Abstract
Aims
Allergic airway disease manifestation is induced by lysophosphatidylcholine (LPC) through CD1d-restricted Natural killer T (NKT) cells. Choline chloride (ChCl) and LPC both have the “choline” moiety in their structure and this may interplay the effect in allergic airway disease pathway.
Main methods
To test the hypothesis, mice were sensitized with cockroach extract (CE); challenged with CE or exposed to LPC and were given ChCl 1hr later.
Key findings
A significant increase in Airway hyperresponsiveness (AHR), total and differential cell count, Th2 cytokines, 8-isoprostanes level in bronchoalveolar lavage fluid (BALF) and inflammation score based on lung histology were observed on challenge with CE or exposure to LPC (p < 0.05) indicating LPC induced airway disease manifestation in mice. These parameters were reduced significantly after administering mice with ChCl (p < 0.05). The inflammatory parameters were significantly increased in LPC exposed mice, not sensitized with CE, which were significantly decreased when mice were administered with ChCl demonstrating its role in the inhibition of LPC induced allergic airway disease manifestation. Docking of CD1d with LPC and ChCl indicated the competitive inhibition of LPC induced effect by ChCl. This was validated in vivo in the form of decreased CD1d-restricted NKT cells in BALF and lung of the immunized mice on ChCl administration. There was no effect of ChCl administration on CD1d expression in BALF and lung cells.
Significance
This study shows that ChCl attenuates the allergic response by inhibiting the LPC induced- NKT cell mediated AHR, inflammation and oxidative stress by competitive inhibition to LPC in binding to CD1d.
Keywords: Allergic airway disease, Competitive inhibition, Choline chloride, Lysophosphatidylcholine, CD1d, And NKT
Graphical abstract
Highlights
-
•
ChCl down regulates LPC (critical for allergic manifestation) induced response.
-
•
Results were validated in cockroach extract immunized mice model.
-
•
In silico studies indicate competitive inhibition to LPC by ChCl in binding to CD1d.
-
•
In silico results were also validated in vivo in terms of CD1d-restricted NKT cells.
-
•
Study explains the mechanism of ChCl action against allergic disease.
1. Introduction
Choline plays an important role in normal physiology of the body like mobilization of fats in liver; synthesis of acetylcholine; formation of phosphatidylcholine (PC) by de novo pathway (Blusztajn, 1998; Pelech and Vance, 1984). It is present in food in free form or as phosphatidylcholine in egg yolk, animal fat and various vegetables (Zeisel, 2000). It helps in maintaining the structural integrity of the cell membranes and involves in cholinergic neurotransmission, methyl group metabolism, lipid and cholesterol transportation and metabolism, and trans-membrane signalling. Cells show apoptosis in choline deficient medium (Yen et al., 1999). Choline deficiency also leads to increased oxidative stress causing oxidative and mitochondrial damage in liver and plasma (Ossani et al., 2007; Yoshida et al., 2006). Choline has shown anti-inflammatory effects in animal model of arthritis (Ganley et al., 1958). It also shows the antinociceptive effects against inflammatory pain (Wang et al., 2005). Mehta et al. showed choline chloride's role in reduction of inflammation and oxidative stress in mice model of allergic airway disease (Mehta et al., 2007),(Mehta et al., 2009). Choline has the therapeutic potential in asthma management (Mehta et al., 2010). Although, the mechanism underlying the modulating effect of choline is yet not clear.
Allergy is known as a hypersensitive response of the immune system. Allergen challenge induces phospholipase A2 secretion by various airway cells including mast cells, alveolar macrophages and neutrophils (Chilton et al., 1996; Touqui et al., 1994; Triggiani et al., 2009; Degousee et al., 2002; Giannattasio et al., 2009).
Lung mast cells store secretory phospholipase A2 (sPLA2) in its granules. Cross-linking of IgE on allergen challenge induces degranulation of mast cells releasing sPLA2 in the extracellular fluid in the early phase of allergic reaction (Pniewska and Pawliczak, 2013; Triggiani et al., 2009). sPLA2 hydrolyses the phospholipids of the cell membrane at the sn-2 position of the ester bond resulting in LPC and free fatty acid or arachidonic acid (Ghesquiere et al., 2005). Increased LPC level is observed in various pathophysiology conditions like cardiovascular complications in diabetes, atherosclerosis, ischemia (Shi et al., 1999a, 1999b), endometriosis (Murphy et al., 1998), psoriasis (Ryborg et al., 1995), hyperlipidemia (Zhang et al., 2006), renal failure during hemodialysis (Sasagawa et al., 1998); rheumatoid arthritis (Fuchs et al., 2005), asthma and rhinitis (Mehta et al., 1990). It is reported to involve in acute lung injury due to damage of alveolar type I cellular membranes (Niewoehner et al., 1987). It acts as a natural adjuvant and involves in humoral and cellular immune responses (Perrin-Cocon et al., 2006).
LPC level increases in the plasma and BALF of asthma and rhinitis patients (Agrawal and Nath, 1978; Chilton et al., 1996; Mehta et al., 1990; Wadehra et al., 1987).The role of LPC has been seen in desensitization of β-adrenergic receptor by Ca2+ sensitization in tracheal smooth muscle cells (Kume et al., 2001). It involves in eosinophils infiltration and bronchoconstriction (Nishiyama et al., 2004; Nobata et al., 2005). A study by Yoder et al. also showed increased level of LPC in the lung lining fluid of asthmatics (Yoder et al., 2014).
LPC is reported critical for allergic manifestation. It increases airway hyperresponsiveness (AHR), Th2 type cytokines, airway inflammation and oxidative stress. It does so via CD1d-restricted LY49C+TCRβ+ natural killer T (NKT) cells (Bansal et al., 2016). CD1d is major histocompatibility complex (MHC) class-I like molecule present on the surface of antigen presenting cells (APCs) and involves in lipid presentation (Oleinika et al., 2018). Blocking of Cd1d by monoclonal antibody does not allow the allergen or LPC induced allergic manifestation (Bansal et al., 2016).
Lysophosphatidylcholine and choline both have the similar “choline” moiety in structures. Therefore, we hypothesised that choline chloride might be competing with LPC in binding to CD1d; thereby inhibiting the LPC mediated response.
2. Material and methods
The immunization protocol was approved by the animal ethics committee of CSIR-Institute of Genomics and Integrative Biology. The experiments were done following the guidelines of committee for the purpose of control and supervision of experiments on animals (CPCSEA), Govt. of India. The experiments were repeated and data presented here belongs to one of the two independent experiments with similar results.
2.1. Preparation of Cockroach extract (CE)
Cockroach extract was prepared from whole body cockroaches by the method described earlier (Thangam, V et al., 2007). Briefly, male and female cockroaches were taken and freeze dried using a freeze dryer (Martin Christ Freeze Dryers, Germany) and ground to fine powder. The powder was defatted with 4–5 changes of diethyl ether at 4 °C. It was dissolved in 125 mM ammonium bicarbonate (NH4HCO3) buffer of pH 8.0, containing EDTA (5 mM) and phenylmethylsulfonyl fluoride (PMSF) (1 mM). It was stirred continuously at 4 °C for 8 h, centrifuged at 6700×g at 4 °C for 1 h, collected the supernatant, dialyzed using distilled water and lyophilized and stored at −20 °C. Protein content of the cockroach extracts was determined by BCA Protein assay kit (Sigma Aldrich). CE was dissolved in phosphate buffer saline (PBS) as per the requirement of the immunization protocol.
