Abstract
Diabetes mellitus, which affects more than 463 million people globally, is caused by the autoimmune ablation or functional loss of insulin-producing β-cells, and prevalence is projected to continue rising over the next decades. Generating β-cells to mitigate the aberrant glucose homeostasis manifested in the disease has remained elusive. Substantial advances have been made in producing mature β-cells from human pluripotent stem cells that respond appropriately to dynamic changes in glucose concentrations in vitro and rapidly function in vivo following transplantation in mice. Other potential avenues to produce functional β-cells include: transdifferentiation of closely related cell types (for example, other pancreatic islet cells such as α-cells, or other cells derived from endoderm); the engineering of non-β-cells that are capable of modulating blood sugar; and the construction of synthetic ‘cells’ or particles mimicking functional aspects of β-cells. This Review focuses on the current status of generating β-cells via these diverse routes, highlighting the unique advantages and challenges of each approach. Given the remarkable progress in this field, scalable bioengineering processes are also discussed for the realization of the therapeutic potential of derived β-cells.
More than 463 million people are affected by diabetes mellitus globally, which is projected to rise to 700 million by 2045 (REF.1). Diabetes mellitus therefore constitutes a global epidemic and is a pressing and growing health problem. The disease not only affects the quality of life of patients and their families but also exerts a tremendous burden on health-care systems across the world. For example, the economic burden in the US alone was estimated at $327 billion in 2017, up from $245 billion in 2012 (REF.2).
Artificial pancreas.
A mechanical device devoid of cells that integrates glucose sensors with insulin pumps to dispense insulin as needed with minimal input from the patient.
Diabetes mellitus is a chronic condition characterized by abnormal glucose metabolism due to insufficient production of the pancreatic hormone insulin. Patients are classified into two main categories: type 1 diabetes mellitus (T1DM) is mediated by an autoimmune destruction of insulin-producing β-cells, whereas type 2 diabetes mellitus (T2DM) ensues when β-cells are unable to meet the increased physiological demand for insulin. Currently, there is no cure for diabetes mellitus and exogenous insulin administration is essential for the treatment of all patients with T1DM and those with late stage T2DM.
Although life saving, the current method of insulin delivery via subcutaneous injection does not mimic the fine temporal glucose control provided by the endogenous insulin-producing β-cells and other islet cells of the pancreas. Large population studies have shown that tight glucose control is essential to prevent not just hypoglycaemia that can result in coma and death3, but also long-term microvascular and macrovascular complications stemming from hyperglycaemia4–6. Technological advances, such as continuous glucose monitoring and the artificial pancreas, have improved patient outcomes7,8, yet these tools still fall short in providing optimal long-term glycaemic control as measured via HbA1C8–10. Results from a multicentre trial indicated that the percentage of time that blood glucose is maintained within the target glycaemic range remains suboptimal, even after adoption of automated methods of insulin delivery in patients with T1DM11. Acceptance of these technological advances is also fairly low, as patients are hesitant to wear several bulky devices such as sensors, pumps and monitors, all at once. Thus, although recombinant insulin provides a life-saving short-term solution, therapies resulting in permanent reconstitution of physiological blood glucose homeostasis are highly desirable in the long-term.
We propose that a central component of therapies for diabetes mellitus should be the restoration of the missing and/or dysfunctional pancreatic β-cells, the underlying cause of both T1DM and T2DM. The remarkable success of islet transplantation, which leads to insulin independence in patients with T1DM for several years12, furnishes the proof-of-principle for cell replacement approaches; however, its widespread application is currently impractical given the scarcity of donor tissue. These results have fuelled efforts to generate functional β-cells, either by inducing endogenous regeneration, or via differentiation of human pluripotent stem cells (hPSCs). In addition to their therapeutic potential, functional β-cells could serve as valuable tools for advancing the still limited knowledge of human β-cell biology.
Here, we review current efforts to generate functional human β-cells from diverse sources. First, we define the key features of a functional β-cell that dictate its identity and highlight differences between β-cells from neonates, children and adults. Next, we discuss several promising avenues to generate functional β-cells. The main focus of this section is on advances in the area of directed differentiation of hPSCs into β-cells; however, other approaches are highlighted as well, including the transdifferentiation of various somatic cells into insulin-producing cells and the engineering of synthetic cells that mimic the functions of β-cells. Finally, we summarize the challenges associated with the mass production of β-cells from hPSCs in scalable bioreactors, the applicability of quality-by-design concepts and downstream processing required to generate the final ‘transplantable product’.
What constitutes a functional β-cell?
Islets of Langerhans are specialized micro-organs residing in the pancreas that are responsible for tightly regulating blood sugar levels through the coordinated release of hormones. The β-cells in islets rapidly sense increases in blood glucose concentrations after a meal and release appropriate amounts of insulin, thereby enabling sugar uptake by the liver and peripheral tissues. Upon the lowering of blood glucose levels, mature β-cells respond by terminating insulin secretion13. By contrast, islet-resident α-cells release glucagon that stimulates hepatic gluconeogenesis and glycogenolysis, thus raising blood glucose levels and preventing dangerous hypoglycaemia. Inhibition of insulin and glucagon secretion is mediated via negative feedback by a third islet hormone, somatostatin, released by adjacent δ-cells14. These finely tuned processes together maintain an average blood glucose concentration at <5.6 mM in a healthy adult.
The machinery in β-cells is primed to sense extracellular glucose and to rapidly secrete insulin in real time, in a glucose concentration-dependent manner. Once glucose enters the β-cell via specialized glucose transporters, the carbohydrate is promptly metabolized via glycolysis to pyruvate, which is shuttled into the mitochondria for oxidative phosphorylation (OxPhos). The ATP generated in the process changes the phosphate potential (ATP to ADP ratio) of the cell leading to closure of ATP-sensitive K+ (KATP) channels, which in turn causes membrane depolarization and the influx of calcium ions (Ca2+) from both the extracellular environment and intracellular stores. The increase in Ca2+ concentration induces translocation of insulin-packed secretory granules to the plasma membrane, vesicle–membrane fusion and release of the hormone. This rapid KATP-dependent insulin secretion is further sustained and augmented by ‘amplifying’ pathways, which are KATP-independent but β-cell metabolism-dependent, thereby leading to the observed biphasic glucose-stimulated insulin secretion (GSIS)15–19.
The metabolic configuration of β-cells is attuned to couple insulin secretion to glucose metabolism. Accurate glucose sensing is facilitated in β-cells by the low-affinity (high KM) glucose sensor — glucokinase (GCK) — instead of other hexokinases (for example, HK1 or HK2) present in other tissues, as well as the low-affinity (high KM) transporter GLUT1 (GLUT2 in rodents). Similarly, proteins that interfere with the stimulus–secretion coupling are suppressed in mature β-cells. Such proteins include lactate dehydrogenase (LDHA), which shunts glucose to lactate instead of pyruvate, or the monocarboxylate transporter 1 (encoded by SLC16A1), which mediates pyruvate and lactate efflux from the cell20,21. Notably, these ‘disallowed genes’ (HK2, HK1, LDHA and SLC16A1) are expressed at higher levels in immature β-cells and/or neonatal islets that hyper-secrete insulin at low glucose concentrations20,21. Thus, a switch in gene expression occurs during maturation of β-cells, where disallowed genes are repressed and those essential for insulin transcription (for example, NEUROD1, NKX6–1, PDX1, PAX6, MAFB, MAFA and GLIS3), processing and packaging (SLC30A8, CHGA, CHGB, PCSK1/3 and PCSK2) and secretion (ABCC8, KCNJ11, KCNK3, GCK and GLUT1) are upregulated22. In particular, the transcription factors NKX6.1, NEUROD1, MAFA and PAX6 facilitate maturation and maintain β-cell identity23–26. Chromogranin-A, chromagranin-B (encoded by CHGA and CHGB, respectively), PCSK1/3, PCSK2 and ZnT8 (encoded by SLC30A8) are localized in the dense core insulin granules. Furthermore, chromogranin-A and chromogranin-B are involved in the generation of insulin granules, ZnT8 is a transporter that specifically imports zinc, which is essential for crystallization of insulin, and PCSK1/3 and PCSK2 are prohormone convertases that process proinsulin to insulin. KATP channels, the upsteam mediators of secretion, consist of four sulfonylurea receptor 1 (encoded by ABCC8) subunits and four KCNJ11 subunits.
Of note, immature β-cells (present in neonatal islets) display a greater sensitivity to calcium at low basal glucose concentrations, which causes increased basal insulin secretion and poor GSIS. Calcium sensitivity itself is plastic and decreases during β-cell maturation in mice, via rising levels of synaptogamin 4 (REF.27). Additional intracellular mechanisms and processes essential for full β-cell functionality are listed in BOX 1.
Box 1 |. Properties of a functional β-cell.
Dynamic biphasic insulin secretion upon stimulation with nutrients147
Responsiveness to signals from the gut and nervous system148,149
Rapid shutdown of insulin secretion upon removal of stimulus147
Expression of the full component of genes regulating insulin synthesis, packaging into granules, glucose sensing, stimulus–secretion coupling and insulin exocytosis150
Repression of disallowed genes that could cause aberrant insulin secretion20
Active mitochondrial redox shuttles for the production of mitochondrial coupling factors152
Formation of SNARE protein complexes involved in docking and exocytosis of insulin granules, and genes regulating proper calcium sensitivity27,153
Emerging evidence of co-secretion of amylin and urocortin 3 with insulin to regulate the activity of other endocrine cells in the islet13,153
β-cell maturation
Neonatal β-cells (from infants <1 year of age) are immature in that they secrete insulin at low glucose concentrations, thus having a reduced glucose threshold for GSIS. Unlike neonatal β-cells, juvenile β-cells (from children between 1 and 9 years of age) behave like cells from adults in dynamic GSIS assays, except that they release lower quantities of insulin28. Like adult β-cells, juvenile β-cells also respond to stimulation with various secretagogues29–31, thereby indicating that human β-cells are functionally mature by 1 year after birth.
Despite their similarities in function, deep sequencing reveals differences between the transcriptomes of juvenile and adult β-cells. For example, although the transcription factor MAFB is expressed at comparable levels in both populations, the other closely related member of the family — MAFA — is highly expressed only in adult cells28. In addition, proliferative markers are reduced and transcription factors such as SIX2 and SIX3 that mediate insulin secretion are enriched in adult β-cells28. Whether transcription factors that are enriched in adult β-cells have other roles besides conferring increased secretory capacity is unclear. Further studies are needed to elucidate additional changes in β-cell functionality and/or physiology that might occur upon assuming the adult state, including the observation made in a 2020 study that adult β-cells contain lipid droplets, whereas juvenile islets do not32.
