Abstract
Background
Asthma is the most common chronic lung condition worldwide, affecting 334 million adults and children globally. Despite the availability of effective treatment, such as inhaled corticosteroids (ICS), adherence to maintenance medication remains suboptimal. Poor ICS adherence leads to increased asthma symptoms, exacerbations, hospitalisations, and healthcare utilisation. Importantly, suboptimal use of asthma medication is a key contributor to asthma deaths. The impact of digital interventions on adherence and asthma outcomes is unknown.
Objectives
To determine the effectiveness of digital interventions for improving adherence to maintenance treatments in asthma.
Search methods
We identified trials from the Cochrane Airways Trials Register, which contains studies identified through multiple electronic searches and handsearches of other sources. We also searched trial registries and reference lists of primary studies. We conducted the most recent searches on 1 June 2020, with no restrictions on language of publication. A further search was run in October 2021, but studies were not fully incorporated.
Selection criteria
We included randomised controlled trials (RCTs) including cluster‐ and quasi‐randomised trials of any duration in any setting, comparing a digital adherence intervention with a non‐digital adherence intervention or usual care. We included adults and children with a clinical diagnosis of asthma, receiving maintenance treatment.
Data collection and analysis
We used standard methodological procedures for data collection. We used GRADE to assess quantitative outcomes where data were available.
Main results
We included 40 parallel randomised controlled trials (RCTs) involving adults and children with asthma (n = 15,207), of which eight are ongoing studies. Of the included studies, 30 contributed data to at least one meta‐analysis. The total number of participants ranged from 18 to 8517 (median 339). Intervention length ranged from two to 104 weeks. Most studies (n = 29) reported adherence to maintenance medication as their primary outcome; other outcomes such as asthma control and quality of life were also commonly reported. Studies had low or unclear risk of selection bias but high risk of performance and detection biases due to inability to blind the participants, personnel, or outcome assessors. A quarter of the studies had high risk of attrition bias and selective outcome reporting. We examined the effect of digital interventions using meta‐analysis for the following outcomes: adherence (16 studies); asthma control (16 studies); asthma exacerbations (six studies); unscheduled healthcare utilisation (four studies); lung function (seven studies); and quality of life (10 studies).
Pooled results showed that patients receiving digital interventions may have increased adherence (mean difference of 14.66 percentage points, 95% confidence interval (CI) 7.74 to 21.57; low‐certainty evidence); this is likely to be clinically significant in those with poor baseline medication adherence. Subgroup analysis by type of intervention was significant (P = 0.001), with better adherence shown with electronic monitoring devices (EMDs) (23 percentage points over control, 95% CI 10.84 to 34.16; seven studies), and with short message services (SMS) (12 percentage points over control, 95% CI 6.22 to 18.03; four studies). No significant subgroup differences were seen for interventions having an in‐person component versus fully digital interventions, adherence feedback, one or multiple digital components to the intervention, or participant age. Digital interventions were likely to improve asthma control (standardised mean difference (SMD) 0.31 higher, 95% CI 0.17 to 0.44; moderate‐certainty evidence) ‐ a small but likely clinically significant effect. They may reduce asthma exacerbations (risk ratio 0.53, 95% CI 0.32 to 0.91; low‐certainty evidence).
Digital interventions may result in a slight change in unscheduled healthcare utilisation, although some studies reported no or a worsened effect. School or work absence data could not be included for meta‐analysis due to the heterogeneity in reporting and the low number of studies. They may result in little or no difference in lung function (forced expiratory volume in one second (FEV1)): there was an improvement of 3.58% predicted FEV1, 95% CI 1.00% to 6.17%; moderate‐certainty evidence); however, this is unlikely to be clinically significant as the FEV1 change is below 12%. Digital interventions likely increase quality of life (SMD 0.26 higher, 95% CI 0.07 to 0.45; moderate‐certainty evidence); however, this is a small effect that may not be clinically significant. Acceptability data showed positive attitudes towards digital interventions. There were no data on cost‐effectiveness or adverse events.
Our confidence in the evidence was reduced by risk of bias and inconsistency.
Authors' conclusions
Overall, digital interventions may result in a large increase in adherence (low‐certainty evidence). There is moderate‐certainty evidence that digital adherence interventions likely improve asthma control to a degree that is clinically significant, and likely increase quality of life, but there is little or no improvement in lung function. The review found low‐certainty evidence that digital interventions may reduce asthma exacerbations. Subgroup analyses show that EMDs may improve adherence by 23% and SMS interventions by 12%, and interventions with an in‐person element and adherence feedback may have greater benefits for asthma control and adherence, respectively. Future studies should include percentage adherence as a routine outcome measure to enable comparison between studies and meta‐analysis, and use validated questionnaires to assess adherence and outcomes.
Keywords: Adult, Child, Humans, Adrenal Cortex Hormones, Asthma, Asthma/drug therapy, Forced Expiratory Volume, Medication Adherence, Quality of Life
Plain language summary
Digital technologies to help people with asthma take their medication as prescribed
Background to the question
Asthma is one of the most common long‐term conditions worldwide. There are effective medicines available to treat symptoms, such as inhalers containing steroids. However, for best effect, maintenance medication need to be taken as prescribed. Many people do not take their medication, due to busy schedules and the belief that medication is only needed short‐term. This is known as 'non‐adherence', which can lead to more symptoms and attacks. Non‐adherence is a major health problem; achieving adherence is very important to prevent attacks and reduce the risk of death. In healthcare there is increasing use of digital interventions such as mobile phones, text messages, and 'smart' inhalers that can feed back information about medication‐taking. However, there is limited evidence on whether these technologies work to improve asthma medication‐taking or improve symptoms.
This review aimed to find out whether digital technologies really work to improve asthma medication‐taking, and whether this improved adherence leads to improvements in asthma symptoms and other benefits.
Study characteristics
We found 40 studies including more than 15,000 adults and children with asthma. Studies ranged from about 2 weeks to 24 months' duration, so we cannot say whether these methods are effective in the long term (a long period of years). We searched multiple information sources to identify relevant studies. This review is current as of June 2020. Looking at the data, we aimed to find out whether digital technologies helped people with asthma to take their medication as prescribed, and whether people who used the technology had better asthma control, and fewer asthma attacks, than those who did not use the technology.
Key results
People with asthma who were given the digital technology to support asthma medication‐taking were better at taking their medication as prescribed compared to people who did not get the technology; 15% more people (likely to be somewhere between 8% and 22%) took their medication as prescribed when they received the digital technology, compared to those who did not (who took their medication on average 45% of the amount prescribed). Importantly, people who got the digital technology had much better asthma control and half the risk of asthma attacks (likely somewhere between 32% and 91%), which has direct benefits for reducing the risk of asthma‐related deaths. We saw improvements in quality of life and lung function, but the effect on lung function was small and may be of limited clinical relevance. No improvements were seen in unscheduled healthcare visits. There was not enough information to tell us about the effect of digital technologies on time off work or school or the cost‐benefits, nor whether there are any harms. Technologies were generally acceptable to patients. Certain types of technologies such as 'smart' inhalers and text messages seemed to be better for improving medication‐taking than other technology types, although the small number of studies means we cannot be certain that these technologies definitely work better than others.
Quality of the information
There is some uncertainty about our results because the studies were quite different from each other. These differences mean that we cannot be completely sure what the real benefit is, as the benefits may be due to other factors not directly related to the technology ‐ for example, being involved in a study can improve medication‐taking. Sometimes the studies did not give us enough information for us to include them with the other studies to work out their effectiveness. We had concerns about a quarter of the studies where people did not finish the study, and we were uncertain whether studies reported everything they measured.
Key message
The studies we found suggest that digital technologies may help people with asthma take their medication better, improve their asthma control, and potentially halve their risk of asthma attacks, compared with people who did not get the technology. Certain types of digital technologies, such as text‐message interventions, may work better than others. However, we have some uncertainties about the quality of the information reported in some studies, and the small number of studies for the different technology types, which means we cannot be 100% certain of their benefits.
Summary of findings
Summary of findings 1. Digital adherence interventions compared to usual care for asthma.
| Digital adherence interventions compared to usual care for asthma | ||||||
| Patient or population: asthma Setting: primary or secondary care Intervention: digital adherence interventions Comparison: usual care | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with usual care | Risk with digital adherence interventions | |||||
| Adherence (percentage of people adhering to their prescribed medication) Follow‐up (weighted mean): 8.0 months (range: 1 to 24 months) |
Weighted mean (44.8%); range (‐4.4% to 82.7%) | MD 14.66 higher (7.74 higher to 21.57 higher) | — | 8885 (16 RCTs) | ⊕⊕⊝⊝ LOW 1 | Digital adherence interventions may increase adherence. |
| Asthma control ‐ change from baseline (various scales; higher scores = better asthma control ‐ standardised for different scales, scale reversed if in opposite direction) Follow‐up (weighted mean): 5.7 months (range 1 to 12 months) |
The mean change from baseline in asthma control in the intervention group compared to the control group was an increase: 0.31 SD higher (0.17 SD higher to 0.44 SD higher) | — | 1638 (15 RCTs) | ⊕⊕⊕⊝ MODERATE 2 | Digital adherence interventions likely increase asthma control when compared to baseline. The SMD describes the difference between the digital intervention and usual care groups adjusted for the different measurement scales used and measurement imprecision (Faraone 2008). The SMD is a Cohen's effect size and can be interpreted as small (< 0.4 = small, 0.4 to 0.7 = moderate, > 0.70 = large) (Undela 2021). For asthma control, the MCID depends on the questionnaire used and the population. An SMD of 0.3 to 0.5 has been used for an MCID when different questionnaires and settings are used (Angst 2017). Here, the SMD suggests that the increase in asthma control with digital adherence interventions is clinically significant. |
|
| Asthma exacerbations ‐ Number of people with one or more exacerbations Follow‐up (weighted mean): 7.5 months (range 3 to 12 months) |
198 per 1000 | 105 per 1000 (63 to 180) | RR 0.53 (0.32 to 0.91) | 678 (6 RCTs) | ⊕⊕⊝⊝ LOW 3 | Digital adherence interventions may result in a reduction in asthma exacerbations. Overall, the number of people with one or more asthma exacerbations halved when receiving digital interventions compared to usual care. |
| Unscheduled healthcare utilisation ‐ number of hospital or GP/ED visits Follow‐up (weighted mean): 10.0 months (range 3 to 12 months) |
199 per 1000 | 147 per 1000 (102 to 211) | RR 0.74 (0.51 to 1.06) | 446 (4 RCTs) | ⊕⊕⊝⊝ LOW 4 | Digital adherence interventions may result in a slight change in unscheduled healthcare utilisation. Overall, the risk of people with unscheduled healthcare visits may be reduced by 25% in those receiving digital interventions compared to usual care, though the interventions may also increase unscheduled healthcare utilisation. |
| Lung function ‐ FEV1 % predicted (change from baseline) Follow‐up (weighted mean): 8.1 months (range 3 to 12 months) |
Weighted mean change from baseline was 1.7%; range (‐4.4% to 7.7%) | The mean change from baseline in FEV1 was 3.58% predicted higher (1% to 6.17% higher) | — | 1052 (7 RCTs) | ⊕⊕⊕⊝ MODERATE 5 | Digital adherence interventions may result in little to no difference in lung function compared to baseline. An increase in FEV1 of 12% after bronchodilator use is considered meaningful (Kaminsky 2019); in children a lower increase of 8% to 9% is considered relevant (Hopp 2016). |
| Quality of life ‐ change from baseline (various scales; higher scores indicate better quality of life) Follow‐up (weighted mean): 6.4 months (range 1 to 12 months) |
The mean change from baseline in quality of life score was an increase: 0.26 SD higher (0.07 SD higher to 0.45 SD higher) | — | 848 (10 RCTs) | ⊕⊕⊕⊝ MODERATE 6 | Digital adherence interventions likely increase quality of life compared to baseline. An SMD of 0.3 to 0.5 would be considered a MCID (Angst 2017). |
|
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). Note follow‐up time for each outcome differs depending on the study duration. CI: confidence interval; FEV1: forced expiratory volume in 1 second; MCID: minimum clinically important difference; MD: mean difference; RCT: randomised controlled trial; RR: risk ratio; SD: standard deviation; SMD: standardised mean difference | ||||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1Downgraded twice due to risk of bias related to allocation concealment in the highly weighted studies (unclear in half of the studies, and of the 16 studies included, we judged nine to be at high risk of bias in at least one domain unrelated to performance bias), and imprecision (heterogeneity is high with I2 = 94%).
2Downgraded once due to high performance and detection bias in studies that have high weighting in this outcome.
3Downgraded twice due to high risk of bias (detection and performance bias) in the studies with high weighting, and the low number of studies included.
4Downgraded twice due to high risk of performance and detection bias, attrition bias, and imprecision of the results with a low number of included studies.
5Downgraded once as most of the studies with high weighting towards this outcome have high risk of bias (detection and performance bias).
6Downgraded once due to high risk of performance and detection bias.
Background
Description of the condition
Asthma is the most common chronic lung condition worldwide, affecting 334 million adults and children globally (Global Asthma Report 2014); it accounts for an estimated 400,000 deaths each year (Soriano 2017). Asthma can cause shortness of breath, chest tightness, and cough and typically presents with wheezing. Many people with asthma experience intermittent worsening of their asthma symptoms, known as 'exacerbations', 'flare‐ups', or 'attacks' (GINA 2017). Attacks can be triggered by common irritants and allergens such as pollution, tobacco smoke, pollen, viral infections, and house dust mites (CDC 2016). Asthma is often incorrectly diagnosed — both overdiagnosed and underdiagnosed — worldwide (Aaron 2017; Looijmans‐van den Akker 2016; Nolte 2006; van Schayck 2000), and treatment remains suboptimal. Most asthma‐related deaths occur in middle‐ and low‐income countries. Poorly controlled asthma places a huge burden on individuals, their families, and society (Normansell 2017; WHO 2013).
Asthma treatment falls into two categories — maintenance preventive treatment for long‐term control of symptoms and prevention of asthma attacks, and more immediate short‐term relief for acute management of symptoms and attacks (BTS/SIGN 2016). This review focuses on maintenance preventive treatment. The mainstay of asthma maintenance treatment for all but the mildest cases consists of regular inhaled corticosteroids (ICSs) (Barnes 1993), which are also commonly referred to as 'preventer' or 'controller' medications (i.e. the intention is that they are used once or twice daily (depending on the preparation), even when the patient is well, to maintain control over symptoms). ICSs, which are delivered directly to a patient's airways via an inhaler or a nebuliser, work by suppressing the multiple inflammatory cascades that are activated in the airways of a person with asthma. Inflammation leads to increased mucus production and airway constriction, which in turn contribute to the symptoms of asthma. Reduction in underlying inflammation through sustained use of an ICS can result in symptom improvement and reduced asthma‐related morbidity and mortality (Barnes 2003; Barnes 2015). Commonly used ICSs include budesonide, beclomethasone, fluticasone (propionate and furoate), mometasone, and ciclesonide. These can be given alone or in combination with other maintenance asthma medications such as long‐acting beta2‐agonists (LABAs), leukotriene receptor antagonists (LTRAs), long‐acting muscarinic antagonists (LAMAs), theophylline, and slow‐release beta2‐agonist tablets (BTS/SIGN 2016). LABAs are add‐on therapies that are used only in combination with an ICS, and work by keeping the airways open and relaxing the muscles of the airways but do not treat any underlying inflammation. Examples of LABAs include formoterol and salmeterol. LTRAs are add‐on therapies to ICS or ICS plus LABA. LTRAs work by blocking the effects of cysteinyl leukotrienes in the airways — these leukotrienes are released during asthma attacks and cause bronchoconstriction. Addition of LTRAs to an ICS may lead to improvements in asthma symptoms and lung function (Joos 2008; NICE 2021). LTRAs are given orally as a tablet formulation; the most common example is montelukast. In adults with asthma who do not respond to ICS and LABA, LAMAs such as tiotropium may be considered as add‐on treatment. Other alternative add‐on maintenance options include theophyllines or slow‐release beta2‐agonist tablets (for adults only), which may improve lung function and symptoms. In patients with a high steroid burden who continue to have frequent asthma attacks, symptoms, and impaired lung function, injectable maintenance treatment with monoclonal antibodies may be considered, such as omalizumab, given as a subcutaneous injection every two to four weeks, or mepolizumab (BTS/SIGN 2016).
Despite the availability of medical treatment, adherence to ICS is suboptimal, with patients needing to take the treatment every day, regardless of whether they have symptoms (Barnes 2015; Lasmar 2009; Williams 2004). 'Adherence' is defined by the World Health Organization (WHO) as “the extent to which a person’s behaviour (such as taking medication) corresponds with the agreed recommendations from a healthcare provider” (WHO 2003). Current adherence rates reported in the literature range from 0% to 100%, varying between and within individuals, but are estimated to average around 50% (McDonald 2002; Nieuwlaat 2014; WHO 2003). Adherence rates are estimated to be even lower in high‐risk populations such as ethnic minority groups (Mathes 2014), as well as in developing countries (McQuaid 2012). Poor adherence to asthma maintenance treatment — in particular ICSs — is associated with increased morbidity and mortality. An estimated 383,000 asthma deaths have been reported worldwide (WHO 2013). In the UK, the National Review of Asthma Deaths found that 67% of asthma deaths were due to avoidable factors such as patients not taking their prescribed asthma medication in the month and/or year before their death (Royal College of Physicians 2014), highlighting non‐adherence as a key modifiable determinant of mortality. Poor adherence is associated with considerable asthma‐related morbidity: the risk of an asthma exacerbation is more than three times higher in patients after cessation of low‐dose inhaled corticosteroids (Ebmeier 2017).
Investigators have identified several reasons for poor adherence, depending on the type of non‐adherence. Broadly speaking, non‐adherence can be classified as unintentional or intentional non‐adherence. In unintentional non‐adherence, patients do not adhere to prescribed treatment owing to factors not directly within their control, such as difficulties with medication‐taking or access to treatment (Clifford 2008; Horne 2005; Kardas 2013). In intentional non‐adherence, the patient makes a conscious decision to not take the medication; the patient chooses not to adhere owing to certain beliefs about treatment or perceptions of asthma (Clifford 2008), such as concerns around side effects of ICSs or lack of perceived personal need for treatment (Cooper 2015; Howell 2008; Menckeberg 2008; Ponieman 2009; Van Steenis 2014).
Description of the intervention
This review focuses on digital adherence interventions. No uniform definition of 'digital' can be found in the literature, and much overlap is evident between different classifications of digital interventions. The categories that are described below are informed by prior literature but are not mutually exclusive or collectively exhaustive, and have a degree of subjectivity. In this review, 'digital' refers to interventions that are delivered via an online (web‐based) platform (e.g. websites, web applications, online forums); a computer‐based platform (e.g. mobile apps, short message service (SMS)‐based interventions, games, interactive voice recognition systems (IVRSs)); or an electronic device of any type (e.g. electronic adherence monitoring devices). Telephone‐based interventions (e.g. health professional phone calls, telemonitoring, telehealth) were outside the scope of this review. Together, digital interventions have benefits of being multi‐functional, including communication and collection of information from users and provision of interactive experiences. Digital interventions provide a platform for delivery of adherence interventions that are considered to be highly customisable to barriers unique to each individual, of low cost, and easily accessible (Dayer 2013). However, challenges remain on the use of digital adherence interventions; engagement rates are often low, with few users downloading and using digital interventions on a regular and long‐term basis, and concerns around privacy and data management remain (Anderson 2016; Krebs 2015). More complex interventions can have difficulties with production and associated high costs (e.g. with computer programs), which can limit their adoption and use in practice (Johnson 2016b).
Online platforms
Online platforms, otherwise known as web‐based platforms, include websites, web‐based apps, and online forums; this term describes any intervention administered through a web browser online usually via a desktop or computer device and requiring Internet connectivity for delivery of the intervention ‐ also often referred to as 'e‐health'. These can be targeted to individuals or groups of individuals.
Computer‐based platforms
This term describes any intervention that is delivered through computer‐based platforms — such as via mobile, tablet, or desktop interfaces — and does not require Internet connectivity for delivery of the intervention (Bussey‐Smith 2007; Johnson 2016b). These generally fall under the category of mobile applications, SMS‐based interventions, or computer programs or games.
Mobile apps
'Mobile apps' refer to software programs designed for smartphones and tablets. Apps are optional add‐ons to mobile devices that interact with users via a set of interfaces (e.g. a visual user interface), also referred to as 'm‐health'. Often Internet connectivity is required but may not be required for full functionality, compared to online interventions (described above), which require Internet connection at all times for functioning. Asthma mobile apps usually aim to promote adherence by supporting overall asthma self‐management skills, as through reminders or feedback on adherence (Marcano Belisario 2013).
Short messaging‐based interventions
Short message services (SMS) and related online messaging platforms such as WhatsApp, LINE, and Viber are increasingly being used worldwide for communications. Most studies that have investigated short message‐based interventions have used SMS (mobile phone text messages) with the aim of improving adherence by sending messages as reminders for medication‐taking (Ali 2014; Kannisto 2014); some interventions use SMS to deliver educational or behavioural messages to mobile phones (Tran 2014). A recent meta‐analysis reported that use of SMS‐based interventions to improve adherence could potentially double the odds of adherence across various chronic diseases (Thakkar 2016). The capability of SMS to relay information to many people without delay was cited by study authors as a key reason for exploring the potential of SMS‐based interventions for adherence (Thakkar 2016).
Computer games or programs
Computer games or programs have been used increasingly as a method of intervention to drive changes in health behaviours (Johnson 2016b). Interactive program‐ or game‐based interventions are postulated to be effective for influencing behaviour through their ability to motivate and stimulate engagement, particularly for children and adolescents. For asthma, game‐based approaches have been used with some success to improve ICS adherence (Bussey‐Smith 2007; Krishna 2003; Mosnaim 2015). These have ranged from simple games to educate and reinforce adherence behaviour (Mosnaim 2015), to complex interactive multimedia programs incorporating animation and scenarios of vignettes targeted to individuals or groups (Krishna 2003).
Interactive voice response systems
Interactive voice response (IVR) systems constitute a type of computer‐linked telephone intervention system that uses several technologies to schedule, make, receive, or record automated phone calls, which can be used to promote adherence (Bender 2010; Reidel 2008). IVR systems can be programmed to make and receive automated phone calls, ask questions, obtain feedback, and provide individualised information. Information can be tailored according to responses given through voice recognition or a touchtone keypad.
Electronic monitoring devices
Electronic adherence monitoring devices (EMDs) have the ability to electronically record doses taken. EMDs can be used with different medication delivery devices including inhalation devices and pill bottles. Most EMDs measure, at minimum, the date and time of dosing, although more sophisticated devices are able to track the GPS location of doses, provide a customisable user interface, wirelessly transmit data to a linked mobile app, and provide dosing reminders (Chan 2013). EMDs can be used in adherence interventions as stand‐alone devices or as part of a wider intervention. EMDs can track adherence patterns over time, and these can be shared with the patient and the healthcare provider via the device or through generated reports. Whilst EMDs can track the time and date of dosing, few can record inhalation or actual medication‐taking. New devices such as the Inhaler Compliance Assessment (INCA) can record the sounds of inhalation (D'Arcy 2014); however the accuracy of this recording, whilst good, is still not perfect (Taylor 2018).
How the intervention might work
Digital interventions offer advantages in terms of adaptiveness, accessibility, reproducibility, and reach. Owing to the widespread use of digital technology, digital interventions can reach many people, particularly in settings where access to either non‐digital materials or face‐to‐face consultations is restricted (Masoli 2004). The ease of accessing digital technologies such as online platforms, websites, and mobile phone apps may promote engagement with the adherence intervention (Baptist 2016; Dayer 2013). This is in line with behavioural economics, or 'nudge' theory, where interventions which make a health behaviour (i.e. medication‐taking) easier or more positive to undertake can be effective (Sunstein 2014). Digital interventions can promote better communication between patients and healthcare providers (Dayer 2013; Eakin 2012). Digital interventions can support monitoring and recording of medication usage, asthma symptoms, or lung function, or all of these. Data can be fed back to patients in real time or communicated to their healthcare provider, thus facilitating a seamless transfer of health information across all interfaces of care (Chan 2013). This enables healthcare providers to gain access to detailed adherence information, which can provide insights into their patient's adherence behaviour that they may not otherwise have. This can add value to consultations by opening up conversations about adherence and drawing on actual, rather than assumed, adherence (Eakin 2012; Riekert 2002). Healthcare providers can be better equipped to provide recommendations personalised to the patient's behaviour. Patients can have the opportunity to reflect on the adherence data and their medication‐taking behaviours, and to see how their adherence may be linked to their asthma control. For example, they may be able to identify patterns in their medication use that may be related to particular adherence barriers, allowing them to understand how this behaviour may be associated with their asthma symptoms.
Digital interventions also offer many interactive opportunities that non‐digital interventions do not. This fact may enhance their effectiveness compared with non‐digital interventions, which have limited interactivity and are primarily static, as patients may find digital media or interactive interfaces more engaging (Johnson 2016b). Compared with traditional paper‐based media, digital interventions can support the delivery of information in a variety of media formats that can be tailored to the patient's information preferences, thus increasing accessibility of the information in different populations (Baptist 2016). Digital interventions also allow 24/7 support which face‐to‐face or in‐person delivery cannot provide. Users can also choose how they want information to be presented to them, such as via a video animation or through text, and what kind of information they want, though more complex information or data review will still require health provider support, which could be delivered via the digital platform. Whilst this does not overcome all adherence barriers (e.g. not barriers due to medication access issues), the ability to tailor digital interventions can help target both unintentional non‐adherence (e.g. through use of personalised reminders tailored to the individual's medication‐taking routine to encourage habit formation (Britto 2012)) and intentional non‐adherence (e.g. through use of messages sent to target and change negative treatment beliefs or perceptions (Petrie 2012)). Digital technologies thus have the potential to deliver accurate information to patients in a timely manner, in a way that can be tailored to patients' healthcare needs and beliefs, and to provide practical medication support such as reminders and alarms. Besides improving engagement, use of different media can help increase the accessibility of health information for patients who may find traditional media (such as patient information leaflets) difficult to engage with — for example, patients with poor health literacy or visual or aural impairments, or those with learning disabilities such as dyslexia (Baptist 2016).
Digital intervention has been found to have issues that need to be considered before these methods are taken up and adopted into practice. These include concerns around data privacy, issues related to information governance such as accountability and liability around identification of non‐adherence, ownership of adherence data, cost, impact on health disparities in terms of differences in ease of digital accessibility, and uncertainties around how best to incorporate digital interventions into existing workflow and health systems and how to train healthcare providers to respond to or use the collected information and how best to engage populations effectively (Anderson 2016; Krebs 2015; Michie 2017).
Why it is important to do this review
Medication non‐adherence is one of the major health challenges facing modern medicine; poor medication adherence is associated with increased morbidity, mortality, and healthcare costs. In asthma, adherence to maintenance treatment such as ICS as the mainstay of treatment averages around 50%, although in some populations it can be as low as 20%, depending on the population and the method used to measure adherence (Normansell 2017; Sulaiman 2016; van Dulmen 2007; WHO 2003).
Poor adherence leads to significant morbidity in the form of poor asthma control, hospitalisations, days off work, and death (Suissa 2000; WHO 2003; Williams 2004). Many studies have highlighted the importance of good adherence in asthma — for example, Suissa et al found that the rate of death from asthma decreased by 21% for each additional canister of ICS used in the previous year (Suissa 2000); likewise Williams et al reported that every 25% increase in ICS use leads to 11% decreased risk of asthma exacerbations (Williams 2011).
In the UK, non‐adherence to preventer treatment has been reported to be a factor contributing to approximately one‐third of asthma deaths in one year (Levy 2014; Royal College of Physicians 2014). Interventions to improve adherence, however, have demonstrated limited effectiveness of adherence and assessment of outcomes (Nieuwlaat 2014). Part of the challenge of non‐adherence is the difficulty involved in measuring adherence accurately and reliably. A range of methods are available to assess adherence directly (e.g. through direct observation of medication‐taking or blood levels) or indirectly (e.g. via prescription or refill records, self‐report, or electronic monitoring devices). However, all of these methods have their own advantages and disadvantages and can be subject to error (Farmer 1999).
Therefore, it remains unclear how delivery of interventions can best support patient adherence to prescribed treatments. A shift within health care suggests that patients increasingly wish to take an active role in self‐managing their own health and making their own healthcare decisions; this shift is driving the need for patients to be fully informed, so they can make informed healthcare choices.
Digital technologies, such as web and mobile platforms and electronic adherence devices, have been used increasingly as part of adherence interventions. Widespread use of smartphones and tablet computers provides a great opportunity for their use in delivery of adherence interventions. Early evidence suggests that certain digital technologies — such as electronic reminder systems (Tran 2014) — may be effective in improving adherence by over 20%, but questions remain around the size of this effect with other types of digital technologies, and whether certain characteristics of digital interventions influence their effectiveness.
A recent Cochrane Review focusing on interventions to improve adherence to ICS in asthma reported that adherence education, electronic trackers or reminders, and simplified regimens showed better adherence than controls (Normansell 2017). This review provided important information highlighting that electronic trackers or reminders may be effective in improving adherence. However, the review classification of 'electronic tracker or reminders' did not allow differentiation between the different types of digital interventions and, likewise, digital interventions (e.g. interactive voice recognition systems) were included under adherence education (Normansell 2017). More information is needed to determine whether digital interventions as a class have an effect on adherence and asthma outcomes, and if certain types of digital interventions are more effective than others. The review was also restricted to only ICS as a medication class; to effectively answer the question around whether digital interventions can be effective for medication adherence behaviour in general, it would be useful to explore all classes of maintenance medication beyond ICS. There is evidence showing that adherence may be different with other maintenance asthma medication than that for ICS, due to the ease of administration of other dosage forms such as oral leukotriene receptor antagonists (Jones 2003), or with injectables such as biologics, as these injections are given every two to eight weeks, often under direct supervision in a healthcare setting (Maddux 2021).
Adherence interventions also vary in terms of whether they are grounded in health psychology theory; recent evidence suggests that interventions that are behaviourally targeted and guided by theory may be more effective than those that are not (Conn 2017; Holmes 2014). Whether this applies to digital‐based interventions remains unknown. Understanding whether use of theory is associated with more effective digital interventions is also important for this review — to inform future intervention development. We are conducting this review to explore this topic.
Objectives
To determine the effectiveness of digital interventions for improving adherence to maintenance treatments in asthma.
Methods
Criteria for considering studies for this review
Types of studies
We included studies that were conducted as randomised controlled trials (RCTs) including cluster‐ and quasi‐randomised trials and abstracts. We excluded cross‐over trials due to difficulties with extracting relevant data pertaining to the intervention, but included studies reported in full text and unpublished data.
Types of participants
We included both adults (aged 18 years and over) and children (under 18 years) with a diagnosis of asthma, as per international or national guidelines, or whose condition was diagnosed by a healthcare professional and are currently prescribed maintenance asthma treatment (via any administration route), given alone or in combination with other controller therapies. We included interventions that were designed for parents or carers who are involved in managing maintenance asthma medication for any participant. We excluded interventions that were targeted at healthcare professionals, as this review relates only to digital interventions for patients.
We excluded participants with the following co‐morbidities/characteristics.
Other respiratory comorbidities such as chronic obstructive pulmonary disease (COPD) or bronchiectasis.
We included studies in which only a subset of participants met the inclusion criteria (asthma diagnosis, prescribed maintenance treatment, or managing maintenance treatment for a participant diagnosed with asthma) if disaggregated data were reported or could be obtained.
Types of interventions
We included studies comparing any interventions with a primary or secondary aim of improving adherence to maintenance asthma treatment (alone or in combination) that uses:
a digital component to deliver the intervention versus non‐digital delivery of the same adherence intervention; or
a digital component to deliver an intervention versus usual care. Usual care is defined as standard asthma care as per evidence‐based guidelines or standard care in the study setting.
Included digital interventions could be completely self‐delivered or could include an 'in‐person' or 'human' element whereby a healthcare professional or a trained peer is involved in the intervention. This can occur at the point of invitation to participate (e.g. introduction of the digital intervention and/or training of the patient to use the digital intervention) or on an ongoing basis (e.g. discussion of data from the digital intervention at regular consultations, use of remote adherence monitoring and feedback to the patient). The interventions could be delivered completely virtually (i.e. completely digital with no 'in‐person' element) or could include some face‐to‐face aspect (i.e. has an 'in‐person' element); delivery could be provided to individuals (e.g. with mobile apps or electronic monitoring) or to groups (e.g. online forums or computer games), and the intervention could be delivered on a one‐off or ongoing basis.
We included the following co‐interventions, provided they were not part of the randomised treatment and were administered equally to all randomised groups:
co‐interventions for which more than one type of digital media is used;
other co‐interventions that are used in asthma management.
When interventions had been described in insufficient detail to determine how the digital intervention was used, or where data were missing or not reported in a way that enabled inclusion in the meta‐analyses, we contacted the authors of identified studies to obtain further information. In the case of non‐response after initial contact, we followed up with study authors twice (over a period of 12 months). Where we received no response after three contacts, we excluded these studies from the review if we were not able to determine eligibility for inclusion or, where data could not be obtained for meta‐analysis, we described the studies narratively.
Types of outcome measures
Primary outcomes
Adherence to maintenance medication as assessed by any objective or validated subjective measure of adherence.
Asthma control as determined by any validated self‐report instrument.
Exacerbations requiring at least oral corticosteroid treatment (prescribed or taken — as measured by self‐report or via objective measurement, e.g. from pharmacy dispensing or prescription records), and/or emergency department visit and/or hospitalisation.
We chose these primary outcomes as these measures are the most likely to be used to assess intervention effect and are clinically important for asthma management.
Secondary outcomes
Unscheduled healthcare utilisation (visits to a healthcare provider/attendance at an emergency department or urgent care centre/hospital admission (i.e. overnight stays)).
Time off school, work, or other commitments due to asthma exacerbations or complications.
Lung function as measured by change compared to baseline in % predicted of forced expiratory volume in one second (FEV1). FEV1 measures the maximum amount of air a person can breathe out/exhale during a forced breath.
Quality of life as assessed by any validated standard instrument.
Acceptability of the digital intervention (using any validated instrument or quantitative measure of acceptability such as dropout rates, proportion of days on which tools were used, satisfaction with the intervention), but excluding qualitative data or patient feedback.
Cost‐effectiveness of the intervention (via reported cost‐effectiveness outcomes such as cost‐benefit analyses or impact hospitalisation costs/length of stay).
All adverse events including severe adverse events, which would be described separately if identified.
If outcomes were reported at multiple time points, we extracted these and included the latest reported time point. We excluded post‐intervention follow‐up data. If multiple measures of adherence were used, we included the most objective measure in the review.
Reporting in the study of one or more of the outcomes listed here was not an inclusion criterion for this review.
Search methods for identification of studies
Electronic searches
We identified studies from the Cochrane Airways Trials Register, with assistance from the Cochrane Airways Information Specialist, as the Register is maintained by the Information Specialist for the Group. The Cochrane Airways Trials Register contains studies identified from several sources.
Monthly searches of the Cochrane Central Register of Controlled Trials (CENTRAL), through the Cochrane Register of Studies Online (crso.cochrane.org).
Weekly searches of MEDLINE Ovid SP (1946 to date).
Weekly searches of Embase Ovid SP (1974 to date).
Monthly searches of PsycINFO Ovid SP (1967 to date).
Monthly searches of Cumulative Index to Nursing and Allied Health Literature (CINAHL) EBSCO (1937 to date).
Monthly searches of Allied and Complementary Medicine (AMED) EBSCO (inception to date).
Handsearches of the proceedings of major respiratory conferences.
Studies contained in the Trials Register are identified through search strategies based on the scope of Cochrane Airways. Details of these strategies, as well as a list of handsearched conference proceedings, are provided in Appendix 1. See Appendix 2 for search terms used to identify studies for this review. These terms have been guided by previous Cochrane Reviews such as the Normansell 2017 review (which identifies asthma adherence reviews, although we did not restrict to inhaled corticosteroids) and the Marcano Belisario 2013 review (which focused on smartphone and tablet apps, although we did not restrict the review to only these two digital media). We conducted the search on 1 June 2020, including a search of the US National Institutes of Health Ongoing Trials Register ClinicalTrials.gov (www.clinicaltrials.gov). A further search was run on 12 October 2021, but studies were not fully incorporated.
The World Health Organization International Clinical Trials Registry Platform (ICTRP) (apps.who.int/trialsearch) was searched on 12 October 2021. Due to the COVID‐19 pandemic, the WHO trials portal was not accessible for the final updates on 1 June 2020 and 12 October 2021. However, a search of CENTRAL was undertaken to cover this, as the WHO trial records feed into CENTRAL.
We searched for studies from the year 2000, as technologies existing before this time are unlikely to be representative of contemporary technologies that support health apps — this is in line with the Cochrane smartphone app review by Marcano Belisario 2013. We did not apply any restrictions on the language of publication.
Searching other resources
We searched the reference lists of all primary studies and review articles to identify if there were any additional references.
We searched on 2 June 2020 for errata or retractions from included studies published in full text on PubMed.
Data collection and analysis
Selection of studies
We used Rayyan, Ouzzani 2016, to screen the titles and abstracts of identified studies based on the aforementioned inclusion criteria. This was done in two stages: four review authors (AC, VW, ADS, CC) split the studies into two equal parts and each pair of authors (AC, VW and ADS, CC) independently screened their half of the titles and abstracts of the search results and coded them as 'include' (eligible or potentially eligible/unclear) or 'exclude'. We retrieved the full‐text study reports/publications of all potentially eligible studies, and split the full‐text studies into two for review. Four review authors in two pairs of two (AC, VW and ADS, LH) independently screened half of the full texts for inclusion, while recording the reasons for exclusion of ineligible studies. We resolved any disagreement through discussion or, if required, consulted a third person/review author at this stage (CC).
We repeated this process in June 2020 following the 2 June 2020 update. In this round, six review authors (AC, SA, NZ, PP, VT, VP) repeated the screening and full‐text review process as described above. Each time, the records were split into three and shared between five review authors (SA, NZ, PP, VT, VP), with overlapping abstracts/full‐texts between the five authors to enable double‐checking of each author's decision (i.e. each author screened and reviewed two‐fifths of the studies so that studies were screened/reviewed twice). AC had oversight of this process and conducted final checks of the full text reviews. We identified and excluded duplicates and collated multiple reports of the same study, so that each study, rather than each report, was the unit of interest in the review. We recorded the selection process in sufficient detail to complete a PRISMA flow diagram and Characteristics of excluded studies table (Moher 2009). A search update was conducted on 12 October 2021 for additional studies, but studies were not fully incorporated. Two review authors (AC, AD) independently screened the search results and included eligible studies in the Studies awaiting classification section.
Data extraction and management
We used an Excel data extraction form that four review authors (AC, VW, ADS, LH) piloted on at least one study in the review. The studies were divided into two and extracted in duplicate by two pairs of two authors (AC, VW and ADS, LH), with one author from each pair double‐checking the other author pair's extraction and resolving any disagreements between the independently extracted data. This process was repeated in August 2020 for the 2 June 2020 update, where five review authors (SA, NZ, PP, VT, VP) independently extracted the study characteristics and outcome data from the updated studies in duplicate by ensuring overlap in the studies (i.e. each author extracted two‐fifths of the studies so that studies were extracted twice). AC double‐checked data extractions for the 2020 search update and resolved any disagreements between the independently extracted data.
The following study characteristics were extracted from included studies:
Methods: date of study, study design and method of randomisation, length of follow‐up, total study duration, details of any 'run‐in' period, number of study centres and locations, study setting (healthcare setting and country), study withdrawals (study dropout and intervention dropout). We attempted to distinguish between study versus intervention dropouts to better understand attrition behaviour, if possible, as per an earlier review (Sohanpal 2012).
Participants: N (baseline and upon completion), mean age, age range, sex, severity of asthma, baseline lung function, smoking history, inclusion criteria, exclusion criteria, and differences between groups at baseline.
Interventions: intervention details, type of intervention (theory‐based versus non‐theory‐based), details of intervention provider, intervention target (primary and secondary), types of digital components used (technologies used), number of digital components, number of intervention sessions, interactivity with patient (i.e. a two‐way flow of information between the digital component and the patient), adherence feedback, concomitant medications, and excluded medications.
Comparison: details of comparison group.
Outcomes: primary and secondary outcomes specified and collected; methods of assessment of outcomes and time points reported.
Notes: funding of trial and notable conflicts of interest of trial authors.
We noted in the Characteristics of included studies table where data were not reported in a usable way. We resolved disagreements by consensus or by involving a third person/review author who had not already extracted the study. One review author (AC) transferred data into the Review Manager file (RevMan 2014). We double‐checked that data had been entered correctly by comparing data presented in the systematic review against study reports. A second review author (SA, PP, VT, VP, or NZ) spot‐checked study characteristics for accuracy against the study report.
Assessment of risk of bias in included studies
As for numerical data extraction, assessment of risk of bias was completed in two stages. Prior to the June 2020 update, assessment of risk of bias was conducted by review authors from the following: AC, VW, ADS, and LH — each author independently assessed risk of bias for half the included studies, so each study was assessed twice. For the June 2020 update, risk of bias was assessed by authors from SA, PP, VT, VP, or NZ for two‐fifths of the studies, so that each study was assessed twice. AC double‐checked assessment of risk of bias for all studies. All review authors used the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We resolved any disagreements by discussion or by involving another review author who had not already assessed the study. We assessed risk of bias according to the following domains similar to previous reviews (Normansell 2017).
Random sequence generation.
Allocation concealment.
Blinding of participants and personnel.
Blinding of outcome assessment.
Incomplete outcome data.
Selective outcome reporting.
Other bias.
We graded each potential source of bias as high, low, or unclear and provided a quote from the study report together with a justification for our judgement in the risk of bias table. We summarised risk of bias judgements across different studies for each of the domains listed. Judgments about overall risk of bias were reached by identifying the key domains that influence these summary assessments through consensus discussion ‐ for example, it was recognised that due to the nature of digital interventions, blinding of participants and personnel may not be possible. Risk of bias in the domains of selection, attrition, and reporting bias are likely to influence outcomes more significantly than performance and detection bias given the nature of the intervention. For assessment of incomplete outcome data, we judged attrition above 20% as high risk of bias, and where the difference in dropout rates between groups was more than 10%, this was deemed to be a large enough difference between intervention and control groups to lead to bias (Babic 2019). We considered blinding separately for different key outcomes when necessary (e.g. for unblinded outcome assessment, risk of bias for all‐cause mortality may be very different from a patient‐reported adherence scale). When information on risk of bias relates to unpublished data or correspondence with a trialist, we noted this in the risk of bias table. When considering treatment effects, we took into account the risk of bias for studies that contribute to that outcome.
For cluster‐RCTs, we considered particular additional biases specific to cluster‐RCTs: (i) recruitment bias; (ii) baseline imbalance; (iii) loss of clusters; (iv) incorrect analysis; and (v) comparability with individually randomised trials (Higgins 2011).
Assessment of bias in conducting the systematic review
We conducted the review according to this published protocol (Chan 2018), and reported any deviations from it in the Differences between protocol and review section of the systematic review.
Measures of treatment effect
We analysed continuous data (data that can take any numerical value) as mean differences (MDs) using a random‐effects model and 95% confidence intervals (CIs). We used MDs rather than standardised mean differences (SMDs) for adherence and lung function as the measures were reported on the same scale and when we included data reported using different methods of measurement, the data were too skewed to use SMDs. We used SMDs for other outcomes that used more than one method of measurement (e.g. asthma control, quality of life). We used the standard deviation (SD) of final (rather than baseline) measurements in the analysis. Although adherence can be presented as dichotomous or continuous, adherence generally is best considered as a continuous variable by nature (to avoid loss of valuable information and use of arbitrary cutoffs), which may be later dichotomised (into two categories), depending on the adherence measurement method used (Saberi 2011). Therefore, we treated adherence as continuous data in this review, as this increased the power to detect a difference. If both change from baseline and endpoint scores were available for continuous data, we used endpoint scores. We transformed reported rate ratios into log‐rate ratios and analysed via a random‐effects model and by generic inverse variance (GIV).
We conducted meta‐analyses only when this was meaningful, that is, when treatments, participants, and the underlying clinical question were similar enough for pooling to make sense, for example, studies using a similar method of digital intervention. We described skewed data, or studies that did not report data in a form that allowed meta‐analysis, narratively (e.g. as medians and interquartile ranges for each group).
When a single study reported multiple trial arms, we included only the relevant arms. If two comparisons (e.g. intervention A versus control and intervention B versus control) were combined in the same meta‐analysis, we combined the active arms or halved the control group to avoid double‐counting. If a study reported outcomes at multiple time points, we used the measure taken at the last follow‐up.
We used intention‐to‐treat (ITT) or 'full analysis set' analyses when they were reported (i.e. those in which data have been imputed for participants who were randomly assigned but did not complete the study) in preference to available case or per‐protocol analyses, if both were reported.
Unit of analysis issues
For dichotomous outcomes (outcomes that have only two possible values), we used participants, rather than events, as the unit of analysis (i.e. number of children with one or more exacerbations rather than number of exacerbations per child). We meta‐analysed data from cluster‐RCTs only if available data had been adjusted (or could be adjusted) to account for the clustering. In keeping with recommendations from the Cochrane Handbook for Systematic Reviews of Interventions, we adjusted cluster‐randomised data by inflating standard errors using a design effect (DE) calculated with an intracluster correlation coefficient (ICC). As per the Normansell 2017 review, the authors adjusted data from Foster 2014 for meta‐analysis using an intracluster correlation coefficient (ICC) of 0.037 (based on the ACT score, kindly supplied by the study author team). However, this adjustment had very little impact on the meta‐analyses, and so the authors from the Normansell 2017 review used the raw unadjusted data, which we have also used.
Dealing with missing data
We contacted investigators to verify key study characteristics where this was unclear, and to obtain missing numerical outcome data when possible (e.g. when a study did not report the data in a way that allowed inclusion in the meta‐analysis). When this was not possible and the missing data were thought to introduce serious bias, we considered this in the GRADE rating for the affected outcome(s).
Assessment of heterogeneity
We used the Chi2 test of homogeneity and the I2 statistic to measure heterogeneity among the studies included in each analysis. If we identified substantial heterogeneity, we reported this and explored the possible causes by performing prespecified subgroup analysis. Higgins et al suggests using an I2 value of 75% and over to indicate high heterogeneity (Higgins 2003).
Assessment of reporting biases
When we were able to pool more than 10 studies, we created and examined a funnel plot to explore possible small‐study and publication biases using Egger's t‐test.
Data synthesis
We used a random‐effects model and performed a sensitivity analysis using a fixed‐effect model.
Subgroup analysis and investigation of heterogeneity
We carried out the following subgroup analyses.
Interventions that have used only one digital component versus interventions with multiple (more than one) digital component.
Different types of digital interventions (i.e. online versus computer‐based versus electronic monitoring devices).
Digital interventions involving adherence feedback versus interventions that do not.
Interventions with an 'in‐person' component versus interventions that are fully digital and self‐delivered.
Adults/adolescents versus children (< 12 years old).
We used the primary outcomes in the subgroup analyses:
Adherence to maintenance medication via any objective or validated subjective measure of adherence.
Asthma control via any validated self‐report instrument.
Exacerbations requiring at least oral corticosteroid treatment.
We used the formal test for subgroup interactions available in Review Manager (RevMan 2014).
Sensitivity analysis
We carried out the following sensitivity analyses while removing these items from primary outcome analyses.
Unpublished data.
Trials with high risk of selection bias.
Trials with subjective adherence outcome measurement methods.
Quasi‐randomised trials.
Non‐English studies.
Commercially funded studies.
We compared results from a fixed‐effect model versus results from a random‐effects model.
Summary of findings and assessment of the certainty of the evidence
We created a summary of findings table using the following outcomes: adherence to maintenance medication; asthma control via any validated self‐report instrument; exacerbations requiring at least oral corticosteroid treatment; and unscheduled healthcare utilisation. We could not create a summary of findings table for: time off school, work, or other commitments due to asthma exacerbations or complications; and any reported adverse events, due to insufficient reported data available for these two outcomes.
We used the five GRADE considerations (risk of bias, consistency of effect, imprecision, indirectness, and publication bias) to assess the quality of a body of evidence as it relates to studies that contribute data for the prespecified outcomes. We used the methods and recommendations described in Section 8.5 and Chapter 12 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011), using GRADEpro software (GRADEpro GDT). We justified all decisions to downgrade or upgrade the quality of studies by using footnotes, and we added comments to aid the reader's understanding of the review when necessary.
Results
Description of studies
Results of the search
We retrieved 1358 records through database searches run on 1 June 2020, and an additional 228 from a search of other resources, including trials registries. Once duplicates had been removed, we had a total of 1469 remaining records to screen. We excluded 1350 references based on a screen of the titles and abstracts. We obtained and reviewed the full text of the remaining 119 records, and excluded 76 articles (64 studies). Eight of the studies are still ongoing (Characteristics of ongoing studies). We included a total of 40 studies, of which 6 are abstracts and 34 full‐text articles.
We conducted an updated search on 12 October 2021, but studies were not fully incorporated. This search identified an additional 321 records, of which 219 remained after duplicates were removed. Of the 219 records screened, 23 met the inclusion criteria and are now listed under Studies awaiting classification. Of note, one of the studies that were originally classified as ongoing is now complete (Riley 2021), and one was a duplicate study of a larger study (Kosse 2019).
For further details of our screening process, see the study PRISMA flow diagram (Figure 1).
1.
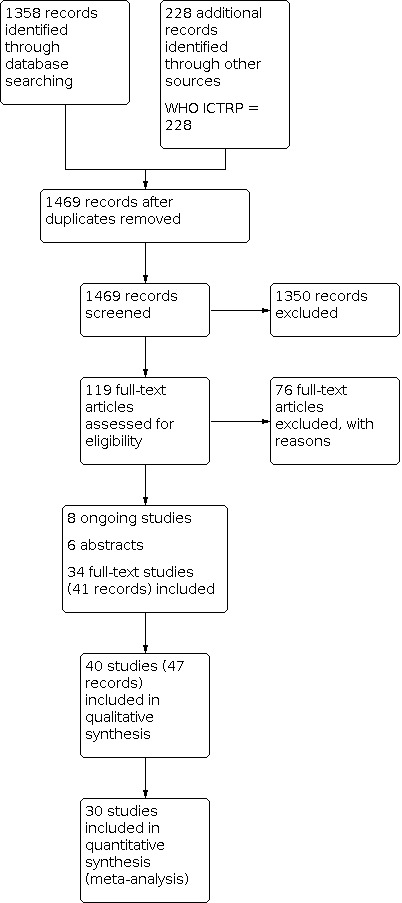
Study flow diagram.
Included studies
We included 40 studies and 30 of these contributed data to at least one meta‐analysis. Ten studies did not report data in a way that could be meta‐analysed for any of the outcomes, so these were reported narratively (Table 2). Reasons why the studies could not be included in the meta‐analysis included missing data or data not provided by the author, or data presented in a way that could not be manipulated for the meta‐analysis.
1. Studies with data that could not be included in the meta‐analysis.
| Outcomes (excluded from forest plots) | Study, year | Results |
| Adherence | Choi 2017 | No significant difference in medication adherence rates between the groups |
| Clerisme‐Beaty 2011 |
Adherence odds ratio: Neutral/montelukast = 1.2 (95% CI 0.4 to 3.8) (rate = 52.0) Enhanced/placebo = 0.5 (95% CI 0.1 to 1.6) (rate = 26.9) Enhanced/montelukast = 4.0 (95% CI 1.1 to 14.3) (rate = 76.0) |
|
| Cvietusa 2012 | Time to first ICS refill was significantly shorter for the SR intervention group (median 52 days) than the control group (median 78 days), HR 1.26 (95% CI 1.12 to 1.42). Proportion of days with medication on hand was greater in the SR intervention than the control group (38% versus 28%, P < 0.0001). |
|
| Davis 2019 | Adherence: 61.30% in intervention, 62.60% in control | |
| Jan 2007 | Adherence percentage to ICS: 63.2 in intervention, 42.1 in control | |
| Johnson 2016a | Adherence change in the last 7 days: 0.611 in intervention, ‐1.345 in control (P = 0.011) | |
| Joseph 2018 | Reported controller medication adherence, adherent ≥ 5 days of the past 7 days: 62.1% in intervention, 50% in control (OR = 1.62) (CI 0.38 to 6.93) | |
| Kim 2016 | Adherence: median 100% in intervention and control | |
| Lv 2012 | Adherence: 80% in SMS, 50% in control (P = 0.113) | |
| Lv 2019 | Adherence: 94.46% in intervention, 92.67% in control (P < 0.05) | |
| Reece 2017 | Adherence: controller medication usage was 86% among app (intervention) users and 90% in the paper group (control) | |
| Searing 2012 | Adherence: intervention group receiving texts reported significantly higher adherence than control group (P = 0.045) | |
| Weinstein 2019 | Adherence: 81% in intervention, not measured in control | |
| Van Sickle 2016 | Adherence: significant improvements in the intervention vs control group (P < 0.001). A 21‐point improvement in adherence reported with the intervention (no units provided). | |
| Zhou 2018 | Adherence rate after 12 weeks: 67.33% in intervention, 40% in control (P < 0.05) | |
| Asthma control | Choi 2017 | No significant difference in asthma control scores between the groups |
| Bender 2015 | Asthma control β2‐agonist canisters mean (SE): mean 3.3 (SE 0.13) in intervention, 3.2 (SE 0.15) in control (P = 0.10) | |
| Charles 2007 | Asthma control mean ACQ score: 0.5 in intervention (95% CI 0 to 1.0), 0.5 in control (95% CI 0.2 to 1.2) (P = 0.33) | |
| Johnson 2016a | Asthma control ACT change scores: 1.65 in intervention, 1.74 in control (change P = 0.728) | |
| Joseph 2018 | Asthma control ACT median change from baseline: 2 in intervention, 2 in control (P = 0.26) | |
| Kenyon 2018 | Asthma control C‐ACT mean change over 30 days: 1.2 in intervention, 3.1 in control (P = 0.16) | |
| Kim 2016 | Asthma control ACT: after 8 weeks, score changed from 22 to 21 points in intervention (P = 0.920), and 22 to 23 points in control (P = 0.571) (not significant in both groups) | |
| Lv 2019 | Asthma control C‐ACT: 24.36 in intervention, 22.44 in control (P < 0.05) | |
| Pernell 2017 |
Asthma control ACT in children improved by a raw score of: 5.1 points in intervention (from 19.2 to 24.3), 2.4 points in control group (from 18.8 to 21.2) Asthma control ACT in adults pre‐ and post‐scores: from 21 to 22 in intervention, from 21.67 to 22.67 in control |
|
| Reece 2017 | ACT scores in the app group improved by a mean of 3.8 points and by 2.4 points in the paper group | |
| Rikkers‐Mutsaerts 2012 | Asthma control ACQ (average weeks per patient): 19.9 in intervention and not reported for control | |
| Sulaiman 2018 | Asthma control mean (SD) ACT: mean 12.5 (SD 4.6) in intervention, 11.7 (SD 4.3) in control (P = 0.25) | |
| Vasbinder 2016 | Asthma control ACT mean score: 21.1 in intervention, 22.2 in control | |
| Van Sickle 2016 | Significant improvements in asthma control in the intervention vs control group (P < 0.001) | |
| Vollmer 2011 | Data not reported | |
| Wiecha 2015 | Asthma control days of wheeze per 2 weeks, mean change at 6 months: ‐1.4 in intervention (P = 0.03), ‐4.2 in control (P = 0.004) | |
| Exacerbations | Bender 2015 | Exacerbations oral steroid bursts: 0.27 (SE 0.18) in intervention, 0.21 (SE 0.23) in control (P = 0.05) |
| Kim 2016 | Exacerbations number of patients treated with short‐term systemic steroid or increased dose of systemic steroid during use of application (8 weeks): 5 patients in intervention (21.7%), 3 patients in control (13.6%), (P = 0.440) | |
| Lv 2019 | Exacerbation frequency: lower frequency post‐enrolment in both intervention and control. Intervention has a lower frequency than control post‐enrolment (graph representation, values not reported in the paper) (P > 0.05 pre‐enrolment, P < 0.05 post enrolment). | |
| Morton 2017 | Exacerbations courses of oral steroids (event rate per 100 child days): 0.411 in intervention, 0.676 in control (95% CI 1.46 to 12.13) | |
| Mosnaim 2013 | Data not reported | |
| Van Sickle 2016 | Significant improvements in asthma‐free days in the intervention vs control group (P < 0.001) | |
| Vasbinder 2016 | Exacerbations yearly rate: 0.23 in intervention, 0.37 in control | |
| Unscheduled healthcare utilisation | Choi 2017 | No significant difference in healthcare utilisation between the groups |
| Bender 2015 | ED visits mean (SE) over 24 months, no./person‐year: 0.06 (SE 0.01) in intervention, 0.04 (SE 0.01) in control (P = 0.23) | |
| Kim 2016 | ED visit due to asthma exacerbation in one year; median (%): 4 (18.1%) in intervention, 3 (13.6%) in control (P = 0.644) | |
| Lv 2012 | ED visits: 18.35 in intervention, 32.7 in control (P = 0.93) | |
| Morton 2017 | GP/ED visits (event rate (per 100 child days): 0.582 in intervention, 0.650 in control (P = 0.316) (95% CI 0.83 to 1.63) | |
| Mosnaim 2013 | Data not reported | |
| Pool 2017 | Number of emergency room visits ‐ mean of change from baseline: ‐0.26 in intervention (CI ‐0.44 to ‐0.08), ‐0.08 in control (CI ‐0.26 to 0.10) (P = 0.17) | |
| Rikkers‐Mutsaerts 2012 | Healthcare provider contacts for asthma, average number per patients (physician visits): 3.6 in intervention, 3.2 in control (95% CI ‐2.3 to 1.7) (P = 0.74) | |
| Van der Meer 2009 | Deterioration in asthma that required emergency treatment or hospitalisation, or the need for oral steroids for 3 days or more: 17 “exacerbations” in intervention, “20 exacerbations” in control (95% CI 0.51 to 2.74) | |
| Wiecha 2015 | Acute asthma‐related PCP or ER visit in prior 2 months, mean change: 1 in intervention (P = 0.18), 1 in control (P = 0.99) | |
| Time off work/school | Chan 2015 | Proportion of days absent from school for any reason over 6 months (based on a standard school day of 193 available school days per year): mean (SD) 1.16 (2.56) in the intervention versus 1.71 (3.44) in the control (P = 0.167) |
| Joseph 2018 | > 2 school or work days missed/30 days (asthma): 9.5% yes in intervention, 90.5% no in intervention; 0% yes in control, 100% no in control | |
| Lv 2019 | Days of school absence (days/year): fewer days of school absence in intervention than control (graph representation, values not given) (P < 0.05) | |
| Morton 2017 | Days off school due to asthma ‐ event rate per 100 child days: 1.365 in intervention, 1.606 in control (P = 0.01) (95% CI 0.97 to 1.39) | |
| Mosnaim 2013 | Data not reported | |
| Wiecha 2015 | Change from baseline ‐ days missed school for asthma over 6 months: mean ‐0.2 (intervention) versus ‐0.4 (control) (P = 0.31) | |
| Lung function | Black 2008 | Data not reported |
| Charles 2007 | Lung function PEF mean: 434 (SD 99) in intervention, 444 (SE 128) in control | |
| Clerisme‐Beaty 2011 |
Lung function FEV1 (L) crude mean change after 4 weeks (95% CI): Neutral/placebo = ‐0.01 (95% CI –0.07 to 0.06) Neutral/montelukast = 0.04 (95% CI –0.02 to 0.09) Enhanced/placebo = 0.03 (95% CI –0.06 to 0.12) Enhanced/montelukast = 0.13 (95% CI 0.02 to 0.24) |
|
| Foster 2014 |
Lung function FEV1 (L) endpoint mean (95% CI): Control = 2.60 (95% CI 2.48 to 2.73) PAD (personalised adherence discussions) = 2.56 (95% CI 2.40 to 2.73) IRF (inhaler reminders and feedback) = 2.60 (95% CI 2.47 to 2.72) IFR + PAD = 2.58 (95% CI 2.43 to 2.72) |
|
| Jan 2007 | Lung function daily variability in PEF change from baseline (mean (SD)): 1.7 (SD 7.5) in intervention, 0.1 (SD 9.9) in control | |
| Kim 2016 | Lung function %FEV1 predicted ‐ change: at baseline, FEV1 was 93% of predicted value as median in the intervention group, and 91% of predicted value as median in control group. This changed to 90% in the intervention group and 100% in controls. Changes in both groups were not significant (P = 0.277 in intervention vs P = 0.217 in control group). | |
| Reece 2017 | PEFRs improved an average of 9.09% in the app group and 7.82% in the paper group | |
| Rikkers‐Mutsaerts 2012 | Lung function FEV endpoint: MD 0.05 (95% CI ‐0.11 to 0.32) between groups | |
| Van der Meer 2009 | Optional daily lung function scores, average days per patient: 107.8 in intervention, NA in control | |
| Weinstein 2019 | Lung function FEV1% change from baseline: 0.760 to 0.776 in intervention, 0.697 to 0.732 in control (no SD reported) | |
| Quality of life | Black 2008 | Data not reported |
| Choi 2017 | No significant difference in quality of life between the groups | |
| Jan 2007 | Quality of life 7‐point Likert scale: 6.5 (SD 0.5) in intervention, 4.3 ± 1.2 in control (P < 0.05) | |
| Johnson 2016a | Quality of life change for mini PAQLQ 7‐point scale: 0.5301 in intervention, 0.0957 in control (P = 0.037) | |
| Joseph 2018 | Data not reported | |
| Kim 2016 | Quality of Life Questionnaire for adult Korean asthmatics: medians (range) ‐ intervention 67 (28 to 81) increasing to 70 (26 to 85) (P = 0.139); control 69 (29 to 85) increasing to 72 (38 to 84) (P = 0.027) | |
| Pool 2017 | Quality of life: improvement in intervention group | |
| Rikkers‐Mutsaerts 2012 | Quality of life PAQLQ endpoint: 6.05 in intervention, 5.93 in control (95% CI ‐0.50 to 0.41) (P = 0.02) | |
| Sulaiman 2018 | Asthma quality of life questionnaire, mean ± SD: 3.7 (SD 1.2) in intervention, 3.6 (SD 1.2) in control (P = 0.53) | |
| Vasbinder 2016 |
Quality of life PAQLQ scores: 6.19 in intervention, 6.25 in control Difference = ‐0.06 (95% CI ‐0.41 to 0.15); P = 0.659 |
|
| Vollmer 2011 | Data not reported | |
| Weinstein 2019 | Quality of life ACQ difference scores in intervention: sleep = ‐0.67, work = ‐0.28, family activities = ‐0.62, recreation = ‐0.41 |
Abbreviations: ACQ: asthma control questionnaire; ACT: asthma control test; C‐ACT: childhood asthma control test; CI: confidence interval; ED: emergency department; ED: emergency room; EMD: electronic monitoring device; FEV: forced expiratory volume; ICS: inhaled corticosteroid; IRF: inhaler reminders and feedback; MD: mean difference; N/A: not available; OR: odds ratio; PAQLQ: Paediatric Asthma Quality of Life Questionnaire PCP: primary care physician; PEFR: peak expiratory flow rate; PAD: personalised adherence discussion; SD: standard deviation; SE: standard error; SMS: short messaging system; SR: speech recognition.
The included studies were conducted in 14 different countries, mostly in the USA and published from 2004 to 2020. The studies included a total of 15,207 participants, who were randomly assigned to comparisons of interest in this review. The largest study was a stratified RCT in 8517 participants who were randomised to receive the interactive voice response (IVR) calls or usual care (Vollmer 2011), and the smallest study was in 18 participants in an ongoing clinical trial of a new device “Turbo+”, an electronic device attached to the Turbohaler, which records whether the patient does the inhalation (La Grutta 2020). The median total number of participants was 339. Investigators reported nine studies only as clinical trial registry records, with no reported study findings (Arain 2020; Jariwala 2018; La Grutta 2020; Landon 2019; Linnhoff 2019; Riley 2021; Schaffer 2004; Simoneau 2018; Zhou 2018), and six as conference abstracts (Black 2008; Choi 2017; Cvietusa 2012; Reece 2017; Searing 2012; Van Sickle 2016). The remainder were full‐text, peer‐reviewed journal articles.
Further details and a summary of the 40 included studies can be found in the Characteristics of included studies table.
Methods
All of our 40 included trials were RCTs that compared a digital intervention to improve asthma maintenance therapy adherence versus usual care or an alternative intervention not specifically designed to improve adherence. Two studies included a cluster‐randomised design (Foster 2014; Kosse 2019), and the remainder (38 studies) were randomised at an individual participant level.
The 40 included studies were conducted in a variety of countries worldwide, though mainly in high‐income, English‐speaking countries. Just under half were conducted in the United States (Bender 2010; Bender 2015; Clerisme‐Beaty 2011; Davis 2019; Johnson 2016a; Joseph 2018; Kenyon 2018; Kolmodin MacDonell 2016; Mosnaim 2013; Simoneau 2018; Riley 2021; Jariwala 2018; Landon 2019; Pernell 2017; Pool 2017; Schaffer 2004; Vollmer 2011; Weinstein 2019; Wiecha 2015). Nine studies were in English‐speaking countries outside of the United States: New Zealand (Black 2008; Chan 2015; Charles 2007; Petrie 2012), Australia (Foster 2014), the UK (Koufopoulos 2016; Morrison 2016; Linnhoff 2019), Canada (Arain 2020) ‐ all high‐income countries. Other studies were undertaken in the Netherlands (Kosse 2019; Rikkers‐Mutsaerts 2012; Van der Meer 2009; Vasbinder 2016), Denmark (Stranbygaard 2010), Ireland (Sulaiman 2018), Italy (La Grutta 2020), Iran (Ebrahimabadi 2019), Taiwan (Jan 2007; Kang‐Cheng Su Su 2015), Korea (Choi 2017; Kim 2016), and China (Lv 2012; Lv 2019; Zhou 2018). Considering the income of these countries, all countries are considered high‐income according to the 2021 World Bank income classification, with the exception of the studies in China, which is classified as an upper‐middle income country and Iran, a lower‐middle income country.
The intervention length varied, with the longest intervention duration being 104 weeks (Bender 2015) and the shortest two weeks (Ebrahimabadi 2019).
Participants
The age of included participants ranged from 2 to 98 years old. Twenty studies only recruited children, 18 studies only recruited adult populations and the remainder recruited both children and adult populations.
Ethnicity breakdowns were not routinely reported in most studies.
Interventions
The studies incorporated a number of digital interventions, including IVR (n = 2), speech recognition (n = 2), electronic monitoring devices (n = 10), web‐based interventions (n = 10), mobile applications (n = 7), SMS‐based interventions (n = 12), video‐based (n = 2), MP3‐player (n = 1), medication dispensing system (n = 1), and audiotape (n = 1). Further details are provided in the Characteristics of included studies table.
Outcomes
Primary outcomes
Twenty‐nine out of the 40 included studies conducted their investigations with adherence as the primary outcome. Adherence was the secondary outcome in 11 studies where five of these also included asthma control as a study outcome (Choi 2017; Foster 2014; Jariwala 2018; Morrison 2016; Morton 2017). The way that adherence was reported was not consistent across studies, though most studies did include some measure of percentage adherence out of 100% (with 100% indicating perfect adherence). Twelve studies did not report adherence using percentage adherence (Choi 2017; Ebrahimabadi 2019; Kolmodin MacDonell 2016; Kosse 2019; Koufopoulos 2016; Morrison 2016; Mosnaim 2013; Pernell 2017; Rikkers‐Mutsaerts 2012; Searing 2012; Van der Meer 2009; Van Sickle 2016). Kang‐Cheng Su Su 2015 investigated an asthma self‐management application providing health information, personalised health assessments, interactive action plans, and adherence reminders, but did not include adherence as an outcome. Overall, 16 studies reported on endpoint % adherence values of the two groups and were included in the meta‐analysis. Studies included objective and subjective measures of adherence and asthma control, ranging from electronic adherence monitoring devices (Bender 2015; Black 2008; Chan 2015; Charles 2007; Clerisme‐Beaty 2011; Foster 2014; Kenyon 2018; Kolmodin MacDonell 2016; Koufopoulos 2016; Morton 2017; Mosnaim 2013; Sulaiman 2018; Vasbinder 2016; Wiecha 2015; Zhou 2018), to pharmacy or prescribing records (Bender 2015; Jan 2007; Johnson 2016a; Pool 2017; Schaffer 2004; Stranbygaard 2010; Vollmer 2011), to validated self‐report measures such as the Morisky Medication Adherence Scale (MMAS) (Ebrahimabadi 2019; Morrison 2016; Pernell 2017), and the Medication Adherence Report Scale (MARS) (Kosse 2019), and also non‐validated measures or self‐report questionnaires not otherwise described (Choi 2017; Davis 2019; Joseph 2018; Kim 2016; Koufopoulos 2016; Lv 2012; Lv 2019; Petrie 2012; Rikkers‐Mutsaerts 2012; Van der Meer 2009). Over a third of the studies reported data at multiple time points — for these studies, we extracted outcome data at the last time point reported to assess enduring effects of the intervention (Chan 2015; Davis 2019; Ebrahimabadi 2019; Johnson 2016a; Kim 2016; Kosse 2019; Morton 2017; Mosnaim 2013; Petrie 2012; Pool 2017; Schaffer 2004; Van der Meer 2009; Vasbinder 2016; Vollmer 2011; Weinstein 2019; Wiecha 2015; Zhou 2018).
Thirty‐one studies reported on whether asthma control was affected when using a digital intervention. Fifteen studies were included in the meta‐analysis. These studies measured the change from baseline in asthma control. However, the method for measuring asthma control varied between studies. Studies used Asthma Control Test (ACT) (n = 5); Childhood Asthma Control Test (n = 1); Asthma Control Questionnaire (ACQ) (n = 7); Control of Allergic Rhinitis and Asthma Test (n = 1) and Perceived Control of Asthma Questionnaire (n = 1).
Thirteen studies reported on asthma exacerbations requiring at least an oral corticosteroid treatment in their intervention and control groups. However, the method of reporting varied from reporting of the number of people with one more exacerbation (Chan 2015; Foster 2014; Kim 2016; Morrison 2016; Zhou 2018), to the number of exacerbations per person‐time (Bender 2015; Lv 2019; Morton 2017; Vasbinder 2016), or both (Rikkers‐Mutsaerts 2012; Van der Meer 2009). One study did not report data on exacerbations (Mosnaim 2013), and one described this outcome as 'asthma‐free days' (Van Sickle 2016). Six studies reporting endpoint scores for the number of people with one or more exacerbations were included in the final meta‐analysis.
Secondary outcomes
Fourteen studies reported on unscheduled healthcare utilisation, but this outcome was inconsistently reported, ranging from event or incidence rate (Bender 2015; Lv 2012; Morton 2017; Pool 2017), to number of visits over time or per patient (Kim 2016; Rikkers‐Mutsaerts 2012; Van der Meer 2009), to number of people with one or more visit to a healthcare provider/attendance to an ED related to asthma (Chan 2015; Joseph 2018; Morrison 2016; Zhou 2018), or not otherwise described (Choi 2017). Data from one study were unclear in terms of whether the data related to number of visits or people (Wiecha 2015), and one study did not report any data (Mosnaim 2013). In line with our protocol, we used participants rather than events as the unit of analysis (i.e. number of people with one or more visits to a healthcare provider/attendance at an emergency department or urgent care centre/hospital admission). Out of the 12 studies, four reported on this and were included in the meta‐analysis.
Six studies reported on how digital adherence interventions affected time off school/work for any reason. However, the reporting of this outcome was inconsistent. Two studies reported an event rate (Joseph 2018; Morton 2017), two studies reported as mean proportions or change from baseline (Chan 2015; Wiecha 2015), and two studies did not report any values (Lv 2019; Mosnaim 2013). Due to the low number of included studies and heterogeneity, a meta‐analysis of the number of days off school due to asthma was not conducted.
Seventeen studies reported on how asthma maintenance medication adherence affected lung function — though the reported unit of analysis ranged from % predicted FEV1 (Choi 2017; Chan 2015; Kim 2016; Kolmodin MacDonell 2016; Lv 2012; Morrison 2016; Morton 2017; Stranbygaard 2010; Weinstein 2019), FEV1 (Black 2008; Clerisme‐Beaty 2011; Foster 2014; Rikkers‐Mutsaerts 2012; Van der Meer 2009), and peak expiratory flow rate (PEFR) (Charles 2007; Jan 2007; Reece 2017). Seven studies reporting on change in predicted % FEV1 from baseline were included in the meta‐analysis.
Twenty‐two studies reported on how digital interventions impacted on quality of life, of which 10 were included in the meta‐analysis. These measured the change from baseline for quality of life with studies using either AQLQ or Paediatric Asthma Quality of Life Questionnaire (PAQLQ). Three studies did not report values for quality of life (Choi 2017; Morton 2017; Pool 2017; Weinstein 2019), and two used non‐validated measures (Kim 2016; Weinstein 2019).
Thirteen studies reported on acceptability. The included studies did not report cost‐effectiveness as an outcome thus data could not be collected. No data were reported on adverse events.
Excluded studies
After conducting full‐text review, we excluded a further 64 studies (Excluded studies). The most common reason for exclusion (n = 21) was that the outcomes did not meet our inclusion criteria — e.g. improving adherence to maintenance asthma medication was not the primary or secondary focus of the study. The second most common reasons for exclusion were incorrect publication types (e.g. systematic review or protocol) (n = 12) or study design did not meet our inclusion criteria (e.g. not RCT) (n = 9); or the intervention did not meet our inclusion criteria (e.g. focused on health professionals) (n = 9). The remainder were excluded as the participants did not meet our inclusion criteria (e.g. diagnosis not asthma) (n = 6), or the study objectives were irrelevant (e.g. study aimed to compare different inhaler regimens/devices, or adherence measurement tools rather than adherence promotion) (n = 4).
Risk of bias in included studies
Overall the risk of bias was low or unclear across most domains, except for performance bias, which was high due to the inability to blind the participants and outcome assessors. For an overall view of our assessments, see Figure 2.
2.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Allocation
We considered most included studies to be at low (n = 20) or unclear (n = 18) risk of bias for the random sequence generation domain, with two studies being considered at high risk. We considered the following studies to be at low risk because the study authors described an accepted method of generating a random sequence (e.g. using a randomisation table, computer‐generated random sequence or central randomisation): Bender 2010; Chan 2015; Charles 2007; Ebrahimabadi 2019; Foster 2014; Joseph 2018; Kenyon 2018; Kolmodin MacDonell 2016; Morrison 2016; Morton 2017; Mosnaim 2013; Pernell 2017; Petrie 2012; Pool 2017; Rikkers‐Mutsaerts 2012; Schaffer 2004; Stranbygaard 2010; Sulaiman 2018; Van der Meer 2009; Vasbinder 2016. We were unable to make a judgement on the following studies considered at unclear risk because the investigators described them as 'randomised' but provided no further details about the method of random sequence generation: Bender 2015; Black 2008; Choi 2017; Clerisme‐Beaty 2011; Cvietusa 2012; Davis 2019; Jan 2007; Johnson 2016a; Kim 2016; Kosse 2019; Koufopoulos 2016; Lv 2012; Reece 2017; Searing 2012; Van Sickle 2016; Vollmer 2011; Wiecha 2015; Zhou 2018. We considered two studies at high risk due to randomisation according to odd and even numbers or via an alternating process respectively (Lv 2019; Weinstein 2019).
In terms of allocation concealment, only 13 included studies described the method of allocation concealment adequately enough to be considered at low risk of bias in this domain (Bender 2010; Chan 2015; Charles 2007; Joseph 2018; Kenyon 2018; Kolmodin MacDonell 2016; Morrison 2016; Morton 2017; Petrie 2012; Pool 2017; Rikkers‐Mutsaerts 2012; Van der Meer 2009; Vasbinder 2016). Accepted methods included use of automatic assignment via the computer or registration software, or use of sequentially numbered, sealed, opaque envelopes. We considered the remaining 27 studies to be at unclear risk, as the investigators did not describe the methods used to conceal allocation.
Blinding
Most studies had a high risk of bias relating to the blinding domain. Due to the nature of the digital intervention, blinding of participants and personnel was not possible in most studies, as it was clear to participants and personnel which participants received the intervention and most studies did not use active controls. Just over half of the included studies (21 out of 40) were deemed to be at overall high risk of performance bias due to a lack of blinding of group allocation (Bender 2010; Black 2008; Choi 2017; Davis 2019; Jan 2007; Kim 2016; Kosse 2019; Lv 2012; Morrison 2016; Morton 2017; Pernell 2017; Reece 2017; Schaffer 2004; Searing 2012; Stranbygaard 2010; Sulaiman 2018; Van der Meer 2009; Van Sickle 2016; Vasbinder 2016; Weinstein 2019; Zhou 2018). We judged 12 studies to be at low risk of performance bias, due to the method of measurement of outcomes (e.g. electronically measured adherence, lung function), as it was deemed less likely to have influenced outcomes as these were more objectively measured and less susceptible to performance bias. In some studies, participants were unaware that they were being monitored (Bender 2015; Chan 2015; Charles 2007; Cvietusa 2012; Ebrahimabadi 2019; Foster 2014; Kenyon 2018; Kolmodin MacDonell 2016; Koufopoulos 2016; Lv 2019; Mosnaim 2013; Pool 2017). For example, in Charles 2007 and Chan 2015, participants were blinded to the adherence measurement, even though not blinded to the intervention, and Kolmodin MacDonell 2016 had an attention control arm.
The remainder of the studies had unclear performance bias (Clerisme‐Beaty 2011; Johnson 2016a; Joseph 2018; Petrie 2012; Rikkers‐Mutsaerts 2012; Vollmer 2011; Wiecha 2015). For example, Johnson 2016a did not describe blinding, but both groups were given online material that could have helped achieve blinding. Similarly, in Rikkers‐Mutsaerts 2012 it was not clear whether participants were aware of the purpose of the intervention as they were exposed to a two‐week baseline run‐in period, so potentially could have been blinded to the purpose of the study (and unaware that the main outcome of interest was adherence) even if not blinded to the allocation group. Adherence may thus be less prone to bias, though asthma control was self‐reported also so may have been subject to bias. We judged this to be an unclear risk of bias overall. Vollmer 2011 did not report on procedures used to blind participants or personnel, but adherence was measured objectively by electronic records, though other outcomes were more subjective and were more prone to bias, such as self‐reported asthma control test, and thus at increased risk of bias. As such, overall, we deemed the study at unclear risk of performance bias.
For detection bias, it was difficult for many studies to blind outcome assessment as most of the outcomes of interest in this review are patient‐reported (e.g. asthma control, quality of life), and the unblinded participant is often the outcome assessor of these self‐report measures. We therefore considered 20 of the included studies to be at high risk of bias in the outcome assessment domain (Black 2008; Choi 2017; Clerisme‐Beaty 2011; Davis 2019; Jan 2007; Johnson 2016a; Kim 2016; Kosse 2019; Morrison 2016; Morton 2017; Pernell 2017; Petrie 2012; Rikkers‐Mutsaerts 2012; Searing 2012; Stranbygaard 2010; Van der Meer 2009; Van Sickle 2016; Vasbinder 2016; Weinstein 2019; Zhou 2018). In these studies, the method of outcome assessment was often reliant on self‐report rather than an objective measure. We judged 15 studies to be at low risk of bias (Bender 2010; Bender 2015; Cvietusa 2012; Ebrahimabadi 2019; Foster 2014; Kenyon 2018; Kolmodin MacDonell 2016; Koufopoulos 2016; Mosnaim 2013; Pool 2017; Schaffer 2004; Sulaiman 2018; Van Sickle 2016; Vollmer 2011; Wiecha 2015). We considered these to be at low risk as the methods of outcomes assessment were objective (e.g. use of electronic adherence monitoring devices or medical records to assess adherence) and were unlikely to be influenced by the outcome assessors' awareness of group allocation. The remainder of the studies were at unclear risk of bias. In these cases, it was unclear whether the outcome assessors were aware of the study objectives and therefore would have been influenced by knowledge of study group allocation. For example, Lv 2019 did not describe any blinding procedures, so it was unclear if the outcome assessors were aware of group allocation — but, importantly, the study did not explain how adherence was measured and whether this method may have been influenced by group allocation awareness. Reece 2017 was available only as an abstract with minimal reported details, so we could not make a judgement.
Incomplete outcome data
We judged most studies as at low or unclear risk of attrition bias indicating that dropout rates were low and balanced between the two groups, and the studies described the reasons for withdrawal of study participants in sufficient detail to make a judgement that withdrawal did not impact on study outcomes. Based on this, we deemed 21 studies to have a low risk of attrition bias (Bender 2010; Bender 2015; Chan 2015; Clerisme‐Beaty 2011; Ebrahimabadi 2019; Jan 2007; Johnson 2016a; Kenyon 2018; Kim 2016; Kolmodin MacDonell 2016; Morton 2017; Mosnaim 2013; Pernell 2017; Pool 2017; Schaffer 2004; Stranbygaard 2010; Sulaiman 2018; Van der Meer 2009; Vasbinder 2016; Weinstein 2019; Wiecha 2015). There were studies with imbalanced dropout rates between arms but if these dropouts were not for reasons that would be related to the outcome of interest, we considered these at low risk. For example, Morton 2017 had higher rates of loss to follow‐up in the intervention group, but these were not for reasons related to adherence — so did not impact on the judgement of bias for the adherence outcome. We considered 10 studies at high risk (Charles 2007; Foster 2014; Joseph 2018; Kosse 2019; Koufopoulos 2016; Lv 2012; Lv 2019; Morrison 2016; Petrie 2012; Rikkers‐Mutsaerts 2012), due to an imbalance of attrition rates between the study arms, or where the reasons for dropout may have impacted on outcomes (e.g. higher dropout rates in the intervention group are likely those who have poor adherence; if those with poor adherence are more likely to drop out, this could falsely inflate the adherence rate in the intervention group) (Kosse 2019). We judged the remaining nine studies to be at unclear risk, usually because most of these studies were only available as abstracts and dropouts were not reported (Black 2008; Choi 2017; Cvietusa 2012; Davis 2019; Reece 2017; Searing 2012; Van Sickle 2016; Vollmer 2011; Zhou 2018).
Selective reporting
We deemed 11 studies to be at high risk of reporting bias. In these cases, the studies reported in the methods that various outcome measures would be collected, but these data were not presented in the findings, representing a deviation from the original stated methodology (Choi 2017; Joseph 2018; Kim 2016; Morton 2017; Mosnaim 2013; Pernell 2017; Pool 2017; Searing 2012; Van Sickle 2016; Vasbinder 2016; Vollmer 2011). For example, Vollmer 2011 stated that ACT, AQLQ and satisfaction with the intervention would be measured, but these were not reported in the results. We judged two studies to be at unclear risk of selective reporting due to the study being only an abstract with minimal details (Black 2008; Cvietusa 2012). The remaining studies were deemed at low risk of reporting bias as the findings reported matched the stated outcomes of interested in the methodology, or we were able to identify a prepublished protocol or prospective trial registration.
Other potential sources of bias
We did not note any additional potential sources of bias in any included studies.
Effects of interventions
See: Table 1
Table 1 summarises the key findings of this review comparing digital adherence interventions versus controls. We describe the key findings under each of the outcome measures in the sections below, and the corresponding subgroup analyses. Due to the limited number of studies reported, we found we could not conduct subgroup analyses for all planned analyses as information for different subgroups was not always available or reported by study authors (see Differences between protocol and review). Because of this, we conducted the following subgroup analyses for our primary outcomes of adherence and asthma control, as the total number of studies reporting exacerbations was low (n = 6):
Digital interventions with one digital component versus interventions with multiple digital components.
Different types of digital interventions (i.e. online versus computer‐based versus electronic monitoring devices).
Digital interventions involving adherence feedback versus interventions without adherence feedback.
Digital interventions with an 'in‐person' component versus self‐directed and fully digitalised interventions.
We added a subgroup analysis comparing studies of adults and adolescents and studies of children, as suggested by our expert advisory group and in line with a similar previous review (Normansell 2017).
Adherence to maintenance medication
Digital interventions may result in a large increase in adherence of 15%: the mean difference (MD) in percentage adherence in those receiving the digital adherence intervention was 15% higher than those in the control group (MD 14.66, 95% confidence interval (CI) 7.74 to 21.57); 8885 participants; 16 studies; low‐certainty evidence; Analysis 1.1; Figure 3).
1.1. Analysis.