2.2. Mice
Female BALB/c mice (4–6 weeks), weighing up to around 20 g were obtained from the National Institute of Nutrition, Hyderabad, India. They were acclimatized for 1 week under standard laboratory conditions, i.e. 25 °C temperature and 60–70% humidity on a 12 h light/dark schedule with access to water and standard diet.
2.3. Immunization and treatment
The groups of mice were sensitized with CE (10μg/100 μl PBS) on day 0, 7th & 14th intraperitoneally (i.p.) whereas a control group was sensitized with PBS (Fig. 1: protocol 1). They were challenged with CE (10 μg/10 μl PBS) or exposed to LPC (1-Palmitoyl-sn-glycero-3-phosphocholine) (Sigma Aldrich) (4.82 μg/10 μl 5%DMSO) intranasally (i.n.) on days 25th, 26th and 27th except the PBS control group, which was challenged with PBS. Mice were treated 1 h after the CE challenge or LPC exposure with choline chloride (ChCl) (Sigma Aldrich) (1 mg/kg) or PBS (i.n). In another experiment (Fig. 1: protocol 2) mice were exposed to LPC (i.n.) on day 0, 7th & 14th and 25th, 26th and 27th and treated 1 h after the LPC exposure on days 25th, 26th and 27th with ChCl (1 mg/kg) or PBS (i.n.). On day 28th, AHR was recorded and mice were sacrificed to collect BALF and lungs. Doses of LPC and ChCl were devised according to the literature (Bansal et al., 2016; Mehta et al., 2007).
Fig. 1.
Schematic presentation of immunization protocol. Protocol 1. Mice were sensitized on days: 0, 7, &14 intraperitoneally (i.p.) with cockroach extract (CE). They were either challenged with CE or exposed to lysophosphatidylcholine (LPC) intranasally (i.n.) on days 25, 26, & 27. 1hr after each challenge or LPC exposure mice were administered i.n. with choline chloride (ChCl). Protocol 2. Mice were exposed to LPC i.n. on days: 0, 7, 14, 25, 26, & 27. They were administered i.n. with ChCl 1hr after LPC exposure on days: 25, 26, & 27.
2.4. Assessment of AHR
AHR was measured on day 28th using different doses of methacholine (2, 4, 8, 12, 16 and 20, 25 and 30 mg) after anaesthetising the mice with xylazine (10 mg/kg) and sodium thiopentone (100 mg/kg) i.p. as per the method described earlier (Bansal et al., 2016).
2.5. Collection of BALF and lungs
Lungs were lavaged three times with 0.5 ml chilled PBS. The bronchoalveolar lavage fluids (BALF) were centrifuged at 400×g for 10 min at 4 °C and supernatants were stored at −80 °C (Saw and Arora, 2016). Cell pellets were resuspended in PBS and used for Total leukocyte count (TLC) and differential leukocyte count (DLC) evaluation. They were also utilised for analysis of CD1d+ cells and LY49/c+ TCRβ+ NKT cells in BALF. Similarly, lungs were collected and processed for histology as described earlier (Bansal et al., 2016) as well as for analysis of CD1d+ cells and LY49/c+ TCRβ+ NKT cells.
2.6. Measurement of 8-isoprostanes
BALF samples were assayed for the measurement of 8-isoprostanes’ levels using enzyme immunoassay kits (Cayman Chemical Co., MI, USA) following the manufacturer's instructions. The detection limit for this assay was 0.8 pg/ml.
2.7. Determination of cytokines by ELISA
Interleukin (IL)-4 and IL-5 levels were determined in BALF by ELISA, using paired antibodies according to the manufacturer's proposal (BD Pharmingen) as described earlier (Bansal et al., 2016). The detection limit for IL-4 and IL-5 assay was 4.0 pg/ml.
2.8. Histopathology
Lungs were fixed in neutral buffered formalin (10%), embedded in paraffin and 4 μm thick sections were cut using microtome. They were stained with haematoxylin–eosin (H&E) and assessed for inflammation score as described elsewhere (Bansal et al., 2014).
2.9. Docking experiment
To understand the binding mechanism of both the molecules, i.e. LPC and ChCl with CD1d, docking studies were performed. CD1d structure was downloaded from the PDB database, whose structure was resolved at 2.8 Å and co-crystallized with internal ligand phosphatidylcholine (Giabbai et al., 2005). Prior to docking study, all water molecules and other ligands were removed and hydrogen and missing atoms were added to the CD1d. Alternative amino acid conformations were also removed. Finally structure was minimized for 1000 steps with steepest descent algorithm (Garg et al., 2010). 3D structure of both the ligands was downloaded from the PubChem Data base, while the minimization was performed using Open Babel software (O'boyle et al., 2011). All the docking analyses were performed using Autodock Vina using the best suitable parameters acquired from the various earlier studies published elsewhere (Trott and Olson, 2010). Exhaustiveness and number of models are very critical parameter for docking. In our case we have used values 50 and 20 for exhaustiveness and number of models, respectively (Chang et al., 2010; Seeliger and De Groot, 2010). Docking results were analyzed and images were generated using pyMol software.
2.10. Flow cytometry to examine NKT and CD1d+ cell population
Lung of each mouse was homogenized and passed through cell strainer of 100μ size to get single cell suspension; centrifuged at 290×g, for 10 min at 4 °C to get the pellet. RBCs were lysed using RBC lysis buffer. BALF cells and lung cells were surface stained for LY49 C/I and TCR-β using PE-labelled anti LY49C/I (clone 14B11) and APC- labelled anti TCR-β(clone H57-597) antibodies (ebioscience, Inc., USA). For CD1d staining, Alexa Fluor ® 488-labelled anti-CD1d (clone1B1) (ebioscience, Inc., USA) antibody was used. The cells were acquired using BD Accuri™ C6 flow cytometer (BD Biosciences) and analyzed using BD Accuri™ C6 software. Dual positive LY49C/I+TCR-β+cells were acquired as NKT cells.
2.11. Statistical analysis
Data were analyzed using Student t-test to examine the differences between control and challenged groups as well as between challenged and treated groups. Differences were considered significant at p < 0.05. Data are presented as mean ± SEM for each group.
