The epithelium-on treatment protocol has been described in previous publications.16,20,21 Corneal anesthesia was obtained with 2–3 drops of topical proparacaine (0.5%, Alcon Laboratories, Fort Worth TX). At the slit lamp, a proprietary, non-disruptive sponge wand (EpiPrep® CXL Ophthalmics, Encinitas, CA) was used to modify surface lipids in the tear film. The patient was then reclined, and a second proprietary round loading sponge (CXL Ophthalmics, Encinitas CA) was placed on the cornea to serve as a reservoir for the fixed drug combination Riboflavin and sodium iodide (RiboStat®; CXL Ophthalmics, Encinitas, CA).16,20,21
After a 20-minute loading period, a slit lamp exam was performed, and the stromal loading was graded using a previously described and validated color scale to confirm riboflavin loading.20 Once adequate loading was confirmed, the eyes were rinsed with BSS for 30 seconds and the patient was reclined and received UVA application without additional riboflavin drops at 4 mW/cm2 for 30 minutes using a pulsed UVA device capable of simultaneous bilateral treatment (CXL Ophthalmics, Encinitas, CA).16 No bandage contact lenses were applied. Topical steroid and antibiotic drops were used 4 times daily for 3 days and the patient generally returned to their normal activities the next day.
Analysis
Minimum thickness was determined by image analysis using automated algorithms (Oculus software Version 6.09r50). Thickness in microns was recorded for each cornea at baseline (pre-operatively), and at follow-up visits at 3 months, 6 months, and 12 months post-op.
Data were compiled and analyzed in Excel v16.52 (Microsoft, Redmond, Washington). The distribution of baseline values was summarized descriptively. The change from baseline was calculated for each subject at each time point. Distributions of changes from baseline were summarized descriptively and a Shapiro–Wilk test was performed to assess their normality. The statistical significance of mean changes from baseline was assessed with a Student’s t-test. A resulting p < 0.05 was considered significant.
Results
One hundred and one (101) eyes met the selection criteria. There were 25 females and 76 males. Average age was 28 ± 10 years. There were 10 patients treated post-refractive surgery. These patients did not differ significantly from the others either in baseline features or any outcomes meaures.
Patients had an average BSCVA of 20/42 (0.32 ± 0.31 logMAR) (range 20/20 – 20/400). Average Kmax was 58.5 ± 9.2, and the D-index from the Pentacam Belin/Ambrosio Display was 10.2 ± 5.7 (range: 1.9–30.1). The baseline minimum thickness averaged 451 microns ± 50 (range: 277–549). Follow-up measurements were available for 98 subjects at 3 months, 90 at 6 months, and 75 at 12 months post-op. All available data were analyzed. The mean (± SD) minimum thickness was 450 ± 46 microns at 3 months, 452 ± 47 microns at 6 months, and 451 ± 48 microns at 12 months post-op.
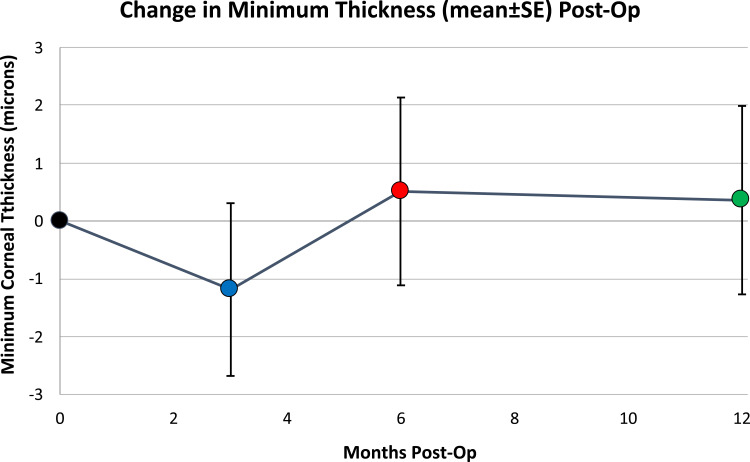
Changes from baseline and associated statistics are found in Table 1. The mean (± standard error) of changes from baseline minimum corneal thickness were −1.2 ± 1.5 microns at 3 months, 0.5 ± 1.6 microns at 6 months, and 0.4 ± 1.6 microns at 12 months post-op (Figure 1). The standard deviations of the distributions of changes were 14.7 microns, 15.4, and 14.1 microns at 3-, 6-, and 12-months post-op, respectively (Figure 2). There was no statistical significance to the average changes from baseline (p > 0.05 in t-tests). The distributions of change from baseline measurements at 6- and 12-months post-op were not significantly different from a normal (Gaussian) distribution, with p-values ≫ 0.05 from the Shapiro–Wilk test. The 3-month values were distributed differently than normal (p < 0.001), noticeable as departures from normality in skewness and kurtosis (Figure 3).
