Abstract
Assessing the quality of one’s own memories is a core cognitive function, but it has been unclear whether rodents possess this ability. Evidence that they do has come from research using a new behavioural paradigm in which rats make temporal bets guided by memory confidence.
Knowing when we know and when we do not is a core cognitive ability that makes possible many kinds of memory-based behaviors1. Imagine, for example, asking someone for directions: an answer without an accompanying confidence that it is correct is of limited use, as it is impossible to know whether the individual simply took a guess or actually knows the answer (and if so, how well). In humans, the memories whose existence can be assessed and verbally described are referred to as declarative memories; indeed, the ‘knowing that we know’ aspect of memory is the defining feature of this class of memory2. The neural circuitry and computational principles that enable confidence judgments for retrieved memories are only beginning to be understood, with many questions remaining unanswered. A noticeable absence has been the lack of a rodent model: in a major step towards establishing such a model, a paper in this issue of Current Biology by Joo et al.3 reports a novel spatial memory task in which rats make continuous memory confidence judgments.
In the new task devised by Joo et al.3, rats made binary choices between two available cued choice ports (which were a subset of the six possible used ports across trials). Rats received reward if they chose the cued port for which most time had elapsed since they visited that particular port last. Making this decision requires memory of a rat’s choice history. The key manipulation depended on how rats made their choices: the length of time for which they remained in the choice port was taken as a ‘bet’ that the decision is correct, with longer times between entering and exiting the choice port leading to more reward (Figure 1A). Reward was only delivered after animals exited the choice port and if the choice was correct, thereby forcing rats to make their bet based on an internal estimate of how certain they were that their choice was correct.
Figure 1. The task used in the work of Joo et al.3 and simple computational model to conceptualize their findings.
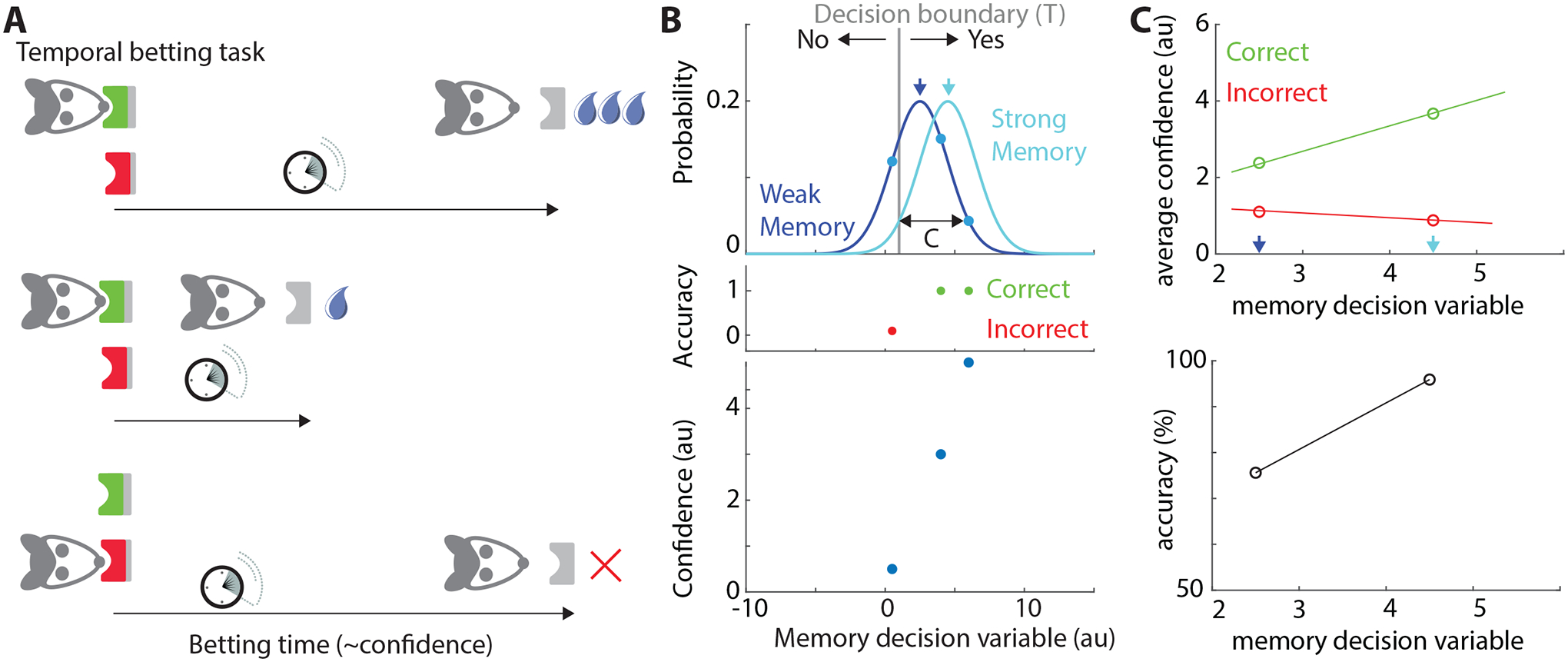
(A) Rats placed temporal bets, with larger bets leading to more reward in correct trials. Correct and incorrect choice ports are indicated in green and red. Shown are two correct trials with long and short temporal bets, and an incorrect trial with a long bet leading to no reward. Confidence is proportional to betting time. (B,C) Toy model that illustrates qualitatively the relationship between confidence, accuracy, and memory strength that the rats exhibited. (B) Distribution of decision variables for weak and strong memories (dark and light blue, mean = 2.5 and 4.5, respectively). The decision is ‘Yes’ if the sampled value falls above the threshold of T = 1 and ‘No’ if otherwise. The confidence is the absolute distance between the data point and the threshold (‘C’). Shown are three example data points from the weak distribution, with accompanying accuracy of the decision (middle) and the associated confidence (bottom). (C) Average confidence (top) and accuracy (bottom) for 1000 randomly sampled datapoints from the distributions shown in (B). Accuracy grows as a function of memory strength. Confidence increases and decreases as a function of memory strength for correct and incorrect choices, respectively. This is because for strong memories, the average distance to the decision boundary is shorter for incorrect decisions. Au=arbitrary units.
Using an impressive combination of careful trial design, behavioral analysis, computational modeling, and machine learning, Joo et al.3 convincingly show that these ‘betting times’ constitute a faithful trial-by-trial behavioral readout of memory confidence. Mathematically, confidence can be defined as the probability of a choice being correct4. Under this model, a readout of confidence would be expected to increase monotonically as a function of choice accuracy. The betting times of the rats indeed increased monotonically as a function of accuracy, therefore showing that they can serve as an indirect readout of confidence.
Confidence varies as a function of both decision difficulty and accuracy (Figure 1B,C). For perceptual or value-based decisions, decision difficulty can be controlled by the experimenter5,6. In contrast, a major difficulty for studying memory confidence is that the underlying decision variable (the memories) is more challenging to control. Rather, what aspects of memory influence a decision has to be inferred from behavior. In visual recognition memory, a fruitful approach has been to show images to many subjects and to define memorability (a form of difficulty) as the proportion of subjects that remember a given image7. But this approach does not capture differences between individuals and trials.
Joo et al.3 propose a new way to solve this problem: they trained a deep neural network to predict choice accuracy (a function of task difficulty) based on a large number of variables that the animal may conceivably retrieve from memory. Note that the authors trained the network to predict whether a decision will be correct or incorrect, rather than what the decision itself is. This is a key difference: trained this way, the network made a ‘meta’ decision about a decision, whereas the latter is the actual choice the animal made (in technical terms, this is a Type 2 versus a Type 1 decision)8. This model reveals which memory-derived variables were used by a given animal to make correct decisions. The synthetic ‘memory decision variable’ that the model provides can then be used much like sensory strength, resolving this major hurdle.
To understand the significance of these new results, it is helpful to consider one of the foundational models of confidence judgments: the ‘balance-of-evidence’ model of Vickers9. In a case where two possible options are available, this model randomly draws one sample value each from the two probability distributions describing the strength of evidence in favor of of that choice. The model then outputs as the choice the option with the larger sampled value and as the confidence the absolute difference between the evidence samples for the two choices (Figure 1B shows a simplified illustration of this model). Thus, confidence is proportional to the difference in the numerical values of the two options, which conforms to the intuition that choices between similar values are made with lower confidence then those with dissimilar values. Variants of this model provide remarkably good fits to behavior in a variety of tasks and species.
Joo et al.3 show that this overall approach can be used to conceptualize how the rats made their confidence judgments. To do so, they constructed a probabilistic model that utilizes the two variables that were together most predictive of accuracy: the time since the last visit to the distractor and the time since the last visit to the target. Based on the behavioral data, the authors estimated the shape of the probability density functions centered on the known time since the last visit of the distractor and target, sampled values from these two distributions and made confidence decisions as described by the balance-of-evidence model. This model mirrors the behavior of the rats remarkably well, thereby providing a simple generative model of how the rats assess their memory confidence.
Indirect retrospective confidence judgments of the kind made by rats in the work of Joo et al.3 have long been one of the principal ways by which experimenters have assessed the ability of non-human primates and rodents to make certainty judgments5,6. This technique has also found applications in humans, including to show that the quality of unconsciously processed information cannot be assessed10 or that pre-verbal infants can make confidence judgments11. While rodents can make post-decision bets for perceptual decisions, this new work now shows that rats can express their confidence indirectly through temporal betting in a graded manner in memory-based decisions much like non-human primates do12,13. A key next step will be to examine whether rats can also make prospective bets (as non-human primates are known to do12).
What are the neural mechanisms that allow rodents to make temporal bets that are indicative of memory strength? While the answer to this question remains unknown, prior work motivates several hypotheses that can now be explored. One possibility is that the rats use the time it took to make the initial choice as a proxy for certainty. However, variance in choice time explained only a minor part of the variance in confidence judgments, indicating that other mechanisms are at work (as is the case in humans14). Neurons signaling memory strength in the human hippocampus15 and posterior parietal cortex16, which are brain areas involved in memory retrieval, carry a graded familiarity signal that is stronger for high-confidence decisions. This is reminiscent of the synthetic memory decision variable, but it remains unknown whether such neurons exist in rats. Other neurons in the same brain areas, on the other hand, signal decision confidence irrespective of the choice16,17. Alternatively, other data indicates that making metamemory decisions requires frontal areas distinct from the underlying memory system13, indicating the need for simultaneous recordings to examine hippocampal-frontal interactions18,19. The availability of a new rat behavioral paradigm now makes it possible to explore these important questions in this model system of memory.
Understanding how a neural or artificial systems can gain insight into the reliability of its own memory is of broad significance. Knowing whether we know drives exploratory behavior, curiosity, and learning (among many other behaviors). In humans, confidence attributed to reports from memories has wide significance in a variety of circumstances that includes the law20. In medicine, deficits in metacognitive ability are thought to be critical factors in neuropsychiatric diseases. Lastly, it is of paramount importance that we endow artificially intelligent systems with the capability to provide confidence judgments. Deciphering the underlying neural circuitry in rodents has the potential to significantly advance these areas of investigation.
References
- 1.Metcalfe J (2008). Metamemory. In Learning and Memory: A Comprehensive Reference Roediger HL, ed. (Elsevier; ), pp. 349–362. [Google Scholar]
- 2.Tulving E, and Markowitsch HJ (1998). Episodic and declarative memory: role of the hippocampus. Hippocampus 8, 198–204. [DOI] [PubMed] [Google Scholar]
- 3.Joo HR, Liang H, Chung JE, Geaghan-Breiner C, Fan JL, Nachman BP, Kepecs A, and Frank LM (2021). Rats use memory confidence to guide decisions. Curr. Biol 31, 4571–4583.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kepecs A, and Mainen ZF (2012). A computational framework for the study of confidence in humans and animals. Phil. Trans. R. Soc. Lond. B 367, 1322–1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kepecs A, Uchida N, Zariwala HA, and Mainen ZF (2008). Neural correlates, computation and behavioural impact of decision confidence. Nature 455, 227–231. [DOI] [PubMed] [Google Scholar]
- 6.Kiani R, and Shadlen MN (2009). Representation of confidence associated with a decision by neurons in the parietal cortex. Science 324, 759–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Khosla A, Raju AS, Torralba A, and Oliva A (2015). Understanding and predicting image memorability at a large scale. In Proc. IEEE Int. Conf. Comput. Vis., pp. 2390–2398. [Google Scholar]
- 8.Fleming SM, and Dolan RJ (2012). The neural basis of metacognitive ability. Phil. Trans. R. Soc. Lond. B 367, 1338–1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vickers D (1979). Decision processes in visual perception (Academic Press; ). [Google Scholar]
- 10.Persaud N, McLeod P, and Cowey A (2007). Post-decision wagering objectively measures awareness. Nat. Neurosci 10, 257–261. [DOI] [PubMed] [Google Scholar]
- 11.Goupil L, and Kouider S (2016). Behavioral and Neural Indices of Metacognitive Sensitivity in Preverbal Infants. Curr. Biol 26, 3038–3045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hampton RR (2001). Rhesus monkeys know when they remember. Proc. Natl. Acad. Sci. USA 98, 5359–5362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Miyamoto K, Osada T, Setsuie R, Takeda M, Tamura K, Adachi Y, and Miyashita Y (2017). Causal neural network of metamemory for retrospection in primates. Science 355, 188–193. [DOI] [PubMed] [Google Scholar]
- 14.Kiani R, Corthell L, and Shadlen MN (2014). Choice certainty is informed by both evidence and decision time. Neuron 84, 1329–1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rutishauser U, Ye S, Koroma M, Tudusciuc O, Ross IB, Chung JM, and Mamelak AN (2015). Representation of retrieval confidence by single neurons in the human medial temporal lobe. Nat. Neurosci 18, 1041–1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rutishauser U, Aflalo T, Rosario ER, Pouratian N, and Andersen RA (2018). Single-neuron representation of memory strength and recognition confidence in left human posterior parietal cortex. Neuron 97, 209–220 e203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Unruh-Pinheiro A, Hill MR, Weber B, Bostrom J, Elger CE, and Mormann F (2020). Single-neuron correlates of decision confidence in the human medial temporal lobe. Curr. Biol 30, 4722–4732. [DOI] [PubMed] [Google Scholar]
- 18.Minxha J, Adolphs R, Fusi S, Mamelak AN, and Rutishauser U (2020). Flexible recruitment of memory-based choice representations by the human medial frontal cortex. Science 368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Spellman T, Rigotti M, Ahmari SE, Fusi S, Gogos JA, and Gordon JA (2015). Hippocampal-prefrontal input supports spatial encoding in working memory. Nature 522, 309–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wixted JT, Mickes L, Dunn JC, Clark SE, and Wells W (2016). Estimating the reliability of eyewitness identifications from police lineups. Proc. Natl. Acad. Sci. USA 113, 304–309. [DOI] [PMC free article] [PubMed] [Google Scholar]


