Abstract
Objective
The inflammatory cascade caused by severe acute respiratory syndrome coronavirus 2 infection may result in arterial thrombosis and acute limb ischemia (ALI) with devastating consequences. The aims of this study were to compare outcomes of ALI in the lower extremities in patients with and without coronavirus disease 2019 (COVID-19), and to determine if ALI development in the context of COVID-19 portends a worse prognosis compared with COVID-19 without ALI.
Methods
Queries were built on TriNetX, a federated network of health care organizations across the United States that provides de-identified patient data. International Classification of Diseases, 10th revision diagnostic codes were used to identify patients with acute limb ischemia of the lower extremities and COVID-19. The study timeframe was defined as January 20, 2020 to May 20, 2021. Statistical analyses, including propensity-score matching, were done through TriNetX’s internal software. Outcomes looked at are rates of mortality, stroke, myocardial infarction, major adverse limb events, re-intervention, respiratory failure, sepsis, mental health complications, and acute renal failure. Baseline cohort characteristics were also collected.
Results
Patients with ALI with COVID-19 (ALI C19+; n = 526) were significantly less likely than patients with ALI without COVID-19 (ALI; n = 14,131) to have baseline comorbidities, including nicotine dependence (18% vs 33%; P < .0001). In contrast, ALI C19+ patients had significantly more comorbidities than hospitalized patients with COVID-19 without ALI (n = 275,903), including nicotine dependence (18% vs 10%; P < .0001). After propensity matching was performed, ALI C19+ patients had significantly higher rates of mortality (24.9% vs 9.2%; P < .0001), major adverse limb events (5.8% vs 2.9%; P = .0223), and acute renal failure (22.2% vs 14.9%; P = .0025) than patients with ALI. Compared with hospitalized patients with COVID-19 without ALI, ALI C19+ patients had higher propensity-matched rates of respiratory failure and being placed on assisted ventilation (32.9% vs 27%; P = .0369), sepsis (16.9% vs 12.2%; P = .0288), acute renal failure (22.1% vs 14.6%; P = .0019), and mortality (24.7% vs 14.4%; P < .0001).
Conclusions
Patients who developed ALI following COVID-19 present with significantly different demographics and comorbidities from those who develop ALI without COVID-19. After controlling for these variables, higher rates of major adverse limb events, acute renal failure, and mortality in patients with ALI with COVID-19 suggest that not only may COVID-19 precipitate ALI, but it may also exacerbate ALI sequelae. Furthermore, development of ALI in COVID-19 portends worse prognosis compared with patients with COVID-19 without ALI.
Keywords: Acute limb ischemia, Arterial thromboembolism, Coronavirus, COVID-19, SARS-CoV-2
Article Highlights.
-
•
Type of Research: Multicenter, retrospective, propensity score-matched study
-
•
Key Findings: Patients with acute limb ischemia (ALI) with coronavirus disease 2019 (COVID-19) (n = 526) were compared with those without COVID-19 (n = 14,131). After propensity matching, patients with ALI with COVID-19 had a higher mortality rate (24.857% vs 9.178%; P < .0001), major adverse limb events (5.763% vs 2.868%; P = .0223), and acute renal failure (22.180% vs 14.914%; P < .0001).
-
•
Take Home Message: These findings suggest that patients with ALI with COVID-19 have significantly different patient demographics and comorbidities than both patients with classical ALI and patients with COVID-19 without ALI and experienced higher rates of adverse clinical outcomes than patients with ALI without COVID-19.
Since the first case of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) was reported in the United States on January 20, 2020, progress has been made in understanding its pathogenicity.
Current understanding of SARS-CoV-2 has proposed that it binds to angiotensin-converting enzyme (ACE) 2 receptors, causing significant inflammation. SARS-CoV-2 bound to these receptors on vascular endothelial cells causes endothelial injury and triggers a pro-inflammatory and hypercoagulable state.1 Abdominal and thoracic aortic thrombosis, mesenteric ischemia, and acute cerebrovascular incident have also been described as manifestations of COVID-19 infection.2 Acute limb ischemia (ALI), a vascular pathology with multifactorial etiology, is a known complication caused by the inflammatory cascade triggered by SARS-CoV-2 viral infection.3 , 4 Although hypercoagulability is a rare cause of limb ischemia, the incidence of thromboembolic events in patients with COVID-19 is as high as 35% to 45%.5 Several observational studies have found that patients with COVID-19 and ALI experience poor outcomes, including high rates of amputation and high failure rates of revascularization.3 , 4 However, the characteristics of patients presenting with ALI following COVID-19 compared with characteristics of patients presenting with ALI alone have not been delineated. Furthermore, the degree to which COVID-19 exacerbates ALI sequelae, and the prognostic value of ALI development in COVID-19 compared with COVID-19 alone, has not been shown. As such, the purpose of this multicenter, retrospective cohort study was to compare the outcomes of ALI in patients with COVID-19 with patients with ALI without COVID-19.
Methods
Data source
Data for this study was obtained from TriNetX’s COVID-19 Research Network platform, a federated research network of electronic health record data from 63 health care organizations (HCOs) across the United States. The network provides access to real-time aggregate data from approximately 83.8+ million patients, including demographics, diagnoses, procedures, medications, lab values, and genomics. The HCOs that comprise the research network include primary care providers, specialists, and hospitals that care for both insured and uninsured patients. The geographical distribution of patients in the database are as follows: 18% from the Northeast, 14% from the Midwest, 26% from the South, and 30% from the West.