3. Results
3.1. AHR, airway inflammation and Th2 type cytokines in CE immunized mice is reduced by ChCl
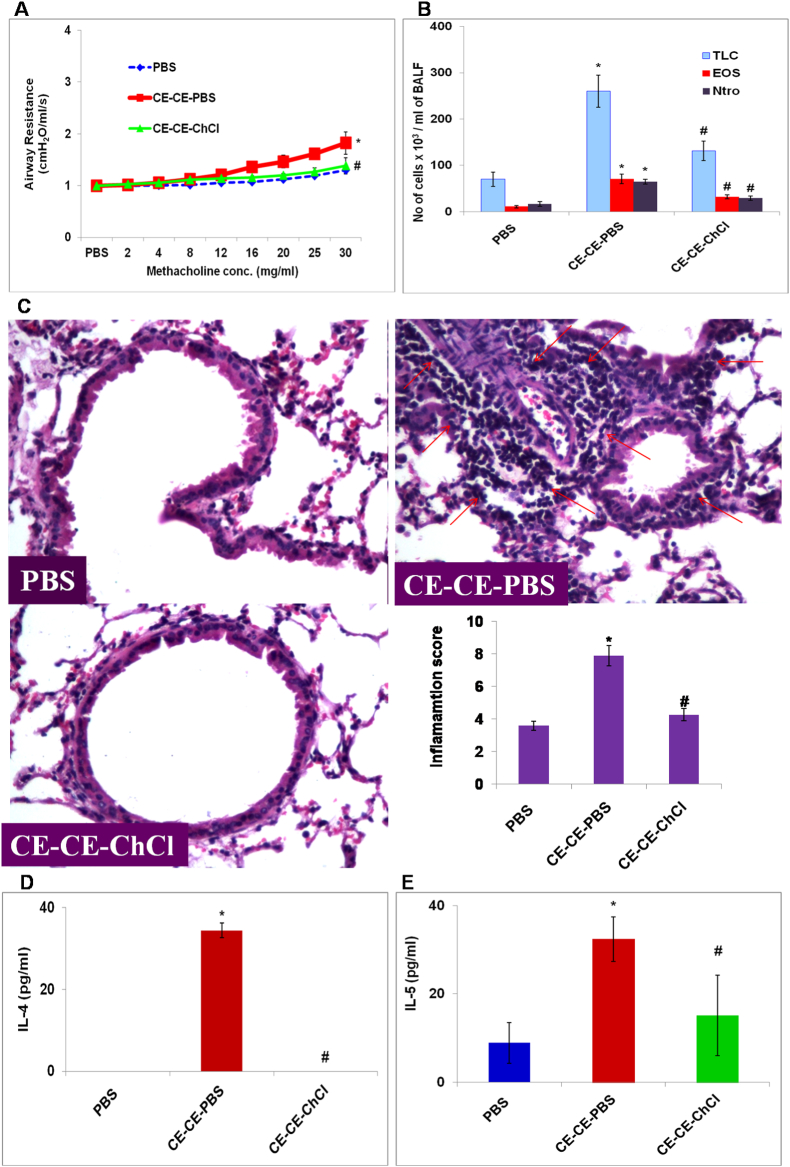
To mimic the natural allergen exposure mice were exposed to cockroach extract and AHR was recorded. At the dose of 30 mg/ml methacholine, there was 1.5 folds increase in AHR as compared to PBS control mice (p < 0.05) (Fig. 2A). A significant airway inflammation was observed in CE immunized mice as compared to PBS control mice as demonstrated by TLC, eosinophils and neutrophils (Fig. 2B), lung histology and inflammation score (Fig. 2C). Th2 type cytokines- IL-4 (p < 0.05) (Fig. 2D) and IL-5 (p < 0.05) (Fig. 2E) were significantly increased in CE immunized mice as compared to PBS control mice. ChCl treatment reduced all these parameters significantly.
Fig. 2.
AHR, TLC, DLC, lung inflammation and Th2 type cytokines in CE immunized mice is reduced by ChCl. A Airway hyperresponsiveness (AHR) and B) total cell count and eosinophil and neutrophil cell count in bronchoalveolar lavage fluid (BALF). C) Lung sections stained with H&E (Power: 40X). Inflammation score was calculated on the basis of cell infiltration in and thickening of airways. D) IL-4 and E) IL-5 in BALF. Concentrations were expressed in pg/ml. Here, PBS- PBS control mice; CE-CE-PBS- Cockroach extract immunized, PBS treated; CE-CE-ChCl- Cockroach extract immunized, choline chloride treated. Data presented here are the means ± SEM of values from 4 mice per group. ∗, p < 0.05 versus PBS; #, p < 0.05 versus CE-CE-PBS.
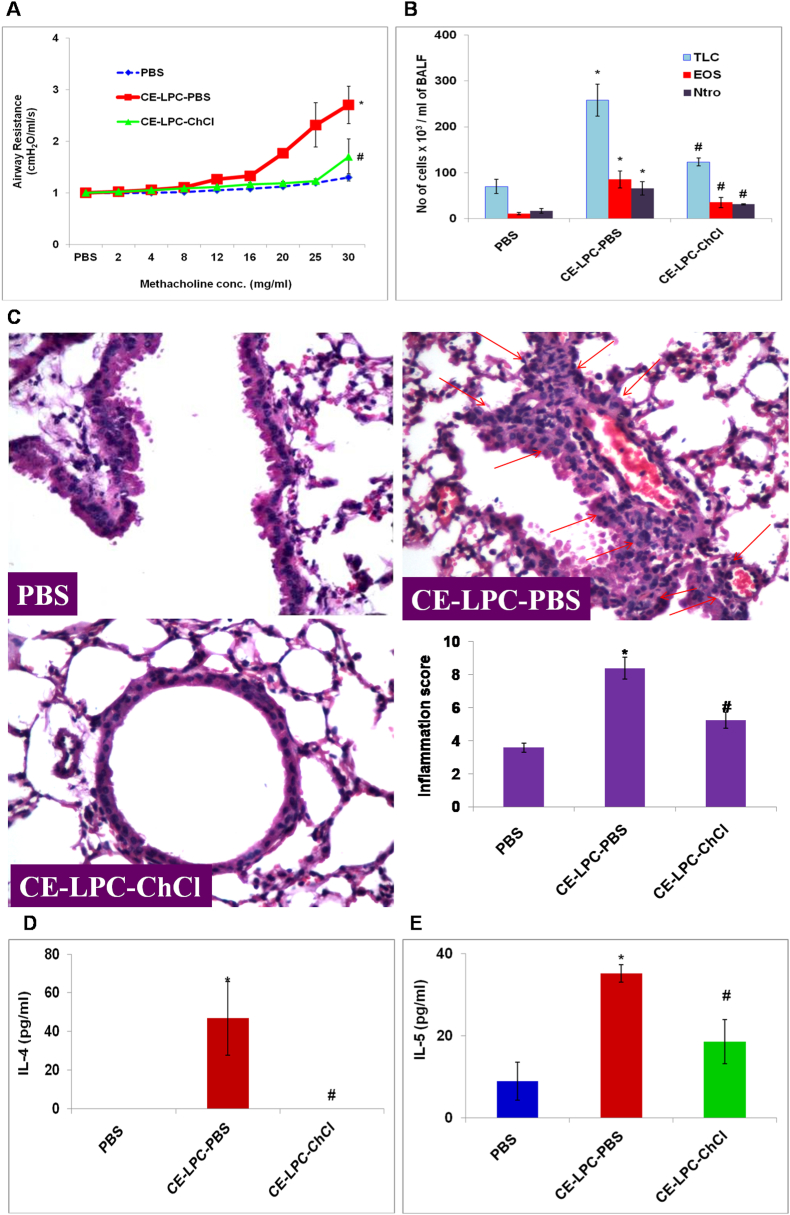
3.2. AHR, airway inflammation, and Th2 type cytokines in CE sensitized and LPC exposed mice is reduced by ChCl
To study the effect of ChCl on LPC exposed mice, mice were exposed to LPC directly after CE sensitization. LPC exposure significantly increased AHR (p < 0.05) (Fig. 3A), TLC (p < 0.05), eosinophils (p < 0.05) and neutrophils (p < 0.05) (Fig. 3B) as compared to PBS control mice. It increased lung inflammation as demonstrated by lung histology and inflammation score (p < 0.05) (Fig. 3C). It also increased IL-4 (p < 0.05) (Fig. 3D) and IL-5 (p < 0.05) (Fig. 3E) as compared to PBS control mice. Treatment with ChCl, 1hr after LPC exposure significantly decreased these parameters as in the case of CE immunized mice demonstrating the ChCl effect in inhibiting the LPC induced airway disease parameters.
