Abstract
Background:
Premature ventricular contractions (PVCs) are associated with heart failure and PVC-Cardiomyopathy. The prevalence of PVC-Cardiomyopathy and outcome benefits of PVC suppression are not clear. We sought to assess the rate and outcomes of PVC-Cardiomyopathy from the CHF-STAT trial, a population with cardiomyopathy (LV ejection fraction <40%) and frequent PVCs (>10 PVCs per hour).
Methods:
A secondary analysis of the CHF-STAT study was performed to compare the rate of successful PVC suppression (≥80% PVC reduction), “LV-recovery” (defined as improvement in LVEF ≥10% points) and PVC-Cardiomyopathy between amiodarone and placebo groups at 6 months. PVC-Cardiomyopathy was defined if both, ≥80% PVC reduction and LVEF improvement ≥10% were present at 6 months. Cardiac events (death or resuscitated cardiac arrest) were compared between PVC-Cardiomyopathy vs. non-PVC-Cardiomyopathy during a 5- year follow-up.
Results:
The rate of successful PVC suppression and LV-recovery were significantly higher in the amiodarone (72 and 39%, respectively) when compared to the placebo group (12 and 16%, respectively; P<0.001), regardless of cardiomyopathy etiology. PVC-Cardiomyopathy was present in 29% and 1.8% of amiodarone and placebo groups, respectively (P<0.001). Similar PVC-Cardiomyopathy rates were found in ischemic (24% amiodarone vs. 2% placebo, P<0.001) and non-ischemic populations (41% amiodarone vs 1.5% placebo, P<0.001). Death and resuscitated cardiac arrest were significantly lower in patients with PVC-Cardiomyopathy and those treated with amiodarone.
Conclusions:
The overall prevalence of PVC-Cardiomyopathy in the CHF-STAT study was significant regardless of ischemic substrate (29% overall population; 41% non-ischemic cardiomyopathy). Treatment of PVC-Cardiomyopathy with amiodarone is likely to improve survival in this high-risk population.
Keywords: Premature ventricular contractions, cardiomyopathy, LV systolic dysfunction, antiarrhythmics, amiodarone
Condensed Abstract:
A secondary analysis of the CHF-STAT study, a randomized double-blind controlled study comparing amiodarone vs. placebo in patients with LVEF <40% and >10 PVCs per hr, was performed to compare the rate and outcomes of PVC-Cardiomyopathy. The prevalence of PVC-Cardiomyopathy in the CHF study was significantly higher in the amiodarone-treated group with successful PVC suppression regardless of cardiomyopathy etiology. Death and resuscitated cardiac arrest were significantly lower in patients with PVC-Cardiomyopathy. This is the first clinical trial to demonstrate survival benefits of PVC suppression with amiodarone in patients with PVC-Cardiomyopathy.
Introduction
Premature ventricular contractions (PVCs) are commonly associated with heart failure (HF), PVC-cardiomyopathy, ventricular arrhythmias and sudden cardiac death (SCD)(1–6). Several studies have attempted to understand the prevalence of PVC-Cardiomyopathy, as well as patient and PVC characteristics that can identify and predict the development of this reversible cardiomyopathy. Unfortunately, most of these studies have significant limitations including a small sample size, and a retrospective or observational non-randomized design. Moreover, PVC suppression with either radiofrequency ablation (RFA) or antiarrhythmics drugs have only demonstrated improvement of LV function and decrease in ICD implant as the sole benefit, but no outcomes data are available(4,7). A secondary analysis of the CHF-STAT study(8) was performed to better understand the prevalence of PVC-Cardiomyopathy in this specific population, and assess the long-term benefit of PVC suppression with amiodarone therapy in patients with potential PVC-Cardiomyopathy.
Methods
Patient Population
In brief, 674 veteran patients with New York Heart Association (NYHA) functional class II, III, or IV CHF of at least 3 months duration were enrolled in a total of 25 Veteran Affair Medical centers as part of the CHF-STAT trial(8). Patients were required to have: 1) dyspnea on exertion or paroxysmal nocturnal dyspnea, 2) left ventricular EF of ≤40% (regardless of etiology: ischemic or non-ischemic) as measured with radionuclide angiography, 3) cardiothoracic ratio on chest radiography of >0.50 or a LV end-diastolic dimension of ≥5.5 cm, and 4) frequent ventricular ectopy (≥10 per hour averaged over a 24-hour period) without symptomatic arrhythmias or sustained ventricular tachycardia. In addition, patients were required to receive vasodilator therapy (angiotensin-converting enzyme inhibitor or hydralazine and non-parenteral nitrates), while heart failure therapy, such as diuretics and digoxin, were given as deemed appropriate by the responsible physician. Ischemic heart disease was defined based on coronary angiographic studies, electrocardiographic changes indicative of myocardial infarction, and chest pain typical of angina with concomitant electrocardiographic changes or reversible defects on radioisotope perfusion scans(8).
CHF-STAT Study Design
The design of the CHF-STAT trial and patient’s characteristics have been described previously(8). CHF-STAT was a prospective multicenter double-blind, placebo-controlled clinical trial that enrolled veterans from September 1989 through 1993, with a subsequent minimal follow-up of 1 year. This study received the proper ethical oversight with institutional approval. Subjects were randomized to either amiodarone or placebo groups. Clinic visits including interim history and complete cardiovascular examination were scheduled monthly. Twenty-four-hour ambulatory Holter monitoring and 12-lead ECGs were performed at baseline, 2 weeks, 1-, 3-, 6-, 9- and 12-months. Patients who permanently discontinued study drug were followed to the end of the trial and analyzed by the intention-to-treat principle. The radionuclide EF determination was repeated at 6, 12, and 24 months.