Comparison 1: Digital intervention versus usual care, Outcome 1: Adherence
3.

Forest plot of comparison: 1 Digital intervention versus usual care, outcome: 1.1 Adherence.
There was high statistical heterogeneity between study results (I2 = 94%), likely due to the underlying variation in the different studies with a large variation in study population and interventions evaluated. There were concerns about risk of biases mainly from unclear allocation concealment in half of the studies, and high risk of performance bias, which downgraded our confidence in the results. We generated a funnel plot to check for publication bias but found none.
For studies that could not be included in the meta‐analysis (Choi 2017; Clerisme‐Beaty 2011; Cvietusa 2012; Davis 2019; Jan 2007; Johnson 2016a; Joseph 2018; Kim 2016; Lv 2012; Lv 2019; Reece 2017; Searing 2012; Van Sickle 2016; Weinstein 2019; Zhou 2018), all studies except Choi 2017, Davis 2019 and Reece 2017 showed that adherence in the digital intervention groups was higher compared to the control group. We were missing data from one study as adherence rate was not measured in the control group (Weinstein 2019). See Table 2 for further information.
Most studies used one digital intervention component as opposed to multiple components. Thirteen studies used one digital intervention component compared to control, and three studies trialled multiple digital interventions. Those using one digital component resulted in improvements in adherence of around 15 percentage points compared to 12 percentage points in those with multiple components (Analysis 1.2) compared to controls (test for subgroup differences: Chi² = 0.21, df = 1 (P = 0.65), I² = 0%).
1.2. Analysis.

Comparison 1: Digital intervention versus usual care, Outcome 2: Adherence ‐ multiple interventions
Looking at all types of digital interventions reported, we categorised these into interventions using electronic monitoring devices (EMDs), short message service (SMS) text messages, web‐based (websites or web applications), or interactive voice response (IVR) or related speech/audio‐based intervention. Of the studies included in the meta‐analysis, seven assessed the effects of EMDs on adherence to asthma maintenance therapy. This subgroup had the largest improvement in adherence with EMD compared to control (MD 22.50, 95% CI 10.84 to 34.16; 932 participants, 7 studies; Analysis 1.3; Figure 4). Four studies assessed the effects of text‐messaging, which found an improvement in adherence compared to controls (MD 12.12, 95% CI 6.22 to 18.03; 391 participants; 4 studies). However, the two studies that assessed the effects of a web‐based intervention found there was little to no improvement in adherence (MD ‐2.68, 95% CI ‐10.07 to 4.71; 368 participants; 2 studies). Four studies that examined interactive voice recognition (IVR) or related interventions found that the intervention may improve adherence to asthma maintenance therapy, but with a smaller magnitude of effect compared to EMD or SMS type interventions (MD 7.60, 95% CI 1.23 to 13.97; 7403 participants, 4 studies), but we note this group also had the largest number of participants due to the inclusion of the Vollmer 2011 study with 3171 participants. The test for subgroup differences suggested that EMDs and SMS messages may be more effective digital interventions in improving adherence to asthma maintenance therapies than other types of digital interventions (test for subgroup differences: Chi² = 15.84, df = 3 (P = 0.001), I² = 81.1%). This effect could be due to there being fewer studies/participants in the web applications and speech‐based intervention studies. Furthermore, subgroup analyses are exploratory and because we have carried out five subgroup analyses per outcome, they are at risk of being significant at random.
1.3. Analysis.

Comparison 1: Digital intervention versus usual care, Outcome 3: Adherence ‐ types of digital
4.

Forest plot of comparison: 1 Digital intervention versus usual care, outcome: 1.3 Adherence ‐ types of digital.
Six studies using digital interventions with adherence feedback compared to control reported a large improvement in adherence (MD 22.60, 95% 8.93 to 36.36; 842 participants; 6 studies), compared to the 10 studies that used digital interventions without adherence feedback (MD 9.05, 95% CI 3.69 to 14.41; 8043 participants; 10 studies), although it should be noted that the number of participants in the subgroup of studies providing no feedback was 10‐fold larger (test for subgroup differences: Chi² = 3.27, df = 1 (P = 0.07), I² = 69.4%) (Analysis 1.4).
1.4. Analysis.

Comparison 1: Digital intervention versus usual care, Outcome 4: Adherence ‐ feedback
Most studies were fully digital with only three having an in‐person component. There were improvements in adherence of a similar magnitude in both subgroups (around 14 percentage points), though it should be noted that there were many more participants in the fully digital subgroup. The 95% CI was wide in both groups (Analysis 1.5). There was no difference in the effect on adherence with a fully digital intervention in comparison to a digital intervention with an in‐person component (test for subgroup differences: Chi² = 0.02, df = 1 (P = 0.89), I² = 0%).
1.5. Analysis.

Comparison 1: Digital intervention versus usual care, Outcome 5: Adherence ‐ in‐person
Two‐thirds of the studies were in adults and adolescents, and the remainder in children. The improvement in adherence in children was large (MD 18.06, 95% CI 3.89 to 32.23; 1489 participants; 6 studies) compared to adults and adolescents (MD 11.04, 95% CI 1.09 to 20.99; 7396 participants; 10 studies) (test for subgroup differences: Chi² = 0.63, df = 1 (P = 0.43), I² = 0%) (Analysis 1.6).
1.6. Analysis.

Comparison 1: Digital intervention versus usual care, Outcome 6: Adherence ‐ age
Asthma control
Thirty‐one studies aimed to assess how digital adherence interventions would affect asthma control. Fifteen studies were included in the meta‐analysis (Analysis 1.7; Figure 5). These studies measured the change from baseline in asthma control. However, the method for measuring asthma control varied between studies. They used the Asthma Control Test (n = 5); Childhood Asthma Control Test (n = 1); Asthma Control Questionnaire (n = 7); Control of Allergic Rhinitis and Asthma Test (n = 1), and Perceived Control of Asthma Questionnaire (n = 1). Overall, digital adherence interventions are likely to improve asthma control compared to the control group (SMD 0.31, 95% CI 0.16 to 0.44, 1638 participants; 15 studies; I2 = 35%; moderate‐certainty evidence; Analysis 1.7; Figure 5). Although the effect size of SMD 0.31 is considered small, this is likely to be clinically significant. We downgraded the evidence due to a high risk of performance and detection bias in the studies that contribute a high weighting to this outcome. We created a funnel plot to check for publication bias and found none.
1.7. Analysis.