Fig. 3.
AHR, TLC, DLC, lung inflammation and Th2 type cytokines in LPC exposed mice is reduced by ChCl. A) Airway hyperresponsiveness (AHR) and B) total cell count and eosinophil and neutrophil cell count in bronchoalveolar lavage fluid (BALF). C) Lung sections stained with H&E (Power: 40X). Inflammation score was calculated on the basis of cell infiltration in and thickening of airways. D) IL-4 and E) IL-5 in BALF. Concentrations were expressed in pg/ml. Here, PBS- PBS control mice; CE-LPC-PBS- Cockroach extract sensitized, lysophosphatidylcholine exposed, PBS treated; CE-LPC-ChCl- Cockroach extract sensitized, lysophosphatidylcholine exposed, choline chloride treated. Data presented here are the means ± SEM of values from 4 mice per group. ∗, p < 0.05 versus PBS; #, p < 0.05 versus CE-LPC-PBS.
3.3. AHR, airway inflammation, and Th2 type cytokines in LPC exposed mice, without CE sensitization is reduced by ChCl
To further demonstrate the ChCl inhibiting effect on LPC induced airway disease manifestation, mice were exposed only to exogenous LPC without CE sensitization. It was found that AHR (p < 0.05) (Fig. 4A), TLC (p < 0.05), eosinophils and neutrophils in BALF (p < 0.05) (Fig. 4B), lung inflammation (p < 0.05) (Fig. 4C), IL-4 (Fig. 4D) and IL-5(p < 0.05) (Fig. 4E) were significantly increased in LPC exposed mice, not sensitized with CE, in similar proportion to CE sensitized, LPC exposed mice. These parameters were significantly decreased by ChCl treatment as compared to LPC exposed mice (p < 0.05).
Fig. 4.
AHR, airway inflammation and Th2 type cytokines are decreased by ChCl in CE unsensitized, LPC exposed mice. A) Airway hyperresponsiveness (AHR) B) TLC, Eosinophil and Neutrophil cell count in bronchoalveolar lavage fluid (BALF). C) Lung sections stained with H&E (Power: 40X). Inflammation score was calculated on the basis of cell infiltration in and thickening of airways. D) IL-4 and E) IL-5 in BALF. Here, vehicle-vehicle control mice; LPC-PBS- Cockroach extract unsensitized, lysophosphatidylcholine exposed, PBS treated; LPC-ChCl- Cockroach extract unsensitized, lysophosphatidylcholine exposed, choline chloride treated. Data here are the means ± SEM of values from 4 mice per group. ∗, p < 0.05 versus Vehicle; #, p < 0.05 versus LPC-PBS.
3.4. ChCl treatment reduces oxidative stress in CE immunized/CE sensitized LPC exposed/LPC exposed, not sensitized with CE mice
CE immunized mice showed a significant increase in oxidative stress marker, 8-isoprostane conc. in BALF (p < 0.05) as compared to PBS control mice (Fig. 5A). There was also a significant increase in 8-isoprostanes in BALF of CE sensitized LPC exposed mice (Fig. 5A) as well as LPC exposed mice, not sensitized with CE (p < 0.05) as compared to PBS control mice (Fig. 5B). There was ∼2 fold increase in 8-isoprostanes level in CE challenged or LPC exposed mice as compared to PBS control mice. ChCl treatment reduced the 8-isoprostanes level in BALF of CE immunized, CE sensitized-LPC exposed mice, and LPC exposed-not sensitized with CE mice significantly (p < 0.05).
Fig. 5.
Oxidative stress decreases by ChCl in CE immunized or CE sensitized, LPC exposed mice or CE unsensitized LPC exposed mice. 8-isoprostanes in bronchoalveolar lavage fluid (BALF). A) Here, PBS- PBS control mice; CE-CE-PBS- cockroach extract immunized, PBS treated; CE-CE-ChCl-cockroach extract immunized, choline chloride treated; CE-LPC-PBS- cockroach extract sensitized, lysophosphatidylcholine exposed, PBS treated; CE-LPC-ChCl- - cockroach extract sensitized, lysophosphatidylcholine exposed, chloride treated. ∗, p < 0.05 versus PBS; #, p < 0.05 versus CE-CE-PBS; $, p < 0.05 versus CE-LPC-PBS.B) Here, vehicle-vehicle control mice; LPC-PBS- cockroach extract unsensitized, lysophosphatidylcholine exposed, PBS treated; LPC-ChCl-cockroach extract unsensitized, lysophosphatidylcholine exposed, choline chloride treated. Data presented in A) and B) are the means ± SEM of values from 4 mice per group.∗, p < 0.05 versus vehicle; #, p < 0.05 versus LPC-PBS.
3.5. Docking shows that both LPC and ChCl have an affinity for binding to CD1d
Docking Result shows that choline and LPC binds within the binding pocket of CD1d with the −3.0 and −7.7 kcal/mol binding energy respectively. This difference in binding energy of both the compounds mainly occurs due the difference in their size. In comparison to LPC, ChCl binds deep inside into the hydrophobic pocket due its small size. Phosphate moiety of LPC forms two hydrogen bonds with the residue Thr 156, while in the case of choline, hydroxyl group contributes two H-bonds with Gly 14 and Ser 28 residues (Fig. 6). Both the compounds form two hydrogen bonds with the CD1d but over all contribution of different intermolecular interactions provide extra stability to the LPC.
Fig. 6.
A) Represents the ball and stick model of docked conformations of LPC and ChCl. Phosphate moiety of LPC (orange stick model) forms two hydrogen bonds with Thr 156 (left side). Hydroxyl group of choline also formed two hydrogen bonds with Glu14 & Ser28 residues. B) Represents the interaction of both the ligands within the binding pocket. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
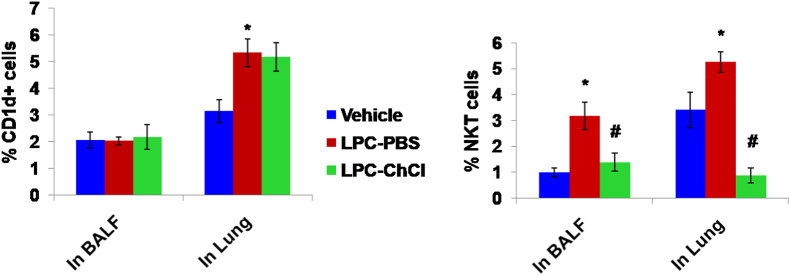
3.6. ChCl treatment does not reduce the CD1d+ cells in BALF and lung
LPC exposed mice does not show any significant change in CD1d+ cells in BALF, but a significant increase is observed in CD1d+ cells in lung of CE sensitized LPC exposed mice as compared to PBS control mice (p < 0.05). However, there is no effect of ChCl administration in LPC exposed mice, on the number of CD1d+ cells in BALF or lung of the mice (Fig. 7).