Interestingly, despite being fully differentiated, adult β-cells are heterogeneous with regard to their insulin secretory capacities, mitochondrial function, calcium signalling and proliferative properties. Maturity is thus not solely defined by the expression of key markers such as PDX1, NKX6.1 and MAFA, or the presence of high insulin expression. For example, a diverse subtype of adult β-cells known as ‘hub’ cells coordinate and synchronize islet-wide calcium and insulin secretory responses. These cells display hyperpolarized mitochondria and high expression of GCK but contain reduced levels of insulin, PDX1 and NKX6.1 (REF.33). Furthermore, two subpopulations of adult β-cells are distinguished by the novel Wnt/PCP effector Flattop (FLTP) and have distinct functional properties. FLTP− β-cells are more proliferative and respond to physiological demands such as pregnancy and convert to FLTP+ β-cells, which are more glucose-responsive34. This plasticity is becoming apparent among various β-cell subtypes and is essential in maintaining islet functionality during metabolic stress and diseased states, as detailed elsewhere35. What controls the plasticity and interconversion of subtypes is less clear and requires further investigation.
Paths to a functional β-cell
Several routes are envisioned to generate functional β-cells from progenitor cells. One option is to replicate the signalling events that control β-cell formation during human pancreas development. Another is to exploit the plasticity of closely related endoderm-derived cell types such as pancreatic non-β-cells, and cells residing in the liver, stomach and intestine, coaxing them to adopt a β-cell phenotype. Lastly, the concept of engineering a synthetic ‘cell’ that possesses the functional properties of a bona fide β-cell is gaining traction, given accumulating advances in biomolecular engineering.
Directed differentiation from hPSCs.
Human PSCs include embryonic stem cells (ESCs) and induced pluripotent stem cells (iPSCs). Human ESCs (hESCs) were first derived from the inner cell mass of blastocysts more than two decades ago and are capable of extensive self-renewal and differentiation to cell types of all three germ layers36. By contrast, iPSCs are obtained by reprogramming somatic cells such as peripheral blood mononuclear cells or dermal fibroblasts to the pluripotent state using defined factors37,38. Human PSCs ushered in the era of regenerative medicine, promising an unlimited source of all types of therapeutic cells, including pancreatic β-cells. Since their discovery, several groups have invested considerable effort and resources in trying to generate functional β-cells from these cells. In early studies, hESCs were cultured as embryoid bodies that allow spontaneous differentiation to occur. Importantly, scattered insulin-positive cells were observed in such cultures, thereby proving the possibility of β-cell formation from hESCs39,40. However, spontaneous differentiation methods also meant that the direction of differentiation was largely uncontrolled.
Later efforts focused on translating the knowledge gleaned from mouse embryonic development and signalling pathways to direct the specification of hESCs towards the pancreatic lineage in a stepwise manner. Directed differentiation entails the exposure of hESCs to physiologically relevant cues, which prompts fate transition through definitive endoderm41 and pancreatic endoderm42 to hormone-expressing cells43,44. Most of the resulting cells generated early on were polyhormonal (for example, simultaneously expressing both insulin and glucagon), thus resembling immature endocrine cells rather than mature islet cells45. Additional signs of immaturity included the absence of essential β-cell markers such as the NKX6.1 transcription factor. Further optimization of directed specification protocols led to the emergence of monohormonal insulin-producing cells, co-expressing NKX6.1 (REFS46–48) through modification of the composition and the timing of addition of the soluble signalling factors, as well as utilization of small molecules with less variable activity than regular growth factors and cytokines. Transplantation of these insulin-positive cells in streptozotocin-treated rodents reversed diabetes within 40 days of engraftment46. This finding was a considerable improvement over previous reports wherein prolonged engraftment of pancreatic progenitor cells for more than four months was necessary to rescue diabetes42,49. However, the β-cells obtained from these protocols were only marginally functional in vitro, exhibiting partial GSIS, and did not resemble fully mature human β-cells46–48.
Single-cell RNA sequencing (scRNA-seq) has provided a more comprehensive view of the wide variety of populations obtained in hPSC differentiations50,51. Surprisingly, in addition to β-cells, two major fractions of cells present were α-like cells expressing markers such as GCG, ARX and IRX2, but also INS and enterochromaffin-like cells normally found in the intestine expressing markers including CHGA, TPH1, LMX1A and SLC18A1. The enterochromaffin cells might represent a previously unknown lineage of the pancreas or their appearance in β-cell cultures indicates the need for stricter control over hPSC commitment to the pancreas versus the intestine to obtain organ-specific endocrine cells. A population of non-endocrine (SOX9+) cells was also detected50. These SOX9+ cells could constitute either uncommitted pancreatic progenitors or cells fated to the pancreatic ductal tree. The heterogeneity in the composition of cells obtained at the end of the stem cell differentiation process, spanning various lineages not present in native human islets, suggests that enrichment strategies to form islet-like clusters as well as additional steps for improving the efficiency of differentiation must be taken.
Exciting advances in the field in 2019 have led to the generation of hESC-derived β-cells that display dynamic insulin secretion properties that largely mirror those of native human islets52,53 (FIG. 1). Islets consist of pseudo-epithelial hormone-producing cells, whose optimal function necessitates close contact with other islet cells. The native architecture of islets is largely recapitulated in 3D rather than 2D differentiation cultures. Moreover, sorting and re-aggregation of stem cell-derived immature endocrine cells at the final stage of differentiation more closely mimic conditions that exist during embryonic islet formation52. In addition to dynamic function, β-cells derived under these conditions exhibit active calcium signalling, functional KATP channels and mitochondrial OxPhos upon glucose stimulation52. Specifically, endocrine cell clustering induces metabolic maturation by activating mitochondrial respiration, a central component of stimulus–secretion coupling in mature β-cells. Another key feature of the latest differentiation approaches is the inclusion of steps to reduce cell cluster size during the process52,53. Elimination of TGFβ inhibitors, usually added to differentiating cultures, following re-aggregation53 was also found to increase maturation. Of note, there are conflicting reports indicating that TGFβ signalling either supports54,55 or impairs GSIS56.
Fig. 1 |. Advances in the generation of mature β-cells from hPSCs and their application for diabetes mellitus cell therapy.
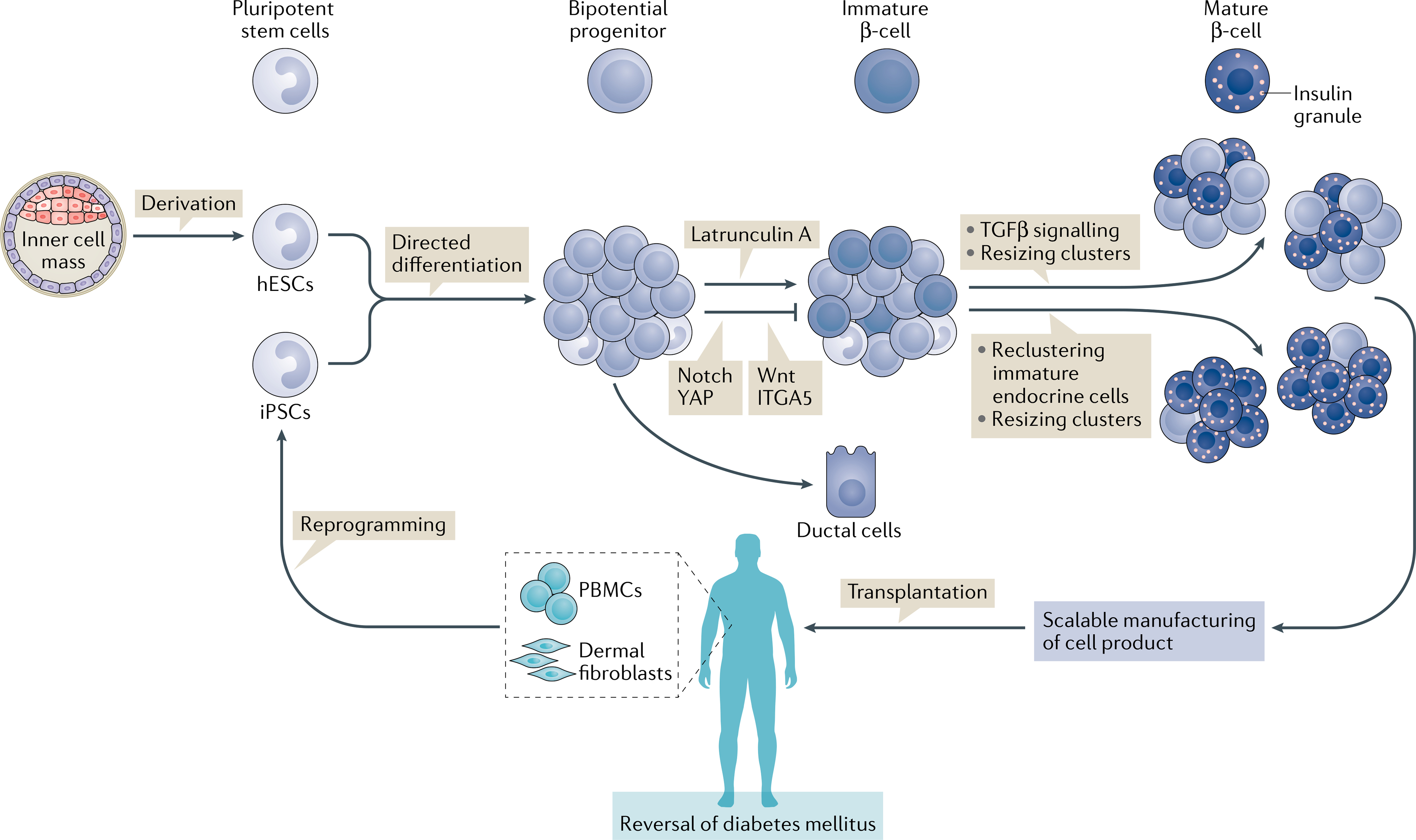
Human pluripotent stem cells (hPSCs) include embryonic stem cells (ESCs) and induced pluripotent stem cells (iPSCs). ESCs are derived from the inner cell mass of blastocyst stage embryos and induced pluripotent stem cells (iPSCs) are obtained by reprogramming somatic cells of patients such as peripheral blood mononuclear cells (PBMCs) or dermal fibroblasts. hPSCs can be converted to mature β-cells by directed differentiation through modulation of signalling pathways active during human pancreas formation. Efforts from 2015 onwards have focused on further promoting endocrine commitment from bipotential progenitors by inhibition of actin polymerization126, YAP125,145, Wnt146, ITGA5125 or Notch, and by adding steps that closely mimic islet formation in vivo to the final stage of differentiation. Isolation of immature endocrine cells and re-aggregation into smaller islet-like assemblies promotes functional and metabolic maturation52. New surface markers have been discovered that allow enrichment of endocrine cells50. Resizing of clusters and removal of TGFβ inhibitor after re-aggregation causes β-cells to acquire dynamic insulin secretion properties53. Ultimately, mature β-cell products need to be manufactured at the clinical scale and transplanted in immunoprotective devices and/or with immunosuppression to reverse diabetes mellitus in patients. hESCs, human ESCs.
Macroencapsulation devices.
Sealed devices constructed out of a selectively permeable membrane that are filled with cells either free floating or in a matrix, wherein the cells can still exert their therapeutic effect.