Comparison 1: Digital intervention versus usual care, Outcome 7: Asthma control ‐ change from baseline
5.

Forest plot of comparison: 1 Digital intervention versus usual care, outcome: 1.7 Asthma control ‐ change from baseline.
Of the studies that could not contribute to the meta‐analysis, asthma control was higher in the intervention groups in all studies except in Choi 2017 and Kim 2016 (see Table 2). One study did not report their data on asthma control (Vollmer 2011) and one study did not report their data on asthma control in the control group (Rikkers‐Mutsaerts 2012).
Studies measuring asthma control in our meta‐analysis used either one digital component (n = 12) or multiple digital components (n = 3). Both achieved similar effects, with no difference between the interventions using one or multiple digital components in their intervention (test for subgroup differences: Chi² = 0.26, df = 1 (P = 0.61), I² = 0%) (Analysis 1.8).
1.8. Analysis.

Comparison 1: Digital intervention versus usual care, Outcome 8: Asthma control ‐ multiple interventions
Similar to our analyses for adherence, we categorised the interventions into four subgroups: EMDs (n = 3), SMS (n = 3), web‐ or app‐based (n = 8), or IVR/speech‐based interventions (n = 2). The largest effects were seen with SMS text messaging (SMD 0.59, 95% CI 0.20 to 0.97; 115 participants; 3 studies) and web‐ or app‐based interventions website (SMD 0.34, 95% CI 0.14 to 0.55; 1112 participants; 8 studies, Analysis 1.9). It should be noted though that there were large variations between studies and study numbers were not balanced between the subgroups with the web/app subgroup having the largest number of participants (test for subgroup differences: Chi² = 3.90, df = 3 (P = 0.27), I² = 23.1%).
1.9. Analysis.

Comparison 1: Digital intervention versus usual care, Outcome 9: Asthma control ‐ types of digital interventions
Studies measuring asthma control in our meta‐analysis either had digital interventions that included adherence feedback (n = 7) or did not have adherence feedback (n = 8) (Analysis 1.10). Both produced similarly large effects on asthma control: having adherence feedback in the intervention (SMD 0.34, 95% CI 0.11 to 0.58; 909 participants; 7 studies) and not having adherence feedback in the intervention (SMD 0.26, 95% CI 0.11 to 0.41; 729 participants; 8 studies) (test for subgroup differences: Chi² = 0.36, df = 1 (P = 0.55), I² = 0%).
1.10. Analysis.

Comparison 1: Digital intervention versus usual care, Outcome 10: Asthma control ‐ adherence feedback
Most digital interventions were fully digital (n = 10) as opposed to having an in‐person component (n = 5). Those interventions that included an in‐person component appeared to achieve a larger effect on asthma control (SMD 0.47, 95% CI 0.13 to 0.81; 536 participants; 5 studies) compared to the subgroup with fully digital interventions (SMD 0.22, 95% CI 0.10 to 0.34; 1102 participants; 10 studies), although as the two subgroups were not balanced in study or participant numbers, this should be interpreted with caution. The SMD size reflects the effect size, with values 0.4 or above considered as a moderate effect size (Undela 2021) (test for subgroup differences: Chi² = 1.96, df = 1 (P = 0.16), I² = 49.0%) (Analysis 1.11).
1.11. Analysis.

Comparison 1: Digital intervention versus usual care, Outcome 11: Asthma control ‐ in‐person component
There did not appear to be a difference in effects on asthma control in studies in adults and adolescents versus children, though digital interventions may have a greater effect on improving asthma control in adults and adolescents (SMD 0.36, 95% CI 0.18 to 0.53; 1165 participants; 12 studies) compared to in children (SMD 0.19, 95% CI 0.01 to 0.37; 473 participants; 3 studies; Analysis 1.12). This should be interpreted with caution as the SMD result was still small (less than 0.4 for both), although the SMD in adults reached clinical significance while the SMD did not in children (Angst 2017). Also, it was not always clear which subgroup the studies belonged to ‐ many comprised mixed groups including early adolescent ages through to adults — e.g. Foster 2014 included participants from 14 to 65 years old. Furthermore, the test for subgroup differences was insignificant (Chi² = 1.76, df = 1 (P = 0.18), I² = 43.1%).
1.12. Analysis.

Comparison 1: Digital intervention versus usual care, Outcome 12: Asthma control ‐ age
Exacerbations
Thirteen studies reported on the number of asthma exacerbations requiring at least oral corticosteroid treatment. Six studies were included in the meta‐analysis; all of these defined exacerbations as requiring at least oral corticosteroid treatment. These measured exacerbations as the number of exacerbations per person per year. Digital interventions may reduce exacerbations by 50% compared to controls (risk ratio (RR) 0.53, 95% CI 0.32 to 0.91; 678 participants; 6 studies; low‐certainty evidence; Analysis 1.13). Statistical heterogeneity was moderate (I2 = 37%). We downgraded the evidence as most studies had high risk of detection and performance bias, with the implicated studies also having a high weighting. Due to the low number of studies, it was not possible to determine publication bias based on funnel plot symmetry; as such, this also downgraded our certainty in the evidence.
1.13. Analysis.

Comparison 1: Digital intervention versus usual care, Outcome 13: Asthma exacerbations ‐ number of people with one or more exacerbations
Seven additional studies measured exacerbations but could not be included in the meta‐analysis due to their method of reporting. These studies found that the frequency of asthma exacerbations in the intervention group was lower than control groups, except one study that reported a higher number of exacerbations in the intervention group (Kim 2016), and another that did not report their exacerbation data (Mosnaim 2013).
We conducted no subgroup analyses for this primary outcome as too few studies could be included.
Unscheduled healthcare utilisation
Fourteen studies reported on whether the intervention group or control group required unscheduled healthcare utilisation related to asthma. Studies reported unscheduled visits in a variety of ways such as hospital visits (Joseph 2018; Morrison 2016; Zhou 2018) or visits to urgent care, general practice (GP), or the emergency department (ED) (Chan 2015; Bender 2015; Kim 2016; Lv 2012; Morton 2017; Mosnaim 2013; Rikkers‐Mutsaerts 2012; Pool 2017; Van der Meer 2009; Wiecha 2015), or this was not described beyond 'healthcare utilisation' (Choi 2017). Out of the 14 studies, four studies were included in the meta‐analysis. One study reported no ED visits for both groups during the study period (Morrison 2016). Out of the three studies where events occurred, we found that digital interventions may slightly reduce unscheduled healthcare utilisation by approximately a quarter, compared to control groups (RR 0.74, 95% CI 0.51 to 1.06; 446 participants; 4 studies; I2 = 0%; low‐certainty evidence; Analysis 1.14; Figure 6), but some reported that the interventions could possibly worsen unscheduled healthcare utilisation. We downgraded the evidence due to imprecision and small sample size, the high risk of performance, detection and attrition biases in the included studies, and the low number of studies, which prevented us from determining publication bias.
1.14. Analysis.

Comparison 1: Digital intervention versus usual care, Outcome 14: Unscheduled healthcare utilisation ‐ number of people with one or more visits to a healthcare provider/attendance at an emergency department or urgent care centre/hospital admission
6.

Forest plot of comparison: 1 Digital intervention versus usual care, outcome: 1.14 Unscheduled healthcare utilisation ‐ number of people with one or more visits to a healthcare provider/attendance at an emergency department or urgent care centre/hospital admission.
Four additional studies found that unscheduled healthcare utilisation rates were lower in intervention groups than control (Table 2) (Lv 2012; Morton 2017; Pool 2017; Van der Meer 2009). Three other studies that investigated the effect on unscheduled healthcare utilisation reported lower rates in the control group (Bender 2015; Kim 2016; Rikkers‐Mutsaerts 2012), one reported equal rates (Wiecha 2015), one reported no significant difference between intervention and control groups (Choi 2017), and one did not report data (Mosnaim 2013).
Time off school or work
Six studies reported on how asthma maintenance medication adherence affected time off school or work for any reason. However, due to the low number of studies and the heterogeneity in reporting methods and author definitions of 'time off school', we performed no meta‐analysis. In the six studies that reported on this outcome, the findings were inconsistent; three reported fewer days of school absence in intervention groups compared to control groups (Chan 2015; Lv 2019; Morton 2017). However, two studies reported that time off school in the intervention group was higher than in the control group (Joseph 2018; Wiecha 2015), and one did not report any data (Mosnaim 2013).
Lung function
Seventeen studies reported on how asthma maintenance medication adherence affected lung function. Seven studies were included in the meta‐analysis. The method by which lung function was measured in those studies in the meta‐analysis was change in baseline lung function measured using FEV1 (% predicted). A single meta‐analysis of the seven studies assessing the effects of digital adherence interventions on lung function showed that digital adherence interventions have little or no effect on lung function compared to controls (MD 3.58, 95% CI 1.00 to 6.17; 1052 participants; 7 studies, I2 = 43%, moderate‐certainty evidence; Analysis 1.15). This 3.58% improvement also falls below the threshold for clinical relevance. For the diagnosis of asthma in adults an increase of FEV1 of 12% after bronchodilator use is considered meaningful; in children a lower threshold of 8% to 9% increase is considered relevant (Hopp 2016). The high risk of performance, detection, and attrition biases reduced our certainty in the evidence.
1.15. Analysis.

Comparison 1: Digital intervention versus usual care, Outcome 15: Lung function ‐ FEV1% predicted (change from baseline)
Lung function was additionally measured in 10 studies, with five studies producing a slight improvement in intervention groups (Clerisme‐Beaty 2011; Jan 2007; Reece 2017; Rikkers‐Mutsaerts 2012; Weinstein 2019). Three studies did not show an improvement of lung function with the intervention (Charles 2007; Foster 2014; Kim 2016). One study did not report lung function in the control group (Van der Meer 2009) and one did not report any outcome data in the abstract (Black 2008).
Quality of life
Twenty‐two studies reported on how digital interventions impacted on quality of life. Ten studies were included in the meta‐analysis. These studies measured the change from baseline for quality of life. However, the method of measuring quality of life varied amongst the studies. They used either the Asthma Quality of Life Questionnaire (AQLQ) (n = 8) or Paediatric Asthma Quality of Life Questionnaire (PAQLQ) (n = 2). Overall, there was moderate‐certainty evidence that digital adherence interventions likely increase quality of life. The SMD suggests that there was a difference in quality of life, with a small effect size favouring the intervention, although the SMD of 0.26 falls short of the 0.3 threshold for clinical significance (Angst 2017) (SMD 0.26, 95% CI 0.07 to 0.45, 848 participants; 10 studies; I2 = 38%; moderate‐certainty evidence; Analysis 1.16). We downgraded the evidence due to concerns about high risk of detection and performance biases.
1.16. Analysis.

Comparison 1: Digital intervention versus usual care, Outcome 16: Quality of life ‐ change from baseline
Ten additional studies measured quality of life but could not be included in the meta‐analysis primarily due to a lack of reporting of standard deviations (SDs). Of these studies, five indicated an improvement in intervention groups (Jan 2007; Johnson 2016a; Pool 2017; Rikkers‐Mutsaerts 2012; Sulaiman 2018); two did not report study data (Joseph 2018; Vollmer 2011), and the remainder did not report improved quality of life in the intervention group (Kim 2016; Vasbinder 2016; Weinstein 2019).
Acceptability of the digital intervention
Acceptability of the digital intervention was reported in 13 studies as dropout rates, proportion of days on which tools were used, or satisfaction with the intervention, with one study reporting data qualitatively (Morrison 2016). Pernell 2017 reported that 20% of participants responded on more than 95% of the days that text messages (the intervention) were sent, but usage varied depending on the day, with an average response rate of 33% (range 21% to 46%). Participants that received once‐daily text message reminders had a higher percentage of days that they responded (median 21.7%, interquartile rage (IQR) 0.0% to 83.3%) than those who received twice‐daily text message reminders (median 3.3%, IQR 0.0% to 85.0%), however this difference was not significant (P = 0.536). Kolmodin 2016 reported that both intervention and control groups rated the intervention favourably with no intervention group participants choosing to opt out of the text message intervention. Intervention group participants had a mean satisfaction score of 3.6 (SD 0.4) out of 4. Control group participants reported a mean score of 3.5 (SD 0.5) overall satisfaction. Overall, satisfaction did not differ significantly across groups. Jan 2007 reported favourable attitudes amongst the caregivers regard to the Internet‐based intervention in an after‐study survey. Similarly, Bender 2015 reported that 42.8% of the intervention group found the intervention programme "helpful" and were highly satisfied, with > 90% agreeing with statements on the usefulness of the intervention for asthma care and 84% stating that their child's asthma was under control due to the intervention. Wiecha 2015 reported strong satisfaction with the website for both the users and the health providers, who stated that the intervention provided useful information. Providers in Foster 2014 rated overall intervention usefulness highly across all groups. Cvietusa 2012 reported that two‐thirds of parents reported the intervention reminder calls as helpful. Kim 2016 reported that patients had a high level of satisfaction with the application, finding the intervention easy to understand, convenient, and easy to use. Searing 2012 reported that 93% of the participants felt the text message intervention helped them take better care of their asthma, but 7% felt that too many messages were sent. Johnson 2016a reported neutral to positive attitudes towards the intervention website, with 78% (18) of users and 86% (18) of nonusers expressing interest in continuing to use the intervention post‐study. Joseph 2018 found overall study compliance for completion of all intervention sessions was 64.5%; at 12 months completion rates were 89.3%, with no significant differences between the intervention and usual care groups. In Kenyon 2018, device data were available for 78% (32/41) of the participants. All participants (32/41) who completed the satisfaction survey reported the text message reminders to be helpful. Caregivers suggested improvements such as use of incentives (35%) or a report of medication use (42%) to support adherence. Morton 2017 reported 50% broken intervention devices in the intervention group compared to 19% in the usual care group.
Cost‐effectiveness of the intervention
The included studies did not report cost‐effectiveness as an outcome thus data could not be collected.
Adverse events
No data were reported on adverse events.
Sensitivity analysis
No unpublished data were included in the analyses, so we found that this sensitivity analysis was not necessary. Similarly, there were no quasi‐randomised trials, nor non‐English studies, so these sensitivity analyses were not conducted. We planned to exclude studies with a high risk of selection bias, however this only affected two studies (Lv 2019; Weinstein 2019). Lv 2019 did not contribute data to any of the meta‐analyses of the primary outcomes and Weinstein 2019 only contributed data for the asthma control meta‐analysis, so this sensitivity analysis was not undertaken as excluding these studies would make little difference to the outcomes.
For the sensitivity analysis comparing studies that measured adherence subjectively versus objectively, the overall conclusions did not change for adherence, asthma control, and asthma exacerbations. For adherence, only one study was excluded (Petrie 2012), so the impact on the overall effect was negligible (MD 14.71, 95% CI 7.28 to 22.13 versus original full dataset MD 14.66, 95% CI 7.74 to 21.57) (Analysis 2.1). For asthma control, three studies were excluded (Kosse 2019; Morrison 2016; Van der Meer 2009). The SMD reduced with the exclusion of the studies but remained in favour of the intervention (SMD 0.25, 95% CI 0.14 to 0.37 versus full dataset SMD 0.31, 95% 0.17 to 0.44) (Analysis 2.2). For exacerbations, three studies were excluded (Morrison 2016; Rikkers‐Mutsaerts 2012; Van der Meer 2009); this reduced the size of the risk ratio but improved the precision (RR 0.35, 95% CI 0.20 to 0.59 versus full dataset RR 0.53, 95% CI 0.32 to 0.91) (Analysis 2.3). Again, the overall effect on reducing the exacerbation rate was not affected by the exclusion of these studies.
2.1. Analysis.

Comparison 2: Digital intervention versus usual care ‐ sensitivity analyses, Outcome 1: Adherence ‐ objective measures
2.2. Analysis.

Comparison 2: Digital intervention versus usual care ‐ sensitivity analyses, Outcome 2: Asthma control ‐ change from baseline ‐ objective measures
2.3. Analysis.

Comparison 2: Digital intervention versus usual care ‐ sensitivity analyses, Outcome 3: Asthma exacerbations ‐ number of people with one or more exacerbations ‐ objective measures
We re‐ran the analyses with a fixed‐effect instead of a random‐effects model for the three primary outcomes. The effect size for adherence reduced, but precision increased with a narrower 95% CI to MD 6.98, 95% CI 5.76 to 8.21 (Analysis 2.4). The effect size for asthma control remained relatively unchanged (SMD 0.30, 95% 0.20 to 0.40) (Analysis 2.5), as did the risk ratio for asthma exacerbations (RR 0.51, 95% CI 0.35 to 0.75) (Analysis 2.6); the intervention group was still favoured across all three outcomes.
2.4. Analysis.

Comparison 2: Digital intervention versus usual care ‐ sensitivity analyses, Outcome 4: Adherence ‐ fixed‐effect
2.5. Analysis.

Comparison 2: Digital intervention versus usual care ‐ sensitivity analyses, Outcome 5: Asthma control ‐ change from baseline ‐ fixed‐effect
2.6. Analysis.

Comparison 2: Digital intervention versus usual care ‐ sensitivity analyses, Outcome 6: Asthma exacerbations ‐ number of people with one or more exacerbations ‐ fixed‐effect
Six studies received full or partial commercial funding (Bender 2010; Charles 2007; Stranbygaard 2010; Sulaiman 2018; Vasbinder 2016; Weinstein 2019); four did not state their funding sources (Black 2008; Choi 2017; Lv 2012; Petrie 2012), and thus were also excluded for these sensitivity analyses. For adherence, seven studies were excluded (Black 2008; Bender 2010; Charles 2007; Petrie 2012; Stranbygaard 2010; Sulaiman 2018; Vasbinder 2016); this had no effect on the adherence outcome (MD 14.34, 95% CI 3.60 to 25.08 versus full dataset MD 14.66, 95% CI 7.74 to 21.57) (Analysis 2.7), although this greatly reduced the precision of the analysis with a much wider CI. For asthma control, four studies were excluded (Bender 2010; Lv 2012; Stranbygaard 2010; Weinstein 2019). This slightly reduced the SMD effect size, but the difference was only slight (SMD 0.28, 95% CI 0.13 to 0.43 versus full dataset SMD 0.31, 95% 0.17 to 0.44) (Analysis 2.8). This analysis did not impact on the outcome for asthma exacerbations as none of the affected studies had contributed data to the meta‐analysis (Analysis 2.9).
2.7. Analysis.

Comparison 2: Digital intervention versus usual care ‐ sensitivity analyses, Outcome 7: Adherence ‐ no commercial funding
2.8. Analysis.

Comparison 2: Digital intervention versus usual care ‐ sensitivity analyses, Outcome 8: Asthma control ‐ change from baseline ‐ no commercial funding
2.9. Analysis.