Any data on the TriNetX platform in aggregate form only contains de-identified data, adhering to the standard defined in Section §164.514(a) of the Health Insurance Portability and Accountability Act of 1996 (HIPAA) Privacy Rule. TriNetX’s de-identification process was attested through a formal determination by a qualified expert as defined in Section §164.514(b)(1) of the HIPAA Privacy Rule, superseding the need for TriNetX’s previous waiver from the Western Institutional Review Board.6 Written patient consent was waived. Because this study was not involved in the collection, use, or transmittal of individually identifiable data, this study was exempted from Albert Einstein College of Medicine and Montefiore Medical Center Institutional Review Board approval. This multicenter, retrospective cohort study followed the REporting of studies Conducted using Observational Routinely-collected Data (RECORD) guidelines.7
Study protocol
The study timeframe was defined as January 20, 2020, to May 20, 2021. The first reported case of SARS-CoV-2 in the United States was on January 20, 2020, and the first reported case of the Delta variant in the United States was towards the end of May 2021.8 , 9 The emergence of the Delta variant was set as the end point of the study because of its markedly different pathogenicity from its parent strains.8
International Classification of Diseases, 10th Revision (ICD-10) codes were used to identify eligible patients as seen in Supplementary Table I (online only). ICD-10 codes were linked to the dates the events occurred. Mainly, ALI of the lower extremities was defined as thrombosis of the arteries of the lower extremities (I74.3), iliac artery (I74.5), or saddle embolus of the abdominal aorta (I74.01). COVID-19 positivity was defined as having a record of a positive SARS-CoV-2 test (9088) or diagnosis of COVID-19 (U07.1). Furthermore, patients were identified as having COVID-19 if they had records of unspecified coronavirus infection within the study timeframe (B34.2), pneumonia due to SARS-associated coronavirus (J12.81), and coronavirus as the cause of diseases classified elsewhere (B97.29). Previous electronic health record studies on COVID-19 included the latter ICD-10 codes (ie, B34.2, J12.81, B97.29) because there was no established code for COVID-19 early in the pandemic.
Patients with COVID-19 who developed ALI (ALI C19+) were identified by looking at the temporal relationship between the ICD-10 codes for ALI and COVID-19. Namely, these patients must have had a diagnosis of ALI either 1 day before, or within 1 week after COVID-19 diagnosis/positivity. This temporal relationship was decided upon because a prior study found ALI to develop around 1 week after COVID-19.10 Furthermore, patients may have had an incidental COVID-19 finding if they were initially admitted for ALI; hence, including patients with diagnosis of ALI 1 day before COVID-19 diagnosis/positivity. Patients who developed ALI without concurrent COVID-19 were identified by excluding all patients who had a record of COVID-19. This meant that patients who had COVID-19 months prior to diagnosis of ALI were also excluded, reasoning that the long-term effects of COVID-19 have not been fully studied. Patients who were hospitalized for COVID-19 and did not develop ALI were identified by excluding any instance of ALI after COVID-19 diagnosis, again reasoning that the long-term effects of COVID-19 have not been fully studied.
The ALI C19+ and ALI without COVID-19 cohorts were stratified into those who had an arterial revascularization procedure performed and those who did not. Arterial revascularization procedures included endovascular, bypass, and embolectomy/thrombectomy/endarterectomy techniques (Supplementary Table I, online only). We defined late surgical intervention as patients who did not have record of intervention within 1 week of ALI, but subsequently had an intervention during the follow-up period of 180 days. Because this was a rare outcome, we defined late surgical intervention as a composite of endovascular, bypass, and embolectomy/thrombectomy/endarterectomy techniques (Supplementary Table I, online only). The follow-up period was defined as 180 days, and outcomes followed were mortality, stroke, myocardial infarction, major adverse cardiovascular events (composite of mortality, stroke, myocardial infarction), major adverse limb events (MALE; amputations), acute renal failure, reintervention rates, respiratory failure or assisted ventilation, sepsis, and mental health complications (Supplementary Table I, online only).
Statistical methods
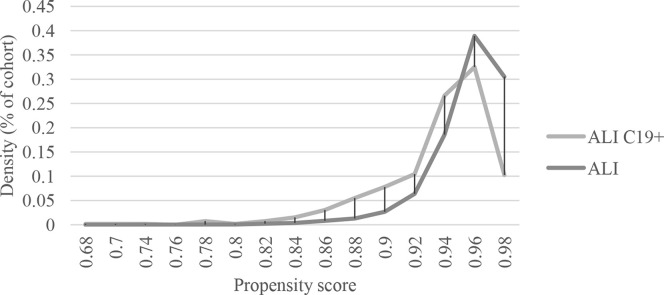
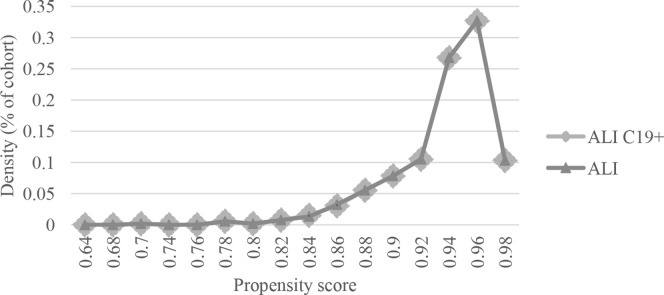
All statistical analyses, including 1:1 propensity-score matching, were performed with TriNetX’s internal software, which uses R 3.4.4 (R Foundation for Statistical Computing, Vienna, Austria) and Python 3.6.5 (Python Software Foundation, Centrum voor Wiskunde en Informatica Amsterdam, The Netherlands). Greedy nearest neighbor matching with a caliper width of 0.1 pooled standard deviations of the logit of the propensity scores in aggregate was used; standard difference less than 0.1 was considered well match.11 Propensity matching was performed for age, sex, ethnicity, medications, and comorbidities (Supplementary Table II, online only). Propensity score distributions before and after matching were reported (Figs 1 and 2 ). Descriptive statistics were expressed as means with standard deviations. Unpaired t tests were used to compare means between the cohorts. Odds ratios (ORs) with 95% confidence intervals (CIs) were reported, and P-values < .05 were considered statistically significant.
Fig 1.
Propensity score density function of patients with acute limb ischemia with coronavirus 2019 (ALI C19+) vs patients with acute limb ischemia (ALI) before matching.
Fig 2.
Propensity score density function of patients with acute limb ischemia with coronavirus 2019) ALI C19+ vs patients with acute limb ischemia (ALI) after matching.