A serious concern with stem cell-derived therapeutic products is the presence of undifferentiated or partially differentiated cells that might interfere with the activity of the desired cell types or even be tumorigenic. Optimizing the generation of desired cell populations, whilst minimizing that of unwanted cell types during in vitro differentiation, is critical for clinical translation. Clinical trials investigating the use of transplantation of pancreatic endoderm have been initiated in patients with T1DM; however, in vivo maturation of cells from such early stages of differentiation in animal models is highly variable and depends on the site of transplantation, delivery device and the circulatory microenvironment57–59. Preliminary results from the first clinical trial with pancreatic endodermal cells in immunoprotective macroencapsulation devices highlighted the difficulties for this approach with minimal tissue engraftment and differentiation into insulin-producing cells60. In a subsequent trial, modification of the device allowing direct vascularization of the engrafted cells resulted in better differentiation; however, the level of insulin production and the number of insulin-positive cells remained low61. Grafts in mice from further differentiated hormone-expressing populations can also form cysts, which are structures consisting of cells with duct properties that might continue to grow over time, as long as progenitor cells are present in the final mix52,62. These observations argue for the need to generate fully mature endocrine cells for cell therapies.
Given the inability to differentiate cells at 100% efficiency, sorting strategies to yield cell clusters that contained only differentiated cell types resembling native islets were implemented in studies published in 2019. For example, using a GFP reporter under the control of the insulin promoter or antibodies against the cell surface marker CD49a (otherwise known as ITGA1), stem cell-derived β-cells can be enriched by more than 80–90%50,52. Although the strategies were designed to purify β-cells, they in fact resulted in enrichment of immature pan-endocrine cells that are capable of giving rise to other hormone-producing islet cell types as well. Increasing evidence points to intricate regulation of glucose via the interplay of α-cells, β-cells and δ-cells63. Arguably, generating the full spectrum of islet endocrine cells, rather than just β-cells, is likely to improve glucose control upon transplantation into patients with diabetes mellitus. Directed differentiation of hESCs towards α-cells, albeit immature, has been reported64, and similar efforts are underway to generate δ-cells (BOX 2). The ultimate goal of assembling the various islet cell types into a functional unit with defined size, architecture and composition that resembles the endogenous human islet, would benefit from the identification and use of cell surface antibodies specific to each endocrine cell type.
Box 2 |. Advances in the generation of non-β-cell islet cell types from hPSCs.
Human embyronic stems cells (hESCs) have been differentiated into α-cells via a 4-week, six-stage protocol64. About 65% of cells were monohormonal for glucagon at the end of the differentiation process. Cells also expressed ARX, a key α-cell transcription factor.
Low glucose levels, arginine, potassium chloride or carbachol triggered glucagon secretion. Conversely, treatment with a somatostatin analogue or high glucose levels suppressed glucagon release.
Glucagon secretion was induced in vivo upon arginine challenge and transplanted cells maintained the α-cell phenotype in grafts.
The production of GLP1 and GLP2 derived from proglucagon indicates the immature state of hESC-derived α-cells.
Efforts to develop protocols to generate δ-cells, ε-cells and pancreatic polypeptide cells are underway.
hPSCs, human pluripotent stem cells
In conclusion, the aforementioned advances in stem cell-derived β-cell differentiation protocols underscore the need to finely tune fate decisions, in order to obtain highly pure populations of endocrine cells. The latest protocols permit the generation of other islet hormone-producing cells in addition to β-cells, and reconstructing the functional equivalents of human islets from stem cells for cell therapy seems within reach.
Transdifferentiation from closely related cell types.
The pancreas arises from the posterior foregut region of the developing embryo, an area that also gives rise to the posterior stomach, liver and proximal gut65. Given the close ontogenetic relationship among these tissues, the potential for interconversion between cell types from these regions is not surprising (FIG. 2). Plasticity is especially pronounced between pancreatic α-cells and β-cells66–70; for example, inhibition of ARX or overexpression of PAX4, MAFA and PDX1 in mice induces α-cell to β-cell conversion, suggesting a possible alternative approach to replacing β-cells in diabetes mellitus. A 2019 study demonstrated such plasticity in human α-cells by lineage tracing and reprogramming with MAFA and PDX1. The converted human insulin-producing cells retained α-cell features as evidenced by transcriptome and proteome analysis, including the expression of ARX but secreted insulin and reversed diabetes for 6 months in mice71.
Fig. 2 |. Transdifferentiation of closely related endoderm-derived somatic cells to β-like cells.
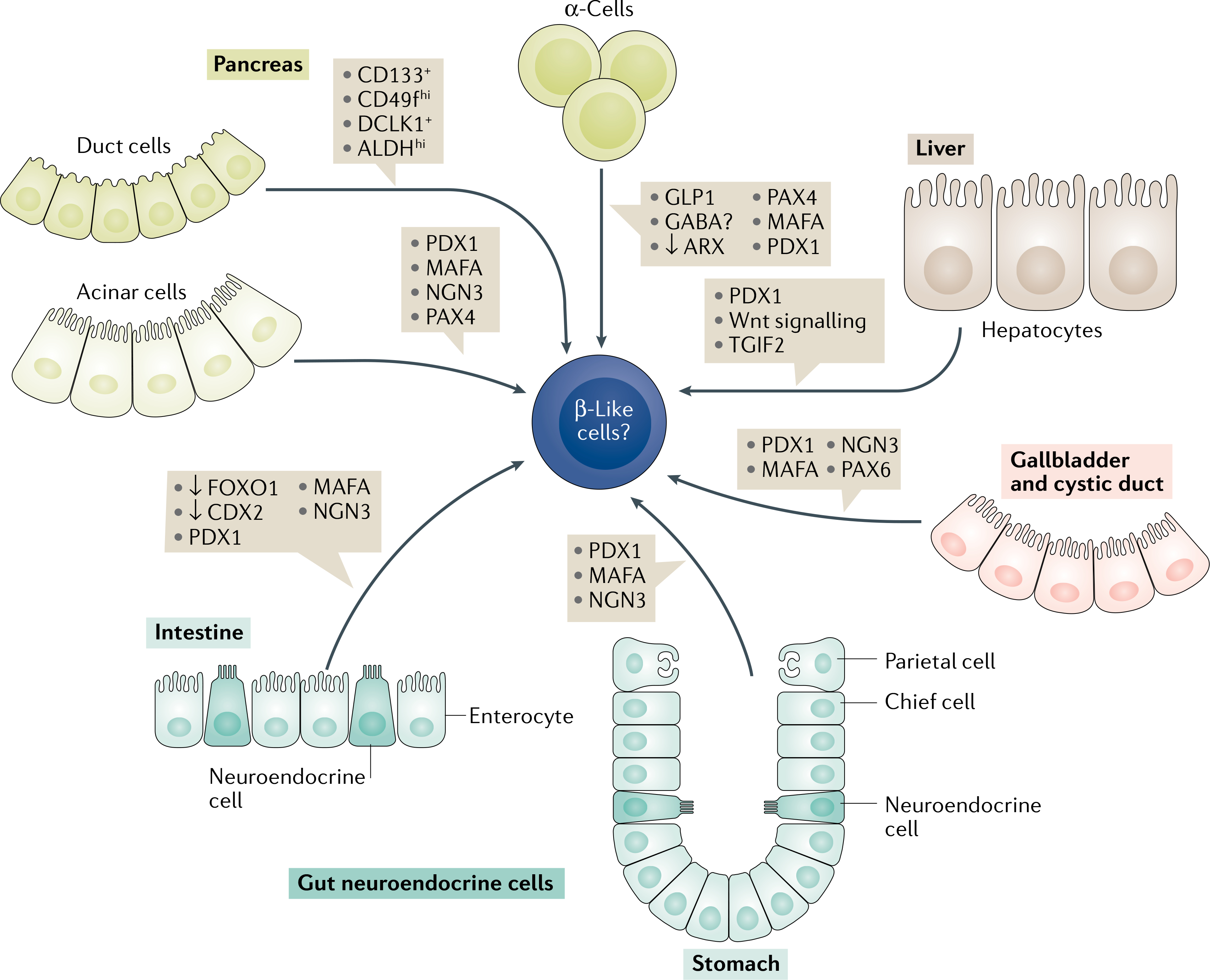
β-Like cells can be derived from other pancreatic cell types such as: acinar cells, by adenoviral reprogramming with PDX1, MAFA, NGN3 (referred to as the PMN-cocktail) and PAX4 (REFS85–87); from α-cells by overexpression of PAX4, MAFA, PDX1 and inhibition of ARX70,75,76; or from progenitor cells expressing CD133, CD49fhi, DCLK1 and ALDHhi lining the ductal tree84. Hepatic and associated extrahepatic tissues share similar developmental programmes with the adjacent pancreas, and hence activation of few key pancreatic markers such as PDX1 (REFS88,89) or TGIF2 (REF.92) or Wnt signalling91 is sufficient to convert liver or gallbladder tissue to β-like cells93. Another source of β-like cell generation are the gut neuroendocrine cells that highly resemble pancreatic endocrine cells. Treatment of gastric endocrine or enteroendocrine cells with the PMN cocktail96,97 in addition to FOXO1 (REF.94) and CDX2 inhibition induces transdifferentiation into cells that possess β-cell characteristics.
Of note, α-cell to β-cell transdifferentiation was also reported to occur naturally upon β-cell loss in mice from puberty to adulthood, although almost complete ablation of β-cells is required to elicit this response72. By contrast, prior to puberty, β-cell loss upon injury is compensated for by conversion of somatostatin-producing δ-cells73. β-Cells also originate from α-cells located at distinct niches within the periphery of rodent islets74. Furthermore, treatment of mice with GLP1-expressing adenovirus, or an α-cell line with GLP1 agonists led to proliferation of α-cells and their conversion to new β-cells75. These studies were, however, conducted in rodents and hence the applicability of the findings to human cells needs to be ascertained.
Long-term administration of γ-aminobutyric acid (GABA) mediated the neogenesis of β-cells from human islet α-cells transplanted in mice, paving the way towards clinical trials76. A supporting report showed that artemisinins, an anti-malarial class of drugs, disrupt α-cell identity in immortalized rodent cell lines by inhibition of ARX and by increasing GABA signalling77. Unfortunately, the success of this approach is not yet clear, as other studies confirmed the inhibition of ARX after artemether (a derivative of artemisinin) treatment but did not find any α-cell to β-cell conversion in primary mouse islets78,79. The contradictory results could be due to GABA-stimulated neogenesis of endocrine cells from ducts rather than direct α-cell to β-cell transdifferentiation. Moreover, artemisinins were used on immortalized cell lines rather than primary islet cells in the initial study77. These discrepancies warrant efforts towards the discovery of novel regulators for efficient and reliable human α-cell to β-cell transdifferentiation.
Similarly, the exocrine cells of the pancreas retain a degree of plasticity. Several groups have proposed the existence of progenitors among the ductal epithelial tree that serve as a source of islet tissue expressing markers, such as CD133, CD49fhi, DCLK1 and ALDHhi in humans80–83. Details on various factors that induce duct to β-cell transdifferentiation is reviewed elsewhere84. In vivo reprogramming of acinar cells to β-cells was also found following injection of an adenovirus delivering a cocktail of the key β-cells genes Pdx1, MafA and Ngn3 (referred to here as PMN-cocktail) into mice85. In addition, experiments with human acinar cells showed promising results upon addition of PAX4 to the PMN-cocktail and suppression of ARX; however, the conversion rate remained low86,87.