Comparison 2: Digital intervention versus usual care ‐ sensitivity analyses, Outcome 9: Asthma exacerbations ‐ number of people with one or more exacerbations ‐ no commercial funding
Discussion
Summary of main results
This review aimed to determine the effects of digital adherence interventions on asthma maintenance therapy and other clinical outcomes. Overall, our findings support the effectiveness of digital interventions in improving adherence to asthma maintenance therapy and clinical outcomes. Specifically, based on our certainty of the evidence, we found that digital interventions may increase adherence and reduce exacerbations, and are likely to improve asthma control and quality of life. The effects on adherence and asthma control in particular are clinically relevant, based on the magnitude of effect seen from the meta‐analyses. However, digital interventions did not show clear benefit in reducing time off school, unscheduled healthcare utilisation, or improving lung function, likely because of the smaller number of studies that could be included in the meta‐analysis. For the subgroup analyses, we found benefits for improving adherence for availability of adherence feedback and for intervention type, with EMDs and SMS messages being more effective for improving adherence to maintenance therapies than other types of digital interventions. This effect could be due to there being fewer studies/participants for the web applications and speech‐based interventions. For asthma control, we found that presence of an in‐person component had benefits for improving asthma control. However, subgroup analyses are exploratory and because we have carried out five subgroup analyses per outcome, they are at risk of being significant at random.
Overall, this systematic review included 40 randomised controlled trials (RCTs), of which 30 contributed data to at least one meta‐analysis. The studies included a total of 15,207 participants who were randomly assigned to the comparisons of interest in this review. The studies incorporated a number of digital interventions, including interactive voice recognition (IVR), speech recognition, electronic monitoring devices, web‐based interventions, mobile applications, short message service (SMS), videos, MP3‐players, medication dispensing systems, and audiotapes. Outcomes reported were not consistent across studies, and did not always use validated scales, particularly for self‐reported adherence measures. Almost all included studies reported some measure of adherence, usually as a percentage, but how this was measured and reported varied greatly between studies. Studies were generally at low or unclear risk of selection bias, but at high risk of bias associated with blinding due to the nature of the intervention (although this varied by outcome). Overall, just under half of these studies were at high risk of performance and detection bias and about a quarter at high risk of attrition bias and selective outcome reporting.
Overall, pooled results for our primary outcomes of adherence, asthma control, and exacerbations favoured digital adherence interventions versus control, although the certainty of this evidence was low, moderate, and low respectively. These results remained positive in favour of the intervention in our sensitivity analyses, when adherence was measured objectively or when all measures were considered; with fixed‐effect and random‐effects models; and excluding studies with commercial funding. Adherence improved overall with the use of digital interventions in our review. There was a difference in mean percentage adherence between the two groups of almost 15% for those receiving the digital adherence intervention (mean difference (MD) 14.66, 95% CI 7.74 to 21.57; 8885 participants; 16 studies; low‐certainty evidence). In asthma, an adherence percentage of 80% is often cited as the threshold required for good asthma outcomes and control (Lasmar 2009). Considering this, a percentage increase of 15% could be clinically significant, as it could shift an individual from suboptimal adherence (e.g. 65%) to optimal (80% and over). Even for individuals whose adherence is only 50%, an increase to 65% could represent important improvements in asthma control and reduction in exacerbation risk (Engelkes 2015).
However, it is unclear whether the interventions themselves mitigated non‐adherence or whether the participants improved their adherence while being observed during the study (Hawthorne effect) (McCambridge 2014). Our confidence in the evidence was also reduced by unclear allocation concealment, performance and detection biases, and the imprecision observed in the adherence outcome, which had high heterogeneity due to the large variation in study design, participants, study setting, and intervention.
Interventions that use multiple digital components did not appear to be better for improving adherence and asthma control compared to those that only had one digital component. Whilst there was no benefit seen in combining interventions, the review did not evaluate whether combinations of digital interventions with non‐digital interventions, such as financial incentives, would be more effective. There is some suggestion based on the direction of effect that interventions with adherence feedback, and an in‐person element, may achieve a larger effect on adherence and asthma control; however, neither of the analyses had wide confidence intervals, and the subgroups were imbalanced, so this should be interpreted with caution. There was some suggestion that interventions in adults had a greater effect on asthma control, but the adherence improvement achieved in children was of a greater magnitude. There were significant associations found between adherence and outcomes in certain study settings and populations, however these were not consistent findings. Of note, the interventions that were included in this review were heterogeneous in their mode of delivery, as would be expected of digital interventions, but this may affect the interpretation of this evidence as there may be components within each of these interventions that may or may not have contributed to their efficacy but could not be identified and/or isolated due to the nature of the intervention and intervention reporting.
Overall completeness and applicability of evidence
While our meta‐analyses had 30 studies that reported on outcomes, there were findings from 10 studies that could not be included in any of the meta‐analyses as they did not have the data in the format required for analysis or had missing numerical values. Furthermore, there are eight ongoing studies, which indicate research on other digital interventions that aim to improve adherence but have not reported any outcomes yet. Consequently, as data from certain studies or trials could not be included, our findings are limited to those studies that had data that could be synthesised into our meta‐analyses.
Overall, the included studies in our meta‐analyses had small sample sizes, short study durations, and limited data on proposed outcomes. Small sample sizes hindered our ability to generalise our findings to a wide population. Short study durations prevented long‐term intervention effectiveness and sustainability evaluation. Most of the included studies were predominantly conducted in the United States, and predominantly in English‐speaking countries. Importantly, most studies were conducted in high‐income countries, with just two studies in an upper‐middle income country (China) (Lv 2012; Lv 2019), and one in a low‐middle income country (Ebrahimabadi 2019). How these interventions work in ethnic minority groups within low‐income countries or countries with a predominantly European ethnic majority, or in rural or vulnerable populations, and the potential impact on health equity, is not yet known, and should be a priority for future research. There is limited information about how adherence drivers differ in low‐income countries compared to higher‐income economies, and how digital interventions may play a role in overcoming adherence barriers. However, data that do exist suggest that whilst there may be unique adherence barriers such as medicines cost and availability, there remain barriers that are common to both high‐ and low‐income countries such as asthma knowledge, beliefs, and lack of habit formation (Desalu 2021; Mazumdar 2015). Whilst digital interventions cannot overcome non‐adherence due to issues of medicines access, they can help improve non‐adherence that is driven by poor knowledge, treatment beliefs, and lack of routine ‐ for example, through personalised text messages to address belief barriers (Petrie 2012) and use of inhaler reminders (Chan 2015). Whilst we were able to conduct a subgroup analysis by age, there were no studies that were conducted in the very elderly population, who may potentially respond differently to younger participants in terms of digital acceptability and literacy. Studies of digital interventions in older adult populations are needed. Finally, the small number of studies reporting on our outcomes of interest, such as exacerbations, meant that significant effects on outcomes may not have been detected. This also prevented us from undertaking more subgroup comparisons (e.g. interactive versus non‐interactive digital interventions, or comparing theory‐ versus non‐theory‐based interventions), hence preventing identification and exploration of the specific effective components that might explain how and what elements of digital adherence interventions are most important for improving adherence. Future studies should describe interventions in sufficient detail to allow identification of the effective components of the intervention.
Of the subgroup analyses, only the type of digital intervention analysis showed significant subgroup differences. The classifications used for conducting the subgroup analyses had a subjective element as these were determined by the author team, which may impact on the results. However, the classifications were rigorously independently checked by several members of the author team. In terms of adherence, electronic monitoring devices (EMDs) and SMS text‐messaging appear to hold the most potential in improving adherence, potentially through their real‐time reminders and ease of integration into existing daily routines of patients. EMDs are advantageous as they track and record patients’ data (Chan 2013). This can provide feedback to develop personalised solutions to overcome non‐adherence barriers. Similarly, the most recent SMS text‐messaging studies explored how adherence can be improved by creating personalised messages (Kolmodin MacDonell 2016; Petrie 2012). The personalisation potential of SMS, coupled with the customisable and reminder functionalities of EMDs, may ensure responsiveness to individual patient needs, however the studies included were limited to SMS text messaging; how this applies to online messaging platforms remains unknown. Other reviews also noted the potential in these digital technologies; Biblowitz 2018 and Bonini 2018 concluded that EMDs are one of the most effective interventions for improving adherence to maintenance therapy. This was supported by the previous Cochrane Review of adherence interventions in asthma (Normansell 2017). The potentially low cost of implementation and simplicity in the usability of SMS messaging makes this an attractive option for further exploration in supporting asthma management (Tran 2014).
The benefits of EMDs were translated to benefits in asthma control, though this may be because of the small number of studies as only half of the studies that investigated EMDs and adherence also measured asthma control. The converse was seen with web‐based interventions, where fewer studies measured effects on adherence but more assessed impact on asthma control, which may account for the benefits seen. More importantly, whilst the type of digital intervention may have effect, the more important determinant is likely how these technologies are used and the content (Horne 2005; Horne 2019; WHO 2013). Few studies were reported in sufficient detail for us to determine the detail of the intervention content, which would likely have influenced its overall effectiveness. Additionally, the most common comparator for the digital intervention was reported as 'usual or 'standard' care. The degree that usual care relates to non‐digital delivery of the same digital intervention is uncertain as studies varied in the detail of their reporting; some reported use of an attention control (Mosnaim 2013; Pool 2017), or a deactivated version of the digital intervention (Chan 2015; Charles 2007; Kenyon 2018; Morton 2017; Vasbinder 2016), or paper‐based delivery of the same information (Reece 2017).
We did attempt to extract data on asthma severity, as it was hypothesised that patients with more severe asthma might benefit more from any adherence intervention. However, the studies did not report on asthma severity consistently, with many studies providing little or no information on asthma severity. This is a limitation of the data available for the review and precluded analyses based on severity. In terms of study designs, we chose to exclude cross‐over trials due to the likely carryover effects of digital interventions. It is possible that future reviews can consider including cross‐over studies and limiting data extraction to only the first phase of the study prior to the cross‐over (Normansell 2017). For exacerbations, we saw a reduction, but mortality or asthma‐related deaths per se was not measured in any of the included studies, likely because the studies were generally too short in duration to assess mortality benefits, ranging from 8 weeks to 52 weeks. The long‐term feasibility and sustainability of digital interventions in improving adherence, asthma control, and any effects on mortality remains to be determined. Widespread and long‐term clinical application of any new intervention requires us to consider the APEASE (Affordability, Practicability, Effectiveness and cost‐effectiveness, Acceptability, Side effects/safety and Equity) criteria (Michie 2017); our review provides evidence on effectiveness and acceptability, but not the other aspects that may need to be considered for successful implementation in practice. Further research into areas of affordability, practicability, and impacts on equity is needed.
Quality of the evidence
The overall certainty of the evidence for the outcomes that we graded was low to moderate. The findings for asthma control had moderate‐certainty evidence. However, whilst the majority of the other outcomes had a lower certainty of evidence, the key driver of this is the small number of included studies under each outcome rather than poorly designed studies. The small number of studies led to a combination of concerns regarding risk of bias, inconsistency, and imprecision. However, as the number of studies in this area increases, the certainty of the evidence is likely to improve over time.
Risk of bias
Studies generally had low or unclear risk of selection bias and reporting bias (Figure 2). Most studies were randomised with transparent methods, and allocation was adequately concealed to personnel. This ensured that the population would have a range of baseline characteristics representative of the general population. In some cases, we assessed blinding, or lack of blinding, as associated with a different level of risk, depending on the outcome in question — for example, if adherence was measured via self‐report and participants were not blinded to the intervention allocation, then there would be a risk of bias in adherence but potentially not for objective measures such as hospitalisation. We factored this into our GRADE decisions for these outcomes (e.g. a study at high risk of detection bias for patient‐reported outcomes, such as quality of life, might be at lower risk for other, more objective outcomes, such as electronically monitored adherence).
Of note, the methods used in some studies in terms of randomisation and allocation were unclear. For example, the study may not have stated how “randomisation” occurred, i.e. computer generation (Lv 2012), or it was unclear whether personnel were aware of what the allocation was. Furthermore, we had concerns about reporting biases in 11 studies; for instance, either they did not report on all outcomes specified in the trial protocols (Mosnaim 2013; Pool 2017; Vasbinder 2016), or the study findings could not be determined based on the data reported by the authors (Kim 2016; Pernell 2017). These were all key pieces of information that could have contributed to our understanding in this review.
Performance and detection bias were high with digital interventions where nearly half of the studies were at high risk, as blinding of participants and personnel was not always possible due to the type of digital intervention. For example, a video and prompt list intervention meant that participants in that group would be aware of what was being assessed, leading to a high risk of performance bias (Davis 2019). High detection bias was generally because of the self‐reported nature for outcomes such as asthma control and quality of life (Jan 2007). However, it was also because studies often monitored how the digital interventions influenced the change in outcome over a time period; hence, intervention groups would be aware of being monitored or tracked. If participants were aware of whether they were in the intervention or control group, and outcomes measures took place in participant homes, it would be easy for allocation to be revealed to the researcher, leading to biased recording of results (Morrison 2016). For example, when researchers contacted patients, there was a risk that the allocation group could be revealed (Morrison 2016; Petrie 2012). There was low reporting bias in most of our included studies, with outcomes in 27 out of 40 studies reported as per methods or protocol. Eleven studies were at high risk for reporting bias as they did not present all data in the results despite being stated as an outcome. There was moderately high attrition bias in about a quarter of the included studies, which could be due to the higher dropout rates and unbalanced dropout rates with high withdrawals for certain digital interventions in the intervention group compared to control groups. The studies with a high risk of attrition bias used a range of digital interventions and the study lengths were varied. This may reflect participants’ lack of desirability to engage in a clinical trial rather than with digital interventions.
Inconsistency
Overall heterogeneity was low across outcomes except for studies measuring endpoint values for adherence (I2 = 94%). As seen in Figure 3, there is an outlier in the adherence forest plot which inflated the heterogeneity (Chan 2015). When removed, the heterogeneity was reduced but still considerable (I2 = 83%). Most studies showed better adherence in the intervention group, but some outliers showed limited differences or effects in the opposite direction, which reduced our confidence in the findings. This high heterogeneity has been noted by other meta‐analyses to be due to the variety of digital interventions examined, underlying differences between the populations chosen and methodological differences such as method of adherence measurement (McLean 2016; Normansell 2017). Additionally, this review included a large number of studies, which may have contributed to the high heterogeneity, but is likely to increase the generalisability of the results.
In terms of consistency of results across outcomes, we found improvements in asthma control and reductions in attacks, which are consistent in the same direction. We did also find reductions in unscheduled healthcare utilisation, however the confidence interval crossed the no effect line (RR 0.74, 95% CI 0.51 to 1.06). This is likely because of the limited number of studies that we could include in the meta‐analysis (446 participants, 4 studies). However, the direction of change was consistent with the other improvements in outcomes we saw in adherence, asthma control, exacerbations, and quality of life.
Imprecision, indirectness and publication bias
Meta‐analyses for secondary outcomes (except lung function and quality of life) had small participant populations (< 500 people in total). This led to wide confidence intervals and reduced certainty about the intervention effect on outcomes, as compared to larger pooled sample sizes. Indirectness was less of an issue due to the inclusion criteria. Publication bias was also not detected for any of the outcomes, however we note that this could not be assessed reliably for certain outcomes (e.g. exacerbations) due to the small number of studies that could be included in the meta‐analysis.
Potential biases in the review process
This review was conducted according to the methods described in the published protocol (Chan 2018), and recorded deviations from the protocol are detailed under Differences between protocol and review. We could not perform certain planned subgroup or sensitivity analyses for some outcomes because of a lack of detail, or a limited number of studies reporting on the outcome of interest. We note that published reports may not have included all the data that they collected, thus there is a possibility that the analysis could be incomplete. However, it is unlikely that eligible studies were missed by the searches conducted because multiple sources were used and were checked in duplicate. We limited our review by omitting studies that only presented data in an abstract. While some studies had results in their abstracts, we chose to omit them from our systematic review because without full‐text articles, we could not evaluate potential risks of bias. Similarly, we excluded non‐English and cross‐over studies. The language barrier and nature of cross‐over studies meant that it was difficult to elucidate the effect of digital interventions. However, there was only one study not in English and three studies that were cross‐over studies that we found during our extraction process. Consequently, the omission should not drastically influence our overall findings. Additionally, we were unable to adjust for design effects for the outcomes in the cluster‐RCTs as no suitable intracluster correlation coefficient (ICC) was found or reported. This could mean that the standard errors (SEs)/standard deviations (SDs) for those trials may be a little larger then stated; however, this would only affect the outcomes of two studies (Foster 2014; Kosse 2019), so is unlikely to affect the overall findings. The latest search was conducted in October 2021, but the data extraction and analysis relate to the search conducted on 1 June 2020. In the most recent search we found 23 Studies awaiting classification, which have not been included in the review. As such, the findings could potentially change if the most recent studies are included. This will be the subject of future updates of this review.
A key barrier to meta‐analysing the data from all studies was the large number of ways that studies measured the outcomes of interest. Whilst we used the standardised mean difference (SMD) to account for studies with different scales or measures for asthma control, the way that data were reported also varied, which prevented synthesis in a meta‐analysis for some outcomes. For example, studies reporting on asthma exacerbations or ED visits reported the data as either number of events or number of people presenting with the event of interest, but data were not available in both formats to allow synthesis. As the unit of analysis was different, we were not able to combine the studies and some studies had to be excluded from the analysis. Furthermore, another limitation is how we defined each outcome. For instance, we only included exacerbations if they had oral steroids, and we only included FEV1% predicted rather than absolute FEV1. These definitions may have limited the number of studies included and the findings.
Agreements and disagreements with other studies or reviews
Several systematic reviews have investigated the effect of digital adherence interventions for asthma maintenance therapy. A major strength of our review was the broadness of our research question, targeting a range of digital interventions in asthma. Within the included studies, there was a large age range of participants (2 to 98 years) in a variety of settings (included both primary and secondary settings) and conducted in different countries. Consequently, the range of studies included improved the generalisability of our findings. Furthermore, methods were either carried out as a group, or by a single member and corroborated for faults and improvements. This ensured accuracy and increased the certainty in our results. Finally, what differentiates our review from others is that we considered a variety of digital interventions in improving adherence and performed several subgroup analyses for adherence and asthma control to provide insight into how these digital interventions improved those respective outcomes.
In line with Bonini 2018 and Biblowitz 2018 our systematic review found that EMDs were the most effective intervention for improving adherence to maintenance therapy (Biblowitz 2018; Bonini 2018). Similar to Tran 2014, our systematic review also found positive effects with reminder systems. Text messages were the most commonly sighted reminder platform in both Tran 2014 and our current systematic review due to the low cost of implementation and simplicity in usability (Tran 2014). The literature on digital adherence interventions for paediatric populations is minimal, therefore our systematic review included a wide age range. Individual included studies also had wide sample ages and demonstrated positive intervention effects for younger populations, a finding similar to Ramsey 2020. However, as suggested by Tran 2014, subgroup analysis should be performed on different population groups to determine adherence patterns.
We found in our review that interventions with adherence feedback may achieve a larger effect on adherence and asthma control, similar to findings in Normansell 2017, which reported that interventions incorporating adherence feedback may be more effective. Our review findings suggest that there is no clear relationship between adherence and outcomes, as improvements in adherence were not consistently translatable into clinical benefits at an individual study level, similar to previous review findings (Normansell 2017). In terms of intervention impact on outcomes (regardless of adherence), the pooled analyses showed benefits on asthma control, exacerbations, lung function, and quality of life, unlike the findings from Normansell 2017. However, the evidence for all of these outcomes was low certainty, except for asthma control. These findings support other existing literature that demonstrates adherence and potentially other asthma outcomes can improve with the use of digital interventions in asthma patients (Biblowitz 2018; Bonini 2018; Jeminiwa 2019; Normansell 2017; Tran 2014).
Previous research had mixed results for the effect of digital interventions on asthma control. Two reviews have found an improvement of asthma control (McLean 2016; Mosnaim 2016), which contrasts with evidence from other studies where no difference was found (Morrison 2014; Normansell 2017). In our review, digital interventions had a positive impact on asthma control. An improvement was likely due to the inclusion of 16 studies with a range of maintenance therapies, whereas Normansell 2017 included only seven studies focusing on inhaled corticosteroid (ICS) use. Furthermore, our review only included studies with validated instruments measuring asthma control while Morrison 2014 included unvalidated scales. The range of studies, breadth of maintenance therapies, and objective methods in evaluating asthma control confers confidence and applicability of our findings to the general population. Consequently, it is likely that in practice digital interventions can improve asthma control.
As adherence and asthma control improved, the risk of exacerbations also decreased with the use of digital interventions. This is because poor medication adherence can increase risk of asthma exacerbations (Williams 2011). However, other reviews showed that exacerbation rates did not decrease (Morrison 2014); or showed conflicting results (Dima 2015). The varying results may be explained by the variety of ‘exacerbation’ definitions across reviews. Morrison 2014 and Dima 2015 did not clearly state their definitions of exacerbations, while McLean 2016 only included one study reporting on exacerbations, skewing its conclusion to that study’s findings. As we meta‐analysed six studies under the same definition of ‘exacerbations’, we are confident in our interpretation that digital interventions can reduce the risk of exacerbations in asthma patients taking maintenance therapies.
Our review found that digital interventions could improve the quality of life for asthma patients. All but one study, Kim 2016, included in the quality of life meta‐analysis also measured asthma control. As asthma control improved, patients achieved day‐to‐day symptom control and hence their quality of life also improved. Recent systematic reviews also similarly noted how digital interventions improve quality of life (Morrison 2014). However, what distinguishes our review from other reviews is that we performed meta‐analysis. This helped us to assess heterogeneity between the different studies and allows us to reach a more conclusive synthesis on the outcome.
The effect on unscheduled healthcare utilisation, number of days absent from school/work, and improvement in lung function were smaller, largely due to the small number of studies available and short study durations. Additionally, the variability in how studies reported unscheduled healthcare utilisation also prevented us from observing sizeable effects. For example, they included hospital visits, urgent care, or ED visits, making it difficult to interpret the effect of the outcome. Another possible explanation for no difference in ED visits is due to the rarity of uncontrolled asthma requiring such a visit. Thus, it can be difficult to use as an outcome measure. Subjective methods may have under‐reported the actual events if days were not recorded by parents or forgotten. For example, days off school/work were based on subjective reports such as Chan 2015 using parental reports and Wiecha 2015 using a validated questionnaire. Finally, lung function may not be solely dependent on medication adherence. Even though the studies were randomised, there may potentially be lifestyle (e.g. smoking) and living environment factors or differences in patients’ individual lung anatomy that could influence lung function (Barroso 2018), which may not have been controlled for in the studies. Ultimately, the limited number of studies prevented the review from concluding with certainty a benefit for these outcomes.
What separates us the most from other systematic reviews is that our review found that digital adherence interventions improved asthma control. A previous Cochrane systematic review on adherence interventions to improve ICS use was unable to conclude that there were significant improvements in asthma control (Normansell 2017). In comparison to our review, Normansell 2017 included a handful of studies and assessed asthma control using mean differences by questionnaire type (e.g. Asthma Control Questionnaire (ACQ) or asthma control test (ACT)). In our review, to pool data across studies using different validated questionnaires, we used the SMD and found an improvement in asthma control. By streamlining our methods and using the SMD to allow synthesis of data across a larger number of studies, this conferred greater certainty in our evidence. Biblowitz 2018 concluded that mobile applications improved asthma control. Alquran 2018 and Unni 2018 further added asthma self‐management applications as having the greatest benefit on asthma control. Contrary to the three systematic reviews, our review found no improvements in asthma control with web or smartphone applications (Alquran 2018; Biblowitz 2018; Unni 2018). A potential reason for this could be the fact that we could only meta‐analyse 15 of the 31 studies that reported on asthma control because it was difficult to group or compare the others due to the various measures of asthma control. Asthma control is important, as self‐perceived improvements in disease management can facilitate further intervention use and increase adherence to maintenance therapy as per the benefit‐risk theory.
The final key difference in our review compared to others is the generally low heterogeneity amongst studies. A consensus exists within the literature highlighting the issue of high heterogeneity amongst the included studies (Normansell 2017). The differences between studies are largely due to variations in the interventions used and the measures of adherence. Moderate to high heterogeneity prevents organic comparisons between studies and limits our confidence in conclusions on the efficacy of various platforms. However, our review has ensured that each outcome has the same scales (i.e. reported or measured their outcomes similarly), in order for it to be meta‐analysed. Consequently, heterogeneity was reduced amongst almost all outcomes. This confers greater certainty of our evidence as we can extrapolate and apply our information to the general population. However, our review also aimed to provide clarity on the differences between digital interventions by performing various subgroup analyses for adherence, asthma control, and exacerbations. The novelty of the current review lies in its inclusion of a variety of digital interventions rather than focusing on one particular type of intervention for improving adherence. We also performed several subgroup analyses to improve understanding of the applications of digital technology in asthma care. However, the limited number of studies restricted our ability to confidently comment and highlight the components of digital interventions required for success.
Authors' conclusions
Implications for practice.
Our review examined in‐depth the effectiveness of digital interventions and found positive effects on asthma medication adherence and asthma control, asthma exacerbations, and quality of life. For the effect on medication adherence, the review found low‐certainty evidence that digital interventions may increase adherence by 15% compared to controls, but there was uncertainty due to risk of bias and imprecision. For the effect on asthma outcomes, our review found moderate‐certainty evidence that participants using digital adherence interventions likely had increased asthma control that is clinically significant, based on a magnitude of increase of a standardised mean difference (SMD) of 0.31, and likely had an increase in quality of life, but had only little or no improvement in lung function, with the certainty of evidence downgraded mainly due to risk of performance and detection bias in the included studies. The review found low‐certainty evidence that digital interventions may reduce asthma exacerbations by 50% compared to controls — a reduction that is much larger than what has previously been reported with other asthma management interventions, although our certainty in this evidence was low due to risk of bias and the small number of studies included. Similarly, there was low‐certainty evidence that digital interventions may slightly reduce unscheduled healthcare utilisation by 25%, although there was evidence that the interventions could possibly worsen unscheduled healthcare utilisation too, with considerable uncertainty arising from risk of bias and imprecision of the results.
Together, these findings can have important implications for everyday practice and policy, particularly during pandemic times or when healthcare resources are stretched, as novel methods of healthcare delivery in remote and contact‐less ways become increasingly necessary. In practice, there is a great emphasis on improving adherence to maintenance therapy, with asthma guidelines recently recommending using a combined long‐acting beta‐agonist (LABA)/inhaled corticosteroid (ICS) inhaler as both preventer and reliever medication (Asthma Foundation NZ; GINA 2019). Digital interventions hold promise in aiding adherence and potentially improving asthma outcomes, however implementation and long‐term sustainability need to be considered.
Whilst our findings suggest possible effectiveness, our review did not find any studies that reported on affordability/cost‐effectiveness or on how interventions may impact equitable access to asthma treatment and management. Our review suggests that short messaging system (SMS) text‐messaging and electronic monitoring devices (EMDs) show the most promise for improving adherence, with the customisability of these technologies and ability to adapt functionalities to individual patients. However, a key limitation of EMDs is their cost, which could impact usage in routine practice (Chan 2013). In comparison, mobile phones are widely prevalent in modern society, with approximately five billion mobile phone users worldwide (Huang 2019). Consequently, it is likely that SMS‐based interventions are more affordable and easily accessible to the general population, including remote and rural populations, as mobile phones are ubiquitous across low‐, middle‐, and high‐income countries (Bastawrous 2013). However, our review is limited by studies that largely assume that these digital interventions are easily available to patients. This is evident as certain studies required participants to independently own a mobile phone (Kenyon 2019; Petrie 2012; Vasbinder 2016). How practical it is to use SMS‐based and other digital adherence interventions with lower socio‐economic groups, who may have poorer asthma control, remains largely undetermined. Of note, none of the included studies were conducted in rural or low socio‐economic populations where access to digital technologies may be difficult. Future studies need to be conducted in high‐risk vulnerable groups and focus on sharing acceptability and economic data to understand its implications on accessibility for patients.
Our review also concluded that fully digitalised interventions (i.e. that do not have an in‐person component) can be effective for improving adherence and some asthma outcomes. Fully digitalised interventions may reduce access barriers such as lack of transportation, inability to get the time off work, or medical consultation costs (Ramsey 2020), and financial and human resource required for asthma management. However, in the short term there may be a financial burden on healthcare systems when shifting to a technology‐based healthcare system due to the required expansion of information technology infrastructure to support digital innovations. Maintenance costs and costs associated with technology disruptions can place a substantial financial burden on health organisations (Blakey 2018). Future studies need to examine the practicability and cost‐effectiveness of integrating digital interventions into the healthcare system, particularly as increasingly national and international asthma guidelines recommend using web‐based systems for self‐management to monitor asthma control (Asthma Foundation NZ).
In terms of acceptability, our findings showed that overall intervention acceptability was rated favourably by participants, though of the included studies, acceptability was only measured in 11 studies, which could limit the generalisability of the findings. Additionally, as satisfaction was assessed primarily by surveys, there could be potential for a positive bias in the reporting. Further research into the views and attitudes of both patient and healthcare providers in terms of acceptability and ease of use of digital interventions in everyday asthma management is needed. Future policy development should focus on the place of digital interventions as part of overall asthma patient care, particularly as data from other studies suggest that data privacy and regulation could affect patient acceptability of digital interventions (Biblowitz 2018; Blakey 2018).
Implications for research.
Future research should focus on the acceptability of digital interventions. The high attrition bias reflects that patient engagement and sustained use is questionable for digital interventions and needs to be further explored. While patient acceptability is an important consideration, there were a limited number of studies included in the review that examined this as an outcome. Future studies should explore the acceptability of digital interventions in a clinical setting amongst various population groups. Acceptability is important to understand for the long‐term feasibility of interventions and the components required to ensure ease of implementation into routine asthma care.
Future research needs to be geared towards understanding the specific components of digital interventions that make them successful in improving adherence. The certainty of evidence was reduced by the heterogeneity in how studies measured and reported adherence. Consequently, in future studies, it is best to streamline how all outcomes are measured, particularly ensuring that validated tools are used for all outcomes. Finally, aside from the type of digital intervention and the effect of an in‐person component on the effectiveness of the intervention, other subgroup analyses either could not be conducted or had statistically insignificant results. This suggests that further research is needed to clearly identify the components of effective digital adherence interventions, and highlights also the importance of clear reporting of any intervention tested in research trials, to allow researchers to identify and classify the most effective aspects of digital adherence interventions.
Additionally, the long‐term feasibility of these findings cannot be confirmed due to the short trial lengths of these randomised controlled trials (RCTs). Future trials will should be designed to be of at least six months' duration, ideally with longer‐term follow‐up to see if intervention effects are sustained beyond the study period. We aimed to make this review an all‐inclusive review by including all types of asthma maintenance medication (not only ICS), but there were limited interventions designed to improve adherence to non‐ICS medication such as oral or biologic therapies. Future studies would benefit from exploring digital interventions designed to support adherence to other types of asthma maintenance medication. Despite this lack of evidence, the observed benefits from digital interventions suggest that there is opportunity for future development of technologies to further improve both adherence and asthma‐related outcomes.
What's new
| Date | Event | Description |
|---|---|---|
| 24 June 2022 | Amended | Author byline and sources of support amended. |
| 22 June 2022 | Amended | Author by‐line amended. |
History
Protocol first published: Issue 5, 2018 Review first published: Issue 6, 2022
Acknowledgements
The Background and Methods sections of this review are based on a standard template used by Cochrane Airways. The first Background section of this protocol has been based on the published Cochrane Review “Interventions to improve adherence to inhaled steroids for asthma” (Normansell 2017).
The electronic searches were undertaken and updated by the Cochrane Airways Information Specialist, Elizabeth Stovold.
The authors of this work are affiliated with the Asthma UK Centre of Applied Research (AUKCAR), Queen Mary University of London, and University College London. This work is carried out with the support of the Asthma UK Centre for Applied Research [AUK‐AC‐2012‐01 and AUK‐AC‐2018‐01].
The authors and Cochrane Airways’ Editorial Team are grateful to the following peer and consumer reviewers for their time and comments: Sandra Moroz (Canada), Ahmadreza Abedi (Iran), and Tjalling W de Vries (the Netherlands).
This project was supported by the National Institute for Health and Care Research, via Cochrane Infrastructure funding to Cochrane Airways. The views and opinions expressed therein are those of the review authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS, or the Department of Health and Social Care.
Appendices
Appendix 1. Sources and search methods for the Cochrane Airways Trials Register
Electronic searches: core databases
| Database | Dates searched | Frequency of search |
| CENTRAL (via the Cochrane Register of Studies (CRS)) | From inception | Monthly |
| MEDLINE (Ovid) | 1946 onwards | Weekly |
| EMBASE (Ovid) | 1974 onwards | Weekly |
| PsycINFO (Ovid) | 1967 onwards | Monthly |
| CINAHL (EBSCO) | 1937 onwards | Monthly |
| AMED (EBSCO) | From inception | Monthly |
Handsearches: core respiratory conference abstracts
| Conference | Years searched |
| American Academy of Allergy, Asthma and Immunology (AAAAI) | 2001 onwards |
| American Thoracic Society (ATS) | 2001 onwards |
| Asia Pacific Society of Respirology (APSR) | 2004 onwards |
| British Thoracic Society Winter Meeting (BTS) | 2000 onwards |
| Chest Meeting | 2003 onwards |
| European Respiratory Society (ERS) | 1992, 1994, 2000 onwards |
| International Primary Care Respiratory Group Congress (IPCRG) | 2002 onwards |
| Thoracic Society of Australia and New Zealand (TSANZ) | 1999 onwards |
Asthma search
1. exp Asthma/
2. asthma$.mp.
3. (antiasthma$ or anti‐asthma$).mp.
4. Respiratory Sounds/
5. wheez$.mp.
6. Bronchial Spasm/
7. bronchospas$.mp.
8. (bronch$ adj3 spasm$).mp.
9. bronchoconstrict$.mp.
10. exp Bronchoconstriction/
11. (bronch$ adj3 constrict$).mp.
12. Bronchial Hyperreactivity/
13. Respiratory Hypersensitivity/
14. ((bronchial$ or respiratory or airway$ or lung$) adj3 (hypersensitiv$ or hyperreactiv$ or allerg$ or insufficiency)).mp.
15. ((dust or mite$) adj3 (allerg$ or hypersensitiv$)).mp.
16. or/1‐15
Filter to identify RCTs
1. exp "clinical trial [publication type]"/
2. (randomised or randomised).ab,ti.
3. placebo.ab,ti.
4. dt.fs.
5. randomly.ab,ti.
6. trial.ab,ti.
7. groups.ab,ti.
8. or/1‐7
9. Animals/
10. Humans/
11. 9 not (9 and 10)
12. 8 not 11
The MEDLINE strategy and RCT filter are adapted to identify studies in other electronic databases.
Appendix 2. Search strategy to identify relevant studies from the Cochrane Airways Trials Register
| #1 | AST:MISC1 |
| #2 | MeSH DESCRIPTOR Asthma Explode All |
| #3 | asthma*:ti,ab |
| #4 | #1 or #2 or #3 |
| #5 | MESH DESCRIPTOR Web Browser |
| #6 | MESH DESCRIPTOR Patient Portals |
| #7 | MESH DESCRIPTOR Online Systems EXPLODE ALL |
| #8 | MESH DESCRIPTOR Internet EXPLODE ALL |
| #9 | (online* OR web* OR browser OR portal OR internet* OR virtual*):ti,ab,kw |
| #10 | MESH DESCRIPTOR Cell Phones EXPLODE ALL |
| #11 | MESH DESCRIPTOR MP3‐Player |
| #12 | MESH DESCRIPTOR Computer Systems EXPLODE ALL |
| #13 | MESH DESCRIPTOR Mobile Applications |
| #14 | ((cell* or mobile*) near3 phone*):ti,ab,kw |
| #15 | (handheld* or hand‐held*):ti,ab,kw |
| #16 | (smartphone* or smart‐phone*):ti,ab,kw |
| #17 | (personal* near3 digital*):ti,ab,kw |
| #18 | (PDA OR "Palm OS" or "Palm Pre classic" OR blackberry OR nokia OR symbian OR INQ OR HTC OR sidekick OR android* OR iphone* OR ipod* OR ipad* OR samsung OR Huawei OR sony OR LG OR pixel OR (windows* near3 (mobile* or phone*)) OR (tablet near3 (device* or comput*))):ti,ab,kw |
| #19 | (app* near3 (smartphone* or smart‐phone or mobile* or phone* or tablet* or computer*)):ti,ab,kw |
| #20 | MESH DESCRIPTOR Text Messaging |
| #21 | (sms OR mms):ti,ab,kw |
| #22 | ((text* OR short*) NEAR3 messag*):ti,ab,kw |
| #23 | texting:ti,ab,kw |
| #24 | MESH DESCRIPTOR Reminder Systems EXPLODE ALL |
| #25 | ((electronic* OR medication*) NEAR3 (reminder* OR monitor* or record* OR system* OR device*)):ti,ab,kw |
| #26 | (reminder NEAR3 (text* or system* or messag*)):ti,ab,kw |
| #27 | alert*:ti,ab,kw |
| #28 | wearable*:ti,ab,kw |
| #29 | MESH DESCRIPTOR Speech Recognition Software EXPLODE ALL |
| #30 | ((interact* OR speech* OR voice* or touchtone) NEAR3 (recogni* OR respon*)):ti,ab,kw |
| #31 | IVR:ti,ab,kw |
| #32 | automat* NEAR3 (phone* or telephone* or call* OR system*):ti,ab,kw |
| #33 | MESH DESCRIPTOR Communications Media EXPLODE ALL |
| #34 | ("social media" OR Facebook OR Twitter OR Instagram OR Snapchat OR YouTube OR WhatsApp):ti,ab,kw |
| #35 | (video* OR television OR radio OR media* OR multimedia OR multi‐media OR audio* OR webinar* OR podcast* OR wiki* OR interactive OR digital* OR tech*) :ti,ab.kw |
| #36 | MESH DESCRIPTOR Telemedicine EXPLODE ALL |
| #37 | MESH DESCRIPTOR Telenursing EXPLODE ALL |
| #38 | (telehealth* or tele‐health* or telecare* or tele‐care*):ti,ab,kw |
| #39 | (mhealth or m‐health or "m health" or "mobile health"):ti,ab,kw |
| #40 | (e‐health or ehealth or "e health"):ti,ab,kw |
| #41 | #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21 OR #22 OR #23 OR #24 OR #25 OR #26 OR #27 OR #28 OR #29 OR #30 OR #31 OR #32 OR #33 OR #34 OR #35 OR #36 OR #37 OR #38 OR #39 OR #40 |
| #42 | #41 AND #4 |
| #43 | INREGISTER |
| #44 | #43 AND #42 |
Data and analyses
Comparison 1. Digital intervention versus usual care.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Adherence | 16 | 8885 | Mean Difference (IV, Random, 95% CI) | 14.66 [7.74, 21.57] |
| 1.2 Adherence ‐ multiple interventions | 16 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.2.1 One digital intervention | 13 | 8566 | Mean Difference (IV, Random, 95% CI) | 15.39 [7.40, 23.39] |
| 1.2.2 Multiple digital interventions | 3 | 319 | Mean Difference (IV, Random, 95% CI) | 11.81 [‐1.25, 24.86] |
| 1.3 Adherence ‐ types of digital | 16 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.3.1 Electronic monitoring devices | 7 | 932 | Mean Difference (IV, Random, 95% CI) | 22.50 [10.84, 34.16] |
| 1.3.2 SMS texts | 4 | 391 | Mean Difference (IV, Random, 95% CI) | 12.12 [6.22, 18.03] |
| 1.3.3 Website/web applications | 2 | 368 | Mean Difference (IV, Random, 95% CI) | ‐2.68 [‐10.07, 4.71] |
| 1.3.4 IVR/speech‐based | 4 | 7403 | Mean Difference (IV, Random, 95% CI) | 7.60 [1.23, 13.97] |
| 1.4 Adherence ‐ feedback | 16 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.4.1 Yes | 6 | 842 | Mean Difference (IV, Random, 95% CI) | 22.60 [8.93, 36.26] |
| 1.4.2 No | 10 | 8043 | Mean Difference (IV, Random, 95% CI) | 9.05 [3.69, 14.41] |
| 1.5 Adherence ‐ in‐person | 16 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.5.1 In‐person component | 3 | 337 | Mean Difference (IV, Random, 95% CI) | 14.51 [6.11, 22.90] |
| 1.5.2 Fully digital | 13 | 8548 | Mean Difference (IV, Random, 95% CI) | 13.58 [3.22, 23.95] |
| 1.6 Adherence ‐ age | 16 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.6.1 Adults and adolescents | 10 | 7396 | Mean Difference (IV, Random, 95% CI) | 11.04 [1.09, 20.99] |
| 1.6.2 Children | 6 | 1489 | Mean Difference (IV, Random, 95% CI) | 18.06 [3.89, 32.23] |
| 1.7 Asthma control ‐ change from baseline | 15 | 1638 | Std. Mean Difference (IV, Random, 95% CI) | 0.31 [0.17, 0.44] |
| 1.8 Asthma control ‐ multiple interventions | 15 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.8.1 One | 12 | 1472 | Std. Mean Difference (IV, Random, 95% CI) | 0.29 [0.14, 0.45] |
| 1.8.2 Multiple | 3 | 166 | Std. Mean Difference (IV, Random, 95% CI) | 0.38 [0.07, 0.69] |
| 1.9 Asthma control ‐ types of digital interventions | 15 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.9.1 Electronic monitoring devices | 3 | 387 | Std. Mean Difference (IV, Random, 95% CI) | 0.20 [0.00, 0.41] |
| 1.9.2 SMS texts | 3 | 115 | Std. Mean Difference (IV, Random, 95% CI) | 0.59 [0.20, 0.97] |
| 1.9.3 Website/web or phone applications | 8 | 1112 | Std. Mean Difference (IV, Random, 95% CI) | 0.34 [0.14, 0.55] |
| 1.9.4 IVR/speech‐based | 2 | 73 | Std. Mean Difference (IV, Random, 95% CI) | 0.10 [‐0.36, 0.56] |
| 1.10 Asthma control ‐ adherence feedback | 15 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.10.1 Yes | 7 | 909 | Std. Mean Difference (IV, Random, 95% CI) | 0.34 [0.11, 0.58] |
| 1.10.2 No | 8 | 729 | Std. Mean Difference (IV, Random, 95% CI) | 0.26 [0.11, 0.41] |
| 1.11 Asthma control ‐ in‐person component | 15 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.11.1 In‐person component | 5 | 536 | Std. Mean Difference (IV, Random, 95% CI) | 0.47 [0.13, 0.81] |
| 1.11.2 Fully digital | 10 | 1102 | Std. Mean Difference (IV, Random, 95% CI) | 0.22 [0.10, 0.34] |
| 1.12 Asthma control ‐ age | 15 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.12.1 Adults and adolescents | 12 | 1165 | Std. Mean Difference (IV, Random, 95% CI) | 0.36 [0.18, 0.53] |
| 1.12.2 Children | 3 | 473 | Std. Mean Difference (IV, Random, 95% CI) | 0.19 [0.01, 0.37] |
| 1.13 Asthma exacerbations ‐ number of people with one or more exacerbations | 6 | 678 | Risk Ratio (M‐H, Random, 95% CI) | 0.53 [0.32, 0.91] |
| 1.14 Unscheduled healthcare utilisation ‐ number of people with one or more visits to a healthcare provider/attendance at an emergency department or urgent care centre/hospital admission | 4 | 446 | Risk Ratio (M‐H, Random, 95% CI) | 0.74 [0.51, 1.06] |
| 1.15 Lung function ‐ FEV1% predicted (change from baseline) | 7 | 1052 | Mean Difference (IV, Random, 95% CI) | 3.58 [1.00, 6.17] |
| 1.16 Quality of life ‐ change from baseline | 10 | 848 | Std. Mean Difference (IV, Random, 95% CI) | 0.26 [0.07, 0.45] |
Comparison 2. Digital intervention versus usual care ‐ sensitivity analyses.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 Adherence ‐ objective measures | 14 | 8730 | Mean Difference (IV, Random, 95% CI) | 14.71 [7.28, 22.13] |
| 2.2 Asthma control ‐ change from baseline ‐ objective measures | 12 | 1153 | Std. Mean Difference (IV, Random, 95% CI) | 0.25 [0.14, 0.37] |
| 2.3 Asthma exacerbations ‐ number of people with one or more exacerbations ‐ objective measures | 3 | 354 | Risk Ratio (M‐H, Random, 95% CI) | 0.35 [0.20, 0.59] |
| 2.4 Adherence ‐ fixed‐effect | 15 | 8854 | Mean Difference (IV, Fixed, 95% CI) | 6.98 [5.76, 8.21] |
| 2.5 Asthma control ‐ change from baseline ‐ fixed‐effect | 15 | 1638 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.30 [0.20, 0.40] |
| 2.6 Asthma exacerbations ‐ number of people with one or more exacerbations ‐ fixed‐effect | 6 | 678 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.51 [0.35, 0.75] |
| 2.7 Adherence ‐ no commercial funding | 9 | 8149 | Mean Difference (IV, Random, 95% CI) | 14.34 [3.60, 25.08] |
| 2.8 Asthma control ‐ change from baseline ‐ no commercial funding | 11 | 1483 | Std. Mean Difference (IV, Random, 95% CI) | 0.28 [0.13, 0.43] |
| 2.9 Asthma exacerbations ‐ number of people with one or more exacerbations ‐ no commercial funding | 6 | 678 | Risk Ratio (M‐H, Random, 95% CI) | 0.53 [0.32, 0.91] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Bender 2010.
| Study characteristics | ||
| Methods |
Design: individually randomised, double‐blind controlled trial Duration: endpoint at 10 weeks Setting: recruited from 1 tertiary care centre; trial carried out in the United States |
|
| Participants |
Population: 50 participants were randomised to receive an IVR telephone call (n = 25) or usual care (n = 25) Age: range from: 18 to 65 years old. Mean age in IVR group was 39.6 years; SD = 13. Mean age in usual care group was 43.5 years; SD = 14. Proportion of male participants: IVR group were 40% male; usual care group were 32% male Proportion of white ethnic participants: IVR group were 56% white; usual care were 60% white Inclusion criteria: daily prescribed corticosteroids, asthma diagnosis Exclusion criteria: any significant disease or disorder that, in the opinion of the investigator, might influence the results of the study or the patient’s ability to participate in the study (this included other chronic health disorders, current substance abuse or dependence, mental retardation, or psychiatric disorder); and current participation in any other asthma‐related research or clinical trial Percentage withdrawn: none withdrew Allowed medication: none recorded Disallowed medication: none recorded |
|
| Interventions |
IVR group: participants received IVR call from Denver Interactive Asthma Learning system program. Most groups had 2 calls; third call given 2 weeks later if symptoms persisted but refill needed. There was two‐way interaction with patient. No adherence feedback. No co‐interventions used (one digital component used). Not a theory‐based intervention. No in‐person component. Usual care group: usual care; no call |
|
| Outcomes |
Primary: Adherence to maintenance medication Secondary: Asthma quality of life Asthma control test Beliefs about medication questionnaire |
|
| Notes |
Type of publication: peer‐reviewed Funding: AstraZeneca COI: none |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation table determined group assignment |
| Allocation concealment (selection bias) | Low risk | IVR system ensured allocation concealment and computer‐generated randomisation |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants were unblinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Investigators remained blind to allocation until final data set |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing data + power calculation determined group size |
| Selective reporting (reporting bias) | Low risk | All data presented as per methods; no study protocol |
Bender 2015.
| Study characteristics | ||
| Methods |
Design: parallel, individually randomised controlled trial Duration: endpoint at 104 weeks Setting: recruited from Kaiser Permanente Colorado ‐ 18 primary care and 2 special care and 2 hospitals; trial carried out in the United States |
|
| Participants |