PVC-Cardiomyopathy Population
To identify patients with a potential diagnosis of PVC-Cardiomyopathy, we required to have at least the first 6-month repeat LVEF assessment and ambulatory Holter monitor. Successful PVC suppression was defined as ≥80% decrease in PVCs in a repeat ambulatory Holter recording. “LV-recovery” was defined as absolute LVEF improvement ≥10% at 6-month follow up, regardless of group assignment. The diagnosis of PVC-Cardiomyopathy was defined as patients with LV-recovery and successful PVC suppression at 6 months, regardless of treatment randomization. Cardiac event was defined as death or resuscitated cardiac arrest. This secondary analysis did not require ethical or IRB approval since all data was obtained in early 1990s and was de-identified upon collection. This secondary analysis was performed with ethical oversight from original data collected with de-identified personal health information.
Statistical Analysis
Descriptive categorical data are presented as percentages; continuous measures are reported as mean±1 SD and median (IQR). Differences between treatment groups in categorical and continuous variables were detected with the χ2 test, and the Student’s t test and/or the Wilcoxon-Mann-Whitney test, respectively. Changes in scores from baseline to timepoints of interest were compared between groups using the Student’s t-test and/or the Wilcoxon-Mann-Whitney test. Kaplan-Meier survival techniques were used to examine differences between treatment groups in the time from randomization to a specific event. Univariable and multivariable logistic regression modeling was performed. Candidate multivariable models were limited to two-term interactions, and alternative models were identified using stepwise and forward model building. In all cases, a two-sided α level of .05 was considered statistically significant; no adjustment was made for multiple comparisons.
Results
A total of 421 out of 674 subjects enrolled on the CHF-STAT study had LVEF assessment by MUGA scan and 24-hour ECG monitor at baseline and 6-month (Figure 1). The mean and median PVC burden in the overall population was 5,400 and 2832 per day, respectively. Supplemental Table 1 summarizes demographics and baseline parameters of 421 patients (mean age 65 years old) based on randomization group (amiodarone vs. placebo). While most patients were male (99%, reflective of the US veteran population), only 21% were of non-white race. Almost 3/4 of patients had ischemic cardiomyopathy (amiodarone, n=147; placebo n=153), while remaining 121 subjects had non-ischemic cardiomyopathy (amiodarone n=56; placebo n=65 patients).
Figure 1.
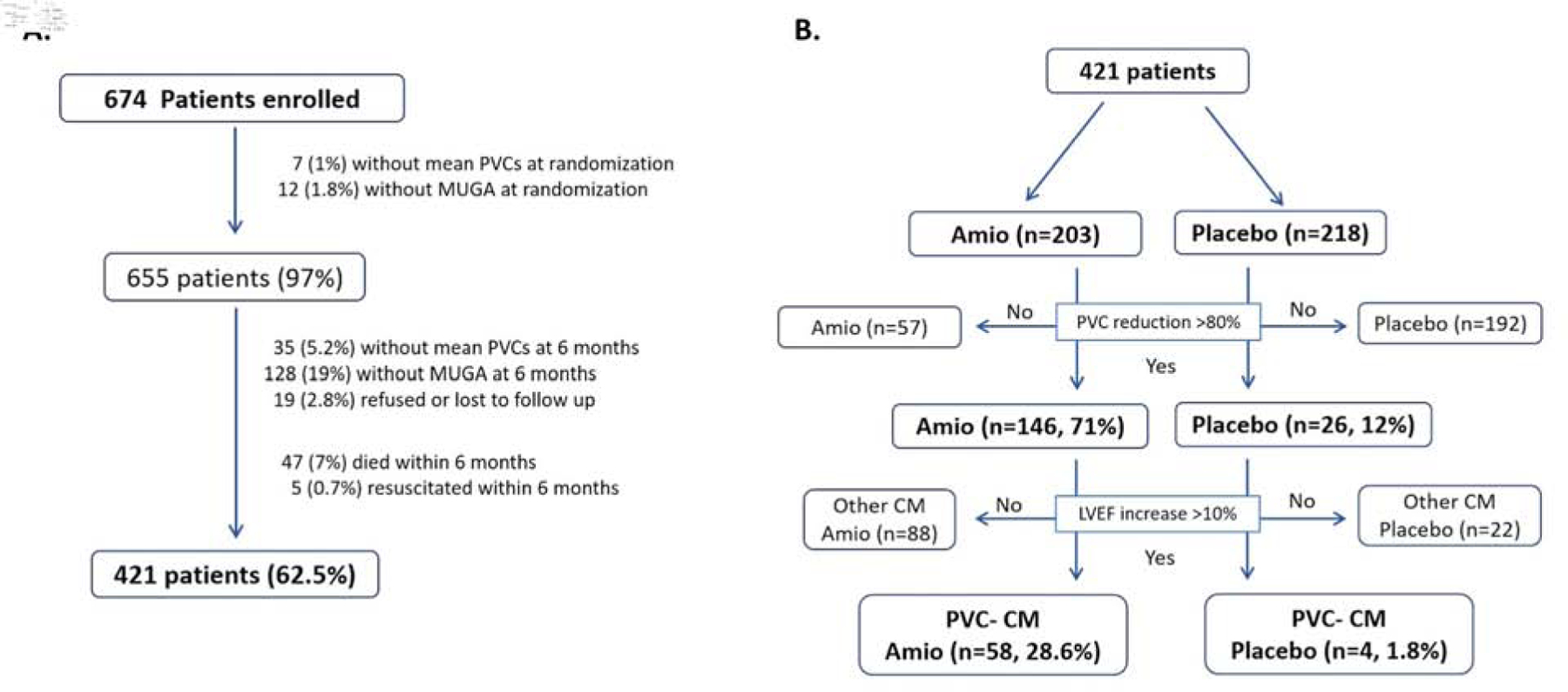
Enrollment and distribution of PVC-Cardiomyopathy in CHF-STAT study. (A) Enrollment of CHF-STAT study and rationale for final 421 patients included in secondary analysis. (B) Prevalence of PVC-Cardiomyopathy (PVC-CM) by treatment randomization including PVC suppression and improvement in LVEF.
