Abstract
Background
Persons with advanced cancer and their relatives experience physical, emotional, and psychosocial consequences of the illness. Most of the time, they must deal with these themselves. While peer self-management support programs may be helpful, there is little evidence on their value for this population. We present the research protocol of our SMART study that will evaluate the effectiveness of the “Living with Cancer” peer self-management support program, aimed at improving self-management behaviors, self-efficacy, and health-related quality of life of persons with advanced cancer and their relatives.
Methods
We will conduct a non-randomized stepped wedge study in the Netherlands. We will include 130 persons with advanced cancer and 32 relatives. Participants can choose to either start the program within 4 weeks after inclusion or after eight to 10 weeks. The “Living with Cancer” is a peer self-management support program, based on the Chronic Disease Self-Management Program. It consists of six 1,5 hours video-conferencing group meetings with eight to 12 participants, preceded by two or three preparatory audio clips with supportive text per session. The program has the following core components: the learning of self-management skills (action-planning, problem-solving, effective communication, and decision-making), discussing relevant themes (e.g. dealing with pain and fatigue, living with uncertainty, and future planning), and sharing experiences, knowledge, and best practices. The primary outcome for both persons with advanced cancer and relatives is self-management behavior assessed by the subscale “constructive attitudes and approaches” of the Health Education Impact Questionnaire. Secondary outcomes are other self-management behaviors, self-efficacy, health-related quality of life, symptoms, depression and anxiety, and loneliness. Participants complete an online questionnaire at baseline, and after eight and 16 weeks. After each session, they complete a logbook about their experiences. Group meetings will be video recorded.
Discussion
SMART aims to evaluate an innovative program building on an evidence-based self-management program. New features are its use for persons with advanced cancer, the inclusion of relatives, and the video-conferencing format for this population. The use of both quantitative and qualitative analyses will provide valuable insight into the effectiveness and value of this program.
Trial registration
This study was registered in the Dutch Trial Register on October 2021, identifier NL9806.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12904-022-00994-5.
Keywords: Self-management program, Peer support, Peer-led, Advanced Cancer, Relatives, Video-conferencing, Non-randomized stepped wedge
Background
In recent years, there has been a shift towards participatory healthcare [1, 2]. Persons with advanced cancer are increasingly expected to take up more responsibility for their health and care at home. Together with their relatives, they have to deal with physical, emotional, psychosocial, complex treatment regimens, and lifestyle consequences of the illness [3, 4]. Due to new treatments, several cancer types are evolving towards a chronic condition, and many patients live with their illness for years [4, 5]. As a result, persons with advanced cancer and their relatives are faced with long term uncertainties, for instance, whether the illness will progress or how they should best deal with the illness [6]. In addition, relatives have a high risk of ‘caregiver burden’ [7]. They can sometimes be in the conflicting position of both giving support to the person with advanced cancer, while also having to deal with the consequences of advanced cancer in their own lives [8, 9].
Self-management is about how a person with a long term condition deals with medical, role, and emotional issues [10]. A key factor in improving self-management is self-efficacy, which refers to people’s beliefs in their capabilities to perform specific behavior [10]. Self-management of persons with an advanced illness has been defined as “the strategies to manage the physical, psychosocial and existential consequences of living with a progressive, life-threatening disease and its treatment.” [11]. Key to this definition is that self-management involves more than symptom management alone and includes the management of problems in other domains, such as psychosocial problems [11]. Therefore, self-management support interventions ideally include both persons with long term conditions and their relatives [9]. Most self-management studies are developed for persons with chronic diseases and are found to reduce the severity of symptoms and improve quality of life [12–14].
One way to support self-management and enhance self-efficacy is through peer support and modeling. This is the support of persons who share their experiential knowledge, and/or emotional, social or practical support to others in similar conditions [15]. Peer support can be provided one-to-one or in a group, it can be professional-led or peer-led, and it can be delivered face-to-face or online [16]. Online peer support can be asynchronous (such as online discussion boards) or synchronous (through video-conferencing). For persons with cancer, a recent review indicates that peer-led peer support has positive effects on coping, self-efficacy and cancer-related knowledge, regardless of the mode, duration and format of the intervention [16]. Most studies in this review were conducted among persons with breast cancer [16]. Despite its potential value, few studies have been conducted addressing the effectiveness of peer support among persons with advanced cancer or their relatives [17–19].
After examining several programs, we identified the Chronic Disease Self-Management Program (CDSMP), developed in the USA by Lorig et al. [20], as among the most effective and potentially relevant given its use in diverse populations, in different countries and cultures, and its peer-led format [12, 13]. A meta-analysis of 23 studies of the CDSMP demonstrated improved self-efficacy, health behaviors (such as exercise, cognitive symptom management and communication with physicians) and physical health outcomes (energy, shortness of breath, fatigue, pain and self-rated health) [13]. The original CDSMP has several adapted versions for various populations [21]. We developed an adapted version of the CDSMP to meet the needs of persons with advanced cancer and their relatives in the context of the Netherlands. Given the increasing symptom burden, limited energy of this population, and the Covid-19 pandemic, we have chosen to deliver the program via video-conferencing [22].