Population: 1187 children were randomised to receive speech recognition reminders for overdue medication (n = 590) or usual care (n = 597) Age: range from 3 to 12 years. Mean age in reminders group was 8.2 years; SD: 0.13. Mean age in usual care group was 8.1 years; SD: 0.13. Proportion of male participants: reminders group was 67.3% male; usual care group was 67.1% male Proportion of white ethnic participants: reminders group was 43.3% white; usual care group was 44.1% white Inclusion criteria: persistent asthma; 1 or more ICS script in last 6 months; enrolled in Kaiser Permanente Colorado for 1 year prior Exclusion criteria: life‐threatening co‐morbidity; sibling enrolled in study; parent decline; ICS prescribed to betaken intermittently or as required; medication not bought at KPCO pharmacy Percentage withdrawn: Withdrawal from reminders group was 23.39%; withdrawal from usual care group was 25.13% Allowed medication: none recorded Disallowed medication: none recorded |
|
| Interventions |
Reminders group: speech recognition reminders for overdue medication group with 1 to 3 calls depending on whether patient has overdue medication or historically has. Two‐way interaction with patient. No adherence feedback. No co‐interventions used (one digital component involved only). Not a theory‐based intervention. No in‐person component. Usual care group: usual care |
|
| Outcomes |
Primary: Adherence (ICS proportion of days covered over 24 months) Secondary: Beta‐agonist use, oral steroid use, asthma‐related primary health care visits, ED visits, hospitalisations, after‐hours visits on weekends or weekdays, asthma related visits, parent satisfaction questionnaire |
|
| Notes |
Type of publication: peer‐reviewed Funding: not stated COI: none |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | States that it was randomised but no information about process |
| Allocation concealment (selection bias) | Unclear risk | Unclear what method of concealment was used |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | No blinding described but unlikely to have influenced outcome |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | No blinding described but unlikely to impact the outcome |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Intention‐to‐treat analysis performed |
| Selective reporting (reporting bias) | Low risk | Study protocol available; results specified as per methods |
Black 2008.
| Study characteristics | ||
| Methods |
Design: randomised controlled study Duration: 2 months Setting: trial carried out in New Zealand |
|
| Participants |
Population: 40 children with asthma Age: 7 to 17 years with symptomatic asthma despite ICS treatment Inclusion criteria: symptomatic asthma despite ICS treatment Exclusion criteria: not stated Percentage withdrawn: not recorded Allowed medication: none recorded Disallowed medication: none recorded |
|
| Interventions |
Intervention: inhaler alarm intervention (n = 20) Control: usual care (n = 20) |
|
| Outcomes | AQLQ, pre‐bronchodilator FEV1, use of salbutamol, adherence to inhaled steroid | |
| Notes | Type of publication: abstract only | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details |
| Allocation concealment (selection bias) | Unclear risk | No details |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding of participants or personnel |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinding of outcome assessor described; adherence measured by device but assessor may be aware of group allocation |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No data on attrition |
| Selective reporting (reporting bias) | Unclear risk | Conference abstract; no trial registration identified. Study reported only as a conference abstract from 2008. Therefore, limited details about methods and outcomes, in particular, no measure of variance for the AQLQ. |
Chan 2015.
| Study characteristics | ||
| Methods |
Design: parallel, double‐blinded, block‐randomised controlled trial Duration: endpoint at 26 weeks Setting: recruited from ED in Auckland; trial carried out in New Zealand Details of run‐in period: 4 weeks |
|
| Participants |
Population: 220 participants were randomised to receive electronic monitoring device (EMD) with audiovisual reminder (n = 110) or EMD without audiovisual reminder (n = 110) Age: range from 6 to 15 years old. Mean age in EMD with audiovisual reminder group was 8.9 years; SD: 2.5. Mean age in was EMD group without audiovisual reminder was 8.9 years; SD: 2.6. Proportion of male participants: EMD with audiovisual reminder group was 50% male; EMD without audiovisual reminder group was 53% male Proportion of white ethnic participants: EMD with audiovisual reminder was 38% white; EMD without audiovisual reminder group was 53% white Asthma severity: severe Inclusion criteria: acute asthma; twice‐daily ICS Exclusion criteria: chronic lung disease; congenital heart disease; lived outside of Auckland; severe chronic medical disorder Percentage withdrawn: Withdrawal from EMD with audiovisual reminder group was 1.82%; withdrawal from EMD without audiovisual reminder group was 4.55% Allowed medication: changed to fluticasone preventer inhaler and albuterol reliever Disallowed medication: none recorded |
|
| Interventions |
EMD with audiovisual reminder group: electronic device with 3 intervention sessions and only one‐way interaction with patient (reminders only). No adherence feedback. No co‐interventions (one digital component involved). Not a theory‐based intervention. No in‐person component. EMD without audiovisual reminder group: electronic device with 3 intervention sessions and no interaction with patient. No adherence feedback. No co‐interventions. |
|
| Outcomes |
Primary: Adherence (proportion of preventer doses relative to the number prescribed) Absence from school Secondary: Childhood ACT and asthma morbidity score, lung function, ED attendance, carer absence, asthma exacerbations, SABA use |
|
| Notes |
Type of publication: peer‐reviewed Funding: Health Research Council of New Zealand and Cure Kids COI: EAM Cure Kids grant received |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Block randomisation used |
| Allocation concealment (selection bias) | Low risk | Sealed, opaque envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Study personnel blinded; unknown to participants monitoring was occurring |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding occurred but unclear if aware of allocation when assessing outcome |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing data and analysis with intention‐to‐treat applied |
| Selective reporting (reporting bias) | Low risk | Results specified as per methods |
Charles 2007.
| Study characteristics | ||
| Methods |
Design: parallel, single‐blinded, individually randomised controlled trial Duration: endpoint at 26 weeks Setting: recruited from clinical trial facility in Wellington; trial carried out in New Zealand Details of run‐in period: 2 weeks |
|
| Participants |
Population: 110 participants were randomised to receive audiovisual reminder function (AVRF) with Smartinhaler group (n = 55) or Smartinhaler group (n = 55) Age: range from: 13 to 65 years. Mean age in reminder + Smartinhaler group was 39 years. Mean age in Smartinhaler group was 35 years. Proportion of male participants: reminder + Smartinhaler group was 50.90% male; Smartinhaler group was 40% male Inclusion criteria: regular ICS at fixed dose; no exacerbations in previous 1/12 or in run‐in period; not pregnant/lactating; using contraception Exclusion criteria: COPD, LABA use or history of clinically significant disease Percentage withdrawn: Withdrawal from AVRF + Smartinhaler group was 20%; withdrawal from Smartinhaler group was 16.36% Allowed medication: SABA Disallowed medication: LABA |
|
| Interventions |
Audiovisual reminder function + Smartinhaler group: electronic device which allows for covert adherence monitoring. Alarm sounding twice daily for 60 minutes until dose taken. 5 intervention sessions. One‐way interaction with patient with reminders only given. No adherence feedback. No co‐interventions (one digital component involved). Not a theory‐based intervention. No in‐person component. SmartInhaler group: covert adherence monitoring |
|
| Outcomes |
Primary: Adherence; proportion of medication taken as prescribed over the latter half of the trial Secondary: ACQ, PEF, questionnaire on adherence |
|
| Notes |
Type of publication: peer‐reviewed Funding: GlaxoSmithKline research fund COI: Beasley served as a medical advisor for Nexus6 Ltd |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random code |
| Allocation concealment (selection bias) | Low risk | Sealed envelope |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants were blinded to adherence measurement, not blinded to intervention |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinded to allocation but unclear if knew group after allocation |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Lower sample size than power calculation, as‐treated analysis |
| Selective reporting (reporting bias) | Low risk | All data presented as per methods; no study protocol |
Choi 2017.
| Study characteristics | ||
| Methods |
Design: pilot randomised controlled trial Duration: 1 year follow‐up Setting: asthma patients who visited outpatient clinics located within a university hospital during the time period of 1 July 2015 to 30 June 2016 |
|
| Participants |
Population: 290 in the intervention group vs 303 in the control group Age: not stated |
|
| Interventions |
Intervention: pharmacist education and teaching, and regular text messages to encourage medication taking Control: usual care |
|
| Outcomes | Patients' pulmonary functions; asthma control scores; medication adherence rates; quality of life; healthcare utilisation; lung function | |
| Notes | Type of publication: abstract only | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants do not appear blinded; likely aware of the group allocation |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinding of outcome assessors; likely aware of group allocation and adherence assessed by self‐report and medical records |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not reported |
| Selective reporting (reporting bias) | High risk | Details not reported for non‐significant outcomes |
Clerisme‐Beaty 2011.
| Study characteristics | ||
| Methods |
Design: parallel randomised controlled trial Duration: endpoint at 4 weeks Setting: recruited from 19 centres participating in American Lung Association Asthma Clinical Research Centres; trial carried out in United States Details of run‐in period: 2 weeks |
|
| Participants |
Population: 99 participants were randomised to receive montelukast enhanced (n = 25 number), placebo enhanced group (n = 26), montelukast neutral (n = 25), placebo neutral (n = 23) Age: participants were at least 15 years old. Mean age in montelukast enhanced was 33.2 years; SD = 2.8. Mean age in placebo enhanced group was 33.3 years; SD = 2.9. Mean age in montelukast neutral was 33.1 years; SD = 2.8. Mean age in placebo neutral group was 39.6 years; SD = 3.2. Proportion of male participants: In montelukast enhanced group 16% were male. In placebo enhanced group 47% were male. In montelukast neutral 24% were male. In placebo neutral group 26% were male. Proportion of white ethnic participants: In montelukast enhanced group 52% were white. In placebo enhanced group 50% were white. In montelukast neutral 80% were white. In placebo neutral group 70% were white. Asthma severity: moderate Baseline lung function (FEV1) Montelukast enhanced group: 81 Placebo enhanced group: 82.8 Montelukast neutral group: 83.3 Placebo neutral group: 82.2 Smoking history (former smokers) Montelukast enhanced group: 12% Placebo enhanced group: 12% Montelukast neutral group: 24% Placebo neutral group: 22% Inclusion criteria: asthma diagnosis, regular asthma medications in past year, 1 or more poor asthma control indicators (> 1.5 ACQ, SABA 2+/week, nocturnal symptoms 1+/week) Exclusion criteria: serious health problems, montelukast use or previous intolerance Percentage withdrawn: 0 Allowed medication: LABA, ICS, oral anti‐leukotriene Disallowed medication: none recorded |
|
| Interventions |
Montelukast enhanced group: web app with scripted introduction + multimedia presentation + commercial on montelukast Placebo enhanced group: web app with scripted introduction + multimedia presentation + commercial on montelukast Montelukast neutral group: web app with scripted introduction + multimedia presentation but no discussion on benefits of montelukast Placebo neutral group: web app with scripted introduction + multimedia presentation but no discussion on benefits of montelukast All had 2 intervention sessions. One‐way interactivity with patient (educational multimedia session). No adherence feedback. No co‐intervention (one digital component involved). This was a theory‐based intervention: social cognitive theory states that an individual's expectations on a outcome can act as an incentive. This was used to increase outcome expectancy by using a multimedia presentation increasing a patient's expectations of treatment benefit, with an additive effect of presentation mode and drug assignment on medication adherence. No in‐person component. |
|
| Outcomes |
Primary: Adherence based on EMD Secondary: Outcome expectancy, PEF, FEV1, ACQ, asthma QOL questionnaire |
|
| Notes |
Type of publication: peer‐reviewed Funding: NHLBI R01HL073494 & American Lung Association COI: Bartlett is chair for behavioural sciences assembly |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation occurred but no detail given on how |
| Allocation concealment (selection bias) | Unclear risk | No allocation concealment described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Drug concealment described; unclear if concealment of electronic element |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinding described; adherence monitored electronically and via pill counts, which could have influenced outcome |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss of follow‐up; power calculation suggested n = 100, n = 99 so should have power |
| Selective reporting (reporting bias) | Low risk | All data presented as per methods; no study protocol |
Cvietusa 2012.
| Study characteristics | ||
| Methods |
Design: randomised controlled trial Duration: 1 year follow‐up Setting: children with persistent asthma |
|
| Participants |
Population: total 1393 children: 290 in the intervention group vs 303 in the control group Age: 3 to 12 years old |
|
| Interventions |
Intervention: received up to 3 tailored speech recognition reminder calls when they were due to refill their ICS. The calls provided information about asthma, facilitated a rapid ICS refill, and offered an opportunity to receive a call back from an asthma nurse specialist Control: usual care (no reminders) |
|
| Outcomes | Time to first ICS refill; proportion of days with medication on hand | |
| Notes | Type of publication: abstract only | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation occurred but no detail given on how |
| Allocation concealment (selection bias) | Unclear risk | No allocation concealment described |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Blinding unlikely to have been achieved but unlikely to have affected ICS refill |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | No blinding of outcome assessors described; adherence monitored by prescription refill so unlikely affected by knowledge of intervention group |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No loss of follow‐up data reported |
| Selective reporting (reporting bias) | Unclear risk | Methods did not describe what outcomes were planned |
Davis 2019.
| Study characteristics | ||
| Methods |
Design: open‐label randomised controlled trial Duration: endpoint at 52 weeks Setting: recruited from 4 paediatric clinics; trial carried out in the United States |
|
| Participants |
Population: 359 participants were randomised to receive digital intervention (n = 164 number) or control group (n = 155) Age: range from 11 to 17. Mean age in digital intervention was 13.1; SD 1.9. Mean age in control group was 13.2; SD 1.9. Proportion of male participants: digital intervention was 59.1% male; control group was 58.7% male Proportion of white ethnic participants: digital intervention was 32.3% white; control group was 40% white Asthma severity: mixed (mild to severe) Inclusion criteria: 11 to 17 years, spoke and read English or Spanish, had persistent asthma, were present for an acute or follow‐up asthma visit or a well‐child visit, and had previously visited the clinic at least once for asthma |
|
| Interventions |
Digital intervention: participants received a question prompt list and watched a short video about asthma self‐management before seeing the provider. 1‐page question prompt list is co‐intervention. There were 2 intervention sessions. No digital interactivity with patient. No adherence feedback. Control group: received usual care (medical visits) |
|
| Outcomes | Primary: self‐reported medication adherence | |
| Notes |
Type of publication: peer‐reviewed Funding: Patient‐Centered Outcomes Research institute CDR‐1402‐09777, the National Center for Advancing Translational Sciences (NCATS), National INstitutes of Health through grant award number UL1TR002489 COI: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Stated "were randomised" but no information on how participants were randomised |
| Allocation concealment (selection bias) | Unclear risk | No information given on how groups were allocated and whether researchers knew of upcoming allocations (did not state if the researchers were blinded and if medical practitioners were informed) |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Unlikely that blinding was achieved as the adolescents in the intervention group received a short video and prompt list, and no mention of blinding procedures being in place |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Self‐reported outcome and participants unblinded; adherence was measured using a VAS, potentially affected by knowledge of allocation group |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | There were no data stating the number of N completed in each group; no information on dropouts ‐ results only from GEE analyses |
| Selective reporting (reporting bias) | Low risk | Outcome measures appear to all be reported as per methods |
Ebrahimabadi 2019.
| Study characteristics | ||
| Methods |
Design: single‐blinded, randomised controlled trial Follow‐up duration: 2 weeks and 1 month after intervention Setting: recruited from 2 University Hospitals in Arak; trial carried out in Iran |
|
| Participants |
Population: 80 participants were randomised to infograph group (n = 41) or video group (n = 39) Age: range from: 20 to 65 years; SD: 41.12 in infograph group; SD: 39.46 in video group Proportion of male participants: 39% male in infograph group; 41% male in video group Asthma severity: severe Inclusion criteria: asthmatic patients 20 to 65 years, native Persian speakers capable of reading and writing with no visual or hearing problems Exclusion criteria: inaccessibility, home displacement, previous involvement in or attendance on a training session Percentage withdrawn: 6.81% withdrew from infograph group; 4.87% withdrew from video group Allowed medication: none recorded Disallowed medication: none recorded |
|
| Interventions |
Infograph: educational content delivered through illustrated material. Sessions delivered to groups of 3 to 5 participants on the second and third days of hospitalisation. Nurse not engaged in asthma care or the trial conducted the intervention. Video: educational content delivered through a film. Sessions delivered to groups of 3 to 5 participants on the second and third days of hospitalisation. Nurse not engaged in asthma care or the trial conducted the intervention. Film provided by the Islamic Republic of Iran Office of Non‐communicable Diseases within the Ministry of Health and Education Training.
|
|
| Outcomes | Asthma medication adherence | |
| Notes |
Type of publication: peer‐reviewed Funding: Vice Chancellor for research at Arak University of Medical Sciences COI: none stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A computerised random number generator performed the randomisation of assignment |
| Allocation concealment (selection bias) | Unclear risk | Researchers began the randomisation by assigning an identification number to each participant ‐ but unclear if the researchers had awareness of the group allocation |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Single‐blinded study; no information given on whether this was participants or researchers; however, participant awareness of allocation less likely to significantly alter outcome data as the same information was presented to each arm of the study for the same duration of time by a nurse unrelated to the study. A nurse, who was not engaged in asthma care and unconnected to the investigators to minimise bias, conducted the intervention in both groups ‐ as not connected to the investigators likely to not have been aware of allocation. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Nurse unrelated to the intervention completed collection of adherence data so likely blinded to the group for outcome assessment which was via MMAS8; unlikely patients knew of intervention group |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Intention‐to‐treat analysis performed. Only 2 participants from the video group and 3 participants from the infograph discontinued the study after randomisation, these numbers are balanced between each group and less likely to impact on adherence data obtained. |
| Selective reporting (reporting bias) | Low risk | All outcomes reported as per methods |
Foster 2014.
| Study characteristics | ||
| Methods |
Design: parallel, cluster‐randomised controlled trial Duration: endpoint at 26 weeks Setting: recruited from 4 primary care organisations; trial carried out in Australia |
|
| Participants |
Population: 143 participants were randomised to receive personalised adherence discussions (PAD) (n = 24), inhaler reminders and feedback (IRF) (n = 35), IRF + PAD (n = 41) or usual care (n = 43) Age: range from 14 to 65 years. Mean age in PAD group was 42.3 years; SD 15.6. Mean age in IRF group was 40 years; SD: 13.7. Mean age in PAD + IRF group was 39.7 years; SD = 17.7. Mean age in usual care group was 40 years; SD = 14.1. Proportion of male participants: PAD group was 46% male IRF group was 51% male PAD + IRF group was 22% male Usual care group was 37% male Proportion of white ethnic participants: PAD group was 46% male IRF group was 51% male PAD + IRF group was 22% male Usual care group was 37% male Asthma severity: moderate severity Baseline lung function (FEV1): PAD group was 67.3 IRF group was 84.4 PAD + IRF group was 78 Usual care group was 75.7 Smoking history (current/ex‐smoker/never %) PAD group was 0/27/73 IRF group was 11/26/63 PAD + IRF group was 22/37/41 Usual care group was 23/23/55 Inclusion criteria: ACT < 19; ICS/LABA twice a day for 1/12+ Exclusion criteria: asthma exacerbation in past month; major respiratory disease; serious uncontrolled medical conditions, visual or auditory impairment; shift workers with variable roster; pregnant/lactating women; use of budesonide/formoterol as maintenance and reliever therapy Percentage withdrawn: 9.79% Allowed medication: ICS/LABA Disallowed medication: budesonide/formoterol |
|
| Interventions |
Personalised adherence discussion (PAD) group
PAD allowed patients to identify barriers and strategies to improve adherence. There were 4 intervention sessions. No adherence feedback. No co‐interventions. In‐person component: GPs Electronic device intervention ‐ inhaler reminders and feedback (IRF) group SmartTrack reminders with adherence feedback. They received twice‐daily reminders for missed ICS/LABA doses. One‐way interaction with patient. Website adherence feedback. There were co‐interventions involved; the website with adherence feedback (2 digital components involved). Not a theory‐based intervention. In‐person component: GPs Electronic device intervention ‐ PAD and IRF group SmartTrack reminders with adherence feedback + personalised adherence plans. They received twice‐daily reminders for missed ICS/LABA doses. One‐way interaction with patient. Website adherence feedback. There were co‐intervention involved; the website with adherence feedback and PAD (one digital component involved). Not a theory‐based intervention. In‐person component: GPs Control ‐ usual care group One‐off checking and teaching inhaler techniques and asthma care plans. No interaction with patient. No adherence feedback. No co‐intervention used. In‐person component: GPs. |
|
| Outcomes |
Primary: ACT score Secondary: Mini asthma quality of life questionnaire, Hospital Anxiety and Depression Scale, Medication Adherence Report Scale for Asthma, and FEV1 (asthma control), prednisolone use |
|
| Notes |
Type of publication: peer‐reviewed Funding: National Health and Medical Research council of Australia COI: Foster received funding from GSK and AstraZeneca |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | GP randomisation by computer‐generated random code |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment until training workshop but before seeing patients, stated concealed but not how; no evidence of recruitment bias as recruitment occurred before cluster randomisation. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Although no complete blinding was possible, the GPs in each group both received usual care; and GPs and patients not informed of the monitoring function ‐ so they were blinded to the knowledge of being monitored (and therefore unlikely to have changed behaviour because of this). Note: other interventions were not described to the other GP groups to aid blinding to intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Adherence data collected by EMD and also Medication Adherence Report Scale for Asthma. Objective measure and unlikely to be influenced by knowledge of allocation group. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Intention‐to‐treat analysis performed, power calculation sufficient size however: GPs randomised 15 in each of the 4 groups. Withdrawals were only in the PAD group and the IRF + PAD group. In the PAD + IRF group the reasons were unknown for the 2 dropouts. In the PAD group the reasons were unknown in 2 and for personal reasons in 1. The dropouts were therefore higher when the GPs had to do a personalised adherence discussion. Likewise follow‐up dropout was highest in the PAD groups. Perhaps PAD was not well received by clinicians and this could bias the results in favour of the electronic monitor. Dropouts: 2/43 (UC) vs 2/24 (PAD) vs 0/35 (IRF) vs 7/41 (IRF + PAD). Proportionally more in the PAD groups; but IRF group alone low rates. These may have been due to non‐adherence ‐ unclear reasons for loss to follow‐up) but they are not in the IRF alone group but in the PAD group. |
| Selective reporting (reporting bias) | Low risk | All data presented as per methods |
Jan 2007.
| Study characteristics | ||
| Methods |
Design: parallel, open‐label randomised controlled trial Duration: trial endpoint of 12 weeks Setting: trial conducted in a single paediatric allergy and asthma clinic, in Tainan, Taiwan |
|
| Participants |
Population: 196 participants were randomised to receive a physician managed online interactive monitoring tool (n = 99) or a traditional clinic‐based patient education programme (n = 97) Age: range from: 6 to 12 years. Mean age in online interactive monitoring tool group was 11 years; SD: 2. Mean age in the traditional clinic‐based patient education programme was 10 years; SD: 3. Proportion of male participants: online interactive tool was 40% male; traditional clinic based patient education programme was 37% male Asthma severity: participants had a mix of mild, moderate and severe asthma Inclusion criteria: participants aged between 6 and 12 years old with a diagnosis of persistent asthma based on the GINA clinical practical guidelines with access to the Internet by their caregivers Exclusion criteria: diagnosis of bronchopulmonary dyplasia or other co‐morbidities with potential to affect the participant's quality of life. Percentage withdrawn: 17.7% withdrew from online interactive monitoring tool group; 26.8% withdrew from the traditional clinic‐based patient education programme Allowed medication: none recorded Disallowed medication: none recorded |
|
| Interventions |
Intervention: Physician managed online interactive asthma monitoring tool: Blue Angel for Asthma Kids is a single digital intervention comprising of an Internet‐based interactive asthma education and monitoring programme. Children taught how to monitor and measure peak expiratory flow and monitor asthma symptoms. The components of the programme were (1) basic information on the care of an asthmatic child; (2) an electronic diary; (3) action plan for patients; (4) retrieval analysis system to review patient uploaded data on symptom scores and peak expiratory flow variability. Physicians used a support decision system to asses the available data from patient uploads onto the Internet‐based monitoring tool to instruct the patients by email or telephone to either increase, decrease, or continue their usual treatment. Control: Traditional clinic‐based patient education programme: traditional asthma care plan with written instructions for self‐management. No patient interaction. No asthma feedback. No co‐interventions. Not a theory‐based intervention.
|
|
| Outcomes |
Primary outcomes: adherence,asthma control Secondary outcomes: PEF, quality of life (children and carers) |
|
| Notes |
Type of publication: peer‐reviewed Funding: National Science Council. Bureau of Health Promotion, Department of Health, Taiwan COI: none stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised but no detail on how |
| Allocation concealment (selection bias) | Unclear risk | "Sealed envelopes containing treatment assignment." This statement does not indicate that envelopes without all safeguards were used. "Following the session, the nurse opened a sealed envelope containing the treatment assignment"; no mention of whether the envelopes were opened consecutively. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Families were told that the purpose of the study was to find out if keeping track of asthma symptoms daily during a 12‐week study period would help them and their physicians manage the child’s asthma better. No direct information on adherence but aware there were two methods of tracking being compared. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Open‐label; adherence measured using "therapeutic and diagnostic monitoring". "We defined therapeutic monitoring as outcomes that directly reflect adherence to therapeutic regimens, including controller medication use and test score for dry powder inhaler (DPI) or metered dose inhaler (MDI) with the spacer technique." ACT and asthma quality of life: both self‐report methods PEFR: although objective, variation in measuring technique can reduce accuracy of results. Participants were aware that the purpose of the study was to see if it would help them manage their asthma better ‐ so potential effect of awareness of group allocation of the 2 methods. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 15 participants, 6 from the control group and 9 from the intervention group, were excluded either at the request of the participants themselves or for lack of data due to Internet failure. 93% per cent of the participants (82/88 in the intervention group and 71/76 in the control group) returned for the follow‐up visit at 12 weeks. 7 families who dropped out were unavailable for contact. Similar rates of loss to follow‐up in both groups. |
| Selective reporting (reporting bias) | Low risk | All data presented as per methods |
Johnson 2016a.
| Study characteristics | ||
| Methods |
Design: open‐label, parallel randomised controlled trial Duration: trial endpoint 3 weeks Setting: conducted in paediatric outpatient clinic at an academic medical centre; trial carried out in the United States |
|
| Participants |
Population: 98 participants were randomised to the MyMediHealth (MMH) group (n = 53) or usual asthma care (n = 45) Age: range from: 12 to 17 years. Mean age in MyMediHealth group was 14 years; SD: 2. Mean age in the usual asthma care group was 14 years; SD: 2. Proportion of male participants: MyMediHealth was 48% male; usual asthma care was 53% male Proportion of white ethnic participants: MyMediHealth was 46% white;usual asthma care was 47% white Asthma severity: moderate Inclusion criteria: English‐speaking participants aged between 12 and 17 years prescribed asthma medication with access to the Internet and a cell phone with an SMS plan Exclusion criteria: not stated Percentage withdrawn: 13.2% withdrew from the MyMediHealth group; 4.4% withdrew from the usual asthma care group Allowed medication: none recorded Disallowed medication: none recorded |
|
| Interventions |
Intervention: MyMediHealth (MMH): a 2‐component digital intervention including a website and short messaging service reminder system. MyMediHealth is a web‐based application that allows participants to create and print a structured medication list, attach a dosing schedule to each medication, request a text message reminder for each dose and to visualise medication adherence performance for each medication in the system. The MyMediHealth application also provides features such as 'vacation' that uses prescription information to determine if refills are needed before a certain date. Reminders are sent by text messages based on the requested time. Users can reply to text messages by typing a letter (T)aking, (S)kipping, or (H)olding, the MMH then generates a response based on the text message. When a dose is skipped a note is created in the administration log. For a held dose MMH asks the user when they expect to next take the medication and generates a reminder for this time. Two‐way interactivity with patient. Co‐intervention (website + SMS). No adherence feedback. No in‐person component. Not a theory‐based intervention. Control: usual asthma care, including online education material about asthma medication management |
|
| Outcomes | Primary outcomes: MyMediHealth usage patterns, self‐reported system usability, medication adherence, asthma control, self‐efficacy and quality of life | |
| Notes |
Type of publication: peer‐reviewed Funding: Agency for Healthcare Research and Quality and National Center for Advancing Translational Science COI: none stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Block randomisation used; states automatically but unclear how |
| Allocation concealment (selection bias) | Unclear risk | No allocation concealment described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No blinding described but both groups given online material, which might have helped concealment |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not blinded and participants contacted "approximately" 3 times so this could have affected retention rates and possible affected adherence |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Intention‐to‐treat analysis performed; no power calculation; losses described |
| Selective reporting (reporting bias) | Low risk | All data presented as per methods |
Joseph 2018.
| Study characteristics | ||
| Methods |
Design: parallel, single‐blinded randomised controlled trial Duration: endpoint at 52 weeks Setting: recruited from secondary setting; ED at 2 healthcare systems (Henry Ford Health System and Children's Hospital of Michigan at the Detroit Medical Centre); trial carried out in the United States |
|
| Participants |
Population: 121 participants were randomised to receive website application (n = 65) or standard care (n = 56) Age: range from 13 to 19 years old; mean age was 15.4 years; SD: 1.7 Proportion of male participants: 44.6% male Proportion of white ethnic participants: 3.3% white Asthma severity: mixed Smoking history: mixed Inclusion criteria: 13 to 19 years of age; those who were under 18 had to be accompanied by legal guardian who provided written informed consent from both teen and guardian; physician diagnosis of acute asthma Exclusion criteria: previously participated in the school‐based version of Puff City; English was not preferred language Percentage withdrawn: 0 Allowed medication: none recorded Disallowed medication: none recorded |
|
| Interventions |
Website application group: Standard care + online, computer‐tailored asthma management intervention. The app had behavioural assessments on the 4 education sessions on asthma management, e.g. psychosocial issues (smoking, depression, perceived emotional support, and lack of insurance/primary care physician), controller medication adherence, keeping a rescue inhaler nearby, and smoking reduction/cessation. Behavioural assessments at each of 4 sessions (no less than 1 week apart; within 90 days). Follow‐up at 6 months and 12 months. One‐way interaction with patient. No adherence feedback. No co‐interventions (one digital component). This was theory‐based intervention: behaviour change relevant to asthma control (e.g. Health Belief Model, Attribution Theory, Motivational Interviewing); one of the main theories includes the Transtheoretical Model (TTM), which describes the cognitive and behavioural processes that individuals undergo in relation to changing behaviour ‐ people will not exhibit behavior change without application of strategies more intense than usual. Standard care group: standard care + access to existing asthma informational websites that were non‐tailored and provide generic asthma education. Follow‐up at 6 months and 12 months. |
|
| Outcomes |
Primary: ED visits Secondary: ACT scores, number of symptoms days/week, school days missed, school or work days missed, ASRDI (Adolescent Self‐Regulatory Inventory) at 12 months, treatment adherence, using rescue inhaler |
|
| Notes |
Type of publication: peer‐reviewed Funding: National Institutes of Health, National Heart, Lung, and Blood Institute Grant COI: none Contact: no attempt made to contact study authors |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Online randomisation to the treatment (Puff City) or control group (generic, online asthma education) occurred when participants logged in for session 1 of the intervention/control program |
| Allocation concealment (selection bias) | Low risk | Computer randomisation leads to concealment; study personnel were blinded to treatment assignment |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | The ED and research staff were blinded to the treatment assignment, but the participant and caregiver were unblinded at the time, and after, the patient was randomised into the trial. During follow‐up and retention efforts, the research staff remained blinded to the patient group assignment. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Investigators remained blind to allocation until final data set ‐ however adherence data were self‐reported by the online session surveys. It is not known whether the participants knew that adherence was a study outcome of interest. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | No information given on dropouts however from Table 2 ‐ study compliance, at all time points there were higher % compliance with the treatment vs control group with more patients staying with the intervention than control group. These patients may be highly motivated to stay and therefore also report better adherence. |
| Selective reporting (reporting bias) | High risk | Methods stated secondary outcome would include "quality of life" but not reported in results |
Kenyon 2018.
| Study characteristics | ||
| Methods |
Design: parallel randomised controlled trial Duration: endpoint at 4 weeks Setting: participants recruited from the ED and general paediatric inpatient units at a tertiary care children's hospital, which also serves as a community children's hospital; trial carried out in Philadelphia, United States |
|
| Participants |
Population: 42 participants were randomised to receive automated text message reminders (n = 21) or no text message reminders (n = 20) Age: range from 2 to 13 years old; mean age was 6 years; SD: 2.0 Proportion of male participants: text message reminder group was 57% male. Group with no text reminders was 50% male. Proportion of white ethnic participants: text message reminder group was 5% white; group with no text reminders was 25% white Asthma severity: moderate Inclusion criteria: 2 to 13 years with diagnosis of persistent asthma, prescribed daily ICS or combined ICS + LABA in the year prior to current admission and the ICS prescribed on discharge compatible with the electronic sensor. Residence in or primary care in a Philadelphia ZIP code with high asthma hospitalisation rates. Parents or legal guardians unlimited text messages on a cell phone. Exclusion criteria: co‐morbidities with potential to impact asthma treatment. Prescribed ICS incompatible with electronic sensor. Participants with significant developmental delays, non‐English speaking or with active social services involvement. Percentage withdrawn: 21.96% withdrew from trial Allowed medication: none recorded Disallowed medication: none recorded |
|
| Interventions |
Intervention: automated text message reminders, electronic monitoring: participants received one of 7 rotating automatic text message reminders. Electronic sensor attached to ICS inhalers for data collection only, audiovisual reminder disabled. Sensor was paired to a mobile app or cellular hub and text messages reminders sent on days 3 and 27 to sync sensors with app or cellular hub. 30 intervention sessions, daily interactivity with patient. No co‐interventions, adherence feedback. Not a theory‐based intervention. Control: electronic monitoring: electronic sensor attached to ICS inhalers for data collection only, audiovisual reminder disabled and only 2 text message reminders on days 3 and 27 to sync sensors with app or cellular hub
|
|
| Outcomes |
Primary outcomes: intervention feasibility Secondary outcomes: adherence to prescribed ICS regimen, 30‐day adherence trajectories |
|
| Notes |
Type of publication: peer‐reviewed Funding: National Centre for Advanced Translational Science. Institute for Translational Medicine and Therapeutics at the University of Pennsylvania COI: none stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Block randomisation used and 1:1 scheme based on location |
| Allocation concealment (selection bias) | Low risk | No concealment described; automated text reminders so unlikely to affect outcome |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Both arms given electronic monitoring and text messages |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Not blinded but unlikely to affect outcomes due to use of electronic adherence monitoring devices |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Powered for feasibility. Of the 21 intervention participants, 3 never synced and 3 had equipment issues, and in the 20 controls, 3 never synced. There were high rates of loss in the intervention group due to equipment issues ‐ but unlikely to have affected outcome assessment via electronic monitoring. |
| Selective reporting (reporting bias) | Low risk | Data presented as per methods |
Kim 2016.
| Study characteristics | ||
| Methods |
Design: parallel Duration: endpoint at 8 weeks Setting: participants recruited from Seoul National University Bundang Hospital; trial carried out in Korea |
|
| Participants |
Population: 44 participants were randomised to application user group (n = 22) or application non‐user group (n = 22) Age: range from 19 to 72 years in the application user group, with mean age of 49 years. Range from 34 to 67 years in the application user group, with mean age of 49 years. Proportion of male participants: application user group was 18% white; non‐user group 36% white Asthma severity: moderate (application user group), mild (non‐user group) Baseline lung function (FEV1% predicted): 0.93 (application user group), 0.91 (non‐user group) Inclusion criteria: over 19 years familiar with using a smartphone Exclusion criteria: unfamiliar with using a smartphone and/or not willing to use a smartphone application Percentage withdrawn: 0 Allowed medication: none recorded Disallowed medication: none recorded |
|
| Interventions |
Intervention: smartphone application; snuCare: snuCare is a smartphone application based on a written asthma action plan. Participants were provided with the snuCare application and a peak flow meter and were instructed to record their symptoms and peak expiratory flow twice daily into the application, which then gave daily signals to users on their asthma control and offered action plans. All inputs were sent to the online server in a real time manner, risky signals e.g. 'emergency situation' were sent by short messaging service to the researchers, who then reviewed participant specific uploaded information and made direct calls to participants to assist their self‐management. 112 intervention sessions, two‐way interactivity with patient, no adherence feedback, no co‐interventions. Not a theory‐based intervention. Control: no smartphone application: usual care |
|
| Outcomes | Feasibility of application, lung function, asthma control, asthma medication adherence, quality of life | |
| Notes |
Type of publication: peer‐reviewed Funding: KT‐Seoul National University of Bundang Hospital (SNUBH) Collaborative Research Fund COI: none stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised but no detail on how |
| Allocation concealment (selection bias) | Unclear risk | No concealment described and could have affected allocation |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding and could have affected outcomes |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Contact at 4 weeks, no blinding, therefore could have affected outcomes |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No participants lost to follow‐up but no power calculations stated in terms of how this would affect outcome |
| Selective reporting (reporting bias) | High risk | Not all data presented, only P value of mean difference give; figures could have been useful |
Kolmodin MacDonell 2016.
| Study characteristics | ||
| Methods |
Design: parallel randomised controlled trial Duration: endpoint at 13 weeks Setting: participants recruited from an urban university and an affiliated medical centre; trial carried out in Michigan, United States |
|
| Participants |
Population: 49 participants were randomised to website + text messages (n = 25) or asthma education (n = 24) Age: range from 18 to 29 years. Mean age of 22, SD: 4 in website + text messages group. Mean age of 23, SD: 3 in asthma education group. Proportion of white ethnic participants: website + text messages group was 35% white; asthma education group was 16% white Asthma severity: moderate Baseline lung function (FEV1% predicted): 80% Inclusion criteria: 18 to 29‐year old African‐Americans diagnosed with persistent asthma and prescribed a controlled medication reporting < 80% adherence in past week and an ACT score < 19. Participants with access to a cell phone with text message ability. Exclusion criteria: pregnant, participants with an inability to speak or understand English, or with serious medication conditions needing regular medications and/or an active psychiatric disorder Percentage withdrawn: 8% withdrew from website + text message group Allowed medication: none recorded Disallowed medication: none recorded |
|
| Interventions |
Intervention: computer‐delivered intervention sessions and text message reminders: 2 web‐based intervention sessions delivered 1 month apart by an animated character selected by the participant and involving certain key components: feedback on medication use and symptoms, how to improve adherence, advantages and disadvantages of taking medication and the degree of interest from youth in improving their adherence with optional goal setting. Participants had the choice of receiving personalised text message reminders to take ICS between the sessions. Ecological momentary assessment enabling participants to report symptoms, effect, behaviour, and cognition in 'real‐time' was completed via text messages to ascertain each participant's experience of living with asthma. This information was used to personalise the intervention sessions. Control: asthma education: computer‐delivered asthma education, involving interactive features such as quizzes and polls. Control participants also received standardised text messages with general information on asthma.
|
|
| Outcomes | Asthma medication adherence, asthma control, lung function, patient satisfaction | |
| Notes |
Type of publication: peer‐reviewed Funding: National Institutes of Heath COI: none stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer automatically randomised participants to groups |
| Allocation concealment (selection bias) | Low risk | No concealment but comparison group received a lot of input so unlikely to affect results |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants not blinded but unlikely to affect the results |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | No blinding but data were collected via self‐report via text message EMA for real time outcome assessment rather than retrospective self‐reporting. Unclear if the participant knew of the study objective but an attention control arm existed, so unlikely to affect the results. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No power calculations, but minimal loss to follow‐up: 2 in the intervention group at 3 months, and control group had 1 missing at 1 month and another 1 at 3 months for similar reasons |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
Kosse 2019.
| Study characteristics | ||
| Methods |
Design: cluster‐randomised controlled trial Duration: endpoint at 26 weeks Setting: participants recruited from community pharmacies; trial carried out in the Netherlands |
|
| Participants |
Population: 234 participants were randomised to receive mobile application (n = 148) or control group (n = 95) Age: range from 12 to 18 years. Mean age in digital intervention was 15.0 years; SD 2.0. Mean age in control group was 15.2 years; SD 1.9. Proportion of male participants: digital intervention was 44.8% male; control group was 49% male Inclusion criteria: children aged 12 to 18 years, filling at least 2 prescriptions for ICS or a fixed combination of ICS with a long‐acting beta‐agonist (ICS/LABA) during the previous 12 months, and having a smartphone (iOS or Android) Exclusion criteria: insufficient comprehension of Dutch language or dependent on (in)formal carers to take their medication Percentage withdrawn: Withdrawal from digital intervention group was 0.67% Withdrawal from control group was 8.42% Concomitant medication: montelukast, oral corticosteroids, antibiotics, antiallergic, other |
|
| Interventions |
Digital intervention: Access to ADAPT intervention ‐ smartphone application for patients, securely connected to a desktop application of the patients own community pharmacist. The app had Weekly Control of Allergic Rhinitis and Asthma Test (CARAT) to monitor disease control over time, short educational and motivational movies on asthma‐related topics; medication reminder alarm to prevent forgetting; peer chat function to contact peers participating in the study; pharmacist chat function to facilitate contact; 2 questions once every 2 weeks to monitor non‐adherence: 1 about forgetting (unintentional) and 1 about deciding to miss out a dose (intentional). One digital component (the app) and participants were able to access the app for 6 months. In terms of digital interactivity with patients, there were 2 questions every fortnight to monitor non‐adherence; pharmacists can contact patients or send additional movies. Theoretical approach was used but not stated which one. Control group: Usual care (inhalation instruction) at first dispensing and automated pharmacy information systems that will detect excessive bronchodilatory or insufficient ICS use |
|
| Outcomes |
Primary: adherence to maintenance medication Secondary: asthma control, quality of life |
|
| Notes |
Type of publication: peer‐reviewed Funding: Netherlands Organisation for Health Research and Development COI: none declared |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No mention of type of randomisation, just says participating pharmacies were "randomly divided over the control and intervention group" ‐ via cluster‐RCT. In terms of recruitment bias, unclear if recruitment of study sites occurred before randomisation into control and intervention. |
| Allocation concealment (selection bias) | Unclear risk | The study report did not mention how this was done |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Article states that "due to the nature of the intervention, blinding of group assignments was impossible for both patients and pharmacists" Therefore there could be a chance performance could have been affected, particularly as the primary outcome of adherence was measured by self‐report. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Blinding of outcome assessors was not described ‐ all data collection was done online via online questionnaires so awareness of group could affect the measurement of the outcome |
| Incomplete outcome data (attrition bias) All outcomes | High risk | There were no missing data. Stratifying the data by age, gender, median, MARS score and median CARAT score did not affect the results therefore those results were not shown thus intervention effect was found. However, in terms of follow‐up ‐ there were fewer numbers in the intervention group ‐ as 7 did not download the intervention. These are likely people who have poor adherence so may have falsely inflated the adherence reported in the intervention. |
| Selective reporting (reporting bias) | Low risk | Protocol stated that they would measure "adherence, asthma control, illness perceptions, medication beliefs, and asthma‐related quality of life are measured" ‐ however, illness perceptions and beliefs were not reported in the final study. Nevertheless the outcomes of interest to the review ‐ adherence, asthma control, QOL were reported. Also analyses corrected for the cluster design by authors. |
Koufopoulos 2016.
| Study characteristics | ||
| Methods |
Design: parallel, randomised controlled trial Duration: endpoint at 9 weeks Setting: participants recruited from 40 universities; trial carried out in the United Kingdom |
|
| Participants |
Population: 216 participants were randomised to online community (n = 99) or online diary (n = 117) Age: mean age of 27, SD: 9 in online community group; mean age of 29, SD: 10 in online diary group Proportion of male participants: 30% white in both arms of the study Asthma severity: none stated Inclusion criteria: participants with an asthma diagnosis on regular ICS Exclusion criteria: failed to complete the eligibility questionnaire, baseline measures or informed consent. Not an asthmatic nor prescribed an ICS preventer inhaler for a weekly regime of at least 1 dose per week. Previous participation in the pilot study. Percentage withdrawn: 8% withdrew from website + text message group Allowed medication: none recorded Disallowed medication: none recorded |
|
| Interventions |
Intervention: online community intervention + weekly reminders: participation in 'Asthma Village', an online community. Participants have the ability to create their own profile, add their weekly controlled ICS use in the diary section and see what others are posting in the news feed. A Q+A feature allows participants to ask questions and answers those of their peers. Automated weekly reminders are sent to alert individuals to log into 'Asthma Village' and record controlled use. No patient interactivity, adherence feedback, co‐interventions. Theory‐based intervention. Control: online diary to record ICS use. Participants were unaware of other users and could not see other posts. |
|
| Outcomes | Self‐reported ICS adherence, visits to online community site | |
| Notes |
Type of publication: peer‐reviewed Funding: pilot grant from the University of Leeds School of Psychology; a Fullbright Scholarship from the US‐UK COI: none stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised but no detail on how |
| Allocation concealment (selection bias) | Unclear risk | No allocation concealment described |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | No blinding described but both groups given online material, which might have helped with concealment |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Not blinded and weekly reminders sent to both groups so unlikely to affect outcome |
| Incomplete outcome data (attrition bias) All outcomes | High risk | High dropout rate |
| Selective reporting (reporting bias) | Low risk | All data presented as per methods |
Lv 2012.
| Study characteristics | ||
| Methods |
Design: parallel RCT Duration: 12 weeks Setting: recruited from a single outpatient university hospital; China Detailsofrun‐inperiod: not provided |
|
| Participants |
Population: 150 participants Age: 18 to 65 years Proportion of male participants: intervention: 67%; control: 50% Smoking history: not stated ‐ smokers were excluded Inclusion criteria: older than 18 years of age, physician‐diagnosed asthma according to GINA at least 3 months before recruitment, bronchodilator reversibility test or bronchodilator provocation test positive in the past year, owning a mobile phone, and ability to read and understand the questionnaires Exclusion criteria: respiratory infection within the previous 4 weeks, pregnancy, heart disease, stomach surgery, other lung diseases, or current or past smoking history of > 10 pack‐years Percentage withdrawn: intervention: 40% dropout; control: 72% dropout |
|
| Interventions |