Results
Characteristics of ALI C19+ patients compared with patients with ALI without C19
A total of 526 patients were identified in the group with ALI C19+ and 14,131 in the group with ALI without COVID-19 (Table I ). 120 patients who had ALI following COVID-19 were not included because they did not meet the 1-week criteria. Unpaired t tests were performed between the two cohorts. The mean age for the groups was similar (65.2 ± 14.7 vs 65.6 ± 13.8; P = .5109). The gender distribution was not statistically different between the two groups (64% vs 59% male; P = .0534). Interestingly, there was a higher proportion of Hispanic patients in the ALI C19+ group (11% vs 4%; P < .0001) and a higher proportion of Caucasian patients in the COVID-19 negative group (64% vs 69%; P = .0052). There was no difference in the proportion of African Americans between the two groups (16% vs 13%; P = .0508).
Table I.
Characteristics of patients who developed acute limb ischemia (ALI) from coronavirus 2019 (COVID-19) vs those who developed ALI without COVID-19
| ALI C19+ (n = 526) | ALI (n = 14,131) | P value | |
|---|---|---|---|
| Demographics | |||
| Age, years | 65.2 ± 14.7 | 65.6 ± 13.8 | .5109 |
| Male | 64 | 59 | .0534 |
| Hispanic or Latino | 11 | 4 | <.0001 |
| Black/African American | 16 | 13 | .0508 |
| White | 64 | 69 | .0052 |
| Comorbidities | |||
| Primary hypertension | 51 | 60 | <.0001 |
| Secondary hypertension | 2 | 2 | .6972 |
| Atrial fibrillation and flutter | 17 | 18 | .6773 |
| Type 1 diabetes mellitus | 5 | 4 | .5260 |
| Type 2 diabetes mellitus | 34 | 30 | .0420 |
| Overweight and obesity | 19 | 16 | .1097 |
| Chronic ischemic heart disease | 28 | 37 | .0001 |
| COPD | 14 | 20 | .0004 |
| Asthma | 7 | 7 | .9350 |
| Obstructive sleep apnea | 7 | 9 | .2067 |
| Nicotine dependence | 18 | 33 | <.0001 |
| Mental, behavioral, and neurodevelopmental disorders | 42 | 50 | .0004 |
| Neoplasms | 19 | 27 | <.0001 |
| Medications | |||
| Aspirin | 35 | 49 | <.0001 |
| Atorvastatin | 29 | 38 | <.0001 |
| Simvastatin | 6 | 8 | .1454 |
| Losartan | 12 | 12 | .8458 |
| Oral hypoglycemic agents | 15 | 16 | .2787 |
| Anticoagulants | 56 | 56 | .8458 |
| ACE inhibitors | 20 | 29 | <.0001 |
| Beta blockers | 41 | 49 | .0003 |
ACE, Angiotensin-converting enzyme; C19+, COVID-19-positive; COPD, chronic obstructive pulmonary disease.
Data are presented as percentage or mean ± standard deviation.
Boldface P values indicate statistical significance.
There were many significant differences in baseline comorbid conditions between these two groups. Primary hypertension (51% vs 60%; P < .0001), chronic ischemic heart disease (28% vs 37%; P = .0001), chronic obstructive pulmonary disease (COPD) (14% vs 20%; P = .0004), psychiatric disorders (42% vs 50%; P = .0004), and neoplasms (19% vs 27%; P < .0001) were all seen at significantly higher rates in patients with ALI without COVID-19. In the ALI C19+ patients, significantly increased rate of type 2 diabetes mellitus (34% vs 30%; P = .0420) was seen. Nicotine dependence was seen at a two-fold higher rate in patients with ALI without COVID-19 (18% vs 33%; P < .0001).
Patients with ALI without COVID-19 were more likely to be on baseline aspirin (35% vs 49%; P < .0001, atorvastatin (29% vs 38%; P < .0001), ACE inhibitors (20% vs 29%; P < .0001), and beta blockers (41% vs 49%; P = .0003).
Outcomes of ALI C19+ patients compared with those without COVID-19
Propensity matching was performed in addition to unpaired t tests. Before propensity matching, patients with COVID-19 and ALI had worse outcomes at 180 days (Table II ). There was a three-fold increase in mortality (24.715% vs 8.598%; OR, 3.490; P < .0001), a two-fold increase in MALE (5.894% vs 2.696%; OR, 2.260; P < .0001), and a 2.5-fold increase in acute renal failure (22.053% vs 13.347%; OR, 1.837; P < .0001) in the ALI C19+ group. This trend remained significant after propensity matching, with a three-fold increase in mortality (24.857% vs 9.178%; OR, 3.273; P < .0001), two-fold increase in MALE (5.763% vs 2.868%; OR, 2.061; P = .0223), and a nearly two-fold increase in acute renal failure (22.180% vs 14.914%; OR, 1.626; P = .0025).
Table II.
Outcomes of acute limb ischemia (ALI) in patients with coronavirus 2019 (COVID-19) and without COVID-19
| Before propensity matching |
After propensity matching (for age, sex, ethnicity, comorbidities, and medications) |
|||||
|---|---|---|---|---|---|---|
| 180-day outcomes | ALI C19+, % (n = 526) | ALI, % (n = 14,131) | OR (95% CI); P value | ALI C19+, % (n = 523) | ALI, % (n = 523) | OR (95% CI); P value |
| Mortality | 24.715 (130) | 8.598 (1215) | 3.49 (2.838-4.291); P < .0001 | 24.857 (130) | 9.178 (48) | 3.273 (2.291-4.678); P < .0001 |
| Stroke | 7.985 (42) | 7.077 (1000) | 1.139 (0.826-1.572); P = .4261 | 8.031 (42) | 7.266 (38) | 1.114 (0.706-1.759); P = .6417 |
| Myocardial infarction | 7.034 (37) | 5.902 (834) | 1.206 (0.857-1.697); P = .2808 | 7.057 (37) | 5.163 (27) | 1.399 (0.838-2.333); P = .1970 |
| Major adverse limb event | 5.894 (31) | 2.696 (381) | 2.260 (1.550-3.295); P < .0001 | 5.763 (30) | 2.868 (15) | 2.061 (1.095-3.878); P = .0223 |
| Acute renal failure | 22.053 (116) | 13.347 (1886) | 1.837 (1.486-2.270); P < .0001 | 22.180 (116) | 14.914 (78) | 1.626 (1.184-2.232); P = .0025 |
C19+, COVID-19-positive; CI, confidence interval; OR, odds ratio.