Fig. 7.
ChCl treatment does not reduce the CD1d+ cells but decreases the NKT cells in BALF and lung of mice.
A) CD1d + cells in bronchoalveolar lavage fluid (BALF) and lung B) NKT cells in bronchoalveolar lavage fluid (BALF) and lung. Both are expressed here as % CD1d + or % NKT cells i.e., proportion of CD1d + or NKT cells out of total BALF or lung cells. Here, vehicle-vehicle control mice; LPC-PBS- cockroach extract unsensitized, lysophosphatidylcholine exposed, PBS treated; LPC-ChCl-cockroach extract unsensitized, lysophosphatidylcholine exposed, choline chloride treated. Data here are the means ± SEM of values from 4 mice per group. ∗, p < 0.05 versus Vehicle; #, p < 0.05 versus LPC-PBS.
3.7. LPC exposure increases, while ChCl administration decreases the NKT cells in BALF and lung
A significant increase is observed in LY49/c+ TCR-β+ NKT cells in BALF (p < 0.05) of CE sensitized LPC exposed mice as compared to PBS control mice. Their number considerably reduces on administration of ChCl (p < 0.05). Similarly, LY49/c+ TCR-β+ NKT cells increase in lung of CE sensitized LPC exposed mice significantly as compared to PBS control mice e (p < 0.05), which shows significant decrease on ChCl administration (p < 0.05) (Fig. 7).
4. Discussion
Asthma is a chronic inflammatory disease. It is accompanied with AHR, lung inflammation, Th2 type cytokines and oxidative stress. Many therapies are available for the management of asthma, but corticosteroids is the mainstay therapy and some asthmatics don't respond to these or develop adverse side-effects (Williams et al., 2003). Choline chloride is a potential anti-inflammatory agent and may have an advantage to be used in asthma patients (Ganley et al., 1958, Mehta et al., 2007). In a three month clinical study, Gaur et al. used choline for the management of asthma. It proved as an effective prophylactic drug in asthma (Gaur et al., 1997). Another placebo controlled study of 4 months using two doses of choline (1.5 and 3 g/day) for asthma was carried out in which choline treatment showed significant improvement in symptom score, reduction in drug use and airway conductance (Gupta and Gaur, 1997). In another study, choline chloride given by oral route to patients (15–45 years) of asthma with or without rhinitis patients reduced airway inflammation, AHR, symptoms and drug use and systemic inflammatory markers as well as oxidative stress effectively than pharmacotherapy alone treatment in asthmatics (Mehta et al., 2010). But, what is the mechanism of its action is not known till date.
Bansal et al. demonstrated that LPC increases AHR, airway inflammation, Th2 type cytokines secretion, and oxidative stress via CD1d-restricted NKT cells (Bansal et al., 2016). In the present study, the effect of ChCl on LPC-induced allergic manifestation is studied in a mice model. CE challenge increases AHR, lung inflammation (as demonstrated by cell infiltration in the airways and lung-histology), Th2 type cytokines, and oxidative stress significantly. Similarly, LPC exposure increases these parameters significantly. ChCl treatment in CE immunized or LPC exposed mice decreases the parameters to the level of PBS control mice. CE challenge increases LPC in BALF. LPC increases AHR, airway inflammation and Th2 type cytokines, and oxidative stress (Bansal et al., 2016). CE challenged or LPC exposed mice when given ChCl has shown a decrease in the parameters to the level of PBS control mice in the present study.
To further confirm the role of ChCl in the inhibition of the LPC-induced immune response, mice are exposed to LPC without CE sensitization. AHR, airway inflammation, Th2 type cytokines and levels of 8-isoprostanes increase significantly on LPC exposure. ChCl decreases the parameters significantly indicating its ability to inhibit the LPC-induced allergic manifestation. This may be due to LPC and ChCl having identical “choline” moiety in the structure.
To confirm that we approached in silico docking studies. Docking data suggests that the long fatty acid chain LPC enters into the deep binding cleft and the major contribution of ligand-CD1d complex stability is driven by the hydrophobic interactions. Due to the smaller size of choline the contribution of H-bond and weak interaction is very low and hence it shows less binding energy in comparison to LPC, hence resulting in low affinity towards CD1d. It can be speculated that when both the molecules would be present in a similar concentration, due to the competitive binding, LPC shows a superior binding over choline, while a 4–6 times higher concentration of choline may replace LPC from the CD1d binding side. In the present experiment, the used concentration of ChCl is 4–5 times higher than that of LPC, which is in corroboration of the observed results in the case of ChCl administration in LPC exposed or CE immunized mice. The doses used for LPC and ChCl in this experiment are derived from the literature (Bansal et al., 2016; Mehta et al., 2007; Mehta et al., 2009), which further consolidates the speculation without any hypothesis driven biasness.
LPC induces NKT cell activation or proliferation by binding to CD1d (Bansal et al., 2016). In the present study, we have found that LPC exposure increases NKT cells in the BALF and lung of the mice. NKT cells in BALF and lung, decreases on ChCl administration. It can be speculated that ChCl gives a competitive hindrance to LPC in binding to CD1d and does not allow NKT cells to get activate and proliferate and thus inhibits the allergic manifestation resulting in decreased AHR, lung inflammation, Th2 type cytokines and oxidative stress. Lombardi et al. has shown in mouse model that dipalmitoyl-phosphatidyletahnolamine polyethylene glycol acted as an antagonist to α-galactoceramide and prevented allergen-induced AHR by competing in binding to CD1d (Lombardi et al., 2010).
It has been observed that LPC level is increased in the plasma and bronchoalveolar lavage fluid of asthma and rhinitis patients (Chilton et al., 1996; Mehta et al., 1990; Wadehra et al., 1987; Yoder, Zhuge, Yuan, Holian, Kuo, van, Thomas, and Lum, 2014; Agrawal and Nath, 1978). Gaur et al. has suggested that therapeutic potential of ChCl in asthma patients may be due to its role in lowering of LPC (Gaur et al., 1997). Still no data is reported to confirm this for ChCl effect. In asthmatics, beta-2 adrenergic receptors are present in normal or increased numbers but get uncoupled in severe asthmatics causing their hyporesponsiveness probably by inflammatory mediators (Bai, 1992). LPC involves in desensitization of β-adrenergic receptors (Kume et al., 2001) and in broncho-constriction (Nobata et al., 2005). One of the explanations for ChCl therapeutic effect may be given by that it acts on or binds with beta-2 adrenergic receptor in such a way that does not allow LPC to bind and its desensitization. Although LPC effects are multiple, in recent study it has been reported that LPC is critical for allergic manifestation via CD1d-restricted NKT cells (Bansal et al., 2016). Studies showed a strong affinity between LPC and CD1d (Lopez-Sagaseta et al., 2012). That is the reason we did the docking studies for CD1d-ChCl to confirm whether ChCl could replace LPC at the given concentration. As shown by its structure (Fig. 6), ChCl is smaller than LPC. Because of its smaller size, it binds deep inside into the hydrophobic pocket of CD1d. If ChCl is in 4–5 times concentration of LPC, then probably, ChCl occupies the pockets of CD1d in such a way that LPC would not be able to bind and hence would not allow NKT activation and hence no allergic manifestation.