Digital intervention (asthma education + SMS communication) Verbal asthma education by outpatient physician based on the Global Initiative for Asthma and daily SMS reminder on how to manage asthma. Participants could send asthma‐related questions by text to clinic investigators for answers. Single digital component. No interactive component. There is adherence feedback and is not fully/self‐delivered digital intervention. Control group: Verbal asthma education as above. Participants also provided a PEF meter and received training on correct use. Participants were encouraged to keep asthma diaries with PEF data, medication usage and asthma symptoms. Additionally, they were taught how to adjust asthma action plan based on their recorded information |
|
| Outcomes | PCA Questionnaire (PCAQ‐6), Standard Asthma‐Specific Quality of Life (AQLQ(S)), spirometry, blood and induced sputum cell count, follow‐up compliance rate, medicine compliance rate, emergency department (ED) visits | |
| Notes |
Type of publication: peer‐reviewed Funding: none stated but the work was supported in part by the pulmonary function technicians who work in the Lung Function Department, Nanfang Hospital, Southern Medical University, Guangzhou, China COI: no COI |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information on how randomised ‐ states "randomly allocated" |
| Allocation concealment (selection bias) | Unclear risk | No information on allocation |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Intervention is such that there is unlikely to be blinding ‐ and no blinding indicated |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information on how compliance was measured so uncertain if there was bias in outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | High risk | High rates of withdrawal: 72% from control vs 40% from intervention, meaning the intervention group may have the more adherent patients in that group thus potentially impacting the results |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
Lv 2019.
| Study characteristics | ||
| Methods |
Design: parallel, individually randomised controlled trial Duration: endpoint at 52 weeks Setting: recruited from primary and secondary setting: 2 tertiary hospitals (secondary), and 2 community healthcare centres (primary); trial carried out in China |
|
| Participants |
Population: 152 children were randomised to receive mobile application (n = 77) or nurse‐led group (n = 75) Age: range from 6 to 12 years. Mean age in mobile application group was 7.8 years; SD: 1.4. Mean age in nurse‐led group was 8.1 years; SD: 1.4. Proportion of male participants: mobile application group was 53.2% male; nurse‐led group was 46.7% male Proportion of white ethnic participants: 0% white Asthma severity: mixed Inclusion criteria: age between 6 and 12 years; medical history, symptoms and signs consistent with the diagnosis of asthma; positive asthma predictive index; willingness and ability to correctly use an inhaler; possession of a smartphone and ability to use the mobile application; ability to correctly use the Childhood Asthma Control Test (C‐ACT) Exclusion criteria: severe asthma exacerbation at the time of enrollment; currently suffering from the concurrent acute or chronic rhinosinusitis, obstructive sleep apnoea, or rheumatic diseases; co‐morbidities including bronchopulmonary dysplasia, bronchiectasis, tracheal or bronchial malacia, obliterative bronchiolitis, diffuse panbronchiolitis or tuberculosis; and diagnosed primary immunodeficiency Percentage withdrawn: Withdrawal from mobile application group was 9.09%; withdrawal from nurse‐led group was 2.67% Allowed medication: received fluticasone propionate inhaled aerosol. For those who required long‐term ICS + LABA, salmeterol‐fluticasone dry powder was prescribed (so just routine controller medications). Disallowed medication: none recorded |
|
| Interventions |
Mobile application + nurse‐led group: the mobile application included modules on medication reminders, adherence management, alert of acute asthma exacerbations, assessment of exacerbation severity, treatment recommendation, keeping a health diary, instant communication with healthcare providers and health education. One‐way interaction with patient. There were 12 intervention sessions; monthly reviews.Two weeks after each visit, nurse would call parents to review treatment adherence. No co‐interventions used (one digital component). Not a theory‐based intervention. In‐person component involved: the nurse. Nurse‐led group: nurse‐led asthma management. There were 12 intervention sessions; monthly reviews. Two weeks after each visit, the nurse would call parents to review treatment adherence. |
|
| Outcomes |
Primary: asthma exacerbations Secondary: treatment adherence, C‐ACT scores, number of respiratory infections, days of antibiotic use, requirements for oral steroid intake, days of school absence, days of parental work loss and medical expenses |
|
| Notes |
Type of publication: peer‐reviewed Funding: Science and Technology Research Project of Jinhua, the Natural Science Foundation of China and Zhejiang Provincial Natural Science Foundation Grant COI: none |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Randomisation through a random number table, with odd numbers assigned to experimental group and even numbers assigned to control group |
| Allocation concealment (selection bias) | Unclear risk | No allocation concealment noted |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Healthcare providers informed every participant in the experimental group the exact information the application would collect from their mobile phone. However, participants from both groups did not know how the information would be used. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No blinding described; unclear if knew allocation and this could affect outcome. Patient measured adherence through (total number of days taking ICS over a year/365) x 100. Did not explain how they measured it; however, most children received a fluticasone propionate aerosol. Those requiring a longer‐term therapy with ICS + LABA, a salmeterol + fluticasone DPI was prescribed. Consequently, we can speculate that dose counts were used. But this is unclear. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Below powered sample; higher rate of dropout in intervention group. This can skew data to favour control group data. |
| Selective reporting (reporting bias) | Low risk | Results specified as per methods |
Morrison 2016.
| Study characteristics | ||
| Methods |
Design: parallel, open‐label randomised controlled trial Duration: endpoint at 12 weeks Setting: participants recruited from 20 general practices in Glasgow, Scotland; trial carried out in the United Kingdom |
|
| Participants |
Population: 51 participants were randomised to receive living well with asthma website (n = 25) or usual care group (n = 26) Age: range from 16 to 78 years. Mean age in digital intervention was 44.6, SD:17. Mean age in control group was 46.4; SD: 14. Proportion of male participants: digital intervention was 82% male; control group was 80% male Proportion of white ethnic participants: digital intervention was 24% white; control group was 24% white Smoking history: current 10%, former 35%, never 55% Inclusion criteria: ≥ 16, physician diagnosis of asthma, duration of asthma symptoms 1 year, ACQ score ≥ 1, access to Internet via PC or laptop (iPad/smartphone not sufficient for purpose) Exclusion criteria: unstable asthma defined by any of following in past 4 weeks: asthma hospital admission, A&E attendance, out of hours GP visit, GP visit at home. Presence of other lung disease, mental impairment or language difficulties that made consent impossible, frequent exacerbations with > 4 courses of oral prednisolone in past 12 months, cognitive impairment, terminal illness Percentage withdrawn: Withdrawal from digital intervention group was 20% Withdrawal from control group was 3.84% Allowed medication: none recorded Disallowed medication: none recorded |
|
| Interventions |
Digital intervention: The intervention was a website called 'Living well with Asthma'. It was one digital component whereby participants were able to access the website for 12 weeks. The provider was not stated ‐ assume intervention content developed by research group. There was two‐way interactivity with the patient and no adherence feedback was given. There was no co‐intervention used. It was not stated about whether it was a theory‐based intervention. There was no in‐person component. Control group: The control group had usual care and there was no digital intervention used. It was not stated whether adherence feedback was given. |
|
| Outcomes |
Primary: asthma control, quality of life Secondary: adherence to maintenance medication, lung function (FEV), exacerbations requiring at least OCS, unscheduled healthcare use |
|
| Notes |
Type of publication: peer‐reviewed Funding: Chief Scientist Office, Scottish Government (CAF 11/08) COI: speaker's honoraria and advisory panel payments from various pharmaceutical companies listed. Also received research funding from pharmaceutical companies but not for this project. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation schedule was generated in advance by external group |
| Allocation concealment (selection bias) | Low risk | Third party interactive voice response system (IVRS) ensured allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants were not blinded to group |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Outcome measures assessed in participant homes; easy for group allocation to be known to researcher |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Attrition in intervention group was 20% compared with 4% in control group |
| Selective reporting (reporting bias) | Low risk | Consistent reporting as per methods |
Morton 2017.
| Study characteristics | ||
| Methods |
Design: open‐label, parallel, randomised controlled trial Duration: endpoint at 52 weeks Setting: participants recruited from Sheffield and Rotherham Hospital Clinics; trial carried out in the United Kingdom |
|
| Participants |
Population: 90 participants were randomised to electronic monitoring device + feedback group (n = 47) or deactivated electronic monitoring device group (n = 43) Age: range from: 6 to 16 years. Mean age of 10.4; SD: 2.9 in electronic monitoring device + feedback group. Mean age of 10.2; SD: 2.9 in deactivated electronic monitoring device group. Proportion of male participants: 60% male in electronic monitoring device + feedback group; 52% male in electronic monitoring device group Proportion of white ethnic participants: electronic monitoring device + feedback group was 64% white; deactivated electronic monitoring device group was 57% white Asthma severity: moderate Inclusion criteria: prescribed ICS, with no medication changes in past month; minimum ACQ score of 1.5 Exclusion criteria: non‐English speakers and those with significant other chronic conditions (none stated) Percentage withdrawn: 17.02% withdrew from electronic monitoring device + feedback group; 9.52% withdrew from deactivated electronic monitoring device group Allowed medication: none recorded Disallowed medication: none recorded |
|
| Interventions |
Intervention: electronic monitoring device: electronic monitoring device attached to preventer inhaler with twice daily adherence reminders + feedback on adherence. 720 intervention sessions (twice daily reminders for 360 days). Two‐way interactivity. Adherence feedback to participants and family every 3 months for a period of 12 months, discussions centred around adherence rates and action plans for the following 3 months to improve adherence. No co‐interventions. Not a theory‐based intervention. Control: electronic monitoring device attached to preventer inhaler with alarm DEACTIVATED and NO adherence feedback |
|
| Outcomes | ICS adherence, asthma control, rescue beta‐agonist use and oral steroid doses, unplanned GP/ED visits, time off school, lung function (FEV1%), asthma‐related quality of life, change in BTS steps | |
| Notes |
Type of publication: peer‐reviewed Funding: Sheffield Children's NHS Foundation Trust COI: none stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation |
| Allocation concealment (selection bias) | Low risk | The allocation of participants involved phoning the independent holder of the randomisation code |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Due to the nature of the intervention, neither the participants nor the study team were blinded. In the intervention group adherence data were made available to clinicians if requested, but not in the control group. Likely participants aware of adherence purpose of the study particularly as adherence discussions were conducted with the intervention group. Note controls were told "the devices monitored how much the inhalers were taken, but that these data would not be reviewed" so likely aware of adherence monitoring function of devices. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Unblinded. No mention of outcome assessors being blind to the outcomes ‐ however, note adherence data were collected by EMD. This was calculated as number of doses actually taken/number of doses prescribed × 100. However, likely affected by knowledge of study purpose. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Higher rates of loss to follow‐up in the intervention group; however, not for reasons related to adherence |
| Selective reporting (reporting bias) | High risk | Data for short‐acting beta agonist use, change in BTS stage, mini PAQLQ, BMQ and IPQ not shown ‐ states no differences but not reported |
Mosnaim 2013.
| Study characteristics | ||
| Methods |
Design: parallel randomised controlled trial Duration: endpoint at 10 weeks Setting: participants recruited from primary care practices at Rush University Medical Center Chicago; trial carried out in the USA Detailsofrun‐inperiod: 3 week run‐in period |
|
| Participants |
Population: 68 participants were randomised to receive peer group and mp3 delivered peer asthma messages (n = 34) or attention control group (n = 34) Age: range from: 11 to 16 years; mean age for digital group was 13.3 years and mean age for control group was 13.6 years Proportion of male participants: digital intervention was 50% male; control group was 47.1% male Smoking history: 2.9% current smoker, 7.4% secondary exposure at home Inclusion criteria: 11 to 16 years, African‐American or Hispanic, persistent asthma, prescribed daily ICS Exclusion criteria: co‐morbidities that could interfere with trial, ≥ 48% adherence to ICS in 2‐week run‐in period Percentage withdrawn: withdrawal from digital intervention group was 29.4%; withdrawal from control group was 26.4% |
|
| Interventions |
Digital intervention: Patients recorded messages to encourage ICS adherence, which were selections of patients MP3 players and played at random between music tracks. They also had weekly peer group support sessions led by a social worker (in‐person component). There is one digital component (MP3 player) and the details of the intervention provider is not stated. The number of intervention sessions and digital interactivity with the patient is not stated. No adherence feedback was given. It was a theory‐based intervention ‐ social cognitive theory. Control group: Doctor recorded messages to encourage ICS adherence and this was played on the MP3 player. They also had weekly meetings with the research assistant. No adherence feedback was given. |
|
| Outcomes |
Primary: ICS adherence Secondary: asthma knowledge, ICS knowledge, ICS self‐efficacy, social support, asthma social support and exacerbations |
|
| Notes |
Type of publication: peer‐reviewed Funding: National Heart, Lung and Blood Institute K23 HL092292 and R21 HL098812. Study drug from GlaxoSmithKline (FLV114794). COI: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Blocked group randomisation from a computer‐generated schedule |
| Allocation concealment (selection bias) | Unclear risk | Computer‐generated allocation schedule but no details of how allocated |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants and their caregivers were fully informed that the electronic monitor would record the number of times that they actuated their ICS on a daily basis, but were kept blind to the specific purpose of the monitoring ‐ so were blinded to the study purpose. The research assistant did not engage in conversation with the participants to promote adherence. At each of these sessions, the attention control group adolescents received the same number of iPod messages as their active intervention group counterparts, with content promoting adherence to daily controller medications. Those in the control group met individually with the research assistant; the research assistant did not engage with the participants to encourage adherence. However, as the intervention group met with the social worker, the research assistant would have been able to deduce the allocation |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Adherence monitored by Doser CTs |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Similar rates of loss to follow‐up in both groups |
| Selective reporting (reporting bias) | High risk | Adherence outcomes reported but no other outcomes despite stating in methods that data on asthma exacerbations and unscheduled healthcare utilisation would be collected at 5 and 10 weeks. 8% missing data at 10 weeks reported. |
Pernell 2017.
| Study characteristics | ||
| Methods |
Design: parallel randomised controlled trial Duration: 8.5 weeks Setting: participants recruited from 47 adult and child participants treated at the Vanderbilt‐Meharry Center of Excellence of Sickle Cell Disease; trial carried out in the USA |
|
| Participants |
Population: 47 participants were randomised to receive SMS‐reminder (n = 26) or usual care ‐ no messages (n = 21) Age: range from: 3 to 59 years. Median age in intervention was 20 years old (IQR 11, 25). Median age in control group was 20.5 (IQR 8.75, 25.5). Proportion of male participants: digital intervention was 53.8% male; control group was 47.6% male Inclusion criteria: age 1 to 70 years, confirmed diagnosis of SCD and mild, moderate or severe persistent asthma with prescribed asthma control therapy and/or hydroxyurea therapy (for a minimum of 3 months) Exclusion criteria: none stated Percentage withdrawn: withdrawal from digital intervention group was 15.38%; withdrawal from control group was 23.8% |
|
| Interventions |
Digital intervention: Two‐way SMS medication reminders over 60 days. One digital component was used and it was provided by REDCap Software. Participants twice daily text message reminders at different times (morning and 10 hours later) to mimic medication schedule ‐ for asthma medication. They would reply yes or no depending on whether or not they took their medication. If responded no then would receive an additional SMS reminder to take them and if did not reply at all they would get an additional text one hour later. There was no co‐intervention used or in‐person component. Control group: Usual care ‐ no messages; provider was not stated but assume usual care clinicians |
|
| Outcomes | Primary: adherence, asthma control (ACT) | |
| Notes |
Type of publication: peer‐reviewed Funding: Junior League of Nashville HRSA grant number 5‐U38‐MC2222‐0‐04‐00 and the Trans‐Institutional Programs at Vanderbilt University Medical Center COI: none declared |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants de‐identified and blinded from research assistant who assigned randomisation |
| Allocation concealment (selection bias) | Unclear risk | Not stated what participants were told |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Adherence feedback for intervention group; potential to know what they were being measured on adherence |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Participants would appear to know they were in the intervention group |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition not unusually high and balanced between groups; however, in the tables the numbers between each group are unclear and we do not have clear idea of how many dropped out from each group. Also unclear if endpoint adherence (table 1) is related to those on asthma medication only. Author confirmed: "We had 7 people drop out (from an initial group of 47), the reason for the 38 is the fact that we didn't have data for the pre medication adherence on 2 people so couldn't calculate differences" |
| Selective reporting (reporting bias) | High risk | Reporting is unclear although study is a feasibility trial |
Petrie 2012.
| Study characteristics | ||
| Methods |
Design: parallel, individually randomised controlled trial Duration: endpoint at 18 weeks Setting: Recruited from pamphlets dispensed with medications and targeted marketing website; trial carried out in New Zealand |
|
| Participants |
Population: 147 participants were randomised to receive a tailored text messages (n = 73) or usual care (n = 74) Age: range from: 16 to 45 Proportion of male participants: 22% male Inclusion criteria: age 16 to 45, diagnosed with asthma, not adhering to preventer medication and owns mobile phone capable of receiving text messages Exclusion criteria: non‐English speaker and COPD Percentage withdrawn: withdrawal from text message group was 43.84%; withdrawal from control group was 29.73% Allowed medication: preventer medication Disallowed medication: none recorded |
|
| Interventions |
Text message group: tailored text messages based on baseline measures (Illness Perceptions Questionnaire/Beliefs about Medicines Questionnaire content) delivered via SMS‐based mobile phone. There were 137 intervention sessions. No interactivity with patient. No adherence feedback. Not theory‐based intervention. No in‐person component. Control group: usual care with no text messages |
|
| Outcomes | Primary: adherence | |
| Notes |
Type of publication: peer‐reviewed Funding: not stated COI: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Unsure: Were text messages automated? What was in the participant information sheet? Did patients know they were in the intervention group? |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Telephone calls to assess adherence could reveal group allocation. Also self‐reported measure bias based on group expectation. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Attrition at high level; uneven between groups and reporting inconsistent |
| Selective reporting (reporting bias) | Low risk | Outcome measures appear to all be reported as per methods |
Pool 2017.
| Study characteristics | ||
| Methods |
Design: parallel, single‐blinded, individually randomised controlled trial Duration: endpoint at 52 weeks Setting: recruited from enrolled insurance plan; trial carried out in the USA |
|
| Participants |
Population: 408 participants were randomised to have received online tools designed to prompt users to ask questions related to asthma (n = 203) or active control tool group (n = 204) Age: mean age in intervention group was 47.6; SD 9.1. Mean age in control group was 47.2; SD 9.6. Proportion of male participants: intervention group was 36.4% male; active control group was 37.7% male Proportion of white ethnic participants: intervention group was 81.7% white; active control group was 86.8% white Smoking history: 3.2% smokers (3.5% in intervention group, 2.9% in active control group), 27.1% smoke ≥ 100 cigarettes in lifetime (25.1% in intervention group, 29.1% in active control group) Inclusion criteria: age 21 to 60 years, diagnosed with asthma using HEDIS (Healthcare Effectiveness Data and Information Set) criteria for persistent asthma, medication specific to asthma, emergency room and outpatient visits Exclusion criteria: no asthma diagnosis from a healthcare provider, not able to read/speak English fluently, no Internet access at home, confirmed pregnancy, smoking > 20 pack‐years Percentage withdrawn: withdrawal from intervention group was 22.66%; withdrawal from active control group was 18.63% |
|
| Interventions |
Intervention group: online tool items designed to prompt users to ask questions related to asthma, e.g. tests from healthcare providers and encouragement of specific self‐management behaviours via website. There were 12 intervention sessions. Feedback is not specifically adherence but identifies overuse of rescue medication and feedback that ICS is recommended. Two‐way interactivity with patients. Non‐theory‐based intervention. Control group: active control; online tool items designed to prompt users to ask questions not related to asthma (e.g. cancer screening) from healthcare providers. Two‐way interactivity with patients. |
|
| Outcomes | ACT, quality of life, medication use, healthcare utilisation | |
| Notes |
Type of publication: peer‐reviewed Funding: National Heart, Lung and Blood Institute R01HL088590 and National Institutes of Health and National Center for Advancing Translational Science, NIH UL1RR033184 COI: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation |
| Allocation concealment (selection bias) | Low risk | Participants were blinded to their assignment and allocated via the software |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | All participants engaged in online tool and were unaware of subject matter (asthma vs non‐asthma) differences in content of messages |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | All contact via online tool so potential for detection of randomisation outcome appears to be low; feedback is automatically generated |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition not unusually high and balanced between groups |
| Selective reporting (reporting bias) | High risk | Authors planned to collected QOL measures ‐ mentioned in abstract and paper as secondary outcome but reported as part of asthma control measures not quality of life |
Reece 2017.
| Study characteristics | ||
| Methods |
Design: pilot randomised controlled trial Duration: endpoint at 12 weeks Setting: recruited from the Howard university Faculty Practice Plan; trial carried out in the USA |
|
| Participants |
Population: 33 participants, predominantly African American, were randomised to receive digital intervention or control group Age: range from 13 to 60 years |
|
| Interventions |
Asthmawin mobile iPhone app group: received mobile app with action plan and monitoring and reminder system, developed by CooperSoftInc. Components are a physician‐generated asthma action plan and daily recording of: (a) peak flow measurements, (b) medication usage ‐ documented with a self‐photo, (c) daily symptoms with an automatic reminder to take medications Control group: paper journalling |
|
| Outcomes | Controller medication usage, ACT, PEFR | |
| Notes | Type of publication: abstract only | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation occurred but no detail given on how |
| Allocation concealment (selection bias) | Unclear risk | No allocation concealment described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding described; potential for participants to increase medication use with knowledge of group allocation |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No blinding described; uncertain how adherence monitored |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No loss of follow‐up data reported |
| Selective reporting (reporting bias) | Low risk | All data presented as per methods; no study protocol |
Rikkers‐Mutsaerts 2012.
| Study characteristics | ||
| Methods |
Design: parallel, block‐randomised controlled trial Duration: endpoint at 52 weeks Setting: recruited from Leiden University Medical Center (LUMC); trial carried out in the Netherlands |
|
| Participants |
Population: 90 participants were randomised to receive digital intervention (n = 46) or control (n = 44) Age: range from 12 to 17 years; mean age in digital intervention was 13.4; mean age in control group was 13.8 Proportion of male participants: digital intervention was 43% male; control group was 57% male Asthma severity: mixed (mild to severe) Inclusion criteria: doctor’s diagnosis of mild to severe persistent asthma characterised by a prescription of ICS more than 3 months in the previous year, age 12 to 18 years, access to Internet, and understanding of the Dutch language Exclusion criteria: patients requiring oral steroids as maintenance or patients with relevant co‐morbidity Percentage withdrawn: withdrawal from digital intervention group was 23.91%; withdrawal from control group was 9.09% Disallowed medication: maintenance oral steroids |
|
| Interventions |
Digital intervention was Internet‐based self‐monitoring (IBSM), which is delivered via website. There were 2 educational sessions then reported ACQ and FEV1 weekly into study website. There was interactivity with patient which was face‐to‐face education during first part of intervention. Adherence feedback was weekly feedback on level of asthma control and treatment plan. Control group: Usual care ‐ without Internet‐based self‐monitoring |
|
| Outcomes | QOL via PAQQLQ (Paediatric Asthma Quality of Life Questionnaire), FEV1, ACQ (Asthma Control Questionnaire), number of symptom‐free days, daily ICS dose, exacerbations | |
| Notes |
Type of publication: peer‐reviewed Funding: Netherlands Asthma Foundation, Numbers: 3.4.03.157, 3.4.03.45 COI: N/A |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation |
| Allocation concealment (selection bias) | Low risk | Assigned to each group by using computer‐generated, permuted‐block scheme after collection of baseline data to ensure concealment of allocation |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Participants unable to be blinded and could have affected outcome; however, there was a 2‐week baseline period where participants were asked to monitor their symptoms and lung function (forced expiratory volume in 1 second, FEV1) daily via the website and to continue their usual medication. It was not clear whether participants knew what the purpose of the intervention was so potentially could have been 'blinded' to the purpose of the study but not the allocation group. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Self‐reported measure of adherence and asthma control so may have been biased based on group expectation of what the intervention may have done |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Substantially higher number of dropouts in the intervention group compared to dropouts from the usual care group |
| Selective reporting (reporting bias) | Low risk | Outcome measures appear to all be reported as per methods |
Schaffer 2004.
| Study characteristics | ||
| Methods |
Design: randomised controlled trial Duration: endpoint at 26 weeks Setting: recruited using flyers posted throughout the health science centre campus, within the university student health centre, and in health departments within the county; trial carried out in the USA |
|
| Participants |
Population: 46 participants were randomised to receive digital intervention or control Age: range from 18 to 63; the mean age of total participants was 37 Proportion of male participants: 32.6% of total participants were male Proportion of white ethnic participants: total participants were 72 % white Baseline lung function: FEV1 43% Inclusion criteria: English‐speaking adults aged 18 to 65, reported use of preventive medication for asthma during the 3 months prior to the study, indicated that they had mild persistent to moderate persistent asthma according to the US NAEPP (2002) guidelines Exclusion criteria: daily oral steroid use, diagnosis of COPD or symptomatic cardiac disease Percentage withdrawn: not stated Disallowed medication: daily oral steroid use |
|
| Interventions |
Theoretically based audiotape intervention: participants took educational materials but were not directed to review them. Research assistant recorded answers. 2 intervention sessions at 3 and 6 months after baseline. No interactivity with patients. No adherence feedback. Control group: standard provider education with no audiotape provided |
|
| Outcomes | Self‐reported and pharmacy‐verified adherence to preventive medication, asthma control (ACQ), asthma quality of life (miniAQLQ), asthma self‐efficacy (PCAQ), asthma knowledge | |
| Notes |
Type of publication: peer‐reviewed Funding: University of Florida College of Nursing Biobehavioral Research Center COI: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation |
| Allocation concealment (selection bias) | Unclear risk | Participants were provided with interventions but the study report did not describe how allocation was blinded to researcher or participants |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants met with the researcher to report on adherence and for social desirability might try to please the researcher |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The researcher remained blind to group assignment until the data collection was completed |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition not unusually high and balanced between groups |
| Selective reporting (reporting bias) | Low risk | Outcome measures appear to all be reported as per methods |
Searing 2012.
| Study characteristics | ||
| Methods |
Study design: randomised, prospective, controlled trial Duration: 30 days |
|
| Participants |
Population: 43 adolescent participants with asthma were randomised to receive digital intervention or control Exclusion criteria: participants undergoing changes to their medication regimen at the time of enrollment |
|
| Interventions |
Digital intervention: received randomly generated text messages pertaining to asthma education at variable frequencies (once every other day to twice per day) for 30 days Control group: usual care |
|
| Outcomes | Asthma Control Test, self‐reported adherence and satisfaction with the texting programme graded on a scale of 1 to 5 | |
| Notes | Type of publication: abstract only | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation occurred but no detail given on how |
| Allocation concealment (selection bias) | Unclear risk | No allocation concealment described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding described; potential for participants to increase medication use with knowledge of group allocation |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinding described; adherence monitored by self‐report which could be influenced by knowledge of group allocation |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No loss of follow‐up data reported |
| Selective reporting (reporting bias) | High risk | Methods do not describe all outcomes planned or reported |
Stranbygaard 2010.
| Study characteristics | ||
| Methods |
Design: block randomised controlled trial Duration: endpoint at 8 weeks Setting: recruited from a newspaper advertisement; trial carried out in Denmark |
|
| Participants |
Population: 26 participants were randomised to receive daily text messages (n = 12) or control group (n = 14) Age: range from 18 to 65; mean age in digital intervention group was 34.4; mean age in control group was 30.7 Proportion of male participants: Digital intervention was 50% male; control group was 57% male Asthma severity: mixed Baseline lung function: FEV1 76.39% Inclusion criteria: asthma diagnosis based on clinical history and symptoms, aged 18 to 45 years, positive methacholine test PD20 < 4 µmol Exclusion criteria: co‐morbidities, smoking history > 10 pack‐years Percentage withdrawn: withdrawal from digital intervention group was 16.67%; withdrawal from control group was 14.29% |
|
| Interventions |
Daily text message intervention: SMS‐based text messages delivered from Internet by software company CIM mobility. 1 text message daily, which is a reminder to take their asthma medication. No adherence feedback. No interactivity with patient. Control group: Not receiving text message reminder |
|
| Outcomes | Adherence, medication reimbursements, eNO, lung function, methacholine challenge | |
| Notes |
Type of publication: peer‐reviewed Funding: GlaxoSmithKline COI: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation in blocks by sex |
| Allocation concealment (selection bias) | Unclear risk | It is unclear how participants were allocated into each group |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | All participants were informed of the aim of the study, so aware of the impact on medication taking |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | All participants were instructed to bring their asthma medicine to the following visit for adherence measurement where medicine dose‐count was used from the inhaler device. Pharmacy records were used to verify this, so potential of being influenced by knowledge of group. ACQ and AQLQ were used for assessing control and quality of life. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 26 were randomised, 22 completed at the end of the study (2 lost to follow‐up in SMS group, 2 in control group ‐ balanced) |
| Selective reporting (reporting bias) | Low risk | Outcome measures appear to all be reported as per methods |
Sulaiman 2018.
| Study characteristics | ||
| Methods |
Design: block and stratified randomisation, open‐label randomised controlled trial Duration: endpoint at 12 weeks Setting: recruited from 5 specialist asthma clinics; trial carried out in Ireland |
|
| Participants |
Population: 218 participants were randomised to receive the (bio)feedback guided training based on an INhaler Compliance Assessment (INCA) device attached to their inhaler (n = 111) or were allocated to an intensive education group not based on the INCA device (n = 107) Age: range from: 33 to 66 years old. Mean age in the (bio)feedback group was 48.2 years; SD = 17.0. Mean age in the intensive education group was 50.3 years; SD = 15.9. Proportion of male participants: (bio)feedback group was 70.3% male; intensive education group was 69.9% male Asthma severity: severe Baseline lung function: FEV1%L 2.2 ± 0.9; FEV1% predicted 73.0 ± 22.1; FEV1/FVC% 66.2 ± 12 Smoking history: 8% smokers, 36% ex‐smokers, 56% never smoked Inclusion criteria: patients already attending the specialist clinic, prescribed therapy equivalent to step 3 or higher on the Asthma Management guidelines for more than 3 months, with at least 1 exacerbation that was treated with systemic glucocorticoids in the prior year, and whose condition was not controlled as per GINA definition of uncontrolled asthma Exclusion criteria: unwillingness to participate in clinical study, prior hypersensitivity to salmeterol/fluticasone Percentage withdrawn: 11.5% Withdrawal from (bio)feedback group: 9.91% Withdrawal from intensive education group: 11.21% Allowed medication: none recorded Disallowed medication: none recorded |
|
| Interventions |
(Bio)feedback group: participants received repeated training in inhaler use, adherence and disease management enhanced by the INCA device being attached to their inhaler. A digital audio recording was made each time the inhaler was used to record adherence. Nurses were both providers and the in‐person component in this intervention? There were 3 intervention sessions, which were carried out monthly. Participants were provided with visual (bio)feedback on their specific components of adherence to improve adherence. No co‐interventions used. Not a theory‐based intervention. Intensive education group: also used the INCA device, however did not receive (bio)feedback based on this device. They received repeated training in inhaler use, and adherence and disease management. |
|
| Outcomes |
Primary: rate of actual inhaler adherence Secondary: pre‐defined assessment of clinical outcome |
|
| Notes |
Type of publication: peer‐reviewed Funding: Health Research Board of Ireland, Dublin Clinical Center for Research, GSK COI: none |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Block randomised by an electronic system and stratified by site. Block sizes were random and varied from eight to 12, with a 1:1 allocation. |
| Allocation concealment (selection bias) | Unclear risk | Outcome assessors blinded to group allocation but methods were not stated |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Unblinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessors blinded to group allocation and outcomes were obtained via actual recorded measurements, i.e. not based on assessor‐participant communication |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Difference in follow‐up between groups was small and power calculation was included |
| Selective reporting (reporting bias) | Low risk | All data presented specified as per abstract and methods; no study protocol |
Van der Meer 2009.
| Study characteristics | ||
| Methods |
Design: block and stratified randomisation, open‐label controlled trial Duration: endpoint at 52 weeks Setting: recruited from 37 general practices and 1 academic outpatient department; trial carried out in the Netherlands Details of run‐in period: collected baseline data during a period of 2 weeks |
|
| Participants |
Population: 200 participants were randomised to receive Internet‐based self‐management (n = 101) or usual care (n = 99) Age: range from: 18 to 50 years old; mean age in the Internet group was 36 years; mean age in usual care was 37 Proportion of male participants: Internet group was 32% male; usual care was 29% male Baseline lung function: Internet group ‐ mean FEV1 = 3.08%; usual care ‐ mean FEV1 = 3.13% Smoking history: Internet group ‐ 58% never smoked, 30% former smoker, 12% current smoker; usual care ‐ 53% never smoked, 33% former smokers, 14% current smokers Inclusion criteria: physician‐diagnosed asthma coded according to the International Classification of Primary Care in the electronic medical record, age 18 to 50 years, prescription of inhaled corticosteroids for at least 3 months in the previous year, no serious comorbid conditions that interfered with asthma treatment, access to the Internet at home, and mastery of the Dutch language Exclusion criteria: patients who were receiving maintenance oral glucocorticosteroid treatment Percentage withdrawn: 9.00% Withdrawal from the Internet group: 8.91% Withdrawal from usual care group: 9.09% Allowed medication: none recorded Disallowed medication: maintenance oral glucocorticosteroid treatment |
|
| Interventions |
Internet group: weekly asthma control monitoring and treatment advice, online and group education, and remote web communications with a specialised asthma nurse. Participants had an Internet‐based asthma action plan and undertook weekly completion of the ACQ. After reporting the ACQ, participants instantly received a return message on the website including advice on how to adjust treatment, therefore interactivity was two‐way. Intervention had a 12‐month duration. No adherence feedback. No co‐interventions used. Not a theory‐based intervention. Usual care: usual physician‐provided care according to the Dutch general practice guidelines on asthma management in adults (medical review and treatment adjustment every 2 to 4 weeks in unstable asthma and medical review once or twice yearly for patients whose asthma is under control. |
|
| Outcomes |
Primary: asthma‐related quality of life Secondary: asthma control, symptom‐free days, pre‐bronchodilator FEV1, daily ICS dose, exacerbations |
|
| Notes |
Type of publication: peer‐reviewed Funding: Netherlands Organization for Health Research and Development, ZonMw, and Netherlands Asthma Foundation COI: none |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated, permuted‐block scheme randomisation |
| Allocation concealment (selection bias) | Low risk | Allocation via computer to ensure concealment |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Unblinded |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Unblinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Minimal loss to follow‐up; no power calculation ‐ similar rates of loss to follow‐up |
| Selective reporting (reporting bias) | Low risk | All data presented specified as per abstract and methods; no study protocol |
Van Sickle 2016.
| Study characteristics | ||
| Methods |
Design: randomised controlled study Duration: endpoint at 26 weeks Setting: trial carried out in the United Kingdom |
|
| Participants | Population: 125 participants were randomised to receive electronic inhaler sensors with access to data and functionalities (n = 67) or received sensors without patient or care manager access to data (n = 58) | |
| Interventions |
WITH sensor‐enabled data collection: electronic inhaler sensors could track medication use, provide access to smartphone and online applications that provided patient visualisation of their data, give reminders to promote adherence, give personalised and guidelines‐based education to patients. Clinical care managers provided feedback by viewing patients’ data in an online dashboard to guide care. WITHOUT sensor‐enabled data collection: electronic inhaler sensors were also used in these patients, however no feedback or viewing of data was involved |
|
| Outcomes | Reduction of short‐acting beta‐agonist use Increased asthma‐free days Asthma control Controller medication adherence |
|
| Notes | Type of publication: abstract only | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation occurred but no detail given on how |
| Allocation concealment (selection bias) | Unclear risk | No allocation concealment described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding possible as patients and clinical care managers in the intervention group had access to data whereas control group did not. Possibility for participants to increase medication use with knowledge of group allocation. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | No blinding described, however adherence monitored by electronic inhaler sensors so unlikely affected by intervention |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No loss of follow‐up data reported |
| Selective reporting (reporting bias) | High risk | Methods do not describe all outcomes planned or reported |
Vasbinder 2016.
| Study characteristics | ||
| Methods |
Design: block randomised, open‐label controlled trial Duration: endpoint at 52 weeks Setting: recruited from 5 outpatient clinics; trial carried out in the Netherlands |
|
| Participants |
Population: 209 participants were randomised to receive real‐time medication monitoring/RTMM with short message service/SMS reminders (n = 108) or RTMM alone, i.e. without SMS reminders (n = 111) Age: range from: 4 to 11 years old. Mean age in RTMM with SMS reminders group was 7.8 years; SD = 2.2. Mean age in the RTMM alone group was 7.7 years; SD = 2.1. Proportion of male participants: RTMM with SMS reminders group was 58.42% male; the RTMM alone group was 66.67% male Smoking history: RTMM with SMS reminders group ‐ 16.8% current smokers, 24.8% former smokers, 56.9% never smoked, 1.5% unknown; RTMM alone group ‐ 20.8% current smokers, 28.2% former smokers, 49.5% never smoked, 1.4% unknown Inclusion criteria: 4 to 11 years with doctor diagnosed asthma for > 6 months, visited outpatient clinic in the past 12 months, the use of ICS (fluticasone, fluticasone/salmeterol or beclometasone) delivered via pMDI for > 3 months, and having at least one parent/caregiver with a mobile phone Exclusion criteria: eligible patients unresponsive to telephone calls and patient information leaflets Percentage withdrawn: 4.57% Withdrawal from digital intervention group: 6.48% Withdrawal from control group: 2.70% Allowed medication: none recorded Disallowed medication: none recorded |
|
| Interventions |
RTMM with SMS reminders group: an RTMM device was connected to the pressurised metered‐dose inhaler (pMDI), where the time and date of administered ICS doses were recorded. Data would be immediately sent to the study database via the mobile telephone network. 2 types of digital interventions as “time‐tailored” SMS reminders were also sent to parents and children, if they possessed a mobile phone, when a dose had not been recorded within 15 minutes of the planned time of administration, therefore the digital interactivity was two‐way. This occurred daily for 365 days. No adherence feedback. No co‐interventions used. Not a theory‐based intervention. RTMM alone group: an RTMM device was also used, however there was only 1 type of digital component as there were no SMS reminders with missed doses |
|
| Outcomes |
Primary: adherence to ICS Secondary: asthma control, frequency of severe asthma exacerbations, asthma‐specific quality of life |
|
| Notes |
Type of publication: peer‐reviewed Funding: the Netherlands Organization for Health Research and Development; GlaxoSmithKline; Evalan BV COI: none |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated block randomisation was used per hospital with block size of 16 patients |
| Allocation concealment (selection bias) | Low risk | At registration at the RTMM software interface, children were automatically assigned to the intervention or control group |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Patients unblinded shortly after the start of the study period, when they found out whether they received SMS reminders or not |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Participants regularly interviewed by research assistants about outcomes, potential for outcomes to be influenced |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Difference in follow‐up between groups was small and power calculation was included |
| Selective reporting (reporting bias) | High risk | Outcomes for “healthcare use” not reported as per protocol |
Vollmer 2011.
| Study characteristics | ||
| Methods |
Design: stratified randomised controlled trial Duration: endpoint at 72 weeks Setting: recruited from Kaiser Permanente/KP (a group‐model health maintenance organisation); trial carried out in the United States |
|
| Participants |
Population: 8517 participants were randomised to receive IVR calls (n = 7033) or usual care (n = 7031) Age: range from: 18 to 98 years old. Mean age in IVR group was 53.7 years; SD = 15.3. Mean age in the usual care group was 53.5 years; SD = 15.3. Proportion of male participants: IVR group was 32.2% male; usual care group was 35.3% male Proportion of white ethnic participants: IVR group was 51.5% white; usual care group was 48.4% white Smoking history: 8.2% current smokers, 9.1% former smokers, 43.3% never smoked, 39.5% unknown Inclusion criteria: treatment for asthma during the 12‐month period prior to randomisation, 1 or more dispensation of a respiratory medication (corresponding to Generic Product Identifier (GPI) class 44 (anti‐asthma drugs, including inhaled steroids, leukotriene antagonists, beta2‐agonists, and ipratropium bromide)) at a KPNW (northwest region members) or KPH (Hawai’i region members) outpatient pharmacy during the 12‐month period prior to randomisation, aged 18 years and older as of the time of randomisation, continuous KP membership from the start of the baseline year until the time of randomisation, willing to participate in the study Exclusion criteria: individuals meeting the above criteria were only included in the final analysis sample if they ever received (or for usual care participants would have qualified for) an intervention call Percentage withdrawn: 39.46% Withdrawal from IVR group: 40.38% Withdrawal from usual care group: 38.53% Allowed medication: none recorded Disallowed medication: none recorded |
|
| Interventions |
IVR group: the intervention included 3 basic IVR call types, each of which typically lasted 2 to 3 minutes: a refill reminder call, a tardy refill call, and an initiator/restart call. The electronic medical record (EMR) was used to determine which type of call a participant was eligible for, where calls were carried out monthly for 18 months. There was two‐way interaction with patients. No adherence feedback. No co‐interventions used. Not a theory‐based intervention. Usual care group: usual care; no call |
|
| Outcomes |
Primary: ICS adherence Secondary: asthma morbidity |
|
| Notes |
Type of publication: peer‐reviewed Funding: National Institute of Health (NIH); National Heart, Lung, and Blood Institute (NHLBI) COI: none |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation stratified by region and the clinic facility to which each patient was panelled but no information on how randomised |
| Allocation concealment (selection bias) | Unclear risk | Data not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Data not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Adherence measured objectively by electronic medical records |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No information given except that only 56% of intervention participants completed the follow‐up survey |
| Selective reporting (reporting bias) | High risk | Stated would measure ACT, AQLQ and satisfaction with intervention but not reported in results |
Weinstein 2019.
| Study characteristics | ||
| Methods |
Design: individually randomised, open‐label controlled trial Duration: endpoint at 13.04 weeks Setting: recruited from Allergy/Clinical Immunology and Pulmonary departments; trial carried out in the United States |
|
| Participants |
Population: 39 participants were randomised to use the Asthma Adherence Pathway/AAP Internet Application and an electronic monitoring device/EMD (n = 27) or usual care (n = 23) Age: range from: 23 to 69 years old; mean age in the Asthma Adherence Pathway and EMD group was 41; mean age in usual care was 39 Proportion of male participants: Asthma Adherence Pathway and EMD group was 40% male; usual care was 25% male Asthma severity: moderate to severe asthma Baseline lung function: Asthma Adherence Pathway and EMD group FEV1 = 0.76%; usual care FEV1 = 0.70% Inclusion criteria: suboptimal asthma control (Asthma Control Questionnaire (ACQ) score > 1.0) and prescribed an ICS or an ICS/LABA for at least 1 month before screening Exclusion criteria: intermittent asthma, asthma exacerbation over the past 3 months, serious uncontrolled medical conditions, diagnosis of any other chronic pulmonary disease Percentage withdrawn: 22% Withdrawal from the AAP and EMD group: 19% Withdrawal from usual care: 20% Allowed medication: none recorded Disallowed medication: none recorded |
|
| Interventions |
Asthma Adherence Pathway and EMD group: intervention patients completed the Asthma Adherence Pathway software and were given barrier‐specific motivational interviewing adherence strategies and a SmartTrackdevice to monitor mometasone furoate/formoterol (MF/F) use (where inhalations were taken twice daily). Clinicians in the interventional group received adherence management training. Interventional patients were given feedback regarding adherence findings at each monthly visit, so interactivity with patients was two‐way. No co‐interventions used. Not a theory‐based intervention. Usual care: usual asthma care |
|
| Outcomes | Secondary: quality of life | |
| Notes |
Type of publication: peer‐reviewed Funding: Merck & Co. COI: AG Weinstein is President of Asthma Management Systems, LLC (Newark, Del). The rest of the authors declare that they have no relevant conflicts of interest. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Randomised in an alternating process to intervention or control group |
| Allocation concealment (selection bias) | Unclear risk | No information given on concealment |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding of clinicians and patients to interventions was not possible for this intervention, however the intervention and control group clinicians evaluated participants in different areas of the medical centre and were asked not to discuss study interactions with each other |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Groups were unblinded. Patients in the intervention group were assessed and given MF/F feedback. Adherence performed at follow‐up visits so likely affected behaviour (despite adherence being assessed objectively by EMD). |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Baseline data from the dropout groups were not included in the analysis and power was not calculated but loss to follow‐up rates were similar in both groups |
| Selective reporting (reporting bias) | Low risk | All data presented specified as per abstract and methods |
Wiecha 2015.
| Study characteristics | ||
| Methods |
Design: prospective randomised controlled pilot trial Duration: endpoint at 26 weeks Setting: recruited from Boston community health centres, the Boston Medical Center, and other practices in the Boston area; trial carried out in the United States |
|
| Participants |
Population: 58 participants were randomised to use an interactive asthma website platform, BostonBreathes (n = 37) or usual care (n = 21) Age: range from: 9 to 17 years old. Mean age in the website platform group was 11.9 years; SD = 2.0. Mean age in usual care group was 12.9 years; SD = 3.0 Proportion of male participants: website platform group was 59.5% male; usual care group was 57.1% male Proportion of white ethnic participants: website platform group was 21.6% white; usual care group was 9.5% white Smoking history: website platform group was 22.9% smokers at home; usual care group was 42.9% smokers at home Inclusion criteria: children with a diagnosis of persistent asthma or on a controller‐type medication, caregivers could speak and read English with functioning Internet connection at home Exclusion criteria: not meeting the inclusion criteria, unable to complete screening, declined to participate Percentage withdrawn: 27.59% Withdrawal from website platform group: 24.32% Withdrawal from usual care group: 33.33% Allowed medication: none recorded Disallowed medication: none recorded |
|
| Interventions |
Website platform group: asthmatic children used an interactive, engaging website to promote adherence to asthma and provide a platform for teamwork between caregivers and patients, as well as giving primary care providers up‐to‐date symptom information and data on medication use. Web portal had asthma education with pre‐programmed feedback based on entry of symptoms, peak flow values, and medication use. For 6 months, there was a 2‐monthly review from the paediatric asthma specialist and asthma nurse specialist of data entered by patients via the BB website. A summary of their conclusions and treatment recommendations, based on entered data, was posted to the private discussion board for review by the physician and patient and caregiver (i.e. a two‐way interactivity). No co‐interventions used. Not a theory‐based intervention. Usual care group: patients received an asthma education manual, and peak flow meter, and otherwise usual care from their physicians |
|
| Outcomes | Primary: adherence to maintenance medication | |
| Notes |
Type of publication: peer‐reviewed Funding: The Commonwealth Fund COI: none |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation occurred but no detail given on how |
| Allocation concealment (selection bias) | Unclear risk | No allocation concealment described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Data not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Home visit training and data collection by research assistant means outcomes could have been affected, however adherence was collected objectively by doser |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Stated that there was no significant dependence of dropout on the outcome values, but dropout rates similar between groups |
| Selective reporting (reporting bias) | Low risk | All data presented specified as per abstract and methods |
Zhou 2018.
| Study characteristics | ||
| Methods |
Design: parallel, randomised controlled trial Duration: endpoint at 12 weeks Setting: recruited from secondary setting: hospital;trial carried out in China |
|
| Participants |
Population: 65 children were randomised to receive electronic device with nebuliser group (n = 35) or usual nebulisation (n = 30) Age: maximum age was 5 years old; mean age was 3.15 years Proportion of male participants: 44% male Proportion of white ethnic participants: 0% white Asthma severity: mixed Inclusion criteria: wheezing children; boys or girls under the age of 5 years; children with positive API (Asthma Predictive Index) who came to Renji Hospital with asthma exacerbation for the first time; parents having signed consent forms and agreed to provide information during the 12‐week study period; children with wheezing episodes that were not caused by congestive heart disease, airway deformity, or occlusive bronchitis Exclusion criteria: not stated Percentage withdrawn: 0 Allowed medication: ICS; bronchial dilator, oral steroid, antibiotics, oral antihistamine drug or leukotriene antagonists can also be given Disallowed medication: none recorded |
|
| Interventions |
Electronic device with nebuliser group: smart electronic device connected to nebuliser; with reminder function and collected data on rate of adherence to ICS, frequency of emergency visits or hospitalisations, application of antibiotics or oral steroids, and wheezing progression or improvement; these data were connected to smart phones via an app. Parents could obtain data on time, duration, and frequency of nebulisation. Parents interviewed every 2 weeks via phone call. There was an in‐person component as paediatricians monitored children's progress and adherence to ICS therapy remotely and had real‐time communication with children's parents when necessary; paediatrician interview every two weeks. There was adherence feedback, as paediatrician could remind children to take the nebulisation if they forgot to do so. No digital interaction with patient. There was a co‐intervention: an application (2 digital components involved in this intervention). Not a theory‐based intervention. Conventional nebulisation group: had a paediatrician interview every 2 weeks via phone call |
|
| Outcomes |
Primary: ICS adherence rate Secondary: day and night‐time symptom scores; additional drug usage; therapy cost |
|
| Notes |
Type of publication: peer‐reviewed Funding: Medical and Industrial Cross Research Fund of Shanghai Jiao Tong University COI: none |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding described and could affect outcome |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinding described, adherence monitored via EMD and electronically, which could be influenced |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No loss to follow‐up but no power calculation stated |
| Selective reporting (reporting bias) | Low risk | Results specified as per methods |
Abbreviations: ACQ: asthma control questionnaire; ACT: asthma control test; AQLQ: asthma quality of life questionnaire; BMQ: Beliefs about Medicines Questionnaire; BTS: British Thoracic Society; CARAT: control of allergic rhinitis and asthma test; COPD: chronic obstructive pulmonary disease; EMD: electronic monitoring device; ED: emergency department; FEV1: forced expiratory volume in 1 second; GINA: Global Initiative for Asthma; GP: general practitioner/physician; ICS: inhaled corticosteroid; IPQ: Illness Perceptions Questionnaire; IQR: interquartile range; IRF: inhaler reminders and feedback; IVR: interactive voice response; LABA: long‐acting beta‐agonist; MDI: metered‐dose inhaler; OCS: oral corticosteroid; PAD: personalised adherence discussions; PEF/PEFR: peak expiratory flow rate; PAQLQ: Paediatric Asthma Quality of Life Questionnaire; QOL: quality of life; RCT: randomised controlled trial; RTMM: real‐time medication monitoring; SABA: short‐acting beta agonist; SD: standard deviation; SMS: short messaging system; VAS: visual analogue scale
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Adejumo 2018 | Wrong study design |
| Ahmed 2016 | Wrong study objective ‐ no adherence |
| Ainsworth 2019 | Wrong objective |
| Anderson 2017 | Wrong publication type |
| Apter 2019 | Wrong intervention |
| Bender 2018 | Wrong publication type |
| Beydon 2017 | Wrong objective |
| Biblowitz 2018 | Wrong publication type |
| Bonini 2018 | Wrong publication type |
| Boutopoulou 2018 | Wrong publication type |
| Britto 2017 | Cross‐over study |
| Bruzzese 2021 | Wrong objective |
| Chan 2017 | Wrong study objective ‐ acceptability |
| Chen 2013 | Wrong study objective ‐ no adherence |
| Christakis 2012 | Comparator not non‐digital or usual care |
| Cingi 2015 | Wrong study objective ‐ no adherence |
| Claus 2004 | Wrong study design ‐ not RCT |
| Dermot 2009 | Wrong publication type ‐ protocol |
| Dermot 2012 | Wrong study objective ‐ no adherence |
| Federman 2018 | Wrong intervention |
| Fonseca 2006 | Wrong study objective ‐ no adherence |
| Frémont 2018 | Wrong objective |
| Gregoriano 2017 | Wrong publication type, wrong population |
| Gregoriano 2019 | Wrong population (unable to separate COPD and asthma) |
| Grossman 2017 | Wrong study design |
| Gustafson 2012 | Telephone‐based intervention |
| Halterman 2012 | Wrong study objective ‐ no adherence |
| Halterman 2018 | Wrong intervention ‐ not digital (telemedicine) |
| Hayter 2019 | Wrong publication type |
| Hew 2019 | Wrong publication type |
| Hoch 2019 | Wrong outcome |
| Jeminiwa 2019 | Wrong publication type |
| Joseph 2013 | Wrong study objective ‐ no adherence |
| Katwa 2018 | Wrong publication type |
| Kojima 2005 | Wrong intervention ‐ not digital |
| Koumpagioti 2020 | Wrong intervention |
| Lathy 2009 | Wrong study objective ‐ no adherence |
| Lathy 2019 | Wrong population, wrong intervention |
| Lau 2015 | Wrong study objective ‐ no adherence |
| Licskai 2016 | Wrong study objective ‐ no adherence |
| Lin 2020 | Wrong intervention, wrong outcome |
| Liu 2011 | Wrong study objective ‐ no adherence |
| Lombard 2019 | Wrong study design, wrong objective |
| Makhecha 2019 | Wrong objective |
| McPherson 2006 | Intervention aim not to improve adherence |
| Newhouse 2016 | Wrong study objective ‐ no adherence |
| Normansell 2017 | Wrong study design |
| Ostojic 2005 | Wrong study objective ‐ no adherence |
| Pearce 2018 | Wrong publication type |
| Perry 2017 | Wrong study objective ‐ no adherence |
| Poureslami 2017 | Wrong objective |
| Poureslami 2019 | Wrong study design, wrong outcome |
| Rasmussen 2005 | Wrong study objective ‐ no adherence |
| Real 2019 | Wrong outcome |
| Seid 2012 | Wrong study objective ‐ no adherence |
| Stukus 2018 | Wrong study objective ‐ no adherence |
| Sutherland 2017 | Wrong publication type |
| Teufel 2018 | Wrong study design, wrong objective |
| Unni 2018 | Wrong publication type |
| Van Gaalen 2013 | Wrong study objective ‐ no adherence |
| Voorend‐van Bergan 2015 | Wrong study objective ‐ no adherence |
| Weinstein 2017 | Wrong study objective ‐ no adherence |
| Williams 2010 | Wrong population (health professionals) |
| Yun 2013 | Wrong study design ‐ qualitative |
COPD: chronic obstructive pulmonary disease; RCT: randomised controlled trial
Characteristics of studies awaiting classification [ordered by study ID]
ACTRN12620001006932.
| Methods | Randomised controlled trial embedded within the AIR Algorithm Study |
| Participants | 100 participants |
| Interventions | The tutorials have been designed specifically for this sub‐study. They consist of educational comics followed by interactive short quizzes, which provide feedback. |
| Outcomes | The AIR‐T sub‐study aims to find out if interactive online tutorial resources which introduce patients with asthma to an Anti‐Inflammatory Reliever algorithm (AIR algorithm), a novel self‐management treatment algorithm, can improve participant understanding of and adherence to this algorithm. |
| Notes | — |
Adejumo 2020.
| Methods | Randomised controlled study |
| Participants | Participants with doctor‐diagnosed asthma reporting an exacerbation in the preceding 12 months |
| Interventions | EMD‐based feedback to participants as an intervention to improve adherence and clinical outcomes |
| Outcomes | Asthma control, quality of life and exacerbation data |
| Notes | — |
Almonacid 2021.
| Methods | Prospective, multicentre, randomised, parallel‐group clinical trial conducted in 10 asthma clinics in Spain |
| Participants | 53 patients in the SMS group and 88 patients in the control group |
| Interventions | Motivational messages using short message service (SMS, or text) to improve adherence to inhaled medication |
| Outcomes | Adherence assessed with electronic monitors |
| Notes | — |
Chen 2020.
| Methods | Randomised controlled trial |
| Participants | 96 recruited children (aged 6 months to 3 years) with mild or moderate persistent asthma who were on regular inhaled corticosteroids |
| Interventions | Electronic monitoring combined with instant messaging software (IMS)‐based weekly feedback regarding adherence along with a reminder to keep taking the ICS (intervention group) |
| Outcomes | Mean device‐monitored adherence |
| Notes | — |
CTRI/2021/02/031075.
| Methods | Randomised controlled trial |
| Participants | 18 years on treatment for at least 6 months period with 2 or more select chronic diseases including asthma |
| Interventions | Structured and customised education using booklet with motivational discussion based on barriers identified at baseline ‐ diary provided and explained. Follow‐up on the 15th day, 3rd and 5th month over phone for motivational discussion, measure adherence, ensure use of VITA and to ask for change in any medication in last month.Follow‐up thrice once at 1st, 2nd month and 4th month for motivational discussion. One text message sent every fortnight. |
| Outcomes | Medication adherence |
| Notes | — |
Cvietusa 2020.
| Methods | Pragmatic randomised controlled trial |
| Participants | 7522 adult patients with persistent asthma |
| Interventions | Automated medication reminders ‐ text, email, or phone |
| Outcomes | Medication adherence and asthma outcomes |
| Notes | — |
Ebrahimabadi 2019a.
| Methods | Randomised clinical trial |
| Participants | 80 asthmatics |
| Interventions | Infographics and video |
| Outcomes | Morisky adherence to medication |
| Notes | — |
EUCTR2019‐003082‐17‐DE.
| Methods | Randomised controlled trial |
| Participants | 18 to 65 years with ACT score 19 or less |
| Interventions | Connected Easyhaler |
| Outcomes | Adherence to controller medication (the percentage of doses taken of the doses prescribed) |
| Notes | — |
Gupta 2021.
| Methods | Randomised controlled trial |
| Participants | Caregiver and child dyads with asthma |
| Interventions | Inhaler sensors that allowed for caregiver and clinician electronic monitoring of medications |
| Outcomes | Asthma Control Test scores (≥ 19 indicated asthma control) and asthma health care use. Caregiver quality of life (QoL) and child ICS adherence were also assessed |
| Notes | — |
Henderson 2020.
| Methods | Randomised controlled trial: 1 of 3 arms |
| Participants | Children aged 5 to 12 years who have had either at least 2 hospitalisations or one hospitalisation and one emergency department visit for asthma in the year prior to their enrollment (and their caregivers) |
| Interventions | Participants in arm 1 receive daily text message reminders, feedback, and gain‐framed, nominal financial incentives; participants in arm 2 receive daily text message reminders and feedback only, and participants in arm 3 receive no reminders, feedback, or incentives |
| Outcomes | Inhaled corticosteroid use in patients with high‐risk asthma |
| Notes | — |
Hollenbach 2021.
| Methods | Pilot, randomised, controlled trial |
| Participants | Children with persistent asthma managed with daily inhaled corticosteroids (ICS) |
| Interventions | EMDs (one for ICS and one for rescue) linked via Bluetooth to a mobile application (app) |
| Outcomes | Medication adherence was measured using pharmacy refill records and self‐report, whereas EMD data were used to measure adherence in the intervention group. Secondary outcomes included asthma control, pulmonary function, and quality of life. |
| Notes | — |
Lombard 2019a.
| Methods | Randomised controlled trial |
| Participants | 2 participants with uncontrolled asthma |
| Interventions | INCA‐directed inhaler education and medication adjustment according to objective adherence |
| Outcomes | Adherence and asthma control |
| Notes | — |
Moore 2020.
| Methods | Open‐label, parallel‐group, 6‐month, randomised controlled trial |
| Participants | 437 adults with uncontrolled asthma (Asthma Control Test (ACT) score < 20) on fixed‐dose inhaled corticosteroid/long‐acting beta‐agonist maintenance therapy |
| Interventions | One of 5 CIS study arms (1:1:1:1:1) reflecting the recipient of the data feedback from the sensors: 1) maintenance use to participants and HCPs (N = 87); 2) maintenance use to participants (N = 88); 3) maintenance and rescue use to participants and HCPs (N = 88); 4) maintenance and rescue use to participants (N = 88); 5) no feedback (control) (N = 86) |
| Outcomes | Observed mean adherence (SD) to maintenance therapy |
| Notes | — |
Mosnaim 2020.
| Methods | Randomised controlled trial |
| Participants | Adults with uncontrolled asthma and prescribed ICS and SABA |
| Interventions | Patient self‐monitoring via electronic medication monitoring and smartphone application plus remote clinician feedback |
| Outcomes | Percentage of SABA‐free days and ICS adherence |
| Notes | — |
NCT04401332.
| Methods | Randomised controlled trial |
| Participants | 500 asthma patients |
| Interventions | An mHealth app that can be installed on patients' smartphones that integrates into clinical workflow; and an asthma PRO dashboard in the electronic health record (EHR) for clinicians |
| Outcomes | Patient‐reported asthma quality of life and asthma‐related healthcare utilisation (defined as urgent care and emergency room visits and hospitalisations) |
| Notes | — |
NCT04607681 2020.
| Methods | Randomised controlled trial |
| Participants | Patients diagnosed with asthma who are registered in Family Health Centers in Eskisehir Tepebası and Odunpazarı districts |
| Interventions | Web‐designed asthma education programme for asthma patients |
| Outcomes | COPD and asthma fatigue scale, drug compliance reporting scale and Asthma Control Test (AKT) |
| Notes | — |
NCT04633018 2020.
| Methods | Randomised, single group assignment, controlled trial |
| Participants | Children self/family‐identified as Hispanic or Latino, 2) school‐aged (5 to 12 years) and attends school within the Lancaster County School District, 3) has received a diagnosis of asthma from a healthcare provider and is taking a controller medication, and 4) parents/primary caregiver (e.g. grandparents, extended family) language of preference is Spanish |
| Interventions | AsthmaMD mobile application: asthma management app with Spanish‐language user interface |
| Outcomes | Medication adherence and lung function test; secondary outcome measures include frequency of rescue inhaler use, as well as asthma exacerbations, outpatient clinic visits, and emergency department visits. Lung capacity will be obtained pre‐ and post‐intervention/bronchodilation using spirometry to obtain relevant lung function variables such as FEV1 and FEV1/FVC. Measures from the control group will include medication counts, number of asthma exacerbations, ED/outpatient clinic visits, and spirometry measures on enrollment, during the intervention phase, and again at the end of the intervention. |
| Notes | — |
NCT04677959 2020.
| Methods | Randomised controlled trial |
| Participants | 13 years and older:
The participant is willing to discontinue all other maintenance ICS with LABA medications and rescue medications and replace them with the study‐provided fluticasone propionate/salmeterol (FS) multidose dry powder inhaler with integrated electronic module (eMDPI) and albuterol eMDPI, respectively, for the duration of the trial, if randomised to the Digital System group. All other asthma maintenance medications, except for ICS with LABA, may be continued. |
| Interventions | Digital System (DS) in improving asthma control: eMDPI DS, including inhaler, smart device application (App), DHP (Cloud solution), and dashboard |
| Outcomes | Proportion of participants achieving well‐controlled asthma or clinically important improvement in asthma as indicated by Asthma Control Test (ACT) score |
| Notes | — |
NCT04744272 2021.
| Methods | Randomised, parallel assignment controlled trial |
| Participants |
Any of the following measures of asthma control:
Frequent symptoms and/or:
|
| Interventions | myAsthma ‐ an online digital self‐management application to support asthma patients by offering education, inhaler technique, pulmonary rehabilitation, symptoms and medication usage tracking remotely |
| Outcomes | Change in Asthma Control Test (ACT) scores Assessment of Inhaler Technique using the UK Inhaler Group (UKIG) Standards and Competencies ‐ 7 Steps Exacerbations Change in EuroQol 5D‐5L scores myAsthma patient feedback (intervention arm) |
| Notes |
NCT04869384 2021.
| Methods | Randomised parallel‐assignment controlled trial |
| Participants | Male and female participants with documented diagnosis of asthma, aged 18 to 65 years, ACT score 19 or less at screening, treatment with oral corticosteroids or hospital or emergency department admission due to asthma exacerbation within the past year |
| Interventions | Reminders and feedback to improve their adherence via a sensor attached to Easyhaler inhaler and mobile application |
| Outcomes | Mean weekly adherence to controller medication |
| Notes | — |
Riley 2021.
| Methods | Wait list randomised controlled pilot trial |
| Participants | 29 black adults who self‐reported ICS non‐adherence, had uncontrolled persistent asthma, and a Duke Primary Care provider visit within the past 3 year |
| Interventions | Comprehensive inhaled corticosteroid (ICS) adherence intervention designed to remediate each patient's unique reason for not taking their ICS as prescribed |
| Outcomes | Primary outcomes were feasibility (e.g. process outcomes) and acceptability (e.g. patient exit interviews) measured at 12 weeks. Secondary asthma (e.g. ACT) and adherence outcomes (e.g. DOSE non‐adherence) were measured. |
| Notes | — |
Sportel 2020.
| Methods | Randomised controlled trial lasting 6 weeks |
| Participants | 68 uncontrolled moderate to severe asthmatic children between 6 and 18 years old who receive controller inhalation medication through the Nexthaler(R), Ellipta(R), or Spiromax(R) |
| Interventions | Immediate smart feedback about the performed inhalations via a mobile application |
| Outcomes | Asthma control can be assessed by means of spirometry (both at home and in the hospital) and (c‐)ACT questionnaires |
| Notes | — |
UMIN000042690.
| Methods | Randomised controlled trial |
| Participants | Patients with moderate to severe asthma |
| Interventions | Dubaobao tracker ‐ turbuhaler‐incorporated tracker |
| Outcomes | Treatment difference (post‐ minus pre‐treatment) of FeNO between the tracker and usual care groups |
| Notes | — |
ACT: asthma control test; COPD: chronic obstructive pulmonary disease; EMD: electronic monitoring device; ED: emergency department; FEV1: forced expiratory volume in 1 second; FVC: forced vital capacity; ICS: inhaled corticosteroid; INCA: Inhaler Compliance Assessment; QOL: quality of life; SABA: short‐acting beta agonist; SMS: short messaging system
Characteristics of ongoing studies [ordered by study ID]
Arain 2020.
| Study name | |
| Methods |
Design: parallel, randomised controlled trial Duration: endpoint at 26 weeks Setting: recruiting from East Calgary Family Care Clinic; trial conducted in Canada |
| Participants |
Population: 50 participants will be randomised to the medication dispensing system group or the control group Age: at least 50 years Inclusion criteria: 50 years or older diagnosed with one or more chronic condition(s) including asthma, taking 5 or more prescribed oral medications; English speaker and resident of the city Calgary Exclusion criteria: patients with moderate to sever cognitive impairment |
| Interventions |
Intervention: medication management with medication dispensing system (Spencer) ‐ dispenses medication on time with visual and audio reminders Control: continuation of current medication management regime (e.g. blister packs) |
| Outcomes | Medication adherence |
| Starting date | 1 March 2019 |
| Contact information | Mubashir Aslam Arain, Senior Research and Evaluation Consultant, Alberta Health Services, Calgary |
| Notes |
Type of publication: clinicaltrials.gov registry record only NCT04339296 Funding: Centre of Aging and Brain Health Innovation (CABHI) |
Jariwala 2018.
| Study name | Adapting and expanding the Asthma‐Educator app |
| Methods |
Design: open‐label randomised control trial Duration: endpoint at 16 weeks Setting: recruiting from primary and secondary care: outpatient primary and specialty care sites; trial conducted in United States |
| Participants |
Population: 130 participants will be randomised to receive mobile application or usual care groups Age: range from 15 to 21 years Inclusion criteria: English‐speaking individuals between 15 and 21; persistent asthma (diagnosis made by a healthcare provider); on a daily controller medication; able to give informed consent; smartphone (iOS or Android) access Exclusion criteria: use of oral corticosteroids in the 2 weeks prior to the baseline visit; pregnancy; severe psychiatric or cognitive problems that would prohibit an individual from understanding and completing the protocol; patients that previously received the ASTHMA‐Educator application |
| Interventions |
Intervention: Adapting and Expanding the Algorithmic Software Tool to Help Manage Asthma (ASTHAMXcel) for Youth with Asthma App: includes interactive games, educational videos, quizzes and personalised feedback. One‐way interaction with patient. There is adherence feedback. No co‐interventions (one digital component). Not a theory‐based intervention. Control: usual care group |
| Outcomes |
Primary: asthma control Secondary: patient satisfaction; interface satisfaction; patient usage; ED visits; Asthma QoL; airway obstruction; medication adherence; health literacy; asthma knowledge; asthma symptom perception |
| Starting date | 1 May 2018 |
| Contact information | Contact: Sunit Jariwala, MD (718) 920‐4767 sjariwal@montefiore.org Contact: Obumneme Njeze, BS (973) 216‐1500 obumneme.njeze@einsteinmed.org |
| Notes |
Type of publication: clinicaltrials.gov registry record only NCT03930381 Funding: Montefiore Medical Centre |
Kang‐Cheng Su Su 2015.
| Study name | ICS/LABA combination with integrated dose counter and smartphone app to improve asthma control |
| Methods |
Design: parallel open‐label randomised controlled trial Duration: endpoint at 24 weeks Setting: participants recruited from 1 Taipei Veterans General Hospital; trial carried out in Taiwan |
| Participants |
Population: 112 participants were randomised to receive the mobile application or usual care Age: 20 to 70 years Inclusion criteria: symptomatic asthmatics free of controller medication for at least 3 months, age 20 to 70, lifelong smoking index < 10 pack‐years Exclusion criteria: COPD, clinically overt bronchiectasis, lung cancer, active tuberculosis, or other known specific pulmonary disease, co‐morbidities, alcohol or medication abuse, lower respiratory tract infection or received systemic steroid 4 weeks prior to study commencement, unwilling to comply with protocol, no smartphone |
| Interventions |
Digital intervention: Smartphone (My Asthma App, GlaxoSmithKline, Chinese version, or Line), which provides, multiple function, including health information (real‐time weather condition, air pollution index) at, the point‐of‐living, personalised health assessments (asthma control test, peak flow rate) and interactive action plans (green, yellow, and red light), and regular reminding for controller administration. The details of the provider are not stated. It is one digital component (the app). Control group: Usual care, no digital components |
| Outcomes | Airway inflammation profile including exhaled NO, cell counts, mediator in induced sputum, ACQ, lung function and medication use |
| Starting date | August 2014 |
| Contact information | |
| Notes | Type of publication: clinicaltrials.gov registry record only NCT02556073 |
Kenyon 2019.
| Study name | |
| Methods |
Design: double‐blinded, parallel, randomised controlled trial Duration: endpoint at 3 weeks Setting: recruiting from the Childrens Hospital of Philadelphia; trial conducted in the United States Details of run‐in period: run‐in period to determine eligibility and collect baseline adherence data |
| Participants |
Population: 125 participants will be randomised to intervention arm 1, 2, or the control arm Asthma severity: severe Inclusion criteria: 5 to 12 years with permission from parent or legal guardian if required. Caregivers with smartphone. Participants prescribed daily ICS or ICS + LABA. Minimum of 2 hospitalisations OR 1 hospitalisation + 1 emergency department visit in previous year. Exclusion criteria: controller inhaler not suitable for monitoring device. Smartphone with compatibility for trial‐specific application. Children with significant developmental delays or disability, chronic co‐morbidities with potential to impact asthma management. Families with Department of Human Services Involvement. Non‐English speaking. Parents or guardians with medical recommendation not to participate in trial. Allowed medication: none recorded Disallowed medication: none recorded |
| Interventions |
Intervention arm 1: daily automated text message reminders or push notification reminders to use ICS and fixed‐ratio monetary incentives for each inhaled dose. Every 7 days an automated feedback summary is delivered to the participant by an electronic mobile platform. 90 intervention sessions (1 reminder daily for 90 days). Two‐way interactivity with patient. No co‐interventions. Intervention arm 2: daily automated text message reminders or push notification reminders to use ICS. Every 7 days an automated feedback summary is delivered to the participant by an electronic mobile platform. 90 intervention sessions (1 reminder daily for 90 days). Two‐way interactivity with patient. Arm 3: Control: no text message or push notification reminders to use ICS |
| Outcomes | ICS medication adherence, adherence patterns, asthma control, asthma specific hospitalisations or emergency department visits and associated costs |
| Starting date | 1 September 2019 |
| Contact information | Chen Kenyon, MD Children's Hospital of Philadelphia |
| Notes |
Type of publication: clinicaltrials.gov registry record only NCT03907410 Funding: Childrens Hospital of Philadelphia, National Institute of Health, National Heart, Lung, and Blood Institute |
La Grutta 2020.
| Study name | The use of an innovative device for therapeutic adherence in pediatric asthma |
| Methods |
Design: parallel open‐label randomised controlled trial Duration: endpoint at 18 weeks Setting: recruited from Institute of Biomedicine and Molecular Immunology (IBIM), National Research Council;trial carried out in Italy |
| Participants |
Population: 18 participants were enrolled to receive digital intervention (n = 10 number) or control group (n = 10) Age: range from 6 to 17 Asthma severity: mixed (mild to moderate) Inclusion criteria: 6 to 17 years with uncontrolled mild to moderate persistent asthma Exclusion criteria: acute upper respiratory infections, immunological or metabolic systemic disease, major malformations of the upper airways, active smokers |
| Interventions |
Digital intervention: participants received Symbicort turbuhaler with Turbo+ (electronic device attached to the turbuhaler, which allows to feel whether the patient does the inhalation and to register it on specific application for 3 months. Intervention session is whenever the turbuhaler is used. One‐way digital interaction with patient. Control group: participants received Symbicort turbuhaler without Turbo+ |
| Outcomes | Adherence (MARs), asthma control (c‐ACT, ACT), quality of life (PAQLQ) |
| Starting date | 10 January 2019 |
| Contact information | Stefania La Grutta, MD, Co‐ordinator of the group "Clinical and Environmental Epidemiology of Pulmonary and Allergic Pediatric Diseases". Institute of Biomedicine and Molecular Immunology, IBIM, National Research Council of Palermo, Italy; Istituto per la Ricerca e l'Innovazione Biomedica |
| Notes |
Type of publication: clinicaltrials.gov registry record only NCT03788395 Funding: Istituto per la Ricerca e I'Innovazione Biomedica |
Landon 2019.
| Study name | |
| Methods |
Design: parallel, single‐blind randomised clinical trial Setting: recruited from Pediatric Diagnostic Center; trial carried out in the United States |
| Participants |
Population: participants to be randomised to receive an EMD with active guidance or an EMD without active guidance Age: 8 years old and above Asthma severity: mild to moderate asthma Inclusion criteria: asthma diagnosis, regular MDI user, ACT score 15 to 25, FEV1 between 60% and 80% of predicted, disease severity mild‐moderate, smartphone and Internet access for entire study duration, cognitively able to utilise the device and express interest in participating Exclusion criteria: patients without asthma, developmental disabilities, do not speak English, do not own a smartphone |
| Interventions |
EMD with active guidance: active guidance from CapMedic device on using MDIs correctly and regularly at home. The MDI usage is recorded using CapMedic device with active guidance turned on. EMD without guidance: standard‐of‐care instructions on using MDIs correctly and regularly at home. The MDI usage is recorded using CapMedic device with active guidance turned off. |
| Outcomes | Secondary: lung function, adherence to MDI |
| Starting date | 1 July 2019 |
| Contact information | Contact: Chris Landon, MD 8053401366 chris.landon@ventura.org Contact: Emilie Paronyan 8184393664 emilieparonyann@gmail.com |
| Notes |
Type of publication: clinicaltrials.gov registry record only NCT04250779 Funding: Landon Pediatric Foundation |
Linnhoff 2019.
| Study name | Effect of the use of an add‐on device connected to a smartphone app on difficult‐to‐treat asthmatic patient's adherence (ADVICE) |
| Methods |
Design: parallel, open‐label randomised controlled trial Duration: endpoint at 12 weeks Setting: recruited from Barlow medical centre; trial carried out in the United Kingdom |
| Participants |
Population: 176 participants were enrolled Age: ≥ 18 years Inclusion criteria: male or female patient aged 18 years and above, patient with established diagnosis of asthma for at least 6 months, patient on maintenance therapy (fixed‐dose combination ICS/LABA) with high dose of ICS, patient with ACT score < 20 at screening and at randomisation, non‐ or ex‐smoker who smoked ≤ 10 pack‐years prior to screening, patient must have their own Android® or iPhone operating system (IOS) smartphone, ability to use the pMDI device correctly Exclusion criteria: Asthma exacerbation or respiratory tract infection requiring systemic steroids and/or antibiotics within 1 to 3 months prior to screening, history of near‐fatal asthma, uncontrolled cardiac, hepatic, renal, gastrointestinal, endocrine, metabolic, neurologic, psychiatric, or any other disorder that would put the safety of the participant at risk through participation, or which would affect the analysis, patient not able to be compliant with the study requirements. BMI > 40, participating in the clinical phase of an interventional trial or have done so within the last 30 days prior to screening, who has an already planned major surgery or hospitalisation, pregnant or lactating or who plans to become pregnant in the next 4 months, history of hypersensitivity to any of the components of Foster pMDI |
| Interventions |
Intervention group: full experience using marketed application, with all functionalities enabled. Component used was mobile app provided by Chiesi Farmaceutici S.p.A. Adherence feedback was reminder from the app. One‐way interactivity with patient. Control group: control experience using marketed application to record medication intake without reminders from the app |
| Outcomes |
Primary: adherence rate of doses correctly taken twice daily Secondary: Asthma Control Test score, Test of the Adherence to Inhalers score, percentage of days without intake of rescue medication |
| Starting date | 12 August 2019 |
| Contact information | Annaliese Linnhoff, Research Center for Medical Studies Praxis für Lungen‐ und Bronchialheilkunde |
| Notes |
Type of publication: clinicaltrials.gov registry record only NCT03951714 Funding: Chiesi Farmaceutici S.p.A COI: no COI declared |
Simoneau 2018.
| Study name | |
| Methods |
Design: parallel, open‐label randomised controlled trial Duration: endpoint at 26 weeks Setting: recruited from Connecticut Children's Medical Center; trial carried out in the United States |
| Participants |
Population: 75 participants were randomised to receive digital intervention ‐ the BreatheSmart (n = 50) or control group (n = 25) Age: range from 8 to 17 Inclusion criteria: 8 to 17 years diagnosed with persistent asthma, prescribed ICS for at least 1 month prior to enrollment, use of pressurised MDI compatible with Cohero mHealth Herotracker, parent/child possess a compatible smartphone (iOS 8.0 or higher) Exclusion criteria: presence with other chronic lung conditions/comorbidities, pregnant |
| Interventions |
Breathsmart system group: participants received BreathSmart system which is a mobile application that tracks medication usage and sends real‐time reminders, Herotracker sensor that counts dosage and monitors real time medication adherence, CoheroConnect provider portal that allows the investigator to monitor adherence. There were 3 digital components used (mobile app, Herotracker sensor, CoheroConnect provider portal). Participants could access app whenever for 6 months. Two‐way digital interaction with patient. There was in‐person component for intervention where investigator can monitor adherence via CoheroConnect provider portal. There was adherence feedback; real‐time reminders. Usual care group: participants are reminded to adhere to the prescribed standard of care therapy provided by their clinician during their clinical encounters and when the family calls to report an illness |
| Outcomes |
Primary: medication adherence Secondary: asthma control, lung function, visit for asthma related adverse events, number of missed days of school |
| Starting date | 1 March 2018 |
| Contact information | Tregony Simoneau, MD Connecticut Childrens Medical Center |
| Notes |
Type of publication: clinicaltrials.gov registry record only NCT03734861 Funding: Connecticut Children's Medical Center |
Abbreviations: ACQ: Asthma Control Questionnaire; ACT: Asthma Control Test; BMI: body mass index; COPD: chronic obstructive pulmonary disease; ED: emergency department; EMD: electronic monitoring device; FEV1: forced expiratory volume in 1 second; ICS: inhaled corticosteroid; LABA: long‐acting beta agonist; MDI: metered dose inhaler; PAQLQ: Paediatric Asthma Quality of Life Questionnaire; QOL: quality of life; RCT: randomised controlled trial.
Differences between protocol and review
Data collection and extraction
The World Health Organization International Clinical Trials Registry Platform (ICTRP) (apps.who.int/trialsearch/) was not searched in the June 2020 update as this could not be accessed due to the COVID‐19 pandemic.
The original protocol definition of exacerbations was “exacerbations requiring at least oral corticosteroid treatment”, however this was updated to include at least either oral corticosteroid treatment and/or ED visits and/or hospitalisation.
We originally planned to use Covidence — the Cochrane official systematic review data extraction site (covidence.org) to extract study characteristics and outcome data, however similar to previous reviews (Normansell 2017), we found that this was too time‐consuming so we decided to use an Excel data extraction form used by Cochrane Airways to capture study characteristics, outcome data, and risk of bias information. Four review authors (AC, VW, ADS, LH) piloted the Excel data extraction form on at least one study in the review. We planned to have two review authors to extract the study characteristics and outcome data, but instead the studies were divided into two and extracted in duplicate by two pairs of two authors (AC, VW and ADS, LH). This process was repeated in August 2020 for the 2 June 2020 update, where five review authors (SA, NZ, PP, VT, VP) independently extracted the study characteristics and outcome data from the updated studies in duplicate by ensuring overlap in the studies (i.e. each author extracted two‐fifths of the studies so that studies were extracted twice).
Sex not gender information was collected.
Data collection had to occur in two stages: prior to the June 2020 update, selection of studies, data extraction, and risk of bias assessment were conducted by review authors from the following: AC, VW, ADS, and LH ‐ each author independently conducted this for half the included studies, so each study was assessed twice. For the June 2020 update, study selection, data extraction, and risk of bias assessment was undertaken by five review authors from SA, PP, VT, VP, or NZ for two‐fifths of the studies, so that each study was assessed twice. AC had overall oversight and double‐checked all studies for the phases of data collection. A search update was conducted on 12 October 2021 for additional studies. Two review authors (AC, AD) independently screened the search results and included eligible studies in the Studies awaiting classification section.
Analyses
Use of endpoint data
If both change from baseline and endpoint scores were available for continuous data, we used endpoint scores. We originally planned to use change from baseline, however most studies reported endpoint scores, and additionally endpoint scores allow a health economics analysis to be conducted in the future, as endpoint scores are preferable and more frequently used for health economics modelling. In addition, we did not combine change from baseline and endpoint scores in analyses using SMDs.
Use of mean difference (MD) for adherence and lung function
We used MDs rather than standardised mean differences (SMDs) for adherence and lung function as the measures were reported on the same scale and when we included data reported using different methods of measurement, the data were too skewed to use SMDs. We used SMDs for other outcomes that used more than one method of measurement (e.g. asthma control, quality of life). We used the standard deviation (SD) of final (rather than baseline) measurements in the analysis.
Exacerbations reporting
Exacerbations were not analysed as time‐to‐event data or as rate ratios — but as participants with one or more exacerbations as a risk ratio due to the data reported in the majority of studies.
Time off work or school
A meta‐analysis was not conducted for the outcome of time off school or work due to the limited number of studies and the inconsistency in methods of data reporting. This was still included as an outcome but reported narratively.
Missing data
In terms of handling missing data, when we could not obtain missing data, and missing data were thought to introduce serious bias, we considered this in the GRADE rating for the affected outcome rather than excluding as originally planned.
Use of % FEV1 for lung function outcome
We originally planned for peak expiratory flow rate (PEFR) data to be analysed, however only two studies reported on this (Charles 2007; Jan 2007); most studies reported on % FEV1, so we used this measure in the final meta‐analysis.
Subgroup analyses
We planned to carry out the following subgroup analyses but could not undertake these due to the small number of studies:
Interventions with an interactive component versus non‐interactive interventions.
Theory‐based versus non‐theory‐based digital interventions.
Interventions for ICS versus non‐ICS therapies.
Primary versus secondary care setting (defined in terms of where participants were recruited for the study).
Of note, a separate review focusing specifically on theory‐ versus non‐theory‐based adherence interventions is the subject of another Cochrane Review (PSY‐AST) (Normansell 2018).
We added an additional subgroup analysis comparing studies of adults and adolescents and studies of children, as suggested by our expert advisory group and in line with a similar previous review (Normansell 2017).
We planned to carry out the following sensitivity analyses while removing these items from primary outcome analyses but could not undertake the following:
Unpublished data: no data in this category.
Trials with high risk of selection bias: only Weinstein 2019 was in this category in the meta‐analysis ‐ all others were low risk so there were not enough studies to undertake this.
Quasi‐randomised trials: no trials fitted this category.
Non‐English studies: there were none.
For cluster‐randomised trials, we planned to run the main analyses using more and less conservative estimates of the ICC; however, the ICC values were not available for the cluster‐RCTs.
Of note, we intended to adjust for design effects for cluster‐RCTs by inflating standard errors using a design effect calculated with an ICC. However, as no suitable ICC could be found for the outcomes and populations in the two cluster‐RCTs, only raw study data could be used in the meta‐analyses.
Contributions of authors
Chan AHY: develop and draft review protocol; develop and run search strategy; obtain copies of studies; select which studies to include; extract data from studies, enter data into RevMan; carry out and interpret analysis; assessment of risk of bias in duplicate; evidence grading; writing and editing of final review; overall co‐ordination of author team, supervision of student team members, and oversight of the overall review.
De Simoni A: draft the protocol; develop and run search strategy; obtain copies of studies; select which studies to include; extract data from studies; enter data into RevMan; carry out and interpret analysis; assessment of risk of bias in duplicate; review write‐up.
Wileman V: draft the protocol; develop and run search strategy; obtain copies of studies; select which studies to include; extract data from studies; enter data into RevMan; carry out and interpret analysis; assessment of risk of bias in duplicate; review write‐up.
Holliday L: draft the protocol; develop and run search strategy; obtain copies of studies; select which studies to include; extract data from studies; enter data into RevMan; carry out and interpret analysis; assessment of risk of bias in duplicate; review write‐up.
Newby C: draft the protocol; carry out and interpret analysis; statistical expertise and advice; review write‐up.
Chisari C: draft the protocol; develop and run search strategy; obtain copies of studies; select which studies to include; review write‐up.
Ali S: run search strategy; obtain copies of studies; select which studies to include; extract data from studies; enter data into RevMan; carry out and interpret analysis; assessment of risk of bias in duplicate; review write‐up.
Zhu N: run search strategy; obtain copies of studies; select which studies to include; extract data from studies; enter data into RevMan; carry out and interpret analysis; assessment of risk of bias in duplicate; review write‐up.
Padakanti P: run search strategy; obtain copies of studies; select which studies to include; extract data from studies; enter data into RevMan; carry out and interpret analysis; assessment of risk of bias in duplicate; review write‐up.
Pinprachanan V: run search strategy; obtain copies of studies; select which studies to include; extract data from studies; enter data into RevMan; carry out and interpret analysis; assessment of risk of bias in duplicate; review write‐up.
Ting V: run search strategy; obtain copies of studies; select which studies to include; extract data from studies; enter data into RevMan; carry out and interpret analysis; assessment of risk of bias in duplicate; review write‐up.
Griffiths C: review the protocol and final review write‐up.
Contributions of editorial team
Sally Spencer (Co‐ordinating Editor) edited the review; advised on methodology, interpretation and content; approved the review prior to publication.
Rebecca Fortescue (Co‐ordinating Editor): checked data entry in the review.
Katy Pike (Contact Editor): edited the review; advised on methodology, interpretation, and content.
Emma Dennett (Deputy Co‐ordinating Editor): advised on interpretation and content; edited the review.
Emma Jackson (Managing Editor): co‐ordinated the editorial process; conducted peer review; obtained translations; edited the plain language summary and reference sections of the review.
Elizabeth Stovold (Information Specialist): designed the search strategy; ran the searches; edited the search methods section.
Sources of support
Internal sources
-
University College London, UK
Authors AHYC, VW, and CC were employed by the UCL at the time of inception of the review and have completed this review whilst employed by this institution.
-
Queen Mary University, UK
ADS, LH, CN, and CG are researchers at the Centre for Primary Care and Public Health, Queen Mary University of London, and were completing this review whilst employed by this institution.
-
The University of Auckland, New Zealand
Authors SA, NZ, PP, VP, VT were students at The University of Auckland at the time of conduct of the review. Author AHYC is employed by The University of Auckland and completed the review whilst employed by this institution.
-
The University of Nottingham, UK
Author CN is employed by The University of Nottingham at the time of the review and completed the analyses whilst employed by this institution.
External sources
-
Asthma UK Centre of Applied Research, UK
Authors AHYC, ADS, CN, and CG on this review are researchers affiliated with, and supported by, AUKCAR.
-
National Institute for Health Research (NIHR) Collaboration for Leadership in Applied Health Research and Care (CLARHC) North Thames at Bart's Health NHS Trust, UK
CG is supported by the National Institute for Health Research (NIHR) Collaboration for Leadership in Applied Health Research and Care (CLAHRC) North Thames at Bart’s Health NHS Trust.
The views expressed are those of the review authors and are not necessarily those of the NHS, the NIHR, or the Department of Health.
Declarations of interest
Chan A: Amy Chan has received research grants from A+ charitable trust, Auckland Academic HeaIth Alliance, Chorus, Health Research Council of New Zealand, Maurice Phyllis Paykel Trust, New Zealand Pharmacy and Educational Research Trust, Oakley Mental Health Foundation, Universitas 21, and the University of Auckland. Amy has provided freelance consultancy and received an educational grant from Johnson and Johnson. She is affiliated with the Asthma UK Centre for Applied Research and is supported by Asthma UK (AUK‐AC‐2012‐01 and AUK‐AC‐2018‐01). Amy has received subcontracts from Hong Kong University and from the World Health Organization via University College London to conduct studies unrelated to this review, and provides freelance consultancy to the UCL‐Business spin‐out company Spoonful of Sugar Limited. Amy is also on the board of Asthma NZ and the recipient of the Robert Irwin Foundation fellowship.
De Simoni A: Anna is affiliated with the Asthma UK Centre for Applied Research, and conducted this work with support of the Asthma Research (AUK‐AC‐2012‐01 and AUK‐AC‐2018‐01). Anna has been supported by the National Institute for Health Research. The views expressed are those of the author and not necessarily those of the NHS, NIHR or Department of Health and Social Care.
Wileman V: none known.
Holliday L: Lois is affiliated with the Asthma UK Centre for Applied Research, and conducted this work with the support of the Asthma UK Centre for Applied Research (AUK‐AC‐2012‐01 and AUK‐AC‐2018‐01). Lois has been supported by the National Institute for Health Research. The views expressed are those of the author and not necessarily those of the NHS, NIHR or Department of Health and Social Care.
Newby C: Chris is affiliated with the Asthma UK Centre for Applied Research, and conducted this work with the support of the Asthma UK Centre for Applied Research (AUK‐AC‐2012‐01 and AUK‐AC‐2018‐01). Chris received payment for externally reviewing a PhD Viva for the College of Medicine and Veterinary Medicine.
Chisari C: none known.
Ali S: none known.
Zhu N: none known.
Padakanti P: none known.
Pinprachanan V: none known.
Ting V: none known.
Griffiths C: Chris is supported by the National Institute for Health Research ARC North Thames. The views expressed in this publication are those of the author(s) and not necessarily those of the National Institute for Health and Care Research or the Department of Health and Social Care.
Edited (no change to conclusions)
References
References to studies included in this review
Bender 2010 {published data only}
- Bender BG, Apter A, Bogen DK, Dickinson P, Fisher L, Wamboldt FS, et al. Test of an interactive voice response intervention to improve adherence to controller medications in adults with asthma. Journal of the American Board of Family Medicine 2010;23(2):159-65. [DOI] [PubMed] [Google Scholar]
Bender 2015 {published data only}
- Bender BG, Cvietusa PJ, Goodrich GK, Lowe R, Nuanes HA, Rand C, et al. Pragmatic trial of health care technologies to improve adherence to pediatric asthma treatment: a randomized clinical trial. JAMA Pediatrics 2015;169(4):317-23. [DOI] [PubMed] [Google Scholar]
Black 2008 {published data only}
- Black PN, Garratt E, Arandjus C, Salmon BT, Sutherland G. An inhaler with ring tones improves compliance with inhaled steroids in childhood asthma. In: American Thoracic Society International Conference. Vol. Poster A46. 2008.
Chan 2015 {published data only}
- Chan AHY, Stewart AW, Harrison J, Camargo Jr CA, Black PN, Mitchell EA. The effect of an electronic monitoring device with audiovisual reminder function on adherence to inhaled corticosteroids and school attendance in children with asthma: a randomised controlled trial. Lancet Respiratory Medicine 2015;3(3):210-9. [DOI] [PubMed] [Google Scholar]
Charles 2007 {published data only}
- Charles T, Quinn D, Weatherall M, Aldington S, Beasley R, Holt S. An audiovisual reminder function improves adherence with inhaled corticosteroid therapy in asthma. Journal of Allergy and Clinical Immunology 2007;119(4):811-6. [DOI] [PubMed] [Google Scholar]
Choi 2017 {published data only}
- Choi B, Lee S, Jung J, Suh D. Impact of patient education on medication on health outcomes and adherence in patients with asthma EMT - adult. Allergy: European Journal of Allergy and Clinical Immunology 2017;72:380-1(Abstract 1513). [Google Scholar]
Clerisme‐Beaty 2011 {published data only}
- Clerisme-Beaty EM, Bartlett SJ, Teague WG, Lima J, Irvin CG, Cohen R, et al. The Madison Avenue effect: how drug presentation style influences adherence and outcome in patients with asthma. Journal ofAllergy and Clinical Immunology 2011;127(2):406-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cvietusa 2012 {published data only}
- Cvietusa Pj, Magid Dj, Goodrich G, Wagner N, Lowe R, Nuanes H, et al. A speech recognition (SR) reminder system improves adherence to ICS among pediatric asthma patients [Abstract]. Journal of Allergy and Clinical Immunology 2012;129(2):AB142 (537). [Google Scholar]
Davis 2019 {published data only}
- Davis SA, Carpenter D, Lee C, Garcia N, Reuland DS, Tudor G, et al. Effect of an asthma question prompt list and video intervention on adolescents' medication adherence 12 months later. Annals of Pharmacotherapy 2019;53(7):683-9. [DOI] [PubMed] [Google Scholar]
Ebrahimabadi 2019 {published data only}
- Ebrahimabadi M, Rezaei K, Moini A, Fournier A, Abedi A. Infographics or video; which one is more effective in asthmatic patients' health? a randomized clinical trial. Journal of Asthma 2019;56(12):1306-13. [DOI] [PubMed] [Google Scholar]
Foster 2014 {published data only}
- Foster JM, Usherwood T, Smith L, Sawyer SM, Xuan W, Rand CS, et al. Inhaler reminders improve adherence with controller treatment in primary care patients with asthma. Journal of Allergy and Clinical Immunology 2014;134(6):1260-8.e3. [DOI] [PubMed] [Google Scholar]
Jan 2007 {published data only}
- Jan RL, Wang JY, Huang MC, Tseng SM, Su HJ, Liu LF. An internet-based interactive telemonitoring system for improving childhood asthma outcomes in Taiwan. Telemedicine Journal and e-Health 2007;13(3):257-68. [DOI] [PubMed] [Google Scholar]
Johnson 2016a {published data only}
- Johnson KB, Patterson BL, Xian HY, Qingxia C, Hui N, Davison C L, et al. The feasibility of text reminders to improve medication adherence in adolescents with asthma. Journal of the American Medical Informatics Association 2016;23(3):449-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
Joseph 2018 {published data only}
- Joseph CLM, Mahajan P, Stokes-Buzzelli S, Johnson DA, Duffy E, Williams R, et al. Pilot study of a randomized trial to evaluate a Web-based intervention targeting adolescents presenting to the emergency department with acute asthma. Pilot and Feasibility Studies 2018;4:5. [DOI: 10.1186/s40814-017-0147-6] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kenyon 2018 {published data only}
- Kenyon CC, Gruschow SM, Quarshie WO, Griffis H, Leach MC, Zorc J, et al. Controller adherence following hospital discharge in high risk children: a pilot randomized trial of text message reminders. Journal of Asthma 2019;56(1):95-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kim 2016 {published data only}
- Kim MY, Lee S-Y, Jo E-J, Lee S-E, Kang M-G, Song WJ, et al. Feasibility of a smartphone application based action plan and monitoring in asthma. Asia Pacific Allergy 2016;6(3):174. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kolmodin MacDonell 2016 {published data only}
- Kolmodin MacDonell K, Naar S, Gibson-Scipio W, Lam P, Secord E. The Detroit Young Adult Asthma Project: pilot of a technology-based medication adherence intervention for African-American emerging adults. Journal of Adolescent Health 2016;59(4):465-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kosse 2019 {published data only}
- Kosse R, Bouvy M, De Vries T, Koster E. Mobile health intervention increases adherence in adolescents with asthma: a cluster randomised controlled trial in community pharmacies. Journal of Pharmacy Practice 2018;16:1. [Google Scholar]
- Kosse RC, Bouvy ML, Vries TW, Kaptein AA, Geers Harm CJ, van Dijk Liset, et al. mHealth intervention to support asthma self-management in adolescents: the ADAPT study. Patient Preference and Adherence 2017;11:571-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosse RC, Bouvy ML, Vries TW, Koster ES. Effect of a mHealth intervention on adherence in adolescents with asthma: a randomized controlled trial. Respiratory Medicine 2019;149:45-51. [DOI] [PubMed] [Google Scholar]
Koufopoulos 2016 {published data only}
- Koufopoulos JT, Conner MT, Gardner PH, Kellar I. A web-based and mobile health social support intervention to promote adherence to inhaled asthma medications: randomized controlled trial. Journal of Medical Internet Research 2016;18(6):e122. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lv 2012 {published data only}
- Lv Y, Zhao H, Liang Z, Dong H, Liu L, Zhang D, et al. A mobile phone short message service improves perceived control of asthma: a randomized controlled trial. Telemedicine and E-Health 2012;18(6):420-6. [DOI] [PubMed] [Google Scholar]
Lv 2019 {published data only}
- Lv S, Ye X, Wang Z, Xia W, Qi Y, Wang W, et al. A randomized controlled trial of a mobile application-assisted nurse-led model used to improve treatment outcomes in children with asthma. Journal of Advanced Nursing 2019;75(11):3058-67. [DOI] [PubMed] [Google Scholar]
Morrison 2016 {published data only}
- Morrison D, Wyke S, Saunderson K, McConnachie A, Agur K, Chaudhuri R, et al. Findings from a pilot Randomised trial of an Asthma Internet Self-management Intervention (RAISIN). BMJ Open 2016;6(5):e009254. [DOI] [PMC free article] [PubMed] [Google Scholar]
Morton 2017 {published data only}
- Morton R, Everard M, Elphick H. Randomised control trial to investigate whether electronic adherence monitoring with reminder alarms and feedback can improve clinical outcomes in childhood asthma EMT. In: European Respiratory Journal. OA4772 edition. Vol. 46. 2015.
- Morton RW, Elphick HE, Rigby AS, Daw WJ, King DA, Smith LJ, et al. STAAR: a randomised controlled trial of electronic adherence monitoring with reminder alarms and feedback to improve clinical outcomes for children with asthma. Thorax 2017;72(4):347-54. [DOI] [PubMed] [Google Scholar]
Mosnaim 2013 {published data only}
- Mosnaim G, Li H, Martin M, Richardson DJ, Belice PJo, et al. The impact of peer support and mp3 messaging on adherence to inhaled corticosteroids in minority adolescents with asthma: a randomized, controlled trial. Journal of Allergy and Clinical Immunology 2013;1(5):485-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosnaim G. Using mobile phones to improve adherence to inhaled steroids (ADEPT4). clinicaltrials.gov/ct2/show/NCT01710059 (first received 18 October 2012).
Pernell 2017 {published data only}
- Pernell BM, DeBaun MR, Becker K, Rodeghier M, Bryant V, Cronin RM. Improving medication adherence with two-way short message service reminders in sickle cell disease and asthma: a feasibility randomized controlled trial. Applied Clinical Informatics 2017;8(2):541-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
Petrie 2012 {published data only}
- Petrie KJ, Perry K, Broadbent E, Weinman J. A text message programme designed to modify patients’ illness and treatment beliefs improves self-reported adherence to asthma preventer medication. British Journal of Health Psychology 2012;17(1):74-84. [DOI] [PubMed] [Google Scholar]
Pool 2017 {published data only}
- Pool AC, Kraschnewski JL, Poger JM, Smyth J, Stuckey H L, Craig TJ, et al. Impact of online patient reminders to improve asthma care: a randomized controlled trial. PLOS One 2017;12(2):e0170447. [DOI] [PMC free article] [PubMed] [Google Scholar]
Reece 2017 {published data only}
- Reece ER, Burnette AF, Lewis-Land C. Pilot study of Asthmawin mobile iphone app in the management of asthma. Journal of Allergy and Clinical Immunology 2017;139(2):AB382. [Google Scholar]
Rikkers‐Mutsaerts 2012 {published data only}
- Rikkers-Mutsaerts E, Winters AE, Bakker MJ, Van Stel HF, Van Der Meer V, De Jongste JC, et al. Internet-based self-management compared with usual care in adolescents with asthma: a randomized controlled trial. Pediatric Pulmonology 2012;47(12):1170-9. [DOI] [PubMed] [Google Scholar]
Schaffer 2004 {published data only}
- Schaffer SD, Tian L. Promoting adherence: effects of theory-based asthma education. Clinical Nursing Research 2004;13(1):69-89. [DOI] [PubMed] [Google Scholar]
Searing 2012 {published data only}
- Searing DA, Bender BG. Short message service (SMS) for asthma management: a pilot study utilizing text messaging to promote asthma self-management. Journal of Allergy and Clinical Immunology 2012;129(2 Suppl):AB142. [Google Scholar]
Stranbygaard 2010 {published data only}
- Strandbygaard U, Backer V. Does daily SMS increase adherence to asthma treatment? A three months follow-up study [Abstract]. In: European Respiratory Society Annual Congress, Vienna, Austria, September 12-16. 2009:E4558.
- Strandbygaard U, Thomsen SF, Backer V. A daily SMS reminder increases adherence to asthma treatment: a three-month follow-up study. Respiratory Medicine 2010;104(2):166-71. [DOI] [PubMed] [Google Scholar]
Sulaiman 2018 {published data only}
- Sulaiman I, Greene G, MacHale E, Seheult J, Mokoka M, D’Arcy S, et al. A randomised clinical trial of feedback on inhaler adherence and technique in patients with severe uncontrolled asthma. European Respiratory Journal 2018;51(1):1701126. [DOI] [PubMed] [Google Scholar]
Van der Meer 2009 {published data only}ISRCTN79864465
- Van Der Meer V, Bakker MJ, Van Den Hout WB, Rabe KF, Sterk PJ, Kievit J, et al. Internet-based self-management plus education compared with usual care in asthma a randomized trial. Annals of Internal Medicine 2009;151(2):110-20. [DOI] [PubMed] [Google Scholar]
- Meer V, Stel HF, Bakker MJ, Roldaan AC, Assendelft WJ, Sterk PJ, et al. Weekly self-monitoring and treatment adjustment benefit patients with partly controlled and uncontrolled asthma: an analysis of the SMASHING study. Respiratory Research 2010;11(1):1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Van Sickle 2016 {published data only}
- Van Sickle D, Barrett M, Humblet O, Henderson K, Hogg C. Randomized, controlled study of the impact of a mobile health tool on asthma SABA use, control and adherence. European Respiratory Journal 2016;48:PA1018. [Google Scholar]
Vasbinder 2016 {published data only}
- Goossens LMA, Vasbinder EC, Van den Bemt PMLA, Rutten-van Mölken MPMH. Cost-effectiveness of real-time medication monitoring in children with asthma. Value in Health 2014;17(7):PA329. [DOI] [PubMed] [Google Scholar]
- Vasbinder E, Goossens L, Janssens H, Winter B De, Dijk L Van, Vulto A, et al. E-monitoring of asthma therapy to improve compliance in children (E-MATIC). In: European Respiratory Society Annual Congress 2015; Amsterdam, Netherlands. Vol. 46. European Respiratory Society, 2015:OA4771.
- Vasbinder EC, Goossens Lucas MA, Rutten-Van Mölken M PMH, De Winter BCM, Van Dijk L, Vulto AG, et al. E-monitoring of asthma therapy to improve compliance in children (e-MATIC): a randomised controlled trial. European Respiratory Journal 2016;48(3):758-67. [DOI] [PubMed] [Google Scholar]
Vollmer 2011 {published data only}
- Vollmer WM, Feldstein A, Smith D, Dubanoski J, Waterbury A, Schneider J, et al. Use of health information technology to improve medication adherence. American Journal of Managed Care 2011;17:79-87. [PMC free article] [PubMed] [Google Scholar]
- Vollmer WM, Rand CS, Feldstein AC, Waterbury A, Dubanoski JP, Schneider J, et al. Use of automated phone calls to support inhaled corticosteroids (ICS) adherence [Abstract]. In: American Thoracic Society International Conference 2009 May 15-20; San Diego. A1089 (Poster #518) edition. 2009.
Weinstein 2019 {published data only}
- Weinstein AG, Singh A, Laurenceau JP, Skoner DP, Maiolo J, Sharara R, et al. A pilot study of the effect of an educational web application on asthma control and medication adherence. Journal of Allergy and Clinical Immunology 2019;7(5):1497-506. [DOI] [PubMed] [Google Scholar]
Wiecha 2015 {published data only}
- Wiecha JM, Adams WG, Rybin D, Rizzodepaoli M, Keller J, Clay JM. Evaluation of a web-based asthma self-management system: a randomised controlled pilot trial. BMC Pulmonary Medicine 2015;15(1):17. [DOI: 10.1186/s12890-015-0007-1] [DOI] [PMC free article] [PubMed] [Google Scholar]
Zhou 2018 {published data only}
- Zhou Y, Lu Y, Zhu H, Zhang Y, Li Y, Yu Q. Short-term effect of a smart nebulizing device on adherence to inhaled corticosteroid therapy in asthma predictive index-positive wheezing children. Patient Preference and Adherence 2018;12:861-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
Adejumo 2018 {published data only}
- Adejumo I, Shaw DE. Electronic monitoring devices as an intervention in asthma: the story so far. Current Respiratory Medicine Reviews 2018;14(1):5-22. [Google Scholar]
Ahmed 2016 {published data only}
- Ahmed S, Ernst P, Bartlett SJ, Valois MF, Zaihra T, Paré, et al. The effectiveness of web-based asthma self-management system, My Asthma Portal (MAP): a pilot randomized controlled trial. Journal of Medical Internet Research 2016;18(12):e313. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ainsworth 2019 {published data only}
- Ainsworth B, Greenwell K, Stuart B, Raftery J, Mair F, Bruton A, et al. Feasibility trial of a digital self-management intervention ‘My Breathing Matters’ to improve asthma-related quality of life for UK primary care patients with asthma. BMJ Open 2019;9(11):e032465. [DOI] [PMC free article] [PubMed] [Google Scholar]
Anderson 2017 {published data only}
- Anderson William C III. Incorporating technology to advance asthma controller adherence. Current Opinion in Allergy and Clinical Immunology 2017;17(2):153-9. [DOI] [PubMed] [Google Scholar]
Apter 2019 {published data only}
- Apter AJ, Localio AR, Morales KH, Han X, Perez L, Mullen AN, et al. Home visits for uncontrolled asthma among low-income adults with patient portal access. Journal of Allergy and Clinical Immunology 2019;144(3):846-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bender 2018 {published data only}
- Bender BG. Technology interventions for nonadherence: new approaches to an old problem. Journal of Allergy and Clinical Immunology 2018;6(3):794-800. [DOI] [PubMed] [Google Scholar]
Beydon 2017 {published data only}
- Beydon N, Delclaux C. Digital action plan for asthma exacerbations (PANAME). Revue des Maladies Respiratoires 2017;34(9):1026-33. [DOI] [PubMed] [Google Scholar]
Biblowitz 2018 {published data only}
- Biblowitz K, Bellam S, Mosnaim G. Improving asthma outcomes in the digital era: a systematic review. Pharmaceutical Medicine 2018;32(3):173-87. [Google Scholar]
Bonini 2018 {published data only}
- Bonini M, Usmani OS. Novel methods for device and adherence monitoring in asthma. Current Opinion in Pulmonary Medicine 2018;24(1):63-9. [DOI] [PubMed] [Google Scholar]
Boutopoulou 2018 {published data only}
- Boutopoulou B, Koumpagioti D, Matziou V, Priftis KN, Douros K. Interventions on adherence to treatment in children with severe asthma: a systematic review. Frontiers in Pediatrics 2018;6:232. [DOI: 10.3389/fped.2018.00232] [DOI] [PMC free article] [PubMed] [Google Scholar]
Britto 2017 {published data only}
- Britto MT, Rohan JM, Dodds CM, Byczkowski Tl. A randomized trial of user-controlled text messaging to improve asthma outcomes. Clinical Pediatrics 2017;56(14):1336-44. [DOI] [PubMed] [Google Scholar]
- Dodds CM, Britto MT. Learnings from a pragmatic pilot trial of text messaging for high-risk adolescents with asthma. Annals of Allergy, Asthma and Immunology 2018;120(5):546-7. [DOI] [PubMed] [Google Scholar]
Bruzzese 2021 {published data only}
- Bruzzese J-M, George M, Liu J, Evans D, Naar S, DeRosier ME, et al. The development and preliminary impact of CAMP air: a web-based asthma intervention to improve asthma among adolescents. Patient Education and Counseling 2021;104(4):865-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chan 2017 {published data only}
- Chan AHY, Stewart AW Harrison J, Black PN, Mitchell EA, Foster JM. Electronic adherence monitoring device performance and patient acceptability: a randomized control trial. Expert Review of Medical Devices 2017;14(5):401-11. [DOI] [PubMed] [Google Scholar]
Chen 2013 {published data only}
- Chen SH, Huang JL, Yeh KW, Tsai YF. Interactive support interventions for caregivers of asthmatic children. Journal of Asthma 2013;50(6):649-57. [DOI] [PubMed] [Google Scholar]
Christakis 2012 {published data only}
- Christakis DA, Garrison MM, Lozano P, Meischke H, Zhou C, Zimmerman FJ. Improving parental adherence with asthma treatment guidelines: a randomized controlled trial of an interactive website. Academic Pediatrics 2012;12(4):302-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cingi 2015 {published data only}
- Cingi C, Yorgancioglu A, Cingi C, Oguzulgen K, Muluk N, Ulusoy, et al. The “physician on call patient engagement trial”(POPET): measuring the impact of a mobile patient engagement application on health outcomes and quality of life in allergic rhinitis and asthma patients. International Forum of Allergy and Rhinology 2015;5:487-97. [DOI] [PubMed] [Google Scholar]
Claus 2004 {published data only}
- Claus R, Michael H, Jan‐Torsten T, Marion S. Internet based patient education evaluation of a new tool for young asthmatics. European Respiratory Journal 2004;24(Suppl 48):383s. [Google Scholar]
Dermot 2009 {published data only}
- Ryan D, Pinnock H, Lee AJ, Tarassenko L, Pagliari Cl, Sheikh A, et al. The CYMPLA trial. Mobile phone-based structured intervention to achieve asthma control in patients with uncontrolled persistent asthma: a pragmatic randomised controlled trial. Primary Care Respiratory Journal 2009;18(4):343. [DOI] [PMC free article] [PubMed] [Google Scholar]
Dermot 2012 {published data only}
- Ryan D, Price D, Musgrave SD, Malhotra S, Lee AJ, Ayansina D, et al. Clinical and cost effectiveness of mobile phone supported self monitoring of asthma: multicentre randomised controlled trial. BMJ 2012;344:e1756. [DOI: 10.1136/bmj.e1756] [DOI] [PMC free article] [PubMed] [Google Scholar]
Federman 2018 {published data only}
- Federman A, O'Conor R, Mindlis I, Hauser D, Hoy-Rosas J, Lopez R, et al. A comprehensive self-management support program improves asthma control and quality of life among older adults: results of a randomized controlled trial. American Journal of Respiratory and Critical Care Medicine 2018;197:A2714. [Google Scholar]
Fonseca 2006 {published data only}
- Fonseca J, Costa-Pereira A, Delgado L, Fernandes L, Castel-Branco M. Asthma patients are willing to use mobile and web technologies to support self-management. Allergy 2006;61:389-90. [DOI] [PubMed] [Google Scholar]
Frémont 2018 {published data only}
- Frémont A, Abou TR, Wanin S, Lebras M-N, Ollier V, Nathanson S, et al. Cartoons to improve young children's cooperation with inhaled corticosteroids: a preliminary study. Pediatric Pulmonology 2018;53(9):1193-9. [DOI] [PubMed] [Google Scholar]
Gregoriano 2017 {published data only}
- Gregoriano C, Dieterle T, Dürr S, Arnet I, Hersberger KE, Leuppi JD. Impact of an electronic monitoring intervention to improve adherence to inhaled medication in patients with asthma and chronic obstructive pulmonary disease: study protocol for a randomized controlled trial. JMIR Research Protocols 2017;6(10):e204. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gregoriano 2019 {published data only}
- Gregoriano C, Dieterle T, Breitenstein AL, Dürr S, Baum A, Giezendanner S, et al. Does a tailored intervention to promote adherence in patients with chronic lung disease affect exacerbations? A randomized controlled trial. Respiratory Research 2019;20(1):273. [DOI] [PMC free article] [PubMed] [Google Scholar]
Grossman 2017 {published data only}
- Grossman B, Conner S, Mosnaim G, Albers J, Leigh J, Jones S, et al. Application of human augmentics: a persuasive asthma inhaler. Journal of Biomedical Informatics 2017;67:51-8. [DOI] [PubMed] [Google Scholar]
Gustafson 2012 {published data only}
- Gustafson D, Wise M, Bhattacharya A, Pulvermacher A, Shanovich K, Phillips B, et al. The effects of combining web-based eHealth with telephone nurse case management for pediatric asthma control: a randomized controlled trial. Journal of Medical Internet Research 2012;14(4):e101. [DOI] [PMC free article] [PubMed] [Google Scholar]
Halterman 2012 {published data only}
- Halterman JS, Fagnano M, Montes G, Fisher S, Tremblay P, Tajon R, et al. The school-based preventive asthma care trial: results of a pilot study. Journal of Pediatrics 2012;161(6):1109-15.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Halterman 2018 {published data only}
- Halterman JS, Fagnano M, Tajon RS, Tremblay P, Wang H, Butz A, et al. Effect of the school-based telemedicine enhanced asthma management (SB-TEAM) program on asthma morbidity: a randomized clinical trial. JAMA Pediatrics 2018;172(3):e174938. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hayter 2019 {published data only}
- Haytor V, Ainsworth B. A feasibility study for My Breathing Matters, an asthma self-management website [‘My Breathing Matters’ - A feasibility study of a digital self-management programme designed to improve the quality of life of people with asthma]. trialsearch.who.int/Trial2.aspx?TrialID=ISRCTN15698435 (first received 11 March 2019).
Hew 2019 {published data only}
- Hew M, Reddel HK. Integrated adherence monitoring for inhaler medications. JAMA 2019;321(11):1045-6. [DOI] [PubMed] [Google Scholar]
Hoch 2019 {published data only}
- Hoch H, Kempe A, Brinton J, Szefler S. Feasibility of medication monitoring sensors in high risk asthmatic children. Journal of Asthma 2019;56(3):270-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jeminiwa 2019 {published data only}
- Jeminiwa R, Hohmann L, Qian J, Garza K, Hansen R, Fox Brent I. Impact of eHealth on medication adherence among patients with asthma: a systematic review and meta-analysis. Respiratory Medicine 2019;149:59-68. [DOI] [PubMed] [Google Scholar]
Joseph 2013 {published data only}
- Joseph CLM, Ownby DR, Havstad SL, Saltzgaber J, Considine S, Johnson D, et al. Evaluation of a web-based asthma management intervention program for urban teenagers: reaching the hard to reach. Journal of Adolescent Health 2013;52(4):419-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
Katwa 2018 {published data only}
- Katwa U, Rivera E. Asthma management in the era of smart-medicine: devices, gadgets, apps and telemedicine. Indian Journal of Pediatrics 2018;85(9):757-62. [DOI] [PubMed] [Google Scholar]
Kojima 2005 {published data only}
- Kojima N, Takeda Y, Akashi M, Kamiya T, Matsumoto M, Ohya Y, et al. Interactive education during summer camp for children with asthma improved adherence of self-management. Journal of Allergy and Clinical Immunology 2005;115(2):S115. [Google Scholar]
Koumpagioti 2020 {published data only}
- Koumpagioti D, Boutopoulou B, Priftis KN, Douros K. Effectiveness of an educational program for children and their families on asthma control treatment adherence. Journal of Asthma 2020;57(5):567-73. [DOI] [PubMed] [Google Scholar]
Lathy 2009 {published data only}
- Prabhakaran L, Chee J, Kc C, Mun W. The use of text messaging to improve asthma control: A Study of Short Message Service (SMS): PD 10–01 2009–006. Respirology 2009;14:A217. [Google Scholar]
Lathy 2019 {published data only}
- Prabhakaran L, Chun WY. Effectiveness of the eCARE programme: a short message service for asthma monitoring. BMJ Health and Care Informatics 2019;26(1):e100007. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lau 2015 {published data only}
- Lau AYS, Arguel A, Dennis S, Liaw S-T, Coiera E. “Why didn’t it work?” Lessons from a randomized controlled trial of a web-based personally controlled health management system for adults with asthma. Journal of Medical Internet Research 2015;17(12):e283. [DOI] [PMC free article] [PubMed] [Google Scholar]
Licskai 2016 {published data only}
- Licskai C, Ferrone M, Taite A, Madeley C, Stevens LA, To T, et al. The evaluation of Breathe-a patient mobile health (mHealth) app for adult asthma. American Journal of Critical Care and Respiratory Medicine 2016;193:A1084. [Google Scholar]
Lin 2020 {published data only}
- Lin H-H, Hung Y-P, Weng S-H, Lee P-Y, Sun W-Z. Effects of parent-based social media and moderate exercise on the adherence and pulmonary functions among asthmatic children. Kaohsiung Journal of Medical Sciences 2020;36(1):62-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
Liu 2011 {published data only}
- Liu W-Te, Huang C-D, Wang C-H, Lee K-Y, Lin S-M, Kuo H-P. A mobile telephone-based interactive self-care system improves asthma control. European Respiratory Journal 2011;37(2):310-7. [DOI] [PubMed] [Google Scholar]
Lombard 2019 {published data only}
- Lombard L, Walsh J, Plunkett S, MacHale E, Mulvey C, Greene G, et al. INCA (TM) Technology directs the appropriate treatment path for uncontrolled patients with asthma. Irish Journal of Medical Science 2019;188:263. [Google Scholar]
Makhecha 2019 {published data only}
- Makhecha S, Chan AHY, Pearce CJ, Jamalzadeh A, Fleming L. P163 Assessment of novel electronic adherence monitoring devices in children with asthma. Thorax 2019;74(Suppl 2):A179. [DOI] [PMC free article] [PubMed] [Google Scholar]
McPherson 2006 {published data only}
- McPherson AC, Glazebrook C, Forster D, James C, Smyth A. A randomized, controlled trial of an interactive educational computer package for children with asthma. Pediatrics 2006;117(4):1046-54. [DOI] [PubMed] [Google Scholar]
Newhouse 2016 {published data only}
- Newhouse N, Martin A, Jawad S, Yu L-M, Davoudianfar M, Locock Lo, et al. Randomised feasibility study of a novel experience-based internet intervention to support self-management in chronic asthma. BMJ Open 2016;6(12):e013401. [DOI] [PMC free article] [PubMed] [Google Scholar]
Normansell 2017 {published data only}
- Normansell R, Kew KM, Stovold E. Interventions to improve adherence to inhaled steroids for asthma. Cochrane Database of Systematic Reviews 2017, Issue 4. Art. No: CD012226. [DOI: 10.1002/14651858.CD012226.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ostojic 2005 {published data only}
- Ostojic V, Cvoriscec B, Ostojic SB, Reznikoff D, Stipic-Markovic A, Tudjman Z. Improving asthma control through telemedicine: a study of short-message service. Telemedicine Journal 2005;11(1):28-35. [DOI] [PubMed] [Google Scholar]
Pearce 2018 {published data only}
- Pearce CJ, Fleming L. Adherence to medication in children and adolescents with asthma: methods for monitoring and intervention. Expert Review of Clinical Immunology 2018;14(12):1055-63. [DOI] [PubMed] [Google Scholar]
Perry 2017 {published data only}
- Perry TT, Marshall A, Berlinski A, Rettiganti M, Brown RH, Randle SM, et al. Smartphone-based vs paper-based asthma action plans for adolescents. Annals of Allergy, Asthma and Immunology 2017;118(3):298-303. [DOI] [PubMed] [Google Scholar]
Poureslami 2017 {published data only}
- Poureslami I, Shum J, Gorrin N, Bayat S, Lester RT, Dorscheid D, et al. A randomized controlled trial of a text messaging based intervention versus a written action plan in asthma management: results from a feasibility study. American Journal of Respiratory and Critical Care Medicine 2017;A94:A2637. [Google Scholar]
Poureslami 2019 {published data only}
- Poureslami I, Shum J, Lester RT, Tavakoli H, Dorscheid DR, FitzGerald JM. A pilot randomized controlled trial on the impact of text messaging check-ins and a web-based asthma action plan versus a written action plan on asthma exacerbations. Journal of Asthma 2019;56(8):897-909. [DOI] [PubMed] [Google Scholar]
Rasmussen 2005 {published data only}
- Rasmussen LM, Phanareth K, Nolte H, Backer V. Can internet‐based management improve asthma control? A long term randomised clinical study of 300 asthmatics. European Respiratory Journal 2005;26:400. [DOI] [PubMed] [Google Scholar]
Real 2019 {published data only}
- Francis JR, Beck AF, DeBlasio D, Zackoff M, Henize A, Yingying X, et al. Dose matters: a smartphone application to improve asthma control among patients at an urban pediatric primary care clinic. Games for Health Journal 2019;8(5):357-65. [DOI] [PubMed] [Google Scholar]
Seid 2012 {published data only}
- Seid M, D'Amico EJ, Varni JW, Munafo JK, Britto MT, Kercsmar CM, et al. The in vivo adherence intervention for at risk adolescents with asthma: report of a randomized pilot trial. Journal of Pediatric Psychology 2012;37(4):390-403. [DOI] [PMC free article] [PubMed] [Google Scholar]
Stukus 2018 {published data only}
- Stukus DR, Farooqui N, Strothman K, Ryan K, Zhao S, Stevens JH, et al. Real-world evaluation of a mobile health application in children with asthma. Annals of Allergy, Asthma and Immunology 2018;120(4):395-400.e1. [DOI] [PubMed] [Google Scholar]
Sutherland 2017 {published data only}
- Sutherland G, Sherlock JP. The potential of connected devices for tackling asthma. Journal of Partnership Opportunities in Drug Delivery 2017:34.
Teufel 2018 {published data only}
- Teufel Ii RJ, Patel SK, Shuler AB, Andrews AL, Nichols M, Ebeling MD, et al. Smartphones for real-time assessment of adherence behavior and symptom exacerbation for high-risk youth with asthma: pilot study. JMIR Pediatrics and Parenting 2018;1(2):e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Unni 2018 {published data only}
- Unni E, Gabriel S, Ariely R. A review of the use and effectiveness of digital health technologies in patients with asthma. Annals of Allergy, Asthma & Immunology 2018;121(6):680-91. [DOI] [PubMed] [Google Scholar]
Van Gaalen 2013 {published data only}
- Van Gaalen JL, Beerthuizen T, Meer V, Reisen P, Redelijkheid GW, Snoeck-Stroband JB, et al. Long-term outcomes of internet-based self-management support in adults with asthma: randomized controlled trial. Journal of Medical Internet Research 2013;15(9):e188. [DOI] [PMC free article] [PubMed] [Google Scholar]
Voorend‐van Bergan 2015 {published data only}
- Voorend-van Bergen S, Vaessen-Verberne AA, Brackel HJ, Landstra AM, den Berg NJ, Hop WC, et al. Monitoring strategies in children with asthma: a randomised controlled trial. Thorax 2015;70(6):543-50. [DOI] [PubMed] [Google Scholar]
Weinstein 2017 {published data only}
- Weinstein A, Gentile D, Singh A, Skoner D, Maiolo J, Sharara R, et al. Preliminary evaluation of an adult asthma adherence management program. American Journal of Respiratory and Critical Care 2017;B38:A7619. [Google Scholar]
Williams 2010 {published data only}
- Williams LK, Peterson EL, Wells K, Campbell J, Wang M, Chowdhry VK, et al. A cluster-randomized trial to provide clinicians inhaled corticosteroid adherence information for their patients with asthma. Journal of Allergy and cCinical Immunology 2010;126(2):225-31.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Yun 2013 {published data only}
- Yun T-J, Arriaga RI. A text message a day keeps the pulmonologist away. In: CHI '13: Proceedings of the SIGCHI conference on human factors in computing systems; 2013 27 April -2 May; Paris. 2013.
References to studies awaiting assessment
ACTRN12620001006932 {published data only}
- ACTRN12620001006932. The Anti-Inflammatory Reliever Tutorial (AIR-T) sub-study: can an interactive comic tutorial help adult patients with asthma understand and adhere to an anti-inflammatory reliever therapy regimen? An embedded randomised controlled trial. anzctr.org.au/ACTRN12620001006932.aspx (first received 6 August 2020).
Adejumo 2020 {published data only}
- Adejumo I, Patel M, McKeever TM, Shaw DE. Feedback on inhaler use does not significantly improve inhaled corticosteroid adherence or clinical outcomes. European Respiratory Journal 2020;56(Suppl 64):4804. [Google Scholar]
Almonacid 2021 {published data only}
- Almonacid C, Melero C, López Viña A, Cisneros C, Pérez de Llano L, Plaza V, et al. Effectiveness of text message reminders on adherence to inhaled therapy in patients with asthma: prospective multicenter randomized clinical trial. JMIR Formative Research 2021;5(2):e12218. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chen 2020 {published data only}
- Chen J, Xu J, Zhao L, Zhang J, Yin Y, Zhang F. The effect of electronic monitoring combined with weekly feedback and reminders on adherence to inhaled corticosteroids in infants and younger children with asthma: a randomized controlled trial. Allergy, Asthma and Clinical Immunology 2020;16(1):68. [DOI] [PMC free article] [PubMed] [Google Scholar]
CTRI/2021/02/031075 {published data only}
- CTRI/2021/02/031075. Effect of education and SMS reminders on medication compliance and quality of life in patients with select multimorbidity (people with 2 or more non communicable diseases)-a randomised controlled trial. ctri.nic.in/Clinicaltrials/pmaindet2.php?trialid=31236&EncHid=&userName=CTRI/2021/02/031075 (first received 8 February 2021).
Cvietusa 2020 {published data only}
- Cvietusa PJ, Wagner NM, Shoup JA, Goodrich GK, Shetterly SM, King DK, et al. Digital communication technology: does offering a choice of modality improve medication adherence and outcomes in a persistent asthma population? Permanente Journal 2020;25:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ebrahimabadi 2019a {published data only}
- Ebrahimabadi M, Rezaei K, Moini A, Fournier A, Abedi A. Infographics or video; which one is more effective in asthmatic patients' health? A randomized clinical trial. Journal of Asthma 2019;56(12):1306-13. [DOI] [PubMed] [Google Scholar]
EUCTR2019‐003082‐17‐DE {published data only}
- EUCTR2019-003082-17-DE. How electronic monitoring and feedback affect use of Easyhaler asthma medication. clinicaltrialsregister.eu/ctr-search/search?query=EUCTR2019-003082-17-DE (first received 29 November 2019).
Gupta 2021 {published data only}
- Gupta RS, Fierstein JL, Boon KL, Kanaley MK, Bozen A, Kan K. Sensor-based electronic monitoring for asthma: a randomized controlled trial. Pediatrics 2021;147(1):1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Henderson 2020 {published data only}
- Henderson BR, Flaherty CM, Floyd GC, You J, Xiao R, Bryant-Stephens TC. Tailored medication adherence incentives using mHealth for children with high-risk asthma (TAICAM): protocol for a randomized controlled trial. JMIR Research Protocols 2020;9(8):e16711. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hollenbach 2021 {published data only}
- Hollenbach J, Simoneau T, Sun Y, Becene I, Almeida S, Langton C, et al. Design, methods, and baseline characteristics of a pilot, randomized, controlled trial of the effects of an electronic monitoring device on medication adherence in children with asthma. Contemporary Clinical Trials Communications 2021;21:100706. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lombard 2019a {published data only}
- Lombard L, Walsh J, Plunkett S, MacHale E, Mulvey C, Greene G, et al. INCATM technology directs the appropriate treatment path for uncontrolled patients with asthma. Irish Journal of Medical Science 2019;188(Suppl 10):S263. [Google Scholar]
Moore 2020 {published data only}
- Moore A, Preece A, Sharma R, Heaney LG, Costello RW, Wise RA, et al. A randomised controlled trial of the effect of a connected inhaler system on medication adherence in uncontrolled asthmatic patients. European Respiratory Journal 2020;57(6):2003103. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mosnaim 2020 {published data only}
- Mosnaim GS, Stempel DA, Gonzalez C, Adams B, BenIsrael-Olive N, Gondalia R, et al. The impact of patient self-monitoring via electronic medication monitor and mobile app plus remote clinician feedback on adherence to inhaled corticosteroids: a randomized controlled trial. Journal of Allergy and Clinical Immunology 2020;9(4):1586-94. [DOI] [PubMed] [Google Scholar]
NCT04401332 {published data only}
- NCT04401332. A randomized controlled trial of apps to home monitor your asthma. clinicaltrials.gov/ct2/show/NCT04401332 (first received 26 May 2020).
NCT04607681 2020 {published data only}
- NCT04607681. The effects of web design in educating asthma. clinicaltrials.gov/ct2/show/NCT04607681 (first received 29 October 2020).
NCT04633018 2020 {published data only}
- NCT04633018. A patient-centered asthma management communication intervention for rural Latino children. clinicaltrials.gov/ct2/show/NCT04633018 (first received 17 November 2020).
NCT04677959 2020 {published data only}
- NCT04677959. A 24-week treatment study to compare standard of care versus the eMDPI DS in patients 13 years or older with asthma. clinicaltrials.gov/ct2/show/NCT04677959 (first received 21 December 2020).
NCT04744272 2021 {published data only}
- NCT04744272. Exploring the efficacy of myAsthma in secondary care. clinicaltrials.gov/ct2/show/NCT04744272 (first received 8 February 2021).
NCT04869384 2021 {published data only}
- NCT04869384. Effect of electronic monitoring and feedback on adherence to Easyhaler controller medication in patients with asthma. clinicaltrials.gov/ct2/show/NCT04869384 (first received 3 May 2021).
Riley 2021 {published data only}
- NCT03769519. AdheRence to Inhaled Corticosteroids in Asthma (ARICA). clinicaltrials.gov/show/NCT03769519 (first received 7 December 2018).
- Riley IL, Jackson B, Olsen M, Svetkey L, Que LG, Sanders L, et al. Adherence to inhaled corticosteroids in asthma (ARICA): a randomized controlled pilot study. American Journal of Respiratory and Critical Care Medicine 2021;203(9):A1615. [Google Scholar]
Sportel 2020 {published data only}
- Sportel ET, Oude Wolcherink MJ, Palen J, Lenferink A, Thio BJ, Movig KLL, et al. Does immediate smart feedback on therapy adherence and inhalation technique improve asthma control in children with uncontrolled asthma? A study protocol of the IMAGINE I study. Trials 2020;21(1):801. [DOI] [PMC free article] [PubMed] [Google Scholar]
UMIN000042690 {published data only}
- UMIN000042690. Using a tracker incorporated into budesonide/formoterol turbuhaler to improve asthma control: a randomized controlled study. upload.umin.ac.jp/cgi-open-bin/ctr_e/ctr_view.cgi?recptno=R000048718 (first received 10 December 2020).
References to ongoing studies
Arain 2020 {published data only}
- NCT04339296. Connected healthcare for individuals living at home with chronic conditions [Medication dispensing system to support medication adherence for individuals living at home with chronic conditions: a randomized controlled trial]. clinicaltrials.gov/show/NCT04339296 (first received 20 October2020).
Jariwala 2018 {unpublished data only}
- NCT03930381. Adapting and expanding the asthma-educator app [Adapting and expanding the algorithmic software tool to help manage asthma (ASTHMAXcel) for youth with asthma]. clinicaltrials.gov/show/NCT03930381 (first received 29 April 2019).
Kang‐Cheng Su Su 2015 {published data only}
- NCT02556073. ICS/LABA combination with integrated dose counter and smartphone app to improve asthma control [The use of fluticasone propionate/salmeterol inhaler with integrated dose counter and smartphone self management to improve airway inflammation and asthma control]. clinicaltrials.gov/show/NCT02556073 (first received 22 September 2015).
Kenyon 2019 {published data only}
- NCT03907410. The tailored adherence incentives for childhood asthma medications (TAICAM) trial. clinicaltrials.gov/show/NCT03907410 (first received 9 April 2019).
La Grutta 2020 {published data only}
- NCT03788395. The use of an innovative device for therapeutic adherence in pediatric asthma. clinicaltrials.gov/show/NCT03788395 (first received 27 December 2018).
Landon 2019 {published data only}
- NCT04250779. Evaluating efficacy of smart device in assisting with inhaler technique and adherence (MOMMIASTHMA1). clinicaltrials.gov/show/NCT04250779 (first received 31 January 2020).
Linnhoff 2019 {published data only}
- NCT03951714. Effect of the use of an add-on device connected to a smartphone app on difficult-to-treat asthmatic patient's adherence (ADVICE) [12-wks randomised controlled trial to explore the effect of a smartphone app. Connected to an add-on device system fitted on pMDI inhaler on adherence to medications intake and clinical outcomes in difficult-to-treat asthmatic patients]. clinicaltrials.gov/show/NCT03951714 (first received 15 May 2019).
Simoneau 2018 {published data only}
- NCT03734861. The study is enrolling kids from 7 to 16 years old. The BreathSmart Device attaches to the inhaler to measure adherence [A prospective, randomized, controlled study to assess medication adherence in children with asthma managed on BreatheSmart and feedback]. clinicaltrials.gov/show/NCT03734861 (first received 8 November2018).
Additional references
Aaron 2017
- Aaron SD, Vandemheen KL, FitzGerald J, Ainslie M, Gupta S, Lemière C, et al. Reevaluation of diagnosis in adults with physician-diagnosed asthma. JAMA 2017;317(3):269-79. [DOI] [PubMed] [Google Scholar]
Ali 2014
- Ali AA, Xiao H, Adunlin G. Effectiveness of text message reminders in asthma medication adherence: a systematic review. Value in Health 2014;17(3):A178. [DOI] [PMC free article] [PubMed] [Google Scholar]
Alquran 2018
- Alquran A, Lambert KA, Farouque A, Holland A, Davies J, Lampugnani ER, et al. Smartphone applications for encouraging asthma self-management in adolescents: a systematic review. International Journal of Environmental Research and Public Health 2018;15(11):2403. [DOI] [PMC free article] [PubMed]
Anderson 2016
- Anderson K, Burford O, Emmerton L. Mobile health apps to facilitate self-care: a qualitative study of user experiences. PLOS One 2016;11(5):e0156164. [DOI] [PMC free article] [PubMed] [Google Scholar]
Angst 2017
- Angst F, Aeschlimann A, Angst J. The minimal clinically important difference raised the significance of outcome effects above the statistical level, with methodological implications for future studies. Journal of Clinical Epidemiology 2017;82:128-36. [DOI] [PubMed] [Google Scholar]
Asthma Foundation NZ
- Asthma Foundation NZ. Asthma and Respiratory Foundation NZ Adolescent and Adult Asthma Guidelines 2020. www.asthmafoundation.org.nz/resources/nz-adolescent-and-adult-asthma-guidelines (accessed prior to 14 October 2021).
Babic 2019
- Babic A, Tokalic R, Amílcar Silva Cunha J, Novak I, Suto J, Vidak M, et al. Assessments of attrition bias in Cochrane systematic reviews are highly inconsistent and thus hindering trial comparability. BMC Medical Research Methodology 2019;19(1):76. [DOI] [PMC free article] [PubMed] [Google Scholar]
Baptist 2016
- Baptist AP, Islam N, Joseph CL. Technology-based interventions for asthma - can they help decrease health disparities? Journal of Allergy and Clinical Immunology 2016;4(6):1135-42. [DOI] [PubMed] [Google Scholar]
Barnes 1993
- Barnes PJ. Anti-inflammatory therapy for asthma. Annual Review of Medicine 1993;44(1):229-42. [DOI] [PubMed] [Google Scholar]
Barnes 2003
- Barnes PJ, Adcock IM. How do corticosteroids work in asthma? Annals of Internal Medicine 2003;139(5 Pt 1):359-70. [DOI] [PubMed] [Google Scholar]
Barnes 2015
- Barnes CB, Ulrik CS. Asthma and adherence to inhaled corticosteroids: current status and future perspectives. Respiratory Care 2015;60(3):455-68. [DOI] [PubMed] [Google Scholar]
Barroso 2018
- Barroso AT, Martín EM, Romero LMR, Ruiz FO. Factors affecting lung function: a review of the literature. Archivos de Bronconeumología (English Edition) 2018;54(6):327-32. [DOI] [PubMed] [Google Scholar]
Bastawrous 2013
- Bastawrous A, Armstrong MJ. Mobile health use in low- and high-income countries: an overview of the peer-reviewed literature. Journal of the Royal Society of Medicine 2013;106(4):130-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
Blakey 2018
- Blakey JD, Bender BG, Dima AL, Weinman J, Safioti G, Costello RW. Digital technologies and adherence in respiratory diseases: the road ahead. European Respiratory Journal 2018;52(5):1801147. [DOI] [PMC free article] [PubMed] [Google Scholar]
Britto 2012
- Britto MT, Munafo JK, Schoettker PJ, Vockell AL, Wimberg JA, Yi MS. Pilot and feasibility test of adolescent-controlled text messaging reminders. Clinical Pediatrics 2012;51(2):114-21. [DOI] [PubMed] [Google Scholar]
BTS/SIGN 2016
- British Thoracic Society Scottish Intercollegiate Guidelines Network. SIGN158 British guideline on the management of asthma. www.brit-thoracic.org.uk/quality-improvement/guidelines/asthma/ (accessed 7 February 2022).
Bussey‐Smith 2007
- Bussey-Smith KL, Rossen RD. A systematic review of randomized control trials evaluating the effectiveness of interactive computerized asthma patient education programs. Annals of Allergy, Asthma and Immunology 2007;98(6):507-16. [DOI] [PubMed] [Google Scholar]
CDC 2016
- Centers for Disease Control and Prevention (CDC). Common asthma triggers. www.cdc.gov/asthma/triggers.html (accessed 14 December 2017).
Chan 2013
- Chan AHY, Reddel HK, Apter A, Eakin M, Riekert K, Foster JM. Adherence monitoring and e-health: how clinicians and researchers can use technology to promote inhaler adherence for asthma. Journal of Allergy and Clinical Immunology 2013;1(5):446-54. [DOI] [PubMed] [Google Scholar]
Clifford 2008
- Clifford S, Barber N, Horne R. Understanding different beliefs held by adherers, unintentional nonadherers, and intentional nonadherers: application of the Necessity-Concerns Framework. Journal of Psychosomatic Research 2008;64(1):41-6. [DOI] [PubMed] [Google Scholar]
Conn 2017
- Conn VS, Ruppar TM. Medication adherence outcomes of 771 intervention trials: systematic review and meta-analysis. Preventive Medicine 2017;99:269-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cooper 2015
- Cooper V, Metcalf L, Versnel J, Upton J, Walker S, Horne R. Patient-reported side effects, concerns and adherence to corticosteroid treatment for asthma, and comparison with physician estimates of side-effect prevalence: a UK-wide, cross-sectional study. NPJ Primary Care Respiratory Medicine 2015;25:15026. [DOI] [PMC free article] [PubMed] [Google Scholar]
D'Arcy 2014
- D'Arcy S, MacHale E, Seheult J, Holmes MS, Hughes C, Sulaiman I, et al. A method to assess adherence in inhaler use through analysis of acoustic recordings of inhaler events. PLOS One 2014;9(6):e98701. [DOI] [PMC free article] [PubMed] [Google Scholar]
Dayer 2013
- Dayer L, Heldenbrand S, Anderson Pl, Gubbins PO, Martin BC. Smartphone medication adherence apps: potential benefits to patients and providers. Journal of the American Pharmacists Association 2013;53(2):172-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
Desalu 2021
- Desalu O, Ozoh O. Achieving asthma control in low-middle-income countries: why it is important? Journal of the Pan African Thoracic Society 2021;2(2):59-60. [Google Scholar]
Dima 2015
- Dima AL, Hernandez G, Cunillera O, Ferrer M, Bruin M. Asthma inhaler adherence determinants in adults: systematic review of observational data. European Respiratory Journal 2015;45(4):994-1018. [DOI] [PubMed] [Google Scholar]
Eakin 2012
- Eakin MN, Rand CS. Improving patient adherence with asthma self-management practices: what works? Annals of Allergy, Asthma and Immunology 2012;109(2):90-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ebmeier 2017
- Ebmeier S, Thayabaran D, Braithwaite I, Bénamara Clément, Weatherall M, Beasley R. Trends in international asthma mortality: analysis of data from the WHO Mortality Database from 46 countries (1993–2012). Lancet 2017;390(10098):935-45. [DOI] [PubMed] [Google Scholar]
Engelkes 2015
- Engelkes M, Janssens HM, Jongste JC, Sturkenboom MC, Verhamme KM. Medication adherence and the risk of severe asthma exacerbations: a systematic review. European Respiratory Journal 2015;45(2):396-407. [DOI] [PubMed] [Google Scholar]
Faraone 2008
- Faraone SV. Interpreting estimates of treatment effects: implications for managed care. Pharmacy and Therapeutics 2008;33(12):700-11. [PMC free article] [PubMed] [Google Scholar]
Farmer 1999
- Farmer KC. Methods for measuring and monitoring medication regimen adherence in clinical trials and clinical practice. Clinical Therapeutics 1999;21(6):1074-90. [DOI] [PubMed] [Google Scholar]
GINA 2017
- Global Initiative for Asthma. 2017 GINA Report, Global Strategy for Asthma Management and Prevention. ginasthma.org/wp-content/uploads/2019/04/wmsGINA-2017-main-report-final_V2.pdf (accessed 14 December 2017).
GINA 2019
- Global Initiative for Asthma. 2019 GINA Report, Global Strategy for Asthma Management and Prevention. ginasthma.org/wp-content/uploads/2019/06/GINA-2019-main-report-June-2019-wms.pdf (accessed prior to 15 October 2021).
Global Asthma Report 2014
- Global Asthma Network. The Global Asthma Report 2014. www.globalasthmareport.org/ (accessed 14 December 2017).
GRADEpro GDT [Computer program]
- GRADEpro GDT. Version accessed before 15 December 2017. Hamilton (ON): McMaster University (developed by Evidence Prime), 2015. Available at gradepro.org.
Higgins 2003
- Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ 2003;327(7414):557-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane-handbook.org.
Holmes 2014
- Holmes EA, Hughes DA, Morrison VL. Predicting adherence to medications using health psychology theories: a systematic review of 20 years of empirical research. Value in Health 2014;17(8):863-76. [DOI] [PubMed] [Google Scholar]
Hopp 2016
- Hopp RJ, Pasha MA. A literature review of the evidence that a 12% improvement in FEV1 is an appropriate cut-off for children. Journal of Asthma 2016;53(4):413-8. [DOI: doi: 10.3109/02770903.2015.1108436] [DOI] [PubMed] [Google Scholar]
Horne 2005
- Horne R, Weinman J, Barber N, Elliott R, Morgan M. Concordance, adherence and compliance in medicine taking. Report for the National Coordinating Centre for NHS Service Delivery and Organization R & D (NCCSDO) (2005). www.netscc.ac.uk/hsdr/files/project/SDO_FR_08-1412-076_V01.pdf (accessed April 2018).
Horne 2019
- Horne R, Cooper V, Wileman V, Chan A. Supporting adherence to medicines for long-term conditions: a Perceptions and Practicalities Approach based on an extended Common Sense Model. European Psychologist 2019;24(1):82-96. [Google Scholar]
Howell 2008
- Howell G. Nonadherence to medical therapy in asthma: risk factors, barriers, and strategies for improving. Journal of Asthma 2008;45(9):723-9. [DOI] [PubMed] [Google Scholar]
Huang 2019
- Huang X, Matricardi PM. Allergy and asthma care in the mobile phone era. Clinical Reviews in Allergy and Immunology 2019;56(2):161-73. [DOI] [PubMed] [Google Scholar]
Johnson 2016b
- Johnson D, Deterding S, Kuhn K-A, Staneva A, Stoyanov S, Hides L. Gamification for health and wellbeing: a systematic review of the literature. Internet Interventions 2016;6:89-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jones 2003
- Jones C, Santanello NC, Boccuzzi SJ, Wogen J, Strub P, Nelsen LM. Adherence to prescribed treatment for asthma: evidence from pharmacy benefits data. Journal of Asthma 2003;40(1):93-101. [DOI: DOI: 10.1081/JAS-120017212] [DOI] [PubMed] [Google Scholar]
Joos 2008
- Joos S, Miksch A, Szecsenyi J, Wieseler B, Grouven U, Kaiser T, et al. Montelukast as add-on therapy to inhaled corticosteroids in the treatment of mild to moderate asthma: a systematic review. Thorax 2008;63(5):453-62. [DOI] [PubMed] [Google Scholar]
Kaminsky 2019
- Kaminsky, David A. What is a significant bronchodilator response? Annals of the American Thoracic Society 2019;16(12):1495-7. [DOI] [PubMed] [Google Scholar]
Kannisto 2014
- Kannisto KA, Koivunen MH, Välimäki MA. Use of mobile phone text message reminders in health care services: a narrative literature review. Journal of Medical Internet Research 2014;16(10):e222. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kardas 2013
- Kardas P, Lewek P, Matyjaszczyk M. Determinants of patient adherence: a review of systematic reviews. Frontiers in Pharmacology 2013;4:91. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kolmodin 2016
- Kolmodin MacDonell K, Naar S, Gibson-Scipio W, Lam P, Secord E. The Detroit Young Adult Asthma Project: pilot of a technology-based medication adherence intervention for African-American emerging adults. Journal of Adolescent Health 2016;59(4):465-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
Krebs 2015
- Krebs P, Duncan DT. Health app use among us mobile phone owners: a national survey. MIR mHealth and uHealth 2015;3(4):e101. [DOI] [PMC free article] [PubMed] [Google Scholar]
Krishna 2003
- Krishna S, Francisco BD, Balas EA, König P, Graff GR, Madsen RW. Internet-enabled interactive multimedia asthma education program: a randomized trial. Pediatrics 2003;111(3):503-10. [DOI] [PubMed] [Google Scholar]
Lasmar 2009
- Lasmar L, Camargos P, Champs NS, Fonseca MT, Fontes MJ, Ibiapina C, et al. Adherence rate to inhaled corticosteroids and their impact on asthma control. Allergy 2009;64(5):784-9. [DOI] [PubMed] [Google Scholar]
Levy 2014
- Levy ML. National review of asthma deaths (NRAD). British Journal of General Practice 2014;64(628):564. [DOI] [PMC free article] [PubMed] [Google Scholar]
Looijmans‐van den Akker 2016
- Looijmans-van den Akker I, Luijn K, Verheij T. Overdiagnosis of asthma in children in primary care: a retrospective analysis. British Journal of General Practice 2016;66(644):e152-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Maddux 2021
- Maddux JT, Inselman JW, Jeffery MM, Lam RW, Shah ND, Rank MA. Adherence to asthma biologics: implications for patient selection, step therapy, and outcomes. Chest 2021;159(3):924-32. [PMID: doi: 10.1016/j.chest.2020.10.050.] [DOI] [PMC free article] [PubMed] [Google Scholar]
Marcano Belisario 2013
- Marcano Belisario JS, Huckvale K, Greenfield G, Car J, Gunn LH. Smartphone and tablet self management apps for asthma. Cochrane Database of Systematic Reviews 2013, Issue 11. Art. No: CD010013. [DOI: 10.1002/14651858.CD010013.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Masoli 2004
- Masoli M, Fabian D, Holt S, Beasley R, Global Initiative for Asthma Program. The global burden of asthma: executive summary of the GINA dissemination committee report. Allergy 2004;59(5):469-78. [DOI] [PubMed] [Google Scholar]
Mathes 2014
- Mathes T, Jaschinski T, Pieper D. Adherence influencing factors - a systematic review of systematic reviews. Archives of Public Health 2014;72(1):37. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mazumdar 2015
- Mazumdar S, Ghosh S, Mukherjee S. Non-adherence to asthma medications: relation to socio-economic status and asthma education. European Respiratory Journal 2015;46:OA4792. [DOI: 10.1183/13993003.congress-2015.OA4792] [DOI] [Google Scholar]
McCambridge 2014
- McCambridge J, Witton J, Elbourne DR. Systematic review of the Hawthorne effect: new concepts are needed to study research participation effects. Journal of Clinical Epidemiology 2014;67(3):267-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
McDonald 2002
- McDonald HP, Garg AX, Haynes RB. Interventions to enhance patient adherence to medication prescriptions: scientific review. JAMA 2002;288(22):2868-79. [DOI] [PubMed] [Google Scholar]
McLean 2016
- McLean G, Murray E, Band R, Moffat KR, Hanlon P, Bruton A, et al. Interactive digital interventions to promote self-management in adults with asthma: systematic review and meta-analysis. BMC Pulmonary Medicine 2016;16(1):1-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
McQuaid 2012
- McQuaid EL, Everhart RS, Seifer R, Kopel SJ, Mitchell DK, Klein RB, et al. Medication adherence among Latino and non-Latino white children with asthma. Pediatrics 2012;129(6):e1404-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Menckeberg 2008
- Menckeberg TT, Bouvy M L, Bracke M, Kaptein AA, Leufkens HG, Raaijmakers JA, et al. Beliefs about medicines predict refill adherence to inhaled corticosteroids. Journal of Psychosomatic Research 2008;64:47-54. [DOI] [PubMed] [Google Scholar]
Michie 2017
- Michie S, Yardley L, West R, Patrick K, Greaves F. Developing and evaluating digital interventions to promote behavior change in health and health care: recommendations resulting from an international workshop. Journal of Medical Internet Research 2017;19(6):e232. [DOI] [PMC free article] [PubMed] [Google Scholar]
Moher 2009
- Moher D, Liberati A, Tetzlaff J, Altman D. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLOS Medicine 2009;6(7):e1000097. [DOI: 10.1371/journal.pmed.1000097] [DOI] [PMC free article] [PubMed] [Google Scholar]
Morrison 2014
- Morrison D, Wyke S, Agur K, Cameron EJ, Docking RI, MacKenzie AM, et al. Digital asthma self-management interventions: a systematic review. Journal of Medical Internet Research 2014;16(2):e51. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mosnaim 2015
- Mosnaim G, Li H, Martin M, Richardson DJ, Belice PJ, Avery E, et al. A tailored mobile health intervention to improve adherence and asthma control in minority adolescents. Journal of Allergy and Clinical Immunology 2015;3(2):288-90.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mosnaim 2016
- Mosnaim GS, Pappalardo AA, Resnick SE, Codispoti CD, Bandi S, Nackers L, et al. Behavioral interventions to improve asthma outcomes for adolescents: a systematic review. Journal of Allergy and Clinical Immunology 2016;4(1):130-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
NICE 2021
- National Institute for Health and Care Excellence (NICE). Asthma: diagnosis, monitoring and chronic asthma management. NICE guideline [NG80]. www.nice.org.uk/guidance/ng80 (accessed prior to 15 October 2021).
Nieuwlaat 2014
- Nieuwlaat R, Wilczynski N, Navarro T, Hobson N, Jeffery R, Keepanasseril A, et al. Interventions for enhancing medication adherence. Cochrane Database of Systematic Reviews 2014, Issue 11. Art. No: CD000011. [DOI: 10.1002/14651858.CD000011.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Nolte 2006
- Nolte H, Nepper-Christensen S, Backer V. Unawareness and undertreatment of asthma and allergic rhinitis in a general population. Respiratory Medicine 2006;100(2):354-62. [DOI] [PubMed] [Google Scholar]
Normansell 2018
- Normansell R, Chan AHY, Katzer CB, Kew KM, Mes MA, Newby CJ, et al. Health psychology interventions to improve adherence to maintenance therapies in asthma. Cochrane Database of Systematic Reviews 2018, Issue 10. Art. No: CD013147. [DOI: 10.1002/14651858.CD013147] [DOI] [Google Scholar]
Ouzzani 2016
- Ouzzani M, Hammady H, Fedorowicz Z, Elmagarmid A. Rayyan—a web and mobile app for systematic reviews. Systematic Reviews 2016;5(1):210. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ponieman 2009
- Ponieman D, Wisnivesky JP, Leventhal H, Musumeci-Szabó TJ, Halm EA. Impact of positive and negative beliefs about inhaled corticosteroids on adherence in inner-city asthmatic patients. Annals of Allergy, Asthma and Immunology 2009;103(1):38-42. [DOI] [PubMed] [Google Scholar]
Ramsey 2020
- Ramsey RR, Plevinsky JM, Kollin SR, Gibler RC, Guilbert TW, Hommel KA. Systematic review of digital interventions for pediatric asthma management. Journal of Allergy and Clinical Immunology 2020;8(4):1284093. [DOI] [PMC free article] [PubMed] [Google Scholar]
Reidel 2008
- Reidel K, Tamblyn R, Patel V, Huang A. Pilot study of an interactive voice response system to improve medication refill compliance. BMC Medical Informatics and Decision Making 2008;8(1):46. [DOI] [PMC free article] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Riekert 2002
- Riekert KA, Rand CS. Electronic monitoring of medication adherence: when is high-tech best? Journal of Clinical Psychology in Medical Settings 2002;9:25-34. [Google Scholar]
Royal College of Physicians 2014
- Royal College of Physicians. Why Asthma Still Kills: The National Review of Asthma Deaths (NRAD) Confidential Enquiry Report (2014). www.rcplondon.ac.uk/projects/national-review-asthma-deaths (accessed 20 April 2018).
Saberi 2011
- Saberi P, Johnson MO, McCulloch CE, Vittinghoff E, Neilands TB. Medication adherence: tailoring the analysis to the data. AIDS and Behavior 2011;15(7):1447-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sohanpal 2012
- Sohanpal R, Hooper R, Hames R, Priebe S, Taylor S. Reporting participation rates in studies of non-pharmacological interventions for patients with chronic obstructive pulmonary disease: a systematic review. Systematic Reviews 2012;1(1):66. [DOI] [PMC free article] [PubMed] [Google Scholar]
Soriano 2017
- Soriano JB, Abajobir AA, Abate KH, Abera SF, Agrawal A, Ahmed MB, et al. Global, regional, and national deaths, prevalence, disability-adjusted life years, and years lived with disability for chronic obstructive pulmonary disease and asthma, 1990-2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet Respiratory Medicine 2017;5(9):691-706. [DOI] [PMC free article] [PubMed] [Google Scholar]
Suissa 2000
- Suissa S, Ernst P, Benayoun S, Baltzan M, Cai B. Low-dose inhaled corticosteroids and the prevention of death from asthma. New England Journal of Medicine 2000;343:332-6. [DOI] [PubMed] [Google Scholar]
Sulaiman 2016
- Sulaiman I, Seheult J, MacHale E, D'Arcy S, Boland F, McCrory K, et al. Irregular and ineffective: a quantitative observational study of the time and technique of inhaler use. Journal of Allergy and Clinical Immunology 2016;4(5):900-9.e2. [DOI] [PubMed] [Google Scholar]
Sunstein 2014
- Sunstein CR, Thaler RH. Nudge: Improving Decisions About Health, Wealth, and Happiness. Penguin Books, 2014. [Google Scholar]
Taylor 2018
- Taylor TE, Zigel Y, Egan C, Hughes F, Costello RW, Reilly RB. Objective assessment of patient inhaler user technique using an audio-based classification approach. Scientific Reports 2018;8(1):2164. [DOI] [PMC free article] [PubMed] [Google Scholar]
Thakkar 2016
- Thakkar J, Kurup R, Laba T-L, Santo K, Thiagalingam A, Rodgers A, et al. Mobile telephone text messaging for medication adherence in chronic disease: a meta-analysis. JAMA Internal Medicine 2016;176(3):340-9. [DOI] [PubMed] [Google Scholar]
Tran 2014
- Tran N, Coffman JM, Sumino K, Cabana MD. Patient reminder systems and asthma medication adherence: a systematic review. Journal of Asthma 2014;51:536-43. [DOI] [PubMed] [Google Scholar]
Undela 2021
- Undela K, Goldsmith L, Kew KM, Ferrara G. Macrolides versus placebo for chronic asthma. Cochrane Database of Systematic Reviews 2021, Issue 11. Art. No: CD002997. [DOI: 10.1002/14651858.CD002997.pub5] [DOI] [PMC free article] [PubMed] [Google Scholar]
van Dulmen 2007
- Dulmen S, Sluijs E, Dijk L, Ridder D, Heerdink R, Bensing J. Patient adherence to medical treatment: a review of reviews. BMC Health Services Research 2007;7:55. [DOI] [PMC free article] [PubMed] [Google Scholar]
van Schayck 2000
- Schayck CP, Heijden FMMA, den Boom G, Tirimanna PRS, Herwaarden CLA. Underdiagnosis of asthma: is the doctor or the patient to blame? The DIMCA Project. Thorax 2000;55(7):562-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Van Steenis 2014
- Van Steenis MNA, Driesenaar JA, Bensing JM, Van Hulten R, Souverein PC, Van Dijk L, et al. Relationship between medication beliefs, self-reported and refill adherence, and symptoms in patients with asthma using inhaled corticosteroids. Patient Preference and Adherence 2014;8:83-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
WHO 2003
- World Health Organization. Adherence to Long-term Therapies: Evidence for Action. Geneva: World Health Organization, 2003. [Google Scholar]
WHO 2013
- World Health Organization. Asthma fact sheets. who.int/mediacentre/factsheets/fs307/en/ (accessed 14 December 2017).
Williams 2004
- Williams LK, Pladevall M, Xi H, Peterson EL, Joseph C, Lafata JE, et al. Relationship between adherence to inhaled corticosteroids and poor outcomes among adults with asthma. Journal of Allergy and Clinical Immunology 2004;114:1288-93. [DOI] [PubMed] [Google Scholar]
Williams 2011
- Williams LK, Peterson EL, Wells K, Ahmedani BK, Kumar R, Burchard EG, et al. Quantifying the proportion of severe asthma exacerbations attributable to inhaled corticosteroid nonadherence. Journal of Allergy and Clinical Immunology 2011;128:1185-91.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Chan 2018
- Chan AHY, De Simoni A, Wileman V, Holliday L, Chisari C, Newby CJ, et al. Digital interventions to improve adherence to maintenance medication in asthma. Cochrane Database of Systematic Reviews 2018, Issue 5. Art. No: CD013030. [DOI: 10.1002/14651858.CD013030] [DOI] [PMC free article] [PubMed] [Google Scholar]