Boldface values indicate statistical significance.
Outcomes for ALI C19+ patients versus those with ALI alone undergoing arterial procedures
Unpaired t tests, without propensity matching, were performed. After propensity matching, there were too few patients remaining in the ALI C19+ group for adequate statistical comparison.
There was a small proportion of patients in each group that initially underwent arterial procedures in this subset, with 89 patients in the ALI C19+ group and 2768 patients in the ALI group (Table III ).
Table III.
Outcomes of patients with acute limb ischemia (ALI) with coronavirus 2019 (COVID-19) and without COVID-19, who initially underwent an arterial procedure for revascularization
| 180-day outcomes | ALI C19+, % (n = 89) | ALI, % (n = 2768) | OR (95% CI); P value |
|---|---|---|---|
| Major adverse cardiovascular events (death, myocardial infarction, cerebral infarction) | 25.843 (23) | 19.725 (546) | 1.418 (0.874-2.300); P = .1549 |
| Major adverse limb events (amputation) | 15.730 (14) | 8.02 (222) | 2.141 (1.190-3.850); P = .0093 |
| Acute renal failure | 22.472 (20) | 14.884 (412) | 1.510 (1.016-2.243); P = .0492 |
| Reintervention-endovascular | 17.978 (16) | 13.403 (371) | 1.416 (0.815-2.459); P = .2145 |
| Reintervention-thromboendarterectomy, embolectomy, thrombectomy | 31.461 (28) | 4.986 (138) | 8.748 (5.418-14.124); P < 0001 |
| Reintervention-bypass | 11.236 (10) | 5.636 (156) | 2.119 (1.077-4.173); P = .0262 |
C19+, COVID-19-positive; CI, confidence interval; OR, odds ratio.
Boldface values indicate statistical significance.
One-to-one propensity matching was unable to be performed because of TriNetX’s data obfuscation policy when patient sample sizes decrease below a specific threshold.
ALI C19+ patients were seen to have significantly higher rates of MALE (15.730% vs 8.020%; OR, 2.141; P = .0093) and acute renal failure (22.472% vs 14.884%; OR, 1.510; P = .0492).
Open reintervention with thromboendarterectomy, embolectomy, and/or thrombectomy was over eight-fold higher in ALI C19+ patients (31.461% vs 4.986%; OR, 8.748; P < .0001). Reintervention with bypass surgery was also seen at a significantly higher rate in ALI C19+ patients (11.236% vs 5.636%; OR, 2.119; P = .0262). There was no statistically significant difference in the rate of endovascular reintervention between the two groups.
Outcomes for ALI C19+ patients who did not undergo arterial procedures
Unpaired t tests, without propensity matching, were performed. After propensity matching, there were too few patients remaining in the ALI C19+ group for adequate statistical comparison. Major adverse cardiovascular events were seen at a greater than two-fold higher rate in the ALI C19+ group who did not undergo arterial interventions (33.410% vs 16.700%; OR, 2.503; P < .0001) (Table IV ). In patients with COVID-19 without initial revascularization, there were significantly higher rates of MALE (3.432% vs 1.356%; OR, 2.586; P = .0003), acute renal failure (21.739% vs 12.756%; OR, 1.900; P < .0001). Interestingly, there was a significantly lower rate in ALI C19+ patients needing surgical intervention later (2.746% vs 5.573%; OR, 0.478; P = .0108).
Table IV.
Outcomes of patients with acute limb ischemia (ALI) with coronavirus 2019 (COVID-19) and without COVID-19, who did not initially undergo an arterial procedure for revascularization
| 180-day outcomes | ALI C19+, % (n = 437) | ALI, % (n = 10,186) | OR (95% CI); P value |
|---|---|---|---|
| Major adverse cardiovascular events (death, myocardial infarction, cerebral infarction) | 33.410 (146) | 16.700 (1897) | 2.503 (2.039-3.071); P < .0001 |
| Major adverse limb events (amputation) | 3.432 (15) | 1.356 (154) | 2.586 (1.509-4.433); P = .0003 |
| Acute renal failure | 21.739 (95) | 12.756 (1449) | 1.900 (1.504-2.400); P < .0001 |
| Intervention | 2.746 (12) | 5.573 (633) | 0.478 (0.268-0.854); P = .0108 |
C19+, COVID-19-positive; CI, confidence interval; OR, odds ratio.
Boldface values indicate statistical significance.
One-to-one propensity matching was unable to be performed because of TriNetX’s data obfuscation policy when patient sample sizes decrease below a specific threshold.
Characteristics of ALI C19+ patients compared with hospitalized C19+ patients without ALI
Unpaired t tests were performed between the two cohorts. When compared with hospitalized C19+ patients who did not develop ALI (n = 275,903), ALI C19+ patients were significantly older (65.2 ± 14.7 vs 57.5 ± 19; P < .0001) (Table V ). Furthermore, ALI C19+ patients were more likely to be male (64% vs 45%; P < .0001) and more likely to be white (64% vs 59%; P = .0199). However, a smaller proportion of ALI C19+ patients were Hispanic compared with patients with COVID-19 without ALI (11% vs 15%; P < .0001).
Table V.
Characteristics of patients with coronavirus 2019 (COVID-19) who developed acute limb ischemia (ALI) versus hospitalized patients with COVID-19 who did not develop ALI
| C19+ with ALI (n = 526) | C19+ without ALI (n = 275,903) | P value | |
|---|---|---|---|
| Demographics | |||
| Age, years | 65.2 ± 14.7 | 57.5 ± 19 | <.0001 |
| Male | 64 | 45 | <.0001 |
| Hispanic or Latino | 11 | 15 | <.0001 |
| Black/African American | 16 | 14 | .1426 |
| White | 64 | 59 | .0199 |
| Comorbidities | |||
| Primary hypertension | 51 | 40 | <.0001 |
| Secondary hypertension | 2 | 1 | .2644 |
| Atrial fibrillation and flutter | 17 | 10 | <.0001 |
| Type 1 diabetes mellitus | 5 | 3 | .0010 |
| Type 2 diabetes mellitus | 34 | 22 | <.0001 |
| Overweight and obesity | 19 | 22 | .0533 |
| Chronic ischemic heart disease | 28 | 15 | <.0001 |
| COPD | 14 | 8 | <.0001 |
| Asthma | 7 | 10 | .0165 |
| Obstructive sleep apnea | 7 | 10 | .0751 |
| Nicotine dependence | 18 | 10 | <.0001 |
| Mental, behavioral, and neurodevelopmental disorders | 42 | 35 | .0026 |
| Neoplasms | 19 | 21 | .2981 |
| Medications | |||
| Aspirin | 35 | 26 | <.0001 |
| Atorvastatin | 29 | 18 | <.0001 |
| Simvastatin | 6 | 5 | .1454 |
| Losartan | 12 | 9 | .8458 |
| Oral hypoglycemic agents | 15 | 13 | .2787 |
| Anticoagulants | 56 | 34 | .8458 |
| ACE inhibitors | 20 | 17 | <.0001 |
| Beta blockers | 41 | 30 | .0003 |
ACE, Angiotensin-converting enzyme; C19+, COVID-19-positive; COPD, chronic obstructive pulmonary disease.