Effects of amiodarone on PVC suppression
While baseline PVCs per hour were similar between groups (mean PVCs amiodarone 226 ± 338 vs. placebo 249 ±370, p=0.5; median PVCs amiodarone 84 (IQR 27 to 240) vs. placebo 112 (IQR 36 to 324), p=0.539), PVC reduction was significantly greater at 6 months among patients treated with amiodarone compared to placebo (amiodarone −176 ± 342 vs. placebo −37 ± 313, p<0.001). The median PVC reduction after intervention was −70 (IQR −209 to −22) and −8 (IQR −91 to 8) per day (p<0.001) for amiodarone and placebo groups, respectively. No statistically significant difference in PVC suppression was observed at 12 months (McNemar’s test 6- vs. 12-month, p = 0.370,); 82% of the patients had the same PVC suppression over this analysis period. Similarly, PVC suppression was not statistically different between 6- and 24 months (McNemar’s test, p = 0.199); 77% of the patients had the same PVC suppression over this analysis period. These two long-term comparisons support that PVC suppression was sufficiently maintained over time.
Figure 2A (left panel) summarizes the rate of successful PVC suppression based on treatment randomization. Successful PVC suppression was significantly higher in the amiodarone group (72% amiodarone vs. 12% placebo, p=0.01) regardless of cardiomyopathy type (ischemic or non-ischemic). Surprisingly, spontaneous PVC suppression (greater than 80%) was found in 11.9% (26 out of 218) of the placebo-control group lacking any intervention.
Figure 2.
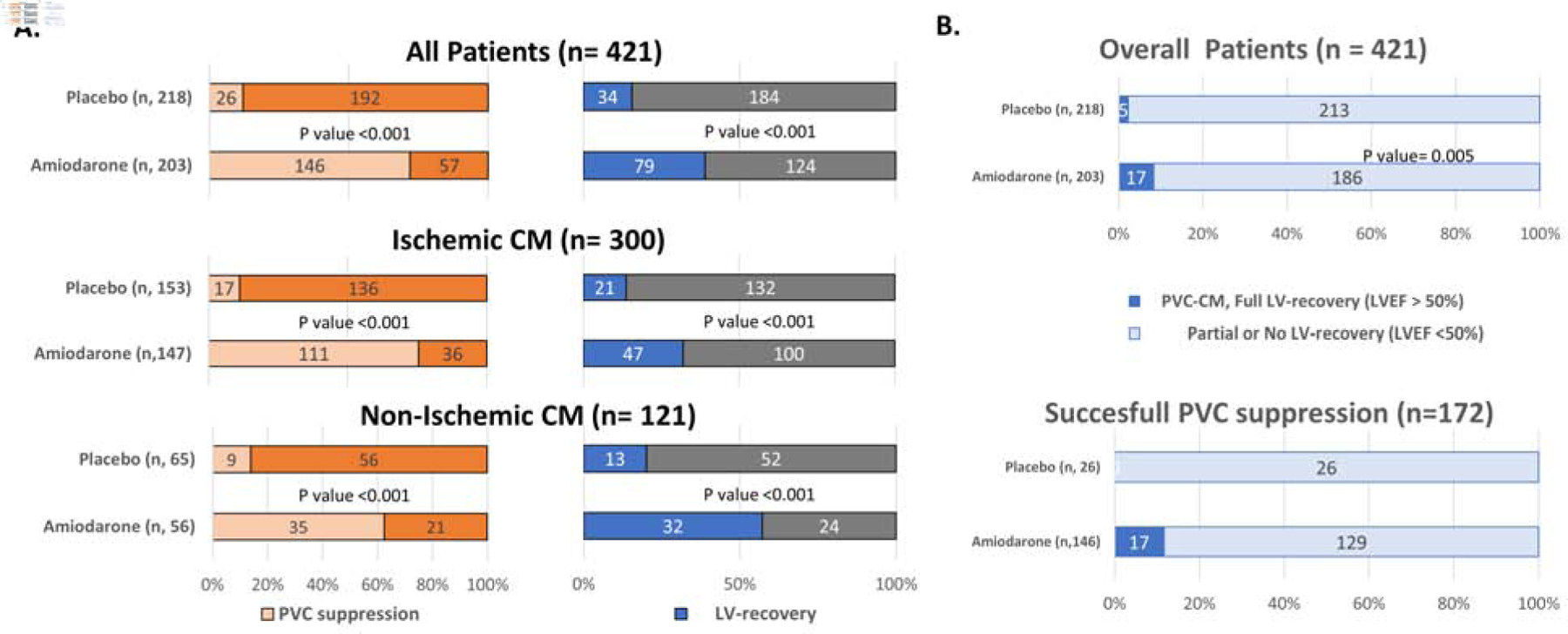
PVC suppression, LV recovery and full LV recovery by treatment randomization. (A). Rate of successful PVC suppression (left panel) and LV-recovery (right panel) based on treatment randomization in overall, ischemic and non-ischemic cardiomyopathy. Successful PVC suppression defined as ≥ 80% reduction of PVC burden at 6-month follow up; LV-recovery defined as ≥ 10% points increase in LVEF at 6-month follow up. (B) Rate of patients with full recovery of LV function (LVEF >50%) in the overall population (upper panel) and those with successful PVC suppression (lower panel) at 6 months. CM, Cardiomyopathy; PVC-CM, PVC-Cardiomyopathy.
Effects of Amiodarone on LV function
Changes in LVEF by MUGA in the overall, ischemic and non-ischemic population is summarized in Supplemental Table 2. As previously reported11, LVEF improved in both amiodarone and placebo groups, however, the increase was significantly greater in the amiodarone treated group. The largest improvement at 6 months was seen in the non-ischemic cardiomyopathy amiodarone group with a mean LVEF change of 11.9 ± 9.9 % points, whereas the mean LVEF change in the ischemic cardiomyopathy amiodarone population improved 7.0 ± 9.5% points. In contrast, the mean change in LVEF in the ischemic and non-ischemic cardiomyopathy population in placebo arm improved only 1.7 ± 6.7 and 3.6 ± 9.7% points, respectively. The change in LVEF between 6 and 12 months was not significant in either group regardless of etiology (ischemic vs. non-ischemic).