The transdifferentiation of liver cells to β-cells following ectopic overexpression of PDX1 has been studied extensively in mice88,89 and in cultured human hepatocytes90. Human hepatocytes can assume a partial β-cell phenotype and ameliorate hyperglycaemia in a mouse model of streptozotocin-induced diabetes mellitus90. Certain subpopulations of liver cells seemingly have a predisposition for transdifferentiation to β-cells and active Wnt signalling is obligatory for retaining this plasticity91. In line with the close lineage association between hepatocytes and pancreatic cells, induced expression of TGF β-induced factor homeobox 2 (TGIF2) activated the pancreatic progenitor programme in mouse adult hepatocytes. TGIF2 induced the expression of markers such as PDX1 and SOX9, and the cells further differentiated into glucagon-producing and insulin-producing cells when co-cultured with mouse embryonic pancreas explants92. Also other tissues of the extrahepatic biliary tree are susceptible to transdifferentiation into β-like cells ex vivo after treatment with the PMN-cocktail and PAX6 overexpression, for example, human gallbladder and cystic duct93.
TGFβ-induced factor homeobox 2.
A transcription factor whose expression separates the pancreatic from the liver lineage early in embryonic development.
The intestinal and antral stomach niches are rich in endocrine cells that possess a high degree of similarity to pancreatic β-cells. Ablating the Forkhead box protein O1 (FOXO1) transcription factor specifically in enteroendocrine cells gave rise to functional β-like cells that could revert streptozotocin-induced diabetes mellitus in mice94. The gastrointestinal tract is considered an immune-privileged site, which raises the possibility that reprogrammed insulin-producing cells in the gut of patients with T1DM could potentially evade immune rejection95. Gut-resident immune cells might also induce systemic tolerance to insulin. Similarly, either in vivo reprogramming of stomach cells with the PMN-cocktail or transplanting in vitro reprogrammed bioengineered stomach-organoids suppressed hyperglycaemia in streptozotocin-induced diabetic mice96. Interestingly, in contrast with reprogrammed intestinal cells, the reprogrammed stomach cells expressed NKX6.1 and were monohormonal97.
Despite these promising findings, transdifferentiating non-β-cells into insulin-producing cells raises several unresolved questions. It is unclear how similar reprogrammed cells are to endogenous β-cells. For example, whether these cells express all the key β-cell factors remains to be elucidated. Furthermore, important functional aspects of reprogrammed cells remain to be determined; such as whether the cells terminate insulin secretion under low glucose conditions, or if the cells respond to various physiological stimuli such as an increased metabolic demand. In addition, further research is required to understand whether reprogrammed cells are locked into their new differentiation state or whether can they relapse to their prior fate. Moreover, the safe adoption of viral reprogramming techniques to the human setting has to be explored further. Small molecules for reprogramming are more attractive than virus-based methodologies, but they are difficult to develop for the regulation of several transcription factors. In addition, a limitation to clinical translation of the transdifferentiation approaches is the low conversion rate to insulin-secreting cells.
Synthetic β-cells.
Beyond the approaches described thus far, an emerging concept centres on forming a β-cell-like cell de novo using just the key elements required for proper functionality. A ‘synthetic cell’ can be defined as a cell or a bioengineered particle with rudimentary components that are sufficient to perform one or several important functions of a specialized cell. β-Cell-mimetic designer cells were developed by engineering human embryonic kidney 293 (HEK293) cells with a glucose-sensing system based simply on glycolysis; glucose sensitivity was conferred by linking an ectopically expressed Ca2+ channel, Cav1.3, to the glucose-sensing system. In turn, Ca2+ entry was coupled to an excitation–transcription system that controls transgenic expression of insulin or GLP1. Remarkably, insulin-expressing and GLP1-expressing glucose-inducible designer cells ameliorated hyperglycaemia and improved insulin secretion in rodent models of T1DM and T2DM, respectively98. Other examples of designer cells include an engineered system with only glucose homeostasis-modulating properties without glucose-inducibility, such as HEK293 cells with light-inducible expression of GLP1. These cells improved glucose tolerance following a glucose challenge in T2DM mice99,100. In summary, although synthetic β-cells are a promising approach, as yet, they have been unable to fully restore normoglycaemia in mouse models. Also, the long-term effects of transplanting these proliferative somatic cells (for example, HEK293) remain to be evaluated.
Simpler than designer cells are acellular bioengineered constructs. These are non-living biomimetic assemblies, for example, vesicles carrying a drug payload or cell membrane-cloaked nanoparticles. Particles delivering insulin dynamically in response to glucose concentrations could theoretically act as β-cell surrogates for diabetes mellitus therapy. Eliciting a specific activity in response to external stimuli is a highly complex and difficult to replicate feature of natural cells. Nonetheless, a multilayered vesicle-in-vesicle superstructure was reported that resembles insulin granules enclosed in a cell101. The inner vesicles were packed with insulin and the outer vesicle was lined with glucose-sensing moieties (GLUT2). Oxidation of glucose into gluconic acid following its entry into the outer vesicle was mediated by glucose oxidase. The glucose concentration-dependent drop in internal pH mediated the fusion of the inner vesicle to the outer membrane, thereby releasing the insulin ‘cargo’ in a glucose-stimulated manner101. It is worth noting that this assembly replicates only the most basic function of a β-cell, whereas insulin synthesis, amplifying signals and fine control of the relative insulin content released are lacking. Yet, such approaches present exciting steps in the direction of building β-cell surrogates that are impervious to autoimmune or alloimmune rejection. Adverse effects such as fibrosis and foreign body-induced response might also be avoided by the selection of appropriate biomaterials.
Bioprocessing and associated challenges
The marked progress in the derivation of functional β-cells from hPSCs has intensified efforts to develop bioprocesses for cellular therapies for diabetes mellitus. However, several challenges must be addressed for the scalable manufacturing of these products at a reasonable cost and of high quality. Present biomanufacturing systems are designed and optimized for making biologics, which differs substantially from the production of cell therapeutics. Whereas in traditional biopharmaceutical processes cells are the means to generate recombinant proteins or vaccines, in stem cell bioprocesses the cells are the actual products. Moreover, the lengthy development of cell lines for high-titre production of biologics might not be applicable to stem cell bioprocessing, which is constrained by time-frames spanning a few weeks; for example, from harvesting cells from patients, to reprogramming and differentiation to the desired progeny.
Stirred suspension bioreactors.
Vessels for cultivation of cells that feature an impeller for mixing, probes for monitoring the culture environment, ports for sampling and exchange of medium, and assemblies for aeration and maintenance of temperature.
Various cultivation modalities.
Most studies on pancreas specification of hPSCs have been carried out in traditional dish or T-flask cultures. Larger 2D systems that comprise multiple parallel plates (for example, cell factories) have also been used for cell expansion prior to differentiation102. Although 2D culture modalities have been employed in clinical protocols103,104, they are characterized by poor mass transfer of soluble factors that becomes more pronounced as the surface area for growth increases. This issue can be partly alleviated by differentiating hPSCs as aggregates in low-adhesion multi-well plates under orbital stirring52,102]. Still, these culture vessels are not well suited for current good manufacturing practice production and are limited by a low surface area-to-volume ratio when compared with other cultivation systems, such as stirred suspension bioreactors (SSBs). Roller bottles, which are utilized in the production of recombinant proteins and vaccines, have been accepted as suitable for the clinical manufacturing of pancreatic endoderm cells from hPSC aggregates105 (FIG. 3). As the scale of roller bottle-based production increases, however, procedures such as medium changes and product harvest become labour intensive with a greater risk of contamination. Another cultivation platform, the wave-agitation bioreactor, is increasingly employed in the production of cellular therapeutics106,107 (Fig. 3). In the wave-agitation bioreactor, cells can be cultured in single-use bags with probes for monitoring the culture environment and in volumes suitable for single-patient batch production. To our knowledge, there are no reports to date on generating islet cells from hPSCs using this platform.
Fig. 3 |. Stem cell bioprocessing for pancreatic islet cell manufacturing.
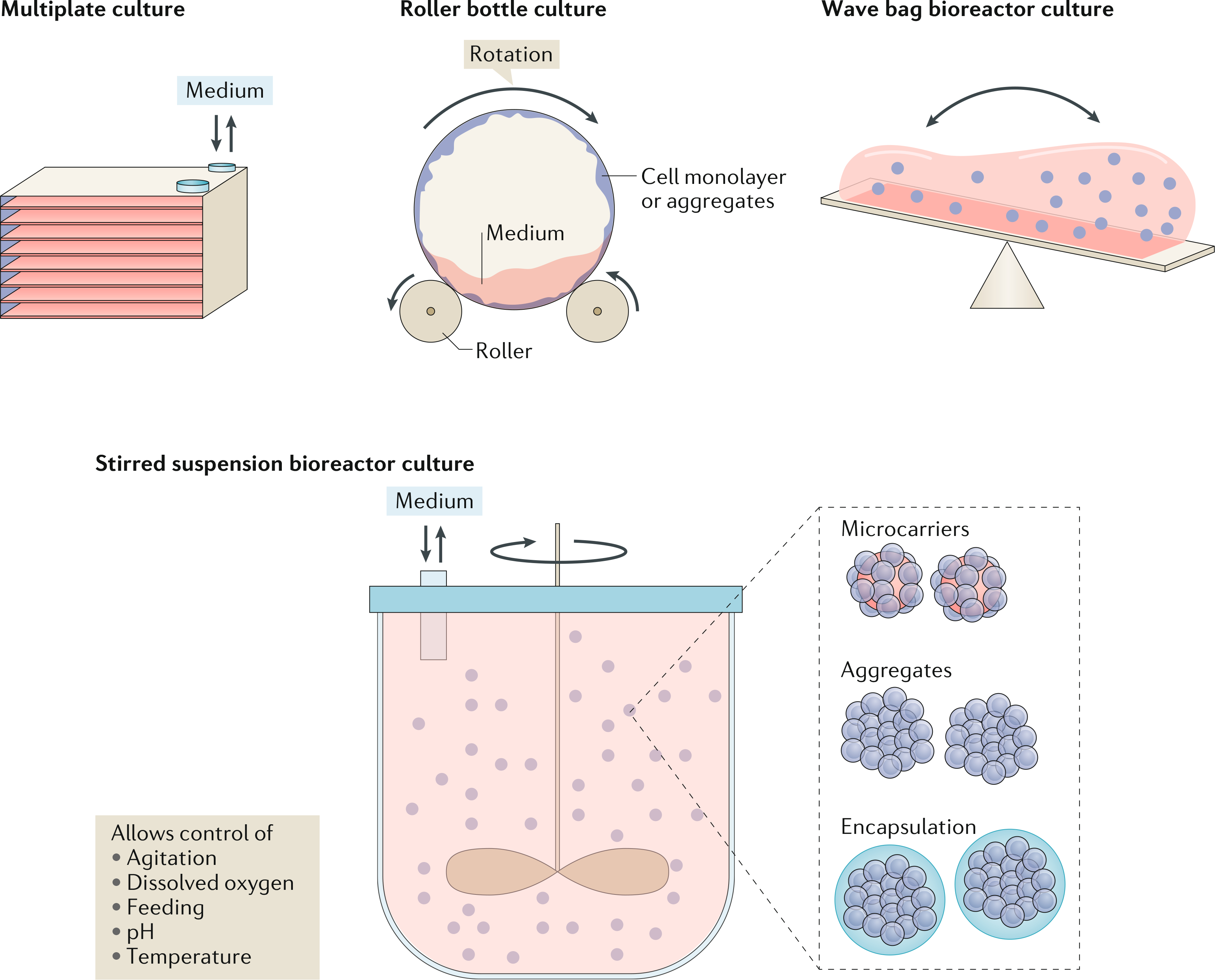
Multiplate systems, roller bottles, bag bioreactors featuring a rocking motion and stirred suspension bioreactors are candidate cultivation modalities for the generation of clinically relevant quantities of islet cells from hPSCs. Stirred suspension bioreactors afford flexibility as cells can be grown and differentiated as aggregates, on microcarriers or following encapsulation. Moreover, these systems allow monitoring and control of the culture environment.