In this article, we present the research protocol of our SMART study to evaluate the “Living with Cancer” program for persons with advanced cancer and their relatives. We used The Standard Protocol Items: Recommendations for Interventional Trials (SPIRIT) to describe relevant aspects of the study [23]. See Additional file 2 for the SPIRIT checklist.
Objectives
The overall aim of this study is to assess the effectiveness of the program on self-management behaviors, self-efficacy and health-related quality of life of persons with advanced cancer, and explore its effectiveness in a smaller group of relatives. Moreover, through qualitative analyses, we will study the experiences of both groups with the program.
Methods
Intervention
Development intervention
The “Living with Cancer” program is a peer self-management support program based on self-efficacy theory [20, 24]. The program was built on two adapted versions of the CDSMP, the “Cancer Thriving and Surviving” program (developed for persons affected by cancer) and the “Building Better Caregivers” program (developed for caregivers of persons with cognitive impairments) [25, 26]. The core of these programs was preserved and some of the content was replaced with relevant themes for our specific group. In close collaboration with the director of the CDSMP (K. Lorig), changes were made based on findings from our systematic review [27] and in-depth interview studies [28, 29], see Additional file 3 for related articles. The content of the “Living with Cancer” program was discussed in reference groups of patients and relatives (N = 10), healthcare professionals (N = 5) and researchers in palliative care (N = 8). A pilot study was conducted to evaluate the initial acceptability and feasibility of the “Living with Cancer” program. In this pilot, 12 participants (seven persons with advanced cancer, three relatives and two potential peer facilitators) completed the program and an online questionnaire (see Table 4). They were interviewed about their experiences. Participants evaluated the program as acceptable and feasible, with a mean satisfaction score of 8.5 on a scale of 0–10.
Table 4.
Measurements tools
| Measurement tools | |
|---|---|
| Concept | Measured by |
| Primary outcome: | |
| Self-management behavior |
Health Education Impact Questionnaire (HEIQ) [30] Patients and relatives: Subscale ‘constructive attitudes and approaches’ (5 items) |
| Secondary outcomes: | |
| Self-management behaviors |
Health Education Impact Questionnaire (HEIQ) [30] Patients: Subscales ‘skill and technique acquisition’ (4 items), ‘health services navigation’ (5 items), ‘social integration and support’ (5 items) Relatives: Subscales ‘health directed behavior’ (4 items), ‘positive and active engagement in life’ (5 items), ‘social integration and support’ (5 items) |
| Quality of life |
Patients: McGill Quality of Life (MQOL) [31, 32] (18 items) Relatives: Quality of Life in Life Threatening Illness Family Carer Version (QOLLTI-F) [33] Subscale ‘overall quality of life’ (1 item) |
| Self-efficacy |
Patients: Self-efficacy for managing chronic disease [24] Adaptation to advanced cancer (6 items) Relatives: Self-efficacy caregivers short adaption [34] Adaptation to advanced cancer (7 items) |
| Symptoms |
Patients and relatives: Fatigue numeric rating scale (NRS) (1 item) Patients: Pain numeric rating scale (NRS) (1 item) Relatives: Stress numeric rating scale (NRS) (1 item), Sleep numeric rating scale (NRS) (1 item) |
| Caregiver burden |
Relatives: Caregiver Reaction Assessment Dutch (CRA-D) [35]; Subscale ‘impact on schedule’ (5 items) |
| Depression and Anxiety |
Patients and relatives: Hospital Anxiety and Depression (HADS) [36] (14 items) |
| Loneliness |
Patients and relatives: University of California, Los Angeles (UCLA) loneliness scale [37] Short version (3 items) |
| Other measures: | |
| Healthcare utilization |
Patients and relatives: Number of contacts with healthcare professionals, number of hospitalization days, reasons for hospitalization, number of visits to accident and emergency departments [38] Self-constructed (6 items) |
| Sociodemographic characteristics |
Patients and relatives: Age, gender, ethnicity, Social Economic Status (SES), marital status, cancer type and time since diagnosis Self-constructed (15 items) Relatives: Hours of caregiving per week (1 item) |
| Comorbidity |
Patients and relatives: Self-Administered Comorbidity Questionnaire (SCQ) [39] (1 item) |
| Health literacy |
Patients and relatives: Degree of understanding medical information, Set of Brief Screening Questions (SBSQ) [40] (3 items) |
| Resilience |
Patients and relatives: Connor-Davidson Resilience Scale (CD-RISC) [41] (10 items) |
| Digital comfort and skills |
Patients and relatives: The comfort and skills of using computer and mobile devices [42] (2 items) |
| Group cohesion |
Patients and relatives: The Group Climate Questionnaire (GCQ-23) [43] (23 items) |
| Evaluation of the program |
Patients and relatives: Experiences with the program Self-constructed (7 items) |
Content and format
The “Living with Cancer” program consists of two parts: six 1,5 hours video-conferencing group meetings with eight to 12 participants, facilitated by two peers, and preparatory audio clips. The latter concerns 15 three-minute preparatory audio clips with supportive text, addressing essential information about the themes that will be discussed in the meetings. As an additional resource, participants receive a workbook containing relevant chapters from the CDSMP book “Living a healthy life with chronic conditions” [44] and links to evidence-based information related to the themes in the program.
The program will be delivered by “Zoom” video-conferencing. To prepare and support participants for the meetings, there is a brief “meeting zero” with every individual participant to test the technical procedures. Technical support is available during the six video-conferencing group meetings.