Data are presented as percentage or mean ± standard deviation.
Boldface P values indicate statistical significance.
Furthermore, ALI C19+ patients had higher rates of primary hypertension (51% vs 40%; P < .0001), atrial fibrillation and flutter (17% vs 10%; P < .0001), type 1 diabetes mellitus (5% vs 3%; P < .0010), type 2 diabetes mellitus (34% vs 22%; P < .0001), chronic ischemic heart disease (28% vs 15%; P < .0001), COPD (14% vs 8%; P < .0001), nicotine dependence (18% vs 10%; P < .0001), and psychiatric disorders (42% vs 35%; P = .0026). However, ALI C19+ patients had lower rates of asthma compared with patients with COVID-19 who did not develop ALI (7% vs 10%; P = .0165).
ALI C19+ patients were more likely to be on baseline aspirin (35% vs 26%; P < .0001), atorvastatin (29% vs 18%; P < .0001), ACE inhibitors (20% vs 17%; P < .0001), and beta blockers (41% vs 30%; P = .0003) than patients with COVID-19 who did not develop ALI.
Outcomes of ALI C19+ patients compared with hospitalized C19+ patients without ALI
t tests with propensity-score matching were utilized. Following propensity matching for age, sex, ethnicity, comorbidities, and medications, ALI C19+ patients had higher 180-day rates of mortality (24.715% vs 14.449%; OR, 1.944; P < .0001), acute renal failure (22.053% vs 14.639%; OR, 1.650; P = .0019), respiratory failure or being placed on assisted ventilation (32.890% vs 26.996%; OR, 1.325; P = .0369), and sepsis (16.920% vs 12.167%; OR, 1.470; P = .0288) (Table VI ). Rates of stroke, myocardial infarction, and psychiatric complications were not significantly different.
Table VI.
Propensity-matched outcomes of patients with coronavirus 2019 (COVID-19) who developed acute limb ischemia (ALI) vs patients with COVID-19 who did not develop ALI
| 180-day outcomes | ALI C19+, % (n = 526) | C19+, % (n = 526) | OR (95% CI); P value |
|---|---|---|---|
| Mortality | 24.715 (130) | 14.449 (76) | 1.944 (1.421-2.660); P < .0001 |
| Stroke | 7.985 (42) | 5.323 (28) | 1.543 (0.941-2.530); P = .833 |
| Myocardial infarction | 7.034 (37) | 6.464 (34) | 1.095 (0.676-1.773); P = .7124 |
| Acute renal failure | 22.053 (116) | 14.639 (77) | 1.65 (1.201-2.267); P = .0019 |
| Mental health complications | 17.300 (91) | 14.449 (76) | 1.239 (0.889-1.726); P = .2057 |
| Respiratory failure or Assisted ventilation | 32.890 (173) | 26.996 (142) | 1.325 (1.017-1.727); P = .0369 |
| Sepsis | 16.920 (89) | 12.167 (64) | 1.470 (1.039-2.080); P = .0288 |
C19+, COVID-19-positive; CI, confidence interval; OR, odds ratio.
Boldface values indicate statistical significance.
Discussion
Prior studies have demonstrated that SARS-CoV-2 is associated with a hypercoagulable state caused by virally-induced vascular endothelial injury.5 , 12 , 13 Due to this procoagulant state, there is a high risk for macro- and micro-thrombi formation in patients with COVID-19.13 , 14 Many of the thromboembolic events associated with COVID-19 are venous in nature, but growing evidence has also shown an increased risk of arterial thrombotic events in patients with COVID-19, especially ALI.3 , 15, 16, 17 Galyfos et al utilized pooled data from multiple case studies to show that COVID-associated ALI presents in patients with low incidence of comorbidities and is associated with a high mortality and amputation risk, but their conclusions were limited by low sample sizes.18 Using national data, our study demonstrates that ALI C19+ patients face worse clinical outcomes compared with patients with ALI without a COVID-19 diagnosis, suggesting that COVID-19 may not only precipitate ALI but may be directly responsible for exacerbating ALI sequelae.