One more possibility for how the ChCl might be acting in vivo may be that ChCl has an effect on CD1d expression, and that is how it may affect activation of NKT cells in vivo and further downstream effects. To inquire that we checked CD1d expression through flow cytometry in BALF and lung of the LPC exposed mice as well as in ChCl administered mice. We find in our study that there is no significant change in BALF cells in CD1d expression on either LPC exposure or ChCl administration. In lung, although there was a significant increase in CD1d expression on LPC exposure, but ChCl administration did not cause any significant decrease in that, ruling out the involvement of any such effect through CD1d expression.
The study demonstrates that use of ChCl decreases AHR, inflammation, Th2 type cytokines and oxidative stress in allergic airway disease by competitively inhibiting the action of LPC. This outcome is yet to be established in allergic airway disease patients where it might probably help in better understanding and managing the disease. The present study might be helpful not only in allergic airway diseases like asthma but also other diseases where LPC plays a critical role (Shi, Yoshinari, Iino, Wakisaka, Iwase, and Fujishima, 1999a; Shi, Yoshinari, Wakisaka, Iwase, and Fujishima, 1999b; Murphy et al., 1998; Ryborg et al., 1995; Zhang et al., 2006; Sasagawa et al., 1998). There are reports in which, LPC has been seen linked with atherosclerosis, acute and chronic inflammation; systemic lupus erythematosus, cardiovascular disease, diabetes and diabetic kidney disease (Schmitz and Ruebsaamen, 2010; Grossmayer et al., 2017; Koenen, 2019; Liu et al., 2020; Yoshioka et al., 2022). As the present study, clearly demonstrated the potential of choline chloride in inhibiting the LPC induced response. It might be promising in managing these diseases.
5. Conclusion
Choline chloride attenuates the allergic response by inhibiting the LPC induced NKT cells activation, proliferation and so AHR, inflammation and oxidative stress by providing competitive inhibition to LPC in binding to CD1d.
CRediT authorship contribution statement
Preeti Bansal: contributed to the conception and design of the experiments; drafting the article for important intellectual content; acquisition, analysis, and interpretation of in vivo, in vitro, and in silico data; and in the final approval of the version Naresh Singh: contributed to design of the experiments; acquisition, analysis and interpretation of in vivo and in vitro data; and in the final approval of the version. Jayadev Joshi: contributed to design of the experiments, acquisition, analysis, and interpretation of in silico data; drafting the article for important intellectual content; and in the final approval of the version. Naveen Arora: contributed to conception and design of the experiments; drafting the article for important intellectual content; and in the final approval of the version. Shailendera N. Gaur: contributed to the conception and design of the experiments; drafting the article for important intellectual content; and in the final approval of the version.This statement has also been added to the manuscript.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The authors thank Mr. Unice Bhat (trainee at CSIR-IGIB), Bharti Arora (Ph.D. Scholar at CSIR-IGIB), and Ekta Nagar (Project assistant at CSIR-IGIB) for their help in mice sacrifice experiments. The authors also thank the Council of Scientific and Industrial Research (CSIR, Govt of India, Delhi) for financial support. One of the authors (Preeti Bansal) has been a ‘Senior Research Fellow’ of the Council of Scientific and Industrial Research, Delhi.
Contributor Information
Preeti Bansal, Email: priiiti1984@gmail.com.
Naresh Singh, Email: nareshsolanki91@gmail.com.
Jayadev Joshi, Email: jaidev53ster@gmail.com.
Naveen Arora, Email: naveen@igib.res.in.
Shailendera N. Gaur, Email: sngaur9@gmail.com, sngaurA@yahoo.com.
References
- Agrawal K.P., Nath P. Raised serum lysolecithin and cholesteroyl ester levels in atopic states. Indian J. Chest Dis. Allied Sci. 1978;20(1):5–10. [PubMed] [Google Scholar]
- Bai T.R. Beta 2 adrenergic receptors in asthma: a current perspective. Lung. 1992;170(3):125–141. doi: 10.1007/BF00174316. [DOI] [PubMed] [Google Scholar]
- Bansal P., Gaur S.N., Arora N. Lysophosphatidylcholine plays critical role in allergic airway disease manifestation. Sci. Rep. 2016;6:27430. doi: 10.1038/srep27430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bansal P., Saw S., Govindaraj D., Arora N. Intranasal administration of a combination of choline chloride, vitamin C, and selenium attenuates the allergic effect in a mouse model of airway disease. Free Radic. Biol. Med. 2014;73:358–365. doi: 10.1016/j.freeradbiomed.2014.05.018. [DOI] [PubMed] [Google Scholar]
- Blusztajn J.K. Choline, a vital amine. Science. 1998;281(5378):794–795. doi: 10.1126/science.281.5378.794. [DOI] [PubMed] [Google Scholar]
- Chang M.W., Ayeni C., Breuer S., Torbett B.E. Virtual screening for Hiv protease inhibitors: a comparison of Autodock 4 and Vina. PLoS One. 2010;5(8) doi: 10.1371/journal.pone.0011955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chilton F.H., Averill F.J., Hubbard W.C., Fonteh A.N., Triggiani M., Liu M.C. Antigen-induced generation of lyso-phospholipids in human airways. J. Exp. Med. 1996;183(5):2235–2245. doi: 10.1084/jem.183.5.2235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degousee N., Ghomashchi F., Stefanski E., Singer A., Smart B.P., Borregaard N., Reithmeier R., Lindsay T.F., Lichtenberger C., Reinisch W., Lambeau G., Arm J., Tischfield J., Gelb M.H., Rubin B.B. Groups Iv, V, and X phospholipases A2s in human neutrophils: role in eicosanoid production and gram-negative bacterial phospholipid hydrolysis. J. Biol. Chem. 2002;277(7):5061–5073. doi: 10.1074/jbc.M109083200. [DOI] [PubMed] [Google Scholar]
- Fuchs B., Schiller J., Wagner U., Hantzschel H., Arnold K. The phosphatidylcholine/lysophosphatidylcholine ratio in human plasma is an indicator of the severity of rheumatoid arthritis: investigations by 31p Nmr and Maldi-Tof Ms. Clin. Biochem. 2005;38(10):925–933. doi: 10.1016/j.clinbiochem.2005.06.006. [DOI] [PubMed] [Google Scholar]
