Abstract
Glycerophospholipids are major components of cell membranes and have enormous variation in the composition of fatty acyl chains esterified on the sn-1 and sn-2 position as well as the polar head groups on the sn-3 position of the glycerol backbone. Phospholipase A2 (PLA2) enzymes constitute a superfamily of enzymes which play a critical role in metabolism and signal transduction by hydrolyzing the sn-2 acyl chains of glycerophospholipids. In human cell membranes, in addition to the conventional diester phospholipids, a significant amount is the sn-1 ether-linked phospholipids which play a critical role in numerous biological activities. However, precisely how PLA2s distinguish the sn-1 acyl chain linkage are not understood. In the present study, we expanded the technique of lipidomics to determine the unique in vitro specificity of three major human PLA2s, including Group IVA cytosolic cPLA2, Group VIA calcium-independent iPLA2, and Group V secreted sPLA2 toward the linkage at the sn-1 position. Interestingly, cPLA2 prefers sn-1 vinyl ether phospholipids known as plasmalogens over conventional ester phospholipids and the sn-1 alkyl ether phospholipids. iPLA2 showed similar activity toward vinyl ether and ester phospholipids at the sn-1 position. Surprisingly, sPLA2 preferred ester phospholipids over alkyl and vinyl ether phospholipids. By taking advantage of molecular dynamics simulations, we found that Trp30 in the sPLA2 active site dominates its specificity for diester phospholipids.
Keywords: phospholipase A2, ether phospholipids, plasmalogens, lipidomics, molecular dynamics simulation
Introduction
Glycerophospholipids are the defining component of cell membranes, and are composed of two acyl chains esterified to the glycerol backbone at the sn-1 and sn-2 positions as well as the polar headgroup on the sn-3 position. Phospholipase A2 (PLA2) comprises a superfamily of enzymes hydrolyzing the acyl-chain of glycerophospholipids at the sn-2 position producing lysophospholipids and free fatty acids [1]. Each PLA2 expresses numerous biological functions, especially in the inflammatory processes, by hydrolyzing membrane glycerophospholipids and producing free fatty acids such as arachidonic acid (20:4, AA) which is an initial substrate of the AA cascade producing bioactive eicosanoids and oxylipins [2]. In addition, importantly PLA2s contribute to membrane remodeling, signal transduction, and “biological surfactants” by producing lysophospholipids which are reacylated with polyunsaturated fatty acids (PUFAs) to produce remodeled phospholipids; lysophospholipids can also be further metabolically converted to ligands for several G-protein coupled receptors (GPCRs); and lysophospholipids as “natural” surfactants/detergents can destabilize membranes exhibiting bacterialcidal effects. These biological functions differ depending on the specific subcellular localization and substrate specificity of each PLA2. Therefore, the substrate specificity and selectivity of each PLA2 is closely correlated with its biological function.
An enormous variety of glycerophospholipids exist based on the combination of acyl chains and head groups and they comprise cell membranes presenting specific morphological and chemical characteristics to the membranes. The enzymatic action of a PLA2 is initiated by its interaction with the lipid-water interface, which is followed by the enzyme extracting a single phospholipid substrate from the membrane to accommodate the specific substrate phospholipid adequately and properly in its active site [3]. Each step contributes to the specific activity of the PLA2, and both the headgroup and the two different acyl chains critically contribute to these steps. For example, Group IVA cytosolic cPLA2 (GIVA cPLA2) shows high activity toward PIP2 containing membranes by interacting specifically with the headgroup at an allosteric site, and Group V secreted sPLA2 (GV sPLA2) preferentially hydrolyzes substrate phospholipids containing phosphatidylglycerol headgroups [4,5]. Also, our previous studies have revealed that the sn-2 acyl chain greatly effects substrate specificity [5,6]. In addition, phospholipids contain a variety of linkages at the sn-1 position, and this should have some effect on the substrate specificity and selectivity of each PLA2. However, the effect of the sn-1 acyl chain on the substrate specificity of each type of PLA2 has not been well understood and this is the focus of this report.
For cellular membranes, sn-1 alkyl ether and sn-1 vinyl ether-linked phospholipids can account for as much as 20% of the phospholipid, and in addition to the conventional diester glycerophospholipids which have been considered as the primary substrate for PLA2s, the sn-1 ether and vinyl ether containing phospholipids can also serve as a substrate for PLA2s. One critical bioactive sn-1 alkyl ether phospholipid is 1-O-hexadecyl-2-acetyl-sn-glycero-3-phosphocholine, which is well known as platelet-activating factor (PAF), and this phospholipid plays a pivotal role in inflammation, platelet activation, and many leucocyte functions [7]. Since lyso-PAF (1-O-alkyl-sn-glycero-3-phosphocholine) is converted to PAF by acylation, PLA2 activity toward the sn-1 alkyl ether phospholipids containing AA and other fatty acids at the sn-2 position as well as toward PAF is important for the synthesis and deactivation of PAF [8]. Indeed, it has been reported that cPLA2 contributes to PAF production [9]. In addition, group VIIA PLA2 (GVIIA PLA2), also known as PAF acetylhydrolase (PAF-AH) as well as being known as lipoprotein-associated PLA2 (LpPLA2), is well recognized as an enzyme responsible for hydrolyzing PAF, and we have demonstrated its substrate specificity and characteristics by taking advantage of lipidomics based techniques and computational approaches [10] (V. Mouchlis et al., manuscript in preparation).
The sn-1 vinyl ether phospholipids which are also known as plasmalogens, are the most common sn-1 ether-containing phospholipids. Plasmalogens exist ubiquitously but are especially enriched in the brain and heart [11]. It has been suggested that subcellular plasmalogens are abundant in the lipid raft microdomains, where signaling molecules accumulate and contribute to raft formation, although the separation and segmentation of lipid rafts within and from cell membranes are still controversial [12,13]. Further, it has been reported that plasmalogens are involving in membrane trafficking, cell differentiation, and phagocytosis in macrophages and are implicating in various diseases [14–19]. It is known that plasmalogens often contain polyunsaturated fatty acids (PUFAs) in their sn-2 acyl chains which are targets for lipid peroxidation. Recently, it has been reported that peroxidation of plasmalogens plays a critical role in cancer ferroptosis [20]. Therefore, regulating ether phospholipids by PLA2 should be critical for many biological responses.
Recently, we have developed lipidomics-based HPLC MS/MS assays using mixed micelles and multiple reaction monitoring (MRM) for various lysophospholipids and demonstrated unique substrate specificity for each of the major groups of human PLA2s including Group IVA cytosolic cPLA2 (GIVA cPLA2), Group VIA calcium-independent iPLA2 (GVIA iPLA2), and Group V secreted sPLA2 (GV sPLA2) focusing on the sn-2 acyl chain [5,6]. To date, several studies have reported the in vitro activity of cPLA2 toward ether phospholipids [21,22]. However, despite the importance of ether phospholipids in various biological processes, precise in vitro specificity and selectivity of PLA2s toward ester and ether phospholipids are still elusive. Herein, we have now applied these lipidomic techniques to sn-1 ether phospholipids and report the effect of the ether linkages on the sn-1 position to the activity and substrate specificity of the three major types of PLA2. Moreover, by also employing molecular dynamics (MD) simulations, we have now explored the molecular basis of the unique specificity of GV sPLA2 toward ester phospholipids.
Methods
Materials
All phospholipids, including the lysophospholipid standards employed in this study, were purchased from Avanti Polar Lipids, Inc. The purity of all lipids was greater than 99%. All other materials were of appropriate and suitable quality for this study.