Our analysis found that the demographics of patients who developed ALI following COVID-19 infection were significantly different from the characteristic demographics of those who presented with ALI alone. Because TriNetX displays aggregate data from institutions throughout the nation, the unmatched comparisons capture the trend that patients who developed ALI following COVID-19 had significantly higher rates of mortality, MALE, and acute renal failure despite lower rates of comorbidities. Even after propensity matching, we found that patients who developed ALI in the setting of COVID-19 had a two-fold higher rate of having MALE, 1.6-fold higher rate of having acute kidney injury, and 3.3-fold higher rate of death (Table II). Although acute kidney injury and mortality are not unique to ALI, major adverse limb events are. We can say then that, independent of comorbidities, patients who developed ALI in the setting of COVID-19 have roughly two-fold higher rates of major amputation compared with patients solely with ALI. These clinical outcomes are consistent with our understanding of how SARS-CoV-2 affects the vascular system and other organs. Patients with COVID-19 have been found to have abnormally elevated coagulation markers including D-dimer, partial thromboplastin time, prothrombin time, fibrinogen, fibrin degradation products, and interleukin-6.5 , 15 , 19 Previous studies note that diffuse, small vessel platelet-fibrin thrombi and intravascular megakaryocytes were found in all major organs of patients with COVID-19, including the heart, lungs, kidney, liver, and mesenteric fat.13 Menter et al also found that post-mortem examination of patients with COVID-19 showed renal tubular injury, interstitial edema, and fibrin thrombi in glomerular capillaries.20 Other studies have also illustrated vascular pathological changes such as vascular endothelial shedding, intimal inflammation, and thrombosis in patients with COVID-19.21 In addition, in the United States, studies have previously shown that with increasing revascularization rates, amputations have drastically decreased in cases of critical limb ischemia.22 , 23 Unfortunately, in the case of patients with COVID-19, amputation may have been the best treatment option due to delayed presentation to medical care or rapidly progressing disease.24
Finally, we found that rates of MALE and acute renal failure were found to be higher in the ALI C19+ cohort that underwent arterial procedures than those without COVID-19 who underwent similar procedures. ALI C19+ patients also had higher rates of open reintervention (thromboendarterectomy, embolectomy, and/or thrombectomy) and bypass surgery. However, there was no difference in rate of endovascular reintervention between the groups. Successful revascularization has been documented to be relatively low in patients with COVID-19 compared with previously reported series.3 Bellosta et al also postulated that their revascularization failure rate of almost 30% was due to the absence of forefoot microvasculature following intervention or potential sudden early recurrent thrombosis in their ALI C19+ patients. In addition, it is possible that poor clinical status of ALI C19+ patients prevented proper recovery following intervention, leading to postoperative complications. Early recognition of ischemic thrombotic events in patients with COVID-19 and more aggressive anticoagulant and thrombolytic treatment may help prevent such serious adverse events in ALI C19+ patients.
Given that COVID-19 appears to exacerbate ALI sequelae, it was important to characterize the ALI C19+ population and compare it with hospitalized patients with COVID-19 who do not develop ALI. Relative to hospitalized patients with COVID-19 who do not develop ALI, ALI C19+ patients notably had higher rates of hypertension and diabetes mellitus. This is consistent with existing knowledge that hypertension and diabetes mellitus are main risk factors for limb ischemia.25 Furthermore, development of ALI appears to suggest worse prognosis in patients with COVID-19. In addition to mortality and acute renal failure, development of ALI led to higher rates of respiratory failure or assisted ventilation and sepsis.
There are certain limitations to this study. The TriNetX platform does not represent the general population, but rather only represents those who sought medical care at the 63 HCOs in the network. Patients who do not receive follow-up care at participating HCOs can also skew occurrence of outcomes. Propensity matching for some of our data set was limited by TriNetX’s internal statistical analysis software and obfuscation policy. Safeguards against queries that could identify small subsets of cohorts are put in place to minimize the risk of patient re-identification.26 There are also always inaccuracies inherent to electronic health record data collection, mainly coding or data entry errors. We attempted to minimize any errors with strict inclusion and exclusion criteria, with particular focus on the temporality between the COVID-19 diagnosis and acute ischemic event. Lastly, the influence of thrombophylactic or therapeutic-anticoagulating regimes prior to development of ALI could not be assessed. As the pandemic progressed, many institutions developed their own guidelines with regards to stratifying patients with COVID-19 to receive thromboprophylaxis, therapeutic anticoagulation, or neither. Future studies should examine whether anticoagulation initiation in patients with COVID-19 prior to development of ALI affects outcomes.
Conclusions
Before and after controlling for covariates, rates of mortality, MALE, and acute renal failure were significantly higher in ALI C19+ patients than in patients with ALI alone. This suggests that COVID-19, independent of the patients’ comorbidities, may directly exacerbate ALI sequelae. Furthermore, development of ALI suggests a worse prognosis in COVID-19 than in COVID-19 alone, with higher rates of mortality, renal failure, sepsis, and respiratory failure. Further studies are warranted to delineate a pathophysiologic link between COVID-19 and development of acute arterial thromboembolic events.
Author Contributions
Conception and design: AP, EG, EL, JI
Analysis and interpretation: AP, AH, EG, EL, JI
Data collection: AP
Writing the article: AP, AH
Critical revision of the article: EG, EL, JI
Final approval of the article: AP, AH, EG, EL, JI
Statistical analysis: Not applicable
Obtained funding: Not applicable
Overall responsibility: JI
From the New England Society for Vascular Surgery
Footnotes
Author conflict of interest: none.
The editors and reviewers of this article have no relevant financial relationships to disclose per the JVS policy that requires reviewers to decline review of any manuscript for which they may have a conflict of interest.
Additional material for this article may be found online at www.jvascsurg.org.
Additional material for this article may be found online at www.jvascsurg.org.
Appendix (online only).
Supplementary Table I (online only).