Similarly, the rate of LV-recovery (improvement of LVEF ≥10% points) was significantly higher in the amiodarone when compared to the placebo group regardless of etiology (ischemic vs. non-ischemic cardiomyopathy, Figure 2A – right panel). Interestingly, the rate of LV-recovery was different between amiodarone and placebo groups (40 vs. 15%) in those patients achieving a successful PVC suppression (58 out of 146 for amiodarone and 4 out of 26 for placebo group).
Prevalence of PVC-Cardiomyopathy
The rate of PVC-Cardiomyopathy (LV-recovery with successful PVC suppression) is summarized in Central Figure (Panel A). PVC-Cardiomyopathy was significantly higher in the amiodarone group (28.6%, 58 out of 203 subjects) when compared to placebo (1.8%, 4 out of 218 patients, P=0.01) in the overall population. Baseline daily PVCs in those patients with PVC-Cardiomyopathy in the amiodarone group ranged from 264 to 55,992 PVCs per day, with a median of 2,832 PVCs per day. The prevalence of PVC-Cardiomyopathy was more pronounced in non-ischemic (41%, 23 out of 56 patients) than ischemic cardiomyopathy (24%; 35 out of 147 patients) treated with amiodarone (Supplemental Table 3). Furthermore, complete recovery of LV function (LVEF >50%) in subjects with successful PVC suppression was greater in amiodarone than placebo treated groups at 6 months (11.6 vs. 0%, respectively, Figure 2B – lower panel). Not surprisingly, the rate of LV-recovery in patients without successful PVC suppression was similar in amiodarone and placebo group (amiodarone 10.3% vs. placebo 13.8%, Central Figure Panel A).
Central Figure.

Rate and Kaplan Meier Curves of PVC-Cardiomyopathy. (A) Rate of PVC-Cardiomyopathy in the overall population based on different treatment groups (amiodarone vs placebo groups) at 6 months. PVC-Cardiomyopathy defined as LV-recovery (LVEF improvement ≥10%) on only those with successful PVC suppression (≥80% PVC reduction). No LV-recovery (LVEF improvement <10%) despite successful PVC suppression represents cardiomyopathy unrelated to PVCs. LV recovery despite lack of PVC suppression represents a reversible CM unrelated to PVCs. Comparison of Event free survival between subjects with PVC-Cardiomyopathy (PVC suppression >80% and LVEF increase >10%) vs. non-PVC-Cardiomyopathy (PVC suppression <80% with or without LV recovery and PVC suppression >80% without LV recovery) in overall (B) and non-ischemic (C) population regardless of treatment randomization. PVC-CM, PVC-Cardiomyopathy.
Factors that predict PVC - Cardiomyopathy
A decrease in PVC burden had a correlation with the improvement in LVEF in the overall and ischemic population (r= - 0.167 and r= - 0.23, respectively; p<0.001). Furthermore, the improvement in LVEF at 6 months was significantly greater among patients who achieve an 80% PVC reduction than those that did not (9.3 ± 9.9% vs. 5.7 ± 9.2%, respectively; p = 0.01). Successful PVC suppression was associated with greater LV recovery rate when compared to those that lack PVC suppression (29% vs. 10%) in the amiodarone-treated population (Central Figure A, upper panel). In contrast, there was no statistical difference in baseline PVC burden between patients with and without LV-recovery analyzed for each treatment arm (amiodarone p=0.5; placebo p=0.1). Based on univariable logistic regression modeling, only amiodarone treatment (OR: 21.4; 95%CI: 7.6 to 60.2; p<0.001) was statistically significant relative to the diagnosis of PVC-Cardiomyopathy. Baseline PVC burden (OR: 1.000; 95%CI: 0.999 to 1.001; p=0.404), QRS duration (OR: 1.01; 95%CI: 0.960 to 1.07; p=0.629), ischemic vs non-ischemic cardiomyopathy (OR: 0.586; 95%CI: 0.334 to 1.03; p=0.062) were not significantly associated with the diagnosis of PVC-Cardiomyopathy. Multivariable logistic regression modeling including two-way interaction terms failed to demonstrate that any of these factors, other than amiodarone treatment, was significantly associated with PVC-Cardiomyopathy.
Outcomes in PVC-Cardiomyopathy
Patients with presumptive diagnosis of PVC-Cardiomyopathy demonstrated a significantly better event-free survival (death and resuscitated cardiac arrest) in the overall (p=0.02, log rank – Central Figure Panel B. Kaplan Meier) and non-ischemic cardiomyopathy population regardless of treatment adjudication (Central Figure Panel C). While outcomes were not significantly different between patients that achieved PVC suppression >80% versus those that did not, event-free survival was significantly better in those subjects with diagnosis of PVC-Cardiomyopathy than those without PVC-Cardiomyopathy in the population treated with amiodarone (p =0.004, log rank - Figure 3). Furthermore, LV recovery had a significant survival benefit as compared to the no-LV-recovery in the overall group and amiodarone group (P=0.04 and P=0.007, respectively) but not in the placebo group (P=0.6) (Supplemental Figure 1). Finally, patients with LV-recovery had better outcomes compared to those without LV-recovery in the overall population with successful PVC suppression (cardiac events: LV-recovery 22% vs. no LV-recovery 39.4%, p=0.02, log rank – Supplemental Figure 2).
Figure 3.
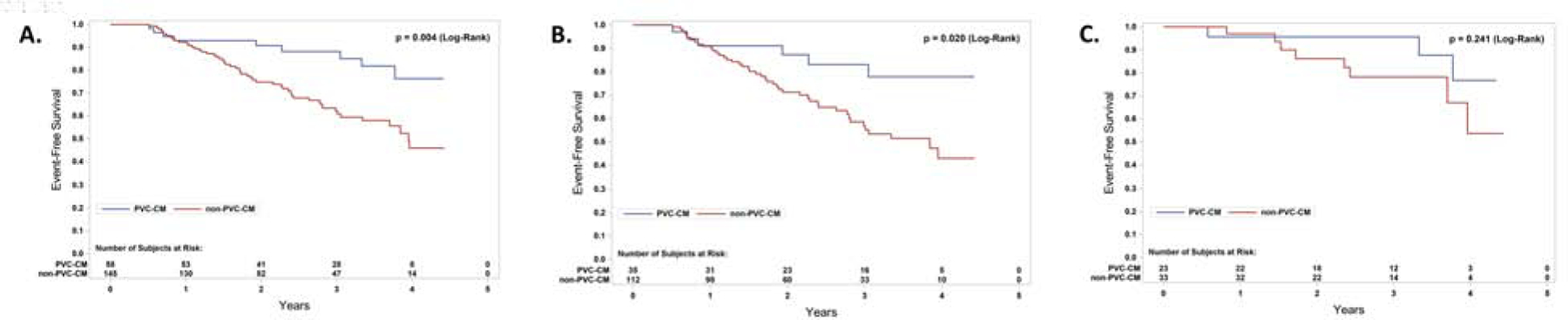
Kaplan Meier Curves in PVC- vs. non-PVC-Cardiomyopathy on amiodarone group arm only. Comparison of Event free survival between subjects with PVC-Cardiomyopathy (PVC suppression >80% and LV recovery) vs. non-PVC-Cardiomyopathy (PVC suppression <80% with or without LV recovery and PVC suppression >80% without LV recovery) in overall (A), ischemic (B) and non-ischemic (C) population within the amiodarone-treated group only. PVC-CM, PVC-Cardiomyopathy.
An Adjudication Committee classified deaths (including resuscitated cardiac events) as either Sudden Cardiac, Non-Sudden Cardiac, or Non-Cardiac. For the 136 deaths for which Adjudication Committee findings were available, 48.5% (66) were Sudden Cardiac, 28.7% (39) were Non-Sudden Cardiac, and 22.8% (31) were Non-Cardiac. Based on Chi Squared or Fisher’s Exact analyses, there was no statistically significant association between cause of death and ≥10% LVEF Improvement (p=0.687), ≥80% PVC Suppression (p=0.620), or PVC-CM (i.e., both ≥10% LVEF Improvement and ≥80% PVC Suppression) (p=0.416).
Discussion
After more than a decade of clinical and translational data, PVC-Cardiomyopathy has been recently recognized as a unique clinical entity(4,5,9,10). Besides improvement in LV function, no outcome benefits have been demonstrated by treating PVC-Cardiomyopathy. This study presents a secondary analysis of the CHF-STAT study(8), one of the largest clinical randomized studies with amiodarone for PVC suppression in patients with LV dysfunction at a time when PVC-Cardiomyopathy was not recognized. The CHF-STAT study was designed with the intention to assess if amiodarone would increase mortality in patients with frequent PVCs (>10 PVC per hour) and cardiomyopathy as shown with encainide and flecainide in the CAST trial(11). In contrast to the CAST trial, the CHF-STAT study showed that amiodarone improved LV function and reduced overall mortality in the non-ischemic cardiomyopathy population(8,12). Moreover, the GESICA trial(13) revealed that amiodarone reduced a combined endpoint of cardiac death and HF hospitalizations in a population with non-ischemic cardiomyopathy, which could not be explained at the time.
The main findings of this secondary analysis are: 1) successful PVC suppression and LV-recovery rate were significantly higher in the amiodarone group when compared to the placebo group; 2) prevalence of PVC-Cardiomyopathy is at least 28% in the overall population (>10 PVCs/hr and LVEF<40%) and up to 41% in the non-ischemic population; 3) the decrease in PVC burden had a weak but statistical correlation with LVEF improvement; 4) amiodarone treatment and non-ischemic cardiomyopathy were predictors for the diagnosis of PVC-Cardiomyopathy; and finally, 5) amiodarone treatment improves outcomes in patients with PVC-Cardiomyopathy.
PVC suppression and LV-recovery on amiodarone
The CHF-STAT study is consistent with previous observational non-randomized studies of frequent PVCs and cardiomyopathy where PVC suppression improves or restores LV function(2,4,6,12). Our analysis demonstrates that amiodarone leads to significant improvement in LV dysfunction after successful PVC suppression in both ischemic and non-ischemic cardiomyopathies. Most of the improvement of LV function after PVC suppression in the amiodarone-treated group occurred within 6 months, without any further improvement at 12 months. Moreover, at least 8% of patients treated with amiodarone had a full recovery of LV function (LVEF >50%, Figure 2B, upper panel) suggesting PVCs as a sole etiology of the cardiomyopathy.
A minimum PVC burden of 10–16% necessary to suspect a diagnosis of PVC-Cardiomyopathy was derived from retrospective studies(2,6) that enrolled only patients referred for PVC ablation. Thus, a selection bias is likely with an unusual high baseline PVC burden (mean 20–33%) when compared to a heart failure population. In contrast, the median and mean PVC frequency in the CHF-STAT study was only 2,832 and 6,600 per day, respectively, which would correspond approximately to 3–6% PVC burden. Likewise, other studies(14–16) have showed an improvement in LV function after PVC suppression in patients with baseline PVC burden as little as 6 to 8%, suggesting that the minimum PVC burden threshold that can cause PVC-Cardiomyopathy may be less than 10% and as low as 4–5%. Moreover, large population-based studies (ARIC and CV health study) have demonstrated an increased risk to develop heart failure even in patients with low PVC burden(1,3). This supports individual susceptibility or idiosyncrasy to develop PVC-cardiomyopathy despite similar and even low PVC burden.
Moreover, spontaneous PVC suppression occurred in 12% of subjects in the placebo group (Figure 2A, left panel) at 6 months, supporting the notion that spontaneous resolution of frequent PVCs without any intervention is possible in a small percentage of patients. In contrast, a similar percentage of subjects in both amiodarone and placebo groups improve their LV function despite the PVC suppression less than 80%, which is likely due to a non-ischemic cardiomyopathy unrelated to PVCs, although we cannot exclude that maybe a lower threshold of PVC suppression is enough to improve LV function in some patients. Only a recent publication supports spontaneous PVC reduction, reporting 44% of cases (baseline PVC >5%) had spontaneous suppression without intervention after a 5-year period(17).
Prevalence of PVC-Cardiomyopathy
The challenge of PVC-Cardiomyopathy is that while non-invasive testing will help us suspect the diagnosis, confirmation can only be made after elimination or suppression of PVCs(4,10). Moreover, because the significant nature of PVC variability from a day to day basis, prior studies assessing the impact of an intervention to successfully suppress PVCs have required at least an 80% reduction of PVC(2,6,18). Thus, the prevalence of PVC-Cardiomyopathy in the CHF-STAT study was solely estimated based on LV-recovery who achieved at least a PVC suppression of 80% at 6 months on the amiodarone group. While patients that improved their LVEF (≥10%) with successful PVC suppression met the diagnostic criteria for PVC-Cardiomyopathy, this diagnosis cannot be excluded among those patients that lack successful PVC suppression despite amiodarone. Thus, the prevalence of PVC-Cardiomyopathy in this population is at least 28% since it is possible that the diagnosis has been missed in some patients that failed to suppress PVCs despite treatment with amiodarone (n=36, Central Figure, Panel A). Moreover, the prevalence of PVC-Cardiomyopathy in the placebo group is only relevant as it allows us to understand the natural history of patients with frequent PVCs and LV dysfunction, as well as the probability of spontaneous recovery of PVC and LV function without any intervention, which in the CHF-STAT study was less than 2% (Central Figure, Panel A). Theoretically, we could speculate that 28% of patients on the placebo arm would have had the diagnosis of PVC-Cardiomyopathy if treated with amiodarone.
While large population-based studies have tried to identify the odds and hazard ratio to develop LV dysfunction with frequent PVCs, they lack the corroboration of the diagnosis of PVC-Cardiomyopathy since no intervention was made(1,3). A retrospective study of 1185 subjects with frequent PVCs (mean daily PVC burden 20 ± 13%) found that 245 patients (21%) had an associated cardiomyopathy (LVEF <50%), of which 67% (164 patients) improved their LVEF more than 10% points after RFA, consistent with a diagnosis of PVC-Cardiomyopathy(6). We could potentially explain the higher prevalence of PVC-Cardiomyopathy in the same population (frequent PVCs and LV dysfunction) due to the higher mean baseline daily PVC burden (20 vs. 5.4%) and LVEF cut-off (less than 50% vs. 40%) when compared to CHF-STAT study.
Predictors of PVC-Cardiomyopathy
Multivariate analysis of the CHF-STAT demonstrated that amiodarone treatment and type of cardiomyopathy were predictors of PVC-Cardiomyopathy. Notably, patients with ischemic cardiomyopathy should also be suspected and treated for a superimposed PVC-Cardiomyopathy (up to 24% of ischemic cardiomyopathy in CHF-STAT, Supplemental Table 3) if frequent PVCs are documented, since its treatment is likely to improve their LVEF and outcomes. In contrast to retrospective and observational studies(2,6,16), baseline PVC burden did not predict the diagnosis of PVC-Cardiomyopathy in the multivariate analysis. This could potentially be explained by a relatively low baseline PVC burden when compared to other studies. Concordant with other studies(19), we found a correlation between decrease in PVC burden and improvement in LVEF in the overall and ischemic population. However, the correlation was weak (r= - 0.167 and r= - 0.23, respectively; p<0.001), suggesting that there are other factors in play that effect change in EF, and that PVC reduction is far from the sole driver. While animal models of PVCs and PVC-Cardiomyopathy have assisted identifying potential triggers and cellular mechanism(s) of PVC-Cardiomyopathy(20–25), future large clinical studies are needed to confirm these predictors.
Outcomes in PVC-Cardiomyopathy
To our knowledge, this is the first study to demonstrate a clear outcome benefit by suppressing PVCs in patients with potential PVC-Cardiomyopathy. An improvement in death and resuscitated cardiac arrest (event-free survival) was noted in those with a diagnosis of PVC-Cardiomyopathy regardless of treatment randomization (Central Figure, Panel B and C). Furthermore, outcomes within the amiodarone-treated group were significantly improved in the patients with a diagnosis of PVC-Cardiomyopathy (improvement of LVEF >10% and PVC burden reduction >80%) than those without PVC-Cardiomyopathy diagnosis (Figure 3). Finally, this outcome benefit was observed only in subjects with successful PVC suppression that achieved LV recovery (Supplemental Figure 2) and amiodarone-treated patients that achieved LV recovery (Supplemental Figure 1). This secondary analysis together with the initial CHF-STAT analysis(8) and the SCD-HeFT trial(26) comparing ICD, amiodarone and placebo in heart failure patients, confirms that the survival benefit is not driven by amiodarone alone but rather a combination of LV recovery and PVC suppression, consistent with a PVC-Cardiomyopathy diagnosis. Only a placebo-control trial can identify outcome and survival benefits of PVC suppression in PVC-Cardiomyopathy. Nowadays, enrollment for such a clinical trial with randomization to a placebo-control arm would not only be difficult, but considered unethical by many.
Limitations:
Secondary analysis of a prospective randomized study has inherent limitations. However, this study prospectively assessed outcomes including a close follow up of ambulatory Holters and LV function with MUGA scan, allowing an accurate diagnosis of PVC-Cardiomyopathy based on response to PVC suppression with amiodarone. We acknowledge that this clinical trial did not have current contemporary HF therapy as it was conducted more than 2 decades ago. Unfortunately, no distribution of medical therapy is available for this secondary analysis (421 subjects). However, based on the original report of 674 patients(8) the use of beta-blockers, ACE inhibitors and diuretics were equally distributed between groups (3.9, 76 and 84% for amiodarone vs. 4.7, 79 and 82% for placebo, respectively). Beta-blockers effect of amiodarone is unlikely to explain the survival benefit since this benefit was absent in those without a final diagnosis of PVC-Cardiomyopathy despite treatment with amiodarone (Figure 3). We cannot exclude that some of patients randomized to amiodarone with likely PVC-CM diagnosis also (n=58) had AF that converted to sinus rhythm during the follow-up period(27). Further statistical analysis (supplemental material) supports that the small fraction of participants with AF included in this study do not significantly changed any findings or conclusions presented in this manuscript. Even though there is a consensus of what is defined as successful PVC suppression (decrease greater than 80%), it is unclear if a PVC suppression of a lesser degree may also improve LVEF and/or outcomes(6,15,16,18). This study was performed with 24-hour recordings at baseline and 6 months, which can limit the assessment of true long-tern PVC burden(28). In addition, multivariate analysis was limited since several PVC characteristics (QRS duration, location and coupling interval) were not available for analysis. Finally, it is unclear if these findings apply to HF patients with less than 10 PVC per hour and women since this veteran population was mostly male.
Together with RFA, antiarrhythmic drugs have been used as a PVC suppression strategy to improve LV dysfunction in patients with frequent PVCs with a presumptive diagnosis of PVC-Cardiomyopathy. Our analysis demonstrates that amiodarone is an effective treatment to improve LV dysfunction in patients with frequent PVCs, even in those with an underlying ischemic cardiomyopathy. Thus, an aggressive approach to suppress PVCs with either RFA or antiarrhythmic drugs is likely to resolve or improve PVC-Cardiomyopathy with subsequent outcome benefits. However, it is unclear if one treatment strategy is superior to the other(4,7), which supports the need for a large randomized clinical trial comparing these treatments. Furthermore, our secondary analysis demonstrates that PVC-Cardiomyopathy is a frequent diagnosis in the population with LV dysfunction and frequent PVCs regardless of the type of cardiomyopathy (ischemic vs. non-ischemic) (28%, 24% and 41% in the overall, ischemic and non-ischemic, respectively). Thus, all patients with LV dysfunction should be screened with an ambulatory cardiac monitor to quantify the PVC burden to exclude or suspect the diagnosis of this potentially reversible form of cardiomyopathy. If frequent PVCs are documented in the setting of a cardiomyopathy, treatment to suppress PVCs should be strongly considered due its potential impact in morbidity and mortality and subsequent ramifications in quality of life and healthcare spending.
Conclusions
This secondary analysis of the CHF-STAT study demonstrates that PVC suppression with amiodarone significantly improves LV systolic function and clinical outcomes in patients with PVC-Cardiomyopathy. A PVC-Cardiomyopathy prevalence of 28% was found in this specific population with frequent PVCs (>10 PVCs per hour) and cardiomyopathy (LVEF <40%), with differences in the presence of ischemic etiology (24% and 41% for ischemic and non-ischemic, respectively). The diagnosis and treatment of PVC-Cardiomyopathy should be strongly considered as it is likely to impact morbidity and mortality in this population.
Supplementary Material
Perspectives.
Competency in Medical Knowledge:
PVC suppression in PVC-cardiomyopathy is paramount not only to improve LV systolic function but most importantly to improves outcomes.
Competency in Patient Care:
PVC-Cardiomyopathy should be suspected in patients with frequent PVCs and LV dysfunction as appropriate treatment is likely to improve survival. Ambulatory ECG monitors should be considered to assess PVC burden in patients with LV systolic dysfunction. However, diagnosis of PVC-cardiomyopathy can only be confirmed if LV function improves after successful PVC suppression.
Translational Outlook 1:
This secondary analysis of the CHF-STAT study compared amiodarone versus placebo in patients with potential PVC-cardiomyopathy. While we assume that any PVC suppression strategy may improve survival outcomes in patients with PVC-cardiomyopathy, future studies should be performed to assess differences in outcomes between radiofrequency ablation and antiarrhythmic drugs.
Translational Outlook 2:
The CHF-STAT study involved mostly males, characteristic of the veteran population, and thus further studies should address if any difference in response to amiodarone exists in gender.
Sources of Funding:
1R01HL139874–01 (PI: Huizar), 5R34HL138110–02 (PI: Huizar); Research grant by Abbott, Inc.
Abbreviation list:
- LV
Left ventricle
- LVEF
LV ejection fraction
- PVC
Premature ventricular contraction
- RFA
Radiofrequency Ablation
- SCD
Sudden cardiac death
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Relationship with Industry: Huizar JF – Research support from Abbott; Kaszala K – Research support from Boston Scientific (BS) and Abbott; Tan A – Research support from BS, MDT and Biotronik. (BTK); Ellenbogen KA – Research support from BS, Biosense Webster (BW), MDT, Abbott, NIH, Consultant for BS, Abbott, Atricure, Medtronic, Honoraria from MDT, BS, BTK, BW and Atricure; the remaining authors have nothing to disclose.
References
- 1.Agarwal SK, Simpson RJ Jr., Rautaharju P et al. Relation of ventricular premature complexes to heart failure (from the Atherosclerosis Risk In Communities [ARIC] Study). Am J Cardiol 2012;109:105–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baman TS, Lange DC, Ilg KJ et al. Relationship between burden of premature ventricular complexes and left ventricular function. Heart Rhythm 2010;7:865–9. [DOI] [PubMed] [Google Scholar]
- 3.Dukes JW, Dewland TA, Vittinghoff E et al. Ventricular Ectopy as a Predictor of Heart Failure and Death. J Am Coll Cardiol 2015;66:101–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huizar JF, Ellenbogen KA, Tan AY, Kaszala K. Arrhythmia-Induced Cardiomyopathy: JACC State-of-the-Art Review. J Am Coll Cardiol 2019;73:2328–2344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huizar JF, Kaszala K, Potfay J et al. Left ventricular systolic dysfunction induced by ventricular ectopy: a novel model for premature ventricular contraction-induced cardiomyopathy. Circ Arrhythm Electrophysiol 2011;4:543–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Latchamsetty R, Yokokawa M, Morady F et al. Multicenter Outcomes for Catheter Ablation of Idiopathic Premature Ventricular Complexes. JACC Clin Electrophysiol 2015;1:116–123. [DOI] [PubMed] [Google Scholar]
- 7.Zhong L, Lee YH, Huang XM et al. Relative efficacy of catheter ablation vs antiarrhythmic drugs in treating premature ventricular contractions: a single-center retrospective study. Heart Rhythm 2014;11:187–93. [DOI] [PubMed] [Google Scholar]
- 8.Singh SN, Fletcher RD, Fisher SG et al. Amiodarone in patients with congestive heart failure and asymptomatic ventricular arrhythmia. Survival Trial of Antiarrhythmic Therapy in Congestive Heart Failure. N Engl J Med 1995;333:77–82. [DOI] [PubMed] [Google Scholar]
- 9.Tan AY, Hu YL, Potfay J et al. Impact of ventricular ectopic burden in a premature ventricular contraction-induced cardiomyopathy animal model. Heart Rhythm 2016;13:755–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bozkurt B, Colvin M, Cook J et al. Current Diagnostic and Treatment Strategies for Specific Dilated Cardiomyopathies: A Scientific Statement From the American Heart Association. Circulation 2016;134:e579–e646. [DOI] [PubMed] [Google Scholar]
- 11.Echt DS, Liebson PR, Mitchell LB et al. Mortality and morbidity in patients receiving encainide, flecainide, or placebo. The Cardiac Arrhythmia Suppression Trial. N Engl J Med 1991;324:781–8. [DOI] [PubMed] [Google Scholar]
- 12.Massie BM, Fisher SG, Radford M et al. Effect of amiodarone on clinical status and left ventricular function in patients with congestive heart failure. CHF-STAT Investigators. Circulation 1996;93:2128–34. [DOI] [PubMed] [Google Scholar]
- 13.Doval HC, Nul DR, Grancelli HO, Perrone SV, Bortman GR, Curiel R. Randomised trial of low-dose amiodarone in severe congestive heart failure. Grupo de Estudio de la Sobrevida en la Insuficiencia Cardiaca en Argentina (GESICA). Lancet 1994;344:493–8. [DOI] [PubMed] [Google Scholar]
- 14.Yarlagadda RK, Iwai S, Stein KM et al. Reversal of cardiomyopathy in patients with repetitive monomorphic ventricular ectopy originating from the right ventricular outflow tract. Circulation 2005;112:1092–7. [DOI] [PubMed] [Google Scholar]
- 15.Sirichand S, Killu AM, Padmanabhan D et al. Incidence of Idiopathic Ventricular Arrhythmias: A Population-Based Study. Circ Arrhythm Electrophysiol 2017;10. [DOI] [PMC free article] [PubMed]
- 16.Sadron Blaye-Felice M, Hamon D, Sacher F et al. Premature ventricular contraction-induced cardiomyopathy: Related clinical and electrophysiologic parameters. Heart Rhythm 2016;13:103–10. [DOI] [PubMed] [Google Scholar]
- 17.Lee AKY, Andrade J, Hawkins NM et al. Outcomes of untreated frequent premature ventricular complexes with normal left ventricular function. Heart 2019;105:1408–1413. [DOI] [PubMed] [Google Scholar]
- 18.Anastasiou-Nana MI, Menlove RL, Nanas JN, Anderson JL. Changes in spontaneous variability of ventricular ectopic activity as a function of time in patients with chronic arrhythmias. Circulation 1988;78:286–95. [DOI] [PubMed] [Google Scholar]
- 19.Mountantonakis SE, Frankel DS, Gerstenfeld EP et al. Reversal of outflow tract ventricular premature depolarization-induced cardiomyopathy with ablation: effect of residual arrhythmia burden and preexisting cardiomyopathy on outcome. Heart Rhythm 2011;8:1608–14. [DOI] [PubMed] [Google Scholar]
- 20.Tan AY, Elharrif K, Cardona-Guarache R et al. Persistent Proarrhythmic Neural Remodeling Despite Recovery From Premature Ventricular Contraction-Induced Cardiomyopathy. J Am Coll Cardiol 2020;75:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kowlgi GN, Ramirez RJ, Kaszala K et al. Post-extrasystolic potentiation as a predictor of premature ventricular contraction-cardiomyopathy in an animal model. Europace 2020. [DOI] [PMC free article] [PubMed]
- 22.Gunda S, Akyeampong D, Gomez-Arroyo J et al. Consequences of chronic frequent premature atrial contractions: Association with cardiac arrhythmias and cardiac structural changes. J Cardiovasc Electrophysiol 2019;30:1952–1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jiang M, Zhang M, Howren M et al. JPH-2 interacts with Cai-handling proteins and ion channels in dyads: Contribution to premature ventricular contraction-induced cardiomyopathy. Heart Rhythm 2016;13:743–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Potfay J, Kaszala K, Tan AY et al. Abnormal Left Ventricular Mechanics of Ventricular Ectopic Beats: Insights Into Origin and Coupling Interval in Premature Ventricular Contraction-Induced Cardiomyopathy. Circ Arrhythm Electrophysiol 2015;8:1194–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang Y, Eltit JM, Kaszala K et al. Cellular mechanism of premature ventricular contraction-induced cardiomyopathy. Heart Rhythm 2014;11:2064–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bardy GH, Lee KL, Mark DB et al. Amiodarone or an implantable cardioverter-defibrillator for congestive heart failure. N Engl J Med 2005;352:225–37. [DOI] [PubMed] [Google Scholar]
- 27.Deedwania PC, Singh BN, Ellenbogen K, Fisher S, Fletcher R, Singh SN. Spontaneous conversion and maintenance of sinus rhythm by amiodarone in patients with heart failure and atrial fibrillation: observations from the veterans affairs congestive heart failure survival trial of antiarrhythmic therapy (CHF-STAT). The Department of Veterans Affairs CHF-STAT Investigators. Circulation 1998;98:2574–9. [DOI] [PubMed] [Google Scholar]
- 28.Loring Z, Hanna P, Pellegrini CN. Longer Ambulatory ECG Monitoring Increases Identification of Clinically Significant Ectopy. Pacing Clin Electrophysiol 2016;39:592–7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