Expression and purification of recombinant human PLA2 enzymes
The human recombinant PLA2s were expressed and purified as described elsewhere [5]. The baculovirus encoding C-terminal 6×His tag conjugated human GIVA cPLA2, and N-terminal 6×His tag conjugated human GVIA iPLA2 were used to infect serum free-cultured Sf9 cells with a MOI of 0.1 and 2.0, respectively. The expression was induced for 72 h at 28°C with shaking. The recombinant protein was purified with Ni-NTA agarose (QIAGEN, Venlo, Netherlands). Elution buffer (25 mM Tris-HCl pH 7.5, 50 mM NaCl, 250 mM imidazole, 30% glycerol, and only for iPLA2, 2 mM ATP was included) was used for elution of recombinant protein. The human GV sPLA2 was expressed using the E. coli expression system and refolded by following and modifying the dilution method reported previously [23]. The C-terminal 6×His tag conjugated human GV sPLA2 was induced for expression with 0.1 mM IPTG for 4 h at 25°C by using BL21 (DE-3) Gold E. coli. The inclusion bodies were collected and denatured in buffer containing 6 M guanidine hydrochloride (GuHCl). Then, the denatured protein was purified with Ni-NTA agarose under the denatured condition. The denatured protein was eluted with elution buffer (25 mM Tris-HCl pH 8.0, 500 mM NaCl, 10 mM β-mercaptoethanol, 250 mM imidazole, 6 M GuHCl), and the eluted solution was dialyzed against dialyzing buffer (50 mM Tris-HCl pH 8.5, 100 mM NaCl, 6 M GuHCl). The protein solution was diluted with refolding buffer (50 mM Tris-HCl pH 8.5, 8.2 mM oxidized glutathione, 9.3 mM reduced glutathione, 12 mM CaCl2 12% glycerol) at 1: 7 dilution rates added dropwise and incubated for 7 days at 4°C. Finally, the solution was dialyzed against enzyme buffer (50 mM Tris-HCl pH 8.0, 50 mM NaCl, 10 mM CaCl2, 20% glycerol) to remove GuHCl completely and used as a GV sPLA2 enzyme solution.
Lipidomics-based LC-MS/MS assay
The lipidomics-based LC-MS/MS assay was performed in accord with our previous method [5]. We prepared a substrate mixture containing 100 μM phospholipid, 400 μM C12E8 surfactant, and 2.5 μM 17:0 lysophosphatidylcholine (LPC) as an internal standard. For cPLA2, 3 μM of porcine brain PIP2 (Avanti polar lipids, Inc., Alabaster, AL, USA) was mixed with 97 μM phospholipid to enhance the activity. When we employed mixtures of several different phospholipid substrates, we used a total of 100 µM phospholipid for iPLA2 and sPLA2, or 97 µM phospholipid plus 3 µM PIP2 (as a specific activator) for cPLA2. The reaction was started by adding 5 μl of enzyme solution to 95 μl of substrate solution in a 96-well plate, and the plate was incubated for 30 min at 40°C with gently shaking. The reaction was quenched by adding 120 µl of methanol/acetonitrile (80/20, v/v), and the sample was directly injected into the HPLC-MS/MS system. Mass spectrometry of lysophospholipids was performed with a 4000 QTRAP® (AB Sciex LLC, Framingham MA, USA). The amount of the products was calculated with a standard curve for each product, and the activity was normalized against the protein concentration of the enzyme solution.
Molecular Dynamics (MD) simulations
The MD simulations were performed by following our previously published methods [5].
Enzyme-substrate complexes.
Initial complexes of GV sPLA2 were generated using our model based on a homology model of GV sPLA2 based on the crystal structure of GIIA sPLA2 [5]. Phospholipids were docked in the active site of each enzyme using the Glide software implemented in the Schrödinger suite using a previously published docking protocol [5,24–26].
Enzyme-membrane systems.
The Membrane Builder implemented in CHARMM-GUI was employed to generate enzyme-membrane models for the MD simulations [27,28]. The membrane patch consisted of 1-palmitoyl-2-arachidonoyl-sn-glycero-3-phosphocholine (POPC), 1-stearoyl-2-arachidonoyl-sn-glycero-3-phosphocholine (SAPC), 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphatidic acid (POPA), 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphatidylethanolamine (POPE), 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphatidylglycerol (POPG), 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphatidylserine (POPS), 1-stearoyl-2-arachidonoyl-sn-glycero-3-phosphatidylinositol-4,5-bisphosphate (SAPI(4,5)P2), and cholesterol. The average ratios of the phospholipids were 0.48 for PC, 0.27 for PE, 0.10 for PI(4,5)P2, 0.06 for PS, and 0.09 for PA and PG. The average cholesterol/lipid ratio was 0.40. The composition of the glycerophospholipids (GPL) portion represents theaverage ratio of the major cellular membranes where the three PLA2s studied are known to be localized and acting. Each system was solvated with TIP3P water molecules and neutralized with 150 mM sodium chloride (NaCl) using the Visual Molecular Dynamics (VMD) package [29].
Equilibration and production runs.
Molecular dynamics simulations were carried out using NAMD 2.12 [30]. The minimization and equilibration protocol were performed as follows: a minimization of 80,000 steps was initially performed by applying harmonic constraints on the enzyme-ligand-membrane that were gradually turned off using a constraint scaling factor, followed by a second 120,000 steps minimization without constraints. An initial equilibration of 10,000 steps was performed by also applying harmonic constraints on the enzyme-ligand-membrane that were gradually turned off using the same constraint scaling factor, followed by a second 10,000 steps equilibration without constraints.
Each system was slowly heated and held to 310 K using temperature reassignment with a reassignment frequency of 500 timesteps (1000 fs) and a reassignment increment of 1 K during the equilibration. The minimization and equilibration protocol were sufficient to induce the appropriate disorder of a fluid-like bilayer, avoid unnatural atomistic positions, and failure of the simulations by atoms moving at exceedingly high velocities. Each system was finally subjected to a 1 µs production run. For each production run, the temperature was maintained at 310 K using the Langevin thermostat with Langevin coupling coefficient of 1/ps [31].
The NPT ensemble was employed and the pressure was kept constant at 1.01325 kPa using the Langevin piston method with the “useGroupPressure,” “useFlexibleCell,” and “useConstantArea” parameters turned on [32]. A time step of 2 fs was used in combination with the SHAKE algorithm to hold the bonds of hydrogen atoms similarly constrained [33]. Nonbonded interactions and full electrostatics were calculated every 1 and 2 time steps, respectively. Switching functions were used to smoothly take electrostatic and van der Waals interactions to zero with a switching distance of 10 Å and a cutoff of 12 Å. Long-range electrostatic forces in the periodic system were evaluated using the Particle Mesh Ewald (PME) Sum method with grid spacing 1/Å [34]. The CHARMM General Force Field (CGenFF) and the CHARMM36 all-atom additive force field and parameters were used for the simulations [35,36].
Statistical analysis
All error bars show the standard deviation (SD). A Student’s t-test was carried out when two groups were compared. A one-way ANOVA followed by the Tukey-Kramer multiple comparison test was carried out when more than three groups were compared using the GraphPad Prism version 5.0. A p-value of less than 0.05 was considered to be significant.
Results
LC-MS/MS assay for ether phospholipids
In contrast to conventional diester phospholipids, sn-1 alkyl ether phospholipids and plasmalogens contain alkyl ether and vinyl ether-linked carbon chains on the sn-1 position, respectively (Figure 1A). In this study, sn-1 alkyl ether-linked 16:0 and sn-1 vinyl ether-linked 18:1 phosphatidylcholine (PC) were used to explore the selectivity of PLA2s toward the sn-1 acyl chain linkage. By modifying the MRM setting, we obtained defined peaks for all lyso products, including 17:0 LPC used as an internal standard (Figure 1B), which resulted in linear standard curves (Figure 1C).
