Abstract
Background
Gallstone disease (GSD) is more commonly presented in aged people.
Purpose
The purpose of the study was to explore the insights of metabolic performance of bacterial species from gut microbiota as well as the clinical background in middle-aged and elderly patients with GSD.
Patients and Methods
This is an observational study concerning 120 research participants. Of those, 90 patients with symptomatic GSD addressed for cholecystectomy, average age 59.83 ± 15.32 years: 45 with cholesterol rich gallstones (CGSs), 45 with pigment gallstones (PGSs) and 30 healthy controls joined this observational study. Clinical examination, lab work-ups, upper and lower digestive video-endoscopies, abdominal ultrasound/CT and gallbladder motility assessment by Dodd’s method were performed. Overall stool dysbiosis (DB) was assessed as 1 = minor, 2 = mild, 3 = severe, species being identified by matrix-assisted laser desorption ionization method. Stool samples from dysbiotic patients were analyzed by a next generation sequencing method with operational taxonomic unit identification.
Results
Patients with GSD presented with a significant high range of overall gut DB (p < 0.0001) when compared with controls. Those with CGSs compared with those having PGSs displayed significant clinical differences related to elderly age, lifestyle and diet particularities, obesity, dyslipidemia, nonalcoholic fatty liver disease, hypertension, type 2 diabetes mellitus or impaired glucose tolerance, as well as motility disturbances of gallbladder with a decrease of the ejection fraction. Significant increase of overall DB range and alterations of several functional bacterial species with a decrease of butyrate, lactate, acetate/propionate and methane producers, mucin degrading bacteria, biodiversity index of microbiota, as well as an increase of lipopolysaccharide positive bacteria were significantly present in patients with CGSs.
Conclusion
Middle-aged and elderly patients with GSD and a clinical background characterized by particular lifestyle, metabolic and gallbladder motility issues displayed significant modifications of biodiversity, overall gut DB and alterations of several functional bacterial species, with a decrease of their metabolic performance.
Keywords: biliary calculi, intestinal functional bacteria, dysbiosis
Graphical Abstract
Introduction
Gallstone disease (GSD) represents the presence of calculi either at the level of gallbladder (GB) or at the level of intrahepatic or extrahepatic bile ducts. GSD is frequently associated to lithogenic conditions such as disorders of GB kinetics, metabolic issues (diabetes mellitus, obesity, dyslipidemia), chronic liver diseases, chronic hemolysis or ileal pathology, resulting in modified chemical composition of bile, that eventually become oversaturated and precipitate, giving life of biliary calculi. Development of GSD is in fact a complex process where could intervene genetic, inherited factors, as well as acquired and environmental ones, that at some point could act synergic and boost GS formation. Age is a major risk factor for GSD and its prevalence tend to proportionally increase in seniors, where patients exhibit either no signs of disease, the diagnosis being incidental or may display various complications defined as inflammation, infections or neoplastic transformation.1 GSD is a relatively frequent digestive condition, affecting about 10–15% of the adult population in Western countries. That is why, among other digestive conditions, GSD is one of the most important causes for patient’s hospitalization. Over the past decades GSs have been reliably classified by clinicians into cholesterol rich GSs (CGSs) and pigment GSs (PGSs) (brown or black), depending on their gross appearance, associated to probable chemical composition.2 Especially in Western countries about 90% of the gallstones found after cholecystectomy were CGSs.3 Diagnosis of GSD is either incidental, when performing a routine abdominal ultrasound, or related to various complications. The treatment of symptomatic GSD being exclusively surgical by laparoscopic cholecystectomy and in selected cases with pure cholesterol gallstones, by orally lithotripsy with biliary acids.4–8 Over the past several years, gut microbiota was extensively studied and many observations regarding its influence in maintaining health on organisms are already well known. This microbial community participating in gut microbiome formation has its own metabolic capabilities that complement the regular activity of the mammalian enzymes, located in the liver cells or gut mucosa, by sharing some enzymes that our genes do not encode and therefore being actively involved in the synthesis of vitamins or helping us to digest some specific nutrients.9 Imbalance of the amount or diversity of gut microbiota could act as a trigger for diverse conditions either functional: irritable bowel syndrome, migraine and chronic pelvic pain, or organic issues related to cardiovascular diseases, metabolic and liver conditions as well as neurodegenerative disease.10–17 Hypotheses and recent evidence related to metabolic pathways influence of microbiota dysbiosis intervention are discussed in the settings of etiopathogenesis of GSD. An important role of gut microbiota is related to its implication in regulation of the enterohepatic bile acid (BA) recycling process that eventually will result in intestinal cholesterol absorption.18 Changes of microbiota characteristics, related to specific phyla, their amount and diversity were observed not only at the level of biliary tract, but also at the gut level.19 Some studies reported that more than 70% of microorganisms seen in bile ducts of patients with GSD were also present in the gut. These observations hypothesized that bile microbiota could in fact originate from gut microbiota.20 Many recent researches focused not only on identifying the microorganisms involved in gut microbiota, but also on better understanding their specific functionality related to human metabolism, especially those associated to dietary components. Following the fermentation of dietary carbohydrates results in bacterial fermentation products represented especially by short chain fatty acids (SCFAs). The most important SCFAs of the normal gut microbiota are acetate propionate and butyrate acids, whose molar ratios range from 3:1:1 to 10:2:1. Their activity is related to energy supply, glucose and cholesterol metabolism, lipogenesis, appetite regulation, antiinflammatory and anticancer capabilities.9 The interactive cross-talk between the host genome and the gut microbiome could modulate a lot of important metabolic pathways, one of these being the BAs metabolism. Butyrate producing bacteria such as Eubacterium spp. are capable of transforming primary BAs to secondary BAs such as: lithocholic acid and deoxycholic acid, which are potentially cytotoxic and have been linked to colorectal cancer and cholesterol GS formation.21 Consecutively to the bacterial deconjugation process a decrease of the efficiency of BAs for the emulsification of dietary lipids and micelle formation would develop. This can result in the alteration of the host digestive abilities, as BAs play a pivotal role regarding the absorption of dietary fats, various nutrients and lipid-soluble vitamins.22
The aim of the study was to explore the insights of metabolic bacterial performance of gut microbiota and its clinical background in patients with GSD.
Materials and Methods
120 research participants joined this observational study. Of those, 90 patients were diagnosed with symptomatic GSD addressed by cholecystectomy and 30 healthy controls were enrolled in this study after the written informed consents were signed. Controls were recruited from apparently healthy siblings of patients or hospital employees. Patients were subjected to a clinical examination including history of alcohol consumption, cigarette smoking, heredity for GSD, associated medical conditions, lifestyle questionnaires about physical activity, occupation/leisure, stress, dietary habits related to amount of calories, amount and frequency of eating five items with fiber (fruits, vegetables, whole grain products, rice, potatoes, pasta, legumes), water intake, assessment of body mass index (BMI) and blood pressure measurement. Laboratory work-ups (blood, urine, stool) included complete cell blood count, total bilirubin, alanine-aminotransaminase, amylase, fast blood glucose, C-reactive protein, creatinine, total cholesterol, triglycerides and H. pylori fecal antigen were performed before cholecystectomy using standardized methods, accredited by the European Community (EC) and Romanian Accreditation Association (RENAR).
Inclusion Criteria
Middle-aged and elderly patients (over 45), average age 59.83 ± 15.32 years, with GSD addressed for cholecystectomy were consecutively included in this observational study based on GS classification. Patients were assigned to two equal groups: 45 patients with cholesterol rich GSs and 45 with pigment GSs.
Exclusion Criteria
Patients with small bowel and colon organic diseases, respiratory, cardiac, liver, and kidney insufficiency; thyroid, oncological, and autoimmune diseases, gastro-esophageal reflux and peptic ulcer disease, H Pylori infection, other infections or inflammations such as: cholecystitis, pancreatitis, peritonitis, as well as patients under bile sequestrants, proton pump inhibitors, antibiotic or probiotic treatment at enrollment or with a recent history of antibiotics/probiotics.