The meetings focus on the development of participants’ self-management skills: action-planning, problem-solving, effective communication and decision-making [10]. In the meetings, these skills are related to relevant themes. Table 1 provides an overview of the program.
Table 1.
Overview of the “Living with Cancer” program
| Meeting 1 | Meeting 2 | Meeting 3 | Meeting 4 | Meeting 5 | Meeting 6 |
|---|---|---|---|---|---|
| Introduction programme | Sharing experiences; Action plan; Problem solving | Sharing experiences; Action plan | Sharing experiences; Action plan | Sharing experiences; Action plan | Sharing experiences; Action plan |
| Introduction participants |
Improving communication Listening activity |
Problem solving Sharing activity |
Decision making Sharing activity |
Communication with ourselves Sharing activity |
Adapting lifestyle Brainstorm |
|
Mind body connection Breathing exercise |
Dealing with difficult emotions Brainstorm |
Living with uncertainty Brainstorm and sharing activity |
Planning the future Brainstorm |
Dealing with pain Brainstorm |
Intimacy/ Sexuality Brainstorm |
|
Dealing with fatigue and prioritizing Brainstorm |
Formulation action plans |
Guided imagery Listening activity |
Communication with healthcare professionals Brainstorm |
Improving communication with family Brainstorm |
Reconnecting to people and getting help Brainstorm |
| Introduction to action plans | Instructions preparation next meeting | Formulation action plans | Formulation action plans | Formulation action plans | Evaluation |
| Instructions preparation next meeting | Instructions preparation next meeting | Instructions preparation next meeting | Instructions preparation next meeting |
The group meetings are structured. The facilitators introduce the activities and themes. After giving information, they initiate conversations between participants by guiding brainstorms and sharing experiences. They lead exercises (e.g. breathing) and listening activities (e.g. relaxation). The facilitators support participants in selecting challenges on which they would like to work, but do not suggest activities (this is called self-tailoring), and encourage them in completing their self-selected goals [20]. For detailed information about the program, see the Template for Intervention Description and Replication Checklist in Additional file 1 [45].
Peer facilitators
Facilitators are peers and are persons with stable (advanced) cancer, cancer survivors, relatives of persons with cancer or bereaved relatives of patients who died at least 6 months before the facilitators’ training. Peer facilitators are trained to follow a structured protocol with standardized scripts, to ensure consistency of delivery and content of the program. This requires a 24-hour online training distributed over 6 weeks, in which they participate in the program and learn how to facilitate it. Two certified master trainers provide the training, consisting of content delivery, adherence to timing and sequence of themes, coverage of the activities as set out in the protocol, and dealing with sensitivities and specific complex situations. Peer facilitators are instructed that they are not allowed to give medical advice. To ensure the quality of the meetings and adequate delivery of the content, fidelity will be checked with the CDSMP fidelity checklist by a selection of the recorded meetings.
Study design
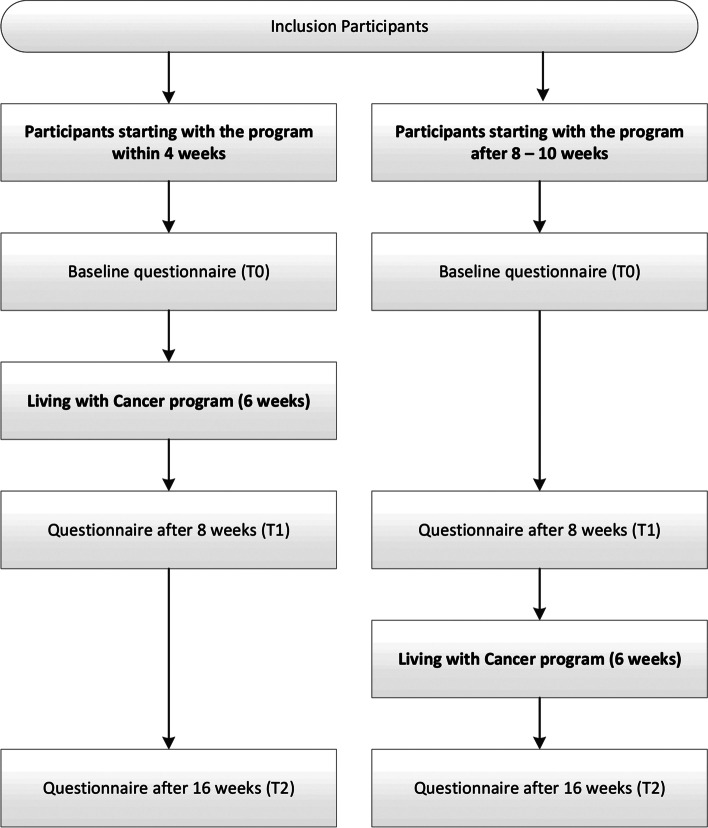
We will conduct a non-randomized stepped wedge study [46]. Participants can choose to either start the program within 4 weeks after inclusion (early starters) or after 8 to 10 weeks (late starters). The late starters allow us to collect control data about participants who have not yet followed the program [47]. See Fig. 1 for an overview of the non-randomized stepped wedge design.
Fig. 1.