International Classification of Disease, 10th Revision (ICD-10) codes used to identify eligible patients
| Outcome/revascularization procedure | Corresponding ICD-10/procedure code | Notes |
|---|---|---|
| Mortality | Registered as deceased | |
| Stroke | I61-I63, G45.9 | Ischemic and hemorrhagic stroke |
| Myocardial infarction | I21 | |
| Major adverse limb events | 1004982, 1005146, 1005298 | Amputation procedures on pelvis, hip, femur, knee, leg and ankle joint |
| Acute renal failure | N17 | |
| Major adverse cardiac events | Deceased, I61-63, G45.9, I21 | Composite of death, stroke, and myocardial infarction |
| Respiratory failure or assisted ventilation | J96, 1014859 | |
| Sepsis | A40, A41, R65.2 | |
| Mental health complications | F43.1, F32, F33, G47, F41.1 | PTSD, depressive episode, major depressive disorder (recurrent), sleep disorders, generalized anxiety disorders |
| Reintervention | Composite of revascularization procedures | |
| Embolectomy or thrombectomy, with or without catheter | 34151 | Renal, celiac, mesentery, aortoiliac artery, by abdominal incision |
| 34201 | Femoropopliteal, aortoiliac artery, by leg incision | |
| 34203 | Popliteal-tibio-peroneal artery, by leg incision | |
| Repair blood vessel, direct | 35221 | Intra-abdominal |
| 35226 | Lower extremity | |
| Repair blood vessel with vein graft | 35251 | Intra-abdominal |
| 35256 | Lower extremity | |
| 35281 | Intra-abdominal | |
| 35286 | Lower extremity | |
| Thromboendarterectomy, including patch graft, when performed | 35302 | Superficial femoral artery |
| 35303 | Popliteal artery | |
| 35304 | Tibioperoneal trunk artery | |
| 35305 | Tibial or peroneal artery, initial vessel | |
| 35306 | Each additional tibial or peroneal artery | |
| 35351 | Iliac | |
| 35355 | Iliofemoral | |
| 35361 | Combined aortoiliac | |
| 35363 | Combined aortoiliofemoral | |
| 35371 | Common femoral | |
| 35372 | Deep femoral | |
| Bypass graft, with vein | 35533 | Axillary-femoral-femoral |
| 35537 | Aortoiliac | |
| 35538 | Aortobi-iliac | |
| 35539 | Aortofemoral | |
| 35540 | Aortobifemoral | |
| 35556 | Femoral-popliteal | |
| 35558 | Femoral-femoral | |
| 35563 | Ilioiliac | |
| 35565 | Iliofemoral | |
| 35566 | Femoral-anterior tibila, posterior tibial, peroneal artery, or other distal vessels | |
| 35570 | Tibial-tibial, peroneal-tibial, or tibial/peroneal trunk-tibial | |
| 35571 | Popliteal-tibial, -peroneal artery or other distal vessels | |
| In-situ vein bypass | 35583 | Femoral-popliteal |
| 35585 | Posterior tibial, or peroneal artery | |
| 35587 | Popliteal-tibial, peroneal | |
| Bypass graft, with other than vein | 35621 | Axillary-femoral |
| 35623 | Axillary-popliteal or -tibial | |
| 35637 | Aortoiliac | |
| 35638 | Aortobi-iliac | |
| 35646 | Aortobifemoral | |
| 35647 | Aortofemoral | |
| 35654 | Axillary-femoral-femoral | |
| 35656 | Femoral-popliteal | |
| 35661 | Femoral-femoral | |
| 35663 | Ilioiliac | |
| 35665 | Iliofemoral | |
| 35666 | Femoral-anterior tibial, posterior tibial, or peroneal artery | |
| 35671 | Popliteal-tibial or peroneal artery | |
| Arterial mechanical thrombectomy | 37184 | Primary percutaneous transluminal mechanical thrombectomy, noncoronary, non-intracranial, arterial or arterial bypass graft, including fluoroscopic guidance and intraprocedural pharmacological thrombolytic injection(s); initial vessel |
| 37185 | Primary percutaneous transluminal mechanical thrombectomy, noncoronary, non-intracranial, arterial or arterial bypass graft, including fluoroscopic guidance and intraprocedural pharmacological thrombolytic injection(s); initial vessel; second and all subsequent vessel(s) within the same vascular family | |
| 37186 | Secondary percutaneous transluminal thrombectomy (eg, nonprimary mechanical, snare basket, suction technique), noncoronary, nonintracranial, arterial or arterial bypass graft, including fluoroscopic guidance and intraprocedural pharmacological thrombolytic injections, provided in conjunction with another percutaneous intervention other than primary mechanical thrombectomy | |
| Revascularization, endovascular, open or percutaneous, iliac artery, unilateral, initial vessel | 37220 | With transluminal angioplasty |
| 37221 | With transluminal stent placement(s), includes angioplasty within the same vessel, when performed | |
| Revascularization, endovascular, open or percutaneous, iliac artery, each additional ipsilateral iliac vessel | 37222 | With transluminal angioplasty |
| 37223 | With transluminal stent placement(s), includes angioplasty within the same vessel, when performed | |
| Revascularization, endovascular, open or percutaneous, femoral, popliteal artery(s), unilateral | 37224 | With transluminal angioplasty |
| 37225 | With atherectomy, includes angioplasty within the same vessel, when performed | |
| 37226 | With transluminal stent placement(s), includes angioplasty within the same vessel, when performed | |
| 37227 | With transluminal stent placement(s) and atherectomy, includes angioplasty within the same vessel, when performed | |
| Revascularization, endovascular, open or percutaneous, tibial, peroneal artery, unilateral, initial vessel | 37228 | With transluminal angioplasty |
| 37229 | With atherectomy, includes angioplasty within the same vessel, when performed | |
| 37230 | With transluminal stent placement(s), includes angioplasty within the same vessel, when performed | |
| 37231 | With transluminal stent placement(s) and atherectomy, includes angioplasty within the same vessel, when performed | |
| Revascularization, endovascular, open or percutaneous, tibial/peroneal artery, unilateral, each additional vessel | 37232 | With transluminal angioplasty |
| 37233 | With atherectomy, includes angioplasty within the same vessel, when performed | |
| 37234 | With transluminal stent placement(s), includes angioplasty within the same vessel, when performed | |
| 37235 | With transluminal stent placement(s) and atherectomy, includes angioplasty within the same vessel, when performed |
Supplementary Table II (online only).
Covariates used in propensity matching
| Covariate | Notes |
|---|---|
| Age | |
| Sex | |
| Ethnicity | |
| Comorbidities | Primary hypertension, secondary hypertension, atrial fibrillation, T1DM, T2DM, overweight/obesity, chronic ischemic heart disease, COPD, asthma, obstructive sleep apnea, nicotine dependence, mental/behavioral/neurodevelopmental disorders, neoplasms |
| Medications | Aspirin, atorvastatin, simvastatin, losartan, oral hypoglycemic agents, anticoagulants, ACE inhibitors, beta blockers |
ACE, Angiotensin-converting enyme; COPD, chronic obstructive pulmonary disease; T1DM, type 1 diabetes mellitus; T2DM, type 2 diabetes mellitus.