- Ganley O.H., Graessle O.E., Robinson H.J. Anti-inflammatory activity on compounds obtained from egg yolk, peanut oil, and soybean lecithin. J. Lab. Clin. Med. 1958;51(5):709–714. [PubMed] [Google Scholar]
- Garg A., Tewari R., Raghava G.P. Kidoq: using docking based energy scores to develop ligand based model for predicting antibacterials. BMC Bioinf. 2010;11:125. doi: 10.1186/1471-2105-11-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaur S.N., Agarwal G., Gupta S.K. Use of Lpc antagonist, choline, in the management of bronchial asthma. Indian J. Chest Dis. Allied Sci. 1997;39(2):107–113. [PubMed] [Google Scholar]
- Ghesquiere S.A., Gijbels M.J., Anthonsen M., Van Gorp P.J., Van D.M., Johansen B., Hofker M.H., De Winther M.P. Macrophage-specific overexpression of group Iia spla2 increases atherosclerosis and enhances collagen deposition. J. Lipid Res. 2005;46(2):201–210. doi: 10.1194/jlr.M400253-JLR200. I. [DOI] [PubMed] [Google Scholar]
- Giabbai B., Sidobre S., Crispin M.D., Sanchez-Ruiz Y., Bachi A., Kronenberg M., Wilson I.A., Degano M. Crystal structure of mouse Cd1d bound to the self ligand phosphatidylcholine: a molecular basis for Nkt cell activation. J. Immunol. 2005;175(2):977–984. doi: 10.4049/jimmunol.175.2.977. [DOI] [PubMed] [Google Scholar]
- Giannattasio G., Lai Y., Granata F., Mounier C.M., Nallan L., Oslund R., Leslie C.C., Marone G., Lambeau G., Gelb M.H., Triggiani M. Expression of phospholipases A2 in primary human lung macrophages: role of cytosolic phospholipase A2-alpha in arachidonic acid release and platelet activating factor synthesis. Biochim. Biophys. Acta. 2009;1791(2):92–102. doi: 10.1016/j.bbalip.2008.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossmayer G.E., Keppeler H., Boeltz S., Janko C., Rech J., Herrmann M., Lauber K., Munoz L.E. Elevated serum lysophosphatidylcholine in patients with systemic lupus erythematosus impairs phagocytosis of necrotic cells in vitro. Front. Immunol. 2017;8:1876. doi: 10.3389/fimmu.2017.01876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta S.K., Gaur S.N. A placebo controlled trial of two dosages of Lpc antagonist--choline in the management of bronchial asthma. Indian J. Chest Dis. Allied Sci. 1997;39(3):149–156. [PubMed] [Google Scholar]
- Koenen R.R. Lysophosphatidylcholine in platelet microvesicles: the grease for cardiovascular disease. Thromb.Haemost. 2019;119(8):1202–1204. doi: 10.1055/s-0039-1693024. [DOI] [PubMed] [Google Scholar]
- Kume H., Ito S., Ito Y., Yamaki K. Role of lysophosphatidylcholine in the desensitization of beta-adrenergic receptors by Ca(2+) sensitization in tracheal smooth muscle. Am. J. Respir. Cell Mol. Biol. 2001;25(3):291–298. doi: 10.1165/ajrcmb.25.3.4364. [DOI] [PubMed] [Google Scholar]
- Liu P., Zhu W., Chen C., Yan B., Zhu L., Chen X., Peng C. The mechanisms of lysophosphatidylcholine in the development of diseases. Life Sci. 2020;247:117443. doi: 10.1016/j.lfs.2020.117443. [DOI] [PubMed] [Google Scholar]
- Lombardi V., Stock P., Singh A.K., Kerzerho J., Yang W., Sullivan B.A., Li X., Shiratsuchi T., Hnatiuk N.E., Howell A.R., Yu K.O., Porcelli S.A., Tsuji M., Kronenberg M., Wilson S.B., Akbari O. A Cd1d-dependent antagonist inhibits the activation of invariant Nkt cells and prevents development of allergen-induced airway hyperreactivity. J. Immunol. 2010;184(4):2107–2115. doi: 10.4049/jimmunol.0901208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Sagaseta J., Sibener L.V., Kung J.E., Gumperz J., Adams E.J. Lysophospholipid presentation by Cd1d and recognition by a human Natural Killer T-cell receptor. EMBO J. 2012;31(8):2047–2059. doi: 10.1038/emboj.2012.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta A.K., Arora N., Gaur S.N., Singh B.P. Choline supplementation reduces oxidative stress in mouse model of allergic airway disease. Eur. J. Clin. Invest. 2009;39(10):934–941. doi: 10.1111/j.1365-2362.2009.02190.x. [DOI] [PubMed] [Google Scholar]
- Mehta A.K., Gaur S.N., Arora N., Singh B.P. Effect of choline chloride in allergen-induced mouse model of airway inflammation. Eur. Respir. J. 2007;30(4):662–671. doi: 10.1183/09031936.00019307. [DOI] [PubMed] [Google Scholar]
- Mehta A.K., Singh B.P., Arora N., Gaur S.N. Choline attenuates immune inflammation and suppresses oxidative stress in patients with asthma. Immunobiology. 2010;215(7):527–534. doi: 10.1016/j.imbio.2009.09.004. [DOI] [PubMed] [Google Scholar]
- Mehta D., Gupta S., Gaur S.N., Gangal S.V., Agrawal K.P. Increased leukocyte phospholipase A2 activity and plasma lysophosphatidylcholine levels in asthma and rhinitis and their relationship to airway sensitivity to histamine. Am. Rev. Respir. Dis. 1990;142(1):157–161. doi: 10.1164/ajrccm/142.1.157. [DOI] [PubMed] [Google Scholar]
- Murphy A.A., Santanam N., Morales A.J., Parthasarathy S. Lysophosphatidyl choline, a chemotactic factor for monocytes/T-lymphocytes is elevated in endometriosis. J. Clin. Endocrinol. Metab. 1998;83(6):2110–2113. doi: 10.1210/jcem.83.6.4823. [DOI] [PubMed] [Google Scholar]
- Niewoehner D.E., Rice K., Sinha A.A., Wangensteen D. Injurious effects of lysophosphatidylcholine on barrier properties of alveolar epithelium. J. Appl. Physiol. 1987;63(5):1979–1986. doi: 10.1152/jappl.1987.63.5.1979. 1985. [DOI] [PubMed] [Google Scholar]
- Nishiyama O., Kume H., Kondo M., Ito Y., Ito M., Yamaki K. Role of lysophosphatidylcholine in eosinophil infiltration and resistance in airways. Clin. Exp. Pharmacol. Physiol. 2004;31(3):179–184. doi: 10.1111/j.1440-1681.2004.03973.x. [DOI] [PubMed] [Google Scholar]
- Nobata K., Kurashima K., Fujimura M., Abo M., Ishiura Y., Kasahara K., Nakao S. Inhaled lysophosphatidylcholine provokes bronchoconstriction in Guinea pigs in vivo. Eur. J. Pharmacol. 2005;520(1–3):150–155. doi: 10.1016/j.ejphar.2005.07.032. [DOI] [PubMed] [Google Scholar]
- O'boyle N.M., Banck M., James C.A., Morley C., Vandermeersch T., Hutchison G.R. Open Babel: an open chemical toolbox. J.Cheminform. 2011;3:33. doi: 10.1186/1758-2946-3-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oleinika K., Rosser E.C., Matei D.E., Nistala K., Bosma A., Drozdov I., Mauri C. Cd1d-dependent immune suppression mediated by regulatory B cells through modulations of inkt cells. Nat. Commun. 2018;9(1):684. doi: 10.1038/s41467-018-02911-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ossani G., Dalghi M., Repetto M. Oxidative damage lipid peroxidation in the kidney of choline-deficient rats. Front. Biosci. 2007;12:1174–1183. doi: 10.2741/2135. [DOI] [PubMed] [Google Scholar]