Overview of the non-randomized stepped wedge design
Study population
Patients and relatives can participate together in the study, in the same or separate groups, as preferred. Patients can also participate without a relative and vice versa. Relatives can be partners, parents, children and other significant others such as close friends or neighbors.
Inclusion and exclusion criteria are described in Table 2 for patients and Table 3 for relatives.
Table 2.
patients’ inclusion and exclusion criteria
| Inclusion criteria | Exclusion criteria |
|---|---|
| Having advanced cancer (defined as having no curatively aimed treatment options available, only life-prolonging or palliative treatments) | Younger than 18 years of age |
| Conform WHO performance status of 0 or 1 (34) | Unable to provide written informed consent |
| Able to read and speak the Dutch language | Not willing to use a camera |
| Access to a computer or laptop and internet |
Table 3.
relatives’ inclusion and exclusion criteria
| Inclusion criteria | Exclusion criteria |
|---|---|
| Relative of a patient with advanced cancer | Younger than 18 years of age |
| Able to read and speak the Dutch language | Unable to provide written informed consent |
| Access to a computer or laptop and internet | Not willing to use a camera |
Recruitment
Participants will be recruited in two ways: via participating hospitals (an academic cancer center and a general hospital) and via self-referral. Self-referral has been described to increase ecological validity, facilitate greater equity of access to psycho-oncology research, and facilitate faster implementation of effective interventions into clinical practice [48]. The study will be advertised through diverse online channels such as a Dutch cancer information website [49], social media (Twitter, LinkedIn and Facebook), newspapers, patient associations and our website: www.smart-onderzoek.nl [50]. Persons with advanced cancer will be asked to invite one of their relatives to participate and vice versa.
Participants will receive the participant information sheet and oral information. They will be given adequate time (at least 1 week) to read and consider participation. Written consent will be obtained without any coercion of participants.
Measurements
In the SMART study, we will take the following measurements:
Questionnaire study. All participants will fill in an online questionnaire at three moments: right after inclusion (baseline), after 8 weeks and after 16 weeks. Completion of a questionnaire will take approximately 20 minutes. A written questionnaire can be provided if preferred. We will use both validated and self-constructed questionnaires (for an overview of the measures, see Table 4).
Logbooks. After each meeting, participants will be asked to answer a set of questions in a logbook: What did you learn during the meeting? In what way was the meeting useful (if at all)? How was the group experience? Which exercises were useful? Participants will be asked to document their action plans.
Recorded video-conferencing sessions. The group meetings will be video recorded with the consent of the participants.
Sample size calculation
To show an effect size of at least 0.5 SD on the HEIQ scale “constructive attitudes and approaches” [51], with a power of 80% and a significance of 0.05, assuming an individual autocorrelation across different time points of 0.7 [51], and assuming an average cluster size of eight patients simulation showed that in total 104 patients would need to be included across a wide range of within-period ICCs. With an expected drop-out rate of 20% (similar to the various CDSMP programs [24–26]), we need to recruit at least 130 patients. Based on our pilot study, we expect that of the participants, 20% will be relatives, therefore, we expect to include at least 32 relatives. In total, we will include 162 participants.
Data management
We will use the online survey tool Lime Survey [52] to send all questionnaires to participants. Gems Tracker will be used to uniformly collect, store and analyze the data [53]. Recordings of the meetings and logbooks will be stored at the Erasmus MC, University Medical Center Rotterdam. Only the research team will have access to the data.
Statistical analysis
We will follow the intention-to-treat principle for the analyses of the primary and secondary outcomes. Descriptive statistics will be used to summarize patient characteristics (age, gender, ethnicity, Social Economic Status, marital status, cancer type and time since diagnosis).
For the patient outcomes, we will use linear mixed models with a random intercept to adjust for repeated measures over time. As fixed effects, we will include time points of measurement (baseline, 8 weeks and 16 weeks) and a variable denoting when the participant received the program at each time point (early versus late starters). To adjust for possible confounding, we additionally will add age, sex, ethnicity, Social Economic Status, cancer type and time since diagnosis as fixed effects to the model.
Furthermore, we will assess whether there is a dose-response relationship between the number of sessions of the program and outcome. We will perform a similar analysis as for the main effect, but include the number of sessions instead of the intervention group.
The quantitative data analyses for relatives will be conducted in a similar way but it will have an explorative nature due to its expected lower number of participants.
Qualitative research
A complementary qualitative study will be carried out to explore the lived experiences of participants with the “Living with Cancer” program. Data concern the recordings of the video-conferencing groups and participants’ logbooks. We will conduct inductive, thematic content analyses [54, 55] describing their experiences with the program, including their perceived value of group-based peer support, the video-conferencing format, and the approach of including both persons with advanced cancer and relatives. We will also explore the perceived working mechanism of the program.
Discussion
Self-management for persons with advanced cancer and their relatives is multifaceted due to multilayered consequences and the uncertainties they face. To our knowledge, there is a lack of research into peer self-management support for this population.
The SMART study aims to fill this gap in knowledge. We will evaluate a self-management program to support persons with advanced cancer and their relatives. The program has been built on the CDSMP, an evidenced-based peer self-management support program for persons with chronic conditions [12, 13]. New features are its use for persons with advanced cancer, the inclusion of relatives, and the video-conferencing format for this population. The use of both quantitative and qualitative analyses will provide valuable insight into the effectiveness and value of this program.