SSBs are an appealing modality for the scalable expansion and differentiation of hPSCs to pancreatic islet cells. Self-renewing hPSCs have been successfully cultivated in SSBs as aggregates108,109, on microcarriers (beads with typical size 100–150 μm made of different materials such as polystyrene, glass, alginate or dextran)110,111 or after encapsulation (for example, in alginate)112, permitting various specification regimes to be accommodated in the same vessel. The use of SSBs presents a considerably lower barrier for translating relevant laboratory-scale differentiation protocols to current good manufacturing practice production of hPSC products in a commercial setting, as this bioreactor type is already the workhorse in biopharmaceutical production facilities.
Several reports have focused on optimizing critical parameters for the propagation of self-renewing hPSCs in SSBs, including the seeding concentration, cell passaging, aggregate size distribution and stirring rate113,114. As a result, hPSC concentrations of 106–107 cells/ml of SSB culture have been reported by several groups. Given that insulin independence in humans is noted with the transplantation of 7,000 islet equivalents (IEQs) per kilogram115, and considering that each IEQ contains ~1,100 β-cells116, ~5 × 108 β-cells/70 kg body weight are needed for reconstituting normal glucose homeostasis in patients with T1DM. The actual numbers could be three-fold to four-fold more than this estimate after considering quality of cells and loss following engraftment. This estimate suggests that culture volumes of several hundred millilitres to a few litres might be adequate for treating a single patient, after adjusting for the efficiencies of differentiation and downstream processes.
Differentiation in SSBs.
In contrast to extensive reports on the expansion of hPSCs in SSBs, fewer studies exist showing coaxing of hPSCs in these systems towards various therapeutically useful cell types (for example, cardiomyocytes109 or neural cells117), particularly to pancreatic cell progeny. Functional human β-cells were generated upon differentiation of ~3 × 108 cells over 4 weeks in 500-ml spinner flasks47, which are convenient as laboratory-scale surrogates of SSBs. Others have also reported the conversion of hPSCs to pancreatic islet cell progeny in spinner flasks118. However, neither continuous monitoring nor active control of bioreactor culture conditions were carried out in these studies, and both are important for reducing batch-to-batch variability. A 2017 study found the growth and differentiation of iPSCs as aggregates towards pancreatic progenitor cells in spinner flasks fitted with sensors for temperature, pH and dissolved oxygen119. The pH was maintained (7.2) through adjustments of the CO2 level inside the incubator and medium exchanges. Dissolved oxygen was regulated by modulating the composition of the gas feed (air, O2 or N2). Cell clumps seeded in spinner flasks were maintained at 60% dissolved oxygen during expansion and differentiation to definitive endoderm (6 days). Subsequent differentiation towards pancreatic progenitors (days 6–17) was carried out under 40% dissolved oxygen resulting in 1.6 × 108 cells/100 ml of culture with 22% of the cells co-expressing PDX1 and NKX6.1 (REF.119).
In contrast to fully instrumented bioreactors, however, spinner flasks are not ideal for testing various feeding strategies120 and continuously surveying and regulating culture parameters. This point is particularly important when considering that differentiation is carried out for 20–30 days. Adaptation of existing differentiation regimens to SSB cultivation will require optimization of bioprocess parameters. For hPSC-derived islet cell manufacturing, the implementation of bioreactor scale-up heuristics121 combined with the rational linking of bioprocess conditions to critical cell product attributes such as identity (for example, expression of β-cell markers), potency (for example, level of GSIS) and purity (for example, minimizing undifferentiated or partially differentiated cells) will be necessary. This process is akin to the quality-by-design framework implemented in the design of processes for the manufacturing of biopharmaceutical products122,123 (BOX 3). The intensified interest by academia and industry in diabetes mellitus cell therapies increases the likelihood of additional breakthroughs in the mass production of β-cells in the not so distant future.
Box 3 |. Quality-by-design attributes for developing bioprocesses for pancreatic cell therapy products.
Quality profile of hPSc-derived β-cells (or islets)
Potency: glucose sensing, GSIS with first-phase and second-phase hormone secretion
Identity: insulin-positive, PDX1+, GLUT1+, MAFA+, CHGA+ (pan-endocrine marker), UCN3+
Safety: exclusion of pluripotent and partially differentiated cells, polyhormonal cells
Cell quantity: approximately 5 × 108 β-cells/70 kg body weight, or >7,000 IEQ/kg body weight. Numbers may be higher subject to quality of cells/islets
Cell transplant: allogeneic (encapsulated cells)
Major process variables and parameters involved in quality-by-design and control of envisaged bioprocesses for manufacturing islet cells from hPSCs
Temperature
pH
Dissolved O2
-
Feeding regimen
Substrate or substrates
Differentiation stimuli
Cell seeding density and/or microcarrier seeding density
Aggregate size distribution
Agitation rate
GSIS, glucose-stimulated insulin secretion; hPSCs, human pluripotent stem cells.
Bioprocess environment and hPSCs.
The expansion of hPSCs in SSBs has largely drawn on protocols used for the cultivation of animal cells (for example, Chinese hamster ovary cells) used for recombinant protein production. Yet, detailed knowledge of the effects of the bioreactor environment on hPSC physiology is essential, as the cells are the products instead of their secreted molecules. For instance, large gaps still exist in our knowledge of how mechanical cues (for example, agitation-induced shear) can affect cell commitment along a particular lineage trajectory124. In 2018, a mechanotransduction cascade was delineated that involves integrin/focal adhesion kinase (FAK) signalling activation of Yes-associated protein 1 (YAP1) as a regulator of bipotent pancreatic progenitors derived from hESCs125. Reduction in FAK signalling in the presence of laminin-enriched or collagen-enriched extracellular matrix resulted in endocrine specification. By contrast, FAK activation of YAP1 suppressed endocrine gene expression and enhanced HES1 expression, yielding ductal cells125 (FIG. 1). Confirming these findings, the depolarized state of the actin cytoskeleton induced by small molecules such as latrunculin A also favoured endocrine differentiation from pancreatic progenitors126. Conceivably, the bioreactor environment can be tuned to facilitate commitment towards pancreatic islet cells, for example with the use of xeno-free laminin peptide-coated or collagen peptide-coated microcarriers127. Xeno-free factors and culture environment are necessary to avoid the transmission of zoonotic diseases to human patients receiving the cells.
Although most cells situated inside clusters can be shielded from external shear in a bioreactor, the exchange of oxygen, nutrients and metabolic products is hindered between the cells within oversized clusters and the bulk of the culture medium, signifying the importance of aggregate size control. Although attenuated in SSBs compared with static cultures, mass transfer limitations for aggregates >200 μm128 still remain a problem, during both the stage of expansion of undifferentiated hPSCs, where proliferation rates are high, and the differentiation to pancreatic endoderm. In fact, increased oxygen tension (pO2) activates β-cell differentiation in cultured pancreatic explants129, and mouse and human PSCs130. These findings warrant the closer examination of how the pO2 level in a bioreactor influences the commitment of hPSCs to pancreatic islet cells, thereby informing strategies for dissolved oxygen control. Besides proliferation, differentiation imparts other changes to cell physiology, many of which are uncharted to date. Undifferentiated hPSCs rely mainly on glycolysis for utilization of glucose, whereas differentiating cells shift to OxPhos131. Even whilst maintained as undifferentiated, cultivation of hPSCs in SSBs induces a switch from glycolysis to OxPhos120. In addition, hPSCs also produce more lactate than differentiated cells132. Accommodating the dynamic metabolic profile and associated hPSC fate decisions in SSBs will require a shift from the techniques employed in traditional cell culture.
Agitation-induced shear.
Shear in the liquid phase of bioreactor cultures arising from spatial gradients of velocity due to stirring.
Chimeric antigen receptors.
(CARs). Novel receptors designed to bind to specific proteins on cells (for example, cancer cells). T cells are engineered with CARs to provide new targeting ability.
Downstream issues.
Downstream processing of hPSC-derived pancreatic cells presents challenges and opportunities for new technologies, since state-of-the-art methods were developed for the separation of molecules (for example, monoclonal antibodies) rather than cells. In fact, the effects of applying current bioprocess procedures for cell separation and retention (for example, acoustic settlers, tangential or alternating tangential flow filtration) to hPSC-derived cells are unknown. Surface markers for sorting β-cells133 or endocrine cell progenitors following hESC differentiation50 have been reported. However, existing methods for sorting cells or islets134 such as fluorescence or magnetic activated cell sorting, lack the throughput necessary for rapid processing of even single-patient cell batches. Cell sorting should also be coupled to online evaluation of functional attributes, for example glucose sensing and biphasic insulin secretion. Incorporation of such online monitoring in the bioproduction will require novel analytical tools given that existing laboratory methods (for example, enzyme-linked immunosorbent assay) for assessing insulin secretion are characterized by long processing times.
Immune modulation
The most obvious application of functional β-cells is in cell-replacement therapy for patients with T1DM or late-stage T2DM. Allograft rejection — and in the case of T1DM, autoimmune rejection — remains a major barrier to clinical translation of therapies derived from stem cell differentiation or transdifferentiation approaches. Despite the remarkable restoration of normoglycaemia upon cadaveric islet transplantation, patients require lifelong immunosuppression with drugs carrying unwanted side effects135. Although graft rejection could be avoided with the use of autologous tissues and patient-specific iPSCs in patients with T2DM, the wide-scale application of this approach would be labour intensive and cost prohibitive. To address these concerns, biobanks of a limited number of iPSC lines with the human leukocyte antigen (HLA) types matching the majority of potential recipients in specific ethnic populations are being considered136–138. However, challenges still exist in differentiating iPSCs as efficiently as hESCs, and the differentiation propensity of iPSC clones derived from even one individual is highly variable139.
In addition to allorejection, autoimmunity in T1DM is a serious challenge. Taking advantage of a combination of immunosuppressive drugs, bioengineering advances and gene-editing tools will be necessary to overcome the barriers posed in T1DM. Bioengineering advances include macroencapsulation in devices made of polymers such as polytetrafluoroethylene or polycaprolactone, and/or microencapsulation of β-cells in materials including alginate, polyacrylate, collagen or agarose for immunoprotection140. These devices prevent immune attack, but poor vascularization of the grafts as well as delayed insulin release kinetics present major limitations. T cell therapies to induce immune tolerance by activating regulatory T cells (Treg) have shown preliminary success in preclinical studies. For example, graft-specific Treg can be isolated, cultured and expanded ex vivo in therapeutically relevant numbers; however, hurdles still exist that prevent high yields being obtained whilst maintaining purity141. The principles used in T cell cancer therapy using chimeric antigen receptors could also be applied to Treg cell therapy to improve tolerogenic outcomes142. Another prospect is engineering β-cells with CRISPR–Cas9 gene editing tools to confer immunoavoidance by dismantling the MHC components that typically present autoantigens and alloantigens to the immune system. Deletion of the highly polymorphic MHC class 1 genes (HLA-A, HLA-B, HLA-C) can render the transplanted cells hypoimmunogenic and maintaining HLA E/G and/or overexpressing CD47/PDL1 can prevent macrophage and natural killer cell-mediated killing143,144. This approach is being pursued as an off-the-shelf or universal cell therapy. However, reducing immunogenicity of the graft also increases the probability of neoplastic growths and pathogenic infections, serious issues confronting the field.
Conclusions
Given the impressive progress in converting hPSCs to β-cells, the path from bench to bedside seems more feasible through the use of hPSC-derived β-cells than transdifferentiation approaches. Improvements in bioprocesses for efficiently manufacturing stable, functionally mature and large quantities of desired cells will accelerate ongoing efforts to develop cell therapy for diabetes mellitus.
Key points.
Recent advances in human stem cell differentiation protocols enable the generation of mature β-cells with dynamic insulin secretion and metabolic properties akin to primary human β-cells.
In addition to β-cells, other hormone-expressing islet cell types are generated under current differentiation protocols.
The unlimited source provided by stem cell-derived β-cells and islet clusters would address the current scarcity in cadaveric donor tissues for islet transplantation, and sophisticated gene-editing tools could be used to cloak them against immune attack.
Transdifferentiation of endogenous non-β-cells to insulin-producing cells could be exploited as an alternative strategy to increase the number of functional β-cell equivalents.
Bioreactors are emerging as technologies for enabling diabetes mellitus cell therapies; these platforms allow precise control of critical cultivation factors for optimized large-scale stem cell differentiation towards functional islet cells.
Acknowledgements
The authors thank S. Puri and N. Kerper of the M. Hebrok laboratory and E. Jacobson of the E. Tzanakakis laboratory for insightful comments during the preparation of the manuscript. G.G.N. was supported by a JDRF postdoctoral fellowship (3-PDF-2016-195-A-N). Research in the M. Hebrok laboratory is supported by grants from the NSF (NSF CBET-1743407) and NIH (R01DK105831). Research in the E. Tzanakakis laboratory is supported by grants from the NSF (NSF CBET-1743367; CBET-1951104). The figures were originally prepared with the help of Biorender and Adobe Illustrator.
Footnotes
Competing Interests
M.H. is affiliated with Semma Therapeutics (Consultant and SAB member) and Encellin Inc. (SAB member, stock holder). M.H. also holds stocks from Viacyte Inc.. The other authors declare no competing interests.
Peer review information
Nature Reviews Endocrinology thanks H. Lickert, A. Stewart and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.International Diabetes Federation. IDF Atlas 9th edn (IDF, 2019). [Google Scholar]
- 2.American Diabetes Association. Economic costs of diabetes in the U.S. in 2017. Diabetes Care 41, 917–928 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cryer PE Mechanisms of hypoglycemia-associated autonomic failure in diabetes. N. Engl. J. Med. 369, 362–372 (2013). [DOI] [PubMed] [Google Scholar]
- 4.Diabetes Control and Complications Trial Research Group, Nathan DM et al. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N. Engl. J. Med. 329, 977–986 (1993). [DOI] [PubMed] [Google Scholar]
- 5.The Diabetes Control and Complications Trial (DCCT)/Epidemiology of Diabetes Interventions and Complications (EDIC) Research Group. Effect of intensive diabetes therapy on the progression of diabetic retinopathy in patients with type 1 diabetes: 18 years of follow-up in the DCCT/EDIC. Diabetes 64, 631–642 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.King P, Peacock I & Donnelly R The UK prospective diabetes study (UKPDS): clinical and therapeutic implications for type 2 diabetes. Br. J. Clin. Pharmacol. 48, 643–648 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tauschmann M et al. Closed-loop insulin delivery in suboptimally controlled type 1 diabetes: a multicentre, 12-week randomised trial. Lancet 392, 1321–1329 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bekiari E et al. Artificial pancreas treatment for outpatients with type 1 diabetes: systematic review and meta-analysis. BMJ 361, k1310 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Foster NC et al. State of type 1 diabetes management and outcomes from the T1D exchange in 2016–2018. Diabetes Technol. Ther. 21, 66–72 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Russell SJ et al. Day and night glycaemic control with a bionic pancreas versus conventional insulin pump therapy in preadolescent children with type 1 diabetes: a randomised crossover trial. Lancet Diabetes Endocrinol. 4, 233–243 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Brown SA et al. Six-month randomized, multicenter trial of closed-loop control in type 1 diabetes. N. Engl. J. Med. 381, 1707–1717 (2019). This report describes results from a multicentre trial evaluating benefits of closed-loop control over sensor-augumented insulin pumps. Closed-loop systems fare better in maintaining the time spent in target glycaemic range (mean ~71%) than sensor-augumented insulin pumps (mean ~59%).
- 12.Barton FB et al. Improvement in outcomes of clinical islet transplantation: 1999–2010. Diabetes Care 35, 1436–1445 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Blum B et al. Functional beta-cell maturation is marked by an increased glucose threshold and by expression of urocortin 3. Nat. Biotech. 30, 261–264 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.van der Meulen T et al. Urocortin3 mediates somatostatin-dependent negative feedback control of insulin secretion. Nat. Med. 21, 769–776 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Henquin JC Triggering and amplifying pathways of regulation of insulin secretion by glucose. Diabetes 49, 1751–1760 (2000). [DOI] [PubMed] [Google Scholar]
- 16.Komatsu M et al. Glucose-stimulated insulin secretion: a newer perspective. J. Diabetes Investig. 4, 511–516 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhao S et al. α/β-Hydrolase domain-6-accessible monoacylglycerol controls glucose-stimulated insulin secretion. Cell Metab. 19, 993–1007 (2014). [DOI] [PubMed] [Google Scholar]
- 18.Ferdaoussi M et al. Isocitrate-to-SENP1 signaling amplifies insulin secretion and rescues dysfunctional β cells. J. Clin. Investig. 125, 3847–3860 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gooding JR et al. Adenylosuccinate is an insulin secretagogue derived from glucose-induced purine metabolism. Cell Rep. 13, 157–167 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pullen TJ et al. Identification of genes selectively disallowed in the pancreatic islet. Islets 2, 89–95 (2010). [DOI] [PubMed] [Google Scholar]
- 21.Thorrez L et al. Tissue-specific disallowance of housekeeping genes: the other face of cell differentiation. Genome Res. 21, 95–105 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lemaire K, Thorrez L & Schuit F Disallowed and allowed gene expression: two faces of mature islet beta cells. Annu. Rev. Nutr. 36, 45–71 (2016). [DOI] [PubMed] [Google Scholar]
- 23.Taylor BL, Liu F-F & Sander M Nkx6.1 is essential for maintaining the functional state of pancreatic beta cells. Cell Rep. 4, 1262–1275 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gu C et al. Pancreatic β cells require NeuroD to achieve and maintain functional maturity. Cell Metab. 11, 298–310 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gosmain Y et al. Pax6 is crucial for β-cell function, insulin biosynthesis, and glucose-induced insulin secretion. Mol. Endocrinol. 26, 696–709 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Aguayo-Mazzucato C et al. Thyroid hormone promotes postnatal rat pancreatic β-cell development and glucose-responsive insulin secretion through MAFA. Diabetes 62, 1569–1580 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huang C et al. Synaptotagmin 4 regulates pancreatic β cell maturation by modulating the Ca2+ sensitivity of insulin secretion vesicles. Dev. Cell 45, 347–361 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Arda HE et al. Age-dependent pancreatic gene regulation reveals mechanisms governing human β cell function. Cell Metab. 23, 909–920 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Henquin J-C & Nenquin M Dynamics and regulation of insulin secretion in pancreatic islets from normal young children. PLoS One 11, e0165961 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hawdon JM et al. The role of pancreatic insulin secretion in neonatal glucoregulation. I. Healthy term and preterm infants. Arch. Dis. Child. 68, 274–279 (1993). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kaye R et al. The response of blood glucose, ketones, and plasma nonesterified fatty acids to fasting and epinephrine injection in infants and children. J. Pediatr. 59, 836–847 (1961). [DOI] [PubMed] [Google Scholar]
- 32.Tong X et al. Lipid droplet accumulation in human pancreatic islets is dependent on both donor age and health. Diabetes 69, 342 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Johnston NR et al. Beta cell hubs dictate pancreatic islet responses to glucose. Cell Metab. 24, 389–401 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bader E et al. Identification of proliferative and mature β-cells in the islets of Langerhans. Nature 535, 430–434 (2016). [DOI] [PubMed] [Google Scholar]
- 35.Nasteska D & Hodson DJ The role of beta cell heterogeneity in islet function and insulin release. J. Mol. Endocrinol. 61, R43–R60 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Thomson JA et al. Embryonic stem cell lines derived from human blastocysts. Science 282, 1145–1147 (1998). [DOI] [PubMed] [Google Scholar]
- 37.Takahashi K & Yamanaka S Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 126, 663–676 (2006). [DOI] [PubMed] [Google Scholar]
- 38.Yu J et al. Induced pluripotent stem cell lines derived from human somatic cells. Science 318, 1917–1920 (2007). [DOI] [PubMed] [Google Scholar]
- 39.Xu X et al. Endoderm and pancreatic islet lineage differentiation from human embryonic stem cells. Cloning Stem Cell 8, 96–107 (2006). [DOI] [PubMed] [Google Scholar]
- 40.Assady S et al. Insulin production by human embryonic stem cells. Diabetes 50, 1691–1697 (2001). [DOI] [PubMed] [Google Scholar]
- 41.D’Amour KA et al. Efficient differentiation of human embryonic stem cells to definitive endoderm. Nat. Biotechnol. 23, 1534–1541 (2005). [DOI] [PubMed] [Google Scholar]
- 42.Kroon E et al. Pancreatic endoderm derived from human embryonic stem cells generates glucose-responsive insulin-secreting cells in vivo. Nat. Biotech. 26, 443–452 (2008). [DOI] [PubMed] [Google Scholar]
- 43.D’Amour KA et al. Production of pancreatic hormone-expressing endocrine cells from human embryonic stem cells. Nat. Biotechnol. 24, 1392–1401 (2006). [DOI] [PubMed] [Google Scholar]
- 44.Xu X, Browning VL & Odorico JS Activin, BMP and FGF pathways cooperate to promote endoderm and pancreatic lineage cell differentiation from human embryonic stem cells. Mech. Dev. 128, 412–427 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Riedel M et al. Immunohistochemical characterisation of cells co-producing insulin and glucagon in the developing human pancreas. Diabetologia 55, 372–381 (2012). [DOI] [PubMed] [Google Scholar]
- 46.Rezania A et al. Reversal of diabetes with insulin-producing cells derived in vitro from human pluripotent stem cells. Nat. Biotech. 32, 1121–1133 (2014). [DOI] [PubMed] [Google Scholar]
- 47.Pagliuca FW et al. Generation of functional human pancreatic β cells in vitro. Cell 159, 428–439 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Russ HA et al. Controlled induction of human pancreatic progenitors produces functional beta-like cells in vitro. EMBO J. 34, 1759–1772 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rezania A et al. Maturation of human embryonic stem cell-derived pancreatic progenitors into functional islets capable of treating pre-existing diabetes in mice. Diabetes 61, 2016–2029 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Veres A et al. Charting cellular identity during human in vitro β-cell differentiation. Nature 569, 368–373 (2019). This is an important study that used single-cell RNA sequencing to elucidate generation of several additional cell types including α-like cells, enterochromaffin cells and non-endocrine cells in stem cell differentiation toward β-cells. Furthermore, the authors identified CD49a as a cell surface marker to sort, re-aggregate and enrich for stem cell-derived β-cells.
- 51.Krentz NAJ et al. Single-cell transcriptome profiling of mouse and hESC-derived pancreatic progenitors. Stem Cell Rep. 11, 1551–1564 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Nair GG et al. Recapitulating endocrine cell clustering in culture promotes maturation of human stem-cell-derived β cells. Nat. Cell Biol. 21, 263–274 (2019). This study demonstrated for the first time that re-aggregation and clustering of stem cell-derived immature β-cells induces maturation by activating mitochondrial respiration. The resulting β-cells closely resemble adult islet β-cells in transcriptome and exhibit similar functional properties such as dynamic insulin secretion.
- 53.Velazco-Cruz L et al. Acquisition of dynamic function in human stem cell-derived β cells. Stem Cell Rep. 12, 351–365 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nomura M et al. SMAD2 disruption in mouse pancreatic beta cells leads to islet hyperplasia and impaired insulin secretion due to the attenuation of ATP-sensitive K+ channel activity. Diabetologia 57, 157–166 (2014). [DOI] [PubMed] [Google Scholar]
- 55.Totsuka Y et al. Stimulation of insulin secretion by transforming growth factor-β. Biochem. Biophys. Res. Commun. 158, 1060–1065 (1989). [DOI] [PubMed] [Google Scholar]
- 56.Lin H-M et al. Transforming growth factor-β/Smad3 signaling regulates insulin gene transcription and pancreatic islet β-cell function. J. Biol. Chem. 284, 12246–12257 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Saber N et al. Sex differences in maturation of human embryonic stem cell-derived β cells in mice. Endocrinology 159, 1827–1841 (2018). [DOI] [PubMed] [Google Scholar]
- 58.Bruin JE et al. Hypothyroidism impairs human stem cell-derived pancreatic progenitor cell maturation in mice. Diabetes 65, 1297–1309 (2016). [DOI] [PubMed] [Google Scholar]
- 59.Motté E et al. Composition and function of macroencapsulated human embryonic stem cell-derived implants: comparison with clinical human islet cell grafts. Am. J. Physiol. Endocrinol. Metab. 307, E838–E846 (2014). [DOI] [PubMed] [Google Scholar]
- 60.Henry RR et al. Initial clinical evaluation of VC-01TM combination product — a stem cell–derived islet replacement for type 1 diabetes (T1D) [abstract 138-OR]. Diabetes 67 (Suppl. 1), A37 (2018). [Google Scholar]
- 61.Shapiro AJ et al. Insulin expression and glucose-responsive circulating C-peptide in type 1 diabetes patients implanted subcutaneously with pluripotent stem cell-derived pancreatic endoderm cells in a macro-device. Preprint at SSRN 10.2139/ssrn.3501034 (2019). [DOI]
- 62.Pepper AR et al. Post-transplant characterization of long-term functional hESC-derived pancreatic endoderm grafts. Diabetes 68, 953–962 (2019). [DOI] [PubMed] [Google Scholar]
- 63.Huising MO et al. The difference δ-cells make in glucose control. Physiology 33, 403–411 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rezania A et al. Production of functional glucagon-secreting α-cells from human embryonic stem cells. Diabetes 60, 239–247 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Nair G & Hebrok M Islet formation in mice and men: lessons for the generation of functional insulin-producing β-cells from human pluripotent stem cells. Curr. Opin. Genet. Dev. 32, 171–180 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Collombat P et al. Embryonic endocrine pancreas and mature β cells acquire α and PP cell phenotypes upon Arx misexpression. J. Clin. Investig. 117, 961–970 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Collombat P et al. Opposing actions of Arx and Pax4 in endocrine pancreas development. Genes Dev. 17, 2591–2603 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Courtney M et al. The inactivation of Arx in pancreatic α-cells triggers their neogenesis and conversion into functional β-like cells. PLoS Genet. 9, e1003934 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chakravarthy H et al. Converting adult pancreatic islet α cells into β cells by targeting both Dnmt1 and Arx. Cell Metab. 25, 622–634 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Xiao X et al. Endogenous reprogramming of alpha cells into beta cells, induced by viral gene therapy, reverses autoimmune diabetes. Cell Stem Cell 22, 78–90 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Furuyama K et al. Diabetes relief in mice by glucose-sensing insulin-secreting human α-cells. Nature 567, 43–48 (2019). This is the first study to show that α-cells can be reprogrammed to insulin-secreting cells. The authors isolated human islet non-β-cells, such as α-cells and γ-cells, from donors with and without diabetes mellitus and reprogrammed them into insulin-secreting cells with PDX1 and MAFA. These cells were able to reverse diabetes mellitus in mice whilst still retaining certain α-cell features.
- 72.Thorel F et al. Conversion of adult pancreatic α-cells to β-cells after extreme β-cell loss. Nature 464, 1149–1154 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Chera S et al. Diabetes recovery by age-dependent conversion of pancreatic δ-cells into insulin producers. Nature 514, 503–507 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.van der Meulen T et al. Virgin beta cells persist throughout life at a neogenic niche within pancreatic islets. Cell Metab. 25, 911–926.e6 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lee Y-S et al. Glucagon-like peptide-1 increases β-cell regeneration by promoting α-to β-cell transdifferentiation. Diabetes 67, 2601–2614 (2018). [DOI] [PubMed] [Google Scholar]
- 76.Ben-Othman N et al. Long-term GABA administration induces alpha cell-mediated beta-like cell neogenesis. Cell 168, 73–85 (2017). [DOI] [PubMed] [Google Scholar]
- 77.Li J et al. Artemisinins target GABA a receptor signaling and impair α cell identity. Cell 168, 86–100 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.van der Meulen T et al. Artemether does not turn α cells into β cells. Cell Metab. 27, 218–225 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ackermann AM, Moss NG & Kaestner KH GABA and artesunate do not induce pancreatic α-to-β cell transdifferentiation in vivo. Cell Metab. 28, 787–792.e3 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Loomans CJM et al. Expansion of adult human pancreatic tissue yields organoids harboring progenitor cells with endocrine differentiation potential. Stem Cell Rep. 10, 712–724 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Gomez DL et al. Neurogenin 3 expressing cells in the human exocrine pancreas have the capacity for endocrine cell fate. PLoS One 10, e0133862 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Westphalen CB et al. Dclk1 defines quiescent pancreatic progenitors that promote injury-induced regeneration and tumorigenesis. Cell Stem Cell 18, 441–455 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sugiyama T et al. Conserved markers of fetal pancreatic epithelium permit prospective isolation of islet progenitor cells by FACS. Proc. Natl Acad. Sci. USA 104, 175–180 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Aguayo-Mazzucato C & Bonner-Weir S Pancreatic β cell regeneration as a possible therapy for diabetes. Cell Metab. 27, 57–67 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zhou Q et al. In vivo reprogramming of adult pancreatic exocrine cells to β-cells. Nature 455, 627–632 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lima MJ et al. Suppression of epithelial-to-mesenchymal transitioning enhances ex vivo reprogramming of human exocrine pancreatic tissue toward functional insulin-producing β-like cells. Diabetes 62, 2821–2833 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lima MJ et al. Generation of functional beta-like cells from human exocrine pancreas. PLoS One 11, e0156204 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Berneman-Zeitouni D et al. The temporal and hierarchical control of transcription factors-induced liver to pancreas transdifferentiation. PLoS One 9, e87812 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ferber S et al. Pancreatic and duodenal homeobox gene 1 induces expression of insulin genes in liver and ameliorates streptozotocin-induced hyperglycemia. Nat. Med. 6, 568–572 (2000). [DOI] [PubMed] [Google Scholar]
- 90.Sapir T et al. Cell-replacement therapy for diabetes: generating functional insulin-producing tissue from adult human liver cells. Proc. Natl Acad. Sci. USA 102, 7964–7969 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Meivar-Levy I & Ferber S Liver to pancreas transdifferentiation. Curr. Diabetes Rep. 19, 76 (2019). [DOI] [PubMed] [Google Scholar]
- 92.Cerdá-Esteban N et al. Stepwise reprogramming of liver cells to a pancreas progenitor state by the transcriptional regulator Tgif2. Nat. Commun. 8, 14127 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Galivo F et al. Reprogramming human gallbladder cells into insulin-producing β-like cells. PLoS One 12, e0181812 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Talchai C et al. Generation of functional insulin-producing cells in the gut by Foxo1 ablation. Nat. Genet. 44, 406–412. (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Spadoni I, Fornasa G & Rescigno M Organ-specific protection mediated by cooperation between vascular and epithelial barriers. Nat. Rev. Immunol. 17, 761–773 (2017). [DOI] [PubMed] [Google Scholar]
- 96. Ariyachet C et al. Reprogrammed stomach tissue as a renewable source of functional β cells for blood glucose regulation. Cell Stem Cell 18, 410–421 (2016). Antral endocrine cells were reprogrammed into insulin+ cells that express NKX6.1 and PC2 at a greater efficiency than enteroendocrine cells; bioengineered stomach mini-organs produced renewable insulin+ cells in vivo and reversed hyperglycaemia in mice.
- 97.Chen Y-J et al. De novo formation of insulin-producing “neo-β cell islets” from intestinal crypts. Cell Rep. 6, 1046–1058 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Xie M et al. β-cell–mimetic designer cells provide closed-loop glycemic control. Science 354, 1296–1301 (2016). [DOI] [PubMed] [Google Scholar]
- 99.Ye H et al. A synthetic optogenetic transcription device enhances blood-glucose homeostasis in mice. Science 332, 1565–1568 (2011). [DOI] [PubMed] [Google Scholar]
- 100.Shao J et al. Smartphone-controlled optogenetically engineered cells enable semiautomatic glucose homeostasis in diabetic mice. Sci. Transl Med. 9, eaal2298 (2017). [DOI] [PubMed] [Google Scholar]
- 101.Chen Z et al. Synthetic beta cells for fusion-mediated dynamic insulin secretion. Nat. Chem. Biol. 14, 86–93 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Schulz TC et al. A scalable system for production of functional pancreatic progenitors from human embryonic stem cells. PLoS One 7, e37004 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Bluestone JA et al. Type 1 diabetes immunotherapy using polyclonal regulatory T cells. Sci. Transl Med. 7, 315ra189 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Marek-Trzonkowska N et al. Administration of CD4+CD25highCD127− regulatory T cells preserves β-cell function in type 1 diabetes in children. Diabetes Care 35, 1817–1820 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Schulz TC Concise review: manufacturing of pancreatic endoderm cells for clinical trials in type 1 diabetes. Stem Cell Transl Med. 4, 927–931 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Somerville RP et al. Clinical scale rapid expansion of lymphocytes for adoptive cell transfer therapy in the WAVE(R) bioreactor. J. Transl Med. 10, 69 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Fraser H et al. A rapamycin-based GMP-compatible process for the isolation and expansion of regulatory T cells for clinical trials. Mol. Ther. Methods Clin. Dev. 8, 198–209 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Krawetz R et al. Large-scale expansion of pluripotent human embryonic stem cells in stirred-suspension bioreactors. Tissue Eng. Part C. Methods 16, 573–582 (2010). [DOI] [PubMed] [Google Scholar]
- 109.Kempf H et al. Controlling expansion and cardiomyogenic differentiation of human pluripotent stem cells in scalable suspension culture. Stem Cell Rep. 3, 1132–1146 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Lock LT & Tzanakakis ES Expansion and differentiation of human embryonic stem cells to endoderm progeny in a microcarrier stirred-suspension culture. Tissue Eng. Part A 15, 2051–2063 (2009). This is the first report of hESC expansion and directed differentiation to definitive endoderm cells in a microcarrier stirred suspension (spinner flask) culture.
- 111.Bardy J et al. Microcarrier suspension cultures for high-density expansion and differentiation of human pluripotent stem cells to neural progenitor cells. Tissue Eng. Part C. Methods 19, 166–180 (2013). [DOI] [PubMed] [Google Scholar]
- 112.Jing D, Parikh A & Tzanakakis ES Cardiac cell generation from encapsulated embryonic stem cells in static and scalable culture systems. Cell Transpl. 19, 1397–1412 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Meng G et al. Optimizing human induced pluripotent stem cell expansion in stirred-suspension culture. Stem Cell Dev. 26, 1804–1817 (2017). [DOI] [PubMed] [Google Scholar]
- 114. Hunt MM et al. Factorial experimental design for the culture of human embryonic stem cells as aggregates in stirred suspension bioreactors reveals the potential for interaction effects between bioprocess parameters. Tissue Eng. Part C. Methods 20, 76–89 (2014). A systematic analysis based on factorial design of experiments was performed to investigate adjustable parameters (for example, agitation rate and cell seeding density) and their interaction on the outcome of hESC cultures in spinner flasks.
- 115.Shapiro AM, Pokrywczynska M & Ricordi C Clinical pancreatic islet transplantation. Nat. Rev. Endocrinol. 13, 268–277 (2017). [DOI] [PubMed] [Google Scholar]
- 116.Pisania A et al. Quantitative analysis of cell composition and purity of human pancreatic islet preparations. Lab. Invest. 90, 1661–1675 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Yan Y et al. Derivation of cortical spheroids from human induced pluripotent stem cells in a suspension bioreactor. Tissue Eng. Part A 24, 418–431 (2018). [DOI] [PubMed] [Google Scholar]
- 118.Yabe SG et al. Induction of functional islet-like cells from human iPS cells by suspension culture. Regen. Ther. 10, 69–76 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Mihara Y et al. Production of pancreatic progenitor cells from human induced pluripotent stem cells using a three-dimensional suspension bioreactor system. J. Tissue Eng. Regen. Med. 11, 3193–3201 (2017). [DOI] [PubMed] [Google Scholar]
- 120. Kropp C et al. Impact of feeding strategies on the scalable expansion of human pluripotent stem cells in single-use stirred tank bioreactors. Stem Cell Transl Med. 5, 1289–1301 (2016). In this elegant study, the authors studied the effect of different feeding strategies on hPSCs cultured in automated SSBs and demonstrated a shift in metabolism from glycolysis to OXPHOS without differentiation.
- 121.Kehoe DE et al. Scalable stirred-suspension bioreactor culture of human pluripotent stem cells. Tissue Eng. Part A 16, 405–421 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Yu LX et al. Understanding pharmaceutical quality by design. AAPS J. 16, 771–783 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Lipsitz YY, Timmins NE & Zandstra PW Quality cell therapy manufacturing by design. Nat. Biotechnol. 34, 393–400 (2016). [DOI] [PubMed] [Google Scholar]
- 124.Vining KH & Mooney DJ Mechanical forces direct stem cell behaviour in development and regeneration. Nat. Rev. Mol. Cell Biol. 18, 728–742 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Mamidi A et al. Mechanosignalling via integrins directs fate decisions of pancreatic progenitors. Nature 564, 114–118 (2018). [DOI] [PubMed] [Google Scholar]
- 126.Hogrebe NJ et al. Targeting the cytoskeleton to direct pancreatic differentiation of human pluripotent stem cells. Nat. Biotechnol. 38, 460–470 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Fan Y, Zhang F & Tzanakakis ES Engineering xeno-free microcarriers with recombinant vitronectin, albumin and UV irradiation for human pluripotent stem cell bioprocessing. ACS Biomater. Sci. Eng. 3, 1510–1518 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Wu J et al. Oxygen transport and stem cell aggregation in stirred-suspension bioreactor cultures. PLoS One 9, e102486 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Heinis M et al. Oxygen tension regulates pancreatic β-cell differentiation through hypoxia-inducible factor 1α. Diabetes 59, 662–669 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Hakim F et al. High oxygen condition facilitates the differentiation of mouse and human pluripotent stem cells into pancreatic progenitors and insulin-producing cells. J. Biol. Chem. 289, 9623–9638 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Cliff TS & Dalton S Metabolic switching and cell fate decisions: implications for pluripotency, reprogramming and development. Curr. Opin. Genet. Dev. 46, 44–49 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Prigione A et al. The senescence-related mitochondrial/oxidative stress pathway is repressed in human induced pluripotent stem cells. Stem Cell 28, 721–733 (2010). [DOI] [PubMed] [Google Scholar]
- 133. Saunders DC et al. Ectonucleoside triphosphate diphosphohydrolase-3 antibody targets adult human pancreatic β cells for in vitro and in vivo analysis. Cell Metab. 29, 745–754 (2019). A novel cell surface biomarker NTPDase 3 was found to be enriched in adult human β-cells. NTPDase 3 antibodies were shown to be applicable to live sorting of β-cells from human islets and in vivo imaging of transplanted β-cells.
- 134.Steffen A et al. Functional assessment of automatically sorted pancreatic islets using large particle flow cytometry. Islets 3, 267–270 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Rangel EB The metabolic and toxicological considerations for immunosuppressive drugs used during pancreas transplantation. Expert. Opin. Drug Metab. Toxicol. 8, 1531–1548 (2012). [DOI] [PubMed] [Google Scholar]
- 136.Nakatsuji N, Nakajima F & Tokunaga K HLA-haplotype banking and iPS cells. Nat. Biotechnol. 26, 739–740 (2008). [DOI] [PubMed] [Google Scholar]
- 137.Taylor CraigJ. et al. Generating an iPSC bank for HLA-matched tissue transplantation based on known donor and recipient HLA types. Cell Stem Cell 11, 147–152 (2012). [DOI] [PubMed] [Google Scholar]
- 138.Gourraud P-A et al. The role of human leukocyte antigen matching in the development of multiethnic “haplobank” of induced pluripotent stem cell lines. Stem Cells 30, 180–186 (2012). [DOI] [PubMed] [Google Scholar]
- 139.Thatava T et al. Intrapatient variations in type 1 diabetes-specific iPS cell differentiation into insulin-producing cells. Mol. Ther. 21, 228–239 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Desai T & Shea LD Advances in islet encapsulation technologies. Nat. Rev. Drug Discov. 16, 338–350 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Tang Q & Bluestone JA Regulatory T-cell therapy in transplantation: moving to the clinic. Cold Spring Harb. Perspect. Med. 3, a015552 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Ferreira LMR et al. Next-generation regulatory T cell therapy. Nat. Rev. Drug Discov. 18, 749–769 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Han X et al. Generation of hypoimmunogenic human pluripotent stem cells. Proc. Natl Acad. Sci. USA 116, 10441–10446 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Deuse T et al. Hypoimmunogenic derivatives of induced pluripotent stem cells evade immune rejection in fully immunocompetent allogeneic recipients. Nat. Biotechnol. 37, 252–258 (2019). In this breakthrough study on the generation of universal ‘off the shelf’ hPSCs, deletion of MHC class I and II genes and overexpression of CD47 rendered both mouse and human iPSCs hypoimmunogenic. The engineered PSCs differentiated to smooth muscle cells, endothelial cells or cardiomyocytes that successfully evaded immune rejection in allogeneic fully MHC-mismatched recipents.
- 145.Rosado-Olivieri EA et al. YAP inhibition enhances the differentiation of functional stem cell-derived insulin-producing β cells. Nat. Commun. 10, 1464 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Sharon N et al. Wnt signaling separates the progenitor and endocrine compartments during pancreas development. Cell Rep. 27, 2281–2291 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Henquin J-C et al. Dynamics of glucose-induced insulin secretion in normal human islets. Am. J. Physiol. Endocrinol. Metab. 309, E640–E650 (2015). [DOI] [PubMed] [Google Scholar]
- 148.Hayes MR et al. Incretins and amylin: neuroendocrine communication between the gut, pancreas, and brain in control of food intake and blood glucose. Annu. Rev. Nutr. 34, 237–260 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Begg DP & Woods SC Interactions between the central nervous system and pancreatic islet secretions: a historical perspective. Adv. Physiol. Educ. 37, 53–60 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Rutter GA et al. Pancreatic β-cell identity, glucose sensing and the control of insulin secretion. Biochem. J. 466, 203–218 (2015). [DOI] [PubMed] [Google Scholar]
- 151.Yoshihara E, et al. ERRγ is required for the metabolic maturation of therapeutically functional glucose-responsive β cells. Cell Metab. 23, 622–634 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Jitrapakdee S et al. Regulation of insulin secretion: role of mitochondrial signalling. Diabetologia 53, 1019–1032 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Vakilian M, Tahamtani Y & Ghaedi K A review on insulin trafficking and exocytosis. Gene 706, 52–61 (2019). [DOI] [PubMed] [Google Scholar]