- Pelech S.L., Vance D.E. Regulation of phosphatidylcholine biosynthesis. Biochim. Biophys. Acta. 1984;779(2):217–251. doi: 10.1016/0304-4157(84)90010-8. [DOI] [PubMed] [Google Scholar]
- Perrin-Cocon L., Agaugue S., Coutant F., Saint-Mezard P., Guironnet-Paquet A., Nicolas J.F., Andre P., Lotteau V. Lysophosphatidylcholine is a natural adjuvant that initiates cellular immune responses. Vaccine. 2006;24(9):1254–1263. doi: 10.1016/j.vaccine.2005.09.036. [DOI] [PubMed] [Google Scholar]
- Pniewska E., Pawliczak R. The involvement of phospholipases A2 in asthma and chronic obstructive pulmonary disease. Mediat. Inflamm. 2013;2013:793505. doi: 10.1155/2013/793505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryborg A.K., Gron B., Kragballe K. Increased lysophosphatidylcholine content in lesional psoriatic skin. Br. J. Dermatol. 1995;133(3):398–402. doi: 10.1111/j.1365-2133.1995.tb02667.x. [DOI] [PubMed] [Google Scholar]
- Sasagawa T., Suzuki K., Shiota T., Kondo T., Okita M. The significance of plasma lysophospholipids in patients with renal failure on hemodialysis. J. Nutr. Sci. Vitaminol. 1998;44(6):809–818. doi: 10.3177/jnsv.44.809. [DOI] [PubMed] [Google Scholar]
- Saw S., Arora N. Pi3k and Erk1/2 kinase inhibition potentiate protease inhibitor to attenuate allergen induced Th2 immune response in mouse. Eur. J. Pharmacol. 2016;776:176–184. doi: 10.1016/j.ejphar.2016.02.050. [DOI] [PubMed] [Google Scholar]
- Schmitz G., Ruebsaamen K. Metabolism and atherogenic disease association of lysophosphatidylcholine. Atherosclerosis. 2010;208(1):10–18. doi: 10.1016/j.atherosclerosis.2009.05.029. [DOI] [PubMed] [Google Scholar]
- Seeliger D., De Groot B.L. Ligand docking and binding site analysis with Pymol and Autodock/Vina. J. Comput. Aided Mol. Des. 2010;24(5):417–422. doi: 10.1007/s10822-010-9352-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi A., Yoshinari M., Iino K., Wakisaka M., Iwase M., Fujishima M. Lysophosphatidylcholine molecular species in low density lipoprotein and high density lipoprotein in alloxan-induced diabetic rats: effect of probucol. Exp. Clin. Endocrinol. Diabetes. 1999;107(6):337–342. doi: 10.1055/s-0029-1212123. [DOI] [PubMed] [Google Scholar]
- Shi A.H., Yoshinari M., Wakisaka M., Iwase M., Fujishima M. Lysophosphatidylcholine molecular species in low density lipoprotein of type 2 diabetes. Horm. Metab. Res. 1999;31(4):283–286. doi: 10.1055/s-2007-978734. [DOI] [PubMed] [Google Scholar]
- Sudha V.T., Arora N., Sridhara S., Gaur S.N., Singh B.P. Biopotency and identification of allergenic proteins in Periplaneta americana extract for clinical applications. Biologicals. 2007;35(2):131–137. doi: 10.1016/j.biologicals.2006.07.004. [DOI] [PubMed] [Google Scholar]
- Touqui L., Herpin-Richard N., Gene R.M., Jullian E., Aljabi D., Hamberger C., Vargaftig B.B., Dessange J.F. Excretion of platelet activating factor-acetylhydrolase and phospholipase A2 into nasal fluids after allergenic challenge: possible role in the regulation of platelet activating factor release. J. Allergy Clin. Immunol. 1994;94(1):109–119. doi: 10.1016/0091-6749(94)90077-9. [DOI] [PubMed] [Google Scholar]
- Triggiani M., Giannattasio G., Calabrese C., Loffredo S., Granata F., Fiorello A., Santini M., Gelb M.H., Marone G. Lung mast cells are a source of secreted phospholipases A2. J. Allergy Clin. Immunol. 2009;124(3):558–565. doi: 10.1016/j.jaci.2009.04.035. 565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trott O., Olson A.J. Autodock Vina: improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010;31(2):455–461. doi: 10.1002/jcc.21334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wadehra N.R., Chhabra S.K., Gaur S.N., Joshi A.P., Agrawal K.P. Abnormalities of lipid metabolism in asthma and rhinitis--a comprehensive study. Indian J. Chest Dis. Allied Sci. 1987;29(3):131–137. [PubMed] [Google Scholar]
- Wang Y., Su D.M., Wang R.H., Liu Y., Wang H. Antinociceptive effects of choline against acute and inflammatory pain. Neuroscience. 2005;132(1):49–56. doi: 10.1016/j.neuroscience.2004.12.026. [DOI] [PubMed] [Google Scholar]
- Williams S.G., Schmidt D.K., Redd S.C., Storms W. Key clinical activities for quality asthma care. Recommendations of the national asthma education and prevention program. MMWR Recomm. Rep. (Morb. Mortal. Wkly. Rep.) 2003;52(Rr-6):1–8. [PubMed] [Google Scholar]
- Yen C.L., Mar M.H., Zeisel S.H. Choline deficiency-induced apoptosis in Pc12 cells is associated with diminished membrane phosphatidylcholine and sphingomyelin, accumulation of ceramide and diacylglycerol, and activation of a caspase. Faseb. J. 1999;13(1):135–142. [PubMed] [Google Scholar]
- Yoder M., Zhuge Y., Yuan Y., Holian O., Kuo S., Van B.R., Thomas L.L., Lum H. Bioactive lysophosphatidylcholine 16:0 and 18:0 are elevated in lungs of asthmatic subjects. Allergy Asthma Immunol.Res. 2014;6(1):61–65. doi: 10.4168/aair.2014.6.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida Y., Itoh N., Hayakawa M., Habuchi Y., Inoue R., Chen Z.H., Cao J., Cynshi O., Niki E. Lipid peroxidation in mice fed a choline-deficient diet as evaluated by total hydroxyoctadecadienoic acid. Nutrition. 2006;22(3):303–311. doi: 10.1016/j.nut.2005.07.020. [DOI] [PubMed] [Google Scholar]
- Yoshioka K., Hirakawa Y., Kurano M., Ube Y., Ono Y., Kojima K., Iwama T., Kano K., Hasegawa S., Inoue T., Shimada T., Aoki J., Yatomi Y., Nangaku M., Inagi R. Lysophosphatidylcholine mediates fast decline in kidney function in diabetic kidney disease. Kidney Int. 2022;101(3):510–526. doi: 10.1016/j.kint.2021.10.039. [DOI] [PubMed] [Google Scholar]
- Zeisel S.H. Choline: needed for normal development of memory. J. Am. Coll. Nutr. 2000;19(5 Suppl. l):528s–531s. doi: 10.1080/07315724.2000.10718976. [DOI] [PubMed] [Google Scholar]
- Zhang B., Fan P., Shimoji E., Itabe H., Miura S., Uehara Y., Matsunaga A., Saku K. Modulating effects of cholesterol feeding and simvastatin treatment on platelet-activating factor acetylhydrolase activity and lysophosphatidylcholine concentration. Atherosclerosis. 2006;186(2):291–301. doi: 10.1016/j.atherosclerosis.2005.07.029. [DOI] [PubMed] [Google Scholar]