Peer support for persons with advanced cancer and their relatives has rarely been researched [17]. Although the concept of peer support dates back several centuries, it is only in the last few decades that it has tracked attention in healthcare. In the field of mental health, there is an international growing trend to adopt peer support [56]. The literature suggests that peer support is beneficial in mental health care, such as improved health-related quality of life and improved patient activation [56]. In this study, we will now explore its value, as part of a self-management support program for persons with advanced cancer and their relatives. Self-management programs for this population with a format of video-conferencing group meetings are also new. A review showed that group interventions delivered by video-conferencing are acceptable and feasible in various populations (such as chronic disease, obesity, caregivers) [22]. In healthcare, telemedicine can be of considerable benefit to patients [57], and induced by the Covid-19 pandemic, it has taken off [58]. Therefore, examining this format for persons with advanced cancer and their relatives is a relevant next step.
Our SMART study has several risks. The study population and the program contain several factors that cannot be fully controlled. Firstly, there will be a considerable risk of drop out, either partly (some meetings) or completely, to disease progression or treatment burden of our vulnerable population [59]. To minimize this, we only include patients with a WHO performance status of 0 or 1 [60], and participants can choose when to start with the program to accommodate their schedule. Secondly, the risk of selection bias cannot be ruled out. Conducting research through self-referral, by online questionnaires and the video-conferencing group format, may primarily attract participants with higher digital literacy [61]. To minimize this risk, we offer technical support before and during the program, and explicitly explain that experience with video-conferencing applications is not necessary. Banbury and colleagues demonstrate that inexperience with video-conferencing or computer use was not a major problem for participation in video-conferencing groups [22]. Thirdly, while a randomized controlled trial (RCT) is preferred as a gold standard for measuring the effects of an intervention, we opt for a non-randomized stepped wedge design to make the study better feasible. Whilst we adjust for possible confounders in the analysis, residual confounding still remains a risk. Fourth, our program is a complex intervention, meaning it contains several interacting components, such as improving self-efficacy and behavior, the self-tailoring format, and group interaction [62]. Therefore, it may be difficult to identify the active components of the program. Combining the quantitative data of this study with the findings of our complementary qualitative study will provide insight into participants’ experiences and the perceived working mechanism of the program.
Supplementary Information
Acknowledgements
Not Applicable.
Abbreviations
- CDSMP
Chronic Disease Self-Management Program
- CD-RISC
Connor-Davidson Resilience Scale
- CRA-D
Caregiver Reaction Assessment Dutch
- GCQ-23
The Group Climate Questionnaire
- HADS
Hospital Anxiety and Depression
- HEIQ
Health Education Impact Questionnaire
- ICC
intraclass correlation coefficient
- MQOL
McGill Quality of Life
- NRS
numeric rating scale
- QOLLTI-F
Quality of Life in Life Threatening Illness Family Carer Version
- RCT
Randomized Controlled Trial
- SBSQ
Set of Brief Screening Questions
- SCQ
Self-Administered Comorbidity Questionnaire
- SES
Social Economic Status
- SPIRIT
Protocol Items: Recommendations for Interventional Trials
- UCLA
University of California, Los Angeles
- WHO
World Health Organization
Authors’ contributions
KLL, FEW, JACR, DN, EMB, LWK, CCDR, KL, AvdH contributed significantly to the design of the study. KLL, FEW and JACR drafted the manuscript with input from all authors. All authors read and approved the final manuscript.
Funding
This study is part of the project “Patient engagement in advanced cancer care: a 21st century myth or miracle?” funded by the Dutch Research Council (NWO, VIDI), file number 91717386. The funder had no role in the design of the study and collection, analysis, and interpretation of data and in writing the manuscript.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
This study (study protocol reference number MEC-2021-0347) is approved by the Medical Ethical Research Committee of the Erasmus MC, University Medical Center Rotterdam, 22 July 2022.