Diagnosis of Entities and Procedures
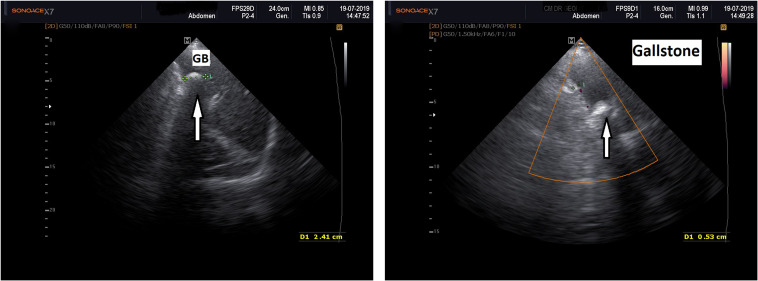
Diagnosis of diabetes mellitus was made according to American Diabetes Association (ADA) criteria and that of dyslipidemia according to Panel III Guidelines, 2004.23,24 Irritable bowel syndrome (IBS) diagnosis was made according to Rome III criteria.25 Stool samples of all research participants (patients and controls) were collected in dedicated 30 mL sterile stool screw cap polypropylene containers for storage at −20 °C, with spoon collectors (Euro Med), then brought to the lab in a maximum 2-hour interval after collection. For overall gut microbiota DB assessment, progressive dilutions in saline water from 10−1 to 10−5 were obtained, and 0.1 mL of the stool samples was placed on different culture media for aerobe, anaerobe, and microaerophilic spp. Different microorganisms were identified based on matrix-assisted laser desorption ionization-time of flight-mass spectrometry (MALDI)-TOF-MS and expressed by colony forming units (CFU)/gram of stool.26 Overall dysbiosis (DB), diagnosed as quantitative and qualitative imbalance of the intestinal flora and assessed as 1 = mild, 2 = moderate and 3 = severe.10 Frozen stool samples from patients who presented dysbiosis were investigated using a 16S rRNA NGS (next generation gene sequencing) method. Workflow steps were: sample collection in 30 mL sterile stool screw cap polypropylene containers with spoon collectors (Euro Med), for storage at −20 °C, preparation for DNA isolation, PCR amplification, mixing, and purifying PCR products, library preparation, sequencing, and data analysis. In this study, DNA was isolated from 43 frozen fecal samples using Sigma’s Gen Elute™ Stool DNA Isolation Kit (Sigma-Aldrich, St Louis, USA), that simultaneously isolated the total genomic DNA from all the various microorganisms found in the frozen stool samples. The kit also removed all traces of humic acid using the provided Bead Tubes and a combination of chemical and physical homogenization and lysis. A spin column procedure was then used to further purify the DNA. The purified genomic DNA was stored at 2–8 °C. (www.sigmaaldrich.com>dnb200). Preparation of the 16S Metagenomic Sequencing Library was based on targeting the variable V3 and V4 regions of the 16S rRNA gene with the Illumina MiSeq System (Illumina, San Diego, CA, USA). This involves a two-step, tailed PCR approach that generates ready-to-pool amplicon libraries. The entire protocol, step by step is illustrated in 16S sample preparation guide from Illumina (www.support.illumina.com>16s). Briefly, the 16S V3 and V4 amplicon workflow consisted of a PCR amplification template from of genomic DNA using region of interest-specific primers with overhang adapters attached as well as Locus-specific primers containing sequence tails that allow for a second PCR step to add Nextera® XT-indexed adapters. Tailed primers increase the melting point, efficiency, and specificity while avoiding the disadvantages of long primers, such as hairpins, self-dimers, primer dimers, and chimeras. The full primer pair sequences according to standard International Union of Pure and Applied Chemistry (IUPAC) nucleotide nomenclature were 16S Amplicon PCR forward primer (50 bp): 5’-TCGTCGGCAGCGTCAGATGTGTATAAGAGACAG-3’ and 16S Amplicon PCR reverse primer (55 bp): 5’-GTCTCGTGGGCTCGGAGATGTGTATAAGAGACAG-3’; attaching the indices, P5Index2 and P7Index1, and Illumina sequencing adapters using the NexteraR XT Index Kit. The Nextera XT Index Kit uses a dual-index strategy involving 12 (i7) and 8 (i5) indexes, for a total of 20 indexes. Together, they can generate 96 (12 × 8) different index combinations. To control the metagenomics sample for 16S rRNA sequencing, a microbial mock community B sample available from BEI Resources (HM-276D) was used. (BEI Resources (www.beiresources.org/Catalog/otherProducts/HM-276D.aspx). Cleaning-up the final library before quantification was performed using AM Pure XP beads, and then the quantification, normalization, and pooling libraries were set. The number of samples pooled per run was adapted for the stool microbiome assessment. For metagenomic samples >100,000 reads/sample were performed to fully survey the bacterial classification, according to the Nextera Low Plex Pooling Guidelines Technical Note. Illumina (2011). (www.illumina.com/documents/products/technotes/technote_nextera_low_plex_pooling_guidelines.pdf). Denaturating the pooled libraries before sequencing on the MiSeq System was performed using V3 reagents, MiSeq, and sample loading. Data analysis was carried out using MiSeq Reporter (v2.3) software (support.illumina.com/documents/documentation/software_documentation/miseqreporter/miseq-reporter-metagenomics-workflow-ref-guide-15042317-c.pdf), and the metagenomic workflow provided a classification of the operational taxonomic units (OTUs) based on the Greengenes database (http://greengenes.lbl.gov/). The specific taxonomical footprint of each sample was identified.27 The final output of this entire workflow provided an elaborate presentation of different taxonomic levels from kingdom and phylum to genus and species as cluster graphs, sample tables, columns, and cluster pie charts, bioindicators including Shannon–Wiener H index of alpha biodiversity, as well as functional phyla assessment.28 GSD diagnostic basically relied on abdominal ultrasound and computed tomography (CT). For the ultrasound procedure patients were examined in a fasting state, in the morning, high resolution ultrasound machines and 3.5–5 MHz phased array probes were used. GB was observed in 2 incidences (longitudinal and transversal). Hyperechoic images inside GB, mobile within gravitation, with acoustic shadows were consistent with the diagnostic of GSs.29 Measurements of the GB dimensions, the wall thickness and the diameters of each clear visualized stone, as well as of intra and extrahepatic bile ducts, liver and spleen, pancreas, kidney were performed in each patient. Figure 1A and B illustrate ultrasound aspects of GSD (personal collection).
Figure 1.
(A) Left: 2D abdominal ultrasound, white arrow pointing at GS. GB=gallbladder. (B) Right: Abdominal duplex ultrasound, white arrow pointing at GS. GS appears as echogenic zone (white) with acoustic shadow (black tail behind the stone).
The GB motility assessment was performed using Dodd’s method with measurement of initial and residual GB volume and calculation of GB ejection fraction (GBEF) after receiving a test meal. The cutoffs for GBEF were 60%.30 The presence of nonalcoholic fatty liver disease (NAFLD) was also reported, based on ultrasound/CT criteria of diagnostic.31 Upper and lower digestive video-endoscopies, abdominal CT, electrocardiograms and thorax X-ray examinations were also performed. Cholecystectomy was performed by retrograde laparoscopic approach in all study participants. Following cholecystectomy all GB pieces were sent to the pathology laboratory, where they were gross examined, then 10% formalin fixated, paraffin embedded and usual hematoxylin-eosin (HE) stained with optical microscopy examination, performed by trained pathologists. In the operation theatre, after the removal of the GB, GSs were gross examined with a magnifier glass by the surgeon, dissected with the scalpel and classified into cholesterol rich (CGSs) or pigment (black or brown) stones (PGSs), depending on their characteristics, according to the Japanese Society of Gastroenterology which associates gross morphology to chemical composition of GSs.32,33 CGSs could be pure when characterized by soft consistence, smooth surface and yellowish color. The surface of the sectional plane of a pure CGS has a radial pattern seen from the center to the edges, evoking a spoke wheel. Combination CGSs are composed by two different zones, clearly visible when sectioned: the middle of cholesterol and the surface of pigment-bilirubin compounds. Mixed CGSs exhibit rough surfaces, display a small pigmented center, a large coat of cholesterol and a radial pattern. PGSs are classified into two groups: brown and black. Brown PGSs contain multiple compounds: calcium bilirubinate, tribasic phosphate and calcium fatty acids; their cut appearance reveals a pattern with concentric circles, like a tree section. Black PGSs are amorphous, formed mostly by calcium bilirubinate and exhibit no pattern at all. Depending on the description of GSs, two equal groups of patients were formed, each with 45 study participants, respectively group 1 with CGSs and group 2 with PGSs.
This study was in accordance with the Helsinki Declaration of Human Rights and has been approved by the Ethical Committee of Scientific Research with the University of Medicine and Pharmacy from Timisoara, number 16/24.05/2021.
Statistical Analysis
Statistical analysis was performed using Graph Pad Prism 9.3.1 (471) version package (Graph Pad Software, Inc., La Jolla, CA, USA). Continuous data were expressed as mean values and standard deviations. Categorical variables were analyzed with the Chi-squared test. Unpaired t test was performed, and p values were calculated. When p was ≤0.05, the results were considered statistically significant, with a confidence interval (CI) of 95%. Histograms of variables distribution were produced.
Results
This is an observational study concerning 120 research participants: 90 patients with symptomatic GSD addressed for cholecystectomy and 30 healthy controls. Table 1 illustrates the demographic and gut microbiota dysbiosis characteristics in patients with GSD and controls. As seen, patients with GSD exhibited statistically significant differences only related to overall DB (p < 0.0001), when compared to the control group. Demographic characteristics were comparable between the study group and the control group.
Table 1.
Demographic and Gut Microbiota Chart in Research Participants
| Variables | Patients with GSD (n=90) | Controls (n=30) | p |
|---|---|---|---|
| Age | 59.83±15.32 | 54.24±10.80 | 0.0670 |
| Gender M/F | 22/68(24.44%/75.56%) | 11/19(36.66%/63.34%) | 0.1961 |
| Location U/R | 53/47(58.88%/41.12%) | 19/11(63.33%/36.67%) | 0.6679 |
| Gut overall DB presence | 49/90 (54.44%) | 2/30(6.66%) | <0.0001* |
Note: *Statistically significant.
Abbreviations: GSD, gallstone disease; n, number; M/F, males/females; U/R, urban/rural; DB, dysbiosis.
Table 2 presents the most important demographic, clinical and biological aspects in study participants (90 patients), divided into two even groups, respectively: 45 patients with cholesterol rich gallstones (CGSs) and 45 patients with pigment gallstones (PGSs).
Table 2.
Demographic, Biological and Clinical Chart in Study Participants
| Variables | CGSs (n=45) | PGSs (n=45) | p |
|---|---|---|---|
| Age | 63.30±14.31 | 53.28±12.34 | 0.0006* |
| Gender ratio M/F | 10/35(22.22%/77.78%) | 12/33(26.66.%/73.34%) | 0.6260 |
| Location U/R | 28/17(62.22%/37.78%) | 25/20(44.44%/55.56%) | 0.0927 |
| Hemoglobin (g/dl) | 12.10±1.49 | 12.03±0.44 | 0.8954 |
| Leukocytes/mm3 | 7.67x103±4.07 x103 | 7.92x103±2.48 x103 | 0.8606 |
| CRP (U/l) | 6.14±1.03 | 3.6±1.57 | <0.0001* |
| ALT (IU/l) | 31.92±15.14 | 34.13±15.08 | 0.7190 |
| Total Bilirubin (mg/dl) | 0.61±0.26 | 0.71±0.21 | 0.3315 |
| Amylase (U/l) | 48.03±21.22 | 45.33±18.76 | 0.7574 |
| FPG (mg/dl) | 118.03±21.45 | 89.36±9.57 | 0.0009* |
| Creatinine (mg/dl) | 0.69±0.27 | 0.58±0.19 | 0.2919 |
| Overall DB percentage | 80% | 28.88% | <0.0001* |
| Overall DB severity | 1.78±1.15 | 0.13±0.35 | 0.0004* |
| Family history for GSD | 51.11% | 46.66% | 0.6745 |
| Cigarettes smoking history | 28.88% | 33.33% | 0.6502 |
| Alcohol drinking history | 11.11% | 15.55% | 0.5378 |
| Diet rich in saturated fat | 77.77% | 31.11% | <0.0001* |
| Low dietary fibers | 62.22% | 31.11% | 0.0033* |
| Sedentary lifestyle | 53.33% | 44.44% | 0.4015 |
| BMI>30 kg/m2 | 66.66% | 12.5% | 0.0078* |
| T2DM/IGT | 42.85% | 4.44% | <0.0001* |
| Oral antidiabetics | 17.77% | 0% | 0.0032* |
| Insulin therapy | 4.44% | 0% | 0.1566 |
| AH | 62.96% | 12.5% | 0.0134* |
| Dyslipidemia | 66.66% | 12.5% | 0.0288* |
| Statins | 37.77% | 4.44% | 0.0001* |
| Statins and fibrates | 15.55% | 0% | 0.0062* |
| NAFLD | 44.44% | 17.77% | 0.0066* |
| Overall chronic liver conditions (except NAFLD) | 6.66% | 22.22% | 0.0368* |
| IBS | 40% | 35.55% | 0.6650 |
| GSD related carcinoma | 2.22% | 0% | 0.6224 |
| GBEF<60% | 26/45(57.77%) | 16/45(35.55%) | 0.0356* |
Note: *Statistically significant.
Abbreviations: CGS, cholesterol rich gallstones; PGS, pigment gallstones; n, number; M/F, males/females; U/R, urban/rural; GS, gallstones; CRP, C-reactive protein; ALT, alanine aminotransaminase; FPG, fast plasma glucose; g/dl, grams/deciliter; mm3, milimetter3; U/L, units/liter; IU/l, international units/liter; mg/dl, milligrams/deciliter; DB, dysbiosis; BMI, body mass index; T2DM/IGT, type 2 diabetes mellitus/impaired glucose tolerance; AH, arterial hypertension; NAFLD, nonalcoholic fatty liver disease; IBS, irritable bowel syndrome; GBEF, gallbladder ejection fraction.