Figure 1. LC-MS/MS analysis of sn-1 ester, alkyl ether, and vinyl ether.
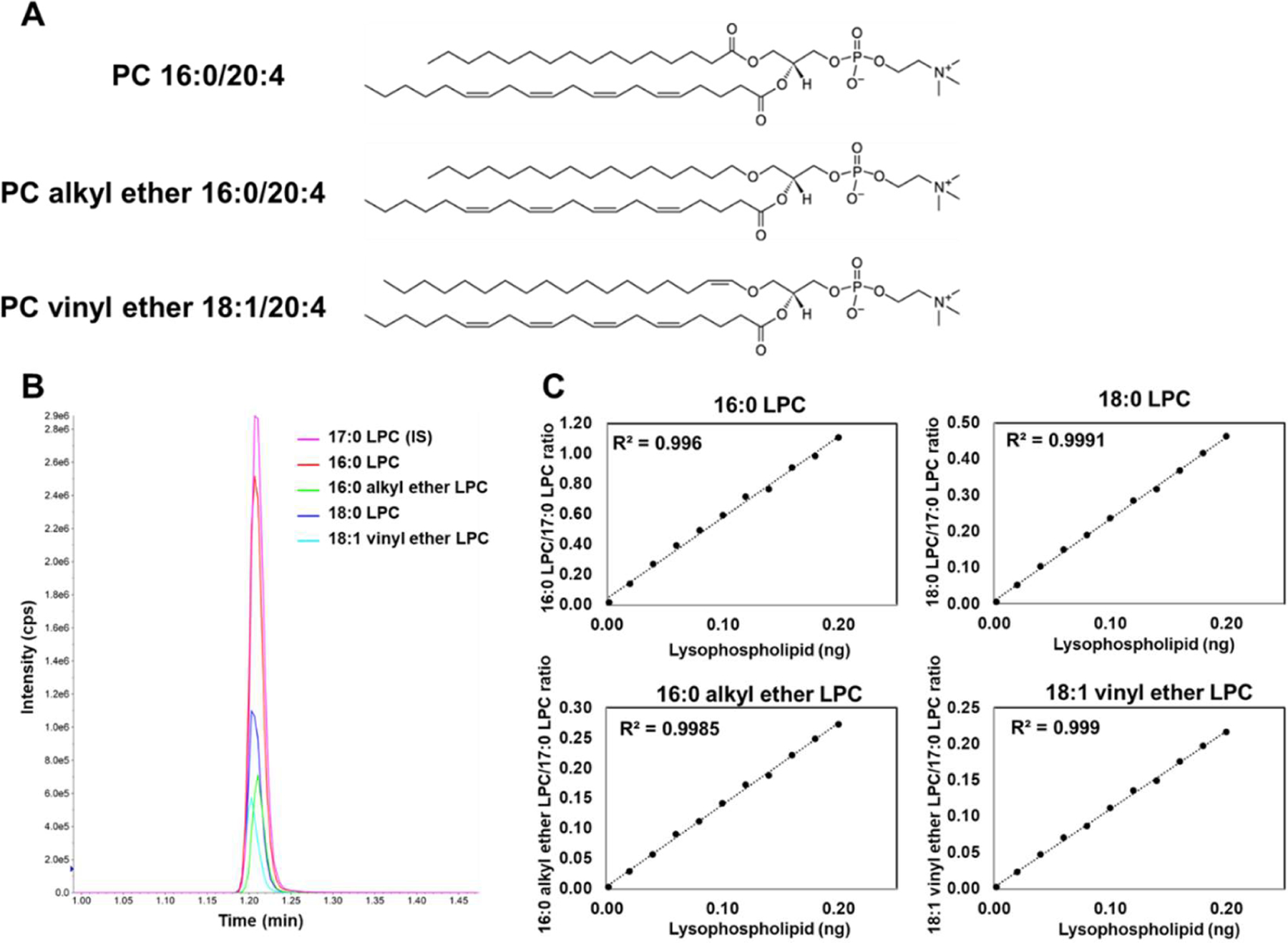
(A) Structures for diester sn-1 16:0, sn-2 20:4 PC (PC 16:0/20:4), sn-1 alkyl ether 16:0, sn-2 20:4 PC (PC alkyl ether 16:0/20:4), and sn-1 vinyl ether, 18:1 sn-2 20:4 (PC vinyl ether 18:1/20:4). (B) Chromatogram of lyso-PC (LPC) products. 17:0 LPC was used as an internal standard (IS). (C) Standard curves for 16:0 LPC, 18:0 LPC, 16:0 alkyl ether LPC, and 18:1 vinyl ether LPC used for quantification of PLA2 activity.
Specificity of PLA2s toward sn-1 alkyl ether phospholipids
First, we tested the activity of PLA2s toward sn-1 alkyl ether PCs and compared the activity with diester PCs which have the same sn-2 acyl chains using the LC-MS/MS assay. The sn-2 acyl chains were 2:0, 18:1, and 20:4. It is well known that cPLA2 is highly specific toward sn-2 20:4 [5] and consistent with that, cPLA2 showed a high specificity toward sn-2 20:4 in both diester and alkyl ether phospholipids (Figure 2A). Also, cPLA2 did not show detectable activity toward sn-1 alkyl 16:0, sn-2 2:0 PC which is known as PAF, whereas it showed a small activity toward the diester PC 16:0/2:0 (Figure 2A). The substrate specificity of iPLA2 is more permissive than that of cPLA2 [5] and showed comparable activity toward sn-2 18:1 and 20:4 in the sn-1 alkyl ether and ester phospholipids (Figure 2B). However, iPLA2 has minimum activity toward sn-2 2:0 in both cases, similar to cPLA2 (Figure 2B). In contrast, sPLA2 showed better activity toward sn-2 2:0 PCs than that of cPLA2 and iPLA2 and high activity toward sn-2 18:1 (Figure 2C). Notably, the sn-2 acyl chain specificity of all PLA2s was not affected by the linkage of the sn-1 chain. More importantly, all PLA2s showed higher activity toward all diester PCs than that toward alkyl ether PCs (Figure 2A, B, C).
Figure 2. Enzyme activity of PLA2s toward sn-1 alkyl ether phospholipids and diester phospholipids.

The activity of (A) GIVA cPLA2, (B) GVIA iPLA2, and (C) GV sPLA2 is shown toward sn-1 alkyl ether or ester 16:0 sn-2 X PC, where X is 2:0, 18:1, or 20:4. (D) The activity of GIVA cPLA2 toward sn-1 alkyl ether or ester 16:0 sn-2 20:4 PC in equal proportions with 100 µM total phospholipid. The activity of (E) GVIA iPLA2 and (F) GV sPLA2 toward sn-1 alkyl ether or ester 16:0 sn-2 18:1 PC in equal proportions with 100 µM total phospholipid. The amount of lyso products were determined and quantified using authentic LPC primary and internal standards. Error bars show standard deviation (SD). N.D.: not detected. Asterisks indicate statistical significance. **P < 0.01.
To eliminate the effect of the shape and characteristics of substrate mixed micelles which might affect the activity of PLA2s, the activity of all three enzymes in equimolar mixtures of diester and sn-1 alkyl ether phospholipids was measured. Mixtures of sn-2 20:4 phospholipids was used for testing cPLA2, and mixtures of sn-2 18:1 phospholipids was used for testing iPLA2 and sPLA2. Even in the mixtures, all PLA2s preferred diester phospholipids (Figure 2D, E, F). Especially, sPLA2 showed a strong preference toward diester phospholipids, and the activity was more than 4 times higher than that toward sn-1 alkyl ether phospholipids (Figure 2F), whereas cPLA2 and iPLA2 showed 1.6 and 1.7 times higher activity toward diesters than that toward alkyl ethers, respectively (Figure 2D, E).
Specificity of PLA2s toward sn-1 vinyl ether phospholipids (plasmalogens)
Next, we tested the preference of all three PLA2s for plasmalogens by comparing the activity toward diester phospholipids. In this experiment, PCs which possess 18 carbon chains (C18) on the sn-1 position and 18:1, 20:4, and 22:6 on the sn-2 position were used. Surprisingly, in contrast to alkyl ether phospholipids, each of the PLA2s tested showed a different preference for plasmalogens. cPLA2 still showed high specificity toward 20:4 in both diester phospholipids and plasmalogens. However, cPLA2 preferred plasmalogens over diester phospholipids (Figure 3A). iPLA2 showed similar activity in both diester phospholipids and plasmalogens with a similar sn-2 acyl chain specificity in both cases (Figure 3B). sPLA2 showed a high preference for diester phospholipids over plasmalogens (Figure 3C). Notably, the vinyl ether linkage in plasmalogens did not significantly affect the sn-2 acyl chain specificity of all three PLA2s, similar to alkyl ether phospholipids (Figure 3A, B, C).
Figure 3. Enzyme activity of PLA2s toward sn-1 vinyl ether phospholipids and diester phospholipids.

The activity of (A) GIVA cPLA2, (B) GVIA iPLA2, and (C) GV sPLA2 is shown toward sn-1 vinyl ether or ester C18 sn-2 X PC, where X is 18:1, 20:4, or 22:6. (D) The activity of GIVA cPLA2 toward sn-1 vinyl ether or ester C18 sn-2 20:4 PC in equal proportions with 100 µM total phospholipid. The activity of (E) GVIA iPLA2 and (F) GV sPLA2 toward sn-1 vinyl ether or ester C18 sn-2 18:1 PC in equal proportions with 100 µM total phospholipid. The amount of lyso products were determined and quantified using authentic LPC primary and internal standards. Error bars show SD. Asterisks indicate statistical significance. **P < 0.01.
Furthermore, even in the mixture of a diester phospholipid and plasmalogen, all three enzymes showed unique preferences. That is, cPLA2 showed double activity toward plasmalogens comparing to diester phospholipids (Figure 3D), while iPLA2 does not have any strong preference between plasmalogen and diester phospholipid (Figure 3E). Of special note is the fact that sPLA2 showed a strong preference for diester phospholipids, namely, 7.5 times higher sPLA2 activity toward diester phospholipids than plasmalogens was detected (Figure 3F).
Specificity of PLA2s in complex mixtures of all substrates
Finally, to confirm a preference for the linkage of the sn-1 acyl chain, we tested complex mixtures of the sn-1 alkyl ether, plasmalogen, and ester phospholipids. We mixed sn-1 16:0 alkyl ether and ester and separately sn-1 C18 vinyl ether and ester phospholipids equally and used them as a substrates in the LC-MS/MS assay. The sn-2 acyl chain of 20:4 was selected for cPLA2, and 18:1 was selected for iPLA2 and sPLA2. Consistent with previous results, cPLA2 showed the highest activity toward plasmalogens over diester and alkyl ether phospholipids and some preference toward diester phospholipids over alkyl ether phospholipids (Figure 4A). iPLA2 also showed a small preference toward diester phospholipids comparing to alkyl ether phospholipids, but there was no significant difference in the activity toward plasmalogen and diester phospholipids (Figure 4B). sPLA2 exhibited a significantly higher preference for diester phospholipids comparing to alkyl ethers and plasmalogens by approximately 3.2 and 5.6-fold, respectively (Figure 4C). From these results, it became clear that the three major types of human PLA2s each exhibit a unique in vitro preference for the linkage of the sn-1 acyl chain in addition to the sn-2 acyl chain. In other words, these results suggest that PLA2s recognize the sn-1 acyl chain linkage in addition to the sn-2 acyl chain and this directly affects substrate specificity.
Figure 4. Enzyme activity of PLA2s in complex mixtures of phospholipids.
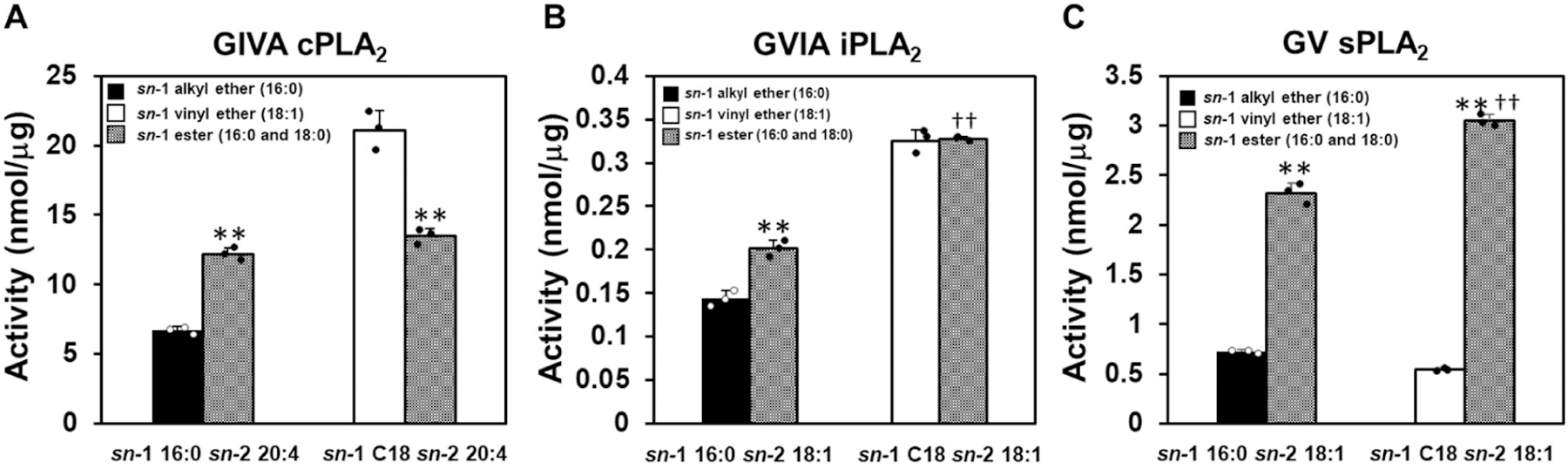
(A) The activity of GIVA cPLA2 toward sn-1 alkyl ether or ester 16:0, sn-2 20:4 (16:0/20:4) and sn-1 vinyl ether or ester C18, sn-2 20:4 (18:0 or 18:1/20:4) PC in equal proportions with 100 µM total phospholipid. The activity of (B) GVIA iPLA2 and (C) GV sPLA2 toward sn-1 alkyl ether or ester 16:0, sn-2 18:1 (16:0/18:1) and sn-1 vinyl ether or ester C18, sn-2 18:1 (18:0 or 18:1/18:1) PC in equal proportions with 100 µM total phospholipid. The amount of lyso products were determined and quantified using authentic LPC primary and internal standards. Error bars show SD. Asterisks indicate statistical significance. **P < 0.01 (comparison between the substrate which has the same number of carbons on the sn-1 position), ††P < 0.01 (comparison between the ester phospholipids).
Binding mode of ether and ester phospholipids in the sPLA2 active site.
To explore the molecular basis by which PLA2 senses the sn-1 acyl chain linkage, we performed 1 µsec MD simulations which analyzed the binding of the sn-1 alkyl ether or ester 16:0, sn-2 18:1 PC in the sPLA2 active site since sPLA2 showed more dramatic selectivity toward the sn-1 linkage than the other PLA2s. We started with the optimal binding mode of the optimal substrate in the sPLA2 active site. After a 1 µsec simulation, although the binding pose of the sn-2 acyl chain was slightly different, both the ether and the ester substrates placed their sn-2 carbonyl groups, which are attacked upon the hydrolysis reaction, near the catalytic histidine (His47) (Figure 5A, and B). The sn-2 acyl chain of the ester PC penetrated the hydrophobic subsite constituted by Leu5, Ile9, Tyr21, Val95, and Leu98 by stretching the chain and appeared stable in the hydrophobic pocket compared to the binding pose of the ether PC (Figure 5A and B). Indeed, the binding free energy of the ester PC calculated by the MM-GBSA method was lower than that of the ether PC (ester: −67.01 and ether: −64.12). For the head group binding, Arg62 mainly contributes to the binding by its hydrogen bonds with high occupancy (ester: 51.85% and ether: 56.96%) in both cases (Figure 5A and B, and Movie 1 and 2). In addition to Arg62, Gly31 takes part in the binding of the head group of the ether PC (Figure 5B and Movie 2). Interestingly, it turned out that Trp30 formed a hydrogen bond with the sn-1 carbonyl oxygen in the case of the ester PC (Figure 5A), while due to lack of the oxygen, the interaction with Trp30 was not part of the binding of the ether PC (Figure 5B). In the simulation of the ester PC, Trp30 seemed to help the precise binding of the substrate in the active site by interacting with the sn-1 carbonyl group at the early steps of the simulation (Movie 1). However, ether PC does not possess the oxygen, and alternative interactions between Trp30 and the ether oxygen appear to trap the substrate away from the catalytic histidine at the early steps of the simulation (Movie 2). Indeed, the distance between the catalytic histidine to the sn-2 carbonyl group of the ester PC reached its optimal distance by 300 ns, but the ether PC took 700 ns to reach the optimal distance (Figure 5C). In other words, thanks to the interaction between the sn-1 carbonyl group and Trp30, ester phospholipids are more rapidly precisely accommodated comparing to ether phospholipids. This appears to contribute to the high preference of sPLA2 toward ester phospholipids.
Figure 5. Binding of ester and alkyl ether PC in the active site of sPLA2.
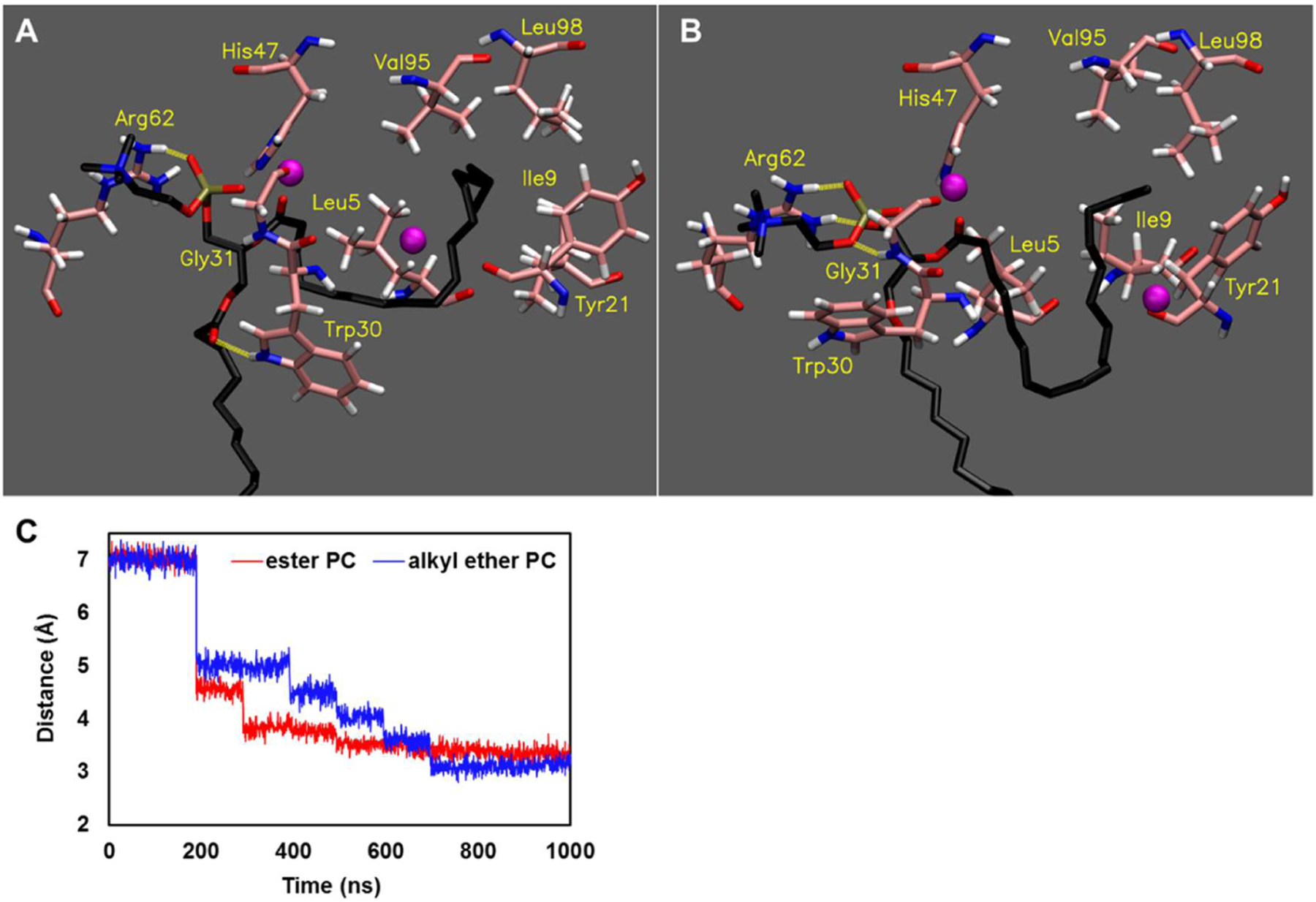
The image of the final frame of the 1 µsec simulations for (A) sn-1 ester 16:0, sn-2 18:1 PC and (B) sn-1 alkyl ether 16:0, sn-2 18:1 PC is shown in the optimal binding mode. Movie 1 and Movie 2 show the result of the entire 1 µs simulation for sn-1 ester 16:0, sn-2 18:1 PC and sn-1 alkyl ether 16:0, sn-2 18:1 PC, respectively. The purple spheres represent Ca2+, and the yellow dashed lines represent hydrogen bonds (distance cutoff: 3.2 Å, angle cutoff: 30°). (C) The distance from His47 to the carbonyl group of each sn-2 acyl chain over the 1 µsec simulations is shown.
Discussion
We have previously explored the substrate specificity of PLA2s and their molecular basis, especially focusing on the variety of head groups and sn-2 acyl chains [5]. Several decades ago, the activity of cPLA2 toward plasmalogens and alkyl ether phospholipids was reported [21,22], and a plasmalogen-selective PLA2 was characterized [37]. However, the precise in vitro selectivity of major human groups of PLA2 toward the precise linkage of the sn-1 acyl chain has been elusive. The present study reveals the unique in vitro preference of three important human PLA2s for the ether as well as the vinyl ether linkage of the sn-1 acyl chain by expanding on our previously developed lipidomics-based LC-MS/MS assay. Moreover, by taking advantage of MD simulations, we can now rationalize the molecular basis of GV sPLA2s unique recognition of the sn-1 acyl chain linkage.
cPLA2 activity toward plasmalogens
Interestingly, the current studies revealed that the in vitro activity of GIVA cPLA2 toward plasmalogens is higher than that toward conventional ester phospholipids. GIVA cPLA2 is highly specific for sn-2 AA and plays a critical role in the production of proinflammatory eicosanoids by providing free AA [2,5]. Typically, polyunsaturated fatty acids, especially AA, occupy the sn-2 acyl chain of plasmalogens [38]. Therefore, plasmalogens can be a major source of AA hydrolyzed by GIVA cPLA2. Also, it has been reported that cPLA2 deficient mice exhibit impaired brain DHA metabolism, implicating cPLA2 in the metabolism of DHA in the brain [39]. It is well recognized that the in vitro activity of cPLA2 toward sn-2 DHA is remarkably low, and our previous study agreed with and documented this conclusion [5]. Therefore, how cPLA2 could contribute to the metabolism of DHA in the brain was enigmatic. The current study revealed that the activity of cPLA2 toward plasmalogen DHA was approximately 4.5-fold higher than that of the diester (Figure 3A). Interestingly, this ratio was much higher than the ratio between plasmalogen AA and diester AA (approximately 1.7-fold). In addition, it is well known that the brain is enriched with both plasmalogens and DHA [11,40].
In the current study, we utilized PC plasmalogens to maintain a constant head group for the three different sn-1 linkages. However, it is well known that phosphatidylethanolamine (PE) is the dominant head group for plasmalogens in the human brain. Our previous study indicated that the head group did not significantly affect the specificity of cPLA2 in a mixture of phospholipids [5]. Therefore, a similar effect for the sn-1 linkage for cPLA2 activity would be expected in PE plasmalogens. Accordingly, cPLA2 is more likely to hydrolyze DHA esterified plasmalogens, rather than diester phospholipids containing DHA, thereby contributing to DHA metabolism in the brain.
In vitro vs in vivo activity of phospholipase A2s
The in vitro preference of PLA2s is not always translatable directly to the in vivo situation. Indeed, a study using a murine macrophage cell line reports that the AA mobilization, which is due to cPLA2, is independent of the plasmalogen content [41]. It has also been reported that cPLA2 contributes to lyso-PAF production, although cPLA2 showed the lowest activity toward sn-1 alkyl ether phospholipids [9]. Also, our data indicates that iPLA2 slightly, but significantly, prefers the sn-1 palmitoyl ester phospholipid over the sn-1 stearoyl ester (Fig. 4B). However, GVIA iPLA2 reportedly utilizes the sn-1 palmitoylated substrate upon zymosan stimulation of mouse macrophages [42], and iPLA2-overexpressed HEK293 cells [43]. These facts indicate that the preference of each PLA2 in vivo can be limited by the availability of the specific phospholipid pool, specific stimulus, and subcellular localization of both substrates and enzyme, although the in vitro preference can show a different underlying preference or selectivity for them to hydrolyze a certain substrate. Indeed, it has been reported that iPLA2 utilizes plasmalogens for AA release in a murine macrophage cell line. However, in plasmalogen-deficient murine macrophage cells, iPLA2 utilizes ester phospholipids without any effect on the amount of released AA, which is consistent with in vitro specificity of iPLA2 [44].
In the current study, we took advantage of detergent/phospholipid mixed micelles as substrate because the vesicle-based assay does not work well for detailed kinetic comparisons (for example, due to lag phases, linearity of the enzyme activity toward varying GPLs is often not seen and phospholipid vesicles of pure phospholipids vary in curvature, and can’t be formed for certain phospholipids). Since the mixed micelles are mainly composed of nonionic surfactant/detergent molecules, the surface where PLA2s interact is an artificial environment, although natural biological membranes are very heterogeneous collections of molecules including proteins and carbohydrates and combinations thereof. However, as described above, the in vivo specificity of PLA2s are limited by many factors including enzyme as well as optimal substrate abundance and localization at the site of enzyme action.
Our in vitro assay reveals the inate catalytic ability of each enzyme toward a certain substrate. Therefore, it is expected that a difference would be observed between the in vitro specificity of each PLA2 toward each specific phospholipid substrate in mixed micelles and specifically formulated phospholipid vesicles, as well as when using natural isolated biological membranes as substrates. Differences would be expected to be especially notable when comparing in vitro specificity results under very controlled and exacting conditions as reported herein with physiological observations in cells and tissues.
sPLA2 preference for diester substrates
Among the three human PLA2s, GV sPLA2 showed remarkable preference toward diester phospholipids. sPLA2 is a 14 kDa small enzyme and has 7 disulfide bonds which make the structure rigid. Due to these characteristics, sPLA2 only accommodates the sn-2 acyl chain and head group in its active site, whereas cPLA2 and iPLA2 accommodate phospholipid substrates entirely [5]. In other words, the tail of the sn-1 acyl chain of phospholipids that are bound to sPLA2 remains in the lipid bilayer and the lipid-water interface. The MD simulation showed that Trp30 critically contributes to the binding pose of substrates by interacting with the carbonyl group of the sn-1 acyl chain and confers a unique preference for the ester phospholipids. However, in addition to this interaction, there is the possibility that the differing chemical properties of ester, ether, and vinyl ethers affects the substrate preference of sPLA2.
Specifically, the carbonyl oxygen on the sn-1 acyl chain can contribute to the stabilization of the sn-1 acyl chain in the lipid-water interface, and this characteristic might help explain the remarkable preference of the GV sPLA2 toward diester phospholipids over ether and vinyl ether phospholipids. Upon the binding of GV sPLA2 to the lipid membrane, Trp30 is localized at the water-membrane interface. It was reported that substitution of the Trp30 of GV sPLA2 by Ala significantly suppressed interfacial binding to the zwitterionic phospholipid surface and its activity [45]. Therefore, it appears that Trp30 is contributing to interfacial binding as well as the sn-1 linkage selectivity and is critical for the function of GV sPLA2. For the MD simulations, we utilized the ester and alkyl ether PC for comparison of the binding assuming that the reason that the addition of the vinylic double bond does not lead to a significant additional activity effect is that sPLA2 fails to recognize the sn-1 double bond.
In this study with substrate-docked GV sPLA2, we have shown the importance of the sn-1 acyl chain linkage in the binding pose of the substrates in the active site. However, it is also important to note that the sn-1 acyl chain linkage contributes to the extraction of substrate. PLA2 extracts a single phospholipid from the cell membrane and stabilizes the orientation of the substrate so as to hydrolyze the substrate. Therefore, each step appears to contribute to the substrate specificity of PLA2s, although further experimental data and modeling studies are needed. Indeed, for example, Lys725 of iPLA2 is suggested to be critical for extracting the substrate into its active site [3]. In the current studies, we performed 1 µsec simulations to show the binding of substrates, and the time seemed to be long enough since the distance from the sn-2 carbonyl group to the catalytic histidine reached and settled at a reasonable distance expected for catalytic function by the end of the simulations. However, the time scale for enzymatic cleavage of the substrate is still elusive and beyond the scope of these computational methods. Of course, the question of whether accommodation of the substrate in its optimal binding mode is the rate-limiting step needs further clarification.
Conclusion
In the present study, we revealed the in vitro selectivity of the three major types of human PLA2 toward the linkage of the sn-1 acyl chain, and this selectivity can be related to their biological function. Moreover, we detailed the molecular basis of the selectivity of GV sPLA2, which shows a dramatic preference toward ester phospholipids over alkyl ethers and vinyl ethers according to MD simulations and suggest that similar types of effects could influence the detailed molecular basis of the selectivity of GIVA cPLA2 and GVIA iPLA2 toward the sn-1 linkage, though they are less extreme. However, this study raised the importance of analyzing ether and vinyl ether containing phospholipids as well as diester phospholipids as substrates for PLA2s in all biological studies if one wants to understand and explore the entire role of PLA2s in vivo.
Supplementary Material
Acknowledgments
This work was supported by NIH grants RO1 GM20501-45 and R35 GM139641-01 (E.A.D.) and a postdoctoral fellowship from The Uehara Memorial Foundation in Japan (D.H.). We sincerely thank Prof. Oswald Quehenberger and Aaron Armando for their support with the LC-MS/MS system in our laboratory.
Footnotes
Conflict of interest
The authors declare no conflicts of interest associated with this manuscript.
References
- [1].Dennis EA, Cao J, Hsu YH, Magrioti V, Kokotos G, Phospholipase A2 enzymes: Physical structure, biological function, disease implication, chemical inhibition, and therapeutic intervention, Chem. Rev 111 (2011) 6130–6185. 10.1021/cr200085w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Dennis EA, Norris PC, Eicosanoid storm in infection and inflammation, Nat. Rev. Immunol 15 (2015) 511–523. 10.1038/nri3859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Mouchlis VD, Bucher D, McCammon JA, Dennis EA, Membranes serve as allosteric activators of phospholipase a2, enabling it to extract, bind, and hydrolyze phospholipid substrates, Proc. Natl. Acad. Sci. U. S. A 112 (2015) E516–E525. 10.1073/pnas.1424651112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Das S, Cho W, Roles of catalytic domain residues in interfacial binding and activation of group IV cytosolic phospholipase A2, J. Biol. Chem 277 (2002) 23838–23846. 10.1074/jbc.M202322200. [DOI] [PubMed] [Google Scholar]
- [5].Mouchlis VD, Chen Y, Andrew McCammon J, Dennis EA, Membrane Allostery and Unique Hydrophobic Sites Promote Enzyme Substrate Specificity, J. Am. Chem. Soc 140 (2018) 3285–3291. 10.1021/jacs.7b12045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Hayashi D, Mouchlis VD, Dennis EA, Omega-3 Versus Omega-6 Fatty Acid Availability is Controlled by Hydrophobic Site Geometries of Phospholipase A2s, J. Lipid Res (2021) 100113. 10.1016/j.jlr.2021.100113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Prescott SM, Zimmerman GA, Stafforini DM, McIntyre TM, Platelet-Activating Factor and Related Lipid Mediators, Annu. Rev. Biochem 69 (2000) 419–445. 10.1146/annurev.biochem.69.1.419. [DOI] [PubMed] [Google Scholar]
- [8].Wykle RL, Malone B, Snyder F, Enzymatic synthesis of 1-alkyl-2-acetyl-sn-glycero-3-phosphocholine, a hypotensive and platelet-aggregating lipid, J. Biol. Chem 255 (1980) 10256–10260. [PubMed] [Google Scholar]
- [9].Bauldry SA, Wooten RE, Leukotriene B4 and platelet activating factor production in permeabilized human neutrophils: role of cytosolic PLA2 in LTB4 and PAF generation, Biochim. Biophys. Acta 1303 (1996) 63–73. [DOI] [PubMed] [Google Scholar]
- [10].Tjoelker LW, Eberhardt C, Unger J, Trong Hai Le, Zimmerman GA, McIntyre TM, Stafforini DM, Prescott SM, Gray PW, Plasma platelet-activating factor acetylhydrolase is a secreted phospholipase A2 with a catalytic triad, J. Biol. Chem 270 (1995) 25481–25487. 10.1074/jbc.270.43.25481. [DOI] [PubMed] [Google Scholar]
- [11].Braverman NE, Moser AB, Functions of plasmalogen lipids in health and disease, Biochim. Biophys. Acta - Mol. Basis Dis 1822 (2012) 1442–1452. 10.1016/j.bbadis.2012.05.008. [DOI] [PubMed] [Google Scholar]
- [12].Pike LJ, Han X, Chung KN, Gross RW, Lipid rafts are enriched in arachidonic acid and plasmenylethanolamine and their composition is independent of caveolin-1 expression: A quantitative electrospray ionization/mass spectrometric analysis, Biochemistry 41 (2002) 2075–2088. 10.1021/bi0156557. [DOI] [PubMed] [Google Scholar]
- [13].Rodemer C, Thai TP, Brugger B, Kaercher T, Werner H, Nave KA, Wieland F, Gorgas K, Just WW, Inactivation of ether lipid biosynthesis causes male infertility, defects in eye development and optic nerve hypoplasia in mice, Hum. Mol. Genet 12 (2003) 1881–1895. 10.1093/hmg/ddg191. [DOI] [PubMed] [Google Scholar]
- [14].Komljenovic D, Sandhoff R, Teigler A, Heid H, Just WW, Gorgas K, Disruption of blood-testis barrier dynamics in ether-lipid-deficient mice, Cell Tissue Res 337 (2009) 281–299. 10.1007/s00441-009-0809-7. [DOI] [PubMed] [Google Scholar]
- [15].Teigler A, Komljenovic D, Draguhn A, Gorgas K, Just WW, Defects in myelination, paranode organization and Purkinje cell innervation in the ether lipid-deficient mouse cerebellum, Hum. Mol. Genet 18 (2009) 1897–1908. 10.1093/hmg/ddp110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Tsukahara T, Tsukahara R, Yasuda S, Makarova N, Valentine WJ, Allison P, Yuan H, Baker DL, Li Z, Bittman R, Parrill A, Tigyi G, Different residues mediate recognition of 1-O-oleyl-lysophosphatidic acid and rosiglitazone in the ligand binding domain of peroxisome proliferator-activated receptor, J. Biol. Chem 281 (2006) 3398–3407. 10.1074/jbc.M510843200. [DOI] [PubMed] [Google Scholar]
- [17].Hossain MS, Mineno K, Katafuchi T, Neuronal orphan G-protein coupled receptor proteins mediate plasmalogens-induced activation of ERK and Akt signaling, PLoS One 11 (2016) 1–14. 10.1371/journal.pone.0150846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Dean JM, Lodhi IJ, Structural and functional roles of ether lipids, Protein Cell 9 (2018) 196–206. 10.1007/s13238-017-0423-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Rubio JM, Astudillo AM, Casas J, Balboa MA, Balsinde J, Regulation of phagocytosis in macrophages by membrane ethanolamine plasmalogens, Front. Immunol 9 (2018) 1–14. 10.3389/fimmu.2018.01723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Zou Y, Henry WS, Ricq EL, Graham ET, Phadnis VV, Maretich P, Paradkar S, Boehnke N, Deik AA, Reinhardt F, Eaton JK, Ferguson B, Wang W, Fairman J, Keys HR, Dančík V, Clish CB, Clemons PA, Hammond PT, Boyer LA, Weinberg RA, Schreiber SL, Plasticity of ether lipids promotes ferroptosis susceptibility and evasion, Nature 585 (2020) 603–608. 10.1038/s41586-020-2732-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Diez E, Louis-Flamberg P, Hall RH, Mayer RJ, Substrate specificities and properties of human phospholipases A2 in a mixed vesicle model, J. Biol. Chem 267 (1992) 18342–18348. [PubMed] [Google Scholar]
- [22].Hanel AM, Schüttel S, Gelb MH, Processive Interfacial Catalysis by Mammalian 85-Kilodalton Phospholipase A2 Enzymes on Product-Containing Vesicles: Application to the Determination of Substrate Preferences, Biochemistry 32 (1993) 5949–5958. 10.1021/bi00074a005. [DOI] [PubMed] [Google Scholar]
- [23].Giordanetto F, Pettersen D, Starke I, Nordberg P, Dahlström M, Knerr L, Selmi N, Rosengren B, Larsson LO, Sandmark J, Castaldo M, Dekker N, Karlsson U, Hurt-Camejo E, Discovery of AZD2716: A Novel Secreted Phospholipase A2 (sPLA2) Inhibitor for the Treatment of Coronary Artery Disease, ACS Med. Chem. Lett 7 (2016) 884–889. 10.1021/acsmedchemlett.6b00188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Mouchlis VD, Limnios D, Kokotou MG, Barbayianni E, Kokotos G, McCammon JA, Dennis EA, Development of Potent and Selective Inhibitors for Group VIA Calcium-Independent Phospholipase A2 Guided by Molecular Dynamics and Structure-Activity Relationships, J. Med. Chem 59 (2016) 4403–4414. 10.1021/acs.jmedchem.6b00377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Friesner RA, Murphy RB, Repasky MP, Frye LL, Greenwood JR, Halgren TA, Sanschagrin PC, Mainz DT, Extra precision glide: Docking and scoring incorporating a model of hydrophobic enclosure for protein-ligand complexes, J. Med. Chem 49 (2006) 6177–6196. 10.1021/jm051256o. [DOI] [PubMed] [Google Scholar]
- [26].Mouchlis VD, Morisseau C, Hammock BD, Li S, McCammon JA, Dennis EA, Computer-aided drug design guided by hydrogen/deuterium exchange mass spectrometry: A powerful combination for the development of potent and selective inhibitors of Group VIA calcium-independent phospholipase A2, Bioorganic Med. Chem 24 (2016) 4801–4811. 10.1016/j.bmc.2016.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Wu EL, Cheng X, Jo S, Rui H, Song KC, Dávila-Contreras EM, Qi Y, Lee J, Monje-Galvan V, Venable RM, Klauda JB, Im W, CHARMM-GUI membrane builder toward realistic biological membrane simulations, J. Comput. Chem 35 (2014) 1997–2004. 10.1002/jcc.23702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Lee J, Cheng X, Swails JM, Yeom MS, Eastman PK, Lemkul JA, Wei S, Buckner J, Jeong JC, Qi Y, Jo S, Pande VS, Case DA, Brooks CL, MacKerell AD, Klauda JB, Im W, CHARMM-GUI Input Generator for NAMD, GROMACS, AMBER, OpenMM, and CHARMM/OpenMM Simulations Using the CHARMM36 Additive Force Field, J. Chem. Theory Comput 12 (2016) 405–413. 10.1021/acs.jctc.5b00935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Humphrey W, Dalke A, Schulten K, VMD: Visual molecular dynamics, J. Mol. Graph 14 (1996) 33–38. 10.1016/0263-7855(96)00018-5. [DOI] [PubMed] [Google Scholar]
- [30].Phillips JC, Braun R, Wang W, Gumbart J, Tajkhorshid E, Villa E, Chipot C, Skeel RD, Kalé L, Schulten K, Scalable molecular dynamics with NAMD, J. Comput. Chem 26 (2005) 1781–1802. 10.1002/jcc.20289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Adelman SA, Doll JD, Generalized Langevin equation approach for atom/solid-surface scattering: General formulation for classical scattering off harmonic solids, J. Chem. Phys 64 (1976) 2375–2388. 10.1063/1.432526. [DOI] [Google Scholar]
- [32].Feller SE, Zhang Y, Pastor RW, Brooks BR, Constant pressure molecular dynamics simulation: The Langevin piston method, J. Chem. Phys 103 (1995) 4613–4621. 10.1063/1.470648. [DOI] [Google Scholar]
- [33].Ryckaert JP, Ciccotti G, Berendsen HJC, Numerical integration of the cartesian equations of motion of a system with constraints: molecular dynamics of n-alkanes, J. Comput. Phys 23 (1977) 327–341. 10.1016/0021-9991(77)90098-5. [DOI] [Google Scholar]
- [34].Essmann U, Perera L, Berkowitz ML, Darden T, Lee H, Pedersen LG, A smooth particle mesh Ewald method, J. Chem. Phys 103 (1995) 8577–8593. 10.1063/1.470117. [DOI] [Google Scholar]
- [35].Vanommeslaeghe K, Hatcher E, Acharya C, Kundu S, Zhong S, Shim J, Darian E, Guvench O, Lopes P, Vorobyov I, Mackerell AD, CHARMM general force field: A force field for drug-like molecules compatible with the CHARMM all-atom additive biological force fields, J. Comput. Chem 31 (2010) 671–690. 10.1002/jcc.21367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Klauda JB, Venable RM, Freites JA, O’Connor JW, Tobias DJ, Mondragon-Ramirez C, Vorobyov I, MacKerell AD, Pastor RW, Update of the CHARMM All-Atom Additive Force Field for Lipids: Validation on Six Lipid Types, J. Phys. Chem. B 114 (2010) 7830–7843. 10.1021/jp101759q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].McHowat J, Liu S, Creer MH, Selective hydrolysis of plasmalogen phospholipids by Ca2 -independent PLA2 in hypoxic ventricular myocytes, Am. J. Physiol 274 (1998) 1727–1737. [DOI] [PubMed] [Google Scholar]
- [38].Gross RW, High Plasmalogen and Arachidonic Acid Content of Canine Myocardial Sarcolemma: A Fast Atom Bombardment Mass Spectroscopic and Gas Chromatography-Mass Spectroscopic Characterization, Biochemistry (1984). 10.1021/bi00296a026. [DOI] [PubMed] [Google Scholar]
- [39].Rosenberger TA, Villacreses NE, Contreras MA, Bonventre JV, Rapoport SI, Brain lipid metabolism in the cPLA2 knockout mouse, J. Lipid Res 44 (2003) 109–117. 10.1194/jlr.M200298-JLR200. [DOI] [PubMed] [Google Scholar]
- [40].Salem N, Litman B, Kim HY, Gawrisch K, Mechanisms of action of docosahexaenoic acid in the nervous system, Lipids 36 (2001) 945–959. 10.1007/s11745-001-0805-6. [DOI] [PubMed] [Google Scholar]
- [41].Lebrero P, Astudillo AM, Rubio JM, Fernández-Caballero L, Kokotos G, Balboa MA, Balsinde J, Cellular Plasmalogen Content Does Not Influence Arachidonic Acid Levels or Distribution in Macrophages: A Role for Cytosolic Phospholipase A2γ in Phospholipid Remodeling, Cells 8 (2019). 10.3390/cells8080799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Gil-de-Gómez L, Astudillo AM, Guijas C, Magrioti V, Kokotos G, Balboa MA, Balsinde J, Cytosolic Group IVA and Calcium-Independent Group VIA Phospholipase A 2 s Act on Distinct Phospholipid Pools in Zymosan-Stimulated Mouse Peritoneal Macrophages, J. Immunol 192 (2014) 752–762. 10.4049/jimmunol.1302267. [DOI] [PubMed] [Google Scholar]
- [43].Murakami M, Masuda S, Ueda-Semmyo K, Yoda E, Kuwata H, Takanezawa Y, Aoki J, Arai H, Sumimoto H, Ishikawa Y, Ishii T, Nakatani Y, Kudo I, Group VIB Ca2+-independent phospholipase A2γ promotes cellular membrane hydrolysis and prostaglandin production in a manner distinct from other intracellular phospholipases A2, J. Biol. Chem 280 (2005) 14028–14041. 10.1074/jbc.M413766200. [DOI] [PubMed] [Google Scholar]
- [44].Gaposchkin DP, Farber HW, Zoeller RA, On the importance of plasmalogen status in stimulated arachidonic acid release in the macrophage cell line RAW 264.7, Biochim. Biophys. Acta - Mol. Cell Biol. Lipids 1781 (2008) 213–219. 10.1016/j.bbalip.2008.01.007. [DOI] [PubMed] [Google Scholar]
- [45].Han SK, Kim KP, Koduri R, Bittova L, Munoz NM, Leff AR, Wilton DC, Gelb MH, Cho W, Roles of Trp31 in high membrane binding and proinflammatory activity of human group V phospholipase A2, J. Biol. Chem 274 (1999) 11881–11888. 10.1074/jbc.274.17.11881. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