This study will be conducted in accordance with the Declaration of Helsinki and local laws, and regulations. Participants provide written informed consent before participation. Patients can withdraw from the study at any time without any consequences.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Bodenheimer T, Lorig K, Holman H, Grumbach K. Patient self-management of chronic disease in primary care. JAMA. 2002;288(19):2469–2475. doi: 10.1001/jama.288.19.2469. [DOI] [PubMed] [Google Scholar]
- 2.Coughlin S, Roberts D, O’Neill K, Brooks P. Looking to tomorrow’s healthcare today: a participatory health perspective. Intern Med J. 2018;48(1):92–96. doi: 10.1111/imj.13661. [DOI] [PubMed] [Google Scholar]
- 3.Rainbird K, Perkins J, Sanson-Fisher R, Rolfe I, Anseline P. The needs of patients with advanced, incurable cancer. Br J Cancer. 2009;101(5):759–764. doi: 10.1038/sj.bjc.6605235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Higginson IJ, Costantini M. Dying with cancer, living well with advanced cancer. Eur J Cancer. 2008;44(10):1414–1424. doi: 10.1016/j.ejca.2008.02.024. [DOI] [PubMed] [Google Scholar]
- 5.Langbaum T, Smith TJ. Time to study metastatic-cancer survivorship. N Engl J Med. 2019;380(14):1300–1302. doi: 10.1056/NEJMp1901103. [DOI] [PubMed] [Google Scholar]
- 6.Verduzco-Aguirre HC, Babu D, Mohile SG, Bautista J, Xu H, Culakova E, et al. Associations of uncertainty with psychological health and quality of life in older adults with advanced cancer. J Pain Symptom Manag. 2021;61(2):369–76. e1. [DOI] [PMC free article] [PubMed]
- 7.Palos GR, Mendoza TR, Liao KP, Anderson KO, Garcia-Gonzalez A, Hahn K, et al. Caregiver symptom burden: the risk of caring for an underserved patient with advanced cancer. Cancer. 2011;117(5):1070–1079. doi: 10.1002/cncr.25695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kristjanson LJ, Aoun S. Palliative care for families: remembering the hidden patients. Can J Psychiatr. 2004;49(6):359–365. doi: 10.1177/070674370404900604. [DOI] [PubMed] [Google Scholar]
- 9.Andersen NI, Nielsen CI, Danbjørg DB, Møller PK, Brochstedt KD. Caregivers' need for support in an outpatient Cancer setting. Oncol Nurs Forum. 2019;46(6):757–767. doi: 10.1188/19.ONF.757-767. [DOI] [PubMed] [Google Scholar]
- 10.Lorig KR, Holman HR. Self-management education: history, definition, outcomes, and mechanisms. Ann Behav Med. 2003;26(1):1–7. doi: 10.1207/S15324796ABM2601_01. [DOI] [PubMed] [Google Scholar]
- 11.Rietjens J, van Dongen S, Witkamp E. Self-management for patients with progressive, life-threatening diseases and their family caregivers. Textbook of Palliative Care: Springer Cham; 2018. p. 1–15.
- 12.Barlow J, Wright C, Sheasby J, Turner A, Hainsworth J. Self-management approaches for people with chronic conditions: a review. Patient Educ Couns. 2002;48(2):177–187. doi: 10.1016/S0738-3991(02)00032-0. [DOI] [PubMed] [Google Scholar]
- 13.Brady TJ, Murphy L, O'Colmain BJ, Beauchesne D, Daniels B, Greenberg M, House M, Chervin D. A metaanalysis of health status, health behaviors, and health care utilization outcomes of the Chronic Disease Self-Management Program. Prev Chronic Dis. 2013;10:120112. [DOI] [PMC free article] [PubMed]
- 14.Allegrante JP, Wells MT, Peterson JC. Interventions to support behavioral self-management of chronic diseases. Annu Rev Public Health. 2019;40:127–146. doi: 10.1146/annurev-publhealth-040218-044008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mead S, Hilton D, Curtis L. Peer support: a theoretical perspective. Psychiatr Rehabil J. 2001;25(2):134. doi: 10.1037/h0095032. [DOI] [PubMed] [Google Scholar]
- 16.Ziegler E, Hill J, Lieske B, Klein J, von dem Knesebeck O, Kofahl C. Empowerment in cancer patients: does peer support make a difference? Psychooncology: A systematic review; 2022. [DOI] [PubMed] [Google Scholar]
- 17.Walshe C, Roberts D. Peer support for people with advanced cancer: a systematically constructed scoping review of quantitative and qualitative evidence. Curr Opin Support Palliat Care. 2018;12(3):308–322. doi: 10.1097/SPC.0000000000000370. [DOI] [PubMed] [Google Scholar]
- 18.Kowitt SD, Ellis KR, Carlisle V, Bhushan NL, Black KZ, Brodar K, et al. Peer support opportunities across the cancer care continuum: a systematic scoping review of recent peer-reviewed literature. Support Care Cancer. 2019;27(1):97–108. doi: 10.1007/s00520-018-4479-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Budhwani S, Wodchis WP, Zimmermann C, Moineddin R, Howell D. Self-management, self-management support needs and interventions in advanced cancer: a scoping review. BMJ Support Palliat Care. 2019;9(1):12–25. doi: 10.1136/bmjspcare-2018-001529. [DOI] [PubMed] [Google Scholar]
- 20.Lorig KR, Sobel DS, Stewart AL, Brown BW Jr, Bandura A, Ritter P, et al. Evidence suggesting that a chronic disease self-management program can improve health status while reducing hospitalization: a randomized trial. Med Care. 1999;37(1):5–14. [DOI] [PubMed]
- 21.Self-management resource center. https://selfmanagementresource.com/ (2022). Accessed 22 Feb 2022.