Table 1.
Baseline Pachymetry and Changes from Baseline to Follow-Up Time Points. Means, Minimum, Maximum, Standard Deviation (SD), and Standard Error of the Mean (SE) are Expressed in Microns. The 95% Confidence Interval is Given for the Pachymetry Results Where Distributions Appeared Normal (at Month 6 and Month 12 Post-Op)
| (Microns) | Baseline | Month 3 | Month 6 | Month 12 |
|---|---|---|---|---|
| Count | 101 | 97 | 90 | 75 |
| Mean | 451.4 | −1.19 | 0.51 | 0.36 |
| SD | 49.6 | 14.71 | 15.40 | 14.11 |
| Maximum | 549 | 52 | 45 | 45 |
| Minimum | 277 | −37 | −42 | −36 |
| SE | 1.49 | 1.62 | 1.63 | |
| Change: p-value (t-test vs baseline) | 0.419 | 0.739 | 0.798 | |
| Normality: p-value (Shapiro–Wilk test) | <0.001 | 0.261 | 0.501 | |
| 95% CI (assumes normality) | (−2.7, 3.7) | (−2.8, 3.5) |
Figure 1.
The means of pachymetry changes from baseline do not vary significantly from zero (p > 0.4).
Figure 2.
Histograms of post-operative pachymetry changes are superimposed. Blue, red, and green bars show the distribution of changes from baseline to the 3-, 6-, and 12-month post-op follow up time points.
Figure 3.
Distributions of change from baseline to 3 months (top), 6 months (middle) and 12 months (bottom) post-op. Q-Q plots (at right) for each distribution show quantiles vs that of a Gaussian distribution of best fit. Only the 3-month post-op distribution departs from normalcy. The 6- and 12-month post-op distributions appear approximately normal.
Discussion
Progressive corneal thinning is commonly associated with progressive keratoconus and post refractive surgery-related corneal ectasia. It is thus useful to study pachymetry as a potentially important metric in ectatic corneal disease. Previous reports of epi-off CXL have shown that pachymetry reaches its thinnest point approximately 3-months post-procedure.8–10 We measured corneal thickness after a novel epithelium-on corneal cross-linking (EpiSmart) procedure. The procedure was previously tested in vivo to confirm adequate loading of riboflavin in corneal stroma,20 which has been elusive with other transepithelial methods.22 It was also confirmed in vivo that the riboflavin solution containing iodide promotes persistence of riboflavin throughout UVA exposure.21 A previous report of clinical application demonstrated significant improvements in visual acuity and corneal topography with a low rate of adverse events.16
In this study, (EpiSmart) epithelium-on CXL was not associated with Scheimpflug tomography-observed corneal thinning during the first year post-op. The change-from-baseline values were normally distributed except for the 3-month post-op values, and in all cases mean TP changes showed very small excursions from zero. The distributions of measurements at 6 and 12 months post-op exclude, with 95% confidence, any mean changes of greater than −2.8 or +3.7 microns. This contrasts with prior reports of epithelium-off CXL where mean corneal thinning was observed,8–10 up to approximately 50 microns at 3-months, and with a mean of about 20 microns of thinning persisting at 12 months post-op.8–10 Only the 3-month distribution of changes observed here differed from a normal distribution, corresponding to the 3-month peak in mean corneal thinning previously observed with epi-off techniques. Further research is needed to elucidate the origin of these effects at 3-months, which may be technique-dependent physiological changes (ie, thinning due to epithelial removal) and/or optical changes (ie, optical density changes during epithelial healing).
The most recent release of the Belin ABCD Progression Display (version III, OCULUS GmbH) displays confidence intervals showing statistically significant change after epithelium-off CXL. The post-CXL confidence intervals appear only on exams at least one-year post-CXL. Earlier testing, prior to one year, showed high measurement noise levels making clinical applications and decision-making problematic (MW Belin, personal communication, August 2020). The lack of post-CXL corneal thinning with epithelium-on CXL may facilitate earlier analysis of CXL efficacy and the possible need for further treatment.9
There are limitations to this study. While the absence of corneal thinning after EpiSmart CXL in a cohort of 101 ectatic eyes is encouraging, the lack of progressive thinning does not prove biomechanical stability or strengthening. Crosslinking efficacy is generally assessed in terms of visual acuity and maximum keratometry (Kmax). In a previous study of the same EpiSmart system, 592 eyes followed up to 2 years demonstrated corneal stability in all 592 treated eyes as defined by no loss of 1 line of CDVA and 1 Diopter increase in Kmax, mean improvement in CDVA, and a substantial proportion of patients with improved CDVA.16 A larger Phase-2 FDA study enrolled 2228 subjects and showed similar vision and Kmax improvements.17 The results of this study do not allow direct comparison of the epi-on technique to epi-off CXL, but do constrain the degree of apparent corneal thinning after a promising epi-on technique to a much lower value than has been observed after epi-off CXL in similar patients.
A less-invasive crosslinking treatment limits patient risk, and this has motivated the development of epi-on crosslinking. Corneal thinning is an important metric in assessing keratoconus progression and safety. This study demonstrates that after EpiSmart epithelium-on crosslinking, corneal thinning within the first 12 months post-op is not observed in a group of patients with keratoconus or post-surgical ectasia. After CXL with this protocol, there were no mean changes in minimum corneal thickness measured by Scheimpflug tomography.
Disclosure
Drs. Majmudar, Belin, Parsons, and Rubinfeld have equity interests in CXL Ophthalmics, LLC, Encinitas, CA. Dr. Parsons is a consultant to CXL Ophthalmics, LLC, Encinitas, CA. Dr. Rubinfeld had a financial interest in CXLUSA, LLC, Bethesda, MD and reports open access fee from CXL Ophthalmics, LLC. Dr. Belin is a consultant to OCULUS GmbH. The authors report no other conflicts of interest in this work.
References
- 1.Helena MC, Baerveldt F, Kim WJ, Wilson SE. Keratocyte apoptosis after corneal surgery. Invest Ophthalmol Vis Sci. 1998;39(2):276–283. PMID: 9477983. [PubMed] [Google Scholar]
- 2.Emre S, Doganay S, Yologlu S. Evaluation of anterior segment parameters in keratoconic eyes measured with the Pentacam system. J Cataract Refract Surg. 2007;33(10):1708–1712. doi: 10.1016/j.jcrs.2007.06.020 [DOI] [PubMed] [Google Scholar]
- 3.Piñero DP, Alió JL, Alesón A, Escaf Vergara M, Miranda M. Corneal volume, pachymetry, and correlation of anterior and posterior corneal shape in subclinical and different stages of clinical keratoconus. J Cataract Refract Surg. 2010;36(5):814–825. doi: 10.1016/j.jcrs.2009.11.012 [DOI] [PubMed] [Google Scholar]
- 4.Belin MW, Jang HS, Borgstrom M. Keratoconus: diagnosis and Staging. Cornea. 2022;41(1):1–11. doi: 10.1097/ICO.0000000000002781 PMID: 34116536. [DOI] [PubMed] [Google Scholar]
- 5.Gomes JA, Tan D, Rapuano CJ, et al. Group of Panelists for the Global Delphi Panel of Keratoconus and Ectatic Diseases. Global consensus on keratoconus and ectatic diseases. Cornea. 2015;34(4):359–369. doi: 10.1097/ICO.0000000000000408 PMID: 25738235. [DOI] [PubMed] [Google Scholar]
- 6.Wollensak G, Iomdina E. Biomechanical and histological changes after corneal crosslinking with and without epithelial debridement. J Cataract Refract Surg. 2009;35(3):540–546. doi: 10.1016/j.jcrs.2008.11.036 PMID: 19251149. [DOI] [PubMed] [Google Scholar]
- 7.Messmer EM, Meyer P, Herwig MC, et al. Morphological and immunohistochemical changes after corneal cross-linking. Cornea. 2013;32(2):111–117. doi: 10.1097/ICO.0b013e31824d701b [DOI] [PubMed] [Google Scholar]
- 8.Pjano MA, Biscevic A, Grisevic S, Gabric I, Salkica AS. Pachymetry and elevation back map changes in keratoconus patients after crosslinking procedure. Med Arch. 2020;74(2):105–108. doi: 10.5455/medarh.2020.74.105-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Danesh Z, Sedaghat MR, Momeni-Moghaddam H, Yekta AA, Belin MW. Corneal stability and visual acuity 1 year after corneal cross-linking assessed using the ABCD keratoconus staging system. J Refract Surg. 2021;37(10):700–706. doi: 10.3928/1081597X-20210712-09. [DOI] [PubMed] [Google Scholar]
- 10.Kandel H, Nguyen V, Ferdi AC, et al. Comparative efficacy and safety of standard versus accelerated corneal crosslinking for keratoconus: 1-year outcomes from the save sight keratoconus registry study. Cornea. 2021;40(12):1581–1589. doi: 10.1097/ICO.0000000000002747 PMID: 33935236. [DOI] [PubMed] [Google Scholar]
- 11.Wollensak G, Spoerl E, Seiler T. Riboflavin/ultraviolet-a-induced collagen crosslinking for the treatment of keratoconus. Am J Ophthalmol. 2003;135(5):620–627. doi: 10.1016/s0002-9394(02)02220-1. [DOI] [PubMed] [Google Scholar]
- 12.Kobashi H, Rong SS, Ciolino JB. Transepithelial versus epithelium-off corneal crosslinking for corneal ectasia. J Cataract Refract Surg. 2018;44(12):1507–1516. doi: 10.1016/j.jcrs.2018.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nath S, Shen C, Koziarz A, et al. Transepithelial versus epithelium-off corneal collagen cross-linking for corneal ectasia: a systematic review and meta-analysis. Ophthalmology. 2021;128(8):1150–1160. doi: 10.1016/j.ophtha.2020.12.023. [DOI] [PubMed] [Google Scholar]
- 14.Mazzotta C, Baiocchi S, Bagaglia SA, Fruschelli M, Meduri A, Rechichi M. Accelerated 15 mW pulsed-light crosslinking to treat progressive keratoconus: two-year clinical results. J Cataract Refract Surg. 2017;43(8):1081–1088. doi: 10.1016/j.jcrs.2017.05.030 PMID: 28917411. [DOI] [PubMed] [Google Scholar]
- 15.Rechichi M, Mazzotta C, Oliverio GW, et al. Selective transepithelial ablation with simultaneous accelerated corneal crosslinking for corneal regularization of keratoconus: STARE-X protocol. J Cataract Refract Surg. 2021;47(11):1403–1410. doi: 10.1097/j.jcrs.0000000000000640 PMID: 33770171. [DOI] [PubMed] [Google Scholar]
- 16.Stulting RD, Trattler WB, Woolfson JM, Rubinfeld RS. Corneal crosslinking without epithelial removal. J Cataract Refract Surg. 2018;44(11):1363–1370. doi: 10.1016/j.jcrs.2018.07.029 [DOI] [PubMed] [Google Scholar]
- 17.NCT03029104. Registry information is available at. Available from: https://clinicaltrials.gov/ct2/show/NCT03029104. Accessed March 11, 2022.
- 18.Villavicencio OF, Gilani F, Henriquez MA, Izquierdo L, Ambrosio RR, Belin MW. Independent Population Validation of the Belin/Ambrósio Enhanced Ectasia Display: implications for Keratoconus Studies and Screening. Int J Keratoconus Ectatic Dis. 2014;3(1):1–8. doi: 10.5005/jp-journals-10025-1069 [DOI] [Google Scholar]
- 19.Belin MW, Villavicencio OF, Ambrosio R. Tomographic parameters for the detection of keratoconus: suggestions for screening and treatment parameters. Eye Contact Lens. 2014;40(6):326–330. doi: 10.1097/ICL.0000000000000077 [DOI] [PubMed] [Google Scholar]
- 20.Rubinfeld RS, Stulting RD, Gum GG, Talamo JH. Quantitative analysis of corneal stromal riboflavin concentration without epithelial removal [published correction appears in J Cataract Refract Surg. 2018 Apr;44(4):523]. J Cataract Refract Surg. 2018;44(2):237–242. doi: 10.1016/j.jcrs.2018.01.010 [DOI] [PubMed] [Google Scholar]
- 21.Rubinfeld RS, Gum GG, Talamo JH, Parsons EC. The effect of sodium iodide on stromal loading, distribution and degradation of riboflavin in a rabbit model of transepithelial corneal crosslinking. Clin Ophthalmol. 2021;15:1985–1994. doi: 10.2147/OPTH.S300886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gore DM, O’Brart DO, French P, Dunsby C, Allan BD. Transepithelial riboflavin absorption in an ex-vivo rabbit corneal model. Invest Ophthalmol Vis Sci. 2015;36:5006–5010. doi: 10.1167/iovs.15-16903 [DOI] [PubMed] [Google Scholar]