References
- 1.Wool G.D., Miller J.L. The impact of COVID-19 disease on platelets and coagulation. Pathobiology. 2021;88:15–27. doi: 10.1159/000512007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Avila J., Long B., Holladay D., Gottlieb M. Thrombotic complications of COVID-19. Am J Emerg Med. 2021;39:213–218. doi: 10.1016/j.ajem.2020.09.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bellosta R., Luzzani L., Natalini G., Pegorer M.A., Attisani L., Cossu L.G., et al. Acute limb ischemia in patients with COVID-19 pneumonia. J Vasc Surg. 2020;72:1864–1872. doi: 10.1016/j.jvs.2020.04.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sánchez J.B., Cuipal Alcalde J.D., Ramos Isidro R., Zuniga Luna C., Samir Cubas W., Coaguila Charres A., et al. Acute limb ischemia in a peruvian cohort infected by COVID-19. Ann Vasc Surg. 2021;72:196–204. doi: 10.1016/j.avsg.2020.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Levi M., Thachil J., Iba T., Levy J.H. Coagulation abnormalities and thrombosis in patients with COVID-19. Lancet Haematol. 2020;7:e438–e440. doi: 10.1016/S2352-3026(20)30145-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.TriNetX Publication guidelines. https://trinetx.com/trinetx-publication-guidelines/ Available at:
- 7.Benchimol E.I., Smeeth L., Guttmann A., Harron K., Moher D., Peersen I., et al. RECORD Working Committee The REporting of studies Conducted using Observational Routinely-collected health Data (RECORD) Statement. PLOS Med. 2015;12:e1001885. doi: 10.1371/journal.pmed.1001885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dougherty K., Mannell M., Naqvi O., Matson D., Stone J. SARS-CoV-2 B.1.617.2 (Delta) variant COVID-19 outbreak associated with a gymnastics facility — Oklahoma, April–May 2021. Morb Mortal Wkly Rep. 2021;70:1004–1007. doi: 10.15585/mmwr.mm7028e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Holshue M.L., DeBolt C., Lindquist S., Lofy K.H., Wiesman J., Bruce H., et al. Washington State 2019-nCoV Case Investigation Team. First case of 2019 novel coronavirus in the United States. N Engl J Med. 2020;382:929–936. doi: 10.1056/NEJMoa2001191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Al-zoubi N., Shatnawi N., Jarbo H. Acute lower limb ischemia in patients infected with COVID-19. Int J Gen Med. 2021;14:833–839. doi: 10.2147/IJGM.S301462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Haukoos J.S., Lewis R.J. The propensity score. JAMA. 2015;314:1637–1638. doi: 10.1001/jama.2015.13480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Singhania N., Bansal S., Nimmatoori D.P., Ejaz A.A., McCullough P.A., Singhania G. Current overview on hypercoagulability in COVID-19. Am J Cardiovasc Drugs. 2020;20:393–403. doi: 10.1007/s40256-020-00431-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fox S.E., Akmatbekov A., Harbert J.L., Li G., Quincy Brown J., Vander Heide R.S. Pulmonary and cardiac pathology in African American patients with COVID-19: an autopsy series from New Orleans. Lancet Respir Med. 2020;8:681–686. doi: 10.1016/S2213-2600(20)30243-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wichmann D., Sperhake J.P., Lütgehetmann M., Steurer S., Edler C., Heinemann A., et al. Autopsy findings and venous thromboembolism in patients with COVID-19: a prospective cohort study. Ann Intern Med. 2020;173:268–277. doi: 10.7326/M20-2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Anwar S., Acharya S., Shabih S., Khabut A. Acute limb ischemia in COVID-19 disease: a mysterious coagulopathy. Cureus. 2020;12:e9167. doi: 10.7759/cureus.9167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Etkin Y., Conway A.M., Silpe J., Qato K., Carroccio A., Manvar-Singh P., et al. Acute arterial thromboembolism in patients with COVID-19 in the New York City Area. Ann Vasc Surg. 2021;70:290–294. doi: 10.1016/j.avsg.2020.08.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goldman I.A., Ye K., Scheinfeld M.H. Lower-extremity arterial thrombosis associated with COVID-19 is characterized by greater thrombus burden and increased rate of amputation and death. Radiology. 2020;297:E263–E269. doi: 10.1148/radiol.2020202348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Galyfos G., Sianou A., Frountzas M., Vasilios K., Vouros D., Theodoropoulos C., et al. Acute limb ischemia among patients with COVID-19 infection. J Vasc Surg. 2022;75:326–342. doi: 10.1016/j.jvs.2021.07.222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tang N., Li D., Wang X., Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost. 2020;18:844–847. doi: 10.1111/jth.14768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Menter T., Haslbauer J.D., Nienhold R., Savic S., Hopfer H., Deigendesch N., et al. Postmortem examination of COVID-19 patients reveals diffuse alveolar damage with severe capillary congestion and variegated findings in lungs and other organs suggesting vascular dysfunction. Histopathology. 2020;77:198–209. doi: 10.1111/his.14134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Varga Z., Flammer A.J., Steiger P., Haberecker M., Andermatt R., Zinkernagel A., et al. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020;395:1417–1418. doi: 10.1016/S0140-6736(20)30937-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hallett J.W., Byrne J., Gayari M.M., Ilstrup D.M., Jacobsen S.J., Gray D.T. Impact of arterial surgery and balloon angioplasty on amputation: a population-based study of 1155 procedures between 1973 and 1992. J Vasc Surg. 1997;25:29–38. doi: 10.1016/s0741-5214(97)70318-5. [DOI] [PubMed] [Google Scholar]
- 23.Nowygrod R., Egorova N., Greco G., Anderson P., Gelijins A., Moskowitz A., et al. Trends, complications, and mortality in peripheral vascular surgery. J Vasc Surg. 2006;43:205–216. doi: 10.1016/j.jvs.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 24.Biswal J.K., Mohanty S.K., Behera S.N., Swain S.K., Sahoo A.K. Acute limb ischemia: a catastrophic COVID-19 sequel leading to amputation. Cureus. 2021;13:e16456. doi: 10.7759/cureus.16456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brooks M., Jenkins M.P. Acute and chronic ischaemia of the limb. Surgery (Oxford) 2008;26:17–20. [Google Scholar]
- 26.Topaloglu U., Palchuk M.B. Using a federated network of real-world data to optimize clinical trials operations. JCO Clin Cancer Inform. 2018;2:1–10. doi: 10.1200/CCI.17.00067. [DOI] [PMC free article] [PubMed] [Google Scholar]