- 22.Banbury A, Nancarrow S, Dart J, Gray L, Parkinson L. Telehealth interventions delivering home-based support group videoconferencing: systematic review. J Med Internet Res. 2018;20(2):e8090. doi: 10.2196/jmir.8090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chan A-W, Tetzlaff JM, Altman DG, Laupacis A, Gøtzsche PC, Krleža-Jerić K, et al. SPIRIT 2013 statement: defining standard protocol items for clinical trials. Ann Intern Med. 2013;158(3):200–207. doi: 10.7326/0003-4819-158-3-201302050-00583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lorig KR, Sobel DS, Ritter PL, Laurent D, Hobbs M. Effect of a self-management program on patients with chronic disease. Eff Clin Pract. 2001;4(6):256–262. [PubMed] [Google Scholar]
- 25.Risendal B, Dwyer A, Seidel R, Lorig K, Katzenmeyer C, Coombs L, et al. Adaptation of the chronic disease self-management program for cancer survivors: feasibility, acceptability, and lessons for implementation. J Cancer Educ. 2014;29(4):762–771. doi: 10.1007/s13187-014-0652-8. [DOI] [PubMed] [Google Scholar]
- 26.Lorig K, Ritter PL, Laurent DD, Yank V. Building better caregivers: a pragmatic 12-month trial of a community-based workshop for caregivers of cognitively impaired adults. J Appl Gerontol. 2019;38(9):1228–1252. doi: 10.1177/0733464817741682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Van Dongen SI, De Nooijer K, Cramm JM, Francke AL, Oldenmenger WH, Korfage IJ, et al. Self-management of patients with advanced cancer: a systematic review of experiences and attitudes. Palliat Med. 2020;34(2):160–178. doi: 10.1177/0269216319883976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.van Dongen SI, Stoevelaar R, Kranenburg LW, Noorlandt HW, Witkamp FE, van der Rijt CCD, et al. The views of healthcare professionals on self-management of patients with advanced cancer: an interview study. Patient Educ Couns. 2022;105(1):136–144. doi: 10.1016/j.pec.2021.05.021. [DOI] [PubMed] [Google Scholar]
- 29.Noorlandt HW, Stoevelaar R, et al. Challenges in selfmanagement of persons living with advanced cancer: A qualitative interview study. Eur J Cancer Care (Engl). forthcoming 2022. [DOI] [PMC free article] [PubMed]
- 30.Maunsell E, Lauzier S, Brunet J, Pelletier S, Osborne RH, Campbell HS. Health-related empowerment in cancer: validity of scales from the health education impact questionnaire. Cancer. 2014;120(20):3228–3236. doi: 10.1002/cncr.28847. [DOI] [PubMed] [Google Scholar]
- 31.Cohen SR, Mount BM, Tomas JJN, Mount LF. Existential well-being is an important determinant of quality of life: evidence from the McGill quality of life questionnaire. Cancer. 1996;77(3):576–586. doi: 10.1002/(SICI)1097-0142(19960201)77:3<576::AID-CNCR22>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 32.De Vrieze T, Coeck D, Verbelen H, Devoogdt N, Tjalma W, Gebruers N. Cross-cultural psychometric evaluation of the Dutch McGill-QoL questionnaire for breast cancer patients. Facts Views Vis ObGyn. 2016;8(4):205. [PMC free article] [PubMed] [Google Scholar]
- 33.Cohen R, Leis AM, Kuhl D, Charbonneau C, Ritvo P, Ashbury FD. QOLLTI-F: measuring family carer quality of life. Palliat Med. 2006;20(8):755–767. doi: 10.1177/0269216306072764. [DOI] [PubMed] [Google Scholar]
- 34.Steffen AM, McKibbin C, Zeiss AM, Gallagher-Thompson D, Bandura A. The revised scale for caregiving self-efficacy: reliability and validity studies. J Gerontol B Psychol Sci Soc Sci. 2002;57(1):P74–P86. doi: 10.1093/geronb/57.1.P74. [DOI] [PubMed] [Google Scholar]
- 35.Nijboer C, Triemstra M, Tempelaar R, Sanderman R, van den Bos GAM. Measuring both negative and positive reactions to giving care to cancer patients: psychometric qualities of the caregiver reaction assessment (CRA) Soc Sci Med. 1999;48(9):1259–1269. doi: 10.1016/S0277-9536(98)00426-2. [DOI] [PubMed] [Google Scholar]
- 36.Spinhoven PH, Ormel J, Sloekers PPA, Kempen G, Speckens AEM, van Hemert AM. A validation study of the hospital anxiety and depression scale (HADS) in different groups of Dutch subjects. Psychol Med. 1997;27(2):363–370. doi: 10.1017/S0033291796004382. [DOI] [PubMed] [Google Scholar]
- 37.Russell DW. UCLA loneliness scale (version 3): reliability, validity, and factor structure. J Pers Assess. 1996;66(1):20–40. doi: 10.1207/s15327752jpa6601_2. [DOI] [PubMed] [Google Scholar]
- 38.Ritter PL, Stewart AL, Kaymaz H, Sobel DS, Block DA, Lorig KR. Self-reports of health care utilization compared to provider records. J Clin Epidemiol. 2001;54(2):136–141. doi: 10.1016/S0895-4356(00)00261-4. [DOI] [PubMed] [Google Scholar]
- 39.Sangha O, Stucki G, Liang MH, Fossel AH, Katz JN. The self-administered comorbidity questionnaire: a new method to assess comorbidity for clinical and health services research. Arthritis Rheum. 2003;49(2):156–163. doi: 10.1002/art.10993. [DOI] [PubMed] [Google Scholar]
- 40.Schlatmann FWM, Hofmeester I, van Balken MR. Met “Ik geef u onze folder mee” heeft een op de tien nóg geen idee. Tijdschr Urol. 2016;6(6):94–96. doi: 10.1007/s13629-016-0131-6. [DOI] [Google Scholar]