As observed in Table 2, statistically significant differences were noted in favor of patients with CGSs related to older age, elevation of CRP, incidence and severity of overall DB, sedentary lifestyle and dietary habits characterized by high saturated fat and low fiber ingestion, as well as to association with several metabolic conditions, such as: obesity, dyslipidemia, NAFLD, hypertension, T2DM or IGT, treatment with oral antidiabetics, hypolipemiants and a decrease of the GB motility. Patients with PGSs displayed significantly younger age and chronic liver conditions others than NAFLD.
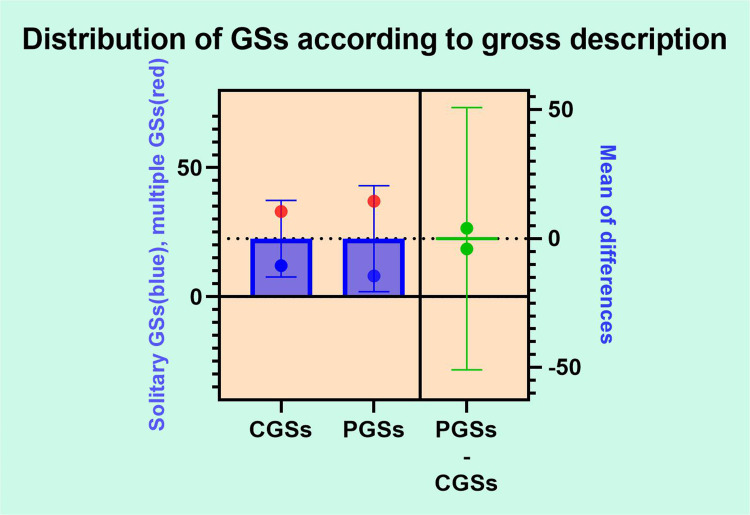
Figure 2 depicts aspects related to gross GSs description after performing the cholecystectomy. Given that, the overall distributions of GSs according to number was 77.77% multiple stones of various size and 22.23% solitary GSs, usually over 1 cm diameter. No significant differences related to number of GSs were noted between the two groups (CGSs vs PGSs).
Figure 2.
GSs distribution according to gross description.
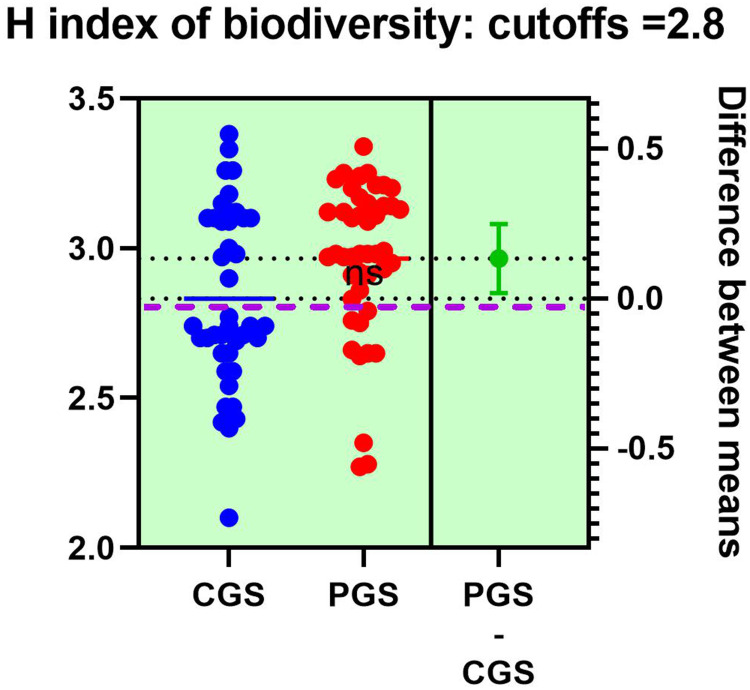
Figure 3 displays alterations of the diversity of entities retrieved in the stool of patients with GSD, respectively, CGSs vs PGSs. The H index of alpha biodiversity significantly decreased (<2.8) in patients with CGSs 58.33% vs PGSs 23.07% (p = 0.03).
Figure 3.
H index of alpha-biodiversity in patients with GSD: CGSs vs PGSs.
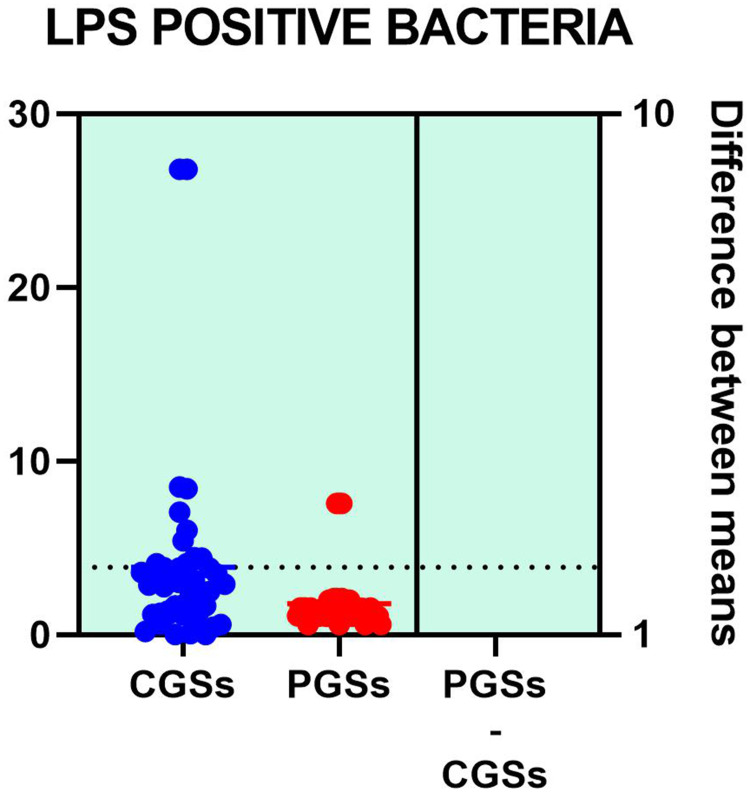
Variation of metabolic bacterial performance of the functional phyla, expressed in percentage above or below the cutoffs, in patients with GSD is illustrated in Table 3. As seen in the table below, significant differences were observed related to butyrate producers where more than half of patients with CGS exhibited an important decrease of these species: 58.33% vs 28.07% (p = 0.031). Decrease of the acetate/propionate producers was significantly noted in patients with CGS: 55.55% vs. 23.07% (p = 0.046). Significant differences were also seen related to an increase of the LPS positive bacteria in patients with CGS: 61.11% vs. 15.38% (p = 0.0051). An important decrease of lactate producers and slight decrease of mucin degrading bacteria were noted in both groups of patients.
Table 3.
Modifications of Stool’s Phyla According to Bacterial Metabolic Performance in Study Participants
| Phylum Alterations | CGSs | PGSs | P |
|---|---|---|---|
| Decrease of butyrate producers | 58.33% | 23.07% | 0.031* |
| Decrease of lactate producers | 80.55% | 76.92% | 0.783 |
| Decrease of acetate/propionate producers | 55.55% | 23.07% | 0.0465* |
| Decrease of mucin degrading bacteria | 22.22% | 15.38% | 0.6037 |
| Increase of LPS positive bacteria | 61.11% | 15.38% | 0.0051* |
Note: *Statistically significant.
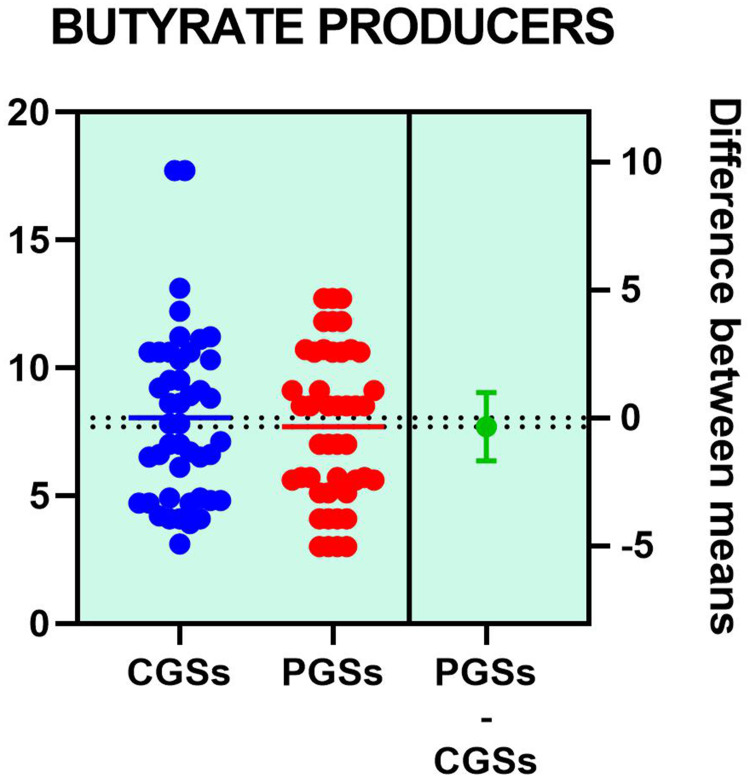
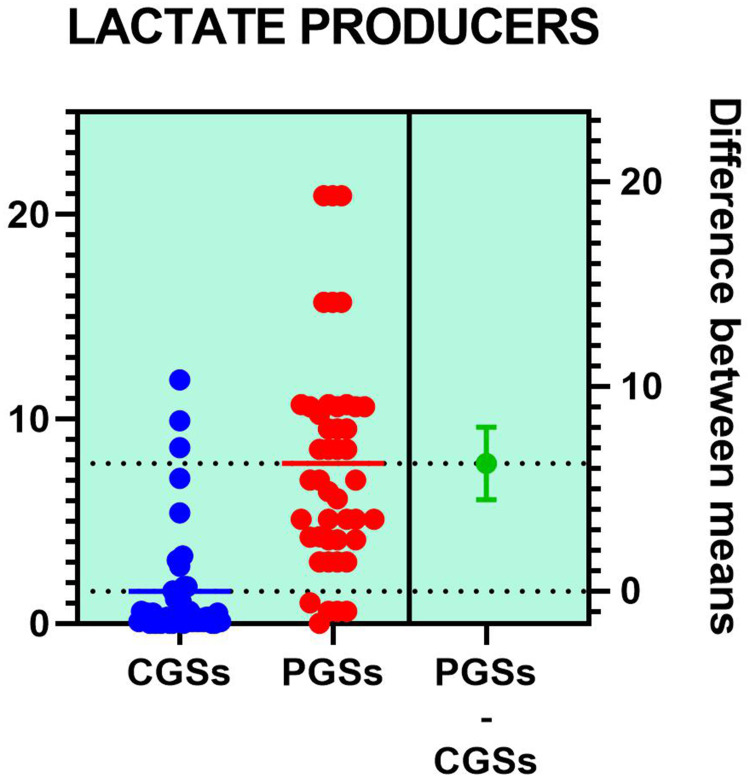
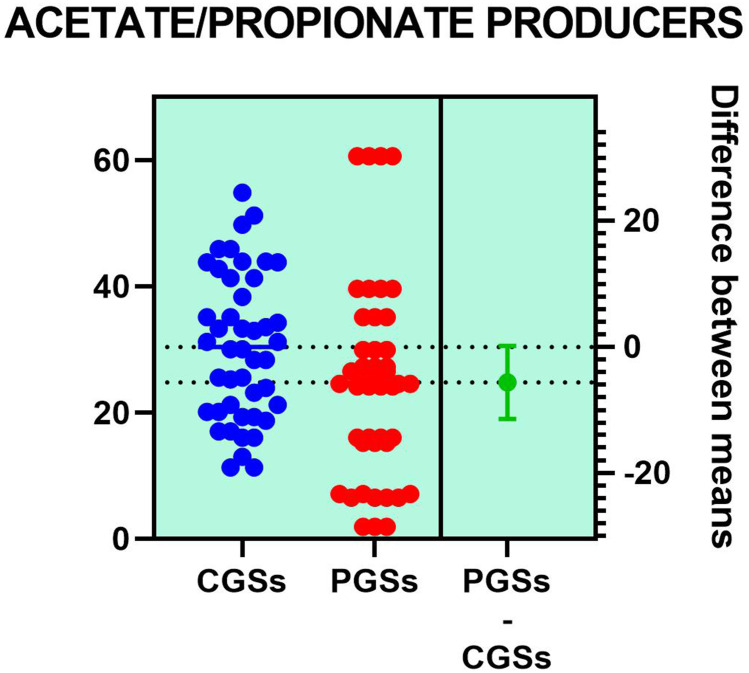
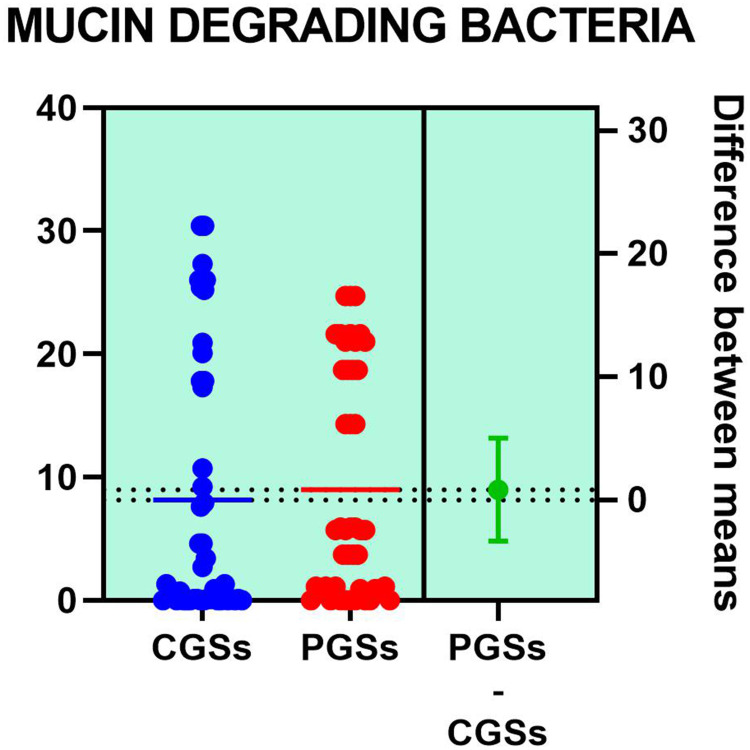
Histograms of the quantitative distribution of the various phyla according to their metabolic performance in patients with GSD are illustrated in Figures 4–8.
Figure 4.
Quantitative distribution of the functional phyla in patient's GSD.
Figure 5.
Quantitative distribution of the functional phyla in patient's GSD.
Figure 6.
Quantitative distribution of the functional phyla in patient's GSD.
Figure 7.
Quantitative distribution of the functional phyla in patient's GSD.
Figure 8.
Quantitative distribution of the functional phyla in patient's GSD.
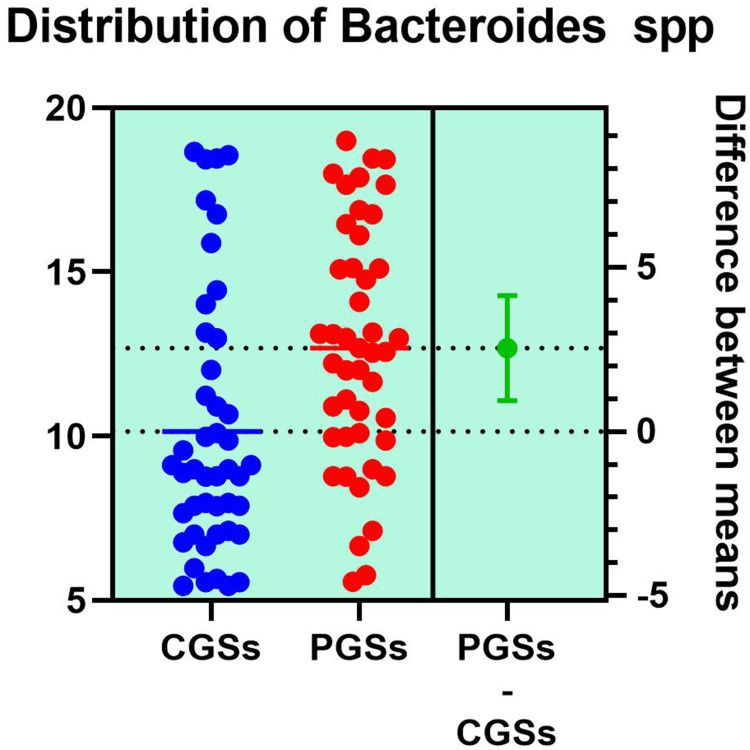
Analyzing the quantitative distribution of functional bacteria, one can see in Figure 5 that lactate producing bacteria were significantly decreased in CGS patients: 1.571 vs 7.835, p < 0.0001 while lipopolysaccharide (LPS) positive bacteria were significantly increased in CGS patients: 2.873 vs. 1.889, p = 0.0013, as depicted in Figure 8. The other quantitative data of functional phyla did not show significant differences between the two groups.
Variation of several functional bacterial species expressed as percentage of decrease below the cutoffs is depicted in Table 4.
Table 4.
Modifications of Functional Bacterial Species in Study Participants
| Bacterial Species Alterations (Decrease) | CGSs | PGSs | p |
|---|---|---|---|
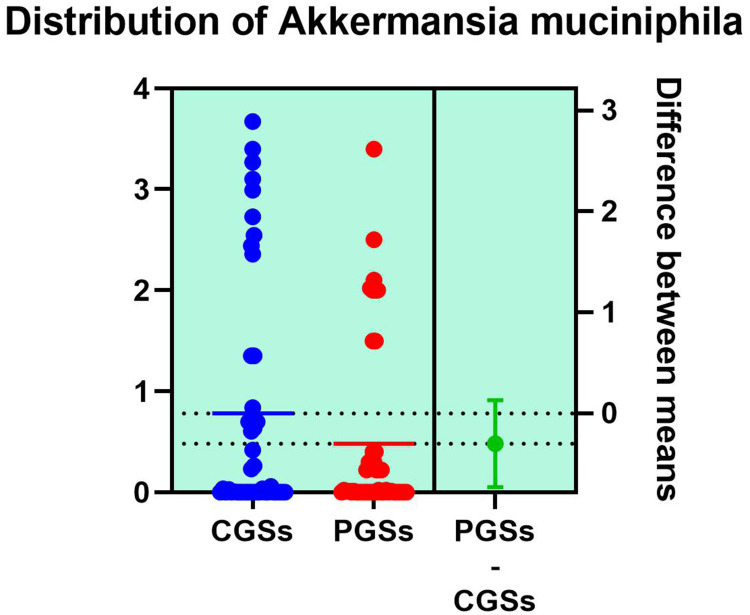
| Akkermansia muciniphila | 66.66% | 33.33% | 0.0017* |
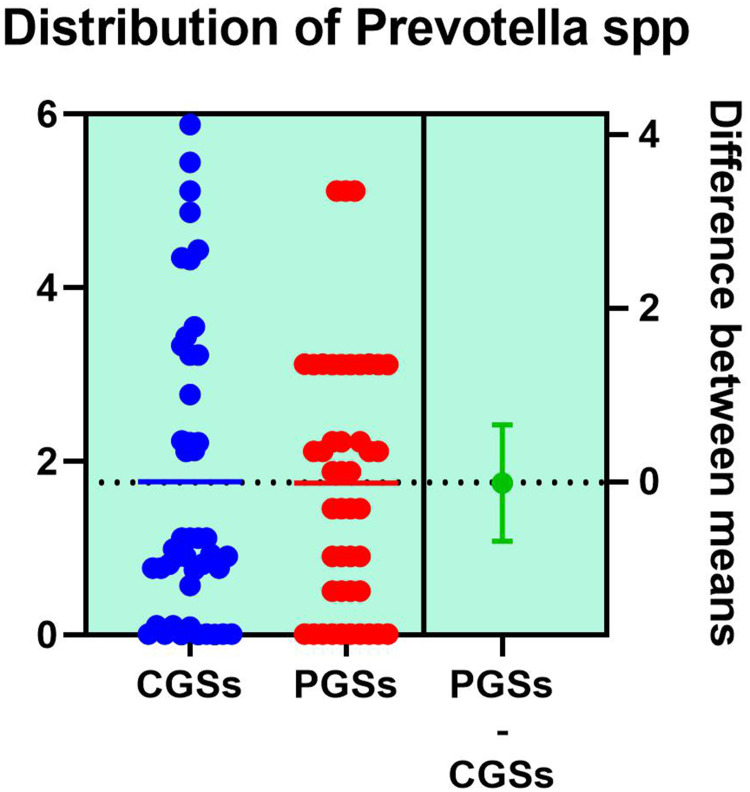
| Prevotella spp. | 27.77% | 7.69% | 0.0131* |
| Desulfuromonas spp. | 5.55% | 7.69% | 0.7784 |
| Bifidobacterium adolescentis | 61.11% | 23.07% | 0.0199* |
| Bifidobacterium dentium | 66.66% | 53.84% | 0.4160 |
| Lactobacillus brevis | 94.44% | 92.30% | 0.7849 |
| Lactobacillus plantarum | 69.44% | 76.92% | 0.6125 |
| Lactobacillus paracasei | 77.77% | 92.30% | 0.2511 |
| Oscillibacter spp. | 86.11% | 84.61% | 0.8957 |
| Alistipes spp. | 55.55% | 15.38% | 0.0135* |
| Bacteroides spp. | 77.77% | 23.07% | 0.0005* |
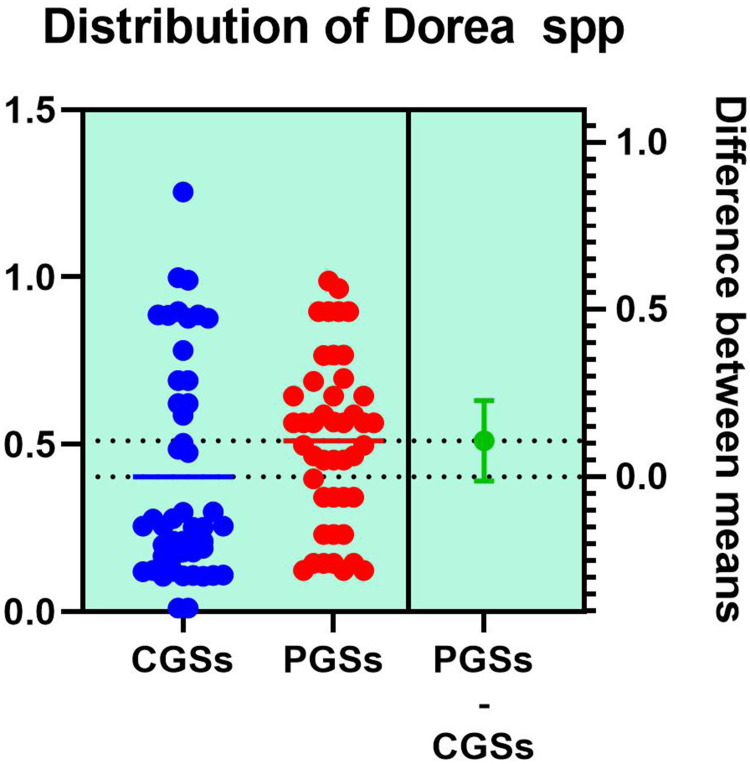
| Dorea spp. | 61.11% | 15.38% | 0.0051* |
| Methanobacteria | 27.77% | 0.00% | 0.0351* |
| Methanobrevibacter smithii | 33.33% | 0.00% | 0.0178* |
| Enterobacter spp. | 22.22% | 15.38% | 0.6037 |
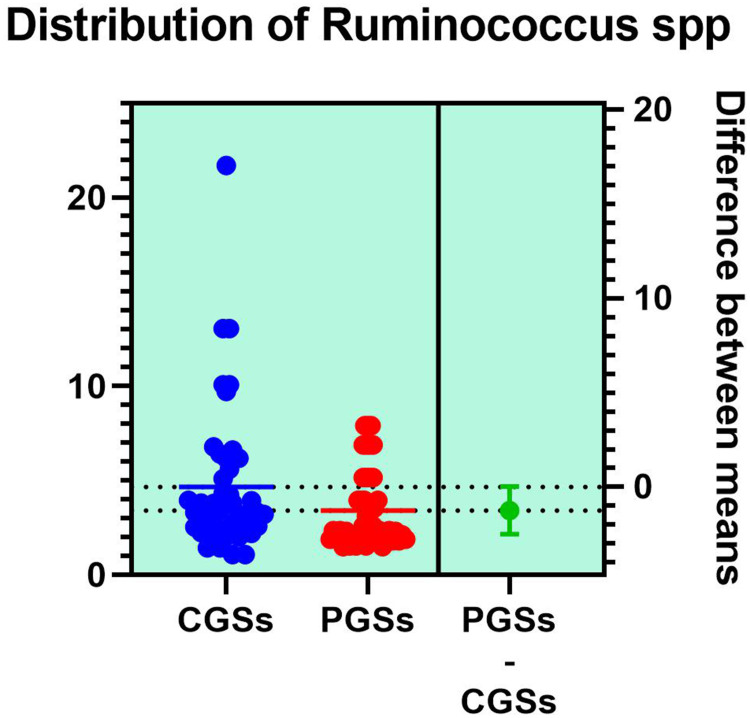
| Ruminococcus spp. | 80.55% | 23.07% | 0.0002* |
| Escherichia spp. | 41.66% | 38.46% | 0.8421 |
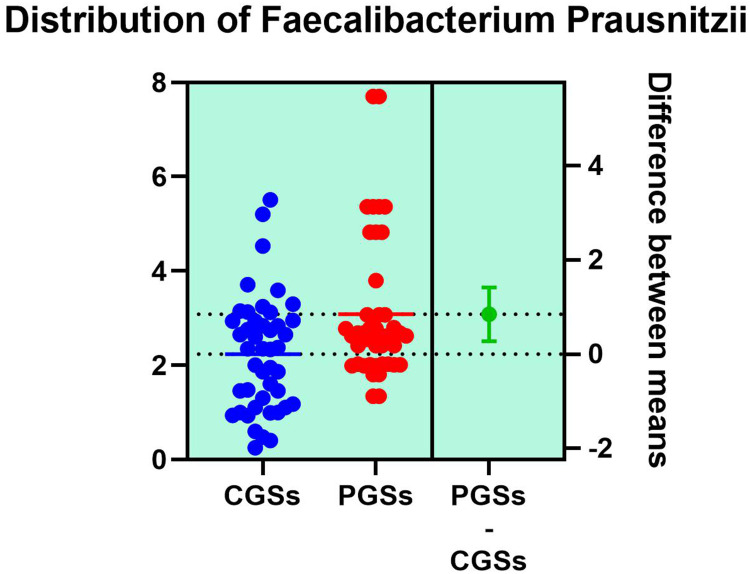
| Faecalibacterium prausnitzii | 55.55% | 15.38% | 0.0135* |
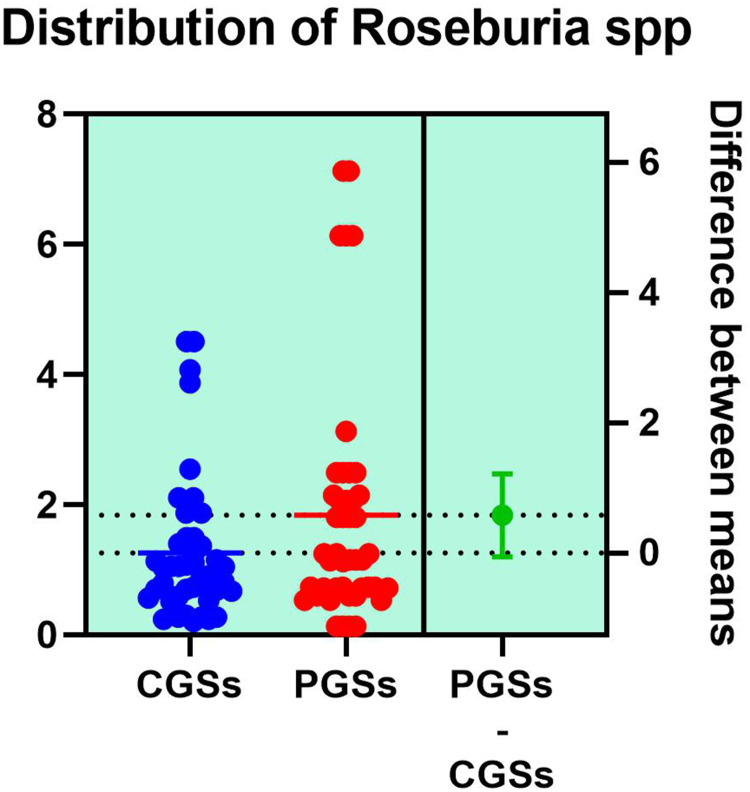
| Roseburia spp. | 22.22% | 7.69% | 0.2500 |
| Lactobacillus spp. | 72.22% | 69.23% | 0.8396 |
Note: *Statistically significant.
Abbreviation: spp, species.
As illustrated in Table 4, patients with CGSs presented a significant decrease of Akkermansia muciniphila (p = 0.0017), Prevotella spp. (p = 0.0131), Bifidobacterium adolescentis (p = 0.0199), Alistipes spp. (p = 0.0135), Bacteroides spp. (p = 0.0005), Dorea spp. (p = 0.0051), Methanobacteria (p = 0.0351), Methanobrevibacter smithii (p = 0.0178), Ruminococcus spp. (p = 0.0002) and Faecalibacterium prausnitzii (p = 0.0135). Both groups exhibited an important decrease of Lactobacillus spp. and slight decrease of Desulfuromonas spp.
Histograms of the quantitative distribution of the most important bacterial species whose functions are associated to butyrate production, in patients with GSD are represented in Figures 9–11.
Figure 9.
Quantitative distribution of the bacterial species producing butyric acid in patients with GSD.
Figure 10.
Quantitative distribution of the bacterial species producing butyric acid in patients with GSD.
As illustrated in Figures 9–11, significant differences between patients with CGSs vs PGSs were observed related to distribution of Faecalibacterium Prausnitzii range (2.223 vs. 3.082, p = 0.048). No significant differences were noted related to Roseburia spp. and Ruminococcus spp. range.
Figure 11.
Quantitative distribution of the bacterial species producing butyric acid in patients with GSD.
Histograms of the quantitative distribution of the bacterial species associated to lactate production, in patients with GSD are represented in Figures 12–14.
Figure 12.
Quantitative distribution of the bacterial species producing lactic acid in patients with GSD.
Figure 13.
Quantitative distribution of the bacterial species producing lactic acid in patients with GSD.
Figure 14.
Quantitative distribution of the bacterial species producing lactic acid in patients with GSD.
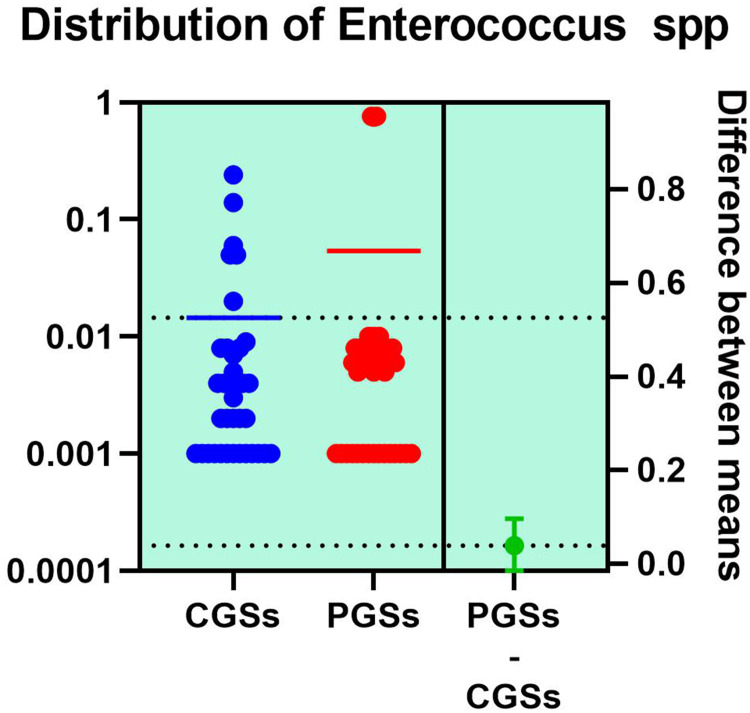
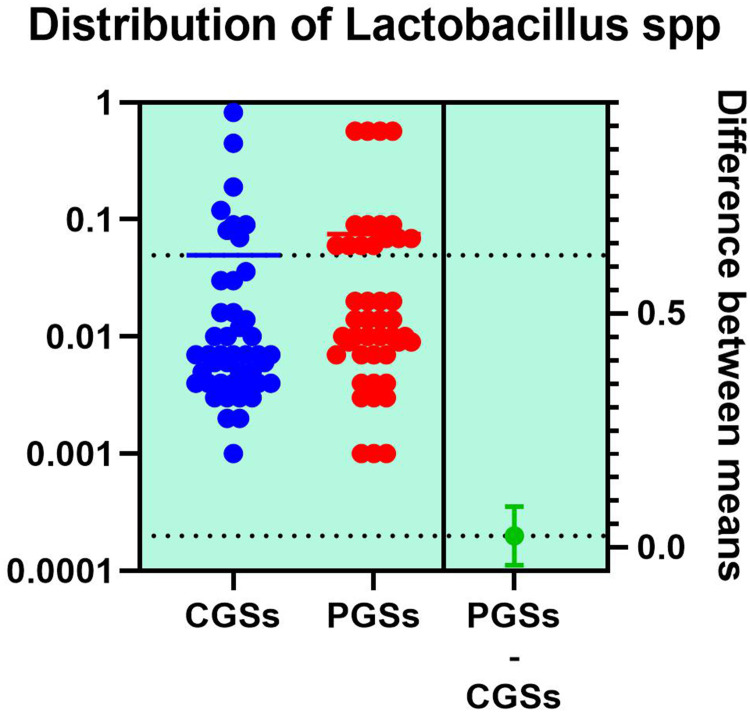
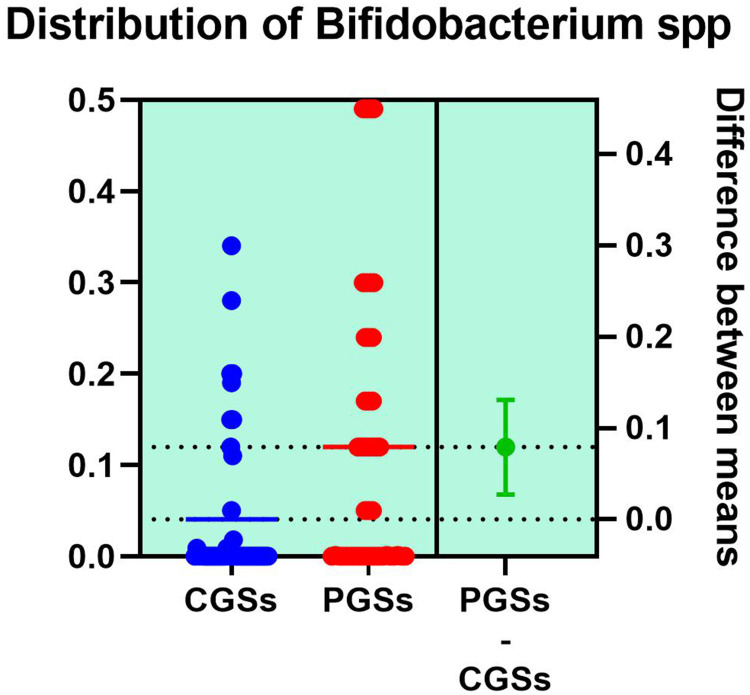
As depicted in Figures 12–14, significant differences were noted between patients with CGSs vs PGSs, related to distribution of Bifidobacterium spp. range (0.04 vs. 0.112, p = 0.0033). No significant differences between the two groups related to Enterococcus spp. and Lactobacillus spp. range were observed.
Histograms of the quantitative distribution of the bacterial species involved in acetate/propionate production, in patients with GSD are represented in Figures 15–17.
Figure 15.
Quantitative distribution of the bacterial species producing acetate/propionate in patients with GSD.
Figure 16.
Quantitative distribution of the bacterial species producing acetate/propionate in patients with GSD.
Figure 17.
Quantitative distribution of the bacterial species producing acetate/propionate in patients with GSD.
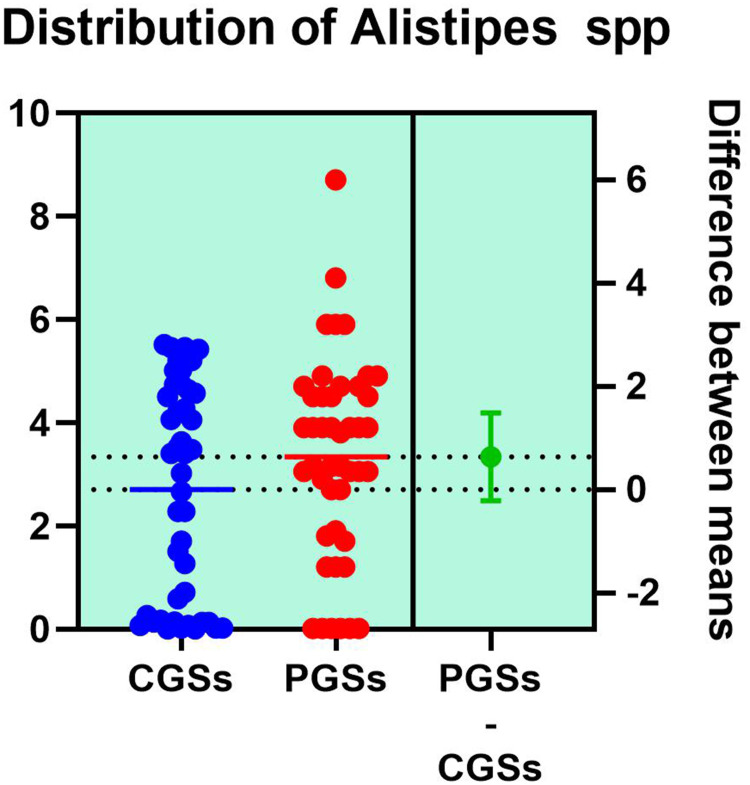
As presented in Figures 15–17, significant differences were observed in patients with CGSs vs. PGSs, related to Bacteroides spp. range distribution (10.15 vs. 13.17, p = 0.0149). No significant differences between the two groups, related to Alistipes spp. and Dorea spp. range were observed.
Histograms of the quantitative distribution of the bacterial species associated with mucin degradation in patients with GSD are represented in Figures 18–19.
Figure 18.
Quantitative distribution of the bacterial species associated with mucin degradation in patients with GSD.
Figure 19.
Quantitative distribution of the bacterial species associated with mucin degradation in patients with GSD.
As observed in Figures 18–19, no significant differences were noted between patients with CGSs vs those with PGSs, related to distribution of Akkermansia muciniphila and Prevotella spp. range.
Discussion
As others previously reported, elderly patients with CGSs are placed in a metabolic issued population, where conditions such as: AH, obesity, T2DM/IGT, dyslipidemia and NAFLD are frequently reported.34,35 Results of the present study highlighted that, by comparing to PGSs population, elderly patients affected by CGSs exhibited in a statistically significant percentage of cases lifestyle particularities, dietary habits with high saturated fat and low fiber ingestion, carbohydrates and lipids metabolic diseases, disturbances of GB motility with a decrease of the GBEF, as well as cardiovascular issues, such as: atherosclerosis, AH and its further complications. GSD and atherosclerosis seem to share some metabolic pathways and the risk for cardiovascular events stays elevated even after cholecystectomy. That is why assessment of cardiovascular risk before intervention is mandatory, while association to AH, obesity and T2DM is often seen in GSD, especially in those having cholesterol stones.36,37 As we observed in past and present studies, the increase of the GB volume with age up to atony of GB was also reported by others in imaging studies, some research noticing the decrease of GB motility in elderly patients with obesity and T2DM, aspects that place GB motility reduction in the risk factors panel for GSD.38,39 A prophylactic approach, related to lifestyle changes including diet customization, together with early diagnostic and prevention of diabetes and other metabolic conditions could substantially improve the overall morbidity and mortality risk associated to GSD.40,41 Besides the cardiovascular risk associated to GSD, another degenerative risk reported in older people with large GSs is GSD related carcinoma. Different kind of approaches are possible when it comes to managing GSD carcinoma risk. Some surgeons proposed prophylactic cholecystectomy especially in patients with morbid obesity and large sized stones.42 Other recent approaches are related to betulinic acid’s antiinflammatory and antiapoptotic properties, that seem to be very promising, at least in preliminary studies.43,44 Gut microbiota alterations could impact the physical characteristics of the BAs and metabolic associated conditions, representing risk factors for GSD, especially for cholesterol type gallstones.45,46 Our study has shown that older patients with GSD extensively exhibited gut DB, when compared to healthy controls. This study highlighted that population with CGSs by comparing to those with PGSs presented significantly older age and higher range of incidence and severity of DB. From the perspective of association of CGSs to various metabolic disorders, where there is lot of evidence for gut DB implication, these findings are not so surprising, but more expected. Some particular bacteria, especially those from the Firmicutes strain, by breaking down undigested food components, can supply the human body with simple carbohydrates and SCFAs, being involved in energy metabolic pathways that can eventually lead to being overweight. The detection of increased relative frequency of Firmicutes and of certain bacteria of the Firmicutes strain, such as Lactobacillus spp., could indicate a higher risk for obesity. By comparing to gut normobiosis, elderly population with CGSs vs those with PGSs exhibited modifications of normal distribution of dominant phyla. This particular microbiota footprint was also recently reported by others.47,48 This microbiota signature in senior patients with CGSs associated in more than half cases type 2 diabetes mellitus, as well as obesity, sedentary lifestyle and dietary particularities with high saturated fat intake and low fiber diet. Over the past decade, beside the effort of identification of various species of gut microbiota, a lot of research was oriented towards understanding the functionality of particular genera and the way they could intervene into various metabolic pathways. That is why functional bacterial groups and metabolic performance of several species came to be extensively studied. Faecalibacterium prausnitzii, member of Clostridium leptum subgroup, representing more than 5% of the total gut microbiota in healthy humans, is one of the most abundant butyric acid producing bacteria of the gut microbiome. It favorably modulates intestinal immune system, oxidative stress and colonocyte metabolism.49,50 Apparently it reduces intestinal inflammatory processes with inhibition of the pro-inflammatory interleukin 8. Production of butyric acid modulates differentiation of regulatory T cells with increasing the anti-inflammatory interleukin 10 and reducing the pro-inflammatory interleukin 12. It seems that butyric acid producing bacteria have also the ability to produce anti-inflammatory substances, like the salicylic acid even in the environmental milieu.51,52 Decrease of Faecalibacterium prausnitzii that was observed in patients with CGSs from the present study, could trigger subclinical gut and systemic inflammation and therefore a lot of pathologic conditions. Some experimental research highlighted the importance of butyric acid producing bacteria by restoration of gut microbiota balance in obese mice, where after treatment with Faecalibacterium prausnitzii an augmentation of adiponectin insulin sensitivity of the subcutaneous and visceral adipose tissue, as well as less inflamed adipose tissue and increased muscle mass linked to enhanced mitochondrial respiration were observed.53
Hepatic hypersecretion of cholesterol related to genetic and acquired dietary factors stays the top reason for CGSs formation. A lot of microbial intestinal factors linked to CGSs are associated to alterations of the bile salts and cholesterol absorption with inhibition of bile salts absorption and augmentation of cholesterol absorption. Therefore, these aspects could play an important role in the formation of gallstones.54,55 Ruminococcus spp., another important butyrate producing bacteria, significantly decreased in patients with CGSs from the present study. Some authors recently proposed that these bacteria to be considered markers for distinguishing patients with GSD from healthy controls.56
Elderly patients with CGSs from the present study displayed significant decrease of Akkermansia muciniphila bacteria, a representative of the mucin degrading bacteria. In healthy human subjects Akkermansia muciniphila was associated to low body weight, low body fat proportion, reduced adipose tissue inflammation and reduced insulin resistance. Abnormal mucin secretion secondary to a decrease of the functional mucin degrading bacteria result not only in many intestinal tumoral diseases, but also in risk factors for GSD. Recent studies highlighted that mucin-4 could be involved in gallstone formation.57,58 Some reviews focused on the role of Akkermansia muciniphila in diabetes, obesity, aortic lesions and atherosclerosis, as well as the use of this bacterium as a promising next-generation probiotic, its abundance assuring the improving of the metabolic condition with reduced metabolic endotoxemia, adiposity, insulin resistance and increase of glucose tolerance.59,60
A significant decrease of Prevotella spp. was also noted in senior patients with CGSs from the present study. Mucosal inflammation mediated by Prevotella spp. could promote systemic dissemination of various pro-inflammatory mediators, as well as bacteria and bacterial products, which in turn may trigger systemic diseases.61 A recent research hypothesized that abundance of Prevotella spp. may play a protective role against gallstones formation by regulation of the BAs composition, reducing metabolic endotoxaemia and protection of the mucosal gut barrier. An interesting question related to the utility of Prevotella spp. as a probiotic treatment for GSD has been asked.62
We noted that elderly patients with CGSs exhibited significant increase of LPS positive bacteria, associated with high levels of CRP, NAFLD and other metabolic issues. Several studies already reported a correlation between intestinal bacteria and the development of NAFLD. A shift in the metabolic function of intestinal bacteria is predominantly caused by dysbiosis. In the intestine, it leads to an increase in the permeability of intestinal mucosa for LPS and ultimately causes chronic inflammation. Concentration of bacterial metabolites in the blood, such as trimethylamine which is metabolized in the liver to trimethylamine-N-oxide (TMAO) correlates with the severity of steatohepatitis.63–65
Interestingly, several bacteria such as: Methanobrevibacter smithii, Bifidobacterium spp., Bifidobacterium animalis, Escherichia coli, Akkermansia muciniphila, Anaerotruncus colihominis and bacteria of the Bacteroidetes strain have the capability to reduce the production of high-calorie substances and therefore also influence the caloric intake. A relatively low frequency of these bacteria correlates with increased body weight, as we have also observed.66–69
The presence of reduced bacterial biodiversity, as we have also noted in the present study, especially in older patients with CGSs, has an additional unfavorable result by promoting inflammation, disruption of the mucosal barrier and dysregulation of BAs composition.70–72 It should be noted that gut microbiota modification is frequently reported in GSD even after cholecystectomy, especially in patients complaining of various dyspeptic complaints.73,74 Given these observations, understanding the insights of gut microbiota DB in elderly patients with GSD could result in new promising probiotic customized therapy.
Limitations are related to possible confounders, a lot of patients with GSD already having T2DM and IBS, conditions known for association to gut DB. Other statistical biases and potential drawbacks could have also intervened, given the cross-sectional study design and relatively small sized research population. Since study of gut microbiota’s insights in patients with GSD could result in new promising next-generation probiotic customized therapy, it is mandatory that these preliminary observations be further validated by larger prospective researches.
Conclusion
Middle-aged and elderly patients with GSD and clinical backgrounds characterized by a particular lifestyle, metabolic and gallbladder motility issues displayed significant modification of biodiversity, overall gut DB and alterations of several functional bacterial species, with a decrease of their metabolic performance.
Acknowledgments
The authors express their gratitude to “Bioclinica” Laboratories from Timisoara for technical support.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Stinton LM, Shaffer EA. Epidemiology of gallbladder disease: cholelithiasis and cancer. Gut Liver. 2012;6(2):172–187. doi: 10.5009/gnl.2012.6.2.172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Watanabe G, Hashimoto M, Matsuda M. Evaluation of new classification of Japanese gallbladder stones (1986) with chemical componential analysis. J-Stage. 2017;31(2):205–213. [Google Scholar]
- 3.Portincasa P, Moschetta A, Palasciano G. Cholesterol gallstone disease. Lancet. 2006;368(9531):230–239. doi: 10.1016/S0140-6736(06)69044-2 [DOI] [PubMed] [Google Scholar]
- 4.Portincasa P, Moschetta A, Petruzzelli M, Palasciano G, Di Ciaula A, Pezzolla A. Gallstone disease: symptoms and diagnosis of gallbladder stones. Best Pract Res Clin Gastroenterol. 2006;20:1017–1029. doi: 10.1016/j.bpg.2006.05.005 [DOI] [PubMed] [Google Scholar]
- 5.Radu D, Olariu S, Marinescu A, Georgescu D, Teodorescu M. Laparoscopic cholecystectomy, rate and predictors for conversion. Surg Endosc. 2011;25:54. [Google Scholar]
- 6.Demehri FR, Alam HB. Evidence-based management of common gallstone-related emergencies. J Intensive Care Med. 2016;31(1):3–13. doi: 10.1177/0885066614554192 [DOI] [PubMed] [Google Scholar]
- 7.Tazuma S, Unno M, Igarashi Y, et al. Evidence-based clinical practice guidelines for cholelithiasis 2016. J Gastroenterol. 2017;52(3):276–300. doi: 10.1007/s00535-016-1289-7 [DOI] [PubMed] [Google Scholar]
- 8.Machado FHF, Castro Filho HF, Babadopulos RFAL, Rocha HAL, Rocha JLC, Moraes Filho MO. Ursodeoxycholic acid in the prevention of gallstones in patients subjected to Roux,-en-Y gastric bypass1. Acta Cir Bras. 2019;14:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rowland I, Gibson G, Heinken A, et al. Gut microbiota functions: metabolism of nutrients and other food components. Eur J Nutr. 2018;57:1–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shanahan F. The colonic microbiota in health and disease. Gastroenterology. 2013;29:49–54. [DOI] [PubMed] [Google Scholar]
- 11.Festi D, Schiumerini R, Eusebi LH, et al. Gut microbiota and metabolic syndrome. WJG. 2014;20(43):16079. doi: 10.3748/wjg.v20.i43.16079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Georgescu D, Iurciuc MS, Petre I, et al. Chronic pelvic pain and irritable bowel syndrome: is subclinical inflammation bridging the gap? Rev Chim. 2019;70(9):3634–3637. doi: 10.37358/RC.19.10.7611 [DOI] [Google Scholar]
- 13.Pittayanon R, Lau JT, Yuan Y, et al. Gut microbiota in patients with irritable bowel syndrome—a systematic review. Gastroenterology. 2019;157:97–108. doi: 10.1053/j.gastro.2019.03.049 [DOI] [PubMed] [Google Scholar]
- 14.Georgescu D, Iurciuc M, Ionita I, et al. Portal vein thrombosis and gut microbiota: understanding the burden. Rev Chim. 2019;70(6):2181–2185. doi: 10.37358/RC.19.6.7301 [DOI] [Google Scholar]
- 15.Ancusa OE, Georgescu D, Iurciuc S, et al. Acute phase inflammatory proteins, gut dysbiosis and nonalcoholic fatty liver disease in patients with type 2 diabetes mellitus. Rev Chim. 2020;71(5):290–298. doi: 10.37358/RC.20.5.8136 [DOI] [Google Scholar]
- 16.Jin M, Qian Z, Yin J, Xu W, Zhou XJ. The role of intestinal microbiota in cardiovascular disease. Cell Mol Med. 2019;23(4):2343. doi: 10.1111/jcmm.14195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Georgescu D, Iurciuc M, Ionita I, et al. Migraine without aura and subclinical atherosclerosis in young females: is gut microbiota to blame? Medicina. 2019;55(12):786. doi: 10.3390/medicina55120786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sayin SI, Wahlström A, Felin J, et al. Gut microbiota regulates bile acid metabolism by reducing the level of tauro-beta muricholic acid, a naturally occurring FXR antagonist. Cell Metab. 2013;17:225–235. doi: 10.1016/j.cmet.2013.01.003 [DOI] [PubMed] [Google Scholar]
- 19.Ridlon JM, Kang DJ, Hylemon PB, Bajaj JS. Bile acids and gut microbiome. Curr Opin Gastroenterol. 2014;30(3):332–338. doi: 10.1097/MOG.0000000000000057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wu T, Zhang Z, Liu B, et al. Gut microbiota dysbiosis and bacterial community assembly associated with cholesterol gallstones in large-scale study. BMC Genomics. 2013;14:669. doi: 10.1186/1471-2164-14-669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ridlon JM, Wolf PG, Gaskins HR. Taurocholic acid metabolism by gut microbes and colon cancer. Gut Microbes. 2016;7:201–215. doi: 10.1080/19490976.2016.1150414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Garcia-Canaveras JC, Donato MT, Castell JV. Lahoz A Targeted profiling of circulating and hepatic bile acids in human, mouse, and rat using a UPLC-MRM-MS-validated method. J Lipid Res. 2012;53:2231–2241. doi: 10.1194/jlr.D028803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.American Diabetes Association diabetes. Diagnostic and classification of diabetes mellitus. Diabetes Care. 2014;37(suppl1):S81–S90. doi: 10.2337/dc14-S081 [DOI] [PubMed] [Google Scholar]
- 24.Grundy SM, Cleeman JI, Merz CNB, et al. Implications of recent clinical trials for the national cholesterol education program adult treatment Panel III guidelines. Circulation. 2004;110(2):227–239. doi: 10.1161/01.CIR.0000133317.49796.0E [DOI] [PubMed] [Google Scholar]
- 25.Lacy BE, Patel NK. Rome criteria and a diagnostic approach to irritable bowel syndrome. J Clin Med. 2017;6(11):99. doi: 10.3390/jcm6110099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hou TY, Chiang-Ni C, Teng SH. Current status of MALDI-TOF mass spectrometry in clinical microbiology. J Food Drug Anal. 2019;27(2):404–414. doi: 10.1016/j.jfda.2019.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ji B, Nielsen J. From next generation sequencing to systemic modeling of the gut microbiome. Front Genet. 2015;6:219. doi: 10.3389/fgene.2015.00219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Strong WL. Biased richness and evenness relationships within Shannon–Wiener index values. Ecoll Ind. 2016;67:703–713. doi: 10.1016/j.ecolind.2016.03.043 [DOI] [Google Scholar]
- 29.Lee JY, Keane MG, Pereira S. Diagnosis and treatment of gallstone disease. Practitioner. 2015;259(1783):15–19. PMID: 26455113. [PubMed] [Google Scholar]
- 30.Dodds W, Groh WJ, Darweesh RMA, Lawson TL, Kishk SMA, Kern MK. Sonographic measurement of gallbladder volume. AJR. 1985;145:1009–1011. doi: 10.2214/ajr.145.5.1009 [DOI] [PubMed] [Google Scholar]
- 31.Chasalani N, Younossi Z, Lanvine JE, et al. The diagnostic and management of NAFLD: practice guidance from AASLD. Hepatology. 2018;67:328–357. doi: 10.1002/hep.29367 [DOI] [PubMed] [Google Scholar]
- 32.Qiao T, Ma RH, Luo XB, Yang LQ, Luo ZL, Zheng PM. The systematic classification of gallbladder stones. PLoS One. 2013;8:e74887. doi: 10.1371/journal.pone.0074887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sook K, Seung-Jae M, Sang SL, Sung-Koo L, Myung-Hwan K. Classification and nomenclature of GS revisited. Yonsei Med J. 2003;44(4):560–570. [DOI] [PubMed] [Google Scholar]
- 34.Shaffer EA. Gallstone disease: epidemiology of GB stone disease. Best Pract Res Clin Gastroenterol. 2006;20:981–996. doi: 10.1016/j.bpg.2006.05.004 [DOI] [PubMed] [Google Scholar]
- 35.Hu KC, Wang HI, Chang WH, et al. Clinical presentation of patients from different age cohorts with biliary tract stone diseases. J Gastroenterol Hepatol. 2014;29:1614–1619. doi: 10.1111/jgh.12581 [DOI] [PubMed] [Google Scholar]
- 36.Wirth J, Giuseppe RD, Wientzek A, et al. Presence of gallstones and the risk of cardiovascular diseases: the EPIC-Germany cohort study. Eur J Prev Cardiol. 2015;22(3):326–334. doi: 10.1177/2047487313512218 [DOI] [PubMed] [Google Scholar]
- 37.Buleu FN, Luca CT, Tudor A, et al. Correlations between vascular stiffness indicators, OPG and 25- OH vitamin D3 status in heart failure patients. Medicina. 2019;55(6):309. doi: 10.3390/medicina55060309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Georgescu D, Basa N, Musta I, Coceala L, Georgescu C. Ultrasonographic study of gallbladder motility in patients with diabetes mellitus and autonomic neuropathy. Ultraschall der Medizin. 2008;29:S52–S53. doi: 10.1055/s-2008-1079943 [DOI] [Google Scholar]
- 39.Kariuki BN, Saidi H, Ndung’u B, Kaisha W, Ogeng’o J. Influence of age on gallbladder morphometry. Anat J of Africa. 2017;6(2):987–994. doi: 10.4314/aja.v6i2.160491 [DOI] [Google Scholar]
- 40.Jurca-Simina IE, Juganaru I, Iurciuc MS, et al. What if body fat percentage association with FINDRISC score leads to a better prediction of type 2 diabetes mellitus? Rom J Morphol Embryol. 2019;60(1):205–210. [PubMed] [Google Scholar]
- 41.Nogueira L, Freedman ND, Engels EA, Warren JL, Castro F, Koshiol J. Gallstones, cholecystectomy, and risk of digestive system cancers. Am J Epidemiol. 2014;179(6):731–739. doi: 10.1093/aje/kwt322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nicolov M, Duse AO, Georgescu D, et al. Preliminary studies on betulinic acid crystals with organic solvents. Rev Chim. 2016;67(7):1411–1414. [Google Scholar]
- 43.Wang H, Dong F, Wang Y, et al. Betulinic acid induces apoptosis of gallbladder cancer via repressing SCD1. Acta Biochim Sin. 2020;52(2):200–206. doi: 10.1093/abbs/gmz148 [DOI] [PubMed] [Google Scholar]
- 44.Wang Q, Hao C, Yao W, et al. Intestinal flora imbalance affects bile acid metabolism and is associated with gallstone formation. BMC Gastroenterol. 2020;20:59. doi: 10.1186/s12876-020-01195-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Grigor’eva IN, Romanova TI. Gallstone disease and microbiome. Microorganisms. 2020;8:835. doi: 10.3390/microorganisms8060835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rezasoltani S, Sadeghi A, Radinnia E, et al. The association between gut microbiota, cholesterol gallstones, and colorectal cancer. Gastroenterol Hepatol Bed Bench. 2019;12(Suppl1):S8–S13. [PMC free article] [PubMed] [Google Scholar]
- 47.Wang Q, Jiao L, He C, et al. Alteration of gut microbiota in association with cholesterol gallstone formation in mice. BMC Gastroenterol. 2017;17:74. doi: 10.1186/s12876-017-0629-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Miquel S, Martin R, Rossi O, et al. Faecalibacterium prausnitzii and human intestinal health. Curr Opin Microbiol. 2013;16:255–261. doi: 10.1016/j.mib.2013.06.003 [DOI] [PubMed] [Google Scholar]
- 49.Huang XL, Zhang X, Fei XY, et al. Faecalibacterium prausnitzii supernatant ameliorates dextran sulfate sodium induced colitis by regulating Th17 cell differentiation. World J Gastroenterol. 2016;22:5201–5210. doi: 10.3748/wjg.v22.i22.5201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Verdier J, Luedde T, Sellge G. Biliary Mucosal Barrier and Microbiome. Viszeralmedizin. 2015;31(3):156–161. doi: 10.1159/000431071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Molinero N, Ruiz L, Milani C, et al. The human gallbladder microbiome is related to the physiological state and the biliary metabolic profile. Microbiome. 2019;7:100. doi: 10.1186/s40168-019-0712-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Munukka E, Rintala A, Toivonen R, et al. Faecalibacterium prausnitzii treatment improves hepatic health and reduces adipose tissue inflammation in high-fat fed mice. ISME J. 2017;11:1667–1679. doi: 10.1038/ismej.2017.24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jia X, Lu S, Zeng Z, et al. Characterization of gut microbiota, bile acid metabolism, and cytokines in intrahepatic cholangiocarcinoma. Hepatology. 2019;71:893–906. doi: 10.1002/hep.30852 [DOI] [PubMed] [Google Scholar]
- 54.Lang S, Fairfied B, Gao B, et al. Changes in the fecal bacterial microbiota associated with disease severity in alcoholic hepatitis patients. Gut Microbes. 2020;12:1785251. doi: 10.1080/19490976.2020.1785251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang W, Zhao J, Gui W, et al. Tauroursodeoxycholic acid inhibits intestinal inflammation and barrier disruption in mice with non-alcoholic fatty liver disease. Br J Pharmacol. 2018;175:469–484. doi: 10.1111/bph.14095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Di Ciaula A, Wang DQ, Portincasa P. An update on the pathogenesis of cholesterol gallstone disease. Curr Opin Gastroenterol. 2018;34:71–80. doi: 10.1097/MOG.0000000000000423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Paone P, Cani PD. Mucus barrier, mucins and gut microbiota: the expected slimy partners? Gut. 2020;69:2232–2243. doi: 10.1136/gutjnl-2020-322260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hu FL, Chen HT, Guo F, et al. Biliary microbiota and mucin 4 impact the calcification of cholesterol gallstones. Hepatobiliary Pancreat Dis Int. 2021;20:61–66. doi: 10.1016/j.hbpd.2020.12.002 [DOI] [PubMed] [Google Scholar]
- 59.Hasani A, Ebrahimzadeh S, Hemmati F, Khabbaz A, Hasani A, Gholizadeh P. The role of Akkermansia muciniphila in obesity, diabetes and atherosclerosis. J Med Microbiol. 2021;70(10). PMID: 34623232. doi: 10.1099/jmm.0.001435 [DOI] [PubMed] [Google Scholar]
- 60.Hagi T, Belzer C. The interaction of Akkermansia muciniphila with host-derived substances, bacteria and diets. Appl Microbiol Biotechnol. 2021;105(12):4833–4841. doi: 10.1007/s00253-021-11362-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Larsen JM. The immune response to Prevotella bacteria in chronic inflammatory disease. Immunology. 2017;151(4):363–374. doi: 10.1111/imm.12760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yang C, Qiang W, Wenqi G, Biao M, Dongbo X, Chenjun H. Changes and correlations of the intestinal flora and liver metabolite profiles in mice with gallstones. Front Physiol. 2021;12:1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Boursier J, Mueller O, Barret M, et al. The severity of nonalcoholic fatty liver disease is associated with gut dysbiosis and shift in the metabolic function of the gut microbiota. Hepatology. 2016;63(3):764–775. doi: 10.1002/hep.28356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Salzman ET, Palacios T, Thomsen M, Vitetta L. Intestinal microbiome shifts, dysbiosis, inflammation and nonalcoholic fatty liver disease. Front Microbiol. 2018;9:61. doi: 10.3389/fmicb.2018.00061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Albhaisi SAM, Bajaj JS. The influence of the microbiome on NAFLD and NASH. Clin LivDis. 2021;17(1):15–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Muscogiuri G, Cantone E, Cassarano S, et al. Gut microbiota: a new path to treat obesity. Int J Obes. 2019;Supp 9:10–19. doi: 10.1038/s41367-019-0011-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Abenavoli L, Scarpellini E, Colica C, et al. Gut microbiota and obesity: a role for probiotics. Nutrients. 2019;11:2690. doi: 10.3390/nu11112690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Aoun A, Darwish F, Hamod N. The influence of the gut microbiome on obesity in adults and the role of probiotics, prebiotics, and synbiotics for weight loss. Prev Nutr Food Sci. 2020;25(2):113–123. doi: 10.3746/pnf.2020.25.2.113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Jie Z, Yu X, Liu Y, et al. The baseline gut microbiota directs dieting-inducing weight loss trajectories. Gastroenterology. 2021;160(5):2029–2042. doi: 10.1053/j.gastro.2021.01.029 [DOI] [PubMed] [Google Scholar]
- 70.Keren N, Konikoff FM, Paitan Y, et al. Interactions between the intestinal microbiota and bile acids in gallstones patients. Environ Microbiol Rep. 2015;7(6):874–880. doi: 10.1111/1758-2229.12319 [DOI] [PubMed] [Google Scholar]
- 71.Petrov WA, Fernández-Peralbo MA, Derks R, et al. Biliary microbiota and bile acid composition in cholelithiasis. Biomed Res Int. 2020:8. doi: 10.1155/2020/1242364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Winston JA, Theriot CM. Diversification of host bile acids by members of the gut microbiota. Gut Microbes. 2020;11(2):158–171. doi: 10.1080/19490976.2019.1674124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yoon WJ, Kim H-N, Park E, et al. The impact of cholecystectomy on the gut microbiota: a case-control study. J Clin Med. 2019;8(1):79. doi: 10.3390/jcm8010079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Georgescu D, Caraba A, Ionita I, et al. Dyspepsia and gut microbiota in female patients with postcholecystectomy syndrome. Int J Women’s Health. 2022;14:41–56. doi: 10.2147/IJWH.S342882 [DOI] [PMC free article] [PubMed] [Google Scholar]