- 41.Markowitz S, Peters ML. Psychometrische evaluatie van de CD-Risc in een Nederlandstalige populatie: een multi-of unifactorieel meetinstrument om veerkracht te meten. Tijdschr Klin Psychol. 2014;44(1):55–68. [Google Scholar]
- 42.Matthys O, De Vleminck A, Dierickx S, Deliens L, Van Goethem V, Lapeire L, et al. Effectiveness of a nurse-delivered (FOCUS+) and a web-based (iFOCUS) psychoeducational intervention for people with advanced cancer and their family caregivers (DIAdIC): study protocol for an international randomized controlled trial. BMC Palliat Care. 2021;20(1):1–18. doi: 10.1186/s12904-021-00895-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Trijsburg RW, Bogaerds H, Letiche M, Bidzjel L, Duivenvoorden HJ. De ontwikkeling van de Group Cohesion Questionnaire (GCQ) 2004. [Google Scholar]
- 44.Lorig K, Laurent D, Gonzalez V, Sobel D, Minor MA, Gecht-Silver M. Living a healthy life with chronic conditions: self-management skills for heart disease, arthritis, diabetes, depression, asthma, bronchitis. Emphysema and Other Physical and Mental Health Conditions: Bull Publishing Company; 2020. [Google Scholar]
- 45.Hoffmann TC, Glasziou PP, Boutron I, Milne R, Perera R, Moher D, et al. Better reporting of interventions: template for intervention description and replication (TIDieR) checklist and guide. BMJ. 2014;348:g1687. [DOI] [PubMed]
- 46.Hu Y, Hoover DR. Non-randomized and randomized stepped-wedge designs using an orthogonalized least squares framework. Stat Methods Med Res. 2018;27(4):1202–1218. doi: 10.1177/0962280216657852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hemming K, Haines TP, Chilton PJ, Girling AJ, Lilford RJ. The stepped wedge cluster randomised trial: rationale, design, analysis, and reporting. BMJ. 2015;350:h391. [DOI] [PubMed]
- 48.Thewes B, Rietjens JAC, van den Berg SW, Compen FR, Abrahams H, Poort H, et al. One way or another: the opportunities and pitfalls of self-referral and consecutive sampling as recruitment strategies for psycho-oncology intervention trials. Psychooncology. 2018;27(8):2056. doi: 10.1002/pon.4780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kanker.nl https://www.kanker.nl/ 2022. Accessed 22 Feb 2022.
- 50.SMART-onderzoek. https://smart-onderzoek.nl/ 2021. Accessed 22 Feb 2022.
- 51.Laursen DH, Christensen KB, Christensen U, Frølich A. Assessment of short and long-term outcomes of diabetes patient education using the health education impact questionnaire (HeiQ) BMC Res Notes. 2017;10(1):1–9. doi: 10.1186/s13104-017-2536-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.LimeSurvey. http://limesurvey.org 2022. Accessed 22 Feb 2022.
- 53.GemsTracker EMC. Equipe Zorgbedrijven. Latest release, 2017, version 1.8. 2, open source (new BSD licence). 2020.
- 54.Braun V, Clarke V. Using thematic analysis in psychology. Qual Res Psychol. 2006;3(2):77–101. doi: 10.1191/1478088706qp063oa. [DOI] [Google Scholar]
- 55.Tong A, Sainsbury P, Craig J. Consolidated criteria for reporting qualitative research (COREQ): a 32-item checklist for interviews and focus groups. Int J Qual Health Care. 2007;19(6):349–357. doi: 10.1093/intqhc/mzm042. [DOI] [PubMed] [Google Scholar]
- 56.Shalaby RAH, Agyapong VIO. Peer support in mental health: literature review. JMIR Ment Health. 2020;7(6):e15572. doi: 10.2196/15572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Almathami HKY, Win KT, Vlahu-Gjorgievska E. Barriers and facilitators that influence telemedicine-based, real-time, online consultation at patients’ homes: systematic literature review. J Med Internet Res. 2020;22(2):e16407. doi: 10.2196/16407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Greenhalgh T, Wherton J, Shaw S, Morrison C. Video consultations for covid-19. British Medical Journal Publishing Group; 2020. [DOI] [PubMed] [Google Scholar]
- 59.Applebaum AJ, Lichtenthal WG, Pessin HA, Radomski JN, Simay Gökbayrak N, Katz AM, et al. Factors associated with attrition from a randomized controlled trial of meaning-centered group psychotherapy for patients with advanced cancer. Psychooncology. 2012;21(11):1195–1204. doi: 10.1002/pon.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jang RW, Caraiscos VB, Swami N, Banerjee S, Mak E, Kaya E, et al. Simple prognostic model for patients with advanced cancer based on performance status. J Oncol Pract. 2014;10(5):e335–ee41. doi: 10.1200/JOP.2014.001457. [DOI] [PubMed] [Google Scholar]
- 61.Kemp E, Trigg J, Beatty L, Christensen C, Dhillon HM, Maeder A, et al. Health literacy, digital health literacy and the implementation of digital health technologies in cancer care: the need for a strategic approach. Health Promot J Austr. 2021;32:104–114. doi: 10.1002/hpja.387. [DOI] [PubMed] [Google Scholar]
- 62.Craig P, Dieppe P, Macintyre S, Michie S, Nazareth I, Petticrew M. Developing and evaluating complex interventions: the new Medical Research Council guidance. BMJ. 2008;337:a1